Abstract
The intersection of metabolic processes and epigenetic regulation during embryogenesis is crucial yet not fully understood. Through a candidate RNAi screen in Caenorhabditis elegans , we identified metabolic enzymes ALDO-2 and PDHB-1 as potential epigenetic regulators. Mild alteration of the chromatin remodeler LET-418 /Mi2 activity rescues embryonic lethality induced by suppressing aldo-2 or pdhb-1 , suggesting a critical role for glucose and pyruvate metabolism in chromatin remodeling during embryogenesis. Given the conservation of central metabolic pathways and chromatin modifiers across species, our findings lay the foundation for future mechanistic investigations into the interplay between epigenetics and metabolism during development and upon disease.
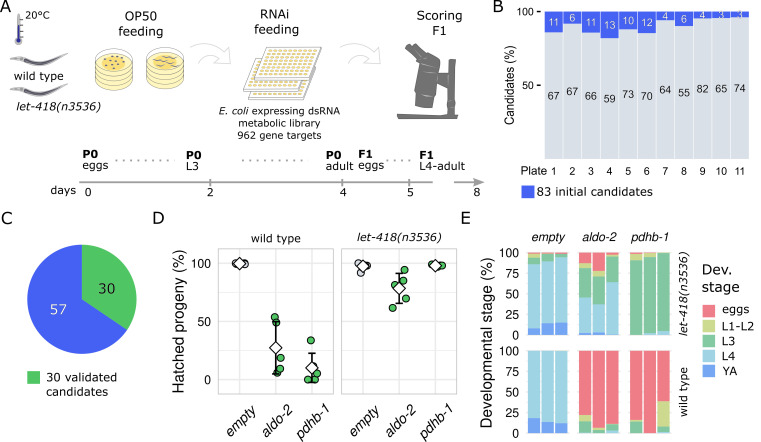
Figure 1. RNAi screening reveals aldo-2 and pdhb-1 metabolic enzymes as potential epigenetic regulators in C. elegans embryonic development .
A. Schematic view of the screening setup. B. Identified candidates across the eleven 96-well screening plates seeded with the RNAi metabolic library, excluding control wells. All clones and results of the primary screen are reported in the extended data. C. Validation of the 83 candidates and confirmation by experimental repetition and sequencing of RNAi clones. D. Quantification of embryonic lethality of F2 wild type and let-418 ( n3536 ) mutant on aldo-2 and pdhb-1 RNAi (in green), compared with the empty vector as RNAi control (L4440) (in gray) . Means are represented by white squares and standard deviations are shown as error bars. E. Quantification of the developmental rate of F2 wild-type and let-418 ( n3536 ) animals on empty vector, aldo-2 , or pdhb-1 RNAi. Colors indicate the proportion of eggs or larval stages L1-L2, L3, L4, and young adults (YA) present 2 days post-egg-laying.
Description
Epigenetic events, i.e. heritable changes in gene function that occur without altering the DNA sequence (Berger et al., 2009; Dupont et al., 2009) , can influence DNA accessibility, transcription, replication, and repair. Thus, epigenetic mechanisms provide robust yet flexible cellular responses. These include adaptation to environmental changes and determination of cell identities during embryo development (Cavalli & Heard, 2019; Wilkinson et al., 2023) . The chromatin landscape is shaped by modifications to DNA nucleotides (Iurlaro et al., 2017) and histone amino acids (Y. Zhang et al., 2021), along with ATP-dependent chromatin remodeling enzymes that organize the position and composition of nucleosomes (Eustermann et al., 2024) . Epigenetic mechanisms are known to be intertwined with cellular metabolism – serving as substrates, co-factors, or regulators, metabolites can directly influence the chromatin structure (Z. Dai et al., 2020; X. Li et al., 2018). The chromatin has recently been proposed to act as a metabolic reservoir, supporting the synergy between cellular metabolism and epigenome (Nirello et al., 2022) . Despite vast research, the causal connection between metabolism and epigenetic regulation remains largely unresolved. This study aimed to identify epigenetic regulators among metabolic genes.
The selection of phenotypes exclusively linked with epigenetic defects is challenging due to the multi-level integration of epigenetic processes within complex cellular networks. Cell pluripotency and differentiation states largely depend on their metabolic and epigenetic profiles (Shyh-Chang & Ng, 2017; Wilkinson et al., 2023) . Therefore, developing embryos represent ideal models to elucidate the interaction between metabolic and epigenetic events. Our goal was to identify metabolic genes specifically linked to the epigenetic regulation of embryogenesis and early development. Caenorhabditis elegans (C. elegans) is particularly suitable for extensive genetic screening because gene loss-of-function via RNA interference (RNAi) can be efficiently induced by feeding C. elegans with bacteria that express double-stranded RNA (dsRNA) homologous to the target gene (Timmons & Fire, 1998) . By specifically suppressing metabolic genes in the temperature-sensitive mutant let-418 ( n3536 ) , we were able to identify genetic interactors of the chromatin remodeler LET-418 , the C. elegans homolog of human CHD3 and CHD4 (Passannante et al., 2010) , respectively known also as Mi2alpha and Mi2beta .
Mi2 proteins are components of the nucleosome-remodeling and deacetylase (NuRD) complex, which modulates epigenetic processes influencing cell pluripotency and differentiation, as well as proper embryonic development (Alendar & Berns, 2021; Reid et al., 2023) . Loss-of-function mutations in the C. elegans Mi2 let-418 leads to sterility (von Zelewsky et al., 2000) ; however, animals bearing the temperature-sensitive allele n3536 generate developmentally arrested progeny at a restrictive temperature of 25 °C, but remain fertile for several generations at a permissive temperature of 20 °C (Käser-Pébernard et al., 2014) . A genome-wide RNAi screen for suppressors of early larval arrest in let-418 ( n3536 ) predominantly identified chromatin regulators, emphasizing the importance of chromatin remodeling during embryonic and larval development (Erdelyi et al., 2017) . Combining let-418 ( n3536 ) mutation with a mutation in the histone H3K4 demethylase spr-5 /LSD1 , which is also fertile for several generations, leads to sterility and germline teratoma at permissive temperature, which enabled the discovery of SPR-5 /LSD1 role in germ cell fate maintenance (Käser-Pébernard et al., 2014) . Inspired by these findings, we employed let-418 ( n3536 ) mutant at 20 °C as a sensitized background, which appears phenotypically wildtype but bears mild alterations in chromatin structure. To identify metabolic regulators of the epigenome, we fed let-418 ( n3536 ) and wild-type C. elegans with an RNAi library targeting 962 metabolic genes, based on Gene Ontology (GO) term annotations (Melo & Ruvkun, 2012) . Starting RNAi exposure from the L4 stage excluded immediate developmental defects and allowed us to monitor the progeny (F1) development for a few days ( Figure 1A ).
Target genes were scored as candidates when either wild-type or let-418 ( n3536 ) animals showed defective reproductive fitness. During the initial screening stage, 83 candidates emerged, evenly distributed among the eleven different 96-well screening plates ( Figure 1B ). Of these initial candidates, 40 were validated through three independent repeats, with 30 confirmed to target the correct gene by plasmid sequencing ( Figure 1C ). To identify candidate genes specifically involved in chromatin remodeling during embryogenesis, we focused on the genes whose suppression exhibited different penetrance between wild-type and let-418 ( n3536 ) C. elegans . We aimed to identify let-418 genetic interactors, excluding general fertility defects unrelated to chromatin remodeling. Suppression of two metabolic enzymes, aldo-2 and pdhb-1 , showed the most notable differences in reproductive fitness between wild-type and let-418 ( n3536 ) . While aldo-2 (fructose bisphosphate aldolase) participates in glycolysis, pdhb-1 (pyruvate dehydrogenase beta) is involved in the conversion of pyruvate into acetyl-CoA. Surprisingly, let-418 ( n3536 ) mutants produced more viable progeny than the wild type when treated with RNAi targeting aldo-2 or pdhb-1 . For the other 28 validated candidates, no clear and reproducible differences in response to the gene suppression were observed between wild-type and let-418 ( n3536 ) animals ( Figure 1C ). However, the inherent variability in RNAi suppression and the slightly different numbers of animals per well posed significant limitations in the screening setup for comparing wild-type and let-418 ( n3536 ) animals. Thus, we focused on two metabolic enzymes aldo-2 and pdhb-1 for further investigations.
To exclude maternal effects, we prolonged the RNAi exposure for one generation and observed strong penetrance of embryonic lethality for F2 wild-type animals: approximately 25% and fewer progeny were viable in response to aldo-2 and pdhb-1 RNAi, respectively ( Figure 1D ). In contrast, let-418 ( n3536 ) mutants showed high success in egg hatching both in the control RNAi and in response to aldo-2 and pdhb-1 RNAi, with over 75% and approximately 100% of the eggs hatching, respectively. Interestingly, despite the high number of hatched eggs, we observed a clear developmental delay of let-418 ( n3536 ) mutants treated with pdhb-1 RNAi, with almost none of the hatched individuals reaching L4 within the observed time frame. In contrast, more let-418 ( n3536 ) mutants reached L4 and YA stages upon aldo-2 RNAi, despite a lower number of hatched eggs ( Figure 1D, 1E). While the striking rescue effect of let-418 ( n3536 ) highlights a novel link between chromatin remodeling and metabolism during embryogenesis, the subsequent developmental defect – more prominent upon pdhb-1 suppression compared to aldo-2 – necessitates future studies to elucidate the mechanistic interplay between let-418 and the two metabolic enzymes. Ideally, these experiments should be confirmed with genetic mutants of aldo-2 and pdhb-1 ; however, in line with their essentiality, to our knowledge no viable mutants are currently available. Future experiments should include the generation of new model systems to partially or reversibly suppress aldo-2 and pdhb-1 , for example through auxin-inducible degron technology (Zhang et al., 2015) . This method would not only allow to validate the results obtained with RNAi, but also give the opportunity to examine tissue- and stage-specific functions.
Besides the possibility that let-418 ( n3536 ) rescue effect derives from downstream regulation of gene expression, this study introduces the fascinating possibility that aldo-2 and pdhb-1 are essential for their non-canonical functions in chromatin remodeling. During embryogenesis, the glycolytic flux is both temporally and spatially regulated, confirming an important role of cellular metabolism in determining cell identity (Johnson et al., 2003; Oginuma et al., 2017) . We hypothesize that the suppression of aldo-2 could impair glycolysis, leading to reduced lactate levels, which are notably high in proliferative cells (Vander Heiden et al., 2010) . More than 50% of glucose is converted into lactate by mouse and human blastocysts, even in the presence of oxygen (Ma et al., 2020) . An increasing number of studies have been revealing a significant role of lactate in epigenetic reprogramming, serving also as a substrate for histone lactylation (D. Zhang et al., 2019). Histone lactylation emerged as a mechanism downstream of increased glycolysis that promotes gene regulatory networks orchestrating pluripotency and tissue determination during embryonic development (S.-K. Dai et al., 2022; Galle et al., 2022; L. Li et al., 2020; Merkuri et al., 2024), as well as immunity and muscle regeneration in adults (Desgeorges et al., 2024; D. Zhang et al., 2019) . Further supporting an epigenetic role of aldolases in embryogenesis, muscular aldolase ALDOA has been reported to localize to the nucleus, interact with DNA, and promote cell proliferation (Mamczur et al., 2013; Ronai et al., 1992) .
Regarding pdhb-1 , we hypothesized that the impaired conversion of pyruvate into acetyl-CoA directly influences histone acetylation. As reported for ALDOA, multiple pyruvate dehydrogenase subunits have been detected within the nucleus, indicating an alternative epigenetic function regulating cell fate decisions through histone acetylation (Kafkia et al., 2022; W. Li et al., 2022; Nagaraj et al., 2017) . Given the potential for defective LET-418 to disrupt the deacetylation capacity of the NuRD complex (Alendar & Berns, 2021; Reid et al., 2023) , the let-418 ( n3536 ) mutation may mitigate the effects of pdhb-1 depletion in C. elegans by compensating for low acetyl-CoA levels with reduced histone deacetylation. This scenario suggests that embryogenesis could potentially depend on the acetylation of specific histones at specific chromatin loci. Alternatively, echoing the hypothesis that chromatin acts as a metabolic reservoir (Nirello et al., 2022) , a higher availability of acetyl groups on the chromatin deriving from reduced NuRD-dependent deacetylation could provide the necessary amount of acetyl-CoA for embryonic development. It remains unknown if histone lactylation or acetylation are affected upon aldo-2 or pdhb-1 suppression and if these changes are heritable. Future studies are required to identify specific chromatin changes that could be affected by impaired glucose and pyruvate metabolism.
In summary, we have identified the metabolic enzymes aldo-2 and pdhb-1 as novel genetic interactors of the chromatin remodeler let-418 . These findings complement previous and recent studies indicating the importance of metabolic enzymes as epigenetic regulators (Boon et al., 2020; Z. Dai et al., 2020; X. Li et al., 2018) . Importantly, core metabolic pathways such as glycolysis and pyruvate metabolism are well conserved from nematodes to humans. Although the components of the NuRD complex have several paralogs in vertebrates compared to lower organisms, the NuRD's function in chromatin remodeling is well conserved from invertebrates to humans (Reid et al., 2023) . Consequently, this study represents a starting point for future research. On one hand, it can help elucidate fundamental mechanisms linking chromatin remodeling and metabolism during embryogenesis. On the other hand, it can uncover specialized molecular pathways that influence tissue-specific functions or pathological conditions, such as metabolic disorders and cancer.
Methods
C. elegans strains and maintenance
C. elegans strains were maintained according to standard procedures (Stiernagle, 2006) , at 15 °C or 20 °C, on nematode growth medium (NGM) agar plates seeded with OP50 E. coli as a food source. Experiments were conducted at 20 °C on the following strains: MT14390 – let-418 ( n3536 ) V and N2 – Bristol strain, which was used as wild-type control.
C. elegans synchronization
C. elegans were synchronized by egg-prep (Stiernagle, 2006) . In brief, mixed C. elegans populations were washed off the plates with M9, bleached with sodium hypochlorite solution, and, after 3 washing steps with M9, the obtained eggs were seeded on a culture plate. Estimation of the number of eggs was done in triplicates with a 2 μl drop of suspension in M9 to seed appropriate amounts of C. elegans in each plate.
Genetic screening
The library of RNAi clones ( HT115 ) targeting metabolic genes was generously shared by Gary Ruvkun to Collin Ewald (Melo & Ruvkun, 2012; Venz et al., 2020) . Bacterial clones were copied from glycerol stocks by growing overnight at 37 °C on 86 x 128 mm LB agar plates supplemented with ampicillin and tetracycline to a final concentration of 50 μg/ml and 12 μg/ml, respectively.
The primary screen was conducted in 96-well plates, each scored at least in 2 independent replicates, as described in (Jongsma et al., 2023) . RNAi against selected candidates was repeated in 24-well plates or 3 cm plates; validated candidates were also confirmed to target the correct gene by plasmid sequencing.
RNA interference (RNAi) was performed following the standard feeding method. Each clone was grown in LB supplemented with ampicillin (50 μg/ml) overnight. The following day, cultures were diluted 1:1 with fresh LB supplemented with ampicillin (50 μg/ml), incubated for 2 hours at 37 °C, and concentrated by centrifugation at 4000 rpm for 10 minutes. Pellets were suspended in fresh LB supplemented with ampicillin (50 μg/ml) and IPTG (1 mM). Bacterial suspension was seeded on NGM plates containing 50 μg/ml ampicillin and 1 mM IPTG (8 μl per well in 96-well plates, 80 μl per well in 24-well plates, 300 μl per 3 cm plates).
Focusing on embryogenesis, parental C. elegans were allowed to develop without RNAi treatment by distributing synchronized eggs on NGM plates seeded with OP50 and incubated at 20 °C until the animals reached the L3 stage. L3 larvae were washed off the plates, resuspended in fresh M9 to remove OP50 , and redistributed to RNAi plates. Progeny viability and health were monitored under a stereomicroscope for multiple days and wells containing arrested larvae (up to L2), non-hatching eggs, or clear developmental defects were recorded as possible candidates. As a control, bacteria expressing the empty vector pPD129.36 were present in at least one well in each 96-well plate.
Embryonic lethality and developmental delay quantification
Synchronized L3 C. elegans were treated with RNAi bacteria (control (L4440), aldo-2 , pdhb-1 ) as described in the “Genetic Screening” section. 10 adults were picked from the progeny (F1), transferred to fresh RNAi plates to lay eggs, and removed after 6 hours. The following day eggs and hatched larvae (L1/L2) were quantified (F2 generation). After 2 days from egg-laying quantification of the developmental stages reached by each of three F2 generations was conducted, distinguishing among unhatched eggs, L1/L2, L3, L4, and young adults (YA).
Reagents
C. elegans strains are available from CGC: N2 – Bristol wild type, MT14390 – let-418 ( n3536 ) V
Extended Data
Description: Complete screening results. Resource Type: Dataset. DOI: 10.22002/ms7c2-j3251
Acknowledgments
Acknowledgments
We thank Gary Ruvkun for the generous gift of the metabolism RNAi library, WormBase (Sternberg et al., 2024) for curated gene and phenotype information. The strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank all members of the von Meyenn, Ewald, and Wicky labs for helpful discussions and feedback.
Funding Statement
Funding from ETH Zurich (to FvM) and the Swiss National Science Foundation Funding from the SNF P3 Project 190072 (to CYE).
References
- Alendar A, Berns A. Sentinels of chromatin: chromodomain helicase DNA-binding proteins in development and disease. Genes Dev. 2021 Nov 1;35(21-22):1403–1430. doi: 10.1101/gad.348897.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009 Apr 1;23(7):781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon R, Silveira GG, Mostoslavsky R. Nuclear metabolism and the regulation of the epigenome. Nat Metab. 2020 Oct 12;2(11):1190–1203. doi: 10.1038/s42255-020-00285-4. [DOI] [PubMed] [Google Scholar]
- Cavalli G, Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019 Jul 24;571(7766):489–499. doi: 10.1038/s41586-019-1411-0. [DOI] [PubMed] [Google Scholar]
- Dai SK, Liu PP, Li X, Jiao LF, Teng ZQ, Liu CM. Dynamic profiling and functional interpretation of histone lysine crotonylation and lactylation during neural development. Development. 2022 Jul 21;149(14) doi: 10.1242/dev.200049. [DOI] [PubMed] [Google Scholar]
- Dai Z, Ramesh V, Locasale JW. The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet. 2020 Sep 9;21(12):737–753. doi: 10.1038/s41576-020-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgeorges T, Galle E, Zhang J, von Meyenn F, De Bock K. Histone lactylation in macrophages is predictive for gene expression changes during ischemia induced-muscle regeneration. Mol Metab. 2024 Mar 22;83:101923–101923. doi: 10.1016/j.molmet.2024.101923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009 Aug 26;27(5):351–357. doi: 10.1055/s-0029-1237423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdelyi P, Wang X, Suleski M, Wicky C. A Network of Chromatin Factors Is Regulating the Transition to Postembryonic Development in Caenorhabditis elegans. G3 (Bethesda) 2017 Feb 9;7(2):343–353. doi: 10.1534/g3.116.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustermann S, Patel AB, Hopfner KP, He Y, Korber P. Energy-driven genome regulation by ATP-dependent chromatin remodellers. Nat Rev Mol Cell Biol. 2023 Dec 11;25(4):309–332. doi: 10.1038/s41580-023-00683-y. [DOI] [PubMed] [Google Scholar]
- Galle E, Wong CW, Ghosh A, Desgeorges T, Melrose K, Hinte LC, Castellano-Castillo D, Engl M, de Sousa JA, Ruiz-Ojeda FJ, De Bock K, Ruiz JR, von Meyenn F. H3K18 lactylation marks tissue-specific active enhancers. Genome Biol. 2022 Oct 3;23(1):207–207. doi: 10.1186/s13059-022-02775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro M, von Meyenn F, Reik W. DNA methylation homeostasis in human and mouse development. Curr Opin Genet Dev. 2017 Mar 2;43:101–109. doi: 10.1016/j.gde.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Mahmood S, Patel MS. Intermediary metabolism and energetics during murine early embryogenesis. J Biol Chem. 2003 Jun 4;278(34):31457–31460. doi: 10.1074/jbc.R300002200. [DOI] [PubMed] [Google Scholar]
- Jongsma E, Goyala A, Mateos JM, Ewald CY. Removal of extracellular human amyloid beta aggregates by extracellular proteases in C. elegans. Elife. 2023 Sep 20;12 doi: 10.7554/eLife.83465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkia E, Andres-Pons A, Ganter K, Seiler M, Smith TS, Andrejeva A, Jouhten P, Pereira F, Franco C, Kuroshchenkova A, Leone S, Sawarkar R, Boston R, Thaventhiran J, Zaugg JB, Lilley KS, Lancrin C, Beck M, Patil KR. Operation of a TCA cycle subnetwork in the mammalian nucleus. Sci Adv. 2022 Aug 31;8(35):eabq5206–eabq5206. doi: 10.1126/sciadv.abq5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käser-Pébernard S, Müller F, Wicky C. LET-418/Mi2 and SPR-5/LSD1 cooperatively prevent somatic reprogramming of C. elegans germline stem cells. Stem Cell Reports. 2014 Mar 27;2(4):547–559. doi: 10.1016/j.stemcr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, Hao Z, Zhang C, Zhang J, Ma B, Liu Z, Yuan H, Liu Z, Long Q, Zhou Y, Qi J, Zhao D, Gao M, Pei D, Nie J, Ye D, Pan G, Liu X. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2020 Aug 24;2(9):882–892. doi: 10.1038/s42255-020-0267-9. [DOI] [PubMed] [Google Scholar]
- Li W, Long Q, Wu H, Zhou Y, Duan L, Yuan H, Ding Y, Huang Y, Wu Y, Huang J, Liu D, Chen B, Zhang J, Qi J, Du S, Li L, Liu Y, Ruan Z, Liu Z, Liu Z, Zhao Y, Lu J, Wang J, Chan WY, Liu X. Nuclear localization of mitochondrial TCA cycle enzymes modulates pluripotency via histone acetylation. Nat Commun. 2022 Dec 2;13(1):7414–7414. doi: 10.1038/s41467-022-35199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Egervari G, Wang Y, Berger SL, Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat Rev Mol Cell Biol. 2018 Sep 1;19(9):563–578. doi: 10.1038/s41580-018-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LN, Huang XB, Muyayalo KP, Mor G, Liao AH. Lactic Acid: A Novel Signaling Molecule in Early Pregnancy? Front Immunol. 2020 Feb 27;11:279–279. doi: 10.3389/fimmu.2020.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamczur P, Gamian A, Kolodziej J, Dziegiel P, Rakus D. Nuclear localization of aldolase A correlates with cell proliferation. Biochim Biophys Acta. 2013 Jul 23;1833(12):2812–2822. doi: 10.1016/j.bbamcr.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012 Apr 13;149(2):452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkuri F, Rothstein M, Simoes-Costa M. Histone lactylation couples cellular metabolism with developmental gene regulatory networks. Nat Commun. 2024 Jan 2;15(1):90–90. doi: 10.1038/s41467-023-44121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R, Sharpley MS, Chi F, Braas D, Zhou Y, Kim R, Clark AT, Banerjee U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell. 2017 Jan 12;168(1-2):210–223.e11. doi: 10.1016/j.cell.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirello VD, Rodrigues de Paula D, Araújo NVP, Varga-Weisz PD. Does chromatin function as a metabolite reservoir? Trends Biochem Sci. 2022 Apr 11;47(9):732–735. doi: 10.1016/j.tibs.2022.03.016. [DOI] [PubMed] [Google Scholar]
- Oginuma M, Moncuquet P, Xiong F, Karoly E, Chal J, Guevorkian K, Pourquié O. A Gradient of Glycolytic Activity Coordinates FGF and Wnt Signaling during Elongation of the Body Axis in Amniote Embryos. Dev Cell. 2017 Feb 27;40(4):342–353.e10. doi: 10.1016/j.devcel.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passannante M, Marti CO, Pfefferli C, Moroni PS, Kaeser-Pebernard S, Puoti A, Hunziker P, Wicky C, Müller F. Different Mi-2 complexes for various developmental functions in Caenorhabditis elegans. PLoS One. 2010 Oct 27;5(10):e13681–e13681. doi: 10.1371/journal.pone.0013681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid XJ, Low JKK, Mackay JP. A NuRD for all seasons. Trends Biochem Sci. 2022 Jul 4;48(1):11–25. doi: 10.1016/j.tibs.2022.06.002. [DOI] [PubMed] [Google Scholar]
- Ronai Z, Robinson R, Rutberg S, Lazarus P, Sardana M. Aldolase-DNA interactions in a SEWA cell system. Biochim Biophys Acta. 1992 Feb 28;1130(1):20–28. doi: 10.1016/0167-4781(92)90456-a. [DOI] [PubMed] [Google Scholar]
- Shyh-Chang N, Ng HH. The metabolic programming of stem cells. Genes Dev. 2017 Mar 17;31(4):336–346. doi: 10.1101/gad.293167.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006 Feb 11;:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg PW, Van Auken K, Wang Q, Wright A, Yook K, Zarowiecki M, Arnaboldi V, Becerra A, Brown S, Cain S, Chan J, Chen WJ, Cho J, Davis P, Diamantakis S, Dyer S, Grigoriadis D, Grove CA, Harris T, Howe K, Kishore R, Lee R, Longden I, Luypaert M, Müller HM, Nuin P, Quinton-Tulloch M, Raciti D, Schedl T, Schindelman G, Stein L. WormBase 2024: status and transitioning to Alliance infrastructure. Genetics. 2024 May 7;227(1) doi: 10.1093/genetics/iyae050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998 Oct 29;395(6705):854–854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, Cantley LC. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010 Sep 17;329(5998):1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venz R, Korosteleva A, Jongsma E, Ewald CY. Combining Auxin-Induced Degradation and RNAi Screening Identifies Novel Genes Involved in Lipid Bilayer Stress Sensing in Caenorhabditis elegans. G3 (Bethesda) 2020 Nov 5;10(11):3921–3928. doi: 10.1534/g3.120.401635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zelewsky T, Palladino F, Brunschwig K, Tobler H, Hajnal A, Müller F. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development. 2000 Dec 1;127(24):5277–5284. doi: 10.1242/dev.127.24.5277. [DOI] [PubMed] [Google Scholar]
- Wilkinson AL, Zorzan I, Rugg-Gunn PJ. Epigenetic regulation of early human embryo development. Cell Stem Cell. 2023 Oct 18;30(12):1569–1584. doi: 10.1016/j.stem.2023.09.010. [DOI] [PubMed] [Google Scholar]
- Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L, Zhao Y. Metabolic regulation of gene expression by histone lactylation. Nature. 2019 Oct 23;574(7779):575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ward JD, Cheng Z, Dernburg AF. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development. 2015 Nov 9;142(24):4374–4384. doi: 10.1242/dev.129635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang Y, Fang Y, Fang D. Overview of Histone Modification. Adv Exp Med Biol. 2021;1283:1–16. doi: 10.1007/978-981-15-8104-5_1. [DOI] [PubMed] [Google Scholar]