In the context of the People's Republic of China, coronary artery disease (CAD) presents a significant clinical challenge, with over 11.3 million patients diagnosed. Traditionally, the diagnosis of CAD has predominantly relied on invasive coronary angiography.[1] However, recent advances in clinical research have revealed a notable trend: a substantial 82% of patients subjected to such invasive diagnostics do not necessitate interventional therapy.[2] In order to standardize the clinical diagnosis and treatment strategy of CAD myocardial ischemia, this revelation has led to a paradigm shift in the diagnostic and management approaches for myocardial ischemia associated with CAD. Current international guidelines have evolved to prioritize stress imaging tests as the primary diagnostic step for patients presenting with chronic chest pain or suspected CAD. Within this framework, stress echocardiography (SE) has gained recognition as a Class I recommended diagnostic protocol, especially for patients exhibiting symptoms of stable angina.[3]
In 1982, Prof. Zhang Yun pioneered the discovery in China that stress M-mode echocardiography, when used to detect abnormal wall motion, surpasses the electrocardiogram exercise test in sensitivity and specificity for diagnosing CAD.[4] The EVAREST study of 2022, a significant real-world study, further substantiated the efficacy of SE. This study reported remarkably high sensitivity (95.4%) and specificity (96%) of SE in predicting obstructive coronary artery lesions, flow-limiting lesions, acute coronary syndromes (ACS), and cardiac-related mortality.[5] The International Guidelines for SE Examination of Ischemic Heart Disease have established the technical and diagnostic criteria for CAD stress ultrasound examination.[6]
In China, despite the prevalent incidence of stable coronary artery disease, the clinical adoption of non-invasive imaging stress tests remains disproportionately low, which has not effectively solved the problem of reliable qualitative and/or quantitative assessment of myocardial ischemia caused by various reasons and related diagnostic issues of cardiac microvascular disease. Reflecting this advancement, the European Society of Cardiology (ESC) introduced in 2019 the concept of chronic coronary syndrome (CCS). This concept encompasses a broader spectrum of CAD subgroups, extending beyond the scope of acute coronary syndromes.[7]
To further the implementation of SE in the diagnosis of CCS within China, this guideline has been collaboratively developed by the Chinese Society of Echocardiograp hy, the Superficial Tissue and Vascular Group of Chinese Society of Ultrasound in Medicine, and the Ultrasound Professional Committee of Chinese Medicine Education Association. Notably, China promulgated a standardized operational guideline for SE in 2017, particularly highlighting exercise stress and dobutamine stress echocardiography in the context of cardiovascular diseases.[8] This current guideline, tailored specifically for the CCS demographic, delineates the prevalent SE methodologies, which are applicable to both major cardiac and microvascular alterations. It underscores the aspects of practicality and universality to facilitate more precise diagnostics and treatment modalities for CCS patients.
To clarify the cognition of clinical usefulness and efficacy of examination modalities, this guideline inherits the international common Class of Recommendation (COR) system:
Class I (COR I): Examinations and/or treatments that have been proven and/or universally recognized to be beneficial, useful and effective.
Class II (COR II): Examinations and/or treatments for which evidence of usefulness and efficacy is conflicting or divergent opinions exist.
Class IIa (COR IIa): Evidence/opinions are in favor of usefulness and/or efficacy. Applying these examinations and/or treatments is reasonable.
Class IIb (COR IIb): Usefulness and/or efficacy have not been fully established by evidence/opinions. These examinations and/or treatments may be considered.
Class III (COR III): Examinations and treatments that have been proven and/or universally recognized to be useless and ineffective, and may be harmful in some cases. Their application is not recommended.
The Level of Evidence (LOE) is graded as:
Level A (LOE A): Evidence from multiple randomized clinical trials or meta-analyses.
Level B (LOE B): Evidence from a single randomized trial or multiple non-randomized controlled studies.
Level C (LOE C): Only expert consensus opinions and/or small clinical trials, retrospective studies, or registry studies.
1. THE PRINCIPLE OF STRESS ECHOCARDIOGRAPHY IN EVALUATING CHRONIC CORONARY SYNDROME
CAD is defined as a clinical syndrome where myocardial ischemia leads to cardiac structural and functional impairments, primarily due to coronary artery lesions. CCS includes six clinical states within the CAD spectrum:[7] (1) patients with suspected CAD and ‘stable’ anginal symptoms, and/or dyspnoea; (2) patients with new onset of heart failure (HF) or left ventricular (LV) dysfunction and suspected CAD; (3) asymptomatic and symptomatic patients with stabilized symptoms < 1 year after an ACS, or patients with recent revascularization; (4) asymptomatic and symptomatic patients > 1 year after initial diagnosis or revascularization; (5) patients with angina and suspected vasospastic or microvascular disease; and (6) asymptomatic subjects in whom CAD is detected at screening. CCS characterizes a state of imbalance between the myocardial blood supply and oxygen demand, which may either remain relatively stable or progressively deteriorate over time.
Epicardial coronary artery stenosis significantly impacts the myocardial blood and oxygen supply. Stenosis exceeding 70% in arteries larger than 2.5 mm in diameter, and over 50% in the left main coronary artery, are generally considered flow-limiting, also termed as obstructive CAD.[9] However, the degree of stenosis in the epicardial coronary artery may not always align with the severity of myocardial ischemia.[10-11] Therefore, evaluating the blood flow beyond the narrowed area is crucial. Coronary flow reserve (CFR) and the coronary flow reserve fraction are pivotal metrics recommended for assessing coronary artery function.[12-14]
Current studies show that approximately 40% of patients undergoing coronary angiography for chest pain do not have obstructive coronary artery lesions.[15-16] This is partly due to the underrecognition of diffuse atherosclerosis in coronary angiography. Moreover, coronary microvascular dysfunction (CMVD) is increasingly recognized as a significant factor in chest pain.[17] CMVD may occur alone or alongside obstructive epicardial coronary artery disease, particularly in female CCS patients.[16] The distribution of microcirculation in the ventricular wall myocardium predisposes the subendocardial myocardium to ischemia. An increase in myocardial oxygen consumption is a key pathophysiological mechanism in CCS, with triggers including elevated heart rate, increased left ventricular afterload (systolic wall stress, arterial elasticity, systolic pressure, etc.), and preload (left ventricular end-diastolic volume, diastolic wall stress, myocardial elasticty, etc).
The process of myocardial ischemia starts with decreased myocardial perfusion, leading to hypoxia and metabolic abnormalities in myocardial cells. This reduction in perfusion results in decreased mitochondrial energy production. Subsequently, local myocardial diastolic function is impaired, followed by contractile function abnormalities, electrocardiographic changes, and clinical angina symptoms. An additional factor is the imbalance caused by increased myocardial oxygen consumption not being met with a proportional increase in oxygen supply, leading to ischemia. The point at which angina or ischemic changes in the electrocardiogram occur, determined by the heart rate and blood pressure product, is termed the "ischemic threshold".[18] In clinical practice, myocardial ischemia is often induced by increasing exercise stress or administering sympathomimetic agents to induce positive inotropic and chronotropic effects and to heighten myocardial oxygen consumption, a key technique in SE examinations (Figure 1).
Figure 1.
Factors determining myocardial oxygen consumption and supply, and the principle of stress testing.
The imbalance between myocardial oxygen consumption and supply is the fundamental cause of myocardial ischemia. Factors determining myocardial oxygen consumption mainly include wall stress, heart rate, and myocardial contractility, where wall stress is determined by myocardial fiber length, ventricular volume, and aortic pressure. Factors determining myocardial oxygen supply include the oxygen-carrying capacity of the blood and coronary artery blood flow, which in turn is determined by coronary artery flow resistance, perfusion pressure, and perfusion duration. The coronary vascular tree, composed of epicardial coronary arteries and micro-vessels, provides blood flow as shown by the blue area in the figure under resting conditions. When myocardial oxygen consumption increases, the coronary vascular tree can dilate to increase blood flow, matching the oxygen supply with the increased demand. When the coronary vascular tree reaches its maximum dilation, the increase in blood flow compared to the resting state represents the coronary flow reserve. Stress testing assesses the reserve capacity of the coronary vascular tree through exercise or pharmacological means or detects any imbalance between myocardial oxygen consumption and supply as the stress increases.
CFR is defined as the maximal capacity of the coronary artery system to augment oxygen supply in response to increased demand. In clinical settings, vasodilators are used to enhance coronary microvasculature dilation, aiding in evaluating blood flow reserve. The simultaneous assessment of left ventricular myocardial perfusion and CFR is achievable through the integration of myocardial contrast echocardiography (MCE) with coronary flow velocity measurement.
Under normal conditions, there is a balance between myocardial oxygen supply and demand. However, an increase in myocardial oxygen consumption or a decrease in oxygen supply can disrupt this balance, leading to the "ischemic threshold", which may induce myocardial ischemia.
In patients with CCS, SE is utilized to assess myocardial systolic and diastolic functions, observe myocardial blood flow perfusion, and evaluate CFR capacity and myocardial viability. SE serves as a valuable visual tool for risk stratification, guiding treatment strategy selection, and prognostic assessment in CCS patients.
2. REQUIREMENTS FOR STRESS ECHOCARDIOGRAPHY PERFORMERS AND EQUIPMENT
2.1. Team Requirements
The SE team should include cardiologists or ultrasound physicians with extensive clinical experience and cardiology knowledge, ultrasound technicians, nurses, and ECG technicians. The protocol is to be executed by trained cardiologists or echocardiographers, who should handle a minimum of 10 SE examinations monthly to maintain expertise, including emergency identification and response.[6,19] The use of ultrasound enhancing agents (UEA) may be necessary, requiring nurses proficient in intravenous injections and personnel trained in managing allergic reactions. Training in UEA use and contrast echocardiography image analysis is vital.[20] While severe adverse reactions during SE are rare, strict medical monitoring and emergency preparedness are crucial, it is still necessary to have medical supervision and emergency response during stress testing.
2.2. Equipment Requirements
For SE examinations, high-quality ultrasound imaging is crucial. It's recommended to use advanced cardiac ultrasound equipment with tissue harmonic imaging and ultrasound contrast features, equipped with 2D/3D probes. A specialized cardiac ultrasound examination bed should be available. Depending on the procedure, a treadmill or supine bicycle, as well as an intravenous infusion pump/micropump, are required. Additionally, cardiac and blood pressure monitors, a resuscitation cart with emergency supplies, and a defibrillator should be readily available in the examination area.
The SE examination analysis software should enable simultaneous display of cardiac ultrasound images at rest and post-stress. It must support various stress protocols, such as exercise or pharmacological stress. Specialized software enhances workflow and accuracy in interpreting image comparisons at different times, crucial when there are baseline wall motion abnormalities. The stress test protocol should display cardiac plane images in a four-quadrant format, with the resting image typically in the upper left quadrant, and the other three quadrants represent images of each stress stage. For contrast echocardiography, storing 10 to 15 cardiac cycles of images per session is recommended.
Recommendations:
(1) Physicians performing SE exams must complete specialized training in both resting and SE techniques and interpretation. This training is essential for effective and accurate conduct of combined echocardiography (COR I, LOE C).
(2) For optimal analysis, SE images at rest and post-stress should be synchronously displayed on the same screen. A four-quadrant format is recommended for each plane, showing rest and stress stage images for efficient interpretation and comparative analysis (COR I, LOE C).
3. STRESS ECHOCARDIOGRAPHY MODALITIES FOR CHRONIC CORONARY SYNDROME AND INDICATIONS/CONTRAINDICATIONS
SE examinations are widely used for both ischemic and non-ischemic cardiovascular diseases, with a primary focus on patients with CCS.
3.1. SE Examination Methods
(1) Exercise stress test: recommended for patients who can tolerate exercise, including treadmill, bicycle, step, and isometric handgrip exercises.
(2) Pharmacological stress test: for patients who cannot tolerate exercise, positive inotropic agents can be used to achieve the target heart rate, or vasodilators can be used to observe myocardial perfusion and coronary reserve. The main drugs include dobutamine, dipyridamole, adenosine, regadenoson, and isoproterenol.
(3) Pacing stress test: in patients with pacemakers, increasing pacing rate can elevate heart rate and myocardial oxygen consumption. Transesophageal atrial pacing is an alternative for non-exercising patients.
(4) Cold pressor stress test: involves cold stimulation to induce vasoconstriction, increasing afterload and myocardial demand, which may induce ischemia.
This guideline mainly addresses exercise stress and pharmacological SE exams. Indications and contraindications for SE are detailed in Table 1.
Table 1. Common indications and contraindications for stress echocardiography testing.
| Exercise Stress Echocardiography Indications | Dobutamine Stress Echocardiography Indications | Vasodilator Stress Echocardiography Indications |
| SE:stress echocardiography; CTA: CT Angiography; CCS: Chronic Coronary Syndrome. 1 mmHg = 0.133 kPa. | ||
| 1. Diagnosis of chronic coronary artery disease. | Same as exercise SE, particularly for patients unable to undergo exercise stress testing or for whom exercise stress testing is not ideal. | 1. CCS patients unable to exercise or with contraindications to exercise, or those with poor resting echocardiographic images (which may complicate image interpretation post-exercise stress). |
| 2. Risk stratification and prognosis assessment in patients with confirmed CCS via CTA or coronary angiography. | 2. Patients with recurrent chest pain, typical exertional angina, or resting angina attacks, and ischemic ST-segment depression during resting or exercise stress test, but with normal or mild stenosis (below 20%) in coronary artery CTA or angiography, suggesting microvascular dysfunction, requiring observation of coronary flow reserve function. | |
| 3. Preoperative risk assessment for non-cardiac surgery in CCS patients. | 3. CCS patients with known coronary artery borderline lesions (30%–70% diameter stenosis) or diameter stenosis below 90%, requiring observation of coronary flow reserve function or myocardial perfusion. | |
| 4. Evaluation of treatment effectiveness or recurrent chest pain post coronary revascularization surgery. | 4. Evaluation of the effectiveness of pharmacological and non-pharmacological treatments. | |
| 5. Assessment of myocardial ischemia location and viability. | 5. Unstable angina, non-ST-segment elevation myocardial infarction, and acute ST-segment elevation myocardial infarction patients requiring observation of coronary flow reserve function or myocardial perfusion during vasodilation, examinations can be performed after 6 days of onset. | |
| 6. Etiological diagnosis of exertional dyspnea (presence or absence of myocardial ischemia). | ||
| 7. Assessment of coronary flow reserve function. | ||
| 8. Evaluation of myocardial ischemic functional reserve. | ||
| Absolute Contraindications | Absolute Contraindications: | Absolute Contraindications |
| 1. Patients with unstable conditions or complications of acute coronary syndrome, myocardial infarction within the last 2 days. | 1. Allergy to dobutamine or atropine. | 1. Active bronchospastic diseases (Adenosine and dipyridamole can cause bronchospasm, regadenoson less likely to cause). |
| 2. Hemodynamically unstable or symptomatic malignant arrhythmias. | 2. Patients with unstable conditions or complications of acute coronary syndrome, myocardial infarction within the last 3 days. | 2. Baseline blood pressure lower than 90/60 mmHg or hypertension (systolic pressure >180 mmHg or diastolic pressure >110 mmHg). |
| 3. Symptomatic severe aortic stenosis with mean transvalvular pressure gradient ≥40 mmHg. | 3. Severe arrhythmias (recurrent sustained supraventricular arrhythmias, new significant ventricular arrhythmias, second or third-degree atrioventricular block). | 3. Severe arrhythmias including hemodynamically significant arrhythmias, severe bradycardic arrhythmias (Second or third-degree atrioventricular block and sick sinus syndrome, except in patients with pacemakers). |
| 4. Severe left ventricular outflow tract obstruction with mean pressure gradient ≥40 mmHg. | 4. Severe hypertension (resting systolic pressure >180 mmHg, diastolic pressure >110 mmHg). | 4. Unstable or complicated acute coronary syndrome. |
| 5. Uncontrolled symptomatic heart failure. | 5. Active myocarditis or endocarditis. | 5. Symptomatic moderate to severe valvular heart disease (Severe aortic stenosis, Adenosine or regadenoson relatively safe). |
| 6. Concomitant acute pulmonary embolism, pulmonary infarction, deep vein thrombosis. | 6. Acute heart failure. | 6. Acute myocarditis or pericarditis, acute aortic dissection, hypertrophic cardiomyopathy or other causes of outflow obstruction, clinically uncontrolled or symptomatic heart failure. |
| 7. Concomitant acute myocarditis or pericarditis. | 7. Left ventricular thrombus. | 7. Severe liver or renal failure, electrolyte disturbances, drug poisoning, drug allergies. |
| 8. Concomitant acute aortic dissection. | 8. Pregnancy, breastfeeding, moderate to severe anemia, hyperthyroidism, mental disorders. | |
| 9. Pregnancy. | ||
| Relative Contraindications | Relative Contraindications | Relative Contraindications |
| 1. Left main coronary artery stenosis. | 1. Paroxysmal supraventricular arrhythmias. | 1. Use of methylene blue. |
| 2. Moderate aortic valve stenosis (mean transvalvular pressure gradient of 20–40 mmHg). | 2. Moderate to severe aortic valve stenosis. | 2. History of allergic respiratory diseases. |
| 3. Electrolyte abnormalities. | 3. Resting left ventricular outflow tract obstruction (maximum pressure gradient >30 mmHg). | 3. Intake of adenosine receptor antagonists and drugs that increase adenosine concentration, effects of adenosine influenced by intake of caffeine, tea, chocolate within <12 hours. |
| 4. Severe hypertension (resting systolic pressure >180 mmHg or diastolic pressure >110 mmHg). | 4. Aortic aneurysm >4.0 cm. | |
| 5. Uncontrollable tachycardia or bradycardia. | ||
3.2. Exercise Stress Echocardiography Examination
This includes two main modes: treadmill exercise and bicycle ergometer exercise.
(1) Operational key points
(a) Treadmill exercise SE examination
The Bruce protocol is the preferred method for treadmill exercise in SE exams.[21] For those with lower exercise capacity, a modified Bruce protocol can be used.[22] Exercise intensity increases every 3 min until reaching the maximum limit or termination criteria.[21] Imaging is conducted at rest, immediately post-exercise, and during the recovery phase. It’s vital to capture images within 90 s after exercise, as wall motion abnormalities may quickly normalize. This includes collecting a comprehensive set of cardiac images, including apical four-chamber, two-chamber, and three-chamber views, and additional short-axis views of the left ventricle as necessary. Proper breathing instructions post-exercise are essential for clear imaging. Rapid heart rate recovery after exercise is indicative of a better prognosis but is not a substitute for a positive treadmill exercise SE result.[6]
(b) Bicycle Ergometer Exercise SE Examination
The initial workload for bicycle ergometer exercise in SE starts at 25 W, increasing every 2-3 min until reaching the endpoint (symptoms, electrocardiogram, and echocardiographic indicators).[6] For the elderly or those with weaker lower limb strength, a 10 W initial phase for 3 min can be added.[23] The operator should collect images at rest, during low-power, peak, and recovery, ensuring image consistency from all views for comparative analysis.
In exercise SE exams, if poor endocardial visualization in ≥ 2 myocardial segments is observed at rest, UEAs are recommended for accurate myocardial wall motion and perfusion observation. Continuous monitoring of the patient's symptoms, heart rate, cardiac rhythm, and blood pressure changes is crucial throughout the test.
(2) Criteria for Test Termination and Standards for Positive Assessment
Criteria for Termination of Exercise SE test:[20] (i) the patient has reached their maximal exercise tolerance or target heart rate (at least 85% of the predicted maximum heart rate for their age); (ii) severe angina; (iii) positive ECG findings (ST segment depression ≥ 0.1 mV compared to baseline, lasting ≥ 2 min, or ST segment elevation); (iv) echocardiographic evidence of ≥ 2 adjacent myocardial segments with abnormal motion; (v) inability to continue the test due to muscle fatigue or other symptoms; (vi) systolic blood pressure > 220 mmHg or diastolic pressure > 120 mmHg (1 mmHg = 0.133 kPa); (vii) symptomatic hypotension (systolic pressure drop > 40 mmHg); (viii) arrhythmias (supraventricular tachycardia, newly developed atrial fibrillation, frequent or complex ventricular ectopic rhythms, ventricular tachycardia, frequent or polymorphic ventricular premature beats).
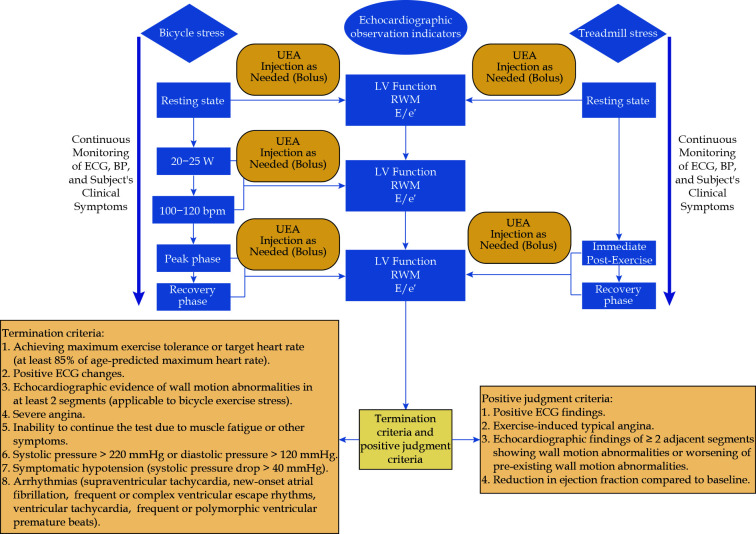
During bicycle ergometer exercise, unlike treadmill exercise, continuous cardiac echocardiographic imaging is possible, allowing for an additional termination criterion: abnormal motion in ≥ 2 segments of the left ventricular wall.[20] The procedure for the exercise SE examination is outlined in Figure 2.
Figure 2.
Exercise stress echocardiography examination flowchart.
BP: blood pressure; ECG: electrocardiogram; E/e': ratio of early diastolic mitral flow velocity (E peak) to the average early diastolic mitral annular velocity (e')); LV: left ventricle; RWM: regional wall motion; UEA: ultrasound enhancing agent.
Criterions for a Positive Assessment include:[6] (i) exercise-induced typical angina pectoris; (ii) positive ECG findings (ST segment depression ≥ 0.1 mV compared to baseline, lasting ≥ 2 min, or ST segment elevation); (iii) echocardiographic emergence of new abnormal motion in ≥ 2 adjacent myocardial segments or an increase in the severity of pre-existing wall motion abnormalities; (iv) a decrease in ejection fraction compared to baseline.
If a positive result is observed in the exercise treadmill electrocardiogram but without corresponding SE wall motion abnormalities, it's still recommended to consider the echocardiographic exercise stress test as positive. Patients in such scenarios should be monitored closely and managed with appropriate medical interventions.[24]
(3) Advantages and Limitations
Exercise SE, which increases myocardial oxygen consumption through physiological skeletal muscle activity, is used to induce myocardial ischemia for echocardiographic assessment. This method reflects the natural physiological and pathological changes in the cardiovascular system and the interplay between cardiac and overall physiological functions. When a patient is capable of exercising, exercise stress is the preferred examination method.[6]
The choice between treadmill and bicycle ergometer SE depends on the medical center's facilities and patient-specific conditions. Treadmill exercise reaches the target heart rate more easily but does not allow continuous imaging. In contrast, the bicycle ergometer permits ongoing heart scanning but slower target heart rate achievement.
Exercise stress is affected by respiration, complicating wall motion observation due to increased heart rate and respiratory movements. Using UEAs is advisable for patients with poor visualization of two or more myocardial segments at rest.[6,25]
(4) Primary value
Exercise SE, compared to exercise stress electrocardiography, demonstrates higher diagnostic accuracy and predictive values for CAD when using coronary angiography as the gold standard.[26]
Studies show that the yearly adverse event rate for patients with normal SE findings is about 0.9%,[27,28] while for those exhibiting segmental wall motion abnormalities, it can reach 6.7%.[29] Positive echocardiographic changes necessitate stringent management and clinical monitoring.[30] Exercise tolerance and heart rate response during SE can be utilized for risk stratification and prognosis.[31-33] Despite high specificity, segmental wall motion abnormalities have lower sensitivity in detecting myocardial ischemia. For CCS patients, the combined use of tissue Doppler imaging, myocardial speckle tracking strain imaging, and myocardial blood flow perfusion imaging is advised for a thorough evaluation (Figure 3).[6-7,34]
Figure 3.
A patient with exertional chest pain without segmental wall motion abnormalities at rest, undergoing treadmill exercise stress echocardiography and myocardial perfusion imaging to assess myocardial ischemia (subsequently confirmed by coronary angiography to have approximately 80% stenosis in the mid-proximal segment of the left anterior descending artery).
(A): Resting state apical four-chamber view, showing good cardiac wall motion and adequate myocardial perfusion; (B): immediate post-exercise, blunting of the left ventricular apical segment with markedly reduced motion amplitude, and significantly decreased perfusion in that segment; (C): recovery phase showing restored motion in the apical segment, but subendocardial perfusion remains poor (yellow arrow indicates reduced myocardial perfusion).
Recommendations:
(i) For CCS patients able to exercise, exercise stress is the preferred method for diagnosing and assessing myocardial ischemia (COR I, LOE A).
(ii) SE can be the initial test for ruling out myocardial ischemia in symptomatic patients where obstructive CAD is not definitively excluded after clinical evaluation (COR I, LOE B).
(ii) Exercise SE is useful for risk stratification in patients with suspected or newly diagnosed CAD (COR I, LOE B).
(iv) In patients with poor endocardial visualization in ≥ 2 cardiac segments at rest, UEAs are recommended for SE (COR I, LOE B).
3.3. Pharmacological Stress Echocardiography Examination
3.3.1. Dobutamine Stress Echocardiography
Dobutamine stress echocardiography (DSE) is a widely used pharmacological stress test. It operates on the principle of dobutamine interacting with β1 receptors, leading to increased heart rate and a strong positive inotropic effect that enhances myocardial contractility. This process significantly raises myocardial oxygen consumption, potentially inducing or exacerbating myocardial ischemia. DSE is effective in detecting segmental or global wall motion abnormalities, thereby assessing ischemic myocardium.
(1) Operational key points
Before a DSE, patients should stop taking any medications that affect myocardial contractility for at least three days. During the exam, patients are monitored with an electrocardiogram and blood pressure monitor, and should be positioned in the left lateral decubitus position. The echocardiography machine is set to pharmacological stress mode, and standard images, including apical four-chamber, two-chamber, three-chamber, and parasternal short-axis views of the left ventricle, are collected at rest and at each dose level of the stress.
Detection of myocardial ischemia: Dobutamine is continuously infused intravenously using a micro-infusion pump. The dosing regimen typically starts at 5 μg·kg-1·min-1, and then increases every 3 min in the following steps: 10 μg·kg-1·min-1, 20 μg·kg-1·min-1, 30 μg·kg-1·min-1, and 40 μg·kg-1·min-1 (Figure 4). When dobutamine alone is insufficient to reach the target heart rate, atropine can be co-administered to increase test sensitivity. Atropine is injected at intervals of one minute at doses of 30 μg·kg-1·min-1 or 40 μg·kg-1·min-1, with doses of 0.25-0.50 mg, up to a total of 1.0-2.0 mg. If it is necessary to use UEAs for the examination, they can be administered during rest, at 10 μg·kg-1·min-1, at peak dose (30 μg·kg-1·min-1 or 40 μg·kg-1·min-1), and during the recovery phase.
Figure 4.
Dobutamine stress echocardiography test protocol.
LV: left ventricle; RWM: regional wall motion; UEA: ultrasound enhancing agent; E/e': ratio of early diastolic mitral flow velocity (E peak) to the average early diastolic mitral annular velocity (e').
Evaluation of myocardial Viability: Low-dose dobutamine stress echocardiography (LDDSE) represents a pivotal technique for evaluating myocardial viability and contractile reserve. The protocol typically involves incremental dosing at 5 μg·kg-1·min-1, 10 μg·kg-1·min-1, and 20 μg·kg-1·min-1, with each dose administered over a period of 3 min. In instances where UEAs are necessary for the examination, they should be administered both at rest and at each incremental stress dose level.
Patients typically exhibit good tolerance to mild adverse reactions during DSE, such as headache, nausea, palpitations, dyspnea, chills, urinary urgency, and anxiety. Notably, cardiovascular adverse reactions like angina, hypotension, and arrhythmias are more prevalent. These reactions generally subside within minutes following the discontinuation of the medication, as indicated in references.[35,36]
(2) Criteria for Test Termination and Standards for Positive Assessment
Criteria for Termination of the Test include: (i) achieving a target heart rate exceeding 85% of the age-predicted maximum; (ii) emergence or exacerbation of wall motion abnormalities; (iii) onset of severe arrhythmias; (iv) a substantial decrease in blood pressure, indicated by a systolic pressure at or below 80 mmHg; (v) a marked increase in blood pressure, with systolic readings reaching or exceeding 220 mmHg; (vi) the patient experiencing intolerable symptoms.
Criterions for a Positive Assessment include[6]: (i) manifestation of typical angina symptoms during the stress examination; (ii) electrocardiographic alterations indicative of ischemia, including ST-segment depression of ≥ 0.1 mV from baseline, persisting for ≥ 2 min, or ST-segment elevation; (iii) echocardiographic detection of abnormal wall motion in ≥ 2 contiguous segments; (iv) a reduction in ejection fraction relative to the baseline state; (v) in LDDSE, observed enhanced motion in a minimum of two adjacent segments with pre-existing regional wall motion abnormalities suggests myocardial viability.
(3) Advantages and limitations
In patients unable to undergo exercise-based stress testing, DSE is the preferred alternative. DSE, notably involving lower incidences of systolic pressure elevation compared to exercise stress tests, is especially advantageous for assessing myocardial viability and contractile reserve. Utilizing dobutamine's potent inotropic properties, which stimulate myocardial contraction at low doses without significantly elevating heart rate or oxygen consumption, LDDSE emerges as an optimal method. The convenience, safety, and high repeatability of DSE render it particularly suitable for critically ill patients in intensive care units requiring stress echocardiography.
A limitation of DSE is its reliance on achieving a target heart rate; failure to do so may diminish its sensitivity in ischemia detection. Atropine co-administration, while aiding in reaching the target heart rate, potentially escalates the risk of adverse drug reactions.
(4) Primary value
DSE functions by inducing myocardial ischemia in areas served by stenotic coronary arteries, thereby increasing myocardial oxygen demand. This process facilitates the identification of wall motion abnormalities, which are often imperceptible under routine resting echocardiographic conditions, thereby substantially enhancing the diagnostic accuracy for coronary artery disease (refer to Figure 5). DSE’s clinical utility is extensive, encompassing the assessment of myocardial ischemia, identification of viable myocardium, evaluation of myocardial contractile reserve, prediction of revascularization outcomes, and prognostication of adverse cardiovascular events.
Figure 5.
Comparison of left ventricular end-systolic diameter during dobutamine stress echocardiography [6].
The images represent the left ventricular end-systolic images at resting state, low dose, pre-peak, and peak dose during the stress test. With the increase in dobutamine dosage, the left ventricular end-systolic diameter significantly increases, indicating the presence of myocardial ischemia. REST: resting state; LOW DOSE: low dose; PRE-PEAK: pre-peak dose; PEAK: peak dose.
LDDSE is the preferred technique for assessing myocardial viability. While it exhibits a slightly lower sensitivity compared to 99mTc perfusion imaging, LDDSE demonstrates superior specificity in the diagnosis of myocardial viability.[37] Moreover, LDDSE is both sensitive and specific in identifying viable myocardium, predicting the recovery of myocardial function post-revascularization, and providing prognostic insights, as supported by references.[38-42]
Recommendations:
In cases where stress imaging is indicated but exercise stress is not feasible, pharmacological stress testing is advised, with DSE being the optimal choice (COR I, LOE B).
LDDSE is the recommended primary method for assessing myocardial viability (COR I, LOE B).
LDDSE is also the preferred approach for evaluating myocardial contractile reserve (COR I, LOE B).
The use of UEAs is advised in patients with unclear display of intima in two or more consecutive ventricular segments (COR I, LOE B).
3.3.2. Vasodilator stress echocardiography examination
In echocardiographic assessments, vasodilators are employed to augment coronary microcirculatory blood flow, thereby evaluating the vasodilatory function and reserve capacity of the vessels. In cases of narrowed epicardial coronary arteries, vasodilation may induce a “steal phenomenon” at these constricted sites (see Figure 6), leading to localized wall motion abnormalities. This, in conjunction with UEAs, further enables the evaluation of myocardial perfusion and augments the imaging of coronary Doppler flow spectra. Vasodilators are predominantly utilized in assessing myocardial perfusion, CFR and patients not suited for exercise or dobutamine stress tests.
Figure 6.
Myocardial Ischemia detection using contrast echocardiography before and after vasodilator administration.
(A): Left ventricular myocardial perfusion at end-diastole under resting conditions; (B): left ventricular myocardial perfusion at end-diastole during adenosine stress; (C): left ventricular myocardial perfusion at end-systole under resting conditions; (D): left ventricular myocardial perfusion at end-systole during adenosine stress. In a patient with moderate to severe stenosis in the left anterior descending artery, adenosine stress leads to a "steal phenomenon," resulting in reduced wall motion and perfusion of the left ventricular anterior wall post-stress (indicated by yellow arrows).
The primary vasodilators used in echocardiography include dipyridamole, adenosine, and regadenoson.[43] These agents primarily function by activating adenosine A2A receptors on coronary artery smooth muscle cells, thereby inducing coronary dilation and enhancing myocardial blood flow.[43-44] The non-selective action of adenosine and dipyridamole on A1, A2B, and A3 receptors contributes to various adverse reactions. Adverse effects include bronchospasm (mediated by A1, A2B, and A3 receptors), atrioventricular conduction block (A1 receptors), and hypotension due to peripheral vasodilation (A2B receptors). Adenosine’s cardiovascular effects at varying doses include sympathetic nervous system stimulation and heart rate increase at low doses, bradycardia and AV block at high doses, arteriolar bed vasodilation excepting for the contraction of the anterior glomerular artery, and hyperventilation through interaction with carotid body chemoreceptors. Regadenoson, a highly selective A2A receptor agonist, offers reduced adverse reactions, comparable efficacy to adenosine, and improved convenience, observation window, safety, and tolerance.[43,45-46]
(1) Operational key points
Pre-Test Medication Protocol: Patients prescribed β-adrenergic blockers, cardiotonic glycosides, calcium channel blockers, or adenosine potentiators, including dipyridamole and ticagrelor, are advised to discontinue these medications a minimum of 24 h before the examination.
Examination Procedure: The test commences with a resting echocardiogram. Continuous monitoring of symptoms, heart rate, blood pressure, and electrocardiogram is essential throughout the procedure. Detailed operational steps and corresponding image illustrations are provided in Figures 7 and 8.
Figure 7.
Vasodilator (Adenosine and Regadenoson) stress echocardiography flowchart.
CFR: coronary flow reserve; LAD: left anterior descending coronary artery; RTMPE: real-time myocardial perfusion echocardiography.
Figure 8.

Color flow and spectral changes in the distal segment of the left anterior descending coronary artery before and after vasodilator administration.
(A): Compared to the resting state, 2 minutes after adenosine stress, the color flow of the distal segment of the left anterior descending coronary artery changes to a mosaic pattern, and pulsed-wave Doppler shows an increase in maximum blood flow during diastole, CFR 3.23; (B): compared to the resting state, 2 minutes after regadenoson stress, the color flow of the distal segment of the left anterior descending coronary artery changes to a mosaic pattern, and pulsed-wave Doppler shows an increase in maximum blood flow during diastole, CFR 2.38. CFR: coronary flow reserve.
Utilizing an infusion pump, the recommended adenosine dosage is 140 μg·kg-1·min-1, administered over 4-6 min, with a maximum limit of 60 mg. The application of UEAs is suggested both at rest and post 3 min of the administration.[6,47] For enhanced diagnostic sensitivity, high-dose adenosine stress echocardiography may commence at 100 μg·kg-1·min-1, increasing up to 140-200 μg·kg-1·min-1 for a duration of 6 min. The peak vasodilatory effect typically occurs at 3 min, and dosage adjustments may be necessary in case of intolerable adverse reactions.[48] A dosage of 140 μg·kg-1·min-1 is generally adequate for assessing myocardial perfusion and Coronary Flow Reserve (CFR), with higher doses potentially inducing localized wall motion abnormalities.
Administration a 0.4 mg solution in 5 mL over 10 s, followed by a 5 mL saline flush. Optimal image acquisition for perfusion imaging should be within 2-10 min post-injection,[49] with recommended image collection intervals at 1-2 min, 2-4 min, and 4-6 min.
(2) Criteria for test termination and standards for positive assessment
Criteria for termination of the test include: (i) two or more newly discovered segmental wall motion abnormalities, including abnormal rates of wall thickening. (ii) Electrocardiographic evidence of ischemia, such as ST-segment depression of ≥ 0.1 mV relative to baseline for ≥ 2 min, or ST-segment elevation. (iii) Excessive heart rate changes, either a rise exceeding 40% from baseline or significant reduction (heart rate ≤ 50 beats/min). (iv) Marked decrease in blood pressure, either a systolic pressure ≤ 80 mmHg or a reduction exceeding 20 mmHg. (v) Severe arrhythmias or conduction block evident on ECG. (vi) Patient presenting intolerable symptoms, for instance, chest pain, nausea, headache, or urinary urgency. (vii) Reaching the maximum dosage of the stress agent.
Criterions for a Positive Assessment include:[6] (i) electrocardiographic indicators of ischemia, such as ST-segment depression ≥ 0.1 mV from baseline for ≥ 2 min, or ST-segment elevation. (ii) Detection of abnormal wall motion in ≥ 2 contiguous segments via echocardiography. (iii) A decrease in ejection fraction relative to baseline measurements. (iv) Reduced CFR with a value < 2.5. (v) New or exacerbated myocardial perfusion abnormalities in comparison to baseline.
(3) Advantages and limitations
Unlike exercise or dobutamine tests, vasodilator SE does not necessitate reaching a target heart rate, making it particularly suitable for patients who are not candidates for exercise or dobutamine stress. The use of vasodilators induces a pronounced coronary hyperemic response, thus establishing this method as the preferred approach for evaluating CFR and myocardial perfusion.
Higher doses of adenosine are effective in augmenting the observation of the 'steal phenomenon' in myocardium served by narrowed coronary arteries, thereby increasing diagnostic sensitivity. However, elevated adenosine levels are correlated with a higher incidence of adverse reactions, such as angina, headache, ECG changes including ST segment depression, chest pain, dyspnea, tachycardia or bradycardia, and atrioventricular block. These adenosine-induced effects can be counteracted by aminophylline. Adenosine's brief half-life of approximately 10 seconds ensures rapid symptom resolution post-discontinuation. Regadenoson, by contrast, typically induces milder adverse reactions, with tachycardia and nausea being more prevalent.
(4) Primary value
Vasodilator SE facilitates the concurrent assessment of coronary blood flow, myocardial perfusion imaging, and wall motion analysis. Adenosine stress demonstrates a sensitivity and specificity for diagnosing local wall motion abnormalities and myocardial viability at 79% and 91.5% respectively, comparable to exercise stress and DSE tests and exhibiting superior specificity relative to SPECT stress imaging.[50] When integrated with UEAs, real-time myocardial perfusion evaluation,[51] and myocardial flow reserve measurement, the diagnostic accuracy for coronary artery disease significantly increases, reaching sensitivity and specificity of 89% and 92% respectively with a cutoff value of 1.94.[52] While research on prognostic value is less extensive, asymptomatic patients with reduced CFR are considered high-risk. A comprehensive analysis incorporating wall motion, myocardial perfusion, and CFR notably augments prognostic insights.
Recommendations:
(1) In CCS patients for whom exercise or dobutamine SE is unsuitable, and who present contraindications, vasodilator SE is the recommended modality (COR I, LOE B).
(2) Vasodilator SE is advised for the assessment of myocardial perfusion in SE examinations (COR I, LOE B).
(3) For the measurement of CFR, vasodilator SE is considered the method of choice (COR I, LOE B).
4. STANDARD ANALYSIS METHODS FOR STRESS ECHOCARDIOGRAPHY EXAMINATION
While various SE examination methods and procedures exhibit certain differences, the underlying principles of assessment remain fundamentally consistent. It is imperative to conduct a thorough analysis of echocardiographic images captured at rest, during stress, and in the recovery phase. This involves a comparative evaluation of wall motion, wall thickening rates, myocardial perfusion, and alterations in both local and global cardiac functions, including left and right ventricular contractions, left ventricular diastolic function, chamber dimensions, and coronary artery flow velocity. Such analyses are crucial to determine myocardial ischemia and viability and may employ qualitative or quantitative methodologies.
Given the segmental or regional nature of coronary artery supply, both qualitative and quantitative assessments should employ a 16-segment or 17-segment left ventricular model for local myocardial function evaluation. The 17-segment model, inclusive of the apical cap, is particularly advantageous for myocardial perfusion assessment and comparative studies with other imaging techniques (refer to Figure 9). Due to the limited segment visualization in standard two-dimensional scanning planes, it is essential to analyze each segment individually across multiple planes. The utilization of techniques like multi-view simultaneous display and real-time three-dimensional imaging significantly enhances the precision and efficiency of wall motion analysis.
Figure 9.
This guideline recommends the use of the 17-segment left ventricular model by the American Heart Association (AHA) (top image), as well as the coronary artery supply segments (bottom image).
4.1. Stress Echocardiography Examination Qualitative Analysis
This guideline advocates a 5-point scoring system for wall motion analysis,[6] categorized as follows: one point for normal or hyperdynamic motion (systolic wall thickening > 50%); two points for reduced motion (systolic wall thickening < 40%); three points for severely reduced or absent motion (systolic wall thickening < 10%); four points for paradoxical motion (systolic wall moving away from the ventricular center); five points for aneurysmal walls (characterized by left ventricular remodeling and localized outward bulging). The Wall motion score index (WMSI) is derived by dividing the sum of segment scores by the total number of segments. Infarction or scarred myocardium is indicated by a left ventricular diastolic wall thickness ≤ 6 mm or less than 70% of the thickness of a normal segment at rest. The guideline recommends qualitative descriptions for these conditions. Interobserver correlation for qualitative wall motion assessment can exceed 0.90.[53] However, due to the influence of adjacent segments (the 'drag effect') and potential delayed activation from bundle branch block, there may be overestimation in segments with reduced motion; hence, emphasis should be placed on segmental wall thickening in qualitative assessments.
Using MCE in myocardial perfusion imaging can enhance diagnostic accuracy in patients with suboptimal image quality, significantly improving the sensitivity of myocardial ischemia detection. However, the qualitative analysis of perfusion defects and their distribution in diagnosing coronary artery disease does not demonstrate greater specificity than basic wall motion analysis.[54]
Transient ischemic left ventricular dilation can be qualitatively judged by visually comparing the size of the left ventricular chamber during stress and at rest. The presence of this sign indicates a more severe and extensive wall motion abnormality, higher WMSI, and a greater likelihood of multi-vessel coronary artery disease.[28]
Recommendations:
(1) Qualitative analysis should primarily observe segmental wall motion, especially new wall motion abnormalities that appear during stress (COR I, LOE B).
(2) During MCE, both the segments with poor wall motion and those with perfusion defects should be described, along with the time and degree of UEA refilling of the wall (COR I, LOE B).
4.2. Quantitative analysis in Stress echocardiography examination
Common quantitative indicators in SE examination include: (1) left ventricular diastolic function: Indicators of left ventricular diastolic function include peak early diastolic mitral flow velocity (E), average of peak early diastolic septal and lateral mitral annular velocity (e'), the E/e' ratio, and peak velocity of tricuspid regurgitant flow. Stress-induced changes in E/e' provide insights into left ventricular diastolic function abnormalities.[55-56]
Impact of Ischemia on Diastolic Function: Ischemia may lead to a decrease in E wave velocity or an increase due to elevated left ventricular filling pressure in cases of extensive ischemia. Patients with myocardial ischemia under stress typically exhibit delayed left ventricular relaxation, reflected by a smaller increase in e' relative to E, resulting in an average E/e' increase >14 or septal E/e' >15. A rise in E/e' by 25% from baseline during stress testing for coronary artery disease is also a positive diagnostic criterion.[57] Normal individuals may experience a rise in pulmonary artery systolic pressure (PASP) up to 40 mmHg post-exercise, with abnormal elevations indicating hemodynamic changes due to diminished left ventricular reserve.[58]
(2) Left ventricular systolic function: (i) assessing left ventricular systolic function: changes in left ventricular ejection fraction (LVEF), end-systolic volume (ESV), and end-diastolic volume (EDV) during stress are key indicators. Notably, an increase in mitral annular displacement ≥ 5 mm and LVEF ≥ 5% post-stress signifies strong systolic reserve. Research has found that the sensitivity and specificity of diagnosing coronary artery triple branch disease are 85% and 90%, respectively, with a stress to rest EDV ratio and ESV ratio>1.12. The specificity is 72% and 84%, respectively.[59] A post-stress increase in ESV is an independent mortality predictor in coronary artery disease, correlating with the disease's severity.[60-61] However, there is no recognized standard for the cutoff values of ESV and EDV change rates. LVEF is influenced by pre - and post load, heart rate, and myocardial synchrony. (ii) Quantitative analysis: left ventricular wall segment motion can be quantitatively assessed using anatomical M-mode.[62] (iii) Strain Analysis with STE: Speckle Tracking Echocardiography (STE) provides more accurate quantitative data. Global longitudinal strain (GLS) changes under stress vary based on ischemia severity and metabolic status (Table 2).[63] However, due to the fact that the STE frame frequency is usually lower than tissue Doppler, peak strain may be underestimated, and under stress conditions, heart rate increases faster, which may be more pronounced. At present, there is no recognized cut-off value for GLS in diagnosing myocardial ischemia. A meta-analysis suggests that the sensitivity and specificity of -16.5% in diagnosing moderate to severe obstructive coronary heart disease are 74.4% and 72.1%, respectively.[64]
Table 2. Relationship between changes in GLS after dobutamine stress and myocardial ischemic status.
| Resting State | Dobutamine Stress | ||
| Low Dose | Peak Dose | ||
| GLS: global longitudinal strain. ↓: decrease, ↑: increase, -: disappearance. The number of arrows represents the degree of change. *In most cases, there is a reduction compared to the low dose. | |||
| Normal Myocardium | Normal | ↑ | No change or ↓* |
| Stunned Myocardium | ↓ | ↑ | ↓↓ |
| Hibernating Myocardium | ↓ | ↑ | ↓ |
| Myocardial Scar | - | - | - |
(3) Right Heart Function Assessment: Assessing right heart function in stress echocardiography involves complex geometrical considerations, as standard measurements for right ventricular volume and ejection fraction are not yet standardized. The assessment primarily focuses on the right ventricle in the four-chamber view. Post-stress measurements like right ventricular area change rate, tricuspid annular plane systolic excursion (TAPSE), and tissue Doppler tricuspid annular systolic myocardial velocity provide insights into overall right ventricular systolic function reserve. According to wall motion analysis, right heart ischemia can only be detected in 2% of patients with right coronary artery stenosis, and the detection rate can be increased to 5% to 10% using TAPSE. The diagnosis of right coronary artery proximal obstruction has good sensitivity when the tricuspid annulus movement decreases by more than 4mm during exercise. A significant exercise-induced decrease in tricuspid annular motion suggests proximal right coronary artery obstruction. Stress-induced right ventricular dilation and overall function decline could indicate extensive multi-vessel coronary artery disease or exercise-induced pulmonary hypertension.
(4) MCE Myocardial Perfusion Quantitative Analysis: recognized parameters include the signal intensity at plateau phase (A) of UEAs, the blood flow rate constant (β), and their product, A × β. These parameters semi-quantitatively reflect relative myocardial blood volume, the rate of contrast passing through the microcirculation, and myocardial blood flow. The stress-rest ratio of A shows limited diagnostic value for coronary artery disease. However, ratios of β and A × β ≥ 2 demonstrate significant positive likelihood ratios for diagnosing abnormal myocardial blood flow reserve, with values of 3.76 and 3.64, respectively.[65]
Recommendations:
(1) Quantitative analysis should include changes in left ventricular diastolic and systolic function, right ventricular function, and myocardial perfusion during baseline, stress, and recovery phases (COR I, LOE C).
(2) Quantitative analysis of left ventricular diastolic function should include changes in E, e', E/e', and peak velocity of tricuspid regurgitant flow (COR I, LOE B).
(3) Quantitative analysis of left ventricular systolic function should at least include LVEF, and it's recommended to add the rate of change in EDV and ESV (COR I, LOE B).
(4) Where analysis conditions permit, quantitative analysis of left ventricular systolic function should include GLS analysis based on STE technology (COR I, LOE B).
(5) Quantitative analysis of right heart function is recommended on the apical four-chamber view, calculating TAPSE, fractional area change (FAC), and right ventricular ejection fraction (Class of Recommendation I, LOE B).
(6) Semi-quantitative analysis of myocardial perfusion assessment using Myocardial Contrast Echocardiography (MCE) technology should include the signal intensity A at the plateau phase of UEA, the blood flow rate constant β, and their product (COR I, LOE B).
4.3. Hemodynamic Changes Under Different Stress Modalities
Stress-induced changes in left ventricular shape, volume, and overall contractile function are key indicators for ischemia assessment (Table 3). Typically, a normal response in DSE is marked by a significant increase in both local and global contractility, accompanied by a substantial decrease in left ventricular volume due to reduced preload and afterload. Unlike DSE or treadmill exercise, bicycle exercise usually results in a less pronounced decrease in end-systolic volume (ESV) in normal individuals. During myocardial ischemia, an increase in left ventricular ESV can be observed in exercise stress testing,[66] and similar patterns are seen in DSE when significant ischemic wall motion abnormalities are present.
Table 3. Hemodynamic and echocardiographic measurement changes under different stress modalities.
| Items | Treadmill Exercise | Supine Bicycle Exercise | Dobutamine | Vasodilator |
| EDV: end-diastolic volume, ESV: end-systolic volume; LVEF: left ventricular ejection fraction. ↓: decrease, ↑: increase, -: abnormality or disappearance. The number of arrows represents the degree of change. | ||||
| Myocardial Motion Amplitude | ||||
| Normal Myocardial Segment Response |
↑↑ | ↑ | ↑↑↑Contractile Speed Increase Dose-Dependent | ↑ |
| Ischemic Myocardial Segment Response |
↓ | ↓ | ↓↓ | ↓ |
| Contractile Speed Decrease Dose-Dependent | ||||
| Heart Rate and Blood Pressure | ||||
| Normal Myocardial Overall Response |
Heart Rate ↑↑ | Heart Rate ↑↑ | Heart Rate ↑↑↑ | Heart Rate ↑↑ |
| Systolic Pressure ↑↑(Increase Can Be >50%) | Systolic Pressure ↑↑(Increase Can Be >50%) | Systolic Pressure↑ | Systolic Pressure↓ | |
| Myocardial Blood Flow | ||||
| Normal Myocardial Overall Response |
↑ | ↑ | ↑↑ | ↑↑↑(3-5 times) |
| Left Ventricular Volume and Contractile Function | ||||
| Normal Myocardial Overall Response |
EDV↓↓ | EDV↓ | EDV↓↓ | EDV↓/- |
| ESV↓ | ESV↓ | ESV↓↓ | ESV↓/- | |
| Stroke Volume↑ | Stroke Volume↑↑ (can be 3 times) |
Stroke Volume↓/- | Stroke Volume- | |
| LVEF↑ | LVEF↑↑ | LVEF↑↑↑ | LVEF↑ | |
| (Myocardial Contractility Increase 4-5 Times) | ||||
| Normal Myocardial Overall Response |
EDV↑ | EDV↑ | EDV -/↑ | EDV -/↑ |
| ESV↑ | ESV↑ | ESV -/↑ | ESV -/↑ | |
| LVEF↓ | LVEF↓ | LVEF↓ | LVEF↓ | |
| (Seen in Left Main or Multivessel Disease) | (Seen in Left Main or Multivessel Disease) | (Seen in Left Main or Multivessel Disease) | (Seen in Left Main or Multivessel Disease) | |
In stress echocardiography, including treadmill exercise, dobutamine, and vasodilator stress, the absence of wall thickening often indicates obstructive coronary artery ischemia. This sign can also manifest in conditions such as microvascular disease, cardiomyopathy, or hypertensive responses to exercise stress. A decrease in LVEF or chamber dilation during exercise stress is more commonly associated with left main or multi-vessel coronary artery disease. Markers like a peak WMSI greater than 1.7 or transient ischemic dilation of the ventricle are significant indicators of a higher risk for cardiovascular events.[28]
4.4. Ischemia and Myocardial Viability Assessment
In assessing myocardial ischemia using wall motion analysis (Table 4), the focus is on identifying normal, ischemic, viable, or necrotic myocardium. For patients who exhibit ischemic wall motion abnormalities during the recovery phase of dobutamine stress but not during the stress phase, it is recommended to perform an additional round of early post-stress imaging. This imaging should be conducted early after dobutamine injection, before reaching peak stress. Additionally, improvement in systolic function post-stress, indicated by an increase in mitral annular displacement during systole by ≥5 mm and an increase in LVEF by ≥5%, suggests good contractile reserve and can predict functional improvement of viable myocardium after revascularization. Studies have shown that preserving contractile reserve in at least four segments is the minimum condition for enhancing LVEF by more than 5% after revascularization[67-69]. In determining myocardial viability or prognosis, the roles of WMSI, LVEF, and the quality of contractile reserve remain to be further clarified[70-72]. Strain and strain rate can also be used to assess myocardial viability, but there is still a lack of recognized diagnostic thresholds.
Table 4. Wall motion changes and analysis of myocardial ischemia and viability.
| Items | Resting Phase | Stress Phase | Recovery Phase |
| Normal Myocardium | Normal | Normal | Normal or Hyperdynamic |
| Reversible Ischemic | Normal | Abnormal | Recovers |
| Severe Ischemia | Normal | Abnormal | Persistently Abnormal |
| Stunned Myocardium | Abnormal | Improves | - |
| Hibernating Myocardium | Abnormal | Biphasic Response | - |
| Necrotic Myocardium | Abnormal | No change | No change |
Stress testing can identify stunned myocardium, hibernating myocardium, and myocardial scarring by assessing myocardial blood flow and reserve, contractile reserve, and viability (Table 5).[73]
Table 5. Stress test identification of stunned myocardium, hibernating myocardium, and myocardial scar.
| Items | CFR | Contractile Reserve | Myocardial Perfusion | Viable Myocardium | Myocardial Fibrosis |
| CFR: coronary flow reserve. ↓: decrease, ↑: increase, +: normal or present, -: abnormal or absent, +/- indicates uncertain. The number of arrows represents the degree of change. | |||||
| Stunned Myocardium | ↓ | + | + | + | - |
| Hibernating Myocardium | ↓↓ | "+/-" | "+/-" | + | - |
| Myocardial Scar | ↓↓↓ | - | - | "+/-" | + |
Recommendations:
(1) Wall motion analysis during stress testing can assess myocardial ischemia, but observations should be made in at least two adjacent segments to enhance diagnostic specificity (COR I, LOE C).
(2) To improve the accuracy of viable myocardium determination, wall motion analysis should be conducted during the recovery phase (COR I, LOE C).
5. APPLICATION OF SPECKLE TRACKING ECHOCARDIOGRAPHY TECHNOLOGY IN STRESS TESTING
Quantitative SE provides an advantage over qualitative analysis by reducing subjectivity in evaluating wall motion abnormalities. STE technology, a component of quantitative SE, measures local myocardial motion speed and deformation, offering critical insights into local myocardial function. This approach significantly enhances the accuracy of coronary artery disease evaluation and improves the detection of subclinical myocardial ischemia when used in conjunction with SE.[6]
5.1. Key Points of SE Combined with Speckle Tracking Technique
Myocardial ischemia affects longitudinal strain earlier than radial strain. Obtaining high-quality two-dimensional echocardiographic images during different phases of SE is fundamental for STE. Key points include:
(1) Due to the interference of exercise stress on image quality, speckle tracking SE is more suitable for pharmacological SE.
(2) The frame rate for two-dimensional images should be set to the maximum possible on the device. The frame rate for capturing images at baseline should be ≥ 60 frames/s, and post-stress, it should be appropriately increased to ensure image quality while accommodating increased heart rates.
(3) Selection of two-dimensional echocardiography planes: this includes apical long-axis, apical four-chamber, and apical two-chamber views.
(4) Angle adjustment: Adjust the scanning angle to display the complete left and right ventricles optimally.
(5) Depth adjustment: Choose a depth that displays the complete left and right ventricles, or completely shows the left atrium, left ventricle, and right atrium, right ventricle.
(6) Acquire dynamic images at the end of the patient's expiration.
(7) Capture continuous dynamic images over five cardiac cycles.
5.2. Image Analysis Methods
(1) Use semi-automatic speckle tracking analysis software to display the peak strain of 17 myocardial segments in a bull's-eye plot format.
(2) Employ dual or multiple image displays to simultaneously show the peak myocardial strain at rest and during peak stress.
5.3. Judgment Criteria
Left ventricular GLS is the most sensitive indicator for assessing myocardial ischemia in speckle tracking SE. In healthy adults, the peak left ventricular GLS during exercise stress is –25.4% ± 2.0%, with no significant difference between ages and genders.[74] Meta-analysis shows that a left ventricular GLS cutoff value of –19.9% diagnoses significant coronary artery narrowing with a sensitivity of 68.6%, specificity of 68.7%, positive predictive value of 53.3%, negative predictive value of 80.7%, and accuracy of 68.7%; a cutoff value of –21.2% diagnoses significant narrowing of the left anterior descending artery with a sensitivity of 72.4%, specificity of 69.9%, positive predictive value of 48.8%, negative predictive value of 86.4%, and accuracy of 71.6%.[75] Regardless of the presence of left anterior descending artery lesions, a peak stress left ventricular GLS > –16% can indicate significant coronary artery narrowing.[76] See Figure 10.
Figure 10.
Changes in left ventricular GLS during treadmill exercise stress test.
(A): Resting left ventricular GLS at -18%; (B): peak left ventricular GLS during treadmill exercise stress at -13.3%; (C): Significant stenosis in the proximal segment of the LAD indicated by the white arrow. GLS: global longitudinal strain; LAD: left anterior descending artery.
5.4. Advantages and Limitations
STE offers an improvement over visual assessment in diagnosing wall motion abnormalities, with enhanced diagnostic accuracy and reduced variability between operators. However, its limitations include challenges with the lower frame rate per cardiac cycle due to increased heart rates during stress testing, which can affect speckle tracking imaging. Obtaining high-quality images at peak stress is critical for accurate speckle tracking, but this can be challenging. Additionally, variability in results can arise due to differences in operator experience and the analysis software of various ultrasound echocardiography manufacturers. Currently, there are no established normal reference ranges for different ages and genders by different manufacturers, making it essential to interpret measurements in conjunction with published guidelines and individual laboratory experience.
5.5. Clinical Value
5.5.1. STE in Diagnosing CCS
Left ventricular longitudinal strain and strain rate are sensitive for assessing myocardial ischemia. In CCS patients, dobutamine stress speckle tracking imaging showing a recovery phase left ventricular GLS > -16% is more sensitive for significant coronary artery stenosis (Figure 11).[75]
Figure 11.
Dobutamine stress echocardiography with speckle tracking imaging in a patient with severe aortic stenosis and heart failure.
(A): Baseline left ventricular GLS at -6.1%, with delayed peak strain time in the interventricular septum and anterior wall, and strain peak time dispersion of 55.9 ms, indicating severe lesion in the LAD; (B): improved left ventricular GLS at -7.3% with 15 μg·kg-1·min-1 of dobutamine, bull's eye plot shows no extension of lesion area, but significant increase in strain peak time dispersion to 103.8 ms; (C): CTA shows significant stenosis in the mid-segment of the LAD. CTA: CT angiography; GLS: global longitudinal strain; LAD: left anterior descending artery.
Patients with coronary artery narrowing > 50% exhibit reduced longitudinal and circumferential strain during stress echocardiography, with left ventricular dyssynchrony (peak contraction time > 33 ms) predicting CCS with 97% sensitivity, 89% specificity, and an AUC of 0.96.[77] Strain-derived myocardial work indices may enhance speckle tracking SE diagnostic value.[78]
Myocardial layer-specific strain during adenosine stress echocardiography independently predicts CCS and correlates with the number of narrowed vessels.[79] Post-PCI, vasodilator SE testing assessing time from aortic valve closure to peak strain rate > 14 ms evaluates local myocardial ischemia with 93% sensitivity and 95% specificity.[76]
5.5.2. Assessment of Viable Myocardium
The evaluation of viable myocardium is crucial for clinical decision-making and treatment, playing a significant role in predicting patient outcomes. Patients with viable myocardium undergoing revascularization have lower perioperative mortality rates, greater improvements in local and global left ventricular function, fewer symptoms of heart failure, and higher long-term survival rates compared to those with extensive non-viable myocardium. The overall strain analysis in LDDSE is superior to the semi-quantitative wall motion analysis in LDDSE.[80] In multivariate analysis, longitudinal strain and strain rate can be considered independent predictors of viable myocardium. A cutoff value of 0.808 for longitudinal strain rate detects viable myocardium with a sensitivity of 80.0% and specificity of 83.7%.
5.5.3. Detection of microvascular dysfunction
In cases where significant coronary artery stenosis is not evident, observed wall motion abnormalities during SE might indicate coronary artery disease stemming from microvascular dysfunction. This scenario is notably relevant in female patients, who may present with chest pain and ECG changes without segmental wall motion abnormalities during SE, potentially pointing to microvascular disease. Additionally, in patients with a LVEF of 45% or higher, the left ventricular GLS reserve (the increase in GLS during stress) proves to be a more effective metric for identifying coronary artery disease when compared to resting left ventricular GLS and the stress/rest LVEF ratio.[77]
Recommendations:
(1) Pharmacological or exercise stress echocardiography combined with STE technology can detect early changes in myocardial ischemia, increasing the sensitivity of myocardial ischemia assessment (COR I, LOE A).
(2) STE enhances the clinical application value of stress echocardiography in diagnosing CCS (COR IIa, LOE B).
6. APPLICATION OF STRESS MYOCARDIAL CONTRAST ECHOCARDIOGRAPHY
The effectiveness of SE hinges on accurate analysis of segmental wall motion, necessitating clear endocardial visualization during systole. In situations where image quality is suboptimal, such as in obese patients, the application of UEAs can significantly improve the visibility of left ventricular segments. This not only enhances inter-observer consistency but also brings the accuracy of left ventricular volume and ejection fraction measurements closer to those obtained by cardiac MRI.[81-83]
Following stress, particularly after exercise and dobutamine stress, rapid breathing and increased heart rate can pose challenges for even skilled sonographers in detecting wall motion abnormalities. To enhance the accuracy of wall motion analysis during such stress testing, the use of UEAs is recommended, especially when two or more segments are not clearly visible during resting echocardiography. This approach aids in improving image clarity and diagnostic accuracy.[6,20]
The utilization of very low mechanical index (VLMI) multi-pulse sequencing technology in stress echocardiography significantly improves the detection sensitivity of microbubbles.[84] This technology allows for clear, artifact-free visualization of the cardiac apex, which is crucial for effectively identifying segmental wall motion abnormalities. Additionally, VLMI technology, when used in conjunction with high mechanical index pulse destruction of microbubbles and subsequent reperfusion of the epicardial layer, generates high-contrast images. This enhanced imaging capability is particularly beneficial in delineating the left ventricular endocardial border and in analyzing wall thickening at this site, thereby aiding in the detection of subendocardial wall thickening abnormalities during stress testing.[85-86]
Combining UEAs with VLMI imaging in the assessment of wall thickening and ischemia, especially in DSE for patients with left bundle branch block, can improve the detection rate of coronary heart disease. This approach also independently predicts mortality and cardiovascular events.[86-87] The use of cardiac perfusion imaging with VLMI technology is recommended to enhance the delineation of the left ventricular endocardial border, particularly useful in identifying minor wall thickening abnormalities due to subendocardial ischemia. General parameter settings for stress myocardial acoustic imaging echocardiography can be found in Table 6.
Table 6. General Settings for Stress Myocardial Contrast Echocardiography.
| Parameters | Settings |
| Focus | Optimal resolution for the entire left ventricle is achieved by setting at the mitral valve level, reducing swirling artifacts at the apex; if the apical resolution is poor, adjust to improve apical visualization. |
| Mechanical index | Low Mechanical Index (MI) Harmonic Imaging: 0.25-0.3. |
| Very Low Mechanical Index Multipulse Imaging: 0.1-0.2. | |
| Gain/time gain compensation | Gain: 60%-70%, balancing imaging quality and signal-to-noise ratio, with slight increase in near-field TGC as needed. |
| Frame rate | Frame rate generally 20-25 fps, can be increased to 25-30 fps for rapid heart rates. |
| Flash | MI setting greater than 0.8, 5-6 frames at rest, 10-15 frames post-stress. |
Using a brief high mechanical index pulse to clear UEAs followed by VLMI observation of microbubble replenishment in myocardial tissue helps to detect myocardial ischemia. In the cascade of ischemic waterfall reactions, myocardial blood flow perfusion abnormalities precede wall motion abnormalities. Compared to wall motion analysis alone, myocardial perfusion analysis enhances the detection rate of coronary artery disease, especially in identifying subendocardial ischemia. Adding perfusion information to SE tests can better define the extent and severity of myocardial ischemia in coronary artery disease, with the incremental prognostic value primarily in detecting perfusion abnormalities in patients without wall motion abnormalities. Reduced perfusion can independently predict future death and non-fatal myocardial infarction events.[33,87-88]
In both exercise and DSE tests, patient cooperation with breathing techniques is crucial for ensuring high-quality image capture. For perfusion assessment, normal replenishment time after high mechanical index pulse destruction should be within 5 s at rest, and less than 2 s under stress conditions. Post-exercise stress, it's important to continuously select a sequence of 10-15 single cardiac cycle images, including one cycle prior to the high mechanical index pulse destruction. The challenges and corrective methods related to myocardial perfusion imaging are detailed in Table 7.
Table 7. Problems in myocardial perfusion imaging and their correction methods.
| Problem | Correction Methods |
| Reduced contrast in the apical myocardium/"dark apex". | Increase near-field Time Gain Compensation (TGC) at rest/try adjusting focus to the apex/use probe tissue pad. |
| Reduced contrast in basal myocardial segments. | Shorten the apical view additionally to obtain basal segments in the near field (do not use this view for wall motion analysis). |
| Overall reduced myocardial contrast/persistent under-infusion or insufficient bolus administration/"swirling" phenomenon. | Check gain settings; check if the Mechanical Index (MI) is set too high; ensure the venous access is unobstructed; if using slow infusion, increase infusion rate or switch to small bolus administration; pay attention to the contrast agent concentration and ensure thorough mixing. |
| Excess contrast agent in the cavity causing shadowing in basal/mid segments. | Slow down the infusion rate or reduce the bolus amount and flushing speed; or wait a few seconds; use high MI for "burst." |
| Rib shadowing in apical two-chamber view obstructing visualization of the basal and mid segments of the anterior wall. | Additional views for shortened anterior wall visualization (do not use this view for wall motion analysis)/parasternal short-axis view for anterior wall examination. |
Vasodilator SE is generally less sensitive in detecting wall motion abnormalities compared to exercise and dobutamine SE. However, its combination with UEAs can enhance the detection of perfusion abnormalities. This method is simpler to perform and usually maintains a lower heart rate, typically not exceeding 100 beats/min. This lower heart rate contributes to better image quality and reduces cardiac translational motion.
Using coronary angiography as a reference, dipyridamole stress MCE shows higher sensitivity compared to SPECT. Unlike vasodilator stress SPECT, which evaluates only capillary blood volume, MCE offers better spatial resolution and assesses both capillary blood volume and flow velocity. Dipyridamole or adenosine stress perfusion echocardiography is more predictive of cardiovascular endpoints than Stress Echocardiography based solely on wall motion analysis.[89-90] Additionally, VLMI imaging can be effectively combined with high mechanical index flash-echo replenishment techniques when using vasodilators.
Recommendations:
(1) UEAs enhance the diagnostic accuracy of segmental wall motion analysis during both resting and stress imaging. VLMI imaging is an ideal technique for segmental wall motion analysis, and the provided perfusion information helps differentiate minor wall thickening abnormalities caused by subendocardial ischemia. It is recommended to use VLMI technology in stress echocardiography for segmental wall motion analysis (COR I, LOE C).
(2) During exercise and dobutamine stress, real-time VLMI imaging using high mechanical index flash-echo replenishment techniques for simultaneous perfusion and wall motion assessment can improve the detection and prognostic prediction accuracy for coronary artery disease (COR IIa, LOE B).
(3) When using vasodilator SE, employing real-time VLMI perfusion imaging technology enhances the sensitivity and specificity for detecting local wall motion and myocardial perfusion abnormalities. Post-vasodilation myocardial perfusion imaging can better predict cardiovascular composite endpoints (COR IIa, LOE B).
(4) In the assessment of SE perfusion imaging, the replenishment of microbubbles in myocardial tissue following high mechanical index flash-echo destruction should be considered abnormal if it takes more than 5 seconds at rest and more than 2 seconds under stress (COR IIa, LOE C).
7. APPLICATION OF CORONARY FLOW VELOCITY MEASUREMENT AND CORONARY FLOW RESERVE
CFR is defined as the maximum capacity of coronary arteries to dilate in response to increased myocardial metabolic demands. CFR is quantified as the ratio of blood flow during hyperemia to the blood flow at rest. The hyperemic state is typically induced using vasodilator drugs, rather than by an increase in myocardial oxygen demand itself.
7.1. Examination Method
To accurately measure coronary artery blood flow velocity, transesophageal echocardiography is used for proximal coronary arteries and transthoracic echocardiography for mid and distal sections, employing pulse wave Doppler. Clinically, the mid and distal segments of the left anterior descending (LAD) coronary artery are commonly examined using transthoracic echocardiography. This involves selecting a coronary artery examination program, using an apical two-chamber view at the interventricular groove, activating color Doppler (velocity settings 10–24 cm/s) to visualize the flow, and then employing pulse wave Doppler to measure the coronary artery's blood flow velocity.
By adjusting the probe's position during echocardiography, different coronary arteries can be visualized. Rotating the probe counterclockwise at the LAD display site allows observation of the posterior descending artery. Similarly, rotating the probe clockwise by 50–80° in the apical four-chamber view displays the proximal and mid-segments of the circumflex artery. The success rate of detecting coronary flow reserve is high in the LAD (over 99%), but lower in the circumflex artery (50%) and the posterior descending artery (60%). The clarity of spectral display is enhanced with the use of UEAs.
7.2. CFR Judgment Criteria
The normal values for CFR are generally consistent across the three main coronary arteries, typically being above 3.0. A CFR value below 2.0 is considered reduced and is associated with an increased risk of mortality. A CFR less than 2.5 indicates a reduced reserve, while values ranging from 2.5 to 3.0 are in a gray area and should be interpreted alongside clinical findings (Figure 12). Athletes may exhibit exceptionally high CFR values, often greater than 4.0.
Figure 12.
Reduced coronary flow reserve.
(A): Coronary blood flow at rest; (B): coronary blood flow under vasodilator-induced hyperemia.
Doppler ultrasound-measured coronary blood flow spectra are biphasic, with systolic flow velocity being the lowest and diastolic velocity the highest. This variation is largely due to myocardial contraction, with systole showing higher extramural vascular resistance and diastole showing lower. Changes in flow velocity reflect total blood flow, assuming vessel lumen diameter remains constant. However, adenosine infusion typically enlarges the diameter of epicardial coronary arteries by about 30%. Accurate flow rate measurement, considering the velocity-time integral and cross-sectional area, is crucial.[91] Not accounting for epicardial artery dilation during hyperemic response can underestimate CFR.
Ultrasound technology currently faces limitations in accurately measuring the diameters of distal coronary arteries at rest and during hyperemia. The increase in blood flow velocity proportional to blood flow volume allows the coronary flow velocity reserve (CFVR), calculated by the ratio of peak diastolic flow velocity during resting and hyperemic states with Doppler, to approximate coronary flow reserve measured by PET.[92] CFVR is thus a practical method for assessing CFR.[93] The absolute flow velocity measurements can be influenced by the angle between the Doppler beam and blood flow, potentially resulting in lower velocity readings. However, since CFR is a ratio of velocities, if the angle between the epicardial coronary artery and the Doppler beam remains consistent, angle correction is typically not required, enhancing the reliability of estimated CFR. This method, with reduced variability, is widely suitable for clinical CFR assessment.[94]
7.3. Advantages and Limitations
Transthoracic echocardiography is a straightforward, repeatable, and cost-effective method for measuring CFR. However, CFVR alone cannot distinguish between microvascular disease and large vessel coronary artery disease.[95] For comprehensive assessment, it requires concurrent epicardial vessel examination. While CFVR measurement is highly successful for the left anterior descending artery, it may not fully represent the overall coronary circulation. Nonetheless, given the LAD's significance in myocardial supply, CFVR assessment in this region is still valuable.
7.4. Clinical Significance
A reduction in CFVR can be linked to significant epicardial coronary artery stenosis and microvascular disease, as well as increased resistance in normal coronary arteries and external compression of these vessels, which may occur in conditions like Syndrome X, dilated or hypertrophic cardiomyopathy, and aortic stenosis. Normal resting wall motion might be observed in epicardial coronary artery stenosis, yet an asymptomatic reduction in CFVR can accompany stenosis greater than 50%. Hemodynamic abnormalities become increasingly apparent with stenosis exceeding 70%, progressively impacting CFVR (Figures 13,14).
Figure 13.

Relationship between coronary artery stenosis degree and CFR.
Coronary artery lumen stenosis degree negatively correlates with CFR capability. When epicardial coronary artery stenosis is about 50%, CFR can still maintain around 4. With stenosis > 70%, hemodynamics become obviously abnormal as the degree of stenosis increases. When stenosis > 90%, the reserve capacity significantly decreases, with CFR < 1. CFR: coronary flow reserve.
Figure 14.
Pathophysiology and prognostic heterogeneity in stress echocardiography with normal wall motion.
The first row shows epicardial coronary arteries; the first two columns have normal vessel structure, the third column shows moderate stenosis, and the fourth column shows moderate-severe stenosis with concurrent anti-ischemic medication treatment. The second row circles represent coronary micro-vessels, with the second column's thickened circle indicating microvascular structural or functional impairment. The third row illustrates coronary flow reserve, showing various degrees of reduction in the last three columns, accompanied by microcirculatory or epicardial vessel structural and functional abnormalities. The fourth row displays left ventricular wall motion, with no significant abnormalities observed. The last row demonstrates the potential prognostic heterogeneity in stress echocardiography with normal wall motion under different pathophysiological states. LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery.
The relationship between coronary artery stenosis and Coronary Flow Velocity Reserve (CFVR) is influenced by several factors: the stenosis's geometric characteristics, presence of coronary collateral circulation, microvascular factors affecting coronary resistance, extra-myocardial factors like left ventricular hypertrophy, the state of myocardial viability or necrosis beyond the stenosis, spasms in large coronary arteries or micro-vessels, and ongoing anti-ischemic treatments. CFVR reductions in ischemic heart disease are not solely due to coronary artery stenosis. In non-obstructive epicardial vessel diseases, reduced CFVR suggests impaired microvascular dilation function. Integrating CFVR with stress wall motion analysis can enhance coronary artery disease diagnosis and risk stratification, with survival rates being notably lower in patients having a CFVR less than 2.0 without myocardial infarction.[96]
8. APPLICATION OF SE TECHNOLOGY AND RISK STRATIFICATION
SE, along with nuclear myocardial imaging, stress magnetic resonance, and coronary artery CT angiography, is highly efficient for diagnosis and prognosis, and is a Class I recommendation for patients with suspected or confirmed stable angina.[6] However, the application of SE in China requires significant enhancement, highlighting the need for training and promotion to improve the expertise of cardiovascular physicians and echocardiographers. The choice of SE modality should be based on the patient's specific condition, the availability of equipment and drugs, and the operator's skill level.
SE is particularly beneficial for patients with a moderate to high pre-test probability of CAD. It enables the assessment of myocardial ischemia through the observation of wall motion and myocardial blood flow perfusion. SE also provides valuable insights into cardiac structure, function, and hemodynamic changes. This comprehensive evaluation is crucial in guiding subsequent treatment strategies, which may include medical therapy or combined coronary revascularization approaches.
Exercise SE, simulating physiological conditions, is often the first choice. Pharmacological stress testing is recommended for patients who cannot perform adequate exercise. Vasodilator perfusion imaging suits those with significant left ventricular dyssynchrony at rest. Clinical studies show that a normal stress test correlates with a < 1% annual incidence of cardiovascular events, akin to healthy populations and patients with normal coronary angiography. Risk stratification based on peak Wall Motion Score Index (WMSI) divides patients into low (WMSI = 1, 0.9% annual event rate), intermediate (WMSI = 1.1–1.7, 3.1%), and high risk (WMSI > 1.7, 5.2%). Patients with extensive and severe wall motion abnormalities can have an annual cardiovascular event rate as high as 6.7%, indicating the importance of assessing the severity of ischemic wall motion abnormalities in accurate prognostication.[27] SE with contrast echocardiography for perfusion enhances detection of subendocardial ischemia and wall motion abnormalities, offering additional prognostic value.[33]
About 20% of patients show ischemia without significant coronary artery stenosis, which could be related to microcirculatory disease or extracardiac factors. SE can identify these cases, and the future incidence of cardiovascular events in these patients is twice as high as those with normal SE results.[97] Adding CFVR assessment to SE provides crucial CFR information. Abnormal CFVR is an independent risk factor for predicting adverse events.[98] Patients with CFVR < 2 face a 14.6% 8-year cardiovascular mortality rate, versus 1.2% in those with CFVR ≥ 2.[99]
Recommendations:
(1) For patients with moderate to high pre-test probability of CAD, it is recommended to use SE to assess wall motion and myocardial blood flow perfusion to guide further coronary angiography and revascularization (COR IIa, LOE B).
(2) SE has a high negative predictive value, and peak WMSI can be used for risk stratification of cardiovascular adverse events (COR IIa, LOE B).
(3) Even in patients without significant coronary artery stenosis or wall motion abnormalities, abnormalities in myocardial blood flow perfusion and CFVR < 2 can predict adverse cardiovascular events (COR IIa, LOE B).
(4) SE ischemic manifestations such as WMSI > 1.7, wall motion abnormalities in > 3 segments, perfusion defects in > 2/17 segments, or CFVR < 2 are considered high-risk; inducement of segmental wall motion abnormalities or perfusion abnormalities but less severe than the high-risk criteria are considered intermediate-risk; absence of ischemic response is considered low-risk (COR IIa, LOE C).
9. CONCLUSION
The diagnostic flowchart in Figure 15 outlines the use of SE in evaluating patients with CCS. SE is crucial for diagnosing CCS, determining the extent and severity of myocardial ischemia, and assessing cardiac reserve function. It guides in selecting treatment plans and evaluating their effectiveness. SE is particularly vital in detecting viable myocardium and further stratifying risk in patients with cardiac dysfunction. The choice between exercise and pharmacological stress tests depends on the patient’s condition and exercise capacity. Vasodilators are recommended for assessing CFR and myocardial perfusion. The application of UEAs, strain, and three-dimensional echocardiography techniques during SE enhances the accuracy of both qualitative and quantitative assessments of myocardial ischemia.
Figure 15.
Chronic coronary syndrome stress echocardiography protocol.
DSE: dobutamine stress echocardiography; WMSI: wall motion score index; CFVR: coronary flow velocity reserve.
This guideline integrates globally published literature, international guidelines and consensus, and expert recommendations. It acknowledges that many recommendations are based on limited evidence, often derived from smaller-scale studies. There is a need for future large-scale, multi-center clinical trials in diverse CCS patient scenarios to strengthen the evidence base for myocardial ischemia assessment. Advances in wearable ultrasound technology and artificial intelligence analysis are expected to simplify SE procedures and enhance measurement accuracy, potentially broadening SE's applicability in clinical practice.[100]
DISCLOSURE
Fundings
National Key Research and Development Program (2022YFC3602400, 2023YFC2506502); Shandong Provincial Key Research and Development Program(2021SFGC0503).
Conflict of Interest:
None.
Expert Committee (Sorted by the number of strokes in the surname in Chinese):
Xiao-Jing MA (Wuhan Asia Heart Hospital);
Chun-Yan MA (The First Hospital of China Medical University);
Xiao-Cong WANG (The First Bethune Hospital of Jilin University);
Wen WANG (Cardiovascular and Cerebrovascular Disease Hospital, General Hospital of Ningxia Medical University);
Shun WANG (The First Affiliated Hospital of Xi'an Jiaotong University);
Yi WANG (Sichuan Provincial People's Hospital);
Hao WANG (Beijing Fuwai Cardiovascular Hospital);
Li-Xue YIN (Sichuan Provincial People's Hospital);
Hong-Ning YIN (The Second Hospital of Hebei Medical University);
You-Bin DENG (Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology);
Yan DENG (Sichuan Provincial People's Hospital);
Jia-Wei TIAN (The Second Affiliated Hospital of Harbin Medical University);
Xin-Qiao TIAN (Henan Provincial People's Hospital, Henan Provincial Cardiovascular Disease Hospital);
Tao CONG (The First Affiliated Hospital of Dalian Medical University);
Tian-Gang ZHU (Peking University People's Hospital);
Hao-Hui ZHU (Henan Provincial People's Hospital);
Mei ZHU (The First Affiliated Hospital of Kunming Medical University);
Wei-Dong REN (Shengjing Hospital of China Medical University);
Li-Wen LIU (Xijing Hospital, Fourth Military Medical University);
Ying-Ying LIU (Shenzhen People's Hospital);
Shuang LIU (The Fourth Affiliated Hospital of China Medical University);
Yong JIANG (Beijing Fuwai Cardiovascular Hospital);
Di XU (Jiangsu Province Hospital);
Hua LI (The Fourth Affiliated Hospital of Xinjiang Medical University);
Jue-Fei WU (Nanfang Hospital, Southern Medical University);
Xiao-Ping LENG (The Second Affiliated Hospital of Harbin Medical University);
Yun ZHANG (Qilu Hospital of Shandong University, National Key Laboratory for innovation and Transformation of Luobing Theory);
Mei ZHANG (Qilu Hospital of Shandong University, National Key Laboratory for innovation and Transformation of Luobing Theory);
Peng-Fei ZHANG (Qilu Hospital of Shandong University);
Li-Sha NA (General Hospital of Ningxia Medical University);
Xiao ZHOU (Chinese PLA General hospital);
Qing ZHOU (Renmin Hospital of Wuhan University, Hubei General Hospital);
Zhe-Lan ZHENG (The First Affiliated Hospital of Zhejiang University School of Medicine);
Hong-Wen FEI (Guangdong Provincial Peoples' Hospital (Guangdong Academy of Medical Sciences), Southern Medical University);
Gui-Hua YAO (Qilu Hospital of Shandong University, Qingdao);
Li-Jun YUAN (Tangdu Hospital, Fourth Military Medical University);
Jian-Jun YUAN (Henan Provincial People's Hospital);
Sheng-Lan GUO (The First Affiliated Hospital of Guangxi Medical University);
Hong TANG (West China Hospital, Sichuan University);
Xian-Hong SHU (Zhongshan Hospital, Fudan University);
Xiang-Dong YOU (The Second Affiliated Hospital, Zhejiang University School of Medicine);
Mingxing Xie (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology);
Yu-Ming MU (The First Affiliated Hospital of Xinjiang Medical University).
Contributor Information
Li-Xue YIN, Email: yinlixue_cardiac@163.com.
Yun ZHANG, Email: yun-zhang@163.com.
Mei ZHANG, Email: daixh@vip.sina.com.
References
- 1.The Writing Committee of the Report on Cardiovascular Health and Diseases in China [Report on Cardiovascular Health and Diseases in China 2021: an Updated Summary] Chinese Circulation Journal. 2022;37:553–578. [Google Scholar]
- 2.Lyu B, Ren X, An Y, et al [Survey of the Application status of cardiovascular imaging modalities and medical ouality report in China] Chinese Circulation Journal. 2020;35:625–633. [Google Scholar]
- 3.Gulati M, Levy PD, Mukherjee D, et al 2021 AHA/ACC/ASE/CHEST/SAEM/ SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Gao D. [Exploration of the diagnostic value of combined submaximal exercise bicycle test for coronary artery disease: a multivariate discriminant analysis of exercise electrocardiogram, vectorcardiogram, echocardiogram, and systolic time intervals]. Shandong Yixueyuan Xuebao 1982: 68–77. [In Chinese].
- 5.Woodward W, Dockerill C, McCourt A, et al Real-world performance and accuracy of stress echocardiography: the EVAREST observational multi-centre study. Eur Heart J Cardiovasc Imaging. 2022;23:689–698. doi: 10.1093/ehjci/jeab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellikka PA, Arruda-Olson A, Chaudhry FA, et al Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J Am Soc Echocardiogr. 2020;33:1–41. doi: 10.1016/j.echo.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Knuuti J, Wijns W, Saraste A, et al 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 8.[Echocardiography Group of Chinese Medical Association Ultrasound Medical Branch Guideline of standardized operation of stress echocardiography] Chinese Journal of Imaging Technology. 2017;33:632–638. [Google Scholar]
- 9.Greenwood JP, Ripley DP, Berry C, et al Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or NICE Guidelines on Subsequent Unnecessary Angiography Rates: The CE-MARC 2 Randomized Clinical Trial. JAMA. 2016;316:1051–1060. doi: 10.1001/jama.2016.12680. [DOI] [PubMed] [Google Scholar]
- 10.Tonino PA, Fearon WF, De Bruyne B, et al Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 11.Layland J, Oldroyd KG, Curzen N, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J 2015; 36: 100-111.
- 12.Taqueti VR, Hachamovitch R, Murthy VL, et al Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echavarria-Pinto M, Escaned ME, Medina GN, et al Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128:2557–2566. doi: 10.1161/CIRCULATIONAHA.112.001345. [DOI] [PubMed] [Google Scholar]
- 14.Xaplanteris P, Fournier S, Pijls NHJ, et al Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250–259. doi: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- 15.Jespersen L, Hvelplund A, Abildstrom SZ, et al Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Peterson ED, Dai D, et al Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basic Research Group of the Chinese Society of Cardiology, Interventional Cardiology Group of the Chinese Society of Cardiology, Women's Heart Health Group of the Chinese Society of Cardiology, et al Chinese expert consensus on the diagnosis and treatment of coronary microvascular disease. Chinese Circulation Journal. 2017;32:421–430. [Google Scholar]
- 18.Garber CE, Carleton RA, Camaione DN, et al The threshold for myocardial ischemia varies in patients with coronary artery disease depending on the exercise protocol. J Am Coll Cardiol. 1991;17:1256–1262. doi: 10.1016/S0735-1097(10)80132-9. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg MJ, Rice S, Schiller NB Guidelines for physician training in advanced cardiac procedures: the importance of case mix. J Am Coll Cardiol. 1994;23:1723–1725. doi: 10.1016/0735-1097(94)90681-5. [DOI] [PubMed] [Google Scholar]
- 20.Porter TR, Mulvagh SL, Abdelmoneim SS, et al Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr. 2018;31:241–274. doi: 10.1016/j.echo.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Lancellotti P, Pellikka PA, Budts W, et al The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17:1191–1229. doi: 10.1093/ehjci/jew190. [DOI] [PubMed] [Google Scholar]
- 22.Bruce RA, Blackmon JR, Jones JW, et al. Exercising testing in adult normal subjects and cardiac patients. Pediatrics 1963; 32: Suppl 742–756.
- 23.Suzuki K, Hirano Y, Yamada H, et al Practical guidance for the implementation of stress echocardiography. J Echocardiogr. 2018;16:105–129. doi: 10.1007/s12574-018-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daubert MA, Sivak J, Dunning A, et al Implications of Abnormal Exercise Electrocardiography With Normal Stress Echocardiography. JAMA Intern Med. 2020;180:494–502. doi: 10.1001/jamainternmed.2019.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardiovascular Imaging Group of the Chinese Society of Cardiology, Expert Consensus Writing Group on the Non-invasive Imaging Examination Pathway for Stable Coronary Artery Disease [Chinese expert consensus of the non-invasive imaging examination pathways of stable coronary artery disease] Chinese Journal of Interventional Cardiology. 2017;25:541–549. [Google Scholar]
- 26.Huang Q, Wang J, Li D, et al Exercise electrocardiography combined with stress echocardiography for predicting myocardial ischemia in adults. Exp Ther Med. 2021;21:130. doi: 10.3892/etm.2020.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marwick TH, Case C, Vasey C, et al Prediction of mortality by exercise echocardiography: a strategy for combination with the duke treadmill score. Circulation. 2001;103:2566–2571. doi: 10.1161/01.CIR.103.21.2566. [DOI] [PubMed] [Google Scholar]
- 28.Yao SS, Qureshi E, Sherrid MV, et al Practical applications in stress echocardiography: risk stratification and prognosis in patients with known or suspected ischemic heart disease. J Am Coll Cardiol. 2003;42:1084–1090. doi: 10.1016/S0735-1097(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 29.Yao SS, Qureshi E, Syed A, et al Novel stress echocardiographic model incorporating the extent and severity of wall motion abnormality for risk stratification and prognosis. Am J Cardiol. 2004;94:715–719. doi: 10.1016/j.amjcard.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 30.From AM, Kane G, Bruce C, et al Characteristics and outcomes of patients with abnormal stress echocardiograms and angiographically mild coronary artery disease (<50% stenoses) or normal coronary arteries. J Am Soc Echocardiogr. 2010;23:207–214. doi: 10.1016/j.echo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Yao SS, Agarwal V, Chaudhry FA Prognostic value of treadmill stress echocardiography at extremes of exercise performance: submaximal <85% maximum predicted heart rate versus high exercise capacity ≥ 10 metabolic equivalents. Echocardiography. 2014;31:340–346. doi: 10.1111/echo.12372. [DOI] [PubMed] [Google Scholar]
- 32.Fine NM, Pellikka PA, Scott CG, et al Characteristics and outcomes of patients who achieve high workload (≥10 metabolic equivalents) during treadmill exercise echocardiography. Mayo Clin Proc. 2013;88:1408–1419. doi: 10.1016/j.mayocp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Elhendy A, Mahoney DW, Khandheria BK, et al Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol. 2003;42:823–830. doi: 10.1016/S0735-1097(03)00832-5. [DOI] [PubMed] [Google Scholar]
- 34.Porter TR, Smith LM, Wu J, et al Patient outcome following 2 different stress imaging approaches: a prospective randomized comparison. J Am Coll Cardiol. 2013;61:2446–2455. doi: 10.1016/j.jacc.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Geleijnse ML, Krenning BJ, Nemes A, et al Incidence, pathophysiology, and treatment of complications during dobutamine-atropine stress echocardiography. Circulation. 2010;121:1756–1767. doi: 10.1161/CIRCULATIONAHA.109.859264. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsui JM, Osório AF, Lario FA, et al Comparison of safety and efficacy of the early injection of atropine during dobutamine stress echocardiography with the conventional protocol. Am J Cardiol. 2004;94:1367–1372. doi: 10.1016/j.amjcard.2004.07.141. [DOI] [PubMed] [Google Scholar]
- 37.Ling LH, Christian TF, Mulvagh SL, et al Determining myocardial viability in chronic ischemic left ventricular dysfunction: a prospective comparison of rest-redistribution thallium 201 single-photon emission computed tomography, nitroglycerin-dobutamine echocardiography, and intracoronary myocardial contrast echocardiography. Am Heart J. 2006;151:882–889. doi: 10.1016/j.ahj.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Schinkel AF, Bax JJ, Poldermans D, et al Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Cornel JH, Bax JJ, Elhendy A, et al Biphasic response to dobutamine predicts improvement of global left ventricular function after surgical revascularization in patients with stable coronary artery disease: implications of time course of recovery on diagnostic accuracy. J Am Coll Cardiol. 1998;31:1002–1010. doi: 10.1016/S0735-1097(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 40.Bax JJ, Poldermans D, Elhendy A, et al Improvement of left ventricular ejection fraction, heart failure symptoms and prognosis after revascularization in patients with chronic coronary artery disease and viable myocardium detected by dobutamine stress echocardiography. J Am Coll Cardiol. 1999;34:163–169. doi: 10.1016/S0735-1097(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 41.Meluzín J, Cerný J, Frélich M, et al Prognostic value of the amount of dysfunctional but viable myocardium in revascularized patients with coronary artery disease and left ventricular dysfunction. Investigators of this Multicenter Study. J Am Coll Cardiol. 1998;32:912–920. doi: 10.1016/s0735-1097(98)00324-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Zhang Y, Xu X, et al [Effect of low dose dobutamine echocardiography on left ventricular systolic function in mitral regurgitation] Chinese Journal of Ultrasound in Medicine. 1998;8:16–18. [Google Scholar]
- 43.Mulvagh S. L, Abdelmoneim S. S, Picano E. Stress Echocardiography. Springer International Publishing, 2015: 237–257.
- 44.Iskandrian AE, Bateman TM, Belardinelli L, et al Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 45.Mahmarian JJ, Cerqueira MD, Iskandrian AE, et al Regadenoson induces comparable left ventricular perfusion defects as adenosine: a quantitative analysis from the ADVANCE MPI 2 trial. JACC Cardiovasc Imaging. 2009;2:959–968. doi: 10.1016/j.jcmg.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Thomas GS, Tammelin BR, Schiffman GL, et al. Safety of regadenoson, a selective adenosine A2A agonist, in patients with chronic obstructive pulmonary disease: A randomized, double-blind, placebo-controlled trial (RegCOPD trial). J Nucl Cardiol 2008; 15: 319–328. D.
- 47.Steeds RP, Wheeler R, Bhattacharyya S, et al Stress echocardiography in coronary artery disease: a practical guideline from the British Society of Echocardiography. Echo Res Pract. 2019;6:G17–G33. doi: 10.1530/ERP-18-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djordjevic-Dikic AD, Ostojic MC, Beleslin BD, et al High dose adenosine stress echocardiography for noninvasive detection of coronary artery disease. J Am Coll Cardiol. 1996;28:1689–1695. doi: 10.1016/S0735-1097(96)00374-9. [DOI] [PubMed] [Google Scholar]
- 49.Porter TR, Adolphson M, High RR, et al Rapid detection of coronary artery stenoses with real-time perfusion echocardiography during regadenoson stress. Circ Cardiovasc Imaging. 2011;4:628–635. doi: 10.1161/CIRCIMAGING.111.966341. [DOI] [PubMed] [Google Scholar]
- 50.Djordjevic-Dikic A, Ostojic M, Beleslin B, et al. Low-dose adenosine stress echocardiography: detection of myocardial viability. Cardiovasc Ultrasound 2003, 1: 7.
- 51.Zhang M, Qu H, Zhang Y, et al [Early diagnosis of myocardial ischemia by adenosine stress real-time myocardial contrast echocardiography] Chinese Journal of Ultrasonography. 2006;15:517–519. [Google Scholar]
- 52.Vogel R, Indermühle A, Meier P, et al Quantitative stress echocardiography in coronary artery disease using contrast-based myocardial blood flow measurements: prospective comparison with coronary angiography. Heart. 2009;95:377–384. doi: 10.1136/hrt.2007.134577. [DOI] [PubMed] [Google Scholar]
- 53.Sierra-Galan LM, Ingkanisorn WP, Rhoads KL, et al Qualitative assessment of regional left ventricular function can predict MRI or radionuclide ejection fraction: an objective alternative to eyeball estimates. J Cardiovasc Magn Reson. 2003;5:451–463. doi: 10.1081/JCMR-120022261. [DOI] [PubMed] [Google Scholar]
- 54.Sicari R, Nihoyannopoulos P, Evangelista A, et al Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur J Echocardiogr. 2008;9:415–437. doi: 10.1093/ejechocard/jen175. [DOI] [PubMed] [Google Scholar]
- 55.el-Said ES, Roelandt JR, Fioretti PM, et al. Abnormal left ventricular early diastolic filling during dobutamine stress Doppler echocardiography is a sensitive indicator of significant coronary artery disease. J Am Coll Cardiol 1994, 24: 1618–1624.
- 56.Peteiro J, Pazos P, Bouzas A, et al. Assessment of diastolic function during exercise echocardiography: annulus mitral velocity or transmitral flow pattern? J Am Soc Echocardiogr 2008; 21: 178–184.
- 57.Mansour MJ, Aljaroudi W, Mroueh A, et al Stress-induced Worsening of Left Ventricular Diastolic Function as a Marker of Myocardial Ischemia. J Cardiovasc Echogr. 2017;27:45–51. doi: 10.4103/jcecho.jcecho_44_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kane GC, Oh JK Diastolic stress test for the evaluation of exertional dyspnea. Curr Cardiol Rep. 2012;14:359–365. doi: 10.1007/s11886-012-0269-7. [DOI] [PubMed] [Google Scholar]
- 59.el-Mahalawy N, Abdel-Salam Z, Samir A, et al Left ventricular transient ischemic dilation during dobutamine stress echocardiography predicts multi-vessel coronary artery disease. J Cardiol. 2009;54:255–261. doi: 10.1016/j.jjcc.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Turakhia MP, McManus DD, Whooley MA, et al Increase in end-systolic volume after exercise independently predicts mortality in patients with coronary heart disease: data from the Heart and Soul Study. Eur Heart J. 2009;30:2478–2484. doi: 10.1093/eurheartj/ehp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu DY, Beyer AT, Pursnani SK, et al Left ventricular end-systolic volume response post-stress echocardiography: Dilation as a marker of multi-vessel coronary artery disease. Echocardiography. 2022;39:215–222. doi: 10.1111/echo.15291. [DOI] [PubMed] [Google Scholar]
- 62.Mastouri R, Mahenthiran J, Kamalesh M, et al Prediction of ischemic events by anatomic M-mode strain rate stress echocardiography. J Am Soc Echocardiogr. 2008;21:299–306. doi: 10.1016/j.echo.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Joyce E, Delgado V, Bax JJ, et al. Advanced techniques in dobutamine stress echocardiography: focus on myocardial deformation analysis. Heart 2015; 101:.
- 64.Liou K, Negishi K, Ho S, et al. Detection of Obstructive Coronary Artery Disease Using Peak Systolic Global Longitudinal Strain Derived by Two-Dimensional Speckle-Tracking: A Systematic Review and Meta-Analysis. J Am Soc Echocardiogr 2016; 29: 724–735. e4.
- 65.Abdelmoneim SS, Dhoble A, Bernier M, et al Quantitative myocardial contrast echocardiography during pharmacological stress for diagnosis of coronary artery disease: a systematic review and meta-analysis of diagnostic accuracy studies. Eur J Echocardiogr. 2009;10:813–825. doi: 10.1093/ejechocard/jep084. [DOI] [PubMed] [Google Scholar]
- 66.Attenhofer CH, Pellikka PA, Oh JK, et al Comparison of ischemic response during exercise and dobutamine echocardiography in patients with left main coronary artery disease. J Am Coll Cardiol. 1996;27:1171–1177. doi: 10.1016/0735-1097(95)00583-8. [DOI] [PubMed] [Google Scholar]
- 67.Yao SS, Supariwala A, Yao A, et al Prognostic Value of Stress Echocardiography in Patients With Low-Intermediate or High Short-Term (10 Years) Versus Low (<39%) or High (≥39%) Lifetime Predicted Risk of Cardiovascular Disease According to the American College of Cardiology/American Heart Association 2013 Cardiovascular Risk Calculator. Am J Cardiol. 2015;116:725–729. doi: 10.1016/j.amjcard.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 68.Cain P, Khoury V, Short L, et al Usefulness of quantitative echocardiographic techniques to predict recovery of regional and global left ventricular function after acute myocardial infarction. Am J Cardiol. 2003;91:391–396. doi: 10.1016/S0002-9149(02)03231-9. [DOI] [PubMed] [Google Scholar]
- 69.Senior R, Kaul S, Lahiri A Myocardial viability on echocardiography predicts long-term survival after revascularization in patients with ischemic congestive heart failure. J Am Coll Cardiol. 1999;33:1848–1854. doi: 10.1016/S0735-1097(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 70.Bountioukos M, Schinkel AF, Bax JJ, et al Pulsed wave tissue Doppler imaging for the quantification of contractile reserve in stunned, hibernating, and scarred myocardium. Heart. 2004;90:506–510. doi: 10.1136/hrt.2003.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joshi K, Alam I, Ruden E, et al Effect of improvement in left ventricular ejection fraction on long-term survival in revascularized patients with ischaemic left ventricular systolic dysfunction. Eur J Echocardiogr. 2011;12:454–460. doi: 10.1093/ejechocard/jer045. [DOI] [PubMed] [Google Scholar]
- 72.Ling LF, Marwick TH, Flores DR, et al Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging. 2013;6:363–372. doi: 10.1161/CIRCIMAGING.112.000138. [DOI] [PubMed] [Google Scholar]
- 73.Ana G Almeida, John-Paul Carpenter, Matteo Cameli, et al Multimodality imaging of myocardial viability: an expert consensus document from the European Association of Cardiovascular Imaging (EACVI) Eur Heart J Cardiovasc Imaging. 2021;22:e97–e125. doi: 10.1093/ehjci/jeab053. [DOI] [PubMed] [Google Scholar]
- 74.Larsen AH, Clemmensen TS, Wiggers H, et al. Left Ventricular Myocardial Contractile Reserve during Exercise Stress in Healthy Adults: A Two-Dimensional Speckle-Tracking Echocardiographic Study. J Am Soc Echocardiogr 2018; 31: 1116–1126. e1.
- 75.d'Entremont MA, Fortin G, Huynh T, et al The feasibility, reliability, and incremental value of two-dimensional speckle-tracking for the detection of significant coronary stenosis after treadmill stress echocardiography. Cardiovasc Ultrasound. 2021;19:27. doi: 10.1186/s12947-021-00259-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mandoli GE, Pastore MC, Vasilijevaite K, et al. Speckle tracking stress echocardiography: A valuable diagnostic technique or a burden for everyday practice? Echocardiography 2020; 37: 2123–2129.
- 77.Hubbard RT, Arciniegas Calle MC, Barros-Gomes S, et al 2-Dimensional Speckle Tracking Echocardiography predicts severe coronary artery disease in women with normal left ventricular function: a case-control study. BMC Cardiovasc Disord. 2017;17:231. doi: 10.1186/s12872-017-0656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin JR, Wu WC, Gao LJ, et al Global myocardial work combined with treadmill exercise stress to detect significant coronary artery disease. J Am Soc Echocardiogr. 2022;35:247–257. doi: 10.1016/j.echo.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 79.Ran H, Zhang PY, Fang LL, et al Clinic value of two-dimensional speckle tracking combined with adenosine stress echocardiography for assessment of myocardial viability. Echocardiography. 2012;29:688–694. doi: 10.1111/j.1540-8175.2012.01690.x. [DOI] [PubMed] [Google Scholar]
- 80.Li L, Wang F, Xu T, et al The detection of viable myocardium by low-dose dobutamine stress speckle tracking echocardiography in patients with old myocardial infarction. J Clin Ultrasound. 2016;44:545–554. doi: 10.1002/jcu.22366. [DOI] [PubMed] [Google Scholar]
- 81.Hoffmann R, von Bardeleben S, Kasprzak JD, et al Analysis of regional left ventricular function by cineventriculography, cardiac magnetic resonance imaging, and unenhanced and contrast-enhanced echocardiography: a multicenter comparison of methods. J Am Coll Cardiol. 2006;47:121–128. doi: 10.1016/j.jacc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Hoffmann R, von Bardeleben S, Barletta G, et al Comparison of two- and three-dimensional unenhanced and contrast-enhanced echocardiographies versus cineventriculography versus cardiac magnetic resonance for determination of left ventricular function. Am J Cardiol. 2014;113:395–401. doi: 10.1016/j.amjcard.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 83.Hoffmann R, Barletta G, von Bardeleben S, et al Analysis of left ventricular volumes and function: a multicenter comparison of cardiac magnetic resonance imaging, cine ventriculography, and unenhanced and contrast-enhanced two-dimensional and three-dimensional echocardiography. J Am Soc Echocardiogr. 2014;27:292–301. doi: 10.1016/j.echo.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Kurt M, Shaikh KA, Peterson L, et al Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;3:02–810. doi: 10.1016/j.jacc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Kaufmann BA, Wei K, Lindner JR Contrast echocardiography. Curr Probl Cardiol. 2007;32:51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 86.Rafter P, Phillips P, Vannan MA Imaging technologies and techniques. Cardiol Clin. 2004;22:181–197. doi: 10.1016/j.ccl.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 87.Xie F, Dodla S, O'Leary E, et al Detection of subendocardial ischemia in the left anterior descending coronary artery territory with real-time myocardial contrast echocardiography during dobutamine stress echocardiography. JACC Cardiovasc Imaging. 2008;1:271–278. doi: 10.1016/j.jcmg.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Baibhav B, Mahabir CA, Xie F, et al Predictive Value of Dobutamine Stress Perfusion Echocardiography in Contemporary End-Stage Liver Disease. J Am Heart Assoc. 2017;6:e005102. doi: 10.1161/JAHA.116.005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaibazzi N, Reverberi C, Lorenzoni V, et al Prognostic value of high-dose dipyridamole stress myocardial contrast perfusion echocardiography. Circulation. 2012;126:1217–1224. doi: 10.1161/CIRCULATIONAHA.112.110031. [DOI] [PubMed] [Google Scholar]
- 90.Senior R, Moreo A, Gaibazzi N, et al Comparison of sulfur hexafluoride microbubble (SonoVue)-enhanced myocardial contrast echocardiography with gated single-photon emission computed tomography for detection of significant coronary artery disease: a large European multicenter study. J Am Coll Cardiol. 2013;62:1353–1361. doi: 10.1016/j.jacc.2013.04.082. [DOI] [PubMed] [Google Scholar]
- 91.Kiviniemi TO, Toikka JO, Koskenvuo JW, et al Vasodilation of epicardial coronary artery can be measured with transthoracic echocardiography. Ultrasound Med Biol. 2007;33:362–370. doi: 10.1016/j.ultrasmedbio.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Radvan J, Marwick TH, Williams MJ, et al Evaluation of the extent and timing of the coronary hyperemic response to dipyridamole: a study with transesophageal echocardiography and positron emission tomography with oxygen 15 water. J Am Soc Echocardiogr. 1995;8:864–873. doi: 10.1016/S0894-7317(05)80010-0. [DOI] [PubMed] [Google Scholar]
- 93.Iliceto S, Marangelli V, Memmola C, et al Transesophageal Doppler echocardiography evaluation of coronary blood flow velocity in baseline conditions and during dipyridamole-induced coronary vasodilation. Circulation. 1991;83:61–69. doi: 10.1161/01.CIR.83.1.61. [DOI] [PubMed] [Google Scholar]
- 94.Wikström J, Grönros J, Gan LM Adenosine induces dilation of epicardial coronary arteries in mice: relationship between coronary flow velocity reserve and coronary flow reserve in vivo using transthoracic echocardiography. Ultrasound Med Biol. 2008;34:1053–1062. doi: 10.1016/j.ultrasmedbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 95.Rigo F, Murer B, Ossena G, et al Transthoracic echocardiographic imaging of coronary arteries: tips, traps, and pitfalls. Cardiovasc Ultrasound. 2008;6:7. doi: 10.1186/1476-7120-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cortigiani L, Rigo F, Gherardi S, et al Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC Cardiovasc Imaging. 2012;5:1079–1085. doi: 10.1016/j.jcmg.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 97.Kutty S, Bisselou Moukagna KS, Craft M, et al. Clinical outcome of patients with inducible capillary blood flow abnormalities during demand stress in the presence or absence of angiographic coronary disease. Circ Cardiovasc Imaging 2018, 11(10): e007483.
- 98.Lee SH, Shin D, Lee JM, et al Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J Am Heart Assoc. 2022;11:e025171. doi: 10.1161/JAHA.121.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gaibazzi N, Picano E, Suma S, et al Coronary flow velocity reserve reduction is associated with cardiovascular, cancer, and noncancer, noncardiovascular mortality. J Am Soc Echocardiogr. 2020;33:594–603. doi: 10.1016/j.echo.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Hu H, Huang H, Li M, et al A wearable cardiac ultrasound imager. Nature. 2023;613:667–675. doi: 10.1038/s41586-022-05498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]