Abstract
Objective
This study was based on MRI features and number of tumor-infiltrating CD8 + T cells in post-operative pathology, in predicting meningioma recurrence risk.
Methods
Clinical, pathological, and imaging data of 102 patients with surgically and pathologically confirmed meningiomas were retrospectively analyzed. Patients were divided into recurrence and non-recurrence groups based on follow-up. Tumor-infiltrating CD8 + T cells in tissue samples were quantitatively assessed with immunohistochemical staining. Apparent diffusion coefficient (ADC) histogram parameters from preoperative MRI were quantified in MaZda. Considering the high correlation between ADC histogram parameters, we only chose ADC histogram parameter that had the best predictive efficacy for COX regression analysis further. A visual nomogram was then constructed and the recurrence probability at 1- and 2-years was determined. Finally, subgroup analysis was performed with the nomogram.
Results
The risk factors for meningioma recurrence were ADCp1 (hazard ratio [HR] = 0.961, 95% confidence interval [95% CI]: 0.937 ~ 0.986, p = 0.002) and CD8 + T cells (HR = 0.026, 95%CI: 0.001 ~ 0.609, p = 0.023). The resultant nomogram had AUC values of 0.779 and 0.784 for 1- and 2-years predicted recurrence rates, respectively. The survival analysis revealed that patients with low CD8 + T cells counts or ADCp1 had higher recurrence rates than those with high CD8 + T cells counts or ADCp1. Subgroup analysis revealed that the AUC of nomogram for predicting 1-year and 2-year recurrence of WHO grade 1 and WHO grade 2 meningiomas was 0.872 (0.652) and 0.828 (0.751), respectively.
Conclusions
Preoperative ADC histogram parameters and tumor-infiltrating CD8 + T cells may be potential biomarkers in predicting meningioma recurrence risk.
Clinical relevance statement
The findings will improve prognostic accuracy for patients with meningioma and potentially allow for targeted treatment of individuals who have the recurrent form.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40644-024-00731-6.
Keywords: Meningioma, Histogram analysis, Magnetic resonance imaging, Recurrence, Tumor microenvironment
Key points
• Currently available methods for determining meningioma recurrence are inaccurate.
• CD8 + T cells counts successfully predicted meningioma recurrence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40644-024-00731-6.
Introduction
Meningiomas are common, slow-growing, primary tumors that originate in arachnoid capillary cells and account for 39.0% of all central nervous system (CNS) tumors [1]. Although more than 80% of meningiomas are benign, a subset become aggressive and are categorized by the World Health Organization (WHO) as grade 2 to 3 meningiomas. These are difficult to completely eradicate surgically, and patients experience early or multiple tumor recurrences. Treatments vary depending on meningioma grade. Grade 1 tumors are monitored, whereas grade 3 tumors require radiotherapy. The treatment for patients with grade 2 meningiomas is less universal; total resection remains controversial regardless of whether the procedure is accompanied by radiotherapy or observation [2]. Partially resected grade 2 tumors are treated with adjuvant radiotherapy. Unfortunately, the 10-year progression-free survival (PFS) rates for grades 1, 2, and 3 meningiomas are 75–90%, 23–78%, and 0%, respectively, reflecting the relative ineffectiveness of available treatments. Indeed, the development of systemic therapies (e.g., immunotherapy) for refractory meningioma is a major clinical challenge for postoperative management [3].
One clear obstacle is the difficulty in predicting meningioma recurrence. The tumor microenvironment (TME) consists of numerous cell types and a variable extracellular matrix that enhance tumor immune tolerance, directly influencing cancer progression and recurrence [4]. A previous study had shown that meningiomas have a large macrophage infiltrate [5]. Tumor-infiltrating lymphocytes (TILs) are important components of the meningioma TME and have a major effect on underlying tumor growth, proliferation, and overall prognosis [6]. In particular, CD8 + TILs exert antitumor effects [7], and their decrease in anaplastic meningiomas suggests the presence of an immunosuppressive tumor microenvironment.
Because meningiomas are intracranial, extracerebral tumors and totally located outside the blood-brain barrier, they are fed by both the meningeal and cerebral arteries. This access to circulating cells underlies their immune infiltrative characteristics [8]. A previous study showed that WHO meningioma grades are negatively correlated with the proportion of CD4+, CD8+, and PD-1 + lymphocytes [9]. One study demonstrated that CD8 + TIL levels correlate with meningioma recurrence and that grade 2–3 tumors are in a state of relative immunosuppression [3]. For immunotherapy to become a realistic therapeutic approach, TILs in meningiomas must be thoroughly characterized, but few studies are available with the necessary data.
Currently, meningioma is mainly diagnosed by radiologists through preoperative MRI. Conventional MRI features, such as tumor location and diameter were thought to be associated with meningioma recurrence [10, 11]. However, these qualitative analyses are limited in predicting meningioma recurrence/progression and risk stratification. In contrast, histogram analysis is a multiparameter image-processing tool that can reflect cellular heterogeneity and microstructural data via extracting first-order histogram features from within a region of interest (ROI) [12]. Apparent diffusion coefficient (ADC) histogram analysis is now widely used for CNS tumors and has excellent outcomes in meningioma grading, subtyping, and differential diagnosis [13, 14]. However, we have little data regarding whether ADC histogram parameters can predict meningioma recurrence preoperatively.
Therefore, this study aimed to investigate the prognostic value of preoperative MRI features (specifically ADC histogram parameters) and tumor-infiltrating CD8 + T cells in risk stratification for meningioma recurrence.
Materials and methods
Patients
This retrospective study was approved by the Ethics Review Committee of our institution (2023 A-169) and the need for informed consent was waived. Preoperative clinical, MRI, and postoperative pathological datas of patients with histopathologically confirmed diagnosis of meningioma from January 2019 to June 2022 in our hospital were retrospectively analysed. Inclusion criteria: (1) resection biopsy specimens were histopathologically confirmed to be meningiomas and immunohistochemical staining for CD8 was available; (2) all patients underwent meningioma resection 1 week after MRI; (3) complete clinical, imaging, and pathological data. Exclusion criteria: (1) patients received radiotherapy, targeted therapy, or other treatments before preoperative MRI scanning; (2) incomplete image sequences or failure to meet the diagnostic requirements; (3) patients with tumor diameter < 1 cm; (4) patients lost to follow-up; and (5) unable to obtain tissue specimens after surgical resection. Finally, a total of 102 patients with meningiomas were included in this study. The flow diagram of patient inclusion and exclusion as showed in Supplementary Material Fig S1.
Follow-up
The primary endpoint was tumor recurrence, defined as the time interval between initial diagnosis (surgery date) and recurrence date. All enrolled patients were followed-up with MRI scans until August 31, 2023. Recurrence was defined as a new lesion in the operated area or a > 10% increase in residual lesion volume on MRI T1C at any time during the follow-up period; otherwise, the patient was categorized as non-recurrence.
MRI scanning protocol
All enrolled patients underwent preoperative MRI examinations (T1-weighted image (T1WI), T2-weighted images (T2WI), DWI, and T1C), using a 3.0-T MRI Scanner (Siemens Verio, Erlangen, Germany) with an 8-channel head coil. The scanning parameters were as follows: (1) gradient echo sequences: T1WI ( repetition time [TR] = 550 ms, time to echo [TE] = 11 ms), slice thickness 5 mm, slice spacing 1.5 mm, field of view (FOV) 260 mm × 260 mm, and matrix 269 × 384; (2) turbo spin echo: T2WI (TR = 2200 ms, TE = 96 ms), echo time 10 ms, and two excitations, and matrix 256 × 256; (3) DWI: (TR = 4500 ms, TE = 102 ms), slice thickness 5 mm, slice spacing 1.5 mm, FOV 260 mm × 260 mm, and matrix 160 × 160, and b values of 0 and 1000 s/mm2; and (4) T1C: gadolinium diethyltriaminopentylacetate (0.1 mmol/kg) was injected through the elbow vein at a rate of 3 mL/s with the same scanning parameters as those for T1WI, and axial, coronal, and sagittal images were obtained.
Analysis of conventional MRI features
The conventional MRI features of all enrolled meningioma patients were independently evaluated by two radiologists (radiologists 1 and 2, with 10 and 8 years of experience in diagnostic neuroradiology, respectively) using a double-blind method to assess the following conventional MRI features: (1) tumor location: skull base, non-skull base; (2) necrosis: present, absent; (3) tumor enhancement: homogeneous, inhomogeneous; (4) tumor shape: round, lobulation; (5) tumor maximum diameter; (6) tumor volume (VT = 4πabc/3); (7) peritumour edema diameter; (8) peritumour edema volume (VE = VT2-high - VT); and (9) edema index (EI = (VE + VT) / VT). Two experienced neurosurgeons, unaware of the imaging datas, retrospectively analysed the Simpson grading of the patients who underwent meningioma resection from the PACS operative records.
ADC histogram analysis
Preoperative ADC images of all enrolled patients were obtained from DWI images after post-processing, and all ADC images were imported into MaZda software (version 4.6, Technical University of Lodz, Institute of Electronics, Łódz, Poland, http://www.eletel.p.lodz.pl/mazda/) after adjusting the window width and window position. Then the largest level of the tumor was selected on the axial ADC images, and the tumor boundary was determined by T1C images, and ROIs were manually sketched along the edges of the tumor on the axial ADC images by the two radiologists mentioned above. In order to better assess the heterogeneity of the tumor, ROIs included cystic and hemorrhage areas of the tumor, but excluded peritumoural edema areas. The sketched ROIs were filled in red and the following parameters were automatically generated: mean, variance, skewness, kurtosis, perc.01%, perc.10%, perc.50%, perc.90%, and perc.99%. Disagreements were resolved by negotiation when they occurred, and the final result was the average of two radiologist measurements.
Pathological analysis
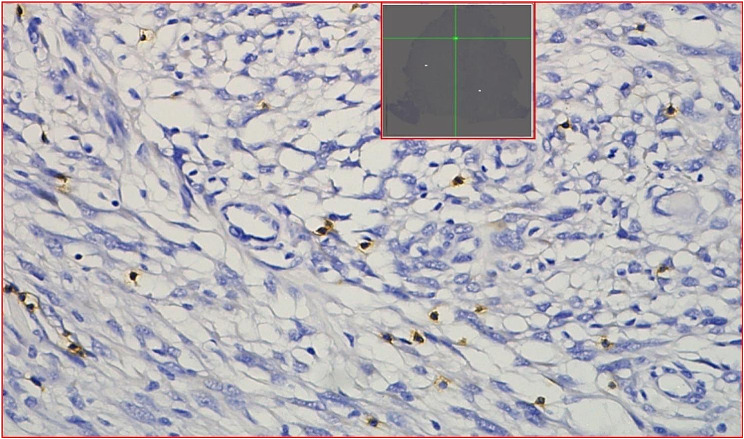
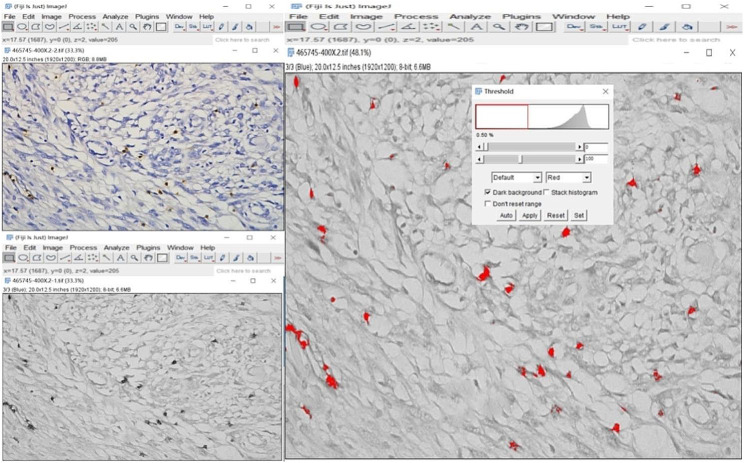
CD8 immunohistochemical staining were performed in 102 patients with meningioma after surgical resection. Paraffin specimens of postoperative meningiomas were cut into 4 μm sections. The CD8 antibody (RMA-0514, 1:100; Maixin) was used for immunohistochemical staining, revealing brownish-yellow cell membranes that suggested the positive surface expression of CD8 protein. All CD8-stained sections were scanned as whole-slide images (WSI) using the Motic EasyScanner digital section scanning system and viewed in DSAssistant software. For each CD8 WSI image, three different high-power fields (HPFs, 400× magnification) were randomly selected as ROIs. Segmented ROI images were analyzed by a neuropathologist in Image J software (version ImageJ2, USA). Final values for CD8 + T cells were the average of three ROIs and expressed as percentages (%), as shown in Figs. 1 and 2.
Fig. 1.
The WSI was checked by DSAssistant software, and 3 hot spots ROI images of CD8 + T cells IHC maps were randomly divided
Fig. 2.
The percentage of CD8 + T cells was obtained by CD8 immunohistochemical staining and gray level transformation and threshold adjustment
Immunohistochemical analysis of Ki-67 was performed using a monoclonal mouse anti-human Ki-67 antibody; brownish-yellow nuclei were considered Ki-67 positive. Regions with the highest concentration of Ki-67-positive cells (number of Ki-67 antibody positives per 1,000 tumor cells) were selected for evaluation. The Ki-67 proliferation index (PI) was calculated as the number of positive cells/total cell count.
Statistical analysis
MedCalc 19.1 (MedCalc, Mariakerke, Belgium) and R software (version 4.2.1; http://www.Rproject.org were used for statistical analysis. Categorical variables were expressed as number of cases (percentage) and were tested using the chi-square test or Fisher’s exact test. The Shapiro-Wilk test was used to test the normal distribution of continuous variables. Conventional MRI features and histogram parameters conforming to the normal distribution were expressed as mean ± standard deviation, and the differences between the groups were compared by the independent samples t-test. The non-normally distributed were expressed using the median ± quartile spacing, and the differences between the groups were compared by the Mann-Whitney U test. A multivariate COX proportional risk model was used to identify independent risk factors for meningioma recurrence and a nomogram was constructed, and validated the model to predict recurrence of WHO grade 1 and WHO grade 2 meningiomas. Recurrence-free survival curves were plotted using the Kaplan-Meier method, and differences between curves were analysed by log-rank tests. The probability of recurrence at 1-year and 2-years was assessed by plotting the receiver operating characteristic (ROC) curve. Interobserver agreement of conventional MRI characteristics and ADC histogram parameters was assessed using the intraclass correlation coefficient (ICC), with good interobserver agreement at ICC > 0.75. p < 0.05 was considered a statistically significant difference.
Results
Basic characteristics and conventional MRI features
The study included 102 patients with meningioma. The recurrence group contained 30 cases (11 men and 19 women) with a mean age of 48.03 ± 14.31 years. The non-recurrence group contained 72 cases (19 men and 53 women) with a mean age of 51.99 ± 13.24 years. The two groups differed significantly (p < 0.05) in the conventional MRI features of enhancement, grade, and Ki-67 PI, but not in basic demographic or clinical characteristics, including sex, age, location, necrosis, tumor shape, Simpson grade, postoperative radiotherapy, tumor diameter, tumor volume, edema diameter, edema volume, and edema index (p > 0.05), Table 1; Figs. 3 and 4.
Table 1.
The comparison of baseline characteristics, conventional MRI features, and CD8 + T cells in recurrence and non-recurrence meningiomas[ case (%)]
| Parameters | Recurrence(n = 30) | Non-recurrence(n = 72) | Statistical values | p-value |
|---|---|---|---|---|
| Age | 48.03 ± 14.31 | 51.99 ± 13.24 | 1.342 | 0.183 |
| Sex | 1.077 | 0.299 | ||
| Female | 19 (63.3%) | 53 (73.6%) | ||
| Male | 11 (36.7%) | 19 (26.4%) | ||
| Location | 0.413 | 0.521 | ||
| Skull base | 8 (26.7%) | 15 (20.8%) | ||
| Non skull base | 22 (73.3%) | 57 ( 79.2%) | ||
| Necrosis | 0.596 | 0.440 | ||
| Yes | 15 (50.0%) | 30 (41.7%) | ||
| No | 15 (50.0%) | 42 (58.3%) | ||
| Grade | 13.909 | 0.001 | ||
| WHO 1 | 12 (40.0%) | 54 (75.0%) | ||
| WHO 2 | 16 (53.3%) | 18 (25.0%) | ||
| WHO 3 | 2 (6.7%) | 0 (0.0%) | ||
| Enhancement | 4.870 | 0.027 | ||
| Homogeneous | 6 (20.0%) | 31 (43.1%) | ||
| Inhomogeneous | 24 (80.0%) | 41 (56.9%) | ||
| Tumor shape | 2.857 | 0.091 | ||
| Round | 12 (40.0%) | 42 (58.3%) | ||
| Lobulation | 18 (60.0%) | 30 ( 41.7%) | ||
| Tumor diameter | 4.24 ± 1.20 | 4.73 ± 1.27 | 1.798 | 0.075 |
| Tumor volume | 208.97 ± 253.11* | 269.69 ± 367.00* | −1.447 | 0.148 |
| Edema diameter | 0.00 ± 3.15* | 1.55 ± 3.80* | −0.912 | 0.362 |
| Edema volume | 48.52 ± 11.62* | 56.22 ± 12.11* | −1.013 | 0.311 |
| Edema index | 1.00 ± 1.80* | 1.00 ± 0.81* | −0.530 | 0.596 |
| Ki−67 PI | 5.00 ± 17.00* | 3.00 ± 5.75* | −2.621 | 0.009 |
| Simpson grade | 2.982 | 0.394 | ||
| Simpson I | 4 (13.3%) | 17 (23.6%) | ||
| Simpson II | 6 (20.0%) | 19 (26.4%) | ||
| Simpson III | 11 (36.7%) | 23 (31.9%) | ||
| Simpson IV | 9 (30.0%) | 13 (18.1%) | ||
| Postoperative therapy | 1.772 | 0.183 | ||
| Yes | 4 (13.3%) | 4 (5.6%) | ||
| No | 26 (86.7%) | 68 (94.4%) | ||
| CD8 + T cells (%) | 0.06 ± 0.13* | 0.17 ± 0.25* | −3.382 | 0.001 |
Continuous variables with a normal distribution are presented as mean ± standard deviation; non-normal variables are reported as median ± interquartile; number of cases (percentage) for categorical data
* non-normal distribution
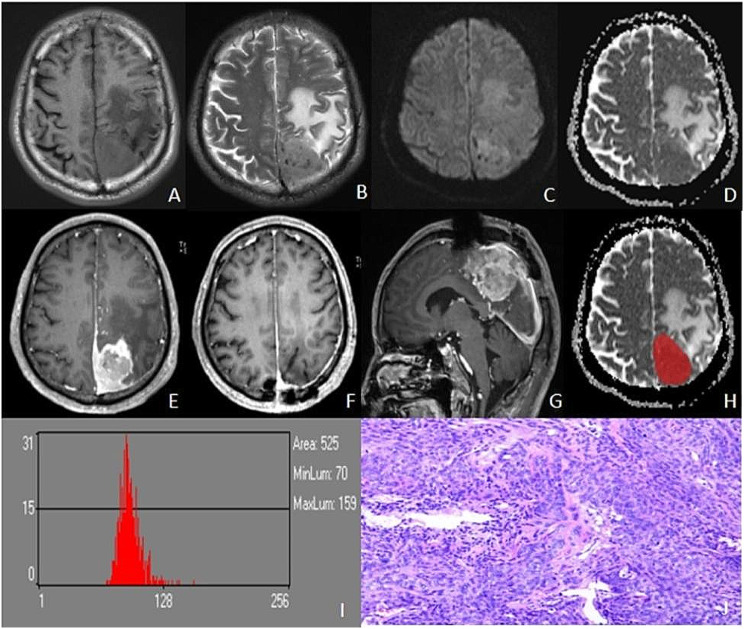
Fig. 3.
Male, 64 years old, left parietal atypical meningioma with recurrence 20 months after surgery. Irregularly shaped iso-T1WI (A) and iso-T2WIsignals in the left parietal, with peritumoural edema (B), slightly hyperintensity in DWI (C), and reduced signals in ADC (D). T1C showed significant inhomogeneous enhancement (E), and there was no recurrence at 3 months after resection (F) and 20 months tumor recurrence (G). Outlined ROI (H), ADC histogram parameters values were as follows: mean, 91.265; variance, 146.88; skewness, -1.3786; kurtosis, 14.874; ADCp1, 70; ADCp10, 81; ADCp50, 91; ADCp90, 103; ADCp99,130 (I). Pathology (HE×100): atypical meningioma, tumor invades brain tissue, multifocal map-like coagulative necrosis, some areas of tightly arranged cells, increased nucleoplasmic proportion of cells, dark staining, pseudo daisy-shaped cluster structure arranged around blood vessels, KI-67% (10–30%) (J)
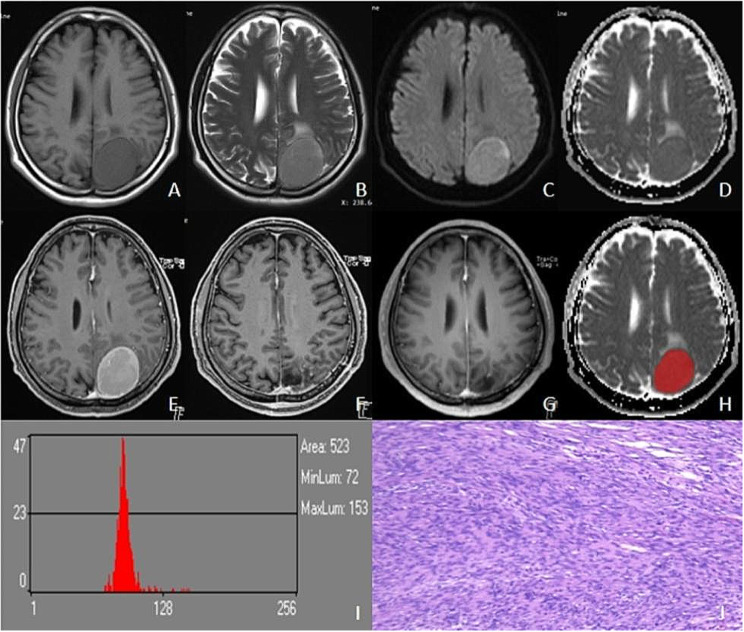
Fig. 4.
Male, 52 years old, left parieto-occipital fibroblastic meningioma with no recurrence till 28 months. The left parieto-occipital region showed a round iso T1WI (A) and slightly longer T2WI signal (B), with signal homogeneity, slightly hyperintensity on DWI (C), and iso-signal on ADC (D). T1C showed significant homogeneous enhancement (E), and there was no recurrence at 3-month (F) and 28-month postoperative review (G). Outlined ROI (H), ADC histogram parameters values were as follows: mean, 94.072; variance, 320.36; skewness, 4.7907; kurtosis, 30.683; ADCp1, 75; ADCp10, 83; ADCp50, 91; ADCp90, 103; ADCp99,184 (I). Pathology (HE×100): fibroblastic meningioma KI-67% was 2% (J)
ADC histogram parameters
The mean, variance, skewness, kurtosis, and all percentile values showed excellent inter-reader agreement (ICC, 0.801–0.951). Differences in ADCp1, ADCp10, ADCp50, ADCp90, and ADCp99 were significant between the two groups, with the recurrence group having lower values (p < 0.05), and we found that ADCp1 had the best predictive efficacy for COX regression analysis further, Table 2.
Table 2.
The comparison of ADC histogram parameters in recurrence and non-recurrence meningiomas [ case (%)]
| Parameters | Recurrence(n = 30) | Non-recurrence (n = 72) | Statistical values | p-value |
|---|---|---|---|---|
| Mean | 97.94 ± 24.44* | 101.73 ± 23.35* | −1.439 | 0.150 |
| Variance | 137.68 ± 151.99* | 131.94 ± 180.98* | −0.481 | 0.630 |
| Skewness | 0.86 ± 1.83* | 1.26 ± 1.54* | −1.061 | 0.289 |
| Kurtosis | 2.49 ± 7.68* | 3.08 ± 8.84* | −0.738 | 0.460 |
| ADCp1 | 76.00 ± 14.50* | 86.00 ± 17.00* | −4.016 | <0.001 |
| ADCp10 | 87.00 ± 14.50* | 94.00 ± 20.75* | −3.130 | 0.002 |
| ADCp50 | 97.50 ± 14.50* | 106.00 ± 24.75* | −2.619 | 0.009 |
| ADCp90 | 110.50 ± 31.25* | 125.00 ± 29.50* | −2.167 | 0.030 |
| ADCp99 | 131.50 ± 45.00* | 150.00 ± 40.00* | −2.163 | 0.031 |
Continuous variables with a normal distribution are presented as mean ± standard deviation; non-normal variables are reported as median ± interquartile; number of cases (percentage) for categorical data
* non-normal distribution
Tumor-infiltrating CD8 + T cells
Tumor-infiltrating CD8 + T cells were lowly expressed in meningiomas overall. However, the recurrence group contained significantly (p = 0.001) fewer CD8 + T cells (0.06 ± 0.13)% than the non-recurrence group (0.17 ± 0.25)%, as shown in Table 1.
Univariate and multivariate COX proportional risk predictions
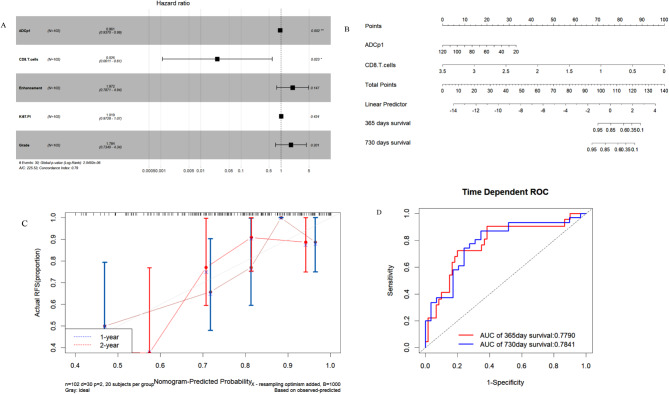
Univariate COX regression revealed that enhancement, grade, Ki-67 PI, ADCp1, and CD8 + T cell levels were significant predictors of meningioma recurrence. Multivariate COX regression further demonstrated that ADCp1 (hazard ratio [HR] = 0.961, 95%CI = 0.937 ~ 0.986, p = 0.002) and CD8 + T cells (HR = 0.026, 95%CI = 0.001 ~ 0.609, p = 0.023) were independent risk predictors influencing recurrence (Table 3; Fig. 5A). We constructed a COX proportional hazards nomogram to predict the probability of recurrence-free survival (RFS) among patients with meningioma (Fig. 5B). Calibration curves showed that model predictions were similar to actual observations (Fig. 5C). Additionally, model AUC values were 0.779 and 0.784 for predicting 1-year and 2-year postoperative RFS, respectively (Fig. 5D).
Table 3.
Univariate and multivariate logistic analysis of conventional MRI features, ADC histogram parameters, and CD8 + T cells in predicting meningiomas recurrence
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| Sex | 1.649 | 0.778 ~ 3.493 | 0.192 | |||
| Age | 0.985 | 0.959 ~ 1.012 | 0.266 | |||
| Location | 0.877 | 0.389 ~ 1.977 | 0.751 | |||
| Necrosis | 1.464 | 0.725 ~ 2.956 | 0.288 | |||
| Grade | 3.318 | 1.769 ~ 6.224 | p<0.001 | 1.784 | 0.734 ~ 4.336 | 0.201 |
| Ki−67 PI | 1.042 | 1.007 ~ 1.079 | 0.019 | 1.019 | 0.973 ~ 1.068 | 0.424 |
| Enhancement | 2.555 | 1.043 ~ 6.262 | 0.040 | 1.972 | 0.787 ~ 4.943 | 0.147 |
| Tumor shape | 1.999 | 0.961 ~ 4.159 | 0.064 | |||
| Simpson grade | 1.374 | 0.954 ~ 1.980 | 0.088 | |||
| Postoperative therapy | 2.810 | 0.970 ~ 8.140 | 0.057 | |||
| Tumor diameter | 0.789 | 0.581 ~ 1.070 | 0.127 | |||
| Tumor volume | 0.999 | 0.997 ~ 1.001 | 0.181 | |||
| Edema diameter | 0.945 | 0.793 ~ 1.126 | 0.525 | |||
| Edema volume | 1.000 | 0.999 ~ 1.001 | 0.477 | |||
|
Edema Index |
1.122 | 0.982 ~ 1.283 | 0.090 | |||
| ADCp1 | 0.960 | 0.941 ~ 0.979 | p<0.001 | 0.961 | 0.937 ~ 0.986 | 0.002 |
| CD8 + T cells | 0.032 | 0.002 ~ 0.591 | 0.021 | 0.026 | 0.001 ~ 0.609 | 0.023 |
HR: Hazard ratio; CI: confidence interval
Fig. 5.
A: Two risk factors were screened out for constructing the nomogram. B: The nomogram was constructed based on Cox proportional hazards regression model, including ADCp1 and CD8 + T cells. C: The 1-year, 2-years calibration curves for the nomogram. D: The 1-year, 2-years ROC curves for the nomogram
Stratified survival analysis using recurrence predictors
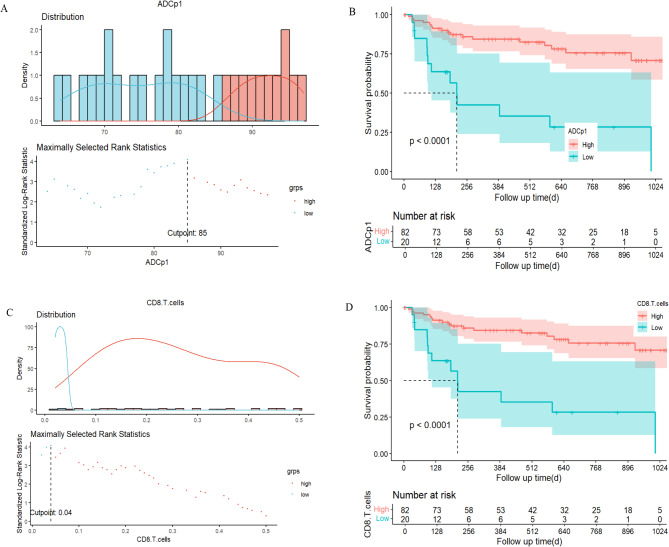
Kaplan-Meier analyses demonstrated that ADCp1 (log-rank test: p < 0.001) and CD8 + T cells (log-rank test: p < 0.001) were significantly correlated with RFS. Patients with low ADCp1 (ADCp1<85) or low CD8 + T cells (CD8 + T cells<0.04) had a higher RFS rate than patients with high ADCp1 or high CD8 + T cells (Fig. 6).
Fig. 6.
A, B: RFS in meningioma patients stratifed by ADCp1 = 85; C, D: RFS in meningioma patients stratifed by CD8 + T cells = 0.04%
Validation of the nomogram to predict recurrence of WHO grade 1 and WHO grade 2 meningiomas
Patients with WHO grade 1, 2, and 3 meningiomas were included in this study as 66, 34, and 2 patients respectively. To determine whether the nomogram worked well in different pathologically grade meningiomas, we performed a validation analysis of WHO grade 1 and WHO grade 2 meningiomas. The results revealed that the nomogram predicted the AUC of 1-year and 2-year recurrence for WHO grade 1 and WHO grade 2 meningiomas to be 0.872 (0.652) and 0.828 (0.751) respectively, Supplementary Material Fig S2.
Discussion
We developed a COX proportional risk prediction model for postoperative RFS in patients with meningioma. The model integrated two independent risk factors, ADCp1 and CD8 + T cells, to predict recurrence probability per patient after 1- and 2-years. The ROC curves, calibration curves, and Kaplan-Meier survival curves demonstrated that the model reliably predicted RFS in patients with meningioma, and the validity of the nomogram in WHO grade 1 and WHO grade 2 meningiomas has also been further validated. Thus, our model should provide guidance for postoperative management of patients with meningioma.
Previous studies have found that meningioma recurrence is often associated with pathological grade, KI-67 PI, tumor diameter, location, necrosis, and peritumoral edema [15–18]. Specifically, prognosis worsens with increasing pathological grade, as that indicates heightened tumor cell proliferation. Furthermore, Khanna et al. [19] used radiomics, a form of machine learning, to profile preoperative meningioma Ki-67 PI. The resultant predictions were successfully applied as a guide for determining the extent of surgical resection and improved prognosis. Similarly, here we demonstrated that tumor grade and Ki-67 PI were statistically significant in predicting meningioma recurrence, but not independent risk factors. However, our findings do not support the existing hypothesis regarding the relationship between tumor size and postoperative recurrence. This hypothesis states that a larger tumor diameter is correlated with greater probability of necrosis and edema, leading to an increase in compression severity on the surrounding brain tissue. As a result, surgical resection becomes more difficult, and the risk of postoperative recurrence rises. The discrepancy between the hypothesis and our results may be attributable to the relatively small sample size. In addition, meningiomas are blood-rich tumors, and heterogenous contrast enhancement, which may be a risk factor for predicting meningioma recurrence. The most likely explanation is that the heterogenous contrast enhancement may indicate the presence of populations of cells with different degrees of malignancy within the tumor, which may have different growth rates and aggressiveness, increasing the risk of recurrence.
In this study, we manually sketched ROIs based on maximum tumor level. Although this method loses some tumor information, it is far less cumbersome than whole-tumor histogram analysis and thus is more practical for clinical diagnosis in hospitals of all levels [20]. The results of comparing ADC histogram parameters revealed that they all differed significantly between recurrence and non-recurrence groups. This outcome is in line with histopathological data showing that high-grade meningiomas exhibit higher mitotic activity and increased nuclear/cytoplasmic ratios of tumor cells. These characteristics in turn lead to lower ADC values and poor prognosis. Moreover, variance is valuable for assessing tumor heterogeneity, as it mainly responds to the degree of data dispersion in histogram parameters [21]. Indeed, we observed that variance was slightly higher in the recurrence group than in the non-recurrence group.
Cytotoxic CD8 + T cells are the most abundant immune cells in the meningioma TME. High-grade meningiomas are immunologically “cold tumors,” with few CD3+, CD8+, and PD-1 + TILs compared with grade 1 tumors, but an elevated number of regulatory T cells [22]. The presence of CD3 + and CD8 + TILs in meningiomas have a positive effect on recurrence free survival, whereas a high percentage of PD1 + T cell infiltration is associated with shorter PFS [23]. Here, we confirmed that the number of CD8 + T cells was significantly lower in the recurrence group than in the non-recurrence group. Likewise, Zhang et al. [3] found that high levels of cytotoxic TILs were associated with improved PFS. Similarly, an association between high CD8 + TIL levels and improved RFS has been observed in patients with non-small cell lung cancer [24, 25] and hepatocellular carcinoma [26]. High CD8 + TIL density is also linked to better prognosis in melanoma [27] and colorectal cancer [28]. Taken together, our results and previous research illustrate the potential of CD8 + TILs as biomarkers for predicting meningioma recurrence.
Our findings suggest that ADCp1 and CD8 + T cells are independent risk factors for meningioma recurrence. This study is the first to integrate imaging, and TME data in a nonogram for predicting postoperative meningioma recurrence. Our model serves as an intuitive visual tool with demonstrable reliability and accuracy, while being an improvement to previous attempts. For example, a prior nomogram was built from clinical data to predict grade 2–3 meningiomas and measure meningioma prognosis [29, 30]. Similarly, Mo et al. [31] constructed a nomogram using independent predictors such as tumor size, Ki-67 index, and resection extent to stratify the recurrence risk of meningiomas. In contrast, we included all meningioma grades to generate a model with broader adaptations. Most importantly, our nomogram incorporated TME data on CD8 + T cells, better reflecting the heterogeneity of meningiomas and thus increasing predictive accuracy.
This study had several limitations. First, this was a single-center retrospective study with a small sample size. Our model must be validated with external data from prospective, large sample-size studies in the future. Secondly, because CD8 + T cells were included, we selected only tumor samples with the most recent paraffin sections, resulting in a shorter overall follow-up time for the patients enrolled. Recent studies have revealed that other TME factors (e.g., CD4 + T cells and PDL-1) are also strongly associated with meningioma recurrence. Thus, these variables should be included in future model-based research to further enhance nomogram predictive efficacy. Finally, for simplicity and feasibility, we chose ROIs based on maximum tumor level, potentially excluding portions of the tumor. Methods such as whole-tumor histogram analysis or machine learning should be explored in future studies to obtain a comprehensive view of the tumor.
Conclusion
In summary, ADCp1 and CD8 + T cells may be potential in vivo biomarkers for predicting meningioma recurrence, providing clinicians with a basis for guidance in personalised treatment and postoperative follow-up of meningioma patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- MRI
Magnetic resonance imaging
- ADC
Apparent diffusion coefficient
- WHO
World Health Organization
- CNS
Central Nervous System.
- DWI
Diffusion-weighted imaging
- TIL
Tumour infiltrating lymphocyte.
- PFS
Progression-free survival
- RFS
Recurrence-free survival
- ROI
Region of interest
- PI
Proliferation index
- ICC
Interclass correlation coefficient
- CI
Confidence intervals
- HR
Hazard ratio
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- T1C
Contrast enhanced T1-weighted imaging
- T2WI
T2-weighted imaging
- T1WI
T1-weighted imaging
- WSI
Whole Slide Image
- TME
Tumour microenvironment
Author contributions
Tao Han: Conceptualization, Methodology, Draft writting; Xianwang Liu: Investigation; Changyou Long: Formal analysis; Shenglin Li: Data processing, Resources; Fengyu Zhou: Data processing; Peng Zhang: Data processing; Bin Zhang: Writing – review; Mengyuan Jing: Formal analysis; Liangna Deng: editing; Yuting Zhang: Investigation; Junlin Zhou: Resources, article revision suggestions, supportive contribution, Project administration.
Funding
This study was supported by grants of Natural Science Foundation of China (No. 82071872, 82371914), Lanzhou University Second Hospital “Cuiying Technology Innovation Plan” Applied Basic Research Project (No. CY2018-QN09), Science and Technology Program of Gansu Province (21YF5FA123), China International Medical Foundation (Z-2014-07-2101), and National Science Foundation of Gansu Province (No. 21JR11RA105).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
This study was approved by the Medical Ethics Committee of the Second Hospital of Lanzhou University (approval number : 2023 A-169) and informed consent was waived.
Consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Han, Xianwang Liu, and Changyou Long contributed equally to this work.
References
- 1.Ostrom QT, Cioffi G, Waite K, et al. CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(12 Suppl 2):iii1–105. doi: 10.1093/neuonc/noab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldbrunner R, Stavrinou P, Jenkinson MD, et al. EANO guideline on the diagnosis and management of meningiomas. Neurooncology. 2021;23(11):1821–34. doi: 10.1093/neuonc/noab150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Wang X, Shi M, Song Y, Yu J, Han S. Programmed death ligand 1 and tumor-infiltrating CD8(+) T lymphocytes are associated with the clinical features in meningioma. BMC Cancer. 2022;22(1):1171. doi: 10.1186/s12885-022-10249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haslund-Vinding J, Møller JR, Ziebell M, Vilhardt F, Mathiesen T. The role of systemic inflammatory cells in meningiomas. Neurosurg Rev. 2022;45(2):1205–15. doi: 10.1007/s10143-021-01642-x. [DOI] [PubMed] [Google Scholar]
- 5.Rossi ML, Cruz Sanchez F, Hughes JT, Esiri MM, Coakham HB. Immunocytochemical study of the cellular immune response in meningiomas. J Clin Pathol. 1988;41(3):314–9. doi: 10.1136/jcp.41.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner CP, McLay J, Hermans IF, et al. Tumour infiltrating lymphocyte density differs by meningioma type and is associated with prognosis in atypical meningioma. Pathology. 2022;54(4):417–24. doi: 10.1016/j.pathol.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Butterfield LH. Cancer vaccines. BMJ (Clinical research ed.). 2015;350:h988. [DOI] [PMC free article] [PubMed]
- 8.Karimi S, Mansouri S, Nassiri F, et al. Clinical significance of checkpoint regulator programmed death ligand-1 (PD-L1) expression in meningioma: review of the current status. J Neurooncol. 2021;151(3):443–9. doi: 10.1007/s11060-020-03584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Z, Abedalthagafi M, Aizer AA, et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget. 2015;6(7):4704–16. doi: 10.18632/oncotarget.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Zheng D, Wang Y, et al. Decision-making tree for surgical treatment in meningioma: a geriatric cohort study. Neurosurg Rev. 2023;46(1):196. doi: 10.1007/s10143-023-02103-3. [DOI] [PubMed] [Google Scholar]
- 11.Spille DC, Adeli A, Sporns PB, et al. Predicting the risk of postoperative recurrence and high-grade histology in patients with intracranial meningiomas using routine preoperative MRI. Neurosurg Rev. 2021;44:1109–17. doi: 10.1007/s10143-020-01301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han T, Liu X, Sun J et al. T2-Weighted imaging and apparent diffusion coefficient Histogram parameters Predict Meningioma consistency. Acad Radiol 2023 Dec 27:S1076–6332(23)00689-X. [DOI] [PubMed]
- 13.Han T, Long C, Liu X, et al. Differential diagnosis of atypical and anaplastic meningiomas based on conventional MRI features and ADC histogram parameters using a logistic regression model nomogram. Neurosurg Rev. 2023;46(1):245. doi: 10.1007/s10143-023-02155-5. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Huang X, Han T et al. Discrimination between microcystic meningioma and atypical meningioma using whole-lesion apparent diffusion coefficient histogram analysis[J]. Clin Radiol. 2022. [DOI] [PubMed]
- 15.Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review) Mol Med Rep. 2015;11(3):1566–72. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 16.Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg. 2016;125(3):551–60. doi: 10.3171/2015.9.JNS15754. [DOI] [PubMed] [Google Scholar]
- 17.Haddad AF, Young JS, Kanungo I, et al. WHO Grade I Meningioma recurrence: identifying high risk patients using histopathological features and the MIB-1 index. Front Oncol. 2020;10:1522. doi: 10.3389/fonc.2020.01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender L, Somme F, Lhermitte B, et al. High risk of recurrence for grade II meningioma: a 10-year multicenter analysis of prognosis factors. Chin Clin Oncol. 2021;10(3):26. doi: 10.21037/cco-20-226. [DOI] [PubMed] [Google Scholar]
- 19.Khanna O, Fathi Kazerooni A, Arif S, et al. Radiomic signatures of meningiomas using the Ki-67 proliferation index as a prognostic marker of clinical outcomes. NeuroSurg Focus. 2023;54(6):E17. doi: 10.3171/2023.3.FOCUS2337. [DOI] [PubMed] [Google Scholar]
- 20.Xue C, Zhou Q, Zhang P, et al. MRI histogram analysis of tumor-infiltrating CD8 + T cell levels in patients with glioblastoma. NeuroImage. Clinical. 2023;37:103353. doi: 10.1016/j.nicl.2023.103353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han T, Liu X, Jing M et al. ADC histogram parameters differentiating atypical from transitional meningiomas: correlation with Ki-67 proliferation index. Acta radiologica (Stockholm, Sweden: 1987). 2023:2841851231205151. [DOI] [PubMed]
- 22.Rapp C, Dettling S, Liu F, et al. Cytotoxic T cells and their activation status are independent prognostic markers in Meningiomas. Clin cancer Research: Official J Am Association Cancer Res. 2019;25(17):5260–70. doi: 10.1158/1078-0432.CCR-19-0389. [DOI] [PubMed] [Google Scholar]
- 23.Li YD, Veliceasa D, Lamano JB, et al. Systemic and local immunosuppression in patients with high-grade meningiomas. Cancer Immunol Immunotherapy: CII. 2019;68(6):999–1009. doi: 10.1007/s00262-019-02342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang B, Liu R, Wang P, Yuan Z, Yang J, Xiong H, et al. CD8(+)CD57(+) T cells exhibit distinct features in human non-small cell lung cancer. J Immunother Cancer. 2020;8(1):e000639. doi: 10.1136/jitc-2020-000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SH, Go SI, Song DH, Park SW, Kim HR, Jang I, et al. Prognostic impact of CD8 and programmed death-ligand 1 expression in patients with resectable non-small cell lung cancer. Br J Cancer. 2019;120(5):547–54. doi: 10.1038/s41416-019-0398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J, et al. Clinicopathologic and prognostic significance of tumor-infiltrating CD8 + T cells in patients with hepatocellular carcinoma: a meta-analysis. Medicine. 2019;98(2):e13923. doi: 10.1097/MD.0000000000013923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–83. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 28.Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory t cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 29.Gao P, Kong T, Zhu X, et al. A clinical prognostic model based on preoperative hematological and clinical parameters predicts the progression of primary WHO grade II meningioma. Front Oncol. 2021;11:748586. doi: 10.3389/fonc.2021.748586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Z, Yan Y, Wang J, et al. Development and validation of prognostic nomogram in patients with WHO grade III meningioma: a retrospective cohort study based on SEER database. Front Oncol. 2021;11:719974. doi: 10.3389/fonc.2021.719974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mo G, Jiang Q, Bao Y, Deng T, Mo L, Huang Q. A Nomogram Model for stratifying the risk of recurrence in patients with Meningioma after surgery. World Neurosurg. 2023;176:e644–50. doi: 10.1016/j.wneu.2023.05.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.