Abstract
Microbes are a worthwhile organism of the earth that could be formulated as consortium which can be utilized as biofertilizers. Consortium-based bioinoculants or biofertilizers are superior to single strain-based inoculants for sustainable agricultural productivity and increased micronutrient content in yield. The aim of present study was to evaluate the effect of different combinations of beneficial bacteria that are more effective than single-based bioinoculants. The current work focuses on the isolation of rhizospheric microorganisms from various cereals and pseudocereal crops and the development of a single inoculum as well as a bacterial consortium which were evaluated on wheat crop. A total 214 rhizospheric bacteria were sorted out and, screened for mineral solubilizing attributes i.e., phosphorus, potassium, zinc and selenium solubilization. Among all the bacterial isolates, four potential strains exhibiting P, K, Zn and Se-solubilizing attributes were identified with the help of 16S rRNA gene sequencing as Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4, respectively. The identified strains formulated as a consortium which were found to improve the plant growth and physiological parameters in comparison to single culture inoculants and control. To the best of our knowledge, the present investigation is the first report that has developed the consortium from bacterial strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4. A combination of bacterial strains could be used as liquid inoculants for cereal crops growing in mountainous regions.
Keywords: Bioformulation, Consortium, P- solubilizer, K-solubilizer, Zn-solubilizer, Se-solubilizer
1. Introduction
Wheat (Triticum aestivum L.) is the second most significant cereal crop in India, which is essential for the food and nutritional security of the country. Approximately 55% of the world population depends on crop of wheat for about 20% of calories consumption. It is the pivotal food grain and staple food crop of the North Indian people which is mostly consumed as breads. Wheat is an important source of carbohydrates, proteins, vitamins and minerals [1]. Wheat is a significant component of the diet due to thier agronomic adaptability, ease of grain storage and effortlessness of grain into flour conversion, which makes wheat edible, digestible, engaging, tasty and satisfying food. Three different types of wheat are grown in India due to the country's varying environmental circumstances and populations eating pattern (bread, durum and dicoccum). Out of them, bread wheat makes up approximately 95% of the overall production, while durum wheat contributes 0.4% and dicoccum wheat close is to 1%.
In India, wheat crop is grown in six diverse agro-climatic zones and its production varies according to nutrient status in soil and fertilizers use. The use of fertilizers is most essential factor to produce wheat and it requires higher quantity of chemical fertilizers of nitrogen, phosphorus and potassium [2]. Inadequate supply of fertilizers can result in stunted growth and reduced yield. On the other hand, excessive application over threshold levels can have negative effects on plant growth which may results in reduced crop production [3]. The use of chemical fertilizers also harms human health and causes methemoglobinemia and damages the respiratory and vascular system in children; therefore, there is an increasing demand for sustainable approaches worldwide. Therefore, the search for sustainable solutions to reduce the negative consequences of intensive farming practices is a more difficult challenge. Hence, researchers worldwide are looking for alternate non-harmful fertilizer sources such as biofertilizers that can maintain the fertility of soils [4], and improve growth of plant and agricultural yield [5].
Microbes play essential function in both aquatic and terrestrial habitats. They colonize the rhizosphere and entire part of the plants (endophytic and phyllospheric region). These microbes enhance the plant growth using direct and indirect mechanismsincluding nitrogen fixation; solubilization of phosphorus, potassium, zinc and selenium; production of siderophores, phytohormones, ammonia, hydrogen cyanide, and hydrolytic enzymes (chitinase, amylase, and protease); and reduction of ethylene content [6]. Microbes increases and improves the plant nutrient availability, and reduce the requirement of agro-chemicals [7]. Nitrogen fixing and mineral (P, K, Zn and Se) solubilizing bacteria used as bioinoculants are important for the plant nourishment as they enhance minerals absorbance of the plants [8,9].
Biofertilizers when added to soil, seeds or plants, the microbes colonize the rhizosphere or interior parts of the plants and stimulate plant growth by increasing the supply of nutrients to the host plant [10]. Bioinoculants are prepared using single or multiple mineral solubilizing and other plant growth prompting (PGP) microbial cultures. The microbial consortium is a better bioinoculants than single microbial bioinoculants as it covers a diverse set of PGP mechanisms of plant growth promotion [11,12]. In a report, consortium of Bacillus megaterium, Arthrobacter chlorophenolicus and Enterobacter sp. was found to promote the growth of wheat by increasing the height of plant, yield, weight of the plant, and N and P uptake [13]. In another report, microbial consortium of Bradyrhizobium japonicum and B. diazoefficiens were inoculated on soybean as a result found to promote the growth of soybean dry weight of root and shoot, seed weight, and grain yield [14]. In the present study, the beneficial rhizospheric microbes were isolated from cereal crops and screened for mineral solubilizing and other growth promoting characteristics and developed as microbial consortium which further evaluated on wheat.
2. Materials and methods
2.1. Sampling, isolation, and characterization of rhizospheric bacteria
The rhizospheric soil samples were collected from diverse cereals and pseudocereal crops i.e., amaranth, buckwheat, goatgrass, maize, millets, and wheat from the foothills of the Shivalik's, Himachal Pradesh. A total of 9 samples were collected separately in a clean plastic bag and stored at 4 °C temperature until isolation. Using serial dilution plating technique, the culturable rhizospheric bacteria on various selective and non-selective growth media such as nutrient agar media, tryptic soy agar, King's B agar, T3A agar, and ammonium mineral salt (AMS) were isolated [15,16]. The isolated strains were morphologically characterized and further the samples were purified (on respective growth media) and preserved on nutrient agar slants and in glycerol stock (25%).
2.2. Screening of bacteria for mineral solubilizing attributes
The isolated microbes were screened for P, K, Zn and Se solubilizing attributes. The qualitative and quantitative estimation of phosphorus solubilization of bacterial samples were done as per the method described by Pikovskaya [17], and Murphy, Riley [18] respectively. The zinc solubilization of microbes were qualitatively and quantitatively determined using Fasim et al. [19] and Francis et al. [20] methods, respectively. The K and Se-solubilizing microbes were qualitatively determined by Hu et al. [21], and Ruanchaiman et al. [22], respectively.
2.3. Identification of selected mineral solubilizing bacterial isolates
The bacteria were selected based on different mineral solubilizing attributes and they were molecularly identified by genomic DNA isolation, followed by 16S rRNA gene amplification. The genomic DNA (gDNA) of the selected efficient bacterial strains were isolated as per the method described by Yadav et al. [23]. Subsequently, isolated genomic DNA of bacterial isolates were then subjected to 16S rRNA gene amplification using pA and pH primers to obtain fragment (nearly 1540-bp). The purification of amplified PCR products using QIA quick purification kit (Qiagen) was done and samples were sent for sequencing to SciGenom lab Ltd., Kochi, Kerala. The identification of bacteria was determined using BLASTn program. The partial 16S rRNA gene sequences were submitted to NCBI GenBank and accession numbers were assigned.
2.4. Development of microbial consortium
The selected four bacterial isolates i.e. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer), were used to develop microbial consortiums. The selected efficient bacterial isolates were assessed for their compatibility by the plate cross streak method as describe earlier Kaur et al. [24]. On freshly prepared nutrient agar media, cross streaking was performed. Strain EU-C3SK2 was firstly seeded on the NA plate after that, EU-A3Rb1, EU-A2RNL1, EU-C3SK2 and EU-WRSe4, were streaked perpendicularly to make a square with colonies in the center and permitted to grown at 30 °C for 24 h. After the incubation period of 24 h, plates were examined for the presence of zones of inhibition at the crossing of the paired strains. The compatible strains were then subjected to inoculums preparation, the selected strains were grown in nutrient broth for 30 °C for 24 h. Afterwards, colony-forming units (CFU) were calculated as 1.85×107 CFU mL−1 (EU-A3Rb1), 2.92×107 CFU mL−1 (EU-A2RNL1), 3.95×107 CFU mL−1 (EU-C3SK2), and 3.68×107 CFU mL−1 (EU-WRSe4). The selected potential liquid inoculums of bacterial culture were mixed in ratio (1:1).
2.5. Evaluation and validation of microbial consortium
Four potential strains with P, K, Zn and Se solubilization attributes were evaluated singly and in combination on wheat cultivar UNNAT PBW-343 in an open field and greenhouse conditions in which seeds were bacterized 1–2 h prior to sowing. In open field conditions, the experiment was carried out in 6.0 × 1.5 m2 plot in which randomize block design was followed. Under greenhouse condition, the experiment was carried out in 30 cm × 30 cm × 26 cm plastic pots filled with non-sterile soil (4 Kg) and in every pot four plants were maintained with 6 cm space. All the pots were spaced appropriately to avoid cross contamination. The experiment was carried out with a total of fourteen treatments in open field conditions and twelve treatments in green house conditions and each experiment was carried out in triplicates. After 30 days microbial consortium and cultures were sprayed with the help of spray bottle in each plant.
2.6. Determination of plant growth and physiological parameters
To determine the plant's growth parameters, plant samples from greenhouse (after 90 days of sowing) and open field conditions (45 and 90 days of sowing) were uprooted in triplicates from each treatment. The plant growth characters i.e., length of shoot/root, and weight of fresh/dry plants were measured. Data was recorded in triplicates. The growth and physiological parameters were determined using the following formula: % increase = final value–starting value/starting value × 100, in which the “final value (consortium treatment) - stating value without inoculation (control) and also calculated in fold.
2.6.1. Chlorophyll and carotenoids content
For the determination of photosynthetic pigment (chlorophyll) and carotenoids content of the wheat, fresh leaves of wheat plant were homogenized in 80% acetone and centrifuged (10 min; 8000 g). Supernatant was used for the determining the content of chlorophyll, and carotenoids by taking absorbance at 645 nm and 663 nm, and 470 nm, respectively. The content of chlorophyll and carotenoids was calculated by applying the equations described by Lichtenthaler [25].
| Chl a (mg g−1 FW) = 12.25 × A663−2.79 × A645 |
| Chl b (mg g−1 FW) = 21.50 × A645−5.10 × A663 |
| Carotenoids = 1000 × A470–1.82 × Ca – 85.02 × Cb /198 |
2.6.2. Total soluble sugar
According to the protocol of Irigoyen et al. [26], wheat plant leaf samples were pulverized in 95% ethanol and reacted with Anthrone reagent to determine total soluble sugar content. Afterwards, sample was put in water bath and kept at 100 °C for 10 min, and the step was followed by its cooling at room temperature. At 625 nm, the absorbance for the total sugar content was measured, and the quantity was given in μg g−1.
2.6.3. Total phenolic content
The modified Folin–Ciocaltu method was used to determine the total phenol concentration of the different extracts [27]. An aliquot of 0.5 mL of each extract 1 mg mL−1 was mixed with 2.5 mL Folin- Ciocalteu reagent (previously diluted with distilled water 1:10 v/v) and 2 mL (75% w/v) of sodium carbonate (Na2CO3). Falcon tubes were vortexed for 15 sec and allowed to settle down at room temperature for 30 min for development of purple color. The absorbance of total phenolic content was measured by the using spectrophotometer (ILB-360 UV-VIS Double Beam Spectrophotometer) at 765 nm. Gallic acid was used as the standard. In order to plot the calibration curve, standard of gallic acid was used. Gallic acid equivalent (μg GAE g−1), which is a typical reference compound, was used to express total phenol values. The experiment was carried out in triplicate, and mean SD values were used to express the results.
2.6.4. Total flavonoids content
Total flavonoid content was measured according to the method described by Patel et al. [27]. One milliliter of aliquots and 1 mg mL−1 of leaves extract sample was taken and 4 mL of distilled water and 0.3 mL of 5% sodium nitrite solution was added into each. After incubation period of 5 min at room temperature, 0.3 mL of 10% aluminum chloride was added. After 6 min of incubation, 2 mL of 1 M sodium hydroxide was added. Finally, the volume was made up to 10 mL with distilled water and mixed thoroughly. The absorbance was taken at 510 nm in spectrophotometer. The blank was implemented using deionized distilled water. The standard was quercetin. The tests on the samples were done in triplicates. Using standard quercetin, the calibration curve was visualized. The data of total flavonoids were expressed as μg QE/g−1.
2.7. Statistical analysis
To determine the degree of significance between values, the collected data were statistically examined using Student's t-test. To compare means, the test of least significant difference (LSD) was used [28]. To evaluate significant differences between different treatments, it was decided to look for significant differences that have a P value less than and critical difference (CD) at a 5% and 1% probability level. The standard error and LSD were calculated.
3. Results
3.1. Sampling, isolation, and characterization of rhizospheric bacteria
A total of 214 rhizospheric bacteria were sorted out from diverse cereal crops. The population of rhizospheric bacterial isolates varied from 3.90×106 CFU g−1 to 9.85×107 CFU g−1. The greatest bacterial diversity was supported by nutrient agar medium (NA) for rhizospheric samples and least population was supported by AMS.
3.2. Screening of bacteria for mineral solubilizing attributes
Among 214 isolated bacteria screened for the mineral solubilizing attributes, 48 exhibited the solubilization of P, whereas 23 were found as K solubilizers, 29 isolates showed Zn solubilization and 32 showed Se-solubilization attribute. The P-solubilization of forty eight isolates ranged from 5.72±0.005 to 194.0±0.05 mg L−1, and twenty nine isolates showed zinc solubilization in the range 12.01±0.05 to 55.6±0.02 mg L−1. Isolate EU-C3SK2 solubilized highest amount of Zn (55.6±0.02 mg L−1), whereas strain EU-A2RNL1, and EU-WRSe4 was found to be efficient K and Se solubilizer via plate assay method (Table 1).
Table 1.
Screening of mineral solubilizing microbes of selected bacterial strains.
| Microbes |
Solubilization |
Production |
||||||
|---|---|---|---|---|---|---|---|---|
| P (mg L−1) | K | Zn (mg L−1) | Se | Sid | HCN | NH3 | IAA mg/L | |
| Rahnella aquatilis EU-A3Rb1 | 194.0±0.05 | + | + | + | – | + | + | – |
| Erwinia aphidicola EU-A2RNL1 | 149.5±0.12 | +++ | + | – | + | + | + | + |
| Brevibacillus brevis C3SK2 | + | + | 55.6±0.02 | – | + | – | – | – |
| Bacillus mycoides EU-WRSe4 | 95.5±0.07 | + | – | + | + | – | – | – |
PSB: Rahnella aquatilis EU-A3Rb1; KSB: Erwinia aphidicola EU-A2RNL1; ZnSB: Brevibacillusbrevis C3SK2; SSB Bacillus mycoides EU-WRSe4; PSB-Phosphorus solubilizing bacteria, K-Potassium solubilizing bacteria, Zn-Zinc solubilizing bacteria, Se-selenium solubilizing bacteria, Sid-Siderophores, NH3-Ammonia, HCN-Hydrogen cyanide, IAA-Indole acetic acid.
3.3. Identification of selected mineral solubilizing bacterial isolates
The 16S rRNA gene sequencing and BLASTn analysis of four selected efficient strains EU-A3Rb1, EU-A2RNL1, EU-C3SK2, and EU-WRSe4 showed <97% similarity with Rahnella aquatilis, Erwinia aphidicola, Brevibacillus brevis, and Bacillus mycoides, respectively. The strains EU-A3Rb1 (OM818476), EU-A2RNL1(OM818480), EU-WRSe4 (OP808361), and EU-C3SK2 (OP808362) were deposited at ICAR-National Bureau of Agriculturally Important Microorganisms (NBAIM) culture-collection facility, Mau Nath Bhanjan, Uttar Pradesh, India.
3.4. Evaluation and validation of microbial consortium
The potential mineral solubilizing microbes including P-solubilizer (R. aquatilis EU-A3Rb1), K-solubilizer (E. aphidicola EU-A2RNL1), Zn-solubilizer (B. brevis EU-C3SK2), and Se-solubilizer (B. mycoides EU-WRSe4), were evaluated as single inoculation and microbial consortium for plant growth promotion of wheat crop. Growth and physiological parameters of the wheat crop were studied under in vitro and in vivo conditions.
3.5. Effect of microbial consortium on plant growth of wheat crop under natural conditions
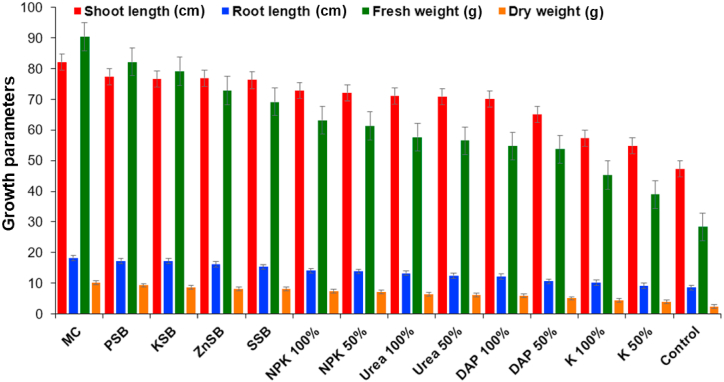
The combined effect of mineral solubilizing bacteria, R. aquatilis EU-A3Rb1, E. aphidicola EU-A2RNL1, B. brevis EU-C3SK2, and B. mycoides EU-WRSe4 was singly and in consortium were inoculated on the wheat crop. The result showed the enhancement of growth and physiological parameters in 45 and 90 days (Table 2; Table 3; Fig. 1, Fig. 2, Fig. 3, Fig. 4). The bacterial consortium incremented the shoot length of wheat plant by 12.8%, 15.4%, 17.2%, 43.5% and 74.0% over the chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control, respectively, in 45 days. Whereas in 90 days, the treatments incremented the shoot length by 23.8%, 25.8%, 29.5%, 36.7% and 52.6% over the chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated plant(control), respectively. The wheat plant root length was increased by 1.0, 1.0, 1.1, 1.1 and 2.1 fold as compared with singly inoculated PSB (P-solubilizer), KSB (K-solubilizer), ZnSB (Zn solubilizer), SSB (Se solubilizer), and untreated control, respectively in 45 days. Root length of wheat plant was also incremented by 1.1, 1.1, 1.0, 1.1 and 1.8 fold as compared with singly inoculated PSB, KSB, ZnSB, SSB, and untreated control, respectively in 90 days. Consortium of EU-A3Rb1, EU-A2RNL1, EU-WRSe4, and EU-C3SK2 increased the fresh root weight of wheat plant by 1.4, 1.5, 1.6 fold, 1.9 and 3.1 fold over the chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control, respectively, in 45 days. The fresh root weight of wheat plant was incremented by microbial consortium up to 1.7-fold, 2.0, 2.4, 2.8 and 5.0 fold over the chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control, respectively, in 90 days. The dry weight of the wheat plant was incremented by the consortium up to 4.8 and 5.0 fold over the untreated control, respectively, in 45 and 90 day.
Table 2.
Effect of microbial consortium on wheat under open field conditions after 45 days.
| Treatments | Shoot length (cm) | Root length (cm) | Fresh weight (g) | Dry weight (g) | Chlorophyll (mg g−1) | Carotenoids (g L−1) | Phenolics (μg g−1) | Flavonoids (μg g−1) | Sugar (μg g−1) |
|---|---|---|---|---|---|---|---|---|---|
| MC | 82.13j**±0.61 | 18.23j**±0.84 | 90.40m**±1.70 | 10.16l**±0.16 | 62.46j**±0.43 | 6.49j±0.70 | 1.75h**±0.05 | 7.08j**±0.45 | 50.63h**±0.94 |
| PSB | 76.50h**±1.19 | 17.27i**±0.95 | 82.20l**±1.88 | 9.33k**±1.36 | 59.02i*8±0.49 | 6.13i±0.43 | 1.39f**±0.02 | 6.67h*±0.04 | 33.89e**±0.16 |
| KSB | 77.40i**±1.53 | 17.20i**±1.21 | 79.13k**±2.61 | 8.77j**±0.24 | 58.04i**±0.35 | 5.87h ± 0.71 | 1.28e**±0.03 | 6.91i*±0.65 | 35.81f**±0.38 |
| ZnSB | 76.23h**±0.69 | 16.20h*±1.06 | 72.83j**±2.72 | 8.25i**±0.14 | 58.87i**±1.24 | 5.60g ± 1.24 | 1.55g*8±0.04 | 6.71h*±0.13 | 43.15g**±0.47 |
| SSB | 76.87h**±1.35 | 15.37g*±1.44 | 69.13i**±1.48 | 8.19i**±0.59 | 57.93i**±0.11 | 6.00h ± 0.28 | 1.29e*±0.02 | 6.31g*±0.06 | 33.51e**±0.53 |
| NPK 100 % | 72.80g**±1.42 | 14.13f±0.45 | 63.17h**±1.44 | 7.47h**±0.12 | 49.26h**±0.39 | 5.54f±0.16 | 1.13d**±0.09 | 6.28g*±0.05 | 31.70d**±0.96 |
| NPK 50 % | 72.17f**±0.25 | 13.87f±0.74 | 61.30g*±2.13 | 7.18g**±0.01 | 48.07g**±1.03 | 5.44f±0.71 | 1.09d**±0.01 | 6.14f±0.39 | 29.11b**±0.35 |
| Urea 100 % | 71.13e**±0.74 | 13.30e±0.54 | 57.63f**±0.31 | 6.40f**±0.09 | 44.81f**±1.53 | 5.16e±0.34 | 1.04c*±0.05 | 5.95e±0.24 | 30.40c**±0.60 |
| Urea 50 % | 70.93e**±2.89 | 12.53d ± 0.86 | 56.47e**±2.05 | 6.11e**±0.25 | 40.98e**±0.45 | 5.12e±0.48 | 1.01c*±0.03 | 5.68d ± 0.01 | 28.78b**±0.23 |
| DAP 100 % | 70.03e**±2.36 | 12.13d ± 0.76 | 54.73d**±1.07 | 5.92e**±0.54 | 36.00d**±1.10 | 4.60d ± 0.45 | 0.90b ± 0.03 | 5.72d ± 0.00 | 29.81c**±0.40 |
| DAP 50 % | 65.00d**±0.62 | 10.60c±0.45 | 53.70d*±80.54 | 5.08d**±0.10 | 33.22c*±0.59 | 4.07c±1.04 | 0.81b ± 0.08 | 5.45c±0.23 | 29.39b**±0.07 |
| K100 % | 57.23c*±0.69 | 10.27c±0.53 | 45.27c*±2.39 | 4.40c±0.17 | 33.19c*±2.77 | 3.97c±1.88 | 0.85b ± 0.06 | 5.40c±0.17 | 29.74c**±0.59 |
| K 50 % | 54.87b ± 0.29 | 9.20b ± 0.83 | 38.90b ± 2.62 | 3.86b ± 0.63 | 31.48b ± 0.77 | 3.11b ± 0.18 | 0.87b ± 0.03 | 5.02b ± 0.44 | 28.51b**±0.55 |
| Control | 47.20a±2.77 | 8.60a±1.06 | 28.43a±0.98 | 2.49a±0.28 | 27.10a±0.26 | 2.31a±0.18 | 0.68a±0.04 | 4.78a±0.15 | 22.80a±0.76 |
| LSD | 1.30 | 0.40 | 2.20 | 0.28 | 1.19 | 0.15 | 0.04 | 0.09 | 0.90 |
| SE | 4.64 | 3.13 | 6.39 | 1.25 | 3.07 | 2.34 | 0.15 | 0.80 | 1.86 |
| CD 5 % | 8.23 | 5.54 | 11.32 | 2.21 | 5.44 | 4.14 | 0.26 | 1.42 | 3.30 |
| CD 1 % | 12.31 | 8.29 | 16.93 | 3.31 | 8.15 | 6.20 | 0.40 | 2.14 | 4.95 |
MC: Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4; PSB: R. aquatilis EU-A3Rb1; KSB: E. aphidicola EU-A2RNL1; ZnSB: B. brevis EU-C3SK2; SSB: B. mycoides EU-WRSe4.
[Numerical values are mean ± Standard deviation of mean (SD) of three independent observations].
Common superscript code on mean values indicate the non-significant differences among derivatives as based on unpaired Student t-test at p < 0.05. On the other hand, different superscript indicates significant differences among lines.
Table 3.
Effect of microbial consortium under open field conditions after 90 days.
| Treatments | Shoot length (cm) | Root length (cm) | Fresh weight (g) | Dry weight (g) | Chlorophyll (mg g−1) | Carotenoids (g L−1) | Phenolics (μg g−1) | Flavonoids (μg g−1) | Sugar (μg g−1) | No of tillers | No of spikes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | 132.70l**±2.05 | 17.53m**±0.53 | 191.80l**±2.66 | 62.00m**±1.10 | 48.21l**±1.25 | 6.77i±0.39 | 0.77e**±0.07 | 9.48g**±0.00 | 48.31j**±1.73 | 13.00k**±0.82 | 8.67n*±0.47 |
| PSB | 115.37j**±0.81 | 15.00j*±1.36 | 163.73k**±1.60 | 52.83k**±1.64 | 43.08k**±1.71 | 5.66h ± 0.32 | 0.45c*±0.07 | 8.02c**±0.08 | 39.71i**±0.06 | 12.00j**±0.83 | 8.33m*±0.94 |
| KSB | 112.60i**±2.49 | 15.53k*±0.90 | 151.70j**±2.43 | 55.23l**±1.25 | 35.31j*±1.41 | 4.48d ± 0.20 | 0.41c*±0.07 | 9.30f**±0.14 | 38.74h**±0.64 | 11.3i**±0.47 | 7.33l*±0.94 |
| ZnSB | 119.73k**±1.13 | 16.20l**±0.78 | 120.67h**±1.28 | 49.37j**±0.77 | 34.42i*±0.99 | 5.53g ± 0.60 | 0.39c*±0.02 | 9.19f**±0.19 | 34.89g**±0.35 | 10.33h**±0.47 | 7.00k*±1.63 |
| SSB | 115.57j**±1.64 | 15.57k*±0.45 | 127.80i**±0.59 | 47.03i**±1.08 | 34.41i*±1.84 | 7.16j±1.50 | 0.55d**±0.02 | 8.74e*±0.04 | 39.99i**±0.55 | 10.33h**±0.47 | 6.00j±1.41 |
| NPK 100 % | 107.13h**±4.35 | 14.90i*±0.45 | 109.27h**±2.92 | 42.70h**±1.91 | 31.44g ± 3.49 | 5.55g ± 0.62 | 0.41c*±0.03 | 8.37d**±0.06 | 34.46g**±0.04 | 9.00g*±0.82 | 5.33i±0.47 |
| NPK 50 % | 96.33c±1.16 | 13.47h ± 0.87 | 97.93g**±1.27 | 41.60h**±1.91 | 30.23f±2.55 | 5.29f±0.81 | 0.33b ± 0.03 | 8.11c**±0.00 | 33.00f**±6.72 | 6.72f*±0.00 | 4.67h ± 1.70 |
| Urea 100 % | 105.43g**±0.62 | 12.97g ± 0.33 | 95.53g**±2.56 | 36.67g**±1.80 | 33.16h*±2.98 | 5.87 ± 0.81 | 0.32b ± 0.01 | 7.72b**±0.15 | 30.80d**±0.45 | 7.67f*±0.47 | 4.33g ± 0.47 |
| Urea 50 % | 99.90e*±1.12 | 12.07f±0.54 | 91.53f**±1.11 | 30.30f**±1.10 | 26.90d ± 2.59 | 4.32c±0.91 | 0.29b ± 0.03 | 8.05c**±0.03 | 30.30d**±0.08 | 7.00e±0.82 | 3.67f±0.94 |
| DAP 100 % | 102.40f*±1.02 | 11.43e±0.82 | 77.93e**±0.76 | 27.70e**±0.92 | 28.55e±1.59 | 3.64b ± 0.72 | 0.24a±0.00 | 7.83b**±0.20 | 26.29c*±0.64 | 6.00d ± 0.82 | 3.00e±0.00 |
| DAP 50 % | 98.07d*±1.56 | 11.00d ± 0.22 | 71.30d**±0.62 | 24.00d*±1.42 | 24.72c±0.74 | 4.67e±1.12 | 0.23a±0.00 | 8.38d**±0.04 | 25.76c*±0.34 | 5.33c±0.47 | 2.33d ± 0.47 |
| K100 % | 97.03c±1.92 | 10.50c±0.43 | 68.30c**±1.49 | 20.63c±1.89 | 24.51b ± 0.20 | 2.84a±0.72 | 0.25a±0.01 | 8.41d**±0.46 | 23.90b*±1.27 | 5.33c±0.47 | 2.00c±0.82 |
| K 50 % | 89.97b ± 1.21 | 10.03b ± 0.52 | 50.67b*±3.04 | 14.40b ± 1.63 | 23.96b ± 0.96 | 3.68b ± 0.31 | 0.19a±0.00 | 8.97e**±0.03 | 22.50b ± 0.96 | 4.33b ± 0.47 | 1.67b ± 0.47 |
| Control | 86.93a±1.19 | 9.60a±0.45 | 37.97a±0.95 | 12.33a±0.81 | 21.40a±1.28 | 4.48 ± 0.27 | 0.18a±0.00 | 6.69a±0.00 | 19.89a±0.37 | 3.00a±0.00 | 1.33a±0.47 |
| LSD | 1.64 | 0.33 | 5.76 | 2.06 | 0.68 | 0.15 | 0.09 | 0.21 | 0.51 | 0.40 | 0.18 |
| SE | 5.94 | 2.31 | 6.22 | 5.13 | 6.30 | 2.48 | 0.11 | 0.38 | 3.98 | 2.34 | 2.99 |
| CD 5 % | 10.53 | 4.09 | 11.01 | 9.09 | 11.16 | 5.02 | 0.19 | 0.68 | 7.06 | 4.14 | 5.31 |
| CD % | 15.76 | 6.13 | 16.48 | 13.61 | 16.70 | 7.52 | 0.29 | 1.03 | 10.56 | 6.20 | 7.94 |
MC: Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4; PSB: R. aquatilis EU-A3Rb1; KSB: E. aphidicola EU-A2RNL1; ZnSB: B. brevis EU-C3SK2; SSB: B. mycoides EU-WRSe4.
[Numerical values are mean ± Standard deviation of mean (SD) of three independent observations].
Common superscript code on mean values indicate the non-significant differences among derivatives as based on unpaired Student t-test at p < 0.05. On the other hand, different superscript indicates significant differences among lines.
Fig. 1.
The effect of the microbial consortium developed from strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4 on growth parameters of wheat under in vivo condition after 45 days. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer); MC: Microbial consortium; PSB: Phosphorus solubilizing bacteria; KSB: Potassium solubilizing bacteria; ZnSB: Zinc solubilizing bacteria; SSB: Selenium solubilizing bacteria; NPK: nitrogen, phosphorus and potassium chemical fertilizer; DAP: Di-ammonium phosphate; K: Potash.
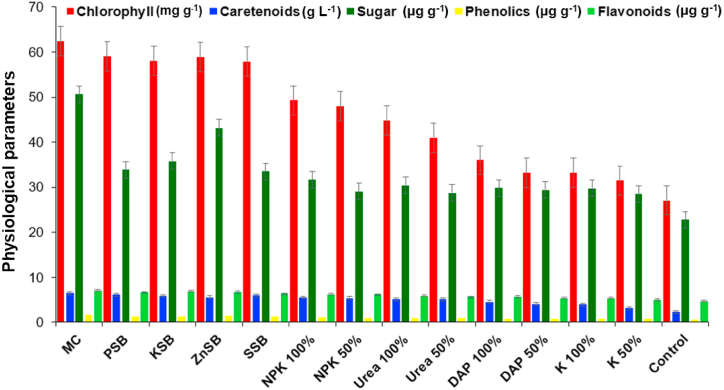
Fig. 2.
The effect of the microbial consortium developed from strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4 on physiological parameters of wheat under in vivo condition after 45 days. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer); MC: Microbial consortium; PSB: Phosphorus solubilizing bacteria; KSB: Potassium solubilizing bacteria; ZnSB: Zinc solubilizing bacteria; SSB: Selenium solubilizing bacteria; NPK: nitrogen, phosphorus and potassium chemical fertilizer; DAP: Di-ammonium phosphate; K: Potash.
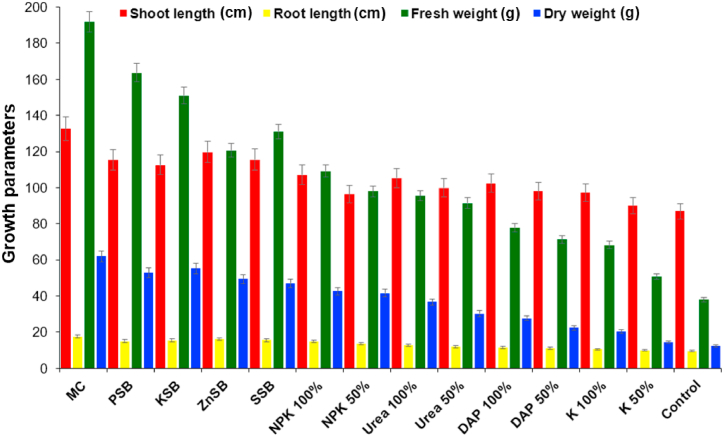
Fig. 3.
The effect of the microbial consortium developed from strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4 on growth parameters of wheat under in vivo condition after 90 days. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer); MC: Microbial consortium; PSB: Phosphorus solubilizing bacteria; KSB: Potassium solubilizing bacteria; ZnSB: Zinc solubilizing bacteria; SSB: Selenium solubilizing bacteria; NPK: nitrogen, phosphorus and potassium chemical fertilizer; DAP: Di-ammonium phosphate; K: Potash.
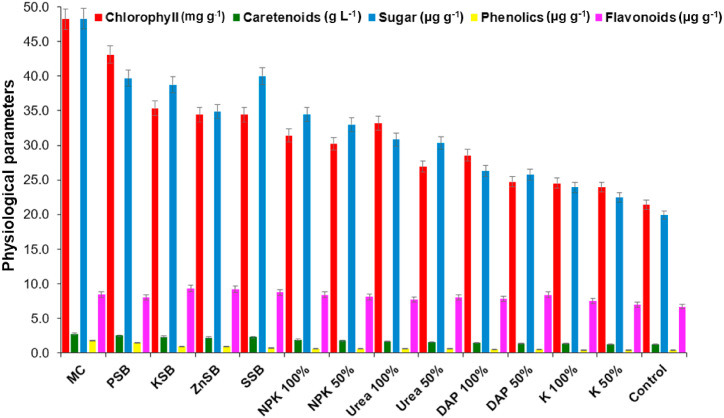
Fig. 4.
The effect of the microbial consortium developed from strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4 on physiological parameters of wheat under in vivo condition after 90 days. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer); MC: Microbial consortium; PSB: Phosphorus solubilizing bacteria; KSB: Potassium solubilizing bacteria; ZnSB: Zinc solubilizing bacteria; SSB: Selenium solubilizing bacteria; NPK: nitrogen, phosphorus and potassium chemical fertilizer; DAP: Di-ammonium phosphate; K: Potash.
The EU-A3Rb1, EU-A2RNL1, EU-WRSe4, and EU-C3SK2 consortium showed highest chlorophyll content up to 1.05, 1.07, 1.06, 1.07 and 2.3 folds over PSB, KSB, ZnSB, SSB, and untreated control, respectively in 45 days and in 90 days showed up to 1.1, 1.3, 1.4, 1.4 and 2.2 folds over PSB, KSB, ZnSB, SSB, and untreated control, respectively. Carotenoids content of the wheat plant was enhanced by the consortium of EU-A3Rb1, EU-A2RNL1, EU-WRSe4, and EU-C3SK2 (6.49±0.70 g L−1), over PSB (6.13±0.43 g L−1), KSB (5.87±0.71 g L−1), ZnSB (5.60±1.24 g L−1), SSB (6.00±0.28 g L−1), and un-inoculated control (2.31±0.18 g L−1) in 45 days. In 90 days, the increase in carotenoid content was observed to be highest in consortium (6.77±0.39 g L−1), over PSB (5.66±0.32 g L−1), KSB (4.48±0.20 g L−1), ZnSB (5.53±0.60 g L−1), SSB (7.16±1.50 g L−1), and un-inoculated control (4.48±0.27 g L−1). The combined effect of bacterial strains EU-A3Rb1, EU-A2RNL1, EU-WRSe4, and EU-C3SK2 was found to enhance the total soluble sugar content by 1.5, 1.6, 1.6, 1.7 and 2.2 fold over chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control, respectively in 45 days. In 90 days, microbial consortium enhanced the sugar content up to 1.4, 1.5, 1.8, 2.0 and 2.4 fold over chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control. The phenolic content of the wheat plant was incremented over PSB, KSB, ZnSB, SSB and untreated control by 1.2,1.3, 1.1, 1.3 and 2.5 fold respectively, in 45 days and in 90 days showed the increment by the microbial consortium up to 1.7, 1.8, 1.9, 1.4 and 4.2 folds over PSB, KSB, ZnSB, SSB, and untreated control, respectively. The content of flavonoids of wheat plant was incremented by the microbial consortium up to 13.2%, 18.9%, 23.7%, 31.1% and 48.4% over the chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control, respectively in 45 days. In 90 days, consortium incremented the flavonoids content of wheat plant by 13.2%, 22.7%, 21.0%, 12.7% and 41.70% over the chemical fertilizers NPK 100%, DAP 100%, urea 100%, potash 100% and untreated control, respectively.
3.6. Effect of microbial consortium on plant growth of wheat crop under green house conditions
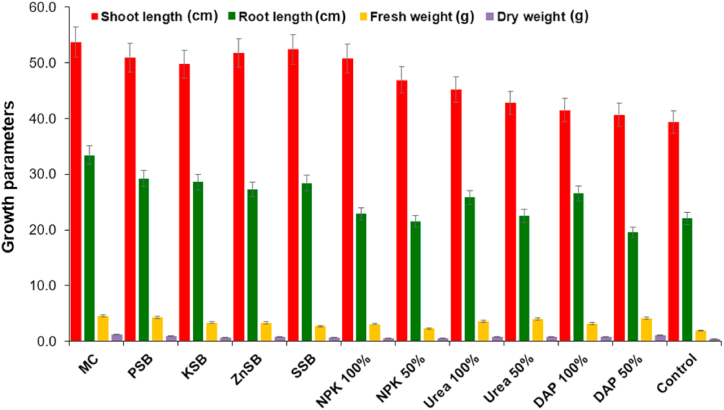
The greenhouse experiment showed that plants inoculated with a diverse combination of microbial consortia with reduced levels of fertilizers were statistically comparable to plants with a recommended dosage of fertilizers. Shoot length of the wheat plant was significantly higher by the treatments of microbial consortium up to 5.7%, 18.7%, 29.3% and 36.4% over recommended dosage of fertilizers i.e., NPK 100%, urea 100%, DAP 100%, and untreated control, respectively, and differing from other treatments (Table 4; Fig. 5, Fig. 6). The root length of wheat plant showed increment up to 14.3%, 16.8%, 22.3%, 17.5% and 70.5% by the consortium of bacterial strains as compared to PSB, KSB, ZnSB, SSB and untreated control. The treatment of microbial consortium showed highest fresh weight of the wheat plant by 1.4, 1.2, 1.4 and, 2.3 fold in comparison to recommended dosage of fertilizers i.e., NPK 100%, urea 100%, DAP 100%, and untreated control, and other treatments, respectively. The bacterial consortium of the mineral solubilizers i.e., PSB, KSB, ZnSB, SSB enhanced the dry biomass of the wheat plant over singly inoculated PSB (1.3 fold), KSB (1.6 fold), ZnSB (1.5 fold), SSB (1.1 fold) and un-inoculated control (3.1 fold).
Table 4.
Effect of microbial consortium on wheat under greenhouse after 90 days.
| Treatments | Shoot length (cm) | Root length (cm) | Fresh weight (g) | Dry weight (g) | Chlorophyll (mg g−1) | Carotenoids (g L−1) | Phenolics (μg g−1) | Flavonoids (μg g−1) | Sugar (μg g−1) |
|---|---|---|---|---|---|---|---|---|---|
| MC | 53.67j**±1.05 | 33.37h**±1.70 | 4.51j*±0.04 | 1.27j**±0.23 | 33.93h**±0.36 | 4.10e±0.52 | 1.17g**±0.02 | 6.76j±0.91 | 54.33l**±0.16 |
| PSB | 50.93h**±1.77 | 29.17g ± 0.40 | 4.33i±0.24 | 0.97h*±0.06 | 30.19g**±0.77 | 4.57f±0.43 | 1.09f**±0.03 | 6.47i±1.23 | 51.07k**±0.17 |
| KSB | 49.73g*±0.31 | 28.57f±1.47 | 3.97g ± 0.41 | 0.78e±0.09 | 26.70f**±10.67 | 3.90d ± 0.25 | 1.06f**±0.09 | 5.82f±0.17 | 46.04i**±0.38 |
| ZnSB | 51.77i**±0.83 | 27.27e±1.75 | 3.32e±0.31 | 0.83g ± 0.15 | 26.26f**±0.94 | 6.12h*±0.27 | 0.83c**±0.03 | 4.90b ± 0.12 | 34.04e**±0.69 |
| SSB | 52.40i**±1.34 | 28.40f±0.86 | 4.17h ± 0.47 | 1.06i*±0.03 | 24.69e**±0.23 | 4.45f±0.44 | 1.05f**±0.03 | 5.40d ± 0.27 | 46.41j**±0.89 |
| NPK 100 % | 50.77h**±0.96 | 22.87c±1.87 | 3.07d ± 0.68 | 0.58c±0.02 | 23.65d**±1.23 | 5.16g*±0.38 | 0.97e**±0.01 | 6.22h ± 0.02 | 39.19h**±3.08 |
| NPK 50 % | 46.90f*±2.03 | 21.50b ± 1.22 | 3.33e±0.31 | 0.51b ± 0.04 | 22.91d**±1.07 | 4.12e±0.90 | 0.83c**±0.05 | 5.21c±0.55 | 36.82g**±0.40 |
| Urea 100 % | 45.20e±2.01 | 25.80d ± 1.39 | 3.57f±0.54 | 0.83g ± 0.09 | 20.52c*±0.49 | 3.68c±0.20 | 0.88d**±0.08 | 6.11g ± 0.76 | 35.58f**±2.50 |
| Urea 50 % | 42.77d ± 1.02 | 22.50c±0.54 | 2.71c±0.29 | 0.66d ± 0.05 | 19.36b*±1.11 | 3.34b ± 0.74 | 0.83c**±0.06 | 5.47d ± 0.55 | 25.32b ± 0.58 |
| DAP 100 % | 41.50c±0.86 | 27.27e±1.75 | 3.17d ± 0.60 | 0.80f±0.17 | 23.40d**±0.67 | 3.81c±0.53 | 0.97e**±0.01 | 5.68e±0.59 | 33.85d*±2.29 |
| DAP 50 % | 40.67b ± 0.40 | 22.03b ± 0.24 | 2.30b ± 0.50 | 0.67d ± 0.02 | 19.38b*±0.31 | 3.46b ± 0.42 | 0.77b**±0.07 | 5.40d ± 1.01 | 27.00c±2.11 |
| Control | 39.33a±0.74 | 19.57a±1.27 | 1.92a±0.44 | 0.40a±0.09 | 14.46a±0.82 | 2.02a±0.74 | 0.30a±0.01 | 4.00a±0.22 | 22.79a±1.57 |
| LSD | 0.79 | 0.62 | 0.13 | 0.03 | 0.81 | 0.15 | 0.04 | 0.11 | 0.08 |
| SE | 3.84 | 3.97 | 1.39 | 0.29 | 2.50 | 1.68 | 0.14 | 1.84 | 4.27 |
| CD 5 % | 6.91 | 7.14 | 2.50 | 0.52 | 4.49 | 3.02 | 0.25 | 3.31 | 7.66 |
| CD 1 % | 10.45 | 10.81 | 3.78 | 0.80 | 6.80 | 4.58 | 0.39 | 5.02 | 11.60 |
MC: Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4; PSB: R. aquatilis EU-A3Rb1; KSB: E. aphidicola EU-A2RNL1; ZnSB: B. brevis EU-C3SK2; SSB: B. mycoides EU-WRSe4.
[Numerical values are mean ± Standard deviation of mean (SD) of three independent observations].
Common superscript code on mean values indicate the non-significant differences among derivatives as based on unpaired Student t-test at p < 0.05. On the other hand, different superscript indicates significant differences among line.
Fig. 5.
The effect of the microbial consortium developed from strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4 on growth parameters of wheat under in vitro condition after 45 days. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer); MC: Microbial consortium; PSB: Phosphorus solubilizing bacteria; KSB: Potassium solubilizing bacteria; ZnSB: Zinc solubilizing bacteria; SSB: Selenium solubilizing bacteria; NPK: nitrogen, phosphorus and potassium chemical fertilizer; DAP: Di-ammonium phosphate; K: Potash.
Fig. 6.
The effect of the microbial consortium developed from strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and Bacillus mycoides EU-WRSe4 on physiological parameters of wheat under in vitro condition after 45 days. EU-A3Rb1 (P-solubilizer), and EU-A2RNL1 (K-solubilizer), EU-C3SK2 (Zn-solubilizers), EU-WRSe4 (Se-solubilizer); MC: Microbial consortium; PSB: Phosphorus solubilizing bacteria; KSB: Potassium solubilizing bacteria; ZnSB: Zinc solubilizing bacteria; SSB: Selenium solubilizing bacteria; NPK: nitrogen, phosphorus and potassium chemical fertilizer; DAP: Di-ammonium phosphate; K: Potash.
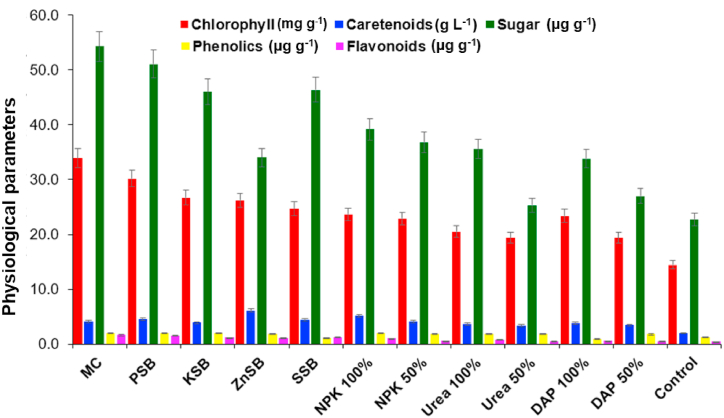
Chlorophyll content of wheat plant was shown to be incremented by the consortium (33.93±0.36 mg g−1) over the other treatments by 23.65±1.23 mg g−1 (100% NPK) 20.52 ± 0.49 mg g−1 (100% urea), 23.40 ± 0.67 mg g−1 (100% potash), and 14.46 ± 0.82 mg g−1 (untreated control). The consortium enhanced carotenoids content in the wheat plant by 2.2 fold over untreated control plant. The content of total soluble sugar in wheat plant was incremented by the consortium of mineral solubilizer up to 1.3, 1.5, 1.6 and2.3 folds as compared to recommended dosage of fertilizers i.e., NPK 100%, urea 100%, DAP 100%, and untreated plant control, and other treatments, respectively. Phenolic content showed the increment by the inoculation of consortium by 1.0 fold (PSB), 1.1 fold (KSB), 1.4 fold (ZnSB), 1.1 fold (SSB), and 3.9 fold (untreated control). The flavonoids content was increased by 93.1% over untreated controls with the application of bacterial consortium. The flavonoids content in the wheat was incremented by the treatment of the consortium. Consortium showed higher carotenoid content over the recommended dosage of chemical fertilizers i.e., NPK 100% (8.6%), urea 100% (10.6%), DAP 100% (19.0%), and untreated control (69%) and other treatments, respectively.
4. Discussion
The world's population mainly depends on cereal crops, which are grown on more than 734.32 million acres of land.
Worldwide, a total of 2980.2 million tonnes of cereal crops are produced. These crops include buckwheat, millets crop, maize, rice, sorghum, triticale, wheat and other cereals. The average productivity of each cereal crops is usually reduced by several factors. In order to increase productivity, advanced farming methods should be combined with balanced and accurate nutrition in cereals. The three main mineral fertilizer N, P, and K, are used globally in 192 million tonnes at a cost of approximately US$ 91,238 million [29]. Additionally to cost, the use of mineral fertilizers is considered the biggest threat to world sustainability. The concerns about cereal crop productivity have increased the potential for the use of microbial consortium as bio-inoculants in a harmonic manner to provide and produce appropriate cereal crops yield.
Microbial inoculation is a time-honored technique for crop improvement, although inoculation of individual strain exhibits unpredictable results in the field by reason of their poor adaptability of individual inoculants to respond changing ecological conditions. Instantaneous studies on plants have shown that microbial consortia, can be used to increase the resilience of bacterial inoculants [30]. Consortium may achieve better enhancement in diverse soils having extensive range of temperature, pH, and humidity for activities including root colonization, phosphorus solubilization, nitrogen fixation, resistance against plant pathogens, secondary metabolic activities, antibiotic, and hormone production [31,32]. Consortium is a more dependable strategy for improving agricultural production and healthy plant growth than other methods because microbial strains work well together as consortia and have a range of functions without the need for genetic engineering [33,34]. On the other hand, the choosing appropriate combinations screened isolates remains a major challenge in the consortium development.
Under natural circumstances, the liquid bacterial inoculums in the rhizosphere may not be successful in promoting crop growth because of a variety of environmental restrictions, such as careless handling that causes cells to be dispersed into the air or groundwater and have the short shelf life. Due to these difficulties farmers are using less biofertilizers as compared to alternative techniques. Therefore, some materials, known as carriers, have the ability to able the growth of microbes and release in the rhizosphere, which are essential for the booming application of inoculants [35]. Depending on their origin, carriers can be either organic (e.g., manure, biogas slurry, crushed corn cobs, charcoal, and peat) or inorganic (e.g., zeolite, perlite, lignite, and talc). The major factors to consider are the cost and availability of the carrier. Another important consideration in choosing carriers is the variation between the microbial strains that are employed. The average number of carrier G1 cells with positive results is 107 CFU g−1 [36].
In the present study, the consortium of mineral solubilizing rhizospheric bacterial strains Rahnella aquatilis EU-A3Rb1, Erwinia aphidicola EU-A2RNL1, Brevibacillus brevis EU-C3SK2, and B. mycoides EU-WRSe4 which was associated wit different cereal crops were developed and evaluated for the growth of wheat plants. These microbes have been already reported as rhizopsheric bacteria associated with different plants except Erwinia aphidicola. In a report, R. aquatilis CF3 was isolated from the rhizosphere of wheat plant [37]. Similarly, R. aquatilis ISL19 from rhizosphere of soybean [38]. In another report, R. aquatilis KSB 39 was reported from the rhizospheric soil of paddy and enhance the availability of potassium in the soils [39]. Li et al. [40] reported, R. aquatilis JZ-GX1 from Masson pine rhizosphere soil significantly improved growth of maize seedlings. Earlier, E. aphidicola was reported from the endophytic region of Phaseolus vulgaris and Pisum sativum in Spain [41]. Similarly, E. aphidicola reported from endophytic regions of commercial bean seeds (Phaseolus vulgaris) [42]. Similarly Kaur et al. [24] reported the Erwinia sp. EU-B2SNL1 from interiors region of wheat plant and improved the growth of barley. In a previous study, Erwinia sp. was reported as an endophyte of coastal sand roots of dune plants [43]. B. mycoides EU-WRSe4 was sorted out from rhizospheric regions of plants growing in Jaisalmer. In a report, B. mycoides B38V isolated from the sunflower rhizosphere [44]. Similarly, Guerrero‐Barajas et al. [45] reported the B. mycoides from rhizospheric soil of avocado plants. In a study, B. brevis [SVC(II)14] was reported from the rhizosphere of the cotton and firstly reported for its PGP potential [46]. Similarly, B. brevis isolated from mangrove sediment soil [47].
In the present study, mineral solubilizing bacteria R. aquatilis EU-A3Rb1, E. aphidicola EU-A2RNL1, B. brevis EU-C3SK2, and B. mycoides EU-WRSe4 as beneficial plant growth promoting bacteria exhibiting P, K, Zn and Se solubilization activity. In the study, R. aquatilis EU-A3Rb1 was found to show highest solubilization of phosphorus (194.0 ± 0.05 mg L−1). The rsults showed proximity with a report in which R. aquatilis was reported for the solubilization of phosphorus [48]. Similarly, R. aquatilis KM977991 was reported for the solubilization of P [49]. Bechtaoui et al. [50], found that the R. aquatilis PGP13 solubilized P. In presnt study, E. aphidicola EU-A2RNL1 was found to be efficient K-solubilizers and B. brevis EU-C3SK2 was able to solubilization of zinc up to 55.6 ± 0.02 mg L−1. In a report, B. brevis HM590700 was reported for the solubilization of zinc compound i.e., ZnCO3 (10.6 ± 0.16 ppm), ZnO (22.4 ± 0.34 ppm), and ZnPO4 (126.6 ± 1.96 ppm) [51]. The present study reported B. mycoides EU-WRSe4 was able to solubilize Se on the basis of qualitative analysis.
In the present research, the microbial consortium was prepared by using different mineral solubilizing bacterial strains i.e., PSB (R. aquatilis EU-A3Rb1), KSB (E. aphidicola EU-A2RNL1), ZnSB (B. brevis EU-C3SK2), and SSB (B. mycoides EU-WRSe4) which were inoculated on the wheat plant for evaluation. This microbial mixture of mineralizing microbes have not yet reported in any study but these bacteria were develped as consortium with other microbes. In a report, microbial consortium was developed using the Serratia marcescens 59, Pseudomonas fluorescens 57, Rahnella aquatilis 36 and B. amyloliquefaciens 63 for the inoculation of chickpea plant [52]. Similarly, Maciag et al. [53] used five combination of microbial strain of S. plymuthica A294, E. amnigenus A167, Rahnella aquatilis H145, S. rubidaea H440, and S. rubidaea H469 used as liquid inoculants on potato. Mahmood et al. [54], developed the microbial consortium using B. mycoides, B. cereus, Bacillus sp., Pseudomonas sp., Micrococcus sp., and B. subtilis. In a report, the consortium was developed using four different mineral solubilizing bacterial strains, Brevibacillus brevis MS1, B. licheniformis MS3, Micrococcus sp. MS4, and Acinetobacter calcoaceticus MS5 and inoculated on Jatropha curcas [55]. Similarly, Vecstaudža et al. [56] reported the consortium of B. brevis, Enterobacter sp., and Pseudomonas sp. for the improvement of the growth of barley.
The present investigation of bacterial consortium showed the enhanced growth of the shoot and root length and fresh/dry weight) and physiological parameters (content of chlorophyll, carotenoids, phenolics, flavonoids, and total soluble sugar) of the wheat crop over single inoculants, chemicals and control. Another studies have also reported that consortium increases the plant growth and physiolgical paramtere more as compare to singel culture, chemical and control. In the plant of Jatropha curcas L. shoot length enhancement was reported to be increased by 61.43% over control by the microbial consortium of A. calcoaceticus MS5, B. brevis MS1, B. licheniformis MS3, and Micrococcus sp. MS4 [55]. The present study found that the microbial consortium of R. aquatilis EU-A3Rb1, E. aphidicola EU-A2RNL1, B. brevis EU-C3SK2, and B. mycoides EU-WRSe4 enhanced the roots length by 2.0 fold. The study revealed that consortium of B. siamensis, R. aceris, Pantoea hericii, and B. paramycoides improved the root length 26% and 37% of wheat plant [57]. In present investigation microbial consortium of EU-A3Rb1, EU-A2RNL1, EU-C3SK2, and EU-WRSe4 increased the wheat fresh weight and dry biomass up to 3.1 and 4.0 folds. The same study have been reported in which Amaranthus plant fresh weight and dry biomass was enhanced by 1.2 and 2.0 fold by the microbial consortium of P. gessardi, Bacillus sp., and E. rhapontici [58]. The content of chlorophyll and carotenoids has been enhanced by the EU-A3Rb1, EU-A2RNL1, EU-C3SK2, and EU-WRSe4 consortium of wheat plant by 2.3and 2.8 fold. Kaur et al. [24], reported the microbial Erwinia sp. EU-B2SNL, Chryseobacterium arthrosphaerae EU-LWNA-37, and P. gessardii EU-MRK-19 increasing the content of chlorophyll by 3.6 fold and carotenoid 3.3 fold in barley plant. In the present study, the bacterial consortium was reported to increase the phenolic and flavonoids content of 2.5, and 1.4 fold of the wheat plant. The similar studies has reported the phenolic and flavonoids content increase by the three compatible microbes including E. persicina EU-A3SK3, P. extremorientalis EU-B1RTR1, and Halomonas aquamarina EU-B2RNL2 in chilli plant [59]. In this research, the microbial consortium has increased the total soluble sugar content (2.2 fold) of the wheat crop. Similarly Khan et al. [60] reported the total soluble sugar content in Sorghum bicolor was increased by 3.61 mg g−1 over control by the bacterial consortium of B. brevis, B. pumilis, and Paenibacillus thiaminolyticus.
In conclusion, the mineral solubilizing microbes R. aquatilis EU-A3Rb1, E. aphidicola EU-A2RNL1, B. brevis EU-C3SK2, and B. mycoides EU-WRSe4 used as consortium enriched the growth of wheat more over single inoculants, chemicals and un-inoculated control. In the present time, reduction in chemical fertilizers is emerging to maintain the environment and agriculture. The use of bacterial consortium can be a suitable bio-formulation for better crop production. In the forthcoming bacterial consortium can be evaluated on different cereal crops. The strains can be genetically modified by genetic engineering for better improvement of crop productivity and growth.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Rubee Devi: Writing – original draft. Tanvir Kaur: Writing – original draft. Rajeshwari Negi: Writing – original draft. Divjot Kour: Writing – review & editing. Sanjeev Kumar: Writing – review & editing. Ashok Yadav: Writing – review & editing. Sangram Singh: Writing – review & editing. Kundan Kumar Chaubey: Writing – review & editing. Ashutosh Kumar Rai: Writing – review & editing. Sheikh Shreaz: Writing – review & editing. Ajar Nath Yadav: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful to the Department of Genetics, Plant Breeding and Biotechnology, Dr. Khem Singh Gill Akal College of Agriculture, Eternal University, Baru Sahib, Sirmour Himachal Pradesh, India and Department of Environment, Science & Technology (DEST), Shimla, Himachal Pradesh, India funded project “Development of microbial consortium as bio-inoculants for drought and low temperature growing crops for organic farming in Himachal Pradesh” for providing the facilities support, to undertake the investigations.
References
- 1.Shewry P.R., Hey S.J. The contribution of wheat to human diet and health. Food Energ. Secur. 2015;4:178–202. doi: 10.1002/fes3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi A., Ahmad E., Khan M.S., Saif S., Rizvi A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: current perspective. Sci. Hortic. 2015;193:231–239. [Google Scholar]
- 3.Negi R., Kaur T., Devi R., Kour D., Yadav A.N. Assessment of nitrogen-fixing endophytic and mineral solubilizing rhizospheric bacteria as multifunctional microbial consortium for growth promotion of wheat and wild wheat relative Aegilops kotschyi. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghazal F., Mahdy E., El-Fattah M., El-Sadany A., Doha N. The use of cyanobacteria as biofertilizer in wheat cultivation under different nitrogen rates. Nat. Sci. 2018;16:234–239. [Google Scholar]
- 5.Kour D., Rana K.L., Yadav A.N., Yadav N., Kumar M., Kumar V., Vyas P., Dhaliwal H.S., Saxena A.K. Microbial biofertilizers: bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020;23 [Google Scholar]
- 6.Idris E.E., Iglesias D.J., Talon M., Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microb. Interact. 2007;20:619–626. doi: 10.1094/MPMI-20-6-0619. [DOI] [PubMed] [Google Scholar]
- 7.Cakmakci R., Dönmez M.F., Erdoğan Ü. The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk. J. Agric. For. 2007;31:189–199. [Google Scholar]
- 8.Çakmakçi R., Dönmez F., Aydın A., Şahin F. Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol. Biochem. 2006;38:1482–1487. [Google Scholar]
- 9.Ekin Z. Performance of phosphate solubilizing bacteria for improving growth and yield of sunflower (Helianthus annuus L.) in the presence of phosphorus fertilizer. Afr. J. Biotechnol. 2010;9:3794–3800. [Google Scholar]
- 10.Kevin Vessey J. Effect of Rhizobacterial biofertilizer on plant growth. Plant Soil. 2003;255:571–586. [Google Scholar]
- 11.Mondal S., Halder S.K., Yadav A.N., Mondal K.C. In: Advances in Plant Microbiome and Sustainable Agriculture: Functional Annotation and Future Challenges. Yadav A.N., Rastegari A.A., Yadav N., Kour D., editors. Springer; Singapore: 2020. Microbial consortium with multifunctional plant growth-promoting attributes: future perspective in agriculture; pp. 219–258. [Google Scholar]
- 12.Santoyo G., Guzmán-Guzmán P., Parra-Cota F.I., Santos-Villalobos S.d.l., Orozco-Mosqueda M.d.C., Glick B.R. Plant growth stimulation by microbial consortia. Agronomy. 2021;11:219. [Google Scholar]
- 13.Kumar A., Maurya B., Raghuwanshi R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.) Biocatal. Agric. Biotechnol. 2014;3:121–128. [Google Scholar]
- 14.Moretti L.G., Crusciol C.A.C., Bossolani J.W., Momesso L., Garcia A., Kuramae E.E., Hungria M. Bacterial consortium and microbial metabolites increase grain quality and soybean yield. J. Soil Sci. Plant Nutr. 2020;20:1923–1934. [Google Scholar]
- 15.Verma P., Yadav A.N., Kazy S.K., Saxena A.K., Suman A. Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:432–447. [Google Scholar]
- 16.Conn V.M., Franco C.M. Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl. Environ. Microbiol. 2004;70:6407–6413. doi: 10.1128/AEM.70.11.6407-6413.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;17:362–370. [Google Scholar]
- 18.Murphy J., Riley J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962;27:31–36. [Google Scholar]
- 19.Fasim F., Ahmed N., Parsons R., Gadd G.M. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol. Lett. 2002;213:1–6. doi: 10.1111/j.1574-6968.2002.tb11277.x. [DOI] [PubMed] [Google Scholar]
- 20.Francis A.J., Dodge C., Chendrayan K. Google Patents; 1988. H.L., Quinby Anaerobic Microbial Dissolution of Lead and Production of Organic Acids. [Google Scholar]
- 21.Hu X., Chen J., Guo J. Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006;22:983–990. [Google Scholar]
- 22.Ruanchaiman S., Kumsopa A., Boontanon N., Prapagdee B. Dispersion of cadmium-resistant bacteria in cadmium-contaminated soils at Mae Sot district, Tak province. Appl. Environ. Res. 2009;31:35–48. [Google Scholar]
- 23.Yadav A.N., Sachan S.G., Verma P., Saxena A.K. vol 54. 2016. pp. 142–150. (Bioprospecting of plant growth promoting psychrotrophic Bacilli from the cold desert of North Western Indian Himalayas). [PubMed] [Google Scholar]
- 24.Kaur T., Devi R., Kumar S., Sheikh I., Kour D., Yadav A.N. Microbial consortium with nitrogen fixing and mineral solubilizing attributes for growth of barley. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenthaler H.K. Chlorophyll fluorescence signatures of leaves during the autumnal chlorophyll breakdown. J. Plant Physiol. 1987;131:101–110. [Google Scholar]
- 26.Irigoyen J., Einerich D., Sánchez‐Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992;84:55–60. [Google Scholar]
- 27.Patel A., Patel A., Patel A., Patel N. Estimation of flavonoid, polyphenolic content and in vitro antioxidant capacity of leaves of Tephrosia purpurea Linn.(Leguminosae) Int. J. Pharm. Sci. Res. 2010;1:66–77. [Google Scholar]
- 28.Clewer A.G., Scarisbrick D.H. John Wiley & Sons; 2013. Practical Statistics and Experimental Design for Plant and Crop Science. [Google Scholar]
- 29.Behera B., Das T., Raj R., Ghosh S., Raza M., Sen S. Microbial consortia for sustaining productivity of non-legume crops: prospects and challenges. Agric. Res. 2021;10:1–14. [Google Scholar]
- 30.Vassilev N., Vassileva M., Lopez A., Martos V., Reyes A., Maksimovic I., Eichler-Löbermann B., Malusa E. Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 2015;99:4983–4996. doi: 10.1007/s00253-015-6656-4. [DOI] [PubMed] [Google Scholar]
- 31.Elkoca E., Turan M., Donmez M.F. Effects of single, dual and triple inoculations with Bacillus subtilis, Bacillus megaterium and Rhizobium leguminosarum bv. Phaseoli on nodulation, nutrient uptake, yield and yield parameters of common bean (Phaseolus vulgaris l. cv.‘elkoca-05’) J. Plant Nutr. 2010;33:2104–2119. [Google Scholar]
- 32.Upadhyay S.K., Singh J.S., Saxena A.K., Singh D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012;14:605–611. doi: 10.1111/j.1438-8677.2011.00533.x. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019;37 doi: 10.1016/j.biotechadv.2019.107423. [DOI] [PubMed] [Google Scholar]
- 34.Zahir Z.A., Ahmad M., Hilger T.H., Dar A., Malik S.R., Abbas G., Rasche F. Field evaluation of multistrain biofertilizer for improving the productivity of different mungbean genotypes. Soil Environ. 2018;37:45–52. [Google Scholar]
- 35.Zafar-ul-Hye M., Bhutta T.S., Shaaban M., Hussain S., Qayyum M.F., Aslam U., Zahir Z.A. Influence of plant growth promoting rhizobacterial inoculation on wheat productivity under soil salinity stress. Phyton. 2019;88:119–129. [Google Scholar]
- 36.Sethi S.K., Adhikary S.P. Cost effective pilot scale production of biofertilizer using Rhizobium and Azotobacter. Afr. J. Biotechnol. 2012;11:13490–13493. [Google Scholar]
- 37.Berge O., Heulin T., Achouak W., Richard C., Bally R., Balandreau J. Rahnella aquatilis, a nitrogen-fixing enteric bacterium associated with the rhizosphere of wheat and maize. Can. J. Microbiol. 1991;37:195–203. [Google Scholar]
- 38.Kim K.Y., Jordan D., Krishnan H.B. Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiol. Lett. 1997;153:273–277. [Google Scholar]
- 39.Yaghoubi Khanghahi M., Pirdashti H., Rahimian H., Nematzadeh G., Ghajar Sepanlou M. Potassium solubilising bacteria (KSB) isolated from rice paddy soil: from isolation, identification to K use efficiency. Symbiosis. 2018;76:13–23. [Google Scholar]
- 40.Li G.-E., Kong W.-L., Wu X.-Q., Ma S.-B. Phytase-Producing Rahnella aquatilis JZ-GX1 promotes seed germination and growth in corn (Zea mays L.) Microorganisms. 2021;9:1647. doi: 10.3390/microorganisms9081647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos M., Diánez F., Miñano J., Marín F., Martínez S., decara M., Tello J. First report of Erwinia aphidicola from Phaseolus vulgaris and Pisum sativum in Spain. Plant Pathol. 2009;58:1171. [Google Scholar]
- 42.Marín F., Santos M., Carretero F., Yau J., Diánez F. Erwinia aphidicola isolated from commercial bean seeds (Phaseolus vulgaris) Phytoparasitica. 2011;39:483–489. [Google Scholar]
- 43.Shin D.S., Park M.S., Jung S.R., Lee M.S., Lee K.H., Bae K.S., Kim S.B. Plant growth-promoting potential of endophytic bacteria isolated from roots of coastal sand dune plants. J. Microbiol. Biotechnol. 2007;17:1361–1368. [PubMed] [Google Scholar]
- 44.Ambrosini A., Stefanski T., Lisboa B., Beneduzi A., Vargas L., Passaglia L. Diazotrophic bacilli isolated from the sunflower rhizosphere and the potential of Bacillus mycoides B38V as biofertiliser. Ann. Appl. Biol. 2016;168:93–110. [Google Scholar]
- 45.Guerrero‐Barajas C., Constantino‐Salinas E.A., Amora‐Lazcano E., Tlalapango‐Ángeles D., Mendoza‐Figueroa J.S., Cruz‐Maya J.A., Jan‐Roblero J. Bacillus mycoides A1 and Bacillus tequilensis A3 inhibit the growth of a member of the phytopathogen Colletotrichum gloeosporioides species complex in avocado. J. Sci. Food Agric. 2020;100:4049–4056. doi: 10.1002/jsfa.10450. [DOI] [PubMed] [Google Scholar]
- 46.Nehra V., Saharan B.S., Choudhary M. Evaluation of Brevibacillus brevis as a potential plant growth promoting rhizobacteria for cotton (Gossypium hirsutum) crop. SpringerPlus. 2016;5:1–10. doi: 10.1186/s40064-016-2584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arumugam T. Optimization of media components for production of antimicrobial compound by Brevibacillus brevis EGS9 isolated from mangrove ecosystem. J. Microbiol. Methods. 2017;142:83–89. doi: 10.1016/j.mimet.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Kim K.Y., Jordan D., Krishnan H.B. Expression of genes from Rahnella aquatilis that are necessary for mineral phosphate solubilization in Escherichia coli. FEMS Microbiol. Lett. 1998;159:121–127. doi: 10.1111/j.1574-6968.1998.tb12850.x. [DOI] [PubMed] [Google Scholar]
- 49.Bakhshandeh E., Pirdashti H., Lendeh K.S. Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol. Eng. 2017;103:164–169. [Google Scholar]
- 50.Bechtaoui N., El Alaoui A., Raklami A., Benidire L., Tahiri A.-i., Oufdou K. Impact of intercropping and co-inoculation with strains of plant growth-promoting rhizobacteria on phosphorus and nitrogen concentrations and yield of durum wheat (Triticum durum) and faba bean (Vicia faba) Crop Pasture Sci. 2019;70:649–658. [Google Scholar]
- 51.Vivas A., Barea J.M., Azcón R. Brevibacillus brevis isolated from cadmium-or zinc-contaminated soils improves in vitro spore germination and growth of Glomus mosseae under high Cd or Zn concentrations. Microb. Ecol. 2005;49:416–424. doi: 10.1007/s00248-004-0044-4. [DOI] [PubMed] [Google Scholar]
- 52.Palmieri D., Vitullo D., De Curtis F., Lima G. A microbial consortium in the rhizosphere as a new biocontrol approach against fusarium decline of chickpea. Plant Soil. 2017;412:425–439. [Google Scholar]
- 53.Maciag T., Krzyzanowska D.M., Jafra S., Siwinska J., Czajkowski R. The Great Five—an artificial bacterial consortium with antagonistic activity towards Pectobacterium spp. and Dickeya spp.: formulation, shelf life, and the ability to prevent soft rot of potato in storage. Appl. Microbiol. Biotechnol. 2020;104:4547–4561. doi: 10.1007/s00253-020-10550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahmood R., Sharif F., Ali S., Hayyat M.U. Enhancing the decolorizing and degradation ability of bacterial consortium isolated from textile effluent affected area and its application on seed germination. Sci. World J. 2015;2015 doi: 10.1155/2015/628195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jha C.K., Saraf M. Evaluation of multispecies plant-growth-promoting consortia for the growth promotion of Jatropha curcas L. J. Plant Growth Regul. 2012;31:588–598. [Google Scholar]
- 56.Vecstaudža D., Seņkovs M., Nikolajeva V., Mutere O. Characteristics of an endophytic microbial consortium and its impact on rhizosphere microbiota of barley. Environ. Exp. Biol. 2018;16:177–183. [Google Scholar]
- 57.Khourchi S., Elhaissoufi W., Loum M., Ibnyasser A., Haddine M., Ghani R., Barakat A., Zeroual Y., Rchiad Z., Delaplace P. Phosphate solubilizing bacteria can significantly contribute to enhance P availability from polyphosphates and their use efficiency in wheat. Microbiol. Res. 2022;262 doi: 10.1016/j.micres.2022.127094. [DOI] [PubMed] [Google Scholar]
- 58.Devi R., Kaur T., Kour D., Yadav A.N. Microbial consortium of mineral solubilizing and nitrogen fixing bacteria for plant growth promotion of amaranth (Amaranthus hypochondrius L.) Biocatal. Agric. Biotechnol. 2022;43 [Google Scholar]
- 59.Devi R., Kaur T., Kour D., Yadav A.N., Suman A. Potential applications of mineral solubilizing rhizospheric and nitrogen fixing endophytic bacteria as microbial consortium for the growth promotion of chilli (Capsicum annum L.) Biologia. 2022:1–11. [Google Scholar]
- 60.Khan S., Bhardwaj U., Iqbal H.M., Joshi N. Synergistic role of bacterial consortium to biodegrade toxic dyes containing wastewater and its simultaneous reuse as an added value. Chemosphere. 2021;284 doi: 10.1016/j.chemosphere.2021.131273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.