Abstract
The availability of an influenza virus NS1 gene knockout virus (delNS1 virus) allowed us to establish the significance of the biological relationship between the influenza virus NS1 protein and double-stranded-RNA-activated protein kinase (PKR) in the life cycle and pathogenicity of influenza virus. Our results show that the lack of functional PKR permits the delNS1 virus to replicate in otherwise nonpermissive hosts, suggesting that the major function of the influenza virus NS1 protein is to counteract or prevent the PKR-mediated antiviral response.
The alpha/beta interferon (IFN-α/β)-induced cellular antiviral response is the first line of defense against a viral infection by the host (31). Major antiviral effectors induced by IFN include Mx (29, 30, 32), the 2′-5′ oligoadenylate synthetase (2, 8), and the double-stranded-RNA (dsRNA)-activated protein kinase (PKR) (25). PKR is a serine/threonine protein kinase, which dimerizes and autophosphorylates upon activation (for reviews, see references 3, 14, 19, and 35). The activated form of PKR is capable of blocking protein synthesis through its ability to phosphorylate the α subunit of eukaryotic translation initiation factor 2 (eIF-2α). This mechanism inhibits viral replication. To counteract the antiviral effects of IFN induction and PKR activation, many eukaryotic viruses have developed strategies to block the activity of PKR (for a review, see reference 11). In the case of influenza A virus, it is assumed that the virus can repress PKR activity by two mechanisms. One of these pathways is characterized by the virus recruiting P58IPK. This cellular protein was suggested to inhibit PKR by binding directly to the kinase (10, 23, 24). The second mechanism of PKR blockage during influenza virus infection involves the viral nonstructural protein NS1 (henceforth NS1). This protein effectively blocks the dsRNA-mediated activation of purified PKR and eIF-2α in vitro. Correspondingly, NS1 blocks the PKR-induced inhibition of translation in reticulocyte lysates. It was therefore postulated that NS1 sequesters dsRNA from activating PKR by binding to dsRNA (21). Other studies suggested that PKR inhibition is also mediated by an RNA-independent mechanism through the formation of complexes between the NS1 protein and PKR (34). However, the direct interaction of NS1 and PKR remains controversial (7). Interestingly, a temperature-sensitive influenza A virus mutant with mutations in the NS1 gene exhibited a defect in protein synthesis at the nonpermissive temperature that correlated with an increased level of phosphorylated PKR and eIF-2α (13).
We recently generated a viable influenza A/PR/8/34 transfectant virus that lacks the entire NS1 gene (termed the delNS1 virus). We showed that the delNS1 virus only replicated efficiently in host systems defective in IFN production or signaling but not in IFN-competent hosts (6, 12). This result demonstrated that the NS1 protein of influenza A virus is dispensable for viral growth in interferon-deficient systems. It also suggested that NS1 protein is a virulence factor that counteracts the interferon-mediated antiviral response. In this study, we have investigated the role of PKR in the IFN-mediated antiviral response in delNS1 virus-infected cells and mice. Analysis of infected cell lysates revealed that PKR phosphorylation was higher in delNS1 virus than in wild-type (wt) influenza virus A/PR/8/34 (PR8)-infected cells, suggesting that the NS1 protein prevents activation of PKR. Cells not permissive for delNS1 virus replication produced infectious particles when the infected cells were incubated with 2-aminopurine (2-AP) (15), a chemical inhibitor of PKR. To analyze the relevance of these observations at an organismic level, we determined the replication properties of delNS1 virus in mice devoid of PKR. While the delNS1 virus failed to replicate in the lungs of wt mice, it grew as efficiently as the PR8 wt virus in PKR knockout mice.
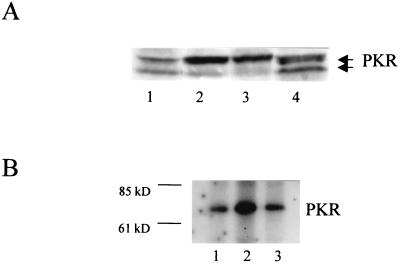
Previously, it was suggested that wt influenza virus is capable of repressing PKR phosphorylation in infected cells (17). If NS1 inhibits PKR activation, delNS1 virus-infected cells should contain higher levels of the activated autophosphorylated form of PKR than cells infected with the wt PR8 virus. Western blot analysis of cell extracts with a PKR-specific antibody allowed us to differentiate between the activated and the nonactivated forms of PKR. A 1:1 ratio of the two activation states was found between wt and mock-infected W138 cell extracts. In contrast, the infection with the delNS1 virus clearly shifted this balance to the activated form of PKR. This shift, initiated by delNS1 virus infection, was almost as pronounced as transfection of W138 cells with dsRNA, the established activator of PKR (Fig. 1A). We further demonstrated this increase of the activated form of PKR in delNS1-infected cells by immunoprecipitation of virus-infected and 32P-labeled cell extracts. This assay selectively detects the activated form of PKR. Again, infection with the delNS1 virus correlated with a higher amount of activated PKR than did mock or wt virus infection. The increase in the autophosphorylated form of PKR by the delNS1 virus was approximately two- to threefold higher than the baseline level (Fig. 1B). It should be noted that we could not do these assays at a high multiplicity of infection (MOI), since the delNS1 virus has a slightly attenuated phenotype. Thus, it is likely that the observed difference in PKR activation between delNS1 virus and PKR is a low estimate. However, the data support the hypothesis that the lack of the NS1 protein prevents the delNS1 virus from inhibiting PKR activation. In turn, this suggests that activation of PKR is at least partly responsible for the inability of the delNS1 virus to form infectious particles in IFN-competent systems.
FIG. 1.
(A) Western blot of PKR in infected W138 cells. Cells were mock treated, transfected with dsRNA, or infected with delNS1 or PR8 virus at an MOI of 2. For dsRNA transfection, 50 μg of poly(I)-poly(C) RNA was transfected using 30 μl of DOTAP transfection reagent according to the manufacturer's protocol (Boehringer, Mannheim, Germany). Twenty-four hours postinfection or posttransfection, respectively, cells were lysed, and equivalent amounts of cell extracts were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. PKR-specific bands were detected by the PKR-specific antibody K-17 (Santa Cruz Biotechnology catalogue no. sc 707; Santa Cruz, Calif.). The upper band corresponds to phosphorylated (active) PKR. The lower band corresponds to unphosphorylated (inactive) PKR (18). The two PKR bands are indicated at the right. Lane 1, mock; lane 2, dsRNA; lane 3, delNS1 virus; lane 4, PR8 virus. (B) Immunoprecipitation of phosphorylated PKR of infected HeLa cells. A total of 106 HeLa cells were mock treated or infected with influenza delNS1 or PR8 virus at an MOI of 0.5. At 5 h postinfection, the cells were washed with a phosphate-free buffer and incubated for 2 h in Dulbecco modified Eagle medium lacking both phosphate and pyruvate (Sigma), containing 500 μCi of [32P]orthophosphate (Amersham). After being labeled, the cells were washed twice with cold phosphate-buffered saline and 10 mM EDTA (without Ca2+ and Mg2+) and lysed for 10 min on ice in lysis buffer. One quarter of the extract was used for immunoprecipitation carried out with 2 μg of PKR antibody B-10 (Santa Cruz Biotechnology catalog no. sc 1215), per ml followed by the addition of 30 μl of protein G-agarose (in a 50/50 ratio) at 4°C. The beads were washed according to the manufacturer's protocol with wash buffer containing PBSTDS (Oncogene, Cambridge, Mass.), heated for 2 min at 95°C and analyzed on a sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis gel. The size of the bands was determined by a size marker (Benchmark, GIBCO-BRL). The bands of phosphorylated PKR were visualized by autoradiography for 7 days and quantified by laser densitometry. Lane 1, mock; lane 2, delNS1 virus; lane 3, PR8 virus. The size marker is indicated at the left. The PKR band is indicated at the right.
To further study this hypothesis, we attempted to rescue replication of delNS1 virus in nonpermissive cells by incubating the infected cells in the presence of 2-AP, an inhibitor of PKR and other serine/threonine protein kinases (15). In this experiment, we had to use a cell line that could withstand the required high doses of 2-AP. We therefore used the human melanoma cell line 518A2 (16), in which the PR8 virus grew to high titers of 5.3 log10 and which was not permissive for the delNS1 virus. Incubation of the infected cells in the presence of 5 mM 2-AP allowed the replication of the delNS1 virus to a titer of 2.6 log10 PFU/ml (data not shown). Although 2-AP is a nonspecific protein kinase inhibitor, this experiment supports the hypothesis that PKR is involved in blocking replication of the delNS1 virus in vivo.
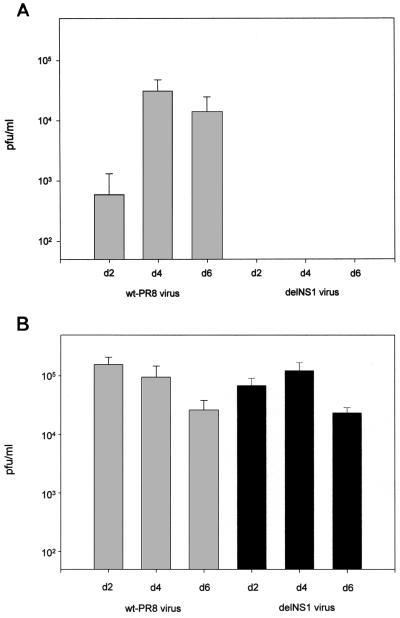
To address the relevance of the antiviral effects of PKR for influenza virus pathogenicity, we took advantage of the availability of PKR knockout (PKR−/−) mice. These mice were derived from C57BL/6 mice by the targeted deletion of PKR (36). C57BL/6 wt (PKR+/+) mice were obtained from Bomholtgard (Ry, Denmark). It should be noted that in untreated PKR-defective mice, the induction of IFN-α/β genes by virus is unimpaired and that antiviral responses appear to be normal (1, 36). Specifically, we analyzed the replication properties of delNS1 and PR8 viruses in mouse lungs. Figure 2 compares the replication properties of delNS1 virus in wt and PKR knockout mice to those of PR8 virus. The mean lung virus titers in wt mice after PR8 infection were 2.8 log10 PFU/ml on day 2, 4.5 log10 PFU/ml on day 4, and 4.1 log10 PFU/ml on day 6 postinfection. After delNS1 infection, the virus titers in lung tissue were less than 50 PFU/ml at each time point analyzed. The lack of detectable replication of delNS1 in wt mice supports the role of the NS1 gene in influenza virus pathogenicity. The mean virus titers in lung tissue in PKR−/− mice after delNS1 infection were 4.8 log10 PFU/ml on day 2, 5.1 log10 PFU/ml on day 4, and 4.4 log10 PFU/ml on day 6 postinfection. These titers were comparable to titers achieved in PKR−/− mice after PR8 virus infection. The latter were 5.2 log10 at day 2, 5.0 log10 at day 4, and 4.3 log10 at day 6 postinfection. The fact that infectious delNS1 virus can be recovered from the lungs of PKR−/− mice establishes the relevance of the PKR protein in mediating an antiviral response against the delNS1 virus. Since the lung virus titers in PKR−/− mice were generally similar for PR8 and delNS1 viruses, this result also suggests that one major function of NS1 protein in the influenza virus life cycle is to counteract the PKR-mediated antiviral response.
FIG. 2.
Titers of influenza PR8 and delNS1 virus in PKR+/+ and PKR−/− mice. Female mice at 7 to 9 weeks of age were used for infection with 105 PFU of wt PR8 or delNS1 virus. Virus was applied intranasally in a volume of 50 μl under ether anesthesia. To determine viral replication in the respiratory tracts, three mice of each group were sacrificed at day 2, 4, or 6 after inoculation. Lungs were removed and homogenized in 3 ml of phosphate-buffered saline. The quantity of virus in homogenates from each mouse was determined by titration on Vero cells. Virus titers are expressed as the number of PFU per milliliter of tissue extract. (A) Titers in PKR+/+ mice. (B) Titers in PKR−/− mice. The standard error of the mean is indicated.
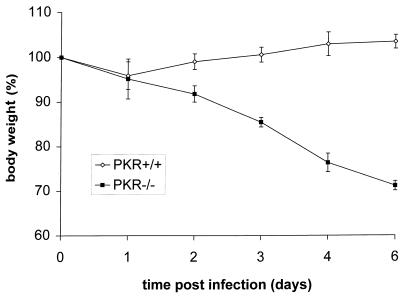
Infection of wt mice by delNS1 virus did not cause weight loss or symptoms of disease, such as ruffled fur. In contrast, approximately 30% weight loss was observed in delNS1 virus-infected PKR−/− mice 6 days after infection (Fig. 3). Correspondingly, all the PKR−/− mice died due to delNS1 virus infection, whereas the PKR+/+ mice survived challenge with this virus. These results reflect the replication data in the mouse lungs and confirm the importance of PKR in viral pathogenicity.
FIG. 3.
Body weights of PKR+/+ and PKR−/− mice which were infected with influenza delNS1 virus. Infection was carried out as described in the legend to Fig. 3. Each group comprised four mice. The standard error of the mean is indicated.
In order to gain information on the role of the PKR-mediated pathway in the IFN-induced antiviral response against influenza virus, we compared the virus titers in lung tissue of the delNS1 virus and PR8 wt virus achieved in the PKR knockout mice to those obtained in STAT1 knockout mice (5). STAT1 is necessary for any IFN-mediated signals, including the transcriptional activation of the PKR gene. In the STAT1 knockout model, the mean virus titer on day 3 postinfection was 4.9 ± 0.3 log10 PFU/ml for the delNS1 virus (n = 3) and 6.3 ± 0.3 log10 PFU/ml for the PR8 virus (n = 3). In wt mice, the PR8 titers were 5.0 ± 0.1 log10, and no infectious particles were recovered after delNS1 virus infection (data not shown). The data indicate that delNS1 virus replication reaches levels similar to those obtained in the PKR−/− knockout mice. This suggests that PKR is the major antiviral effector against influenza virus in the IFN pathway in our system. However, the wt PR8 virus levels are about 1 log unit higher in the STAT1−/− mice than in the PKR−/− mice, suggesting that other IFN-activated genes may also play a role in the antiviral host response. Since the IFN-inducible 2′-5′ oligoadenylate synthetase mRNA was shown to be induced in influenza virus-infected lung cells (30), RNase L might be a likely candidate. Triply deficient RNase L, PKR, and Mx knockout mice and cells which have been recently described (37) should be helpful in addressing this question. It should also be noted that C57BL/6 mice do not express functional Mx, another IFN-induced antiviral gene (32), which was shown to be a very potent antiviral defense. Thus, despite our data demonstrating the potency of PKR to inhibit viral replication, an exclusive role of PKR in the IFN-mediated pathway can certainly not be postulated.
Another important question is the role of the cellular PKR inhibitor P58IPK in influenza virus pathogenicity. Although the ability of P58IPK to inhibit PKR has been clearly shown in vitro and by overexpression in cultured cells, its role in an animal model has never been tested. One interpretation of our observations in the PKR knockout model in connection with P58IPK is that either recruitment of P58IPK by influenza virus plays a minor role in influenza virus pathogenicity in vivo or the NS1 protein is responsible for recruiting P58IPK. The replication properties of the delNS1 virus in the PKR knockout model allow us also to speculate about the in vivo relevance of other NS1 protein-associated functions established by in vitro studies, such as inhibition of host mRNA polyadenylation, mRNA nuclear export, and mRNA splicing (9, 20, 27, 28, 22). Since in the PKR−/− mice the absence of NS1-associated functions did not affect replication capacity in the mouse lungs, it appears that these functions are unlikely to play a major role in influenza virus replication in vivo. Alternatively, the functions ascribed to the NS1 may be redundant and could be taken over by other viral or cellular proteins.
A central role of PKR in the cellular antiviral defense strategies was also demonstrated during reovirus infection. Inhibition of PKR by 2-AP resulted in the growth of the virus in cell lines otherwise not permissive for reovirus replication. Interestingly, the growth of reovirus could also be observed when proteins of the ras signaling pathway, such as EGFR, v-erbB, sos, and ras were overexpressed in cell lines (33). This finding could be explained by the fact that activated ras induces an inhibitor of PKR (26). The potential of reovirus as an oncolytic virus specifically replicating in oncogenic ras-positive tumor cells could be confirmed in a mouse tumor model (4). Since the delNS1 virus has a phenotype similar to that of reovirus with respect to PKR-dependent growth, the delNS1 virus might also be an oncolytic virus that specifically eradicates tumors expressing oncogenic ras. Experiments to test this hypothesis are in progress.
Acknowledgments
This work was supported by Austrian Science Fund grant MOB-12548 (T.M.), the Niarchos Foundation (K.W.), and grants from the National Institutes of Health to A.G.-S. and P.P.
PKR knockout mice were kindly provided by Charles Weissmann from the University of Zürich. We thank Reinhard Fleischhacker and Ingrid Romirer for technical assistance.
REFERENCES
- 1.Abraham N, Stojdl D F, Duncan P I, Methot N, Ishii T, Dube M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 2.Carrol S S, Chen E, Viscount T, Geib J, Sardana M K, Gehman J, Kuo L C. Cleavage of oligoribonucleotides by the 2′-5′-oligoadenylate-dependent ribonuclease L. J Biol Chem. 1996;271:4988–4992. doi: 10.1074/jbc.271.9.4988. [DOI] [PubMed] [Google Scholar]
- 3.Clemens M J, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 4.Coffey M C, Strong J E, Forsyth P A, Lee P W K. Reovirus therapy of tumors with activated ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 5.Durbin J E, Hackenmiller R, Simon M, Levy D E. Targeted disruption of the mouse STAT1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 6.Egorov A, Brandt S, Sereinig S, Romanova J, Ferko B, Katinger D, Grassauer A, Alexandrova G, Katinger H, Muster T. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J Virol. 1998;72:6437–6441. doi: 10.1128/jvi.72.8.6437-6441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcon A M, Fortes P, Marion R M, Beloso A, Ortin J. Interaction of influenza virus NS1 protein and the human homologue of Staufen in vivo and in vitro. Nucleic Acids Res. 1999;27:2241–2247. doi: 10.1093/nar/27.11.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floyd-Smith G, Slattery E, Lengyel P. Interferon action: RNA cleavage pattern of a (2′-5′) oligoadenylate-dependent endonuclease. Science. 1981;212:1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- 9.Fortes P, Beloso A, Ortin J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 1994;13:704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale M, Blakely C M, Hopkins D A, Melville M W, Wambach M, Romano P R, Katze M G. Regulation of interferon-induced protein kinase PKR: modulation of P58IPK inhibitory function by a novel protein, P52rIPK. Mol Cell Biol. 1998;18:859–871. doi: 10.1128/mcb.18.2.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gale M, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 13.Hatada E, Saito S, Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovanessian A G. The double-stranded RNA-activated protein kinase induced by interferon: dsRNA-PK. J Interferon Res. 1989;9:641–647. doi: 10.1089/jir.1989.9.641. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Conway T W. 2-Aminopurine inhibits the double-stranded RNA-dependent protein kinase both in vitro and in vivo. J Interferon Res. 1993;13:323–328. doi: 10.1089/jir.1993.13.323. [DOI] [PubMed] [Google Scholar]
- 16.Jansen B, Schlagbauer-Wadl H, Eichler H-G, Wolff K, van Elsas A, Schrier P I, Pehamberger H. Activated N-ras contributes to the chemoresistence of human melanoma in severe combined immunodeficiency (SCID) mice by blocking apoptosis. Cancer Res. 1997;57:362–365. [PubMed] [Google Scholar]
- 17.Katze M G, Tomita J, Black T, Krug R M, Safer B, Hovanessian A. Influenza virus regulates protein synthesis during infection by repressing autophosphorylation and activity of the cellular 68,000-Mr protein kinase. J Virol. 1988;62:3710–3717. doi: 10.1128/jvi.62.10.3710-3717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Yang Y, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R G. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lengyel P. Tumor-suppressor genes: news about the interferon connection. Proc Natl Acad Sci USA. 1993;90:5893–5895. doi: 10.1073/pnas.90.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Qian X Y, Krug R M. The influenza virus NS1 protein: a novel inhibitor of pre-mRNA splicing. Genes Dev. 1994;8:1817–1828. doi: 10.1101/gad.8.15.1817. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Wambach M, Katze M G, Krug R M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology. 1995;214:222–228. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- 22.Marion R M, Aragon T, Beloso A, Nieto A, Ortin J. The N-terminal half of the influenza virus NS1 protein is sufficient for nuclear retention of mRNA and enhancement of viral mRNA translation. Nucleic Acids Res. 1997;25:4271–4277. doi: 10.1093/nar/25.21.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melville M W, Hansen W J, Freeman B C, Welch W J, Katze M. The molecular chaperone hsp40 regulates the activity of p58IPK, the cellular inhibitor of PKR. Proc Natl Acad Sci USA. 1997;94:97–102. doi: 10.1073/pnas.94.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melville M W, Tan S L, Wambach M, Song J, Morimoto R I, Katze M G. The cellular inhibitor of the PKR protein kinase, P58(IPK), is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J Biol Chem. 1999;274:3797–3803. doi: 10.1074/jbc.274.6.3797. [DOI] [PubMed] [Google Scholar]
- 25.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 26.Mundschau L J, Faller D V. Oncogenic ras induces an inhibitor of double-stranded RNA-dependent eukaryotic initiation factor 2 alpha-kinase activation. J Biol Chem. 1992;267:23092–23098. [PubMed] [Google Scholar]
- 27.Qiu Y, Krug R M. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A) J Virol. 1994;68:2425–2432. doi: 10.1128/jvi.68.4.2425-2432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu Y, Nemeroff M, Krug R M. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6-U2 and U6-U4 snRNA interactions during splicing. RNA. 1995;1:304–316. [PMC free article] [PubMed] [Google Scholar]
- 29.Ronni T S, Sareneva T, Pirhonen J, Julkunen I. Activation of IFN-alpha, IFN-gamma, MxA, and IFN regulatory factor 1 genes in influenza A virus-infected human peripheral blood mononuclear cells. J Immunol. 1994;154:2764–2774. [PubMed] [Google Scholar]
- 30.Ronni T, Matikainen S, Sareneva T, Melen K, Pirhonen J, Keskinen P, Julkunen I. Regulation of IFN-alpha/beta, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human epithelial cells. J Immunol. 1997;158:2363–2374. [PubMed] [Google Scholar]
- 31.Samuel C E. Antiviral actions of interferon-regulated proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 32.Staeheli P, Grob R, Meier E, Sutcliffe J G, Haller O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol. 1988;8:4518–4523. doi: 10.1128/mcb.8.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strong J E, Coffey M C, Tang D, Sabinin P, Lee P W K. The molecular basis of viral oncolysis: usurpation of the ras signaling pathway by reovirus. EMBO J. 1998;12:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan S-L, Katze M. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J Interferon Cytokine Res. 1998;18:757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 35.Williams B R. PKR: a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y-L, Reis L F L, Pavlovic J, Aguzzi A, Schäfer R, Kumar A, Williams B R G, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou A, Paranjape J M, Der S D, Williams B R, Silverman R H. Interferon action in triply deficient mice reveals the existence of alternative pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]