Abstract
Problem
Need to decrease the number of requests for arterial blood gas analysis and increase their appropriateness to reduce the amount of blood drawn from patients, the time wasted by nurses, and the related cost.
Design
Assessment of the impact of a multifaceted intervention aimed at changing requests for arterial blood gas analysis in a before and after study.
Background and setting
Twenty bed surgical intensive care unit of a tertiary university affiliated hospital, receiving 1500 patients per year.
Key measures for improvement
Number of tests per patient day, proportion of tests complying with current guideline, and safety indicators (mortality, incident rate, length of stay). Comparison of three 10 month periods corresponding to baseline, pilot (first version of the guideline), and consolidated (second version of the guideline) periods from March 1997 to August 1999.
Strategies for change
Multifaceted intervention combining a new guideline developed by a multidisciplinary group, educational sessions, and monthly feedback about adherence to the guideline and use of blood gas analysis.
Effects of change
Substantial decrease in the number of tests per patient day (from 8.2 to 4.8; P<0.0001), associated with increased adherence to the guideline (from 53% to 80%, P<0.0001). No significant variation of safety indicators.
Lessons learnt
A multifaceted intervention can substantially decrease the number of requests for arterial blood gas analysis and increase their appropriateness without affecting patient safety.
Introduction
Expenditure due to laboratory testing increases continuously and represents up to 25% of the cost of caring for patients in intensive care units.1 Intensive care medicine accounts for a considerable proportion of hospital resources,2 and its cost rises as new therapeutic and diagnostic methods are developed.3 Blood tests can induce iatrogenic anaemia in patients,4,5 are time consuming for staff, and are costly. Arterial blood gas analysis is the most commonly performed laboratory test in intensive care units.6,7 Indications for analysis are often left to clinical judgment without reference to any written protocol.
Background
Outline of problem
In our surgical intensive care unit, 46 000 arterial blood gas analyses were performed each year. A one week prospective study showed that over half of these tests could not be justified clinically. In addition, 96% of requests were left to the discretion of the nursing staff, while clinical signs such as respiratory rate or altered pattern of breathing were seldom taken into account in deciding whether the test was necessary. Values of percutaneous oxygen saturation from pulse oximetry were rarely used, even though they match arterial measurements. These findings led us to set up an intervention aimed at reducing the number of requests.
Outline of context
The intervention was conducted in the surgical intensive care unit of a 1200 bed tertiary, university affiliated hospital. The 20 bed unit receives 1500 patients per year for postoperative care after major surgery, multiple trauma, neurosurgery, and organ transplantation for a total of 5300 hospital days. The team comprises three senior intensive care specialists, 10 physicians in training, and 71 nurses (69% certified in intensive care nursing, 15% in second year training, and 15% in first year training). Blood gas analysis is performed at the patient's bedside with three Stat Profile Ultra machines (NOVA biomedical, Waltham, MA) located in the unit. All patients are equipped with a pulse oximeter for monitoring of percutaneous oxygen saturation. Capnography is not used in the unit because it is not reliable in critically ill patients.8 Arterial catheters are inserted in most patients as clinically required.
Assessment of problems
Detail of approach
Guidelines and feedback have been proposed in the literature as a means of changing ordering habits for routine blood gas analysis. A guideline specifies the rules to apply when performing analyses and helps the user to make an adequate decision.9–11 Feedback, either concurrent12,13 or retrospective,14 allows users to correct their behaviour. It can be provided on a regular basis12,13 or triggered by thresholds.14 It can focus on adherence to rules13 or on impact on volumes14 or costs12 of laboratory tests.
To improve the use of blood gas analysis, we designed a multifaceted intervention15 that combined a new guideline, educational sessions, and feedback. To achieve consensus of all involved parties and facilitate acceptance of the guideline, this intervention was carried out by a multidisciplinary group.16
Guideline development
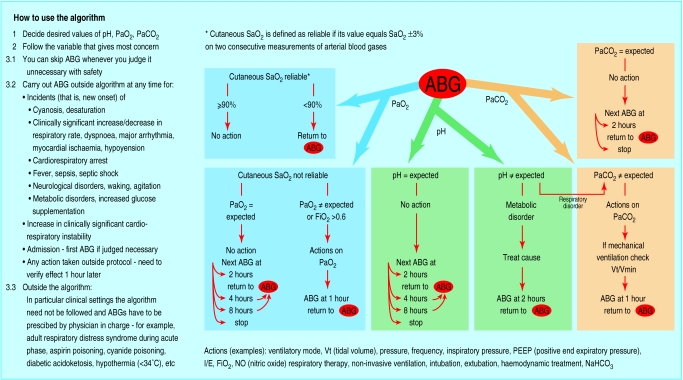
A pilot version of the guideline was designed locally by a group comprising one intensive care consultant, one intensive care senior registrar, three nurses (certified and in training), and one respiratory therapist. From October to December 1997, the group met weekly to define the decision making process underlying the measurement of three blood gas parameters (pH, Pao2, Paco2). The guideline was developed on a consensus basis17 as an extensive literature search in the Cochrane Library and Medline did not identify any suitable guideline.
The pilot guideline, which included a comprehensive time frame and the use of pulse oximetry, was devised as an algorithm based on three pathways corresponding to pH, Pao2, and Paco2 and included a minimum number of three mandatory analyses per day for safety reasons. The guideline was approved by the two other unit consultants.
To incorporate the opinion and the experience of the users we amended the pilot version of the guideline ten months later. In this consolidated version, the time frames were widened and the daily mandatory tests removed. We added a list of clinical settings in which the algorithm should not be applied. The consolidated guideline was validated by four critical care experts from outside the unit (fig 1).
Figure 1.
Algorithm for requests for arterial blood gas analysis (translated from French)
Intervention
Both pilot and consolidated guidelines were distributed as pocket-sized documents and were displayed next to blood gas analysers. They were introduced to all team members in the surgical intensive care unit during 45 minute teaching sessions twice a week over one month. In addition, all new team members were individually instructed when they arrived in the unit.
Feedback was given to the whole team by using two indicators. The adherence indicator revealed deviations from the guideline and the blood gas indicator showed the impact of increased adherence, defined here as appropriateness,11 on the use of blood gas analysis. Both indicators were presented systematically each month, plotted as time series charts, displayed on walls, and published in the unit information bulletin.
Measurement of problem
Intervention assessment and data collection
We compared three consecutive ten month periods (baseline, pilot, consolidated). They were separated by the dissemination of the pilot version and the consolidated version of the guideline, respectively. Data were obtained retrospectively for the baseline period and prospectively for the pilot and consolidation periods and tabulated monthly.
A first set of data was obtained by sampling one day a month (the audit day). For all patients who were in the unit on that day, two investigators independent of the care team collected data on age, the simplified acute physiology score, version 2 (SAPS II)18 as a measurement of the severity of illness, and the type of surgery. They individually assessed the proportion of blood gas analyses that complied with the guideline (adherence). All results were checked between the two observers and each disagreement was discussed to achieve consensus. As undetected severe acidaemia or hypoxaemia might lead to major cardiac arrhythmia, cardiac or respiratory arrests, new episodes of pH <7.25, or systolic systemic blood pressure <80 mm Hg, any such incidents were recorded by the attending nurse on the electronic chart. Two independent reviewers computed the number of incidents per day (incident rate) at the end of the patient's stay in the unit to assess the safety of the implemented procedure. Results were compared between the two reviewers, and all discrepancies were double checked and corrected. For patients who stayed long enough to be involved in two consecutive audits, the incident rate from one audit was used.
A second set of data consisted of aggregates extracted from the hospital information system for the whole unit. We tabulated the monthly average number of blood gas analyses per patient day, the average number of all other tests performed per patient day (other tests), and the average length of stay. In addition, we extracted from the hospital information system, the mortality for the whole unit population for the three periods. For a given month, the average adherence observed during the corresponding audit day served as adherence indicator, whereas number of tests per patient day was used as the arterial blood gas indicator.
Cost-benefit analysis
We calculated benefits associated with increased appropriateness of requests in terms of amount of blood taken (5 ml per test), nurse working days (4 minutes per test), number of analyses per patient day, and corresponding costs (SFr 20 (£8) per test). We assessed the cost of the whole intervention, including meetings, teaching sessions, and reviews, in terms of collaborators' time (number of hours).
Statistical analysis
To perform statistical comparisons, we grouped monthly data together within each period. All variables were compared by the Kruskal-Wallis test except for adherence, mortality, and type of surgery, for which we used Pearson's χ2 test. Data analyses were performed with SPSS release 9.0. P <0.05 was considered significant.
This study was approved by our institution's ethical committee for human research, who authorised a waiving of informed consent. The whole programme was followed and supervised by the Hospital Quality of Care Unit.
Results of assessment
Feedback
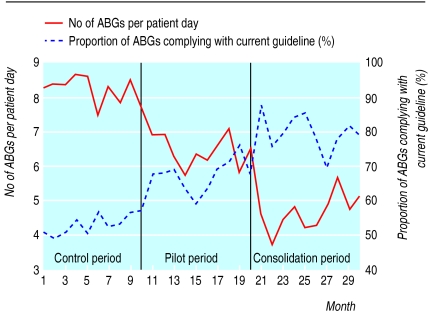
Figure 2 shows the time series chart reported monthly to the whole team. It shows the regular decrease of the blood gas analysis indicator and the concomitant increase of the adherence indicator after the introduction of the pilot and subsequently the consolidated guideline.
Figure 2.
Monthly change in absolute number of arterial blood gas analyses per patient day and proportion of tests adhering to current guideline from baseline (before implementation of pilot guideline), pilot period (after introduction of pilot guideline), and consolidation (after implementation of consolidated guideline)
Impact of the intervention
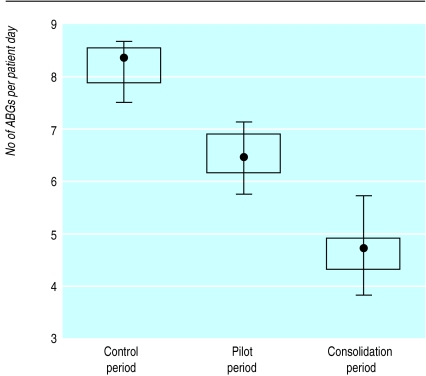
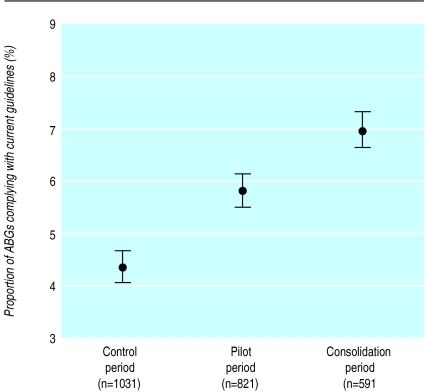
During the three periods, the characteristics of the patient population studied remained unchanged with regard to age, acute physiology score, and type of surgery (table). Between baseline and pilot periods, the average number of blood gas analyses decreased from 8.2 to 6.5 per patient day (fig 3), whereas the average adherence rose from 53% to 68% (fig 4). Between pilot and consolidation periods, the average number of tests further decreased from 6.5 to 4.8 (fig 3) and the average adherence rose from 68% to 80% (fig 4).
Figure 3.
Number of arterial blood gas analyses per patient day per 10 month period (median with minimum and maximum values; 25th and 75th centiles indicated by box plot). P<0.001 for comparison of three periods (Kruskal-Wallis)
Figure 4.
Adherence to guideline per 10 month period (percentage with 95% confidence intervals). P<0.0001 for comparison periods (Pearson's χ2)
The numbers of all other tests performed in the unit did not vary significantly during pilot and consolidation periods (mean (SD) 204, 242, and 222 for the three periods respectively, P=0.05). The incident rate, length of stay, and mortality remained unchanged throughout the study (table).
Cost-benefit analysis
Per patient day, this reduction resulted in savings of 8.5 ml and 17 ml of patients' blood, 6.8 and 13.6 minutes of nurse working time, and SFr 34.8 (£14.15) and SFr 68.4 (£27.81) in test costs for the two periods, respectively. The global reduction in requests for blood gas analysis during the 20 month study therefore resulted in savings equivalent to 112 litres of patients' blood, 187 nurse working days, and SFr 452 600 (£180 369).
The total cost of the intervention was SFr 79 050 (£31 502) (or SFr 9 (£3.60) per patient day), representing 710 hours for nurses, 270 hours for respiratory therapists, and 400 hours for physicians. Follow up of the process—that is, monthly collection and report of the number of tests—can be carried out by one person at negligible cost (four hours a month).
Lessons learnt
Human factor specialists have shown that deficiencies of attention or reasoning are common and that even experienced and responsible professionals can err. To prevent errors, the use of an explicit model such as a guideline is essential in complex decisions.19 However, guidelines can be rejected because of their content or format, especially if users are not involved in their design. Allowing users to participate in the revision of this guideline facilitated its acceptance in our project. By providing monthly feedback, both on use of blood gas analysis and on appropriateness through multiple sources over 20 months, we stimulated learning and education in users. In particular, we were able to show that when the guideline was applied, it could result in a safe reduction in the number of requests. As proposed by others,15 our results favour the combination of multiple approaches in attempts to change professional practice.
The goal of quality improvement projects is not to show without any bias that a specific pinpoint intervention is instrumental in changing practice, especially when already convincing methods are available.15 It aims much more at making this change happen. This requires a managerial approach, combining strategies and dealing with local resources and barriers. We therefore opted for a long term approach that facilitated the implementation of our guideline, using periodic measurements and feedback. As a consequence, less attention was paid to strict control of any bias. For instance, we chose a before and after study design, as well as retrospective data for establishing baseline, and we did not examine the question of inter-rater reliability. However, the decrease in the use of blood gas analysis can hardly be attributed to external independent factors. Indeed, patient characteristics remained stable and the number of all other tests performed in our intensive care unit did not vary.
Key learning points
Acceptance of the guideline was facilitated because it was developed by a local multidisciplinary group; based on the available scientific evidence and local habits; simple and user friendly; disseminated via pocket sized documents, posters, and educational sessions
The impact of the guideline on requests for blood gas analysis was enhanced by combining iterative education and regular information through ongoing feedback from multiple sources (posters, oral presentations, local information bulletins)
Feedback comprising test volume and adherence to the guideline helped the whole team to recognise the relation between the improved use of blood gas analysis and the reduction in the number of requests
Changing professional behaviour requires long term effort and commitment
Next steps
To maintain the benefits of our intervention, a permanent follow up of this project should be engaged. Firstly, we will set up a periodic revision of our guideline. Indeed, despite satisfying the requirements regarding the methodological standards on guideline development and format recently published in two major medical journals,20,21 our guideline was based on a consensus of experts. Therefore it could benefit from regular reviews of the literature, and its rating on the scale of evidence could be progressively increased. Secondly, we will replace the time consuming monthly audit day with continuous low cost monitoring capable of detecting a decrease in appropriateness of requests. This could be done by using the sole arterial blood gas analysis indicator, which can be easily extracted from our hospital computer system. This indicator could be plotted as a control chart22 and trigger a measure of appropriateness based on a review of patients' records each time it exceeds a statistically predefined threshold. Coupled to a measure of appropriateness every six months, we should be able to control deviations permanently.
Table.
Details of introduction and implementation of guideline on use of arterial blood gas analysis in each 10 month period: baseline (before study), pilot (after first version of guideline), and consolidation (after final version of guideline)
| Baseline | Pilot | Consolidation | P value | |
|---|---|---|---|---|
| Data from audit days (patient sample) | ||||
| No of patients | 189 | 176 | 184 | |
| Mean (SD) age (years) | 55 (19) | 58 (17) | 57 (17) | 0.49* |
| Mean (SD) SAPS II | 29 (12) | 30 (12) | 30 (14) | 0.29* |
| Type of surgery (No (%)): | 0.19† | |||
| Cardiovascular | 68 (36) | 74 (42) | 73 (39) | |
| Pulmonary | 20 (10) | 15 (9) | 14 (8) | |
| Digestive | 39 (21) | 29 (16) | 33 (18) | |
| Neurosurgical | 39 (21) | 27 (15) | 46 (25) | |
| Other | 23 (12) | 31 (18) | 18 (10) | |
| Mean incident rate (70th and 90th centiles)‡ | 0.22 (0.20, 0.66) | 0.36 (0.22, 0.88) | 0.19 (0.18, 0.67) | 0.85* |
| Information system data (unit population) | ||||
| No of months | 10 | 10 | 10 | |
| Mean (SD) stay (days) | 4.6 (0.5) | 4.3 (0.4) | 4.3 (0.5) | 0.26* |
| No of patients | 1276 | 1356 | 1403 | |
| Mortality (%) (95% CI) | 7.1 (5.7 to 8.5) | 7.2 (5.8 to 8.6) | 6.6 (5.3 to 7.9) | 0.80† |
SAPS II: simplified acute physiology score, version 2.
Kruskal-Wallis test.
Presented as such because scarcity of incidents induced skewed distribution.
Pearson's χ2.
Acknowledgments
We are grateful to the four experts, Professors Peter Suter and Jean-Claude Chevrolet and Drs Jean-Pierre Revelly and Jean Roeseler, who helped to revising the consolidated guideline, Eric Gremion, Nadia Mehla, Jean-Max Granier for their technical assistance, and the whole surgical intensive care team, who actively participated in this programme.
Footnotes
Funding: Quality of care program, Geneva University Hospital.
Competing interests: None declared.
References
- 1.Klepzig H, Winten G, Thierolf C, Kiesling G, Usadel KH, Zeiher AM. Treatment costs in a medical intensive care unit: a comparison of 1992 and 1997 (in German) Dtsch Med Wochenschr. 1998;123:719–725. doi: 10.1055/s-2007-1024044. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy TW, Konopad E, Shustack A, Johnston R, Grace M. Cost accounting of adult intensive care: methods and human and capital inputs. Crit Care Med. 1996;24:1168–1172. doi: 10.1097/00003246-199607000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Singer M, Myers S, Hall G, Cohen SL, Armstrong RF. The cost of intensive care: a comparison on one unit between 1988 and 1991. Intensive Care Med. 1994;20:542–549. doi: 10.1007/BF01705718. [DOI] [PubMed] [Google Scholar]
- 4.Eyster E, Bernene J. Nosocomial anemia. JAMA. 1973;223:73–74. [PubMed] [Google Scholar]
- 5.Henry ML, Garner WL, Fabri PJ. Iatrogenic anemia. Am J Surg. 1986;151:362–363. doi: 10.1016/0002-9610(86)90468-x. [DOI] [PubMed] [Google Scholar]
- 6.Muakkassa FF, Rutledge R, Fakhry SM, Meyer AA, Sheldon GF. ABGs and arterial lines: the relationship to unnecessarily drawn arterial blood gas samples. J Trauma. 1990;30:1087–1093. [PubMed] [Google Scholar]
- 7.Raffin TA. Indications for arterial blood gas analysis. Ann Intern Med. 1986;105:390–398. doi: 10.7326/0003-4819-105-3-390. [DOI] [PubMed] [Google Scholar]
- 8.Niehoff J, DelGuercio C, LaMorte W, Hughes-Grasberger SL, Heard S, Dennis R, et al. Efficacy of pulse oximetry and capnometry in postoperative ventilatory weaning. Crit Care Med. 1988;16:701–705. doi: 10.1097/00003246-198807000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DE, Bell DD, Ostryzniuk T, Dobson K, Oppenheimer L, Martens D, et al. Eliminating needless testing in intensive care—an information-based team management approach. Crit Care Med. 1993;21:1452–1458. doi: 10.1097/00003246-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Browning JA, Kaiser DL, Durbin CG. The effect of guidelines on the appropriate use of arterial blood gas analysis in the intensive care unit. Resp Care. 1989;34:269–276. [Google Scholar]
- 11.Pilon CS, Leathley M, London R, McLean S, Phang PT, Priestley R, et al. Practice guideline for arterial blood gas measurement in the intensive care unit decreases numbers and increases appropriateness of tests. Crit Care Med. 1997;25:1308–1313. doi: 10.1097/00003246-199708000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Barie PS, Hydo LJ. Learning to not know: results of a program for ancillary cost reduction in surgical critical care. J Trauma. 1996;41:714–720. doi: 10.1097/00005373-199610000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Martin AR, Wolf MA, Thibodeau LA, Dzau V, Braunwald E. A trial of two strategies to modify the test-ordering behaviour of medical residents. N Engl J Med. 1980;303:1330–1336. doi: 10.1056/NEJM198012043032304. [DOI] [PubMed] [Google Scholar]
- 14.Studnicki J, Bradham DD, Marshburn J, Foulis PR, Straumfjord JV. A feedback system for reducing excessive laboratory test. Arch Pathol Lab Med. 1993;117:35–39. [PubMed] [Google Scholar]
- 15.Bero LA, Grilli R, Grimshaw JM, Harvey E, Oxman AD, Thomson MA.on behalf of the Cochrane Effective Practice and Organisation of Care Review Group. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings BMJ 1998317465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318:593–596. doi: 10.1136/bmj.318.7183.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health. 1984;74:979–983. doi: 10.2105/ajph.74.9.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemeshow S, Le Gall JR. Modelling the severity of illness of ICU patients. A systems update. JAMA. 1994;272:1049–1055. [PubMed] [Google Scholar]
- 19.Reason J. Understanding adverse events: human factors. Qual Health Care. 1995;4:80–89. doi: 10.1136/qshc.4.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grilli R, Magrini N, Penna A, Mura G, Liberati A. Practice guidelines developed by speciality societies: the need for a critical appraisal. Lancet. 2000;355:103–106. doi: 10.1016/S0140-6736(99)02171-6. [DOI] [PubMed] [Google Scholar]
- 21.Shaneyfelt TM, Mayo-Smith MF, Rothwangl J. Are guidelines following guidelines? The methodological quality of clinical practice guidelines in the peer-reviewed medical literature. JAMA. 1999;281:1900–1905. doi: 10.1001/jama.281.20.1900. [DOI] [PubMed] [Google Scholar]
- 22.Wilcock PM, Thomson RG. Modern measurement for a modern health service. Qual Health Care. 2000;9:199–200. doi: 10.1136/qhc.9.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]