Abstract
Background
This study aimed to assess the performance of currently available risk calculators in a cohort of patients with malignant peripheral nerve sheath tumors (MPNST) and to create an MPNST-specific prognostic model including type-specific predictors for overall survival (OS).
Methods
This is a retrospective multicenter cohort study of patients with MPNST from 11 secondary or tertiary centers in The Netherlands, Italy and the United States of America. All patients diagnosed with primary MPNST who underwent macroscopically complete surgical resection from 2000 to 2019 were included in this study. A multivariable Cox proportional hazard model for OS was estimated with prespecified predictors (age, grade, size, NF-1 status, triton status, depth, tumor location, and surgical margin). Model performance was assessed for the Sarculator and PERSARC calculators by examining discrimination (C-index) and calibration (calibration plots and observed-expected statistic; O/E-statistic). Internal–external cross-validation by different regions was performed to evaluate the generalizability of the model.
Results
A total of 507 patients with primary MPNSTs were included from 11 centers in 7 regions. During follow-up (median 8.7 years), 211 patients died. The C-index was 0.60 (95% CI 0.53–0.67) for both Sarculator and PERSARC. The MPNST-specific model had a pooled C-index of 0.69 (95%CI 0.65–0.73) at validation, with adequate discrimination and calibration across regions.
Conclusions
The MPNST-specific MONACO model can be used to predict 3-, 5-, and 10-year OS in patients with primary MPNST who underwent macroscopically complete surgical resection. Further validation may refine the model to inform patients and physicians on prognosis and support them in shared decision-making.
Keywords: internal–external validation, malignant peripheral nerve sheath tumors, model performance, neurofibromatosis 1, prognosis
Key Points.
The MPNST-specific MONACO tool predicts overall survival with a good performance.
This is the first prediction tool that incorporated MPNST-specific predictors.
The MONACO tool can predict 3-, 5-, and 10-year survival in resected primary MPNST.
Importance of the Study.
Sarculator and PERSARC are 2 well-known and well-performing generic prediction tools for survival in patients with soft tissue sarcoma. These tools, however, do not include type-specific predictors, such as neurofibromatosis type 1 and triton status for malignant peripheral nerve sheath tumors (MPNSTs). This study developed and internally–externally validated an MPNST-specific prediction tool, the MONACO tool, for overall survival. This is the first MPNST-specific prediction tool that incorporated MPNST-specific predictors. The MONACO tool can be used to predict 3-, 5- and 10-year overall survival in patients with resected primary MPNST and seems to outperform Sarculator and PERSARC. Further validation may enhance the model to inform patients and physicians on prognosis and support them in shared decision-making.
Prognostic tools are important instruments for clinical decision-making in soft tissue sarcomas (STS). STS is a heterogeneous group of malignant tumors with more than 100 different histological types that can affect patients of all age groups.1 Given the heterogeneity of prognosis within the STS spectrum, several classification systems have been developed to classify patients into different risk groups. Traditionally, the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system and the American Joint Committee of Cancer (AJCC) staging system were used to classify STS patients in different risk groups.2,3 However, in the last decade several new prognostic tools have been developed incorporating patient, tumor, and treatment characteristics that generate individual prognosis for patients with STS. Two widely used prognostic tools for STS of the extremities are Sarculator and PERSARC.4,5 These tools can be used for the most common histological types such as leiomyosarcoma, liposarcoma, undifferentiated pleomorphic sarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumors (MPNSTs). Although applicable to a wide range of STSs, these tools are limited to general predictors and do not incorporate type-specific prognostic factors.
MPNST is a rare and aggressive sarcoma type that accounts for 2–6% of all STS.6–8 While most STS arise de novo, MPNSTs can be associated with neurofibromatosis type 1 (NF-1).1 Approximately 30–50% of the MPNSTs are NF-1-associated.9,10 The NF1 gene is commonly affected in MPNSTs which causes loss-of-function of neurofibromin and inhibition of RAS oncogenes.11 Several studies have shown that NF-1 status is a negative predictor for overall survival (OS) and distant metastasis (DM).10,12 In addition, MPNSTs can present with partial rhabdomyoblastic differentiation (triton tumor) which appears to have a poorer prognosis compared with conventional MPNSTs.13
Considering that, in contrast to other STS types, MPNSTs can occur in patients with NF-1 and can present with partial rhabdomyoblastic differentiation, one may argue that the commonly used generic prognostic tools for STS, such as Sarculator and PERSARC,4,5 could be improved by MPNST-specific predictors such as NF-1 and triton status. As shown in a recent study, Sarculator is a good model for predicting survival in patients from the United States with resected STS of the extremities.14 However, the performance in patients with MPNSTs was poorer than in patients with other histological types.14 Furthermore, the Sarculator models were only built on patients of 18 years and older with a retroperitoneal or extremity STS and PERSARC was only built on patients of 18 years and older with high-grade extremity STS.4,5,15 While around 50% of the MPNSTs is located outside the extremities and retroperitoneum and approximately 10% of the patients is younger than 18 years old.16
The first aim of this study is to assess the performance of both Sarculator and PERSARC in an external cohort of MPNST patients. Furthermore, we extend and update these models by developing an MPNST-specific prognostic tool that can be used for a wider range of patients with primary MPNST.
Materials and Methods
Study Design
A retrospective multicenter international cohort study, the MPNST Oncological And Clinical Outcome Consortium (MONACO), was undertaken after approval of the institutional ethical review boards (IRB) of all included centers. IRB waived the need for informed consent. Patients from 11 secondary or tertiary centers diagnosed with histologically proven primary MPNST who were surgically treated with curative intent from 1 January 2000 to 31 December 2019 were included in this study. The following patients were excluded: patients with macroscopic residual disease (R2) after definitive surgery; patients with incorrectly registered time-to-event outcomes; patients with local recurrence (LR) who were previously resected elsewhere; patients with synchronous metastasis, defined as distant disease before date of definitive surgery. The list of participating centers is available in Appendix A.
Study Procedure
Clinical and pathological data were retrieved from medical records or from existing prospective sarcoma databases. All included centers adhere to the clinical guidelines of the European Society for Medical Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN) for STS.17,18 Follow-up usually consists of clinical examination and imaging (CT scan or X-ray of the chest and MRI for local control) once every 4 months for 2 years, every 6 months up to 5 years after definitive treatment, and thereafter yearly.
External Validation and Extension Approach
To validate and extend the prognostic models for MPNSTs, we undertook 3 steps. First, we validated the original PERSARC and Sarculator models for STS of the extremities (eSTS) in a subset of our cohort. This subset included all patients with primary high-grade (II/III) MPNST of the extremities. Model performance was assessed at 5 years from definitive surgery. Secondly, the original models were updated and extended by using the original predictors plus the MPNST-specific predictors. In this model, patients were included without eligibility restrictions on age, location, and grade. Finally, the extended model was internally–externally cross-validated across 7 regions. This means that each region was left out once while models were developed in the remaining 6 regions.19 As this split based on regions is not random, it qualifies as external validation.19 We used regions instead of centers to ensure a sufficient number of events within each split. A list of the specified regions is available in Appendix A.
Time-to-event was defined as the time interval between the date of definitive surgery (T = 0) and death from any cause. The outcomes of interest were OS at 3-, 5-, and 10-years.
The American Joint Committee on Cancer criteria were used for building a high-quality extended prognostic model.20 The Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement was followed for reporting the validation and extension of the prediction models (Appendix B provides TRIPOD checklist).21
Candidate Predictor Variables
The prognostic factors included in the model were prespecified based on Sarculator, PERSARC, and literature review for MPNST-specific predictors.10,12,13,16,22,23 The included predictors were: age, grade, size, NF-1 status, triton status, depth, tumor location, and surgical margin. All possible interaction terms with NF-1, location and triton, were considered clinically plausible.
Age was determined as age at the time of diagnosis. The grade was based on the FNCLCC grading criteria (grades I, II, and III).2 Tumor size was defined as the largest diameter (in cm) on imaging or based on pathology report if imaging was not available. A tumor was categorized as NF-1-associated by confirmed genetic testing of an NF1 mutation or by clinical evaluation.24 Triton status was extracted from pathological reports and was concluded either when stated as such in the report or when MPNST with rhabdomyoblastic differentiation was reported. Depth was assessed on imaging and categorized as superficial or deep in relation to the investing fascia. Tumor location was categorized as extremity (including plexus), central (including thorax, abdomen, pelvis, retroperitoneal, or head), and neck. Tumor margins were classified as negative (R0) or microscopically positive (R1) based on pathology reports. Macroscopically incomplete resections (R2) were excluded. The assessors of the predictors were inherently blinded for the outcome (death from any cause) due to the longitudinal nature of this study. OS was defined as the time interval between definitive surgery and the date of death or date of last follow-up.
Model Validation and Extension
No formal sample size calculation was performed. All available data were used to maximize the robustness of our analyses. We ensured that we had at least 10 events per parameter for modeling prespecified predictors in our full model.25,26 We determined the amount of optimism of the final model using bootstrap resampling (1000 replications).25,26 Shrinkage of regression coefficients was also estimated with this bootstrap validation procedure to improve predictions in future patients by preventing too extreme predictions due to overfitting.25
Multivariable Cox proportional hazard models were used for OS. The proportional hazard assumption was assessed visually with the Schoenfeld residuals. The possible nonlinearity of the continuous variables age and size was modeled using restricted cubic splines (4 knots) in initial univariate analyses. Subsequently, we used simple parametric transformations, based on visual assessment.25,26 The chosen transformation was based on visual inspection and supported by the Akaike information criterion (AIC), which penalizes for model complexity. The full model included all prespecified predictors, the selected parametric transformation for the continuous variables and the potential interaction terms. All clinically plausible interactions were tested using a global test followed by individual testing if the global test was significant.26 A P-value ≤ .20 was considered as a threshold for the selection of interaction terms.27
To make efficient use of the available data, multiple imputation by chained equations was used to fill in missing data for a completed data set.28 The variables included in the imputation model were: age, American Society of Anesthesiologists physical status (ASA) score, NF-1, prior radiotherapy on the same location, nerve type, tumor size, tumor depth, triton, grade, margins, radiotherapy, and chemotherapy (CTX). Furthermore, we included the event indicator and Nelson–Aalen estimator for the cumulative baseline hazard in the imputation model.28 Twenty imputed datasets were created as part of the multiple imputations (m = 20). Estimates from the imputed datasets were combined using Rubin’s rules. For 1 center, no information on grade, depth, and triton was available, as these variables were not included in their database. This center was only included as a validation cohort in the internal–external cross-validation procedure after the imputation of the systematically missing variables. It was not used for model development.
Model performance was assessed by examining discrimination and calibration. Discrimination was measured using the concordance index (C-index). Discrimination of a time-to-event model relates to how well the model could distinguish between patients with a shorter time-to-event from patients with a longer time-to-event. A C-index of 0.5 indicates that the model is no better than chance, whereas a C-index of 1 indicates perfect discrimination.29 Calibration was assessed with the Observed/Expected (O/E) statistic, and visually by plotting the predicted against the observed OS at 3-, 5-, and 10-years. The 45 °C line is a reference for perfect calibration.30
The clinical usefulness of the model was assessed by decision curve analysis (DCA).31 Clinical guidelines recommend to consider perioperative CTX in a selected group of patients based on risk-predicting tools such as the MPNST-specific model.17 For illustrative purposes a decision threshold for treatment with perioperative CTX was set at 34%, based on literature, to calculate the net benefit, sensitivity, and specificity of the prediction tool at this threshold.32 This threshold implies that we allow for overtreatment of approximately 2 patients (who would survive without additional treatment) per correctly treated patient (who would die without additional treatment) since a 1:2 ratio implies a probability threshold of 33%.
To provide individual predictions based on the updated model, a web-based tool was built and published on www.evidencio.com (MONACO prediction tool: Survival after resection of malignant peripheral nerve sheath tumors). An interactive tool in an Excel spreadsheet is available including all estimates to validate, update, and incorporate the predictors in existing or new tools.
Baseline characteristics were described with proportions for categorical variables and medians with interquartile ranges for continuous variables. Median follow-up was assessed with the reverse Kaplan–Meier method. 5-year OS stratified for baseline characteristics was estimated using the Kaplan–Meier method. All statistical tests were two-sided with a statistical significance level set at P ≤ .05. The 95% confidence interval (CI) of the O/E statistic was estimated using bootstrapping (B = 1000). All statistical analyses were performed in R (version 4.1.2) with the packages “mice,” “survival,” “boot,” “rms,” and “dcurves.”33
Results
Study Population
A total of 507 patients with primary MPNST surgically treated with curative intent were included in this study (Appendix C). Among them, 168 patients (33%) had NF-1 and 39 (10%) had a triton tumor. The median follow-up was 8.7 years. Baseline characteristics for the total population are presented in Table 1 and Appendix D.
Table 1.
Baseline Characteristics
| Variable | Overall (N = 507) | 5-yr OS (95% CI) |
|---|---|---|
| Age (years) | ||
| Median (IQR) | 43 (30–57) | <44: 69 (63–75) ≥44: 60 (54–67) |
| Neurofibromatosis type 1 | ||
| No | 336 (66.7%) | 67 (62–72) |
| Yes | 168 (33.3%) | 61 (53–69) |
| Missing | 3 | |
| Location | ||
| Central | 188 (37.1%) | 62 (55–70) |
| Extremity | 266 (52.5%) | 68 (62–75) |
| Head and neck | 53 (10.5%) | 59 (47–74) |
| Size (cm) | ||
| Median (IQR) | 7 (4-11) | <7: 75 (69–81) ≥7: 58 (51–65) |
| Missing | 59 | |
| Depth | ||
| Deep | 267 (70.4%) | 61 (55–67) |
| Superficial | 112 (29.6%) | 80 (72–88) |
| Missing | 128 | |
| Triton | ||
| No | 351 (90.0%) | 68 (63–73) |
| Yes | 39 (10.0%) | 54 (40–74) |
| Missing | 117 | |
| Grade (FNCLCC) | ||
| 1 | 66 (21.9%) | 92 (85–100) |
| 2 | 68 (22.6%) | 71 (61–84) |
| 3 | 167 (55.5%) | 60 (53–69) |
| Missing | 206 | |
| Surgical margin | ||
| R0 | 388 (76.5%) | 68 (62–73) |
| R1 | 119 (23.5%) | 54 (46–65) |
| Radiotherapy | ||
| Adjuvant | 169 (33.8%) | 58 (51–67) |
| Neoadjuvant | 99 (19.8%) | 62 (52–74) |
| No radiotherapy | 232 (46.4%) | 72 (66–78) |
| Missing | 7 | |
| Chemotherapy | ||
| Adjuvant | 31 (6.2%) | 66 (50–87) |
| Neoadjuvant | 89 (17.8%) | 64 (54–75) |
| No chemotherapy | 379 (76.0%) | 65 (60–70) |
| Missing | 8 | |
| Status | ||
| Dead | 211/507 | 65 (61–69) |
Abbreviations: FNCLCC: Fédération Nationale des Centres de Lutte Contre Le Cancer; IQR: interquartile range.
Validation of Sarculator and PERSARC
A subset of 207 patients, that met all the inclusion criteria of both Sarculator for eSTS and PERSARC, was considered to assess the performance of these prediction tools in an MPNST population (Appendix E). Figure 1 of Appendix F depicts the calibration performance (O/E-statistic) and discriminative ability (C-index) of both tools across regions. The C-index was 0.60 for both Sarculator and PERSARC. The predictions by Sarculator were slightly too high (O/E-statistic 0.81, 95%CI 0.71–0.91), and near perfect for PERSARC (O/E-statistic 0.95, 95%CI 0.83–1.05). The calibration plots are presented in Figure 2 of Appendix F.
Figure 1.
Calibration plot and distribution of the predictions based on the MONACO model at 5 years from definitive surgery.
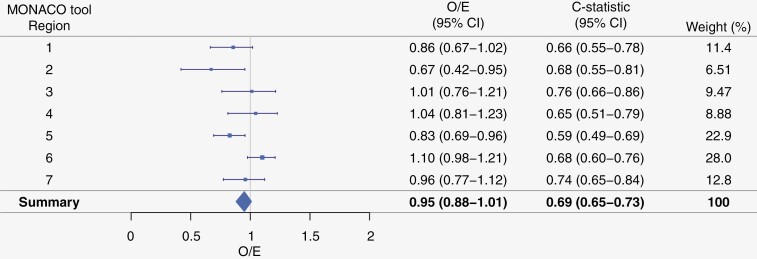
Figure 2.
Discrimination (c-statistic) and calibration (O/E-statistic) of the MONACO model in an internal–external cross-validation procedure across regions (see Appendix A).
Model Extension: The MONACO Tool
The final multivariable Cox model included all main effects, in which tumor size was square root transformed and age was modeled as a linear variable (Table 2). None of the prespecified interaction terms were statistically significant. All regression coefficients were multiplied by a shrinkage factor of 0.88 to account for overfitting in predictions. Appendix G depicts an overview of the model characteristics of the Sarculator, PERSARC, and MONACO tools, respectively.
Table 2.
Results of the Final MONACO Model Before and After Shrinkage (Factor = 0.88)
| HR (95% CI) | HR After Shrinkage | |
|---|---|---|
| Age (per 10 years) | 1.29 (1.17–1.42) | 1.25 |
| Size (per √1 cm) | 1.37 (1.10–1.71) | 1.32 |
| NF1 | ||
| No | 1 | 1 |
| Yes | 1.38 (0.95–2.02) | 1.33 |
| Location | ||
| Central | 1 | 1 |
| Extremity | 0.83 (0.58–1.19) | 0.85 |
| Head and neck | 1.69 (0.93–3.08) | 1.59 |
| Depth | ||
| Deep | 1 | 1 |
| Superficial | 0.49 (0.31–0.78) | 0.53 |
| Triton | ||
| No | 1 | 1 |
| Yes | 1.07 (0.64–1.80) | 1.06 |
| Grade | ||
| 1 | 1 | 1 |
| 2 | 1.63 (0.84–3.17) | 1.54 |
| 3 | 2.71 (1.50–4.90) | 2.39 |
| Margin | ||
| R0 | 1 | 1 |
| R1 | 1.89 (1.32–2.69) | 1.74 |
Model Performance of the MONACO Tool
The C-index for the final model was 0.73 (95% CI 0.69–0.77) and calibration at 5-year OS was adequate (Figure 1). The C-index for the 7 regions ranged from 0.59 to 0.76 with a pooled C-index of 0.69 (95%CI 0.65–0.73, Figure 2). The model was reasonably calibrated across the regions (Appendix H).
Clinical Applicability
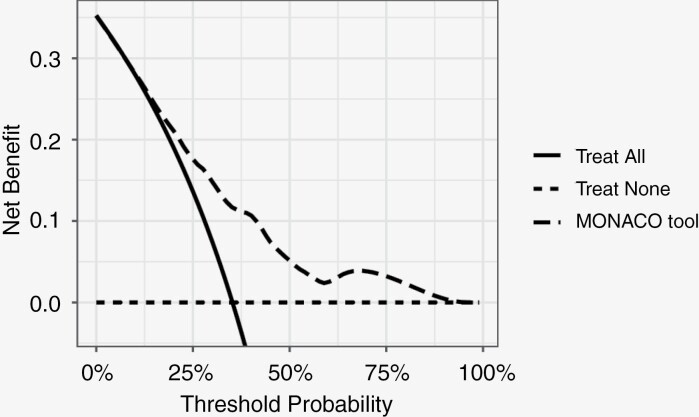
Figure 3 depicts the decision curve of the MONACO model for the total population. This figure illustrates that using the MONACO model is clinically useful if the decision-maker—physician and/or patient—would opt for an intervention if the 5-year risk of death is ≥10%. Applying a risk-based cut-off for perioperative CTX of 34% (5-year OS ≤ 66%) results in a net benefit of 0.12 when using the MPNST-specific MONACO model. This is a higher net benefit compared with treating all or none of the patients with perioperative CTX (Table 3). The net benefit represents the proportion of extra true positives while accounting for false positives, meaning that 12 patients would get CTX recommended and would otherwise die within 5 years, while zero patients would receive unnecessary CTX per 100 patients. At this risk threshold, the sensitivity and specificity of using the extended model were 61% (95% CI 53—68) and 73% (95% CI 68—78%), respectively.
Figure 3.
Decision curve analysis. The y-axis is the net benefit, which is the sum of true positives and a weighted number of false positives. The x-axis is the preference of the patient or physician. The unit of preference is the 5-year probability of death from any cause. The lines represent the different treatment strategies: treating all patients (solid line), treating none (dotted line), or using the MONACO prediction tool to decide which patients to treat or not to treat, with the cutoff for treatment at the threshold probability (dashed line). Preference refers to how one values the harms and benefits of a certain intervention or treatment. This may vary from patient to patient or physician to physician. For example, 1 physician would only want to treat patients with a certain treatment, taking the harms and benefits of the treatment into account, if the patients’ 5-year risk of death is more than 33%. The threshold probability of physician’s preference is then 33%, implying that the overtreatment of 2 patients (unnecessary perioperative chemotherapy) is worth 1 necessary treatment. At this threshold probability, the use of the MONACO model results in a higher net benefit than treating all or none of the patients with the certain treatment.
Table 3.
Calculation of Net Benefit for Different Treatment Strategies
| Strategy | True Positives: Patients Treated with CTX Who Would Otherwise Die Within 5 Years | False Positives: Patients Treated with CTX Who Will Not Die Within 5 Years | Net Benefit |
|---|---|---|---|
| Treat all with CTX | 178 | 329 | 0.02 |
| Treat none with CTX | 0 | 0 | 0 |
| Treat with CTX if 5-year mortality ≥ 34% according to MONACO | 108 | 88 | 0.12 |
Abbreviation: CTX: perioperative chemotherapy.
Discussion
The present study validated and extended existing personalized risk assessment tools for a wider range of patients with MPNSTs based on type-specific predictors. This MPNST-specific model, the MONACO tool, calculates the 3-, 5- and 10-year survival in patients with primary MPNST who underwent macroscopically complete surgical resection with curative intent. This is the first study that assessed the performance of existing generic prognostic tools in an MPNST population and updated the models with type-specific predictors. All estimates have been published online to validate, update, or incorporate the estimated predictors in existing or future prediction tools.
Several prediction models have been developed for patients with primary STS. Most of the externally validated models were built for all histological types and did not include type-specific predictors.4,5,34,35 In this study we assessed the performance of well-known Sarculator and PERSARC calculators in a multicenter cohort of patients with MPNST. Both had a comparable moderate discriminative ability and comparable calibration performance.
As ±50% of the MPNSTs are located outside the extremities and retroperitoneum and±10% of the patients are younger than 18 years, the Sarculator and PERSARC tools may not be applicable for a large proportion of patients with MPNST. In addition, MPNSTs differ from other STS as they are associated with NF-1 and rhabdomyoblastic differentiation, which are common MPNST-specific negative predictors for OS.10,12,13,16,23 By extending the existing models the c-statistic improved from 0.60 for both Sarculator and PERSARC to around 0.70 at external validation.19 Reassessment of the generic predictors and assessment of the MPNST-specific predictors allowed us to further improve the ability to predict survival in patients with MPNST. However, there are several other prognostic markers that could further improve our model, while aiming for the right balance between the prognostic ability of the model and its clinical usability. A recent systematic review provided an overview of all published prognostic molecular and immunohistochemical markers.36 In addition, there are several international initiatives for multi-omics characterization of MPNSTs that could further improve our prognostic performance.37 With this study we intended to initiate an MPNST-specific prognostic model that could be further extended, updated and recalibrated together with the research community. Through Evidencio (MONACO prediction tool: Survival after resection of malignant peripheral nerve sheath tumors), each institution could validate (and recalibrate) the MONACO prediction tool for its own MPNST population.
To our knowledge, only 1 model has previously been developed specifically for patients with MPNSTs.38 However, this nomogram did not include MPNST-specific predictors and important generic predictors such as tumor size and grade. Furthermore, this nomogram was built based on the Surveillance, Epidemiology, and End Results (SEER) database including patients with MPNST diagnosed in 1973. This is an important limitation since treatment and prognosis could be different at that time. In addition, this study included patients with distant disease at the time of presentation.38
Strengths and Limitations
An important strength of the present study is that it is based on large cohorts of patients with MPNST including MPNST-specific predictors. The inclusion of patients from multiple centers allowed for the assessment of performance across a spectrum of settings.19 Other strengths are the easily determinable predictors included in the MONACO model. In addition to being used to obtain personalized survival probabilities and to inform patients and physicians about prognosis for shared decision-making, the MONACO tool can also be used in research settings to adjust for confounders or to assess heterogeneity in treatment effects based on prognosis.39
In this paper, the clinical usefulness of the MONACO tool was illustrated with a decision curve, which is a relatively novel approach to performance assessment (Figure 3). The MONACO prediction tool can have a positive impact on decision-making on perioperative CTX as illustrated by a decision threshold for perioperative CTX of 34% (5-year OS of ≤66%).32 The decision threshold of 34% implies that the benefit of perioperative CTX for a patient who would otherwise die is approximately worth the harm of 2 unnecessary treatments of patients who would survive without perioperative CTX. Obviously, the decision threshold may vary from patient to patient and from physician to physician. The MONACO tool has a positive net benefit across a wide range of possible thresholds, in particular between 25% and 60%.
This study has some limitations. One region did not record data on tumor depth, grade, and triton status. Therefore, we included this region only for validation of the MONACO model. In addition, no information on disease-specific death was available. Also, owing to the retrospective nature of this study loss to follow-up is an important limitation. Longer follow-up would further clarify the prognosis after 5 years. Furthermore, no central pathology review was performed. Although this resembles clinical practice, we recognize that diagnosing MPNST can be challenging due to the lack of specific histologic criteria and overlapping morphologic features with other types of nerve sheath tumors.40 Histologic evaluation sometimes requires correlation with clinical and radiological findings in order to classify a tumor as MPNST. Due to these diagnostic challenges, some MPNSTs might have been misclassified. In line with improved histologic criteria and advances in (molecular) pathology in the last decades, we have restricted our inclusion period from 2000 onwards, to minimize this misclassification bias. In addition, no complete case analysis could be performed to examine important differences between the results based on multiple imputations and the complete case dataset. However, an important advantage of multiple imputations, under the missing at-random assumption, lies in its ability to address biases that can arise in complete case analyses.41
Finally, prediction tools should ideally be updated to improve local validity.42 As reflected in the internal–external cross-validation, model performance differs to some extent across regions.25 In this study, we did not yet update the model with setting-specific estimates. Through Evidencio, one could recalibrate the MONACO prediction tool for a specific population of patients with MPNST.
In conclusion, the survival of patients with primary MPNST surgically treated with curative intent can be predicted by a simple tool including MPNST-specific predictors. The MONACO tool may benefit from further validation and is applicable for a wider range of patients with MPNST compared with the existing generic STS prediction tools. All estimates have been published online to validate, update, or incorporate the estimated predictors in existing or future prediction tools.
Supplementary Material
Contributor Information
Ibtissam Acem, Department of Surgical Oncology and Gastrointestinal Surgery,Erasmus MC Cancer Institute, >Rotterdam, The Netherlands; Department of Orthopedic Oncology, Leiden University Medical Centre, >Leiden, The Netherlands.
Ewout W Steyerberg, Department of Biomedical Data Sciences, Leiden University Medical Centre, >Leiden, The Netherlands.
Marta Spreafico, Department of Medical Statistics, Mathematical Institute, Leiden University, >Leiden, The Netherlands.
Dirk J Grünhagen, Department of Surgical Oncology and Gastrointestinal Surgery,Erasmus MC Cancer Institute, >Rotterdam, The Netherlands.
Dario Callegaro, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, >Milan, Italy.
Robert J Spinner, Department of Neurosurgery, Mayo Clinic, >Rochester, Minnesota, USA.
Courtney Pendleton, Department of Neurosurgery, Stony Brook University School of Medicine, Stony Brook, New York, USA.
J Henk Coert, Department of Reconstructive Surgery, University Medical Centre Utrecht, >Utrecht, The Netherlands.
Rosalba Miceli, Department of Clinical Epidemiology and Trial Organization, Fondazione IRCCS Istituto Nazionale dei Tumori, >Milan, Italy.
Giulia Abruzzese, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, >Milan, Italy.
Uta E Flucke, Department of Pathology, Radboud University Medical Centre, >Nijmegen, The Netherlands.
Willem-Bart M Slooff, Department of Neurosurgery, University Medical Centre Utrecht, >Utrecht, The Netherlands.
Thijs van Dalen, Department of Surgical Oncology and Gastrointestinal Surgery,Erasmus MC Cancer Institute, >Rotterdam, The Netherlands.
Lukas B Been, Department of Surgical Oncology, University Medical Centre Groningen, Groningen, The Netherlands.
Han J Bonenkamp, Department of Surgical Oncology, Radboud University Medical Centre, >Nijmegen, The Netherlands.
Monique H M E Anten, Department of Neurology, Maastricht University Medical Centre, >Maastricht, The Netherlands.
Martinus P G Broen, Department of Neurology, Maastricht University Medical Centre, >Maastricht, The Netherlands.
Marc H A Bemelmans, Department of Surgical Oncology, Maastricht University Medical Centre, >Maastricht, The Netherlands.
Jos A M Bramer, Department of Orthopedic Surgery, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Gerard R Schaap, Department of Orthopedic Surgery, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Arthur J Kievit, Department of Orthopedic Surgery, Amsterdam University Medical Centre, Amsterdam, The Netherlands.
Jos van der Hage, Department of Surgical Oncology, Leiden University Medical Centre, Leiden, The Netherlands.
Winan J van Houdt, Department of Surgical Oncology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Michiel A J van de Sande, Department of Orthopedic Oncology, Leiden University Medical Centre, >Leiden, The Netherlands.
Alessandro Gronchi, Department of Surgery, Fondazione IRCCS Istituto Nazionale dei Tumori, >Milan, Italy.
Cornelis Verhoef, Department of Surgical Oncology and Gastrointestinal Surgery,Erasmus MC Cancer Institute, >Rotterdam, The Netherlands.
Enrico Martin, Department of Reconstructive Surgery, University Medical Centre Utrecht, >Utrecht, The Netherlands.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
No author has any form of disclosure.
Authorship statement
I.A. (Conceptualization, Methodology, Formal analysis, Writing—Original Draft, Project administration, Visualization); E.W.S. (Conceptualization, Methodology, Formal analysis, Validation, Writing—Original Draft, Visualization); M.S. (Conceptualization, Methodology, Formal analysis, Validation, Writing—Original Draft, Visualization); D.J.G. (Conceptualization, Writing—Review & Editing, Resources, Supervision); D.C. (Conceptualization, Methodology, Writing—Review & Editing, Resources); R.J.S. (Conceptualization, Writing—Review & Editing, Resources); C.P. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); J.H.C. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); R.M. (Conceptualization, Methodology, Formal analysis, Validation); G.A. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); U.E.F. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); W.-B.M.S. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); T.D. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); L.B.B. (Conceptualization, Writing—Review & Editing, Resources); H.J.B. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); M.H.M.E.A. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); M.P.G.B. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); M.H.A.B. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); J.A.M.B. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); G.R.S. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); A.J.K. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); J.H. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); W.J.H. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); M.A.J.S. (Conceptualization, Writing—Review & Editing, Resources, Data Curation, Supervision); A.G. (Conceptualization, Writing—Review & Editing, Resources, Data Curation); E.M. (Conceptualization, Methodology, Validation, Writing—Original Draft, Project administration, Resources, Data Curation, Visualization, Supervision); C.V. (Writing—Review & Editing, Resources, Data Curation, Supervision).
Data availability
Deidentified patient data used for this study will be made available upon reasonable request.
References
- 1. WHO Classification of Tumours: Soft Tissue and Bone Tumours. Vol. 3. 5th ed.Lyon, France: International Agency for Research on Cancer; 2020. [Google Scholar]
- 2. Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33(1):37–42. [DOI] [PubMed] [Google Scholar]
- 3. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. Chicago: Springer; 2017. [Google Scholar]
- 4. Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671–680. [DOI] [PubMed] [Google Scholar]
- 5. van Praag VM, Rueten-Budde AJ, Jeys LM, et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: personalised sarcoma care (PERSARC). Eur J Cancer. 2017;83:313–323. [DOI] [PubMed] [Google Scholar]
- 6. Brennan MF, Antonescu CR, Moraco N, Singer S.. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416–21; discussion 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stiller CA, Trama A, Serraino D, et al. ; RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49(3):684–695. [DOI] [PubMed] [Google Scholar]
- 8. Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97(10):2530–2543. [DOI] [PubMed] [Google Scholar]
- 9. Watson KL, Al Sannaa GA, Kivlin CM, et al. Patterns of recurrence and survival in sporadic, neurofibromatosis Type 1-associated, and radiation-associated malignant peripheral nerve sheath tumors. J Neurosurg. 2017;126(1):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miao R, Wang H, Jacobson A, et al. Radiation-induced and neurofibromatosis-associated malignant peripheral nerve sheath tumors (MPNST) have worse outcomes than sporadic MPNST. Radiother Oncol. 2019;137:61–70. [DOI] [PubMed] [Google Scholar]
- 11. Basu TN, Gutmann DH, Fletcher JA, et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356(6371):713–715. [DOI] [PubMed] [Google Scholar]
- 12. Acem I, Martin E, van Houdt WJ, van de Sande MAJ, Grünhagen DJ, Verhoef C, Monaco Collaborators. The association of metastasis pattern and management of metastatic disease with oncological outcomes in patients with malignant peripheral nerve sheath tumors: a multicenter cohort study. Cancers (Basel). 2021;13(20):5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamran SC, Howard SA, Shinagare AB, et al. Malignant peripheral nerve sheath tumors: prognostic impact of rhabdomyoblastic differentiation (malignant triton tumors), neurofibromatosis 1 status and location. Eur J Surg Oncol. 2013;39(1):46–52. [DOI] [PubMed] [Google Scholar]
- 14. Voss RK, Callegaro D, Chiang YJ, et al. Sarculator is a good model to predict survival in resected extremity and trunk sarcomas in US patients. Ann Surg Oncol. 2022;29:4376–4385. [DOI] [PubMed] [Google Scholar]
- 15. Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31(13):1649–1655. [DOI] [PubMed] [Google Scholar]
- 16. Martin E, Coert JH, Flucke UE, et al. A nationwide cohort study on treatment and survival in patients with malignant peripheral nerve sheath tumours. Eur J Cancer. 2020;124:77–87. [DOI] [PubMed] [Google Scholar]
- 17. Gronchi A, Miah AB, Dei Tos AP, et al. ; ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(11):1348–1365. [DOI] [PubMed] [Google Scholar]
- 18. Von Mehren M, Kane JM, Agulnik M, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–563. [DOI] [PubMed] [Google Scholar]
- 19. Steyerberg EW, HarrellFE, Jr. Prediction models need appropriate internal, internal–external, and external validation. J Clin Epidemiol. 2016;69:245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kattan MW, Hess KR, Amin MB, et al. ; members of the AJCC Precision Medicine Core. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin. 2016;66(5):370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins GS, Reitsma JB, Altman DG, Moons KG.. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68(2):134–143. [DOI] [PubMed] [Google Scholar]
- 22. Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107(5):1065–1074. [DOI] [PubMed] [Google Scholar]
- 23. Martin E, Coert JH, Flucke UE, et al. Neurofibromatosis-associated malignant peripheral nerve sheath tumors in children have a worse prognosis: a nationwide cohort study. Pediatr Blood Cancer. 2020;67(4):e28138–e28138. [DOI] [PubMed] [Google Scholar]
- 24. National Institutes of Health Consensus Development Conference. Neurofibromatosis: conference Statement. Arch Neurol. 1988;45(5):575–578. [PubMed] [Google Scholar]
- 25. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer International Publishing; 2019. [Google Scholar]
- 26. Harrell FE. Regression Modeling Strategies. 1st ed.New York: Springer New York, NY; 2001. [Google Scholar]
- 27. Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19(5):640–648. [DOI] [PubMed] [Google Scholar]
- 28. Van Buuren S. Flexible Imputation of Missing Data. 2nd ed.New York: Chapman and Hall/CRC; 2018. [Google Scholar]
- 29. Harrell FE, Jr, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 30. McLernon DJ, Giardiello D, Van Calster B, et al. ; topic groups 6 and 8 of the STRATOS Initiative. Assessing performance and clinical usefulness in prediction models with survival outcomes: practical guidance for Cox proportional hazards models. Ann Intern Med. 2023;176(1):105–114. [DOI] [PubMed] [Google Scholar]
- 31. Vickers AJ, Elkin EB.. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Acem I, van Houdt WJ, Grünhagen DJ, et al. ; PERSARC research group. The role of perioperative chemotherapy in primary high-grade extremity soft tissue sarcoma: a risk-stratified analysis using PERSARC. Eur J Cancer. 2022;165:71–80. [DOI] [PubMed] [Google Scholar]
- 33. R: A Language and Environment for Statistical Computing [Computer Program]. Version 3.6.3. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 34. Kattan MW, Leung DH, Brennan MF.. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–796. [DOI] [PubMed] [Google Scholar]
- 35. Sampo M, Tarkkanen M, Tukiainen E, et al. A web-based prognostic tool for extremity and trunk wall soft tissue sarcomas and its external validation. Br J Cancer. 2012;106(6):1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martin E, Acem I, Grünhagen DJ, Bovée J, Verhoef C.. Prognostic significance of immunohistochemical markers and genetic alterations in malignant peripheral nerve sheath tumors: a systematic review. Front Oncol. 2020;10:594069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller DT, Cortés-Ciriano I, Pillay N, et al. Genomics of MPNST (GeM) consortium: rationale and study design for multi-omic characterization of NF1-associated and sporadic MPNSTs. Genes (Basel). 2020;11(4):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan P, Huang R, Hu P, et al. Nomograms for predicting the overall and cause-specific survival in patients with malignant peripheral nerve sheath tumor: a population-based study. J Neurooncol. 2019;143(3):495–503. [DOI] [PubMed] [Google Scholar]
- 39. Kent DM, Paulus JK, van Klaveren D, et al. The predictive approaches to treatment effect heterogeneity (PATH) statement. Ann Intern Med. 2020;172(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Le Guellec S, Decouvelaere A-V, Filleron T, et al. Malignant peripheral nerve sheath tumor is a challenging diagnosis: a systematic pathology review, immunohistochemistry, and molecular analysis in 160 patients from the French sarcoma group database. Am J Surg Pathol. 2016;40(7):896–908. [DOI] [PubMed] [Google Scholar]
- 41. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Binuya MAE, Engelhardt EG, Schats W, Schmidt MK, Steyerberg EW.. Methodological guidance for the evaluation and updating of clinical prediction models: a systematic review. BMC Med Res Methodol. 2022;22(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified patient data used for this study will be made available upon reasonable request.