Abstract
Cryptococcus is an opportunistic fungal pathogen that can cause disseminated infection with predominant central nervous system involvement in patients with compromised immunity. Biologics are increasingly used in the treatment of neoplasms and autoimmune/inflammatory conditions and the prevention of transplant rejection, which may affect human defense mechanisms against cryptococcosis. In this review, we comprehensively investigate the association between cryptococcosis and various biologics, highlighting their risks of infection, clinical manifestations, and clinical outcomes. Clinicians should remain vigilant for the risk of cryptococcosis in patients receiving biologics that affect the Th1/macrophage activation pathways, such as tumor necrosis factor α antagonists, Bruton tyrosine kinase inhibitors, fingolimod, JAK/STAT inhibitors (Janus kinase/signal transducer and activator of transcription), and monoclonal antibody against CD52. Other risk factors—such as age, underlying condition, and concurrent immunosuppressants, especially corticosteroids—should also be taken into account during risk stratification.
Keywords: autoimmune diseases, biologics, cryptococcosis, hematology, transplant
In this review, we investigated the association between cryptococcosis and various biologics affecting Th1/macrophage activation pathways, such as tumour necrosis factor-a antagonists, Bruton tyrosine kinase inhibitors, fingolimod, JAK/STAT inhibitors, and monoclonal antibody against CD52, highlighting risks of infection, clinical manifestations, and outcomes
Members of the Cryptococcus neoformans/gattii species complex are basidiomycetous fungal pathogens that are environmental saprophytes and the etiologic agents of the potentially fatal human fungal infection cryptococcosis. Clinical manifestation ranges from asymptomatic pulmonary infection to disseminated central nervous system (CNS) infection [1]. Cryptococcosis has become a major global health concern since the HIV pandemic in the 1980s, with most cases occurring in adults infected with HIV who live in sub-Saharan Africa. A recent modeling study estimated 152 000 cases of cryptococcal meningitis occurring among people with HIV per annum, resulting in 112 000 cryptococcosis-related deaths [2]. Besides advanced HIV, other risk factors include hematopoietic stem cell or solid organ transplantation, hematologic malignancies, organ failure, sarcoidosis, primary immunodeficiencies affecting T-cell immunity, autoantibody against the granulocyte-macrophage colony-stimulating factor (GM-CSF), and iatrogenic immunosuppression (eg, corticosteroids) [3].
With advances in the medical treatment of cancer and autoimmune and inflammatory diseases, including wider availability of solid organ and hematopoietic stem cell transplantation and an expanding variety of immunomodulatory agents, the number of patients who are immunocompromised and at risk of opportunistic infections is increasing. In addition, recent modeling studies have demonstrated global warming as a major driver of the expansion in the ecologic niches of pathogenic cryptococci [4]. Coupled with the changing patterns of human behaviors and increasing numbers of susceptible hosts, the incidence of cryptococcosis is expected to rise in the next decades. In this review, we describe the pathobiology of cryptococcosis and review the risks of infection conferred by different biological agents used in clinical practice.
PATHOBIOLOGY
Pathogenesis
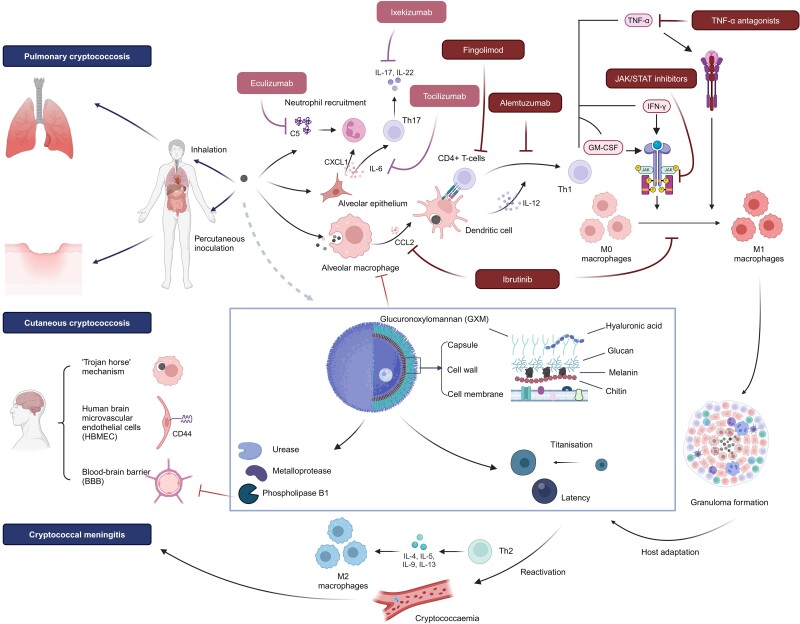
Cryptococcal infection occurs via inhalation of small desiccated yeast cells or basidiospores (1–5 µm), which reach the lower bronchioalveolar tree [1, 5, 6] (Figure 1). Due to the ubiquitous environmental distribution of Cryptococcus, most infections are acquired in early childhood [8]. Primary pulmonary cryptococcosis usually results in asymptomatic or subclinical infection in individuals who are immunocompetent [8, 9] but can result in pneumonia in patients who are immunocompromised [10]. In immunocompetent hosts, cryptococci are either cleared by the immune system after initial infection or establish a latent stage in immune cells, primarily macrophages, that can reactivate later in life due to immune dysregulation [11, 12].
Figure 1.
Cryptococcosis pathogenesis and the impact of major categories of biologics in this review [1, 6, 7]. Pathogenetic cryptococci elaborate various virulence factors to help establish infection and dissemination, especially to the central nervous system. For a detailed description of the impact of specific biologics on cryptococcosis, refer to the corresponding sections on TNF-α blockers, Bruton tyrosine kinase inhibitors, fingolimod, and others. CXCL1, chemokine (C-X-C motif) ligand 1; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL, interleukin; JAK/STAT, Janus kinase/signal transducer and activator of transcription; TNF-α, tumour necrosis factor-α. Image created with BioRender.com.
Besides pulmonary infection, disseminated infection involving the skin, soft tissue, bone, joint, liver, lymph nodes, peritoneum, urogenital tract, adrenal, eyes, and especially the CNS can occur, particularly in immunosuppressed hosts [3]. Cryptococcal entry into the CNS compartment is postulated to occur through 1 or a combination of 3 mechanisms: paracytosis with the aid of fungal metalloproteases, transcytosis through binding between hyaluronic acid and CD44 on the endothelium, and a “Trojan horse” mechanism by hijacking host phagocytes to cross the blood-brain barrier [1].
Virulence Factors
The C neoformans/gattii species complex expresses several virulence factors to enable host invasion and survival. The yeast cells are surrounded by a fungal capsule of various thickness, which is predominantly composed of the polysaccharide glucuronoxylomannan. Glucuronoxylomannan plays a pivotal role in immune modulation through inhibition of phagocytosis, phagosomal acidification, antigen presentation, T-lymphocyte proliferation and humoral response, induction of macrophage apoptosis, and induction of an immune-tolerant state [13–17]. The capsule size determines early macrophage control of infection and subsequent intracellular proliferation [18]. The production of melanin, regulated by the laccase gene, protects against intraphagocytic killing by nitrogen- and oxygen-derived radicals [19, 20]. C neoformans also produces a multitude of other virulence factors to aid systemic dissemination, especially CNS dissemination, including urease, hyaluronic acid, metalloprotease, and phospholipase B1 [21–24].
During in vivo infection, dramatic changes in cryptococcal cellular morphology have been observed, resulting in the formation of “titan cells,” which are 5- to 10-fold larger than typical cryptococcal yeast cells, are polypoid with a thickened cell wall and tightly compacted capsule, and form approximately 5% to 20% of the fungal cells in the infected lungs of mice [25–28]. Titan cell formation impairs phagocytosis and skews the inflammatory response to a Th2-type response [29], promoting the establishment of the initial pulmonary infection, stress adaptation, brain dissemination, and mortality [27–30].
Host Defense
Upon inhalational exposure, cryptococcal interaction with pulmonary epithelium mainly involves adhesion mediated by glucuronoxylomannan, phospholipase B1, and the mannoprotein MP84 [31–33]. Using 2-dimensional human lung organoid derived from human embryonic stem cells, Rossi et al recently demonstrated that C neoformans H99 was able to invade the minilung tissue and alter the expression of surfactants [34]. In addition, pulmonary epithelia respond to cryptococcal adhesion with the production of the proinflammatory interleukin 6 (IL-6), IL-8, and CXCL1 [31, 34, 35], as well as the Th2-inducing cytokine IL-33 [36].
As the predominant resident phagocytic cells in the lung, alveolar macrophages play an essential role in the human immune response to cryptococcal invasion, including receptor-mediated phagocytosis, secretion of chemokines and cytokines, and antigen presentation, as well as serving as a reservoir for latency [37]. The ability of macrophages to contain cryptococcal invasion depends on macrophage polarization and activation status, which are influenced by the cytokine microenvironment [38]. Interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and GM-CSF signaling stimulates M1 polarization, which is essential for macrophage fungicidal activity [38, 39]. Yet, IL-4 stimulation differentially induces M2 polarization, which is associated with deficient anticryptococcal activity and disease progression [40]. Conceptually, treatments that impair M1 polarization, such as antagonists to TNF-α or JAK/STAT inhibitors (Janus kinase/signal transducer and activator of transcription) that impair IFN-γ signaling, are associated with increased risks of cryptococcosis, among a population of patients who are often already predisposed to infection due to their underlying disease or concomitant immunosuppressants.
T-cell responses after cryptococcal infection are stimulated by activated dendritic cells, which respond to fungal pathogen-associated molecular patterns such as β-glucan, chitin, and glucuronoxylomannan. Activated CD4+ T cells secrete IL-12 and IL-23 to activate the T helper 1 (Th1) cells, which in turn produce IFN-γ to “superactivate” macrophages to enhance intraphagocytic killing. However, massive accruement of pathologic cryptococcal antigen-specific Th2 cells was demonstrated in the lungs following in vivo infection, which was coordinated by lung-resident CD11b+ conventional dendritic cells and induced by cleavage of chitin by the host chitotriosidase [41].
ASSOCIATION BETWEEN BIOLOGICS AND CRYPTOCOCCOSIS
We conducted a literature search on PubMed using combinations of an individual drug name and “cryptococcosis,” “cryptococcal,” or “cryptococcus” for publications related to cryptococcosis and biologics [42–154]. Articles containing the relevant search terms that were published from 1990 to 20 January 2024 were included for title and abstract screening. Eligible articles that contained case-level data on at least 1 individual who was receiving biologics and was diagnosed with cryptococcosis were retrieved for full-text review. References of articles containing primary data were also reviewed for additional publications that might contain patient information. Non–English-language articles, cases whose demographic and clinical details were not available, as well as data reported only in abstracts of conference proceedings or scientific meetings were excluded (Supplementary Figure 1). The list of biologics according to therapeutic targets and disease groups is summarized in Supplementary Table 1. Only biologics approved by the US Food and Drug Administration (FDA) as of 20 January 2024 were included. The definitions of proven or probable cryptococcosis followed the 2020 EORTC/MSGERC consensus definitions (European Organization for Research and Treatment of Cancer/Mycoses Study Group Education and Research Consortium) [155]. Infection was deemed “disseminated” if there was fungemia or the infection involved at least 2 noncontiguous sites.
TNF-α Antagonists
TNF-α is a pleiotropic cytokine that is predominantly produced by cells of the monocytic lineage. It is synthesized as membrane-associated or soluble forms, and it signals through TNF receptors 1 and 2 to regulate a range of biologic activities, including inflammation, cell proliferation, host defense, and cell survival [156]. Due to the prominent role of TNF-α in the proinflammatory cascade, therapeutic targeting of the TNF pathway has been harnessed to treat various inflammatory and autoimmune conditions. Despite the revolutionary success in tackling TNF-mediated pathogenesis, the use of TNF-α antagonists has been associated with an increased risk of opportunistic infections. Due to the inhibition of the formation and maintenance of granulomas [157], TNF-α inhibition increases the risk of infection by intracellular pathogens that are normally contained by granulomatous inflammation, most notably, tuberculosis, histoplasmosis, and coccidioidomycosis [158–160].
We identified 33 published cases of proven/probable cryptococcosis associated with TNF-α antagonists: 25 cases associated with infliximab, 6 cases with adalimumab, and 1 case each with etanercept and certolizumab pegol (Table 1). There was a male preponderance (male:female ratio 2.3), and the median age was 56 years (range, 14–87). The most common indication for TNF-α antagonists was Crohn disease (14/33, 43%), followed by rheumatoid arthritis (13/33, 39%). Most patients received other immunosuppressants (27/33, 82%), including 18 (55%) with corticosteroids. The most common manifestation was pulmonary cryptococcosis (18/33, 55%), followed by disseminated cryptococcosis (7/33), cryptococcal meningitis (4/33), and skin and soft tissue infection (4/33). Except for 1 case of primary cutaneous infection associated with etanercept that was caused by Naganishia albida (previously Cryptococcus albidus) [72], all other cases were caused by C neoformans or speciation was not provided. The implicated TNF-α antagonist was resumed in only 2 cases: one that resulted in relapse of pulmonary cryptococcosis [55] and the aforementioned case of N albida primary cutaneous infection in which the patient remained well despite stopping fluconazole [72].
Table 1.
Cases of Cryptococcosis Associated With TNF-α Antagonists
| Agent: First Author | Year | Age, y | Sex | Condition | TNF-α Doses or Durationa | Other ISx | Manifestation | Antifungal | Outcome | Resumption of TNF-α Antagonists |
|---|---|---|---|---|---|---|---|---|---|---|
| Infliximab | ||||||||||
| True [42] | 2002 | 69 | M | RA | 5 | Steroid, MTX | Disseminated | AmB → FLZ | Recovery | … |
| Hage [43] | 2003 | 61 | M | RA | 3 | Steroid, MTX, LFM | Pulmonary | AmB → FLZ | Recovery | … |
| Hrnicek [44] | 2003 | 51 | M | CD | 2 | Steroid, MTX | Pulmonary | AmB → FLZ | Recovery | … |
| Arend [45] | 2004 | 47 | F | RA | 2 | Steroid | Pulmonary | FLZ (5 mo) | Recovery | … |
| Shrestha [46] | 2004 | 65 | M | RA | 3 | MTX, HCQ | Pulmonary | FLZ (28 d) | Recovery | … |
| Muñoz [47] | 2007 | 67 | F | RA | 12 | Steroid, MTX | Meningitis | FLZ | Recovery | … |
| Kozic [48] | 2008 | 57 | M | RA | 2 | MTX | Disseminated | AmB | Death | … |
| Rehman [49] | 2008 | 61 | M | CD | 2.5 y | Steroid, AZA | Pulmonary | AmB + 5FC → FLZ | Recovery | … |
| Arnaud [50] | 2009 | 42 | M | Sarcoidosis | 2 | THD, MTX, ETC (for 11 mo before INX) | Disseminated | AmB + 5FC → FLZ | Recovery | … |
| Kluger [51] | 2009 | 46 | M | Behçet disease | 19 | Steroid, MMF | Meningitis | AmB + 5FC → FLZ | Recovery | … |
| Osawa [52] | 2010 | 53 | M | CD | 3 y | Steroid, AZA | Disseminated | AmB + 5FC → FLZ | Recovery | … |
| Hirai [53] | 2011 | 39 | M | CD | 5 | Nil | Pulmonary | Nil (surgery) | Recovery | … |
| Wingfield [54] | 2011 | 70 | M | RA | 39 | Steroid, RTX, MTX | Meningitis | AmB + 5FC → AmB → VRC (4 mo) | Recovery | … |
| Takazono [55] | 2016 | 35 | M | CD | 8 (initial), 11 (relapse) | Steroid, 5-ASA (initial) | Pulmonary | FLZ (initial, 6 mo); FLZ → ITC + 5FC → ITC (relapse) |
Relapsed after initial episode | 1 mo after initial episode |
| Vasant [56] | 2016 | 74 | F | CD | 3 | Steroid | Disseminated | AmB + 5FC → VRC | Recovery | … |
| Yamada [57] | 2016 | 55 | M | PsO, PsA | 5 | Nil | Pulmonary | FLZ | Recovery | … |
| Asakura [58] | 2017 | 79 | M | UC | 7 | MTX, 5-ASA | Pulmonary | FLZ | Recovery | … |
| Chiriac [59] | 2017 | 72 | F | RA | 20 | Nil | Primary cutaneous | FLZ (3 mo) | Recovery | … |
| Lee [60] | 2017 | 70 | F | CD | 3 | Steroid, AZA | Disseminatedb | AmB → VRC | Death | … |
| Nosaki [61] | 2019 | 65 | F | RA | 4 y | MTX | Meningitis | AmB + 5FC → FLZ | Recovery | … |
| Santo [62] | 2019 | 23 | M | CD | 6 mo | AZA | Pulmonary | FLZ | Recovery | … |
| Hussein [63] | 2021 | 54 | M | CD | 5 | Steroid, MTX | Pulmonary | FLZ (6 mo) | Recovery | … |
| Fang [64] | 2023 | 65 | M | CD | 4 | Nil | Pulmonary | FLZ (6 mo) | Recovery | … |
| 20 | M | CD | 22 | Nil | Pulmonary | FLZ (5 mo) | Recovery | … | ||
| Sha [65] | 2023 | 51 | M | UC | 4 | Steroid | Pulmonary | VRC | Recovery | … |
| Adalimumab | ||||||||||
| Horcajada [66] | 2007 | 69 | F | RA | 26 | Steroid, MTX, CQ, SSZ | Tenosynovitis | AmB + 5FC → FLZ (6 mo) | Survival (amputation) | … |
| Cadena [67] | 2009 | 56 | F | RA | … | MTX | Pulmonary | FLZ → AmB + 5FC → FLZ | Recovery; IRIS | … |
| Iwata [68] | 2011 | 56 | F | RA | 10 | MTX | Pulmonary | Nil (surgery) | Recovery | … |
| Fraison [69] | 2013 | 54 | M | AS, CD | 2 | Steroid, AZA | Pulmonary | AmB + 5FC → FLZ | Recovery | … |
| Gomes [70] | 2013 | 87 | M | RA | … | Steroid | Primary cutaneous | Surgery + FLZ (6 mo) | Recovery | … |
| Yeh [71] | 2021 | 57 | F | CD, SLE | 3 mo | Steroid, HCQ | Pulmonary | AmB + 5FC | Recovery | … |
| Etanercept | ||||||||||
| Hoang [72] | 2007 | 14 | M | PsO | 8 mo | Nil | Primary cutaneousc | FLZ | Recovery | 1 y afterward |
| Certolizumab pegol | ||||||||||
| Wysocki [73] | 2015 | 46 | M | CD | … | AZA, INX (until 5 mo ago) → ADM → CZP | Disseminated | AmB + 5FC → FLZ | Recovery | … |
Abbreviations: 5-ASA, 5-aminosalicylic acid; 5FC, flucytosine; ADM, adalimumab; AmB, amphotericin B; AS, ankylosing spondylitis; AZA, azathioprine; CD, Crohn disease; CQ, chloroquine; CZP, certolizumab pegol; ETC, etanercept; F, female; FLZ, fluconazole; HCQ, hydroxychloroquine; INX, infliximab; IRIS, immune reconstitution inflammatory syndrome; ISx, immunosuppressant; ITC, itraconazole; LFM, leflunomide; M, male; MMF, mycophenolate mofetil; MTX, methotrexate; PsA, psoriatic arthritis; PsO, psoriasis; RA, rheumatoid arthritis; RTX, rituximab; SLE, systemic lupus erythematosus; SSZ, sulfasalazine; THD, thalidomide; TNF-α, tumor necrosis factor α; UC, ulcerative colitis; VRC, voriconazole.
aDoses or duration of TNF-α antagonists before onset.
bMultiple infections with Klebsiella pneumoniae bacteremia and possible pneumocystis pneumonia.
cInfection by Naganishia albida (previously Cryptococcus albidus).
The risk of opportunistic infection is not equally elevated across all TNF-α antagonists. Infliximab binds to monomer and trimer forms of soluble TNF and assembles more stable complexes with soluble and transmembrane TNF, whereas etanercept binding is restricted to the trimer form, creates less stable complexes, and demonstrates lower avidity to transmembrane TNF than infliximab [161]. These differences in pharmacodynamics underlie the lower risk of opportunistic infection conferred by etanercept as compared with antibody-mediated TNF-α neutralizers such as infliximab and adalimumab, as demonstrated by data collected through the Adverse Event Reporting System of the FDA [162]. In addition, patients who develop opportunistic infections while undergoing treatment with infliximab typically manifest earlier than those taking etanercept [163]. The only study that yielded a cryptococcosis-specific risk calculation was a retrospective case-control study conducted among patients with rheumatoid arthritis who developed cryptococcosis from a single center in Taiwan over a 14-year period [164]. Though the number of cryptococcosis cases with current use of TNF-α antagonists was small, exposure to adalimumab (n = 3) was significantly associated with increased risks of cryptococcosis (adjusted odds ratio, 4.50; 95% CI, 1.03–19.66; P = .046) while the crude odds ratio (1.61; 95% CI, .33–7.77; P = .55) for etanercept (n = 2) did not reach statistical significance.
Ibrutinib and Other Bruton Tyrosine Kinase Inhibitors
Ibrutinib is a small molecule inhibitor approved for the treatment of various lymphoid neoplasms, such as chronic lymphocytic leukemia (CLL) [165, 166], Waldenstrom macroglobulinemia [167], mantle cell lymphoma [168], and follicular lymphoma [169]. Early-onset opportunistic fungal infections have been associated with the use of ibrutinib [170], most notably cases of invasive aspergillosis with frequent involvement of the CNS [171].
Susceptibility to infection in patients treated with ibrutinib has been linked to altered B-cell receptor signaling and inhibition of IL-2–inducible kinases [172] as well as to impairments in neutrophil and monocyte functionality [173, 174]. Of note, a significant number of cases of invasive fungal infections, including cryptococcosis, in patients treated with ibrutinib occurred in heavily pretreated cases with relapsed or refractory disease [82, 175]. During experimental C neoformans infection with Bruton tyrosine kinase (BTK)–deficient mice, Szymczak et al found that X-linked immunodeficient mice carrying a Btk mutation were unable to contain C neoformans lung infection after intranasal inoculation and experienced disseminated disease [176]. In contrast, Messina et al found no differences in disease severity among BTK knockout mice as compared with wild type ones [177]. In addition, the administration of ibrutinib at doses replicating human exposure did not affect infection severity [177]. Collectively, these animal models and clinical data suggest that increased susceptibility to cryptococcosis in patients with BTK inhibitors (BTKis) may reflect a high net state of immunosuppression rather than sole linkage to receipt of ibrutinib [178]. Two more recent BTKis with greater specificity, acalabrutinib and zanubrutinib, are increasingly used in the treatment CLL due to better cardiovascular tolerability vs ibrutinib [179, 180]. Whether these newer BTKis are associated with the same off-target effects leading to increased susceptibility to fungal infections such as cryptococcosis is as yet unknown. Of note, however, 7 cases of cryptococcosis were reported in a pooled safety analysis of 6 studies totaling 779 patients receiving zanubrutinib [181].
We identified 28 cases of proven/probable cryptococcosis occurring in patients receiving BTKis, almost exclusively with ibrutinib (2 cases with acalabrutinib and 1 with zanubrutinib; Table 2). Only 2 cases were due to C gattii [88, 91]. The median age was 74 years, and 79% (22/28) were male. The main indication for receipt of BTKis was CLL (17/28, 61%), followed by mantle cell lymphoma (6/28, 21%). The median duration of treatment before onset of cryptococcosis was 4.5 months, and 18 of 28 (64.3%) cases occurred within the first 6 months of treatment. The BTKi was used as first-line therapy after diagnosis in only 29% of cases with available data (7/24) and was given with concurrent immunosuppressive treatment in 25% (6/24) of cases. The main presentations of infection were cryptococcal meningitis (10/28), pulmonary infections (9/28; including single nodule, n = 2; multiple nodules, n = 2; consolidations, n = 3; pleural empyema, n = 1), and disseminated infections (7/28). In these reports, the BTKi was inconstantly discontinued after cryptococcosis in 65% (11/17) of patients with available data, indicating a need for clearer guidelines regarding the management of these biologics after the onset of opportunistic fungal infections. With the increasing treatment options available for these lymphoid neoplasms, discontinuation of BTKis may be a reasonable approach until more data emerge.
Table 2.
Cases of Cryptococcosis Associated With Ibrutinib and Other BTKis
| Agent: First Author | Year | Age, y | Sex | Condition | BTKi as First Line | Other ISx | BTKi Before Onset, mo | Manifestation | Antifungal | Outcome | Resumption of BTKi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ibrutinib | |||||||||||
| Ajam [74] | 2016 | 76 | F | CLL | No | … | … | Primary cutaneous | FLZ | Recovery | … |
| Okamato [75] | 2016 | 68 | F | CLL | No | CHL, steroid | 2 | Disseminated | AmB + 5FC | Recovery | Yes |
| Baron [76] | 2017 | 74 | F | WM | No | CHOP, RTX, F-ara-A, CP, idelalisib, | 2 | Meningitis | AmB | Death | … |
| Kimball [77] | 2017 | 71 | M | MCL | No | RTX, bendamustine, bortezomib | 4 | Disseminated | AmB + 5FC | Death | … |
| Messina [78] | 2017 | 88 | M | LPL | No | RTX, bendamustine | 1 | Meningitis | AmB + 5FC | Recovery | … |
| 54 | M | CLL | No | F-ara-A, CP, RTX | 1 | Disseminated | AmB + 5FC | Death | … | ||
| Sudhakaran [79] | 2017 | 74 | M | MCL | No | RTX, CHOP, bortezomib | 5 | Pulmonary | FLZ | Recovery | … |
| Sun [80] | 2018 | 70 | M | MCL | No | RTX, CHOP, bortezomib | 5 | Meningitis | AmB + 5FC | Recovery | … |
| 78 | M | MCL | No | RTX, CHOP, bortezomib, bendamustine, tositumomab, Len | 24 | Meningitis | AmB + 5FC | Recovery | Yes | ||
| Swan [81] | 2018 | 79 | M | DLBCL | No | RTX, CHOP | 2 | Pulmonary | AmB + 5FC | Recovery | … |
| Varughese [82] | 2018 | 70 | M | CLL | Yes | Nil | 5 | Pulmonary | FLZ | Recovery | … |
| 52 | M | FL | Yes | RTX | 3 | Pulmonary | FLZ | Recovery | … | ||
| 61 | M | CLL | No | RTX, F-ara-A, CP | 7 | Pulmonary | FLZ | Recovery | … | ||
| Abid [83] | 2019 | 83 | M | CLL | No | F-ara-A, CP, RTX | … | Disseminated | AmB + 5FC | Recovery | … |
| Koehler [84] | 2019 | 57 | M | CLL | Yes | Nil | 4 | Pulmonary | FLZ | Recovery | … |
| Peri [85] | 2019 | 82 | F | CLL | Yes | RTX | 8 | Primary cutaneous | FLZ | Recovery | … |
| Stankowicz [86] | 2019 | 66 | M | CLL | No | CHL, RTX, bendamustine | 5 | Meningitis | AmB + 5FC | Recovery | Yes |
| 73 | M | CLL | Yes | Steroid | 2 | Pulmonary | FLZ | Recovery | Yes | ||
| Brochard [87] | 2020 | 67 | M | CLL | … | … | 6 | Disseminated | FLZ | Deatha | Yes |
| 79 | M | CLL | … | … | 15 | Pulmonary | FLZ | Deatha | Yes | ||
| 78 | F | CLL | … | … | 2 | Meningitis | AmB + 5FC | Deatha | … | ||
| Paccoud [88] | 2021 | 88 | F | CLL | No | CHL, RTX, bendamustine | 8 | Meningitis | AmB + 5FC | Recovery | … |
| Van Rooij [89] | 2021 | 75 | M | MCL | Yes | Nil | 6 | Disseminated | AmB + 5FC | Recovery | … |
| Oumayma [90] | 2023 | 69 | M | CLL | No | F-ara-A, CP, RTX | 2 | Meningitis | AmB + 5FC | Death | … |
| Sung [91] | 2023 | 76 | M | CLL | … | Nil | … | Pulmonary | FLZ | Recovery | … |
| Acalabrutinib | |||||||||||
| Wilson [92] | 2019 | 61 | M | CLL | Yes | Nil | 7 | Meningitis | AmB + 5FC | Recovery | … |
| Trivedi [93] | 2022 | 78 | M | MCL | No | RTX, bendamustine | … | Meningitis | AmB + 5FC | Recovery | … |
| Zanubrutinib | |||||||||||
| Patel [94] | 2022 | 75 | M | WM | No | Nil | 4 | Disseminated | AmB + 5FC | Death | … |
Abbreviations: 5FC, flucytosine; AmB, amphotericin B; BTKi, Bruton tyrosine kinase inhibitor; CHL, chlorambucil; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; CLL, chronic lymphocytic leukemia; CP, cyclophosphamide; DLBCL, diffuse large B-cell lymphoma; F, female; F-ara-A, fludarabine; FL, follicular lymphoma; FLZ, fluconazole; ISx, immunosuppressant; Len, lenalidomide; LPL, lymphoplasmacytic lymphoma; M, male; MCL, mantle cell lymphoma; RTX, rituximab; WM, Waldenström macroglobulinemia.
aDeath from unrelated causes.
Fingolimod
Fingolimod (FTY720) is a first-in-class oral disease-modifying medication that was approved by the FDA in 2010 for the treatment of patients with relapsing forms of multiple sclerosis. It acts by interacting with sphingosine 1-phosphate receptors to prevent lymphocyte egress from lymphoid tissues, thereby reducing autoreactive lymphocyte infiltration into the CNS [182]. Fingolimod induces a rapid and reversible reduction in lymphocyte counts, which remains stable during chronic treatment at 28% and 24% of baseline values at 24 months with 0.5 and 1.25 mg, respectively [183]. Specifically, patients treated with fingolimod showed a significant reduction in circulating CD4+ T cells, and activation of T cells in the presence of fingolimod led to a subinflammatory phenotype with reduced production of IFN-γ, granzyme B, IL-17, GM-CSF, and TNF-α [184]. These perturbations in lymphocyte number and function, which predominantly impair the activation of Th1 pathways, may underlie the increased risk of cryptococcosis in patients with multiple sclerosis treated by fingolimod.
Our literature search identified 25 published cases of proven/probable cryptococcosis associated with fingolimod treatment at a median interval of 5 years (range, 1.4–12) after the initiation of therapy (Table 3). The most common presentation was cryptococcal meningitis, which occurred in 11 patients (44%), followed by disseminated infections (7/25, 28%), primary cutaneous cryptococcosis (5/25, 20%), osteomyelitis (1/25), and isolated pulmonary cryptococcosis (1/25). The median absolute lymphocyte count upon presentation was 0.3 × 109/L (range, 0.09–2.39 × 109/L); where available, the CD4 count ranged from 5 to 145/µL. In reported cases where the speciation of Cryptococcus was provided, all were caused by C neoformans. Fingolimod was discontinued in all cases except 1 with primary cutaneous cryptococcosis [116]. Immune reconstitution inflammatory syndrome was reported in 3 cases of cryptococcal meningitis, including a fatal case [95, 97, 104]. A search of the Novartis safety database for cases with cryptococcal meningitis between January 2006 and February 2020 identified 60 case reports, with an estimated incidence of 8 per 100 000 patient-years (95% CI, 6.0–10.0), including 13 cases with fatal outcomes [185]. Although there is currently no lymphocyte cutoff that mandates the cessation of fingolimod therapy in the prescribing information, temporary drug interruption with lymphopenia <0.2 × 109/L is recommended to allow for immune reconstitution [186]. Fingolimod can be restarted when the lymphocyte count is ≥0.6 × 109/L [187].
Table 3.
Cases of Cryptococcosis Associated With Fingolimod Therapy
| Manifestation: First Author | Year | Age, y | Sex | Condition | Fingolimod Duration, y | Other ISx | ALC x 109/L | CD4/µL | Antifungal | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Cryptococcal meningitis | ||||||||||
| Achtnichts [95] | 2015 | 40s | M | RRMS | 2 | Nil | 0.4 | 56 | AmB + 5FC → FLZ (13 mo) | Recovery; IRIS |
| Grebenciucova [96] | 2016 | 62 | M | RRMS | 3 | Nil | 0.34 | … | AmB + 5FC → FLZ | Recovery |
| Ward [97] | 2016 | 67 | F | RRMS | 3.4a | Nil | 2.39 | … | AmB → FLZ | Death; IRIS |
| Pham [98] | 2017 | 61 | F | RRMS | 3 | Nil | 0.12 | 5 | AmB + 5FC → FLZ (12 mo) | Recovery |
| Anene-Maidoh [99] | 2018 | 61 | F | RRMS | 4.8 | Nil | 0.3 | 69 | AmB + 5FC | Death |
| Chong [100] | 2019 | 40 | F | RRMS | 2.3 | Nil | 0.2 | … | AmB + 5FC | Recovery |
| Ma [101] | 2020 | 58 | M | RRMS | 7 | Nil | 0.9 | … | AmB + 5FC → FLZ | Recovery |
| Aoki [102] | 2021 | 41 | M | RRMS | 6 | Nil | 0.18 | … | AmB + 5FC → FLZ (1 y) | Recovery |
| Baghbanian [103] | 2021 | 41 | F | MS | 5 | Nil | 0.25 | … | AmB + FLZ (4 wk) | Recovery |
| Cuascut [104] | 2021 | 48 | F | RRMS, RA | 7.6 | Abatacept, HCQ | 0.21 | … | AmB + 5FC → FLZ | Recovery; IRIS |
| Nasir [105] | 2023 | 21 | F | RRMS | 5 | Nil | 0.53 | 6 | AmB + 5FC → FLZ | Recovery |
| Disseminated cryptococcosis | ||||||||||
| Huang [106] | 2015 | 50 | M | MS | 3.5 | Nil | 0.5 | … | AmB + 5FC → FLZ | Recovery |
| Seto [107] | 2016 | 63 | M | MS | 2 | Nil | 0.3 | 145 | AmB + 5FC → FLZ | Recovery |
| Kaur [108] | 2020 | 34 | M | RRMS | 5 | Nil | … | 61 | AmB + 5FC → FLZ (2 y) | Recovery |
| Wienemann [109] | 2020 | 49 | F | RRMS | 5.5 | Nil | 0.09 | 77 | AmB + FLZ → AmB + 5FC → FLZ | Recovery |
| Kammeyer [110] | 2022 | 61 | M | RRMS | 7.5 | Nil | 0.3 | … | AmB + 5FC → FLZ | Recovery |
| Chey [111] | 2023 | 56 | F | MS | 3.8 | Nil | … | … | AmB + FLZ (12 mo) | Recovery |
| Zhou [112] | 2023 | 67 | M | RRMS | 6 | Nil | 0.2 | … | … | … |
| Primary cutaneous cryptococcosis | ||||||||||
| Forrestel [113] | 2016 | 62 | F | MS | 3 | Nil | 0.65 | 56 | FLZ | Recovery |
| Carpenter [114] | 2017 | 47 | M | RRMS | 1.4 | Nil | 0.3 | 73 | FLZ (12 mo) | Recovery |
| Patil [115] | 2020 | 63 | M | MS | 7 | Nil | … | 13 | FLZ (6 mo) | Recovery |
| Dahshan [116] | 2021 | 49 | M | MS | 9 | Nil | 0.3 | … | FLZ (4 mo) | Recoveryb |
| Gibson [117] | 2024 | 33 | F | RRMS | 5 | Nil | 0.22 | … | … | … |
| Osteomyelitis | ||||||||||
| Carpenter [118] | 2022 | 46 | F | RRMS | 12 | Nil | 0.3 | … | AmB + 5FC → FLZ | Recovery |
| Pulmonary cryptococcosis | ||||||||||
| Samudralwar [119] | 2019 | 45 | M | RRMS | 3 | Nil | 0.68 | … | FLZ | Recovery |
Abbreviations: 5FC, flucytosine; ALC, absolute lymphocyte count; AmB, amphotericin B; CD4, CD4+ T-lymphocyte count; FLZ, fluconazole; HCQ, hydroxychloroquine; IRIS, immune reconstitution inflammatory syndrome; ISx, immunosuppressant; MS, multiple sclerosis; RA, rheumatoid arthritis; RRMS, relapsing remitting multiple sclerosis.
aFingolimod stopped 6 to 8 weeks before onset of cryptococcosis.
bFingolimod not discontinued.
Other Biologics Associated With Cryptococcosis
In addition to the aforementioned biologics that have been shown to be associated with major risks of cryptococcosis, we identified several other biologics with ≥3 cases of treatment-associated cryptococcosis reported (Table 4). These include inhibitors of the JAK/STAT pathway, anti-CD52 antagonists, anti-CD20 antagonists, and IL-6 inhibitors. The JAK/STAT signaling pathway functions downstream of >50 cytokines and growth factors, including key players in anticryptococcal immunity, such as IFN-γ and GM-CSF [188]. STAT1 deletion resulted in a shift from Th1 to Th2 cytokine response, pronounced lung inflammation, and defective classical macrophage activation in murine models of cryptococcosis [189]. There have been 12 cases of ruxolitinib-associated cryptococcosis; most of them (8/12, 67%) did not receive other concomitant immunosuppressants, indicating that ruxolitinib per se leads to increased susceptibility to cryptococcosis. Consistent with this, in a retrospective cohort study, baricitinib (odds ratio, 12.4; 95% CI, 6.4–24.1; P < .0001), not dexamethasone, was associated with the development of cryptococcosis [190].
Table 4.
Biologics With at Least 3 Cases of Treatment-Associated Cryptococcosis Reported in the Literature
| Agent: First Author | Year | Age, y | Sex | Condition | Biologic Duration Before Onset | Other ISx | Manifestation | Antifungal | Outcome | Resumption of Biologic |
|---|---|---|---|---|---|---|---|---|---|---|
| JAK/STAT inhibitors | ||||||||||
| • Ruxolitinib | ||||||||||
| Wysham [120] | 2013 | 66 | M | PV, MF | 18 mo | Steroid | Pulmonary | FLZ (5 mo) | Recovery | 5 mo later |
| Chen [121] | 2016 | 69 | F | MF | 46 mo | Nil | Meningitis | AmB + FLZ | Recovery | … |
| Hirano [122] | 2017 | 79 | M | MF | 6 mo | Nil | Pulmonary | FLZ → VRC | Recovery | … |
| Dioverti [123] | 2018 | 70 | M | MF, cHL, HLH | 12 wk | Nil | Disseminated | … | Death | … |
| Liu [124] | 2018 | 71 | M | CMML | 3 cycles | Azacitidine, ara-C, HU | Disseminated | MIC + FLZ | Death | … |
| Prakash [125] | 2019 | 51 | M | PV | 18 mo | Nil | Meningitisa | AmB + 5FC → ISA | Recovery | … |
| Tsukui [126] | 2020 | 76 | M | MF | 5 mo | Nil | Meningitis | AmB → FLZ | Recovery | … |
| Kasemchaiyanun [127] | 2021 | 56 | F | MF | 10 mo | Nil | Pulmonary | AmB + 5FC → FLZ | Recovery | … |
| Sayabovorn [128] | 2021 | 70 | M | MF | 4 y | Nil | Disseminatedb | AmB + FLZ → FLZ | Death | Continued |
| Ciochetto [129] | 2022 | 82 | M | MF | 4 y | Steroid | Disseminated | AmB + 5FC | Death | … |
| Ogai [130] | 2022 | 71 | M | MF | 30 mo | Nil | Disseminatedc | Nil | Death | … |
| Kobe [131] | 2023 | 77 | F | NSCLC, MF | 2 y | Erlotinib, ramucirumab | Pulmonary | FLZ | Recovery | … |
| • Tofacitinib | ||||||||||
| Kremer [132] | 2013 | 68 | F | RA | 247 d | SSZ | Pulmonary | AmB → FLZ | Recovery | … |
| Seminario-Vidal [133] | 2015 | 65 | M | PsO, PsA | 6 mo | Steroid | Pulmonary | FLZ (6 mo) | Recovery | … |
| Li [134] | 2024 | 64 | F | RA | 2 mo | Steroid | Disseminated | ITC, FLZ, VRC | Recovery | … |
| Anti-CD52 antagonist | ||||||||||
| • Alemtuzumab | ||||||||||
| Dilhuydy [135] | 2007 | 44 | M | CLL | 6 wk | F-ara-A | Disseminated | AmB + 5FC | Death | … |
| Ingram [136] | 2007 | 55 | M | T-PLL | 26 doses | F-ara-A, CP | Disseminated | AmB | Recovery, IRIS at 10 mo | … |
| Bassetti [137] | 2009 | 70 | M | CLL | 6 wk | F-ara-A, CP, RTX, cyclosporin, THD | Disseminated | AmB | Death | … |
| Henn [138] | 2014 | 42 | M | CLL | 4 doses | Steroid, F-ara-A, AC, CP, RTX | Disseminated | AmB + 5FC | Death | … |
| Martin-Blondel [139] | 2014 | 60 | M | CLL | … | Steroid, F-ara-A, CP, RTX | Disseminated | AmB + 5FC | Recovery | … |
| Cruz [140] | 2019 | 57 | F | AITL | … | Steroid, RTX, CP, AC, cyclosporin, Len | Meningitis | AmB + 5FC | Recovery | … |
| Anti-CD20 antagonist | ||||||||||
| • Rituximab | ||||||||||
| Ahmed [141] | 2009 | 75 | F | CLL | 2 cycles | Steroid, CP | Meningitis | AmB + 5FC → FLZ | Recovery | … |
| Hirai [142] | 2011 | 65 | F | DLBCL | 3 cycles | CHOP | Disseminated | FLZ → AmB + 5FC → FLZ | Recovery | 52 mo later |
| Wingfield [54] | 2011 | 70 | M | RA | 2 doses | Steroid, INX, MTX | Meningitis | AmB + 5FC → AmB → VRC (4 mo) | Recovery | … |
| Hamerschlak [143] | 2012 | 62 | M | DLBCL | 3 cycles | CHOP | Pulmonary | FLZ | Recovery | Yes |
| Marchand [144] | 2013 | 69 | M | CLL | 2 cycles | F-ara-A, CP | Disseminated | AmB + 5FC → FLZ | Death due to disease progression | 6 mo later |
| AlMutawa [145] | 2016 | 72 | M | CLL, ITP | 3 cycles | Steroid, F-ara-A, CP, vincristine | Disseminated | AmB + 5FC + FLZ → FLZ | Recovery | … |
| Patel [146] | 2016 | 68 | M | CLL | 18 mo | Nil | Oral | ITC | Death due to disease progression | … |
| Reis [147] | 2016 | 17 | F | SLE | 1 dose | Steroid, MMF | Disseminated | AmB + FLZ | Recovery | 50 d later |
| Fontana [148] | 2018 | 16 | F | SLE | 2 doses | Steroid, CP, HCQ, MMF | Meningitis | AmB + 5FC → FLZ | Recovery | … |
| Swan [81] | 2018 | 79 | M | DLBCL | 2 cycles | CHOP, ibrutinib | Pulmonary | AmB + 5FC → FLZ (12 mo) | Recovery | Yes |
| Zhang [149] | 2021 | 5 | M | X-ALD, post-UCBT d130 | 3 doses | Cyclosporin | Meningitis | AmB + 5FC → FLZ (12 mo) | Recovery | … |
| Edupuganti [150] | 2023 | 40s | F | Myositis and diffuse alveolar hemorrhage | … | Steroid | Disseminated | AmB + 5FC | Death | … |
| Interleukin-6 inhibitor | ||||||||||
| • Tocilizumab | ||||||||||
| Nishioka [151] | 2018 | 55 | M | Castleman disease | 5 y | Steroid, cyclosporin | Disseminated | AmB → FLZ | Recovered | 15 mo later |
| Khatib [152] | 2021 | 60 | M | COVID-19 | 3 doses | Steroid | Disseminated | AmB + 5FC | Death | … |
| Thota [153] | 2022 | 76 | F | COVID-19 | 1 dose | Steroid | Disseminated | AmB + 5FC → FLZ | Unresponsive | … |
| Tran [154] | 2023 | 48 | M | RA, PMR | … | Steroid, MTX | Disseminated | AmB + 5FC → FLZ | Death due to CVD | … |
Abbreviations: 5FC, flucytosine; AC, anthracycline; AITL, angioimmunoblastic T-cell lymphoma; AmB, amphotericin B; ara-C, cytarabine; cHL, classical Hodgkin lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisolone; CLL, chronic lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; CP, cyclophosphamide; CVD, cardiovascular disease; DLBCL, diffuse large B-cell lymphoma; F-ara-A, fludarabine; FLZ, fluconazole; HCQ, hydroxychloroquine; HLH, hemophagocytic lymphohistiocytosis; HU, hydroxyurea; INX, infliximab; IRIS, immune reconstitution inflammatory syndrome; ISA, isavuconazole; ISx, immunosuppressant; ITC, itraconazole; ITP, immune thrombocytopenia; JAK, Janus kinase; Len, lenalidomide; MF, myelofibrosis; MIC, micafungin; MMF, mycophenolate mofetil; MTX, methotrexate; NSCLC, non–small cell lung cancer; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; PsO, psoriasis; PV, polycythemia vera; RA, rheumatoid arthritis; RTX, rituximab; SLE, systemic lupus erythematosus; SSZ, sulfasalazine; STAT, signal transducer and activator of transcription; THD, thalidomide; T-PLL, T-cell prolymphocytic leukemia; UCBT, umbilical cord blood transplant; VRC, voriconazole; X-ALD, X-linked adrenoleukodystrophy.
aDual infection with disseminated histoplasmosis.
bDual infection with Mycobacterium haemophilum.
cDual infection with Mycobacterium tuberculosis.
The anti-CD52 agent alemtuzumab is indicated in the treatment of CLL, T-cell lymphoma, and relapsing-remitting multiple sclerosis and has been used in solid organ and hematopoietic stem cell transplantation for induction therapy and acute organ rejection [191–195]. Alemtuzumab selectively targets CD52, which is expressed on the surface of B and T lymphocytes, leading to sustained lymphocyte depletion [196]. Use of alemtuzumab has been associated with a range of opportunistic infections in patients with hematologic malignancies and solid organ transplantation [197–200]. Among 547 patients with solid organ transplantation who received alemtuzumab for induction or rejection therapy, 62 (11%) experienced at least 1 opportunistic infection at a median 84 days after treatment initiation, including 2 cases of cryptococcosis [199]. Among 357 patients with CLL or cutaneous T-cell lymphoma, 33 experienced opportunistic fungal infections, including 2 cases of cryptococcosis [200]. In our review of 6 reported cases of cryptococcosis with individual case details, all occurred in heavily pretreated patients with hematologic malignancies (including 4/6 with CLL), and 5 of 6 presented with disseminated disease. Similarly, although we identified several cases of cryptococcosis in patients being treated with rituximab (anti-CD20) and tocilizumab (anti–IL-6), almost all of them received concomitant corticosteroids and/or chemotherapeutic agents, suggesting that susceptibility to cryptococcosis in these populations more likely reflected an overall degree of immunosuppression instead of the independent effect of the biologics. Other biologics with rare cases of treatment-associated cryptococcosis are included in Supplementary Table 2.
Role of Steroid and Other Immunosuppressants in Cryptococcosis Associated With Biologics
As previously stated, a significant percentage of patients in this review received concomitant immunosuppressants, most notably corticosteroids. Increased susceptibility to infection caused by corticosteroid use is multifactorial and is influenced by corticosteroid dose and duration, as well as the underlying disease [201]. Corticosteroid use affects innate and adaptive immune responses. Specifically, corticosteroids reduce T-cell responses, particularly Th1 responses, by promoting T-cell apoptosis, suppressing T-cell activation and proliferation, and preventing cytokine production [201]. Corticosteroid use is commonly reported among specific subgroups of individuals with cryptococcosis who are immunocompromised, particularly in patients with malignancy, solid organ transplant, and autoimmune conditions [202–205]. Among HIV-seronegative cohorts with cryptococcosis, prior corticosteroid use was reported in up to 28% to 48% of patients [206–209], although the dose and duration were often not specified. Prior high-dose corticosteroid use, defined as the equivalent of ≥20 mg/d of prednisone for ≥60 days prior to diagnosis of cryptococcosis, has been associated with a higher likelihood of dissemination (41% vs 18%, P = .002) among patients with pulmonary cryptococcosis [210], and corticosteroid usage was associated with a higher 30-day mortality in a recent observational study from Japan [208].
Since biologics are most likely to be initiated in patients with autoimmune conditions, neoplasms, and transplantation, other immunosuppressants and immunomodulatory agents, especially those affecting the T-cell activation and proliferation pathways, play a role in mediating the risk of cryptococcosis. For example, in our identified cases, purine analogues such as fludarabine and cytarabine were often given to patients with hematologic malignancies. In addition to corticosteroids, transplant recipients are likely receiving calcineurin inhibitors, mycophenolate mofetil, and/or mTOR inhibitors (mammalian target of rapamycin), all of which affect T-cell activation and differentiation [211–213]. Therefore, the overall risk of infection is a product of the interaction between biologics and the host, as well as between biologics and Cryptococcus species.
There are limitations to this study. First, cases whose demographics and clinical details were not available were excluded from the analysis. Second, there is inherent difficulty in attributing causality to the biologics, as many patients in the literature review had underlying hematologic or rheumatologic conditions that impaired the immunity and they received concomitant or recent immunosuppressants, which all contributed to the increased risk of infection. The current study did not aim to address the causality of each biologic from a mechanistic point of view. Third, the manifestations of cryptococcosis may mimic other conditions. As noted in our series, different groups of biologics appeared to be associated with specific manifestations, such as the relatively high percentage of pulmonary cryptococcosis with TNF-α antagonists and skin and soft tissue infections with fingolimod. Disseminated disease most often occurred in patients receiving concomitant immunosuppressants and those with advanced age. However, there were significant differences in the exhaustivity in the diagnostic workup, which was based on the discretion of treating physicians and limited by the systematic availability of diagnostic tools. The apparent high percentage of some non-CNS forms of cryptococcosis associated with certain biologics may be partially attributed to the heterogeneity of the diagnostic workup.
CONCLUSION
In conclusion, biologics, especially those blocking the Th1-macrophage activation pathways, impart a substantially increased risk of cryptococcosis among patient populations who are already susceptible to opportunistic infections due to their underlying conditions or concomitant immunosuppressants. With the increasing number and variety of biologics—expanding from the treatment of autoimmune diseases and neoplasms to novel therapeutics for atopy and metabolic diseases—clinicians must be vigilant of the risks, as lack of suspicion may lead to diagnostic delays and poorer outcomes. Knowledge of the association between biologic therapies and cryptococcosis, including the underlying mechanism of immune susceptibility and clinical manifestations, will help clinicians stratify the risks of cryptococcal infection and individualize the management plans for their patients. More data are needed to guide the management of cryptococcal infection in patients receiving biologic therapy, especially regarding the continuation or resumption of biologics during and after antifungal therapy.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Xin Li, Department of Infectious Diseases and Tropical Medicine, Université Paris Cité, Necker-Enfants Malades University Hospital, Assistance Publique–Hôpitaux de Paris, IHU Imagine, Paris, France; Department of Microbiology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China.
Olivier Paccoud, Department of Infectious Diseases and Tropical Medicine, Université Paris Cité, Necker-Enfants Malades University Hospital, Assistance Publique–Hôpitaux de Paris, IHU Imagine, Paris, France.
Koon-Ho Chan, Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China.
Kwok-Yung Yuen, Department of Microbiology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China.
Romain Manchon, Department of Infectious Diseases and Tropical Medicine, Université Paris Cité, Necker-Enfants Malades University Hospital, Assistance Publique–Hôpitaux de Paris, IHU Imagine, Paris, France.
Fanny Lanternier, Department of Infectious Diseases and Tropical Medicine, Université Paris Cité, Necker-Enfants Malades University Hospital, Assistance Publique–Hôpitaux de Paris, IHU Imagine, Paris, France; Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Mycology Translational Research Group, Mycology Department, Université Paris Cité, Paris, France.
Monica A Slavin, Department of Infectious Diseases, Peter MacCallum Cancer Centre, Melbourne, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Australia; Victorian Infectious Diseases Service, Royal Melbourne Hospital, Melbourne, Australia.
Frank L van de Veerdonk, Department of Internal Medicine, Radboud Center for Infectious Diseases, Radboudumc, Nijmegen, the Netherlands.
Tihana Bicanic, Institute of Infection and Immunity, St George's University of London, London, UK.
Olivier Lortholary, Department of Infectious Diseases and Tropical Medicine, Université Paris Cité, Necker-Enfants Malades University Hospital, Assistance Publique–Hôpitaux de Paris, IHU Imagine, Paris, France; Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Mycology Translational Research Group, Mycology Department, Université Paris Cité, Paris, France.
Notes
Author contributions. X. L.: methodology, investigation, writing–original draft. O. P.: methodology, investigation, writing–original draft. K.-H. C.: writing–review and editing. K.-Y. Y.: writing–review and editing, supervision. R. M.: investigation. F. L.: supervision. M. A. S.: writing–review and editing. F. L. v. d. V.: writing–review and editing. T. B.: writing–review and editing. O. L.: conceptualization, methodology, writing–review and editing, supervision.
Patient consent statement. This study exclusively uses existing published data and thus does not require ethical approval.
References
- 1. May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 2016; 14:106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 2022; 22:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am 2016; 30:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cogliati M. Global warming impact on the expansion of fundamental niche of Cryptococcus gattii VGI in Europe. Environ Microbiol Rep 2021; 13:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 2009; 77:4345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elsegeiny W, Marr KA, Williamson PR. Immunology of cryptococcal infections: developing a rational approach to patient therapy. Front Immunol 2018; 9:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rogers K. Ibrutinib and fungus: an invasive concern. Blood 2018; 131:1882–4. [DOI] [PubMed] [Google Scholar]
- 8. Goldman DL, Khine H, Abadi J, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 2001; 107:E66. [DOI] [PubMed] [Google Scholar]
- 9. Ye F, Xie JX, Zeng QS, Chen GQ, Zhong SQ, Zhong NS. Retrospective analysis of 76 immunocompetent patients with primary pulmonary cryptococcosis. Lung 2012; 190:339–46. [DOI] [PubMed] [Google Scholar]
- 10. Batungwanayo J, Taelman H, Bogaerts J, et al. Pulmonary cryptococcosis associated with HIV-1 infection in Rwanda: a retrospective study of 37 cases. Aids 1994; 8:1271–6. [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol 1999; 37:3204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saha DC, Goldman DL, Shao X, et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin Vaccine Immunol 2007; 14:1550–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccioni M, Monari C, Kenno S, et al. A purified capsular polysaccharide markedly inhibits inflammatory response during endotoxic shock. Infect Immun 2013; 81:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Leon-Rodriguez CM, Fu MS, Çorbali MO, Cordero RJB, Casadevall A. The capsule of Cryptococcus neoformans modulates phagosomal pH through its acid-base properties. mSphere 2018; 3:e00437-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villena SN, Pinheiro RO, Pinheiro CS, et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol 2008; 10:1274–85. [DOI] [PubMed] [Google Scholar]
- 16. Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel TR. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun 1998; 66:664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syme RM, Bruno TF, Kozel TR, Mody CH. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect Immun 1999; 67:4620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bojarczuk A, Miller KA, Hotham R, et al. Cryptococcus neoformans intracellular proliferation and capsule size determines early macrophage control of infection. Sci Rep 2016; 6:21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun 1995; 63:3131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salas SD, Bennett JE, Kwon-Chung KJ, Perfect JR, Williamson PR. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 1996; 184:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olszewski MA, Noverr MC, Chen GH, et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 2004; 164:1761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jong A, Wu CH, Gonzales-Gomez I, et al. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem 2012; 287:15298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vu K, Tham R, Uhrig JP, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio 2014; 5:e01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maruvada R, Zhu L, Pearce D, et al. Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood-brain barrier. Cell Microbiol 2012; 14:1544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zaragoza O, García-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog 2010; 6:e1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okagaki LH, Strain AK, Nielsen JN, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 2010; 6:e1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gerstein AC, Fu MS, Mukaremera L, et al. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio 2015; 6:e01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dambuza IM, Drake T, Chapuis A, et al. The Cryptococcus neoformans titan cell is an inducible and regulated morphotype underlying pathogenesis. PLoS Pathog 2018; 14:e1006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. García-Barbazán I, Trevijano-Contador N, Rueda C, et al. The formation of titan cells in Cryptococcus neoformans depends on the mouse strain and correlates with induction of Th2-type responses. Cell Microbiol 2016; 18:111–24. [DOI] [PubMed] [Google Scholar]
- 30. Crabtree JN, Okagaki LH, Wiesner DL, Strain AK, Nielsen JN, Nielsen K. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun 2012; 80:3776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barbosa FM, Fonseca FL, Figueiredo RT, et al. Binding of glucuronoxylomannan to the CD14 receptor in human A549 alveolar cells induces interleukin-8 production. Clin Vaccine Immunol 2007; 14:94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teixeira PA, Penha LL, Mendonça-Previato L, Previato JO. Mannoprotein MP84 mediates the adhesion of Cryptococcus neoformans to epithelial lung cells. Front Cell Infect Microbiol 2014; 4:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganendren R, Carter E, Sorrell T, Widmer F, Wright L. Phospholipase B activity enhances adhesion of Cryptococcus neoformans to a human lung epithelial cell line. Microbes Infect 2006; 8:1006–15. [DOI] [PubMed] [Google Scholar]
- 34. Rossi SA, García-Barbazán I, Chamorro-Herrero I, Taborda CP, Zaragoza Ó, Zambrano A. Use of 2D minilungs from human embryonic stem cells to study the interaction of Cryptococcus neoformans with the respiratory tract. Microbes Infect 2023; 26:105260. [DOI] [PubMed] [Google Scholar]
- 35. Guillot L, Carroll SF, Badawy M, Qureshi ST. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir Res 2008; 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heyen L, Müller U, Siegemund S, et al. Lung epithelium is the major source of IL-33 and is regulated by IL-33-dependent and IL-33-independent mechanisms in pulmonary cryptococcosis. Pathog Dis 2016; 74:ftw086. [DOI] [PubMed] [Google Scholar]
- 37. McQuiston TJ, Williamson PR. Paradoxical roles of alveolar macrophages in the host response to Cryptococcus neoformans. J Infect Chemother 2012; 18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davis MJ, Tsang TM, Qiu Y, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio 2013; 4:e00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Castro-Dopico T, Fleming A, Dennison TW, et al. GM-CSF calibrates macrophage defense and wound healing programs during intestinal infection and inflammation. Cell Rep 2020; 32:107857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, Wormley FL Jr. STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect Immun 2015; 83:4513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiesner DL, Specht CA, Lee CK, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog 2015; 11:e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. True DG, Penmetcha M, Peckham SJ. Disseminated cryptococcal infection in rheumatoid arthritis treated with methotrexate and infliximab. J Rheumatol 2002; 29:1561–3. [PubMed] [Google Scholar]
- 43. Hage CA, Wood KL, Winer-Muram HT, Wilson SJ, Sarosi G, Knox KS. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest 2003; 124:2395–7. [DOI] [PubMed] [Google Scholar]
- 44. Hrnicek MJ, Young RL. Immunomodulatory therapy in Crohn's disease as a cause of Cryptococcus neoformans pneumonia. Am J Gastroenterol 2003; 98:S162. [Google Scholar]
- 45. Arend SM, Kuijper EJ, Allaart CF, Muller WH, Van Dissel JT. Cavitating pneumonia after treatment with infliximab and prednisone. Eur J Clin Microbiol Infect Dis 2004; 23:638–41. [DOI] [PubMed] [Google Scholar]
- 46. Shrestha RK, Stoller JK, Honari G, Procop GW, Gordon SM. Pneumonia due to Cryptococcus neoformans in a patient receiving infliximab: possible zoonotic transmission from a pet cockatiel. Respir Care 2004; 49:606–8. [PubMed] [Google Scholar]
- 47. Muñoz P, Giannella M, Valerio M, et al. Cryptococcal meningitis in a patient treated with infliximab. Diagn Microbiol Infect Dis 2007; 57:443–6. [DOI] [PubMed] [Google Scholar]
- 48. Kozic H, Riggs K, Ringpfeil F, Lee JB. Disseminated Cryptococcus neoformans after treatment with infliximab for rheumatoid arthritis. J Am Acad Dermatol 2008; 58:S95–6. [DOI] [PubMed] [Google Scholar]
- 49. Rehman T, Ali J, Lopez FA. A 61-year-old man with asymptomatic, bilateral lung masses. J La State Med Soc 2008; 160:309–14. [PubMed] [Google Scholar]
- 50. Arnaud L, Sene D, Costedoat-Chalumeau N, Cacoub P, Chapelon-Abric C, Piette JC. Disseminated cryptococcal infection and anti-tumor necrosis factor-alpha treatment for refractory sarcoidosis: an expected association? J Rheumatol 2009; 36:462–3. [DOI] [PubMed] [Google Scholar]
- 51. Kluger N, Poirier P, Guilpain P, Baixench MT, Cohen P, Paugam A. Cryptococcal meningitis in a patient treated with infliximab and mycophenolate mofetil for Behcet's disease. Int J Infect Dis 2009; 13:e325. [DOI] [PubMed] [Google Scholar]
- 52. Osawa R, Singh N. Colitis as a manifestation of infliximab-associated disseminated cryptococcosis. Int J Infect Dis 2010; 14:e436–40. [DOI] [PubMed] [Google Scholar]
- 53. Hirai F, Matsui T, Ishibashi Y, et al. Asymptomatic pulmonary cryptococcosis in a patient with Crohn's disease on infliximab: case report. Inflamm Bowel Dis 2011; 17:1637–8. [DOI] [PubMed] [Google Scholar]
- 54. Wingfield T, Jani M, Krutikov M, et al. Cryptococcal meningitis in an HIV-negative patient with rheumatoid arthritis treated with rituximab. Rheumatology (Oxford) 2011; 50:1725–7. [DOI] [PubMed] [Google Scholar]
- 55. Takazono T, Sawai T, Tashiro M, et al. Relapsed pulmonary cryptococcosis during tumor necrosis factor α inhibitor treatment. Intern Med 2016; 55:2877–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vasant DH, Limdi JK, Borg-Bartolo SP, Bonington A, George R. Posterior reversible encephalopathy syndrome and fatal cryptococcal meningitis after immunosuppression in a patient with elderly onset inflammatory bowel disease. ACG Case Rep J 2016; 3:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamada S, Kajihara I, Johno T, et al. Symptomless pulmonary cryptococcosis in a psoriatic arthritis patient during infliximab therapy. Ann Dermatol 2016; 28:269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asakura T, Arai D, Ishii M, et al. Pulmonary cryptococcosis developed from a nodule after treatment with infliximab for arthritis associated with ulcerative colitis. Ann Am Thorac Soc 2017; 14:603–5. [DOI] [PubMed] [Google Scholar]
- 59. Chiriac A, Mares M, Mihaila D, et al. Primary cutaneous cryptococcosis during infliximab therapy. Dermatol Ther 2017; 30:e12405. [DOI] [PubMed] [Google Scholar]
- 60. Lee WS, Azmi N, Ng RT, et al. Fatal infections in older patients with inflammatory bowel disease on anti-tumor necrosis factor therapy. Intest Res 2017; 15:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nosaki Y, Ohyama K, Watanabe M, et al. Simultaneous development of progressive multifocal leukoencephalopathy and cryptococcal meningitis during methotrexate and infliximab treatment. Intern Med 2019; 58:2703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Santo P, Zaltman C, Santos P, et al. Association of cryptococcosis and tuberculosis in a patient with Crohn's disease—a challenging diagnosis. Am J Gastroenterol 2019; 114:S29. [Google Scholar]
- 63. Hussein M, Haq IU, Hameed M, et al. Isolated pulmonary cryptococcosis in a patient with Crohn's disease treated with infliximab: a case report and literature review. Respir Med Case Rep 2021; 33:101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fang YF, Cao XH, Yao LY, Cao Q. Pulmonary cryptococcosis after immunomodulator treatment in patients with Crohn's disease: three case reports. World J Gastroenterol 2023; 29:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sha S, Shi H, Wu J, et al. Case report: unusual cause of fever in ulcerative colitis treated with infliximab. J Inflamm Res 2023; 16:1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horcajada JP, Peña JL, Martínez-Taboada VM, et al. Invasive cryptococcosis and adalimumab treatment. Emerg Infect Dis 2007; 13:953–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cadena J, Thompson GR 3rd, Ho TT, Medina E, Hughes DW, Patterson TF. Immune reconstitution inflammatory syndrome after cessation of the tumor necrosis factor alpha blocker adalimumab in cryptococcal pneumonia. Diagn Microbiol Infect Dis 2009; 64:327–30. [DOI] [PubMed] [Google Scholar]
- 68. Iwata T, Nagano T, Tomita M, et al. Adalimumab-associated pulmonary cryptococcosis. Ann Thorac Cardiovasc Surg 2011; 17:390–3. [DOI] [PubMed] [Google Scholar]
- 69. Fraison JB, Guilpain P, Schiffmann A, et al. Pulmonary cryptococcosis in a patient with Crohn's disease treated with prednisone, azathioprine and adalimumab: exposure to chicken manure as a source of contamination. J Crohns Colitis 2013; 7:e11–4. [DOI] [PubMed] [Google Scholar]
- 70. Gomes RM, Cerio DR, Loghmanee C, et al. Cutaneous cryptococcoma in a patient on TNF-α inhibition. J Clin Med 2013; 2:260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yeh H, Wu RC, Tsai WS, et al. Systemic lupus erythematosus complicated by Crohn's disease with rectovaginal fistula. BMC Gastroenterol 2021; 21:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoang JK, Burruss J. Localized cutaneous Cryptococcus albidus infection in a 14-year-old boy on etanercept therapy. Pediatr Dermatol 2007; 24:285–8. [DOI] [PubMed] [Google Scholar]
- 73. Wysocki JD, Said SM, Papadakis KA. An uncommon cause of abdominal pain and fever in a patient with Crohn's disease. Gastroenterology 2015; 148:e12–3. [DOI] [PubMed] [Google Scholar]
- 74. Ajam T, Hyun G, Blue B, Rajeh N. Primary cutaneous cryptococcosis in a patient with chronic lymphocytic leukemia: a case report. Ann Hematol Onco 2016; 3:1082. [Google Scholar]
- 75. Okamoto K, Proia LA, Demarais PL. Disseminated cryptococcal disease in a patient with chronic lymphocytic leukemia on ibrutinib. Case Rep Infect Dis 2016; 2016:4642831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baron M, Zini JM, Challan Belval T, et al. Fungal infections in patients treated with ibrutinib: two unusual cases of invasive aspergillosis and cryptococcal meningoencephalitis. Leuk Lymphoma 2017; 58:2981–2. [DOI] [PubMed] [Google Scholar]
- 77. Kimball CD, Cruse A, Craig L, et al. Petechial, purpuric, and ecchymotic presentation of cutaneous Cryptococcus in mantle cell lymphoma. JAAD Case Rep 2017; 3:53–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Messina JA, Maziarz EK, Spec A, Kontoyiannis DP, Perfect JR. Disseminated cryptococcosis with brain involvement in patients with chronic lymphoid malignancies on ibrutinib. Open Forum Infect Dis 2017; 4:ofw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sudhakaran S, Bashoura L, Stewart J, Balachandran DD, Faiz SA. Pulmonary cryptococcus presenting as a solitary pulmonary nodule. Am J Respir Crit Care Med 2017; 196:1217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sun K, Kasparian S, Iyer S, Pingali SR. Cryptococcal meningoencephalitis in patients with mantle cell lymphoma on ibrutinib. Ecancermedicalscience 2018; 12:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Swan CD, Gottlieb T. Cryptococcus neoformans empyema in a patient receiving ibrutinib for diffuse large B-cell lymphoma and a review of the literature. BMJ Case Rep 2018; 2018:bcr-2018-224786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Varughese T, Taur Y, Cohen N, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis 2018; 67:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Abid MB, Stromich J, Gundacker ND. Is ibrutinib associated with disseminated cryptococcosis with CNS involvement? Cancer Biol Ther 2019; 20:138–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Koehler AB, Vijayvargiya P, Ding W. Probable invasive pulmonary cryptococcosis and possible cryptococcal empyema in CLL treated with frontline ibrutinib. Mayo Clin Proc 2019; 94:915–7. [DOI] [PubMed] [Google Scholar]
- 85. Peri AM, Rossio R, Tafuri F, et al. Atypical primary cutaneous cryptococcosis during ibrutinib therapy for chronic lymphocytic leukemia. Ann Hematol 2019; 98:2847–9. [DOI] [PubMed] [Google Scholar]
- 86. Stankowicz M, Banaszynski M, Crawford R. Cryptococcal infections in two patients receiving ibrutinib therapy for chronic lymphocytic leukemia. J Oncol Pharm Pract 2019; 25:710–4. [DOI] [PubMed] [Google Scholar]
- 87. Brochard J, Morio F, Mahe J, et al. Ibrutinib, a Bruton’s tyrosine kinase inhibitor, a new risk factor for cryptococcosis. Med Mal Infect 2020; 50:742–5. [DOI] [PubMed] [Google Scholar]
- 88. Paccoud O, Bougnoux ME, Desnos-Ollivier M, Varet B, Lortholary O, Lanternier F. Cryptococcus gattii in patients with lymphoid neoplasms: an illustration of evolutive host-fungus interactions. J Fungi (Basel) 2021; 7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Van Rooij N, Johnston J, Mortimore R, Robertson I. A case of disseminated cryptococcal disease after Bruton tyrosine kinase inhibitor therapy: a brief review in the Australian context. JAAD Case Rep 2021; 13:43–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Oumayma H, Mahtat EM, Moussa Bouh H, Elmaaroufi H, Doghmi K. Fatal cryptococcal meningitis in a patient with chronic lymphocytic leukemia treated with ibrutinib. Cureus 2023; 15:e37891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sung D, Singh S, Goswami SK. Cryptococcal pneumonia in a patient on tyrosine kinase inhibitor therapy: how common is it? Cureus 2023; 15:e47884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wilson P, Melville K. Disseminated cryptococcal infection in a patient receiving acalabrutinib for chronic lymphocytic leukemia. Infect Dis Clin Pract 2019; 27:1. [Google Scholar]
- 93. Trivedi K, Patel K, Patel N, Ahmed M, Dylan M, Shaaban H. Rare case of cryptococcal meningitis in non-HIV patient with mantle cell lymphoma associated with acalabrutinib (tyrosine kinase inhibitor). J Infect Dis Case Rep 2022; 3:1–3. [Google Scholar]
- 94. Patel D, Sidana M, Mdluli X, Patel V, Stapleton A, Dasanu CA. A fatal disseminated cryptococcal infection in a patient treated with zanubrutinib for Waldenström's macroglobulinemia. J Oncol Pharm Pract 2022; 28:1917–21. [DOI] [PubMed] [Google Scholar]
- 95. Achtnichts L, Obreja O, Conen A, Fux CA, Nedeltchev K. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol 2015; 72:1203–5. [DOI] [PubMed] [Google Scholar]
- 96. Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord 2016; 9:158–62. [DOI] [PubMed] [Google Scholar]
- 97. Ward MD, Jones DE, Goldman MD. Cryptococcal meningitis after fingolimod discontinuation in a patient with multiple sclerosis. Mult Scler Relat Disord 2016; 9:47–9. [DOI] [PubMed] [Google Scholar]
- 98. Pham C, Bennett I, Jithoo R. Cryptococcal meningitis causing obstructive hydrocephalus in a patient on fingolimod. BMJ Case Rep 2017; 2017:bcr-2017-220026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Anene-Maidoh TI, Paschall RM, Graham RS. Refractory cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod: a case report. Interdiscip Neurosurg 2018; 12:8–9. [Google Scholar]
- 100. Chong I, Wang KY, Lincoln CM. Cryptococcal meningitis in a multiple sclerosis patient treated with fingolimod: a case report and review of imaging findings. Clin Imaging 2019; 54:53–6. [DOI] [PubMed] [Google Scholar]
- 101. Ma SB, Griffin D, Boyd SC, Chang CC, Wong J, Guy SD. Cryptococcus neoformans var grubii meningoencephalitis in a patient on fingolimod for relapsing-remitting multiple sclerosis: case report and review of published cases. Mult Scler Relat Disord 2020; 39:101923. [DOI] [PubMed] [Google Scholar]
- 102. Aoki R, Mori M, Suzuki YI, et al. Cryptococcal meningitis in a fingolimod-treated patient: positive antigen test a year before onset. Neurol Clin Pract 2021; 11:e549–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baghbanian SM, Amiri MRM. Cryptococcal meningoencephalitis in a multiple sclerosis patient after fingolimod discontinuation—a case report. Neurol Sci 2021; 42:1175–7. [DOI] [PubMed] [Google Scholar]
- 104. Cuascut FX, Alkabie S, Hutton GJ. Fingolimod-related cryptococcal meningoencephalitis and immune reconstitution inflammatory syndrome in a patient with multiple sclerosis. Mult Scler Relat Disord 2021; 53:103072. [DOI] [PubMed] [Google Scholar]
- 105. Nasir M, Galea I, Neligan A, Chung K. Cryptococcal meningoencephalitis in multiple sclerosis treated with fingolimod. Pract Neurol 2023; 23:512–5. [DOI] [PubMed] [Google Scholar]
- 106. Huang D. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology 2015; 85:1001–3. [DOI] [PubMed] [Google Scholar]
- 107. Seto H, Nishimura M, Minamiji K, et al. Disseminated cryptococcosis in a 63-year-old patient with multiple sclerosis treated with fingolimod. Intern Med 2016; 55:3383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kaur P, Lewis A, Basit A, Cyr NS, Muhammad Z. Increased risk of disseminated cryptococcal infection in a patient with multiple sclerosis on fingolimod. IDCases 2020; 22:e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wienemann T, Müller AK, MacKenzie C, et al. Cryptococcal meningoencephalitis in an IgG(2)-deficient patient with multiple sclerosis on fingolimod therapy for more than five years—case report. BMC Neurol 2020; 20:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kammeyer JA, Lehmann NM. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol 2022; 14:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chey SY, O'Sullivan NA, Beer T, Leong WK, Kermode AG. Cutaneous presentation of cryptococcal infection with subclinical central nervous system involvement secondary to fingolimod therapy. Mult Scler J Exp Transl Clin 2023; 9:20552173231197132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhou DJ, Situ-Kcomt M, McLaughlin MT. Cryptococcal meningoencephalitis mimicking a multiple sclerosis flare in a patient taking fingolimod. Neurohospitalist 2023; 13:325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Forrestel AK, Modi BG, Longworth S, Wilck MB, Micheletti RG. Primary cutaneous Cryptococcus in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol 2016; 73:355–6. [DOI] [PubMed] [Google Scholar]
- 114. Carpenter AF, Goodwin SJ, Bornstein PF, Larson AJ, Markus CK. Cutaneous cryptococcosis in a patient taking fingolimod for multiple sclerosis: here come the opportunistic infections? Mult Scler 2017; 23:297–9. [DOI] [PubMed] [Google Scholar]
- 115. Patil SM, Beck PP, Arora N, Acevedo BA, Dandachi D. Primary cutaneous cryptococcal infection due to fingolimod—induced lymphopenia with literature review. IDCases 2020; 21:e00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Dahshan D, Dessie SA, Cuda J, Khalil E. Primary cutaneous cryptococcosis in a patient on fingolimod: a case report. Cureus 2021; 13:e16444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gibson S, McGraw C. Teaching neuroImage: Cryptococcus in a woman with multiple sclerosis on fingolimod. Neurology 2024; 102:e208027. [DOI] [PubMed] [Google Scholar]
- 118. Carpenter K, Etemady-Deylamy A, Costello V, et al. Cryptococcal chest wall mass and rib osteomyelitis associated with the use of fingolimod: a case report and literature review. Front Med (Lausanne) 2022; 9:942751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Samudralwar RD, Spec A, Cross AH. Case report: fingolimod and cryptococcosis: collision of immunomodulation with infectious disease. Int J MS Care 2019; 21:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wysham NG, Sullivan DR, Allada G. An opportunistic infection associated with ruxolitinib, a novel janus kinase 1,2 inhibitor. Chest 2013; 143:1478–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen CC, Chen YY, Huang CE. Cryptococcal meningoencephalitis associated with the long-term use of ruxolitinib. Ann Hematol 2016; 95:361–2. [DOI] [PubMed] [Google Scholar]
- 122. Hirano A, Yamasaki M, Saito N, et al. Pulmonary cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis. Respir Med Case Rep 2017; 22:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dioverti MV, Abu Saleh OM, Tande AJ. Infectious complications in patients on treatment with ruxolitinib: case report and review of the literature. Infect Dis (Lond) 2018; 50:381–7. [DOI] [PubMed] [Google Scholar]
- 124. Liu J, Mouhayar E, Tarrand JJ, Kontoyiannis DP. Fulminant Cryptococcus neoformans infection with fatal pericardial tamponade in a patient with chronic myelomonocytic leukaemia who was treated with ruxolitinib: case report and review of fungal pericarditis. Mycoses 2018; 61:245–55. [DOI] [PubMed] [Google Scholar]
- 125. Prakash K, Richman D. A case report of disseminated histoplasmosis and concurrent cryptococcal meningitis in a patient treated with ruxolitinib. BMC Infect Dis 2019; 19:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tsukui D, Fujita H, Suzuki K, Hirata K. A case report of cryptococcal meningitis associated with ruxolitinib. Medicine (Baltimore) 2020; 99:e19587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kasemchaiyanun A, Suwatanapongched T, Incharoen P, Plumworasawat S, Bruminhent J. Combined pulmonary tuberculosis with pulmonary and pleural cryptococcosis in a patient receiving ruxolitinib therapy. Infect Drug Resist 2021; 14:3901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sayabovorn N, Chongtrakool P, Chayakulkeeree M. Cryptococcal fungemia and Mycobacterium haemophilum cellulitis in a patient receiving ruxolitinib: a case report and literature review. BMC Infect Dis 2021; 21:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ciochetto Z, Wainaina N, Graham MB, Corey A, Abid MB. Cryptococcal infection with ruxolitinib in primary myelofibrosis: a case report and literature review. Clin Case Rep 2022; 10:e05461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ogai A, Yagi K, Ito F, Domoto H, Shiomi T, Chin K. Fatal disseminated tuberculosis and concurrent disseminated cryptococcosis in a ruxolitinib-treated patient with primary myelofibrosis: a case report and literature review. Intern Med 2022; 61:1271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kobe H, Yokoe S, Ishida T. Incidental diagnosis of pulmonary cryptococcosis by rebiopsy for epidermal growth factor receptor T790M mutation: a case report. Thorac Cancer 2023; 14:210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kremer J, Li ZG, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013; 159:253–61. [DOI] [PubMed] [Google Scholar]
- 133. Seminario-Vidal L, Cantrell W, Elewski BE. Pulmonary cryptococcosis in the setting of tofacitinib therapy for psoriasis. J Drugs Dermatol 2015; 14:901–2. [PubMed] [Google Scholar]
- 134. Li Z, Shan Y, Dong J, Mei H, Kong Q, Li Y. Disseminated cryptococcosis presenting with generalized cutaneous involvement in a rheumatoid arthritis patient receiving tofacitinib: a case report. J Dermatol 2024; 51:e39–41. [DOI] [PubMed] [Google Scholar]
- 135. Dilhuydy MS, Jouary T, Demeaux H, Ravaud A. Cutaneous cryptococcosis with alemtuzumab in a patient treated for chronic lymphocytic leukaemia. Br J Haematol 2007; 137:490. [DOI] [PubMed] [Google Scholar]
- 136. Ingram PR, Howman R, Leahy MF, Dyer JR. Cryptococcal immune reconstitution inflammatory syndrome following alemtuzumab therapy. Clin Infect Dis 2007; 44:e115–7. [DOI] [PubMed] [Google Scholar]
- 137. Bassetti M, Repetto E, Mikulska M, et al. Cryptococcus neoformans fatal sepsis in a chronic lymphocytic leukemia patient treated with alemtuzumab: case report and review of the literature. J Chemother 2009; 21:211–4. [DOI] [PubMed] [Google Scholar]
- 138. Henn A, Mellon G, Benoît H, et al. Disseminated cryptococcosis, invasive aspergillosis, and mucormycosis in a patient treated with alemtuzumab for chronic lymphocytic leukaemia. Scand J Infect Dis 2014; 46:231–4. [DOI] [PubMed] [Google Scholar]
- 139. Martin-Blondel G, Ysebaert L. Images in clinical medicine: disseminated cryptococcosis. N Engl J Med 2014; 370:1741. [DOI] [PubMed] [Google Scholar]
- 140. Cruz D, Costa P, Sagüés M. Meningeal cryptococcosis in a patient with angioimmunoblastic lymphoma treated with alemtuzumab. Med Clin (Barc) 2019; 152:e19–20. [DOI] [PubMed] [Google Scholar]
- 141. Ahmed I, Powell S, Hoth M, Javed A, Moen SK, Haehn MR. Cryptococcal meningitis presenting with recurrent syncope in a patient with chronic lymphoid leukemia: a case report. Cases J 2009; 2:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hirai Y, Ainoda Y, Shoji T, et al. Disseminated cryptococcosis in a non-Hodgkin's lymphoma patient with late-onset neutropenia following rituximab-CHOP chemotherapy: a case report and literature review. Mycopathologia 2011; 172:227–32. [DOI] [PubMed] [Google Scholar]
- 143. Hamerschlak N, Pasternak J, Wagner J, Perini GF. Not all that shines is cancer: pulmonary cryptococcosis mimicking lymphoma in [(18)] F fluoro-2-deoxy-D-glucose positron emission tomography. Einstein (Sao Paulo) 2012; 10:502–4. [DOI] [PubMed] [Google Scholar]
- 144. Marchand T, Revest M, Tattevin P, et al. Early cryptococcal meningitis following treatment with rituximab, fludarabine and cyclophosphamide in a patient with chronic lymphocytic leukemia. Leuk Lymphoma 2013; 54:643–5. [DOI] [PubMed] [Google Scholar]
- 145. AlMutawa F, Leto D, Chagla Z. Disseminated cryptococcal disease in non-HIV, nontransplant patient. Case Rep Infect Dis 2016; 2016:1725287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Patel S, Navas M, Batt C, Jump RL. Oral cryptococcosis in a patient with chronic lymphocytic leukemia. Int J Infect Dis 2016; 50:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Reis J, Aguiar F, Brito I. Anti CD20 (rituximab) therapy in refractory pediatric rheumatic diseases. Acta Reumatol Port 2016; 41:45–55. [PubMed] [Google Scholar]
- 148. Fontana F, Alfano G, Leonelli M, et al. Efficacy of belimumab for active lupus nephritis in a young Hispanic woman intolerant to standard treatment: a case report. BMC Nephrol 2018; 19:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang N, Chen K, Zhu JS, Li H, Shao JB, Jiang H. Sequential infection of Epstein-Barr virus and cryptococcal encephalitis after umbilical cord blood transplantation in a child with X-linked adrenoleukodystrophy. Pediatr Transplant 2021; 25:e13956. [DOI] [PubMed] [Google Scholar]
- 150. Edupuganti S, Yadav D, Upadhyay M, Benck AR, Nika A. A rare presentation of myositis and diffuse alveolar hemorrhage associated with disseminated Cryptococcus neoformans infection. Cureus 2023; 15:e42062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nishioka H, Takegawa H, Kamei H. Disseminated cryptococcosis in a patient taking tocilizumab for Castleman’s disease. J Infect Chemother 2018; 24:138–41. [DOI] [PubMed] [Google Scholar]
- 152. Khatib MY, Ahmed AA, Shaat SB, Mohamed AS, Nashwan AJ. Cryptococcemia in a patient with COVID-19: a case report. Clin Case Rep 2021; 9:853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Thota DR, Ray B, Hasan M, Sharma K. Cryptococcal meningoencephalitis during convalescence from severe COVID-19 pneumonia. Neurohospitalist 2022; 12:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tran DH, Verceles AC, Marciniak ET. Disseminated cryptococcosis in an immunocompromised patient with altered mental status and a lung nodule. J Community Hosp Intern Med Perspect 2023; 13:34–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2019; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 2016; 12:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 2002; 168:4620–7. [DOI] [PubMed] [Google Scholar]
- 158. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2016; 64:e1–33. [DOI] [PubMed] [Google Scholar]
- 159. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007; 45:807–25. [DOI] [PubMed] [Google Scholar]
- 160. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 2016; 63:e112–46. [DOI] [PubMed] [Google Scholar]
- 161. Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002; 301:418–26. [DOI] [PubMed] [Google Scholar]
- 162. Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis 2004; 39:1254–5. [DOI] [PubMed] [Google Scholar]
- 163. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004; 38:1261–5. [DOI] [PubMed] [Google Scholar]
- 164. Liao TL, Chen YM, Chen DY. Risk factors for cryptococcal infection among patients with rheumatoid arthritis receiving different immunosuppressive medications. Clin Microbiol Infect 2016; 22:815.e1–3. [DOI] [PubMed] [Google Scholar]
- 165. Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014; 371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015; 373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med 2015; 372:1430–40. [DOI] [PubMed] [Google Scholar]
- 168. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bartlett NL, Costello BA, LaPlant BR, et al. Single-agent ibrutinib in relapsed or refractory follicular lymphoma: a phase 2 consortium trial. Blood 2018; 131:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ruchlemer R, Ben-Ami R, Bar-Meir M, et al. Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: an observational study. Mycoses 2019; 62:1140–7. [DOI] [PubMed] [Google Scholar]
- 171. Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018; 131:1955–9. [DOI] [PubMed] [Google Scholar]
- 172. Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013; 122:2539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Blez D, Blaize M, Soussain C, et al. Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica 2020; 105:478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ferrarini I, Rigo A, Montresor A, Laudanna C, Vinante F. Monocyte-to-macrophage switch reversibly impaired by ibrutinib. Oncotarget 2019; 10:1943–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis 2018; 66:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Szymczak WA, Davis MJ, Lundy SK, Dufaud C, Olszewski M, Pirofski LA. X-linked immunodeficient mice exhibit enhanced susceptibility to Cryptococcus neoformans infection. mBio 2013; 4:e00265-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Messina JA, Giamberardino CD, Tenor JL, et al. Susceptibility to Cryptococcus neoformans infection with Bruton’s tyrosine kinase inhibition. Infect Immun 2023; 91:e0004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Maschmeyer G, De Greef J, Mellinghoff SC, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology: a position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia 2019; 33:844–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol 2021; 39:3441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2023; 388:319–32. [DOI] [PubMed] [Google Scholar]
- 181. Tam CS, Dimopoulos M, Garcia-Sanz R, et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv 2022; 6:1296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010; 33:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Francis G, Kappos L, O’Connor P, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler 2014; 20:471–80. [DOI] [PubMed] [Google Scholar]
- 184. Mazzola MA, Raheja R, Murugaiyan G, et al. Identification of a novel mechanism of action of fingolimod (FTY720) on human effector T cell function through TCF-1 upregulation. J Neuroinflammation 2015; 12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Del Poeta M, Ward BJ, Greenberg B, et al. Cryptococcal meningitis reported with fingolimod treatment: case series. Neurol Neuroimmunol Neuroinflamm 2022; 9:e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Grebenciucova E, Pruitt A. Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr Neurol Neurosci Rep 2017; 17:88. [DOI] [PubMed] [Google Scholar]
- 187. Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord 2021; 14:17562864211039648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Schwartz DM, Bonelli M, Gadina M, O’Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 2016; 12:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Wormley FL Jr. STAT1 signaling is essential for protection against Cryptococcus neoformans infection in mice. J Immunol 2014; 193:4060–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Chastain DB, Kung VM, Golpayegany S, et al. Cryptococcosis among hospitalised patients with COVID-19: a multicentre research network study. Mycoses 2022; 65:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007; 25:5616–23. [DOI] [PubMed] [Google Scholar]
- 192. Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene 2007; 26:3644–53. [DOI] [PubMed] [Google Scholar]
- 193. Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359:1786–801. [DOI] [PubMed] [Google Scholar]
- 194. Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood 2004; 103:2920–4. [DOI] [PubMed] [Google Scholar]
- 195. Hale G, Jacobs P, Wood L, et al. CD52 antibodies for prevention of graft-versus-host disease and graft rejection following transplantation of allogeneic peripheral blood stem cells. Bone Marrow Transplant 2000; 26:69–76. [DOI] [PubMed] [Google Scholar]
- 196. Lundin J, Porwit-MacDonald A, Rossmann ED, et al. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukaemia. Leukemia 2004; 18:484–90. [DOI] [PubMed] [Google Scholar]
- 197. Silveira FP, Husain S, Kwak EJ, et al. Cryptococcosis in liver and kidney transplant recipients receiving anti-thymocyte globulin or alemtuzumab. Transpl Infect Dis 2007; 9:22–7. [DOI] [PubMed] [Google Scholar]
- 198. Nath DS, Kandaswamy R, Gruessner R, Sutherland DE, Dunn DL, Humar A. Fungal infections in transplant recipients receiving alemtuzumab. Transplant Proc 2005; 37:934–6. [DOI] [PubMed] [Google Scholar]
- 199. Peleg AY, Husain S, Kwak EJ, et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis 2007; 44:204–12. [DOI] [PubMed] [Google Scholar]
- 200. Thursky KA, Worth LJ, Seymour JF, Miles Prince H, Slavin MA. Spectrum of infection, risk and recommendations for prophylaxis and screening among patients with lymphoproliferative disorders treated with alemtuzumab. Br J Haematol 2006; 132:3–12. [DOI] [PubMed] [Google Scholar]
- 201. Chastain DB, Spradlin M, Ahmad H, Henao-Martínez AF. Unintended consequences: risk of opportunistic infections associated with long-term glucocorticoid therapies in adults. Clin Infect Dis 2023; 78:e37–56. [DOI] [PubMed] [Google Scholar]
- 202. Pagano L, Fianchi L, Caramatti C, et al. Cryptococcosis in patients with hematologic malignancies: a report from GIMEMA-infection. Haematologica 2004; 89:852–6. [PubMed] [Google Scholar]
- 203. Madigan V, Smibert O, Chen S, Trubiano JA, Slavin MA, Teh BW. Cryptococcal infection in patients with haematological and solid organ malignancy in the era of targeted and immune-based therapies. Clin Microbiol Infect 2020; 26:519–21. [DOI] [PubMed] [Google Scholar]
- 204. Singh N, Dromer F, Perfect JR, Lortholary O. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis 2008; 47:1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chen HS, Tsai WP, Leu HS, Ho HH, Liou LB. Invasive fungal infection in systemic lupus erythematosus: an analysis of 15 cases and a literature review. Rheumatology (Oxford) 2006; 46:539–44. [DOI] [PubMed] [Google Scholar]
- 206. Marr KA, Sun Y, Spec A, et al. A multicenter, longitudinal cohort study of cryptococcosis in human immunodeficiency virus–negative people in the United States. Clin Infect Dis 2020; 70:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus–negative patients in the era of effective azole therapy. Clin Infect Dis 2001; 33:690–9. [DOI] [PubMed] [Google Scholar]
- 208. Namie H, Takazono T, Hidaka Y, et al. The prognostic factors for cryptococcal meningitis in non–human immunodeficiency virus patients: an observational study using nationwide database. Mycoses 2024; 67:e13658. [DOI] [PubMed] [Google Scholar]
- 209. Guan ST, Huang YS, Huang ST, Hsiao FY, Chen YC. The incidences and clinical outcomes of cryptococcosis in Taiwan: a nationwide, population-based study, 2002–2015. Med Mycol 2024; 62:myad125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis 2008; 27:937–43. [DOI] [PubMed] [Google Scholar]
- 211. Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 2010; 115:2989–97. [DOI] [PubMed] [Google Scholar]
- 212. He X, Smeets RL, Koenen HJPM, et al. Mycophenolic acid-mediated suppression of human CD4+ T cells: more than mere guanine nucleotide deprivation. Am J Transplant 2011; 11:439–49. [DOI] [PubMed] [Google Scholar]
- 213. Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol 2012; 12:325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.