Abstract
Rta, the gene product of Kaposi's sarcoma-associated herpesvirus (KSHV) encoded mainly in open reading frame 50 (ORF50), is capable of activating expression of viral lytic cycle genes. What was not demonstrated in previous studies was whether KSHV Rta was competent to initiate the entire viral lytic life cycle including lytic viral DNA replication, late-gene expression with appropriate kinetics, and virus release. In HH-B2, a newly established primary effusion lymphoma (PEL) cell line, KSHV ORF50 behaved as an immediate-early gene and autostimulated its own expression. Expression of late genes, ORF65, and K8.1 induced by KSHV Rta was eliminated by phosphonoacetic acid, an inhibitor of viral DNA polymerase. Transfection of KSHV Rta increased the production of encapsidated DNase-resistant viral DNA from HH-B2 cells. Thus, introduction of an ORF50 expression plasmid is sufficient to drive the lytic cycle to completion in cultured PEL cells.
The switch from latency to lytic cycle viral gene expression of Kaposi's sarcoma-associated herpesvirus (KSHV) is an important component of the pathogenesis of Kaposi's sarcoma (KS). Sera of patients with KS contain high titers of antibodies to both latent and lytic cycle viral products (1, 8, 9, 15, 17, 22, 27, 35). Although the majority of tumor cells are latently infected, some spindle cells and monocytic cells in the KS lesions express lytic cycle products, including chemokines and proinflammatory cytokines encoded by KSHV (4, 24, 25, 36, 37, 41). The molecular mechanism of KSHV lytic cycle activation can be analyzed in cultured lymphoid cells derived from primary effusion lymphoma (PEL), another AIDS-associated malignancy (2, 5, 6, 12, 20, 21). The kinetic class of KSHV genes has been determined in two biologically distinct PEL cell lines, BC-1 and BCBL1 (6, 21, 22, 31, 34, 41, 44). Five viral genes are expressed in PEL cells during latency (10). These are the latent nuclear antigen, LANA (open reading frame 73 [ORF73]) (3, 11, 30), Kaposin A (K12), and K15 (which are transmembrane proteins) (10, 23, 26, 33, 36, 44), vFLIP (ORF72) (42), and K1, an integral membrane protein (13, 14, 28). Following addition of stimuli that disrupt latency in PEL cells, an orderly progression of expression of viral lytic cycle genes ensues (34, 41). Four immediate-early genes have been identified (45); of these, the only gene with a known function is ORF50, which encodes a transcriptional activator (18, 40), provisionally designated KSHV ORF50/Rta (replication and transcription activator).
When plasmids that constitutively express ORF50 are introduced into the BC-1 or BCBL-1 PEL cell lines, they activate expression of early viral lytic cycle genes, such as the abundant polyadenylated nuclear RNA (PAN) (39, 43), viral interleukin 6 (IL-6), K8, and ORF59 (7), as well as late genes, such as sVCA (ORF65) and K8.1 (15, 17). The effects of KSHV ORF50/Rta are specific: (i) when ORF50 is cloned in reverse orientation, or in a vector lacking a strong eukaryotic promoter, the KSHV lytic cycle is not activated; (ii) a mutant of ORF50 with a stop codon at amino acid (aa) 134 likewise does not activate the KSHV lytic cycle; and (iii) in BC-1 cells that are dually infected with KSHV and Epstein-Barr virus (EBV), KSHV ORF50 protein activates KSHV lytic gene expression but not EBV lytic gene expression; conversely, EBV Rta does not activate KSHV lytic gene expression (40). Recently, a C-terminal truncation mutant of KSHV Rta has been shown exert a dominant-negative phenotype (19).
The present group of experiments addresses several questions that remained unanswered in earlier functional studies of the ORF50 protein (18, 19, 40). Although transcripts of a late gene, ORF65, were previously shown to be activated by ORF50/Rta, it was not determined whether they were activated directly by the ORF50 product, bypassing a requirement for DNA synthesis, or were stimulated with appropriate kinetics consequent to the ability of ORF50 to activate DNA replication (29). Previous studies did not address the ability of ORF50/Rta to induce lytic viral DNA replication or to promote release of encapsidated viral DNA. The difficulties in answering these questions were related to the cell-virus systems under study. BCBL-1 cells have a high background of lytic cycle replication against which it is difficult to assess the effects of ORF50 overexpression. Moreover, gene transfer into many PEL cell lines is relatively inefficient, again making it difficult to score the effects of ORF50/Rta. Even after lytic cycle activation by tetradecanoyl phorbol acetate (TPA) or n-butyrate, BC-1 cells did not release significant amounts of KSHV DNA, thus making it impossible to determine whether ORF50/Rta promoted viral release (21). This report describes the biologic effects downstream of the KSHV ORF50/Rta activator in HH-B2, a newly isolated PEL cell line which overcomes some of these obstacles.
Characterization of the HH-B2 PEL cell line.
In a suitable cell-virus system in which to investigate whether the ORF50 protein could activate the entire KSHV lytic cycle, there should be a low rate of spontaneous entry into the lytic cycle, KSHV should be inducible to produce virions, and dual infection with EBV should not present a confounding factor. A new variant PEL cell line, HH-B2, established from the pleural fluid of a patient with AIDS by coculture with autologous peripheral blood mononuclear cells, met these requirements. The HH-B2 cell line contained KSHV DNA but not EBV DNA. There was little spontaneous late gene expression, but HH-B2 could be induced by treatment with TPA to express a late protein, sVCA, the product of ORF65. Furthermore, TPA treatment led to the release of readily detectable amounts of DNase-resistant KSHV DNA from the HH-B2 cell line (data not shown).
When the HH-B2 PEL cell line was surveyed for markers that are found on the surfaces of lymphocytes, monocytes, dendritic cells, natural killer cells, and macrophages, only two markers were represented on the majority of cells, namely CD45, a marker for human lymphocytes, and CD38, an activation marker usually found on human T cells or plasma cells (Table 1). Many fewer HH-B2 cells expressed CD2 (a T-cell marker), CD23 (an activation marker found on many different cell types), and HLA-DR (a class II major histocompatibility complex antigen) (Table 1). In keeping with previous studies of PEL cell lines, HH-B2 did not express classical B-cell markers, such as CD19 or CD20.
TABLE 1.
Cell surface characteristics of the HH-B2 cell line as determined by fluorescence-activated cell sortinga
| Marker | Cells expressing marker | % Cells positive | Interpretation |
|---|---|---|---|
| CD45 | Lymphocytes | 76 | Positive |
| CD19 | B cells | <0.5 | Negative |
| CD20 | B cells | <0.5 | Negative |
| CD2 | T cells | 3.2 | Positive |
| CD3 | T cells | <0.5 | Negative |
| CD4 | T cells | <0.5 | Negative |
| CD5 | T cells | <0.5 | Negative |
| CD7 | T cells | <0.5 | Negative |
| CD8 | T cells | <0.5 | Negative |
| CD13 | Monocytes | <0.5 | Negative |
| CD14 | Monocytes | <0.5 | Negative |
| CD16 | Monocytes and NK cells | <0.5 | Negative |
| CD33 | Monocytes | <0.5 | Negative |
| CD1a | Langerhans; dendritic cells | <0.5 | Negative |
| CD1b | Myeloid, NK cells | <0.5 | Negative |
| CD23 | Activation (B, Mφ, Eos, and FDC cells) | 4.7 | Positive |
| CD30 | Activation (B, T, and NK cells) | <0.5 | Negative |
| CD38 | Activation (T and plasma cells) | 98.6 | Positive |
| CD10 | Differentiation (B and T cells) | <0.5 | Negative |
| CD34 | Differentiation (stem cells) | <0.5 | Negative |
| IL-6-Rα | IL-6 receptor | <0.5 | Negative |
| HLA-DR | MHC II | 11 | Positive |
Antigens present on the cell surface were detected by fluorescence-activated cell sorting using commercially available antibodies conjugated to a fluorochrome, either phycoerythrin or fluorescein isothiocyanate. Reactions were compared with those of mouse immunoglobulin isotype controls. MHC, major histocompatibility complex.
ORF50 behaves as an immediate-early gene in HH-B2 cells.
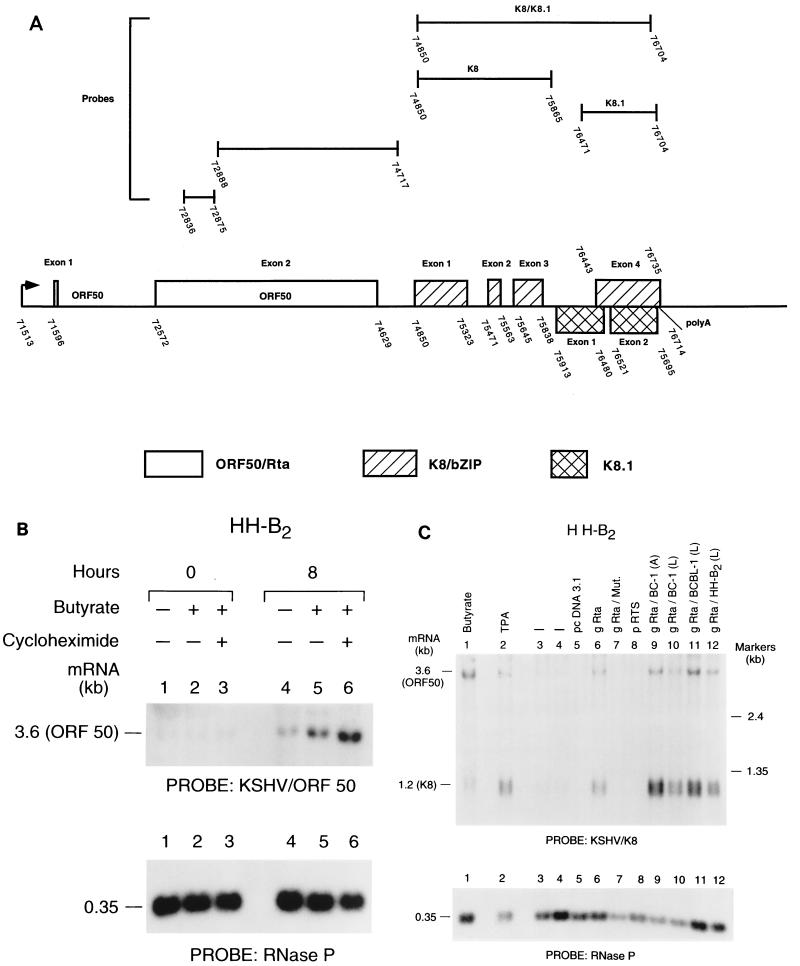
Following induction of the KSHV lytic cycle in BC-1 cells, approximately 20% of the 3.6-kb ORF50 mRNA remains if the chemical inducing stimulus is applied in the presence of cyclohexamide (CHX), an inhibitor of protein synthesis (41). Remarkably, in HH-B2 cells, the abundance of the 3.6-kb ORF50 mRNA increased two- to threefold at 8 and 12 h following treatment with n-butyrate in the presence of CHX (Fig. 1B). Identical results were obtained whether the Northern blots were probed with ORF50 itself or with K8, which shares the 3.6-kb bicistronic transcript with ORF50 (data not shown; Fig. 1A). Use of an antisense oligonucleotide probe confirmed that the CHX-resistant 3.6-kb mRNA was transcribed in the rightward direction (Fig. 1B). By 20 h after chemical induction by n-butyrate, the abundance of the 3.6-kb ORF50 mRNA decreased in the CHX-treated culture relative to its abundance in the absence of CHX (data not shown). These findings indicated that at 8 and 12 h after chemical induction in HH-B2 cells, ORF50 mRNA behaved with immediate-early kinetics, but at later times it was under control of newly made proteins.
FIG. 1.
Expression of ORF50 mRNA in HH-B2 cells. (A) Exon map of the region of KSHV DNA encompassing ORF50, K8, and K8.1. Arrow indicates a transcriptional start mapped in reference 40. The locations of the exons of ORF50 are defined in references 19, 40, and 45, of K8 in reference 16, and of K8.1 in reference 27. Numbers below the line are numbers in the KSHV sequence (32). Not shown is ORF49, located between exon 1 and exon 2 of ORF50 and transcribed in the opposite direction (32). polyA, polyadenylation site. Probes used to detect ORF50, K8, and K8.1 are shown above the map. (B) CHX resistance of the 3.6-kb ORF50 mRNA in HH-B2 cells. Cells were untreated or were treated for 8 h with 3 mM n-butyrate in the presence or absence of 33 μg of CHX/ml. At the indicated times, RNA was prepared and analyzed by probing a Northern blot with a single-stranded oligonucleotide complementary to the 3.6-kb ORF50 mRNA. (C) Autostimulation of expression of 3.6-kb ORF50 mRNA by transfection of KSHV Rta. HH-B2 cells were treated with chemical inducing agents (lanes 1 and 2), were untreated without (lane 3) or with (lane 4) electroporation, or were transfected with the plasmids indicated in lanes 5 to 12. KSHV gRta constructs derived from different KSHV strains beginning at nucleotide 71505 are designated with (L) for leader. KSHV gRta constructs beginning at nucleotide 71588 are designated with (A) for ATG. Twenty hours after transfection, total cellular RNA was harvested and analyzed by Northern blotting with a probe for K8 which detects both the K8 and ORF50 mRNAs.
Since a probe comprised of ORF K8 detects both a bicistronic mRNA containing ORF50 and K8, as well as a monocistronic mRNA containing only K8 (Fig. 1A), the kinetic behavior of the ORF50 and K8 genes could be compared using a single probe derived from K8. This experiment showed that at 8 h following chemical induction by butyrate, the 3.6-kb ORF50 mRNA increased 20-fold in abundance relative to uninduced cells in the presence of CHX while the abundance of the 1.2-kb K8 mRNA decreased to 60% of the value of uninduced cells (data not shown). These experiments confirm the conclusion that ORF50, but not K8, is an immediate-early gene of KSHV.
Autostimulation of the ORF50 mRNA by KSHV Rta.
To determine whether KSHV Rta derived from several different strains of KSHV could activate the viral lytic cycle in HH-B2 cells, aliquots of cells were transfected with a group of ORF50 expression plasmids. These plasmids, containing genomic viral DNA with or without portions of a 5′ untranslated leader upstream of the ATG initiator codon in exon 1 of the ORF50 gene, were derived from three KSHV strains present in the BC-1, BCBL-1, and HH-B2 PEL cell lines. All the ORF50 expression plasmids activated lytic gene expression, as evidenced by the appearance of the mRNAs of the K8 early lytic cycle gene and PAN RNA (data not shown). The level of induction of K8 mRNA by the ORF50 expression plasmids was at least as high or higher than was achieved by treating HH-B2 cells with chemical inducers of lytic cycle gene expression, such as n-butyrate or TPA.
The 3.6-kb ORF50 mRNA itself was also induced by all the ORF50 expression plasmids (Fig. 1C, lanes 6 and 9 to 12). Since the probe for the Northern blot, derived from ORF K8, detects both the monocistronic early 1.2-kb K8 mRNA and the bicistronic immediate-early 3.6-kb mRNA containing ORF50 (Fig. 1A), this result indicates that ORF50 expression plasmids autostimulated expression of ORF50 mRNA.
The level of ORF50 protein made following transfection was compared with the level expressed following treatment with chemical inducing stimuli (data not shown). At 48 h after transfection, the abundance of ORF50 protein was similar in transfected and chemically treated cells (not shown). Transfection of the two plasmids containing ORF50/BC-1(A) and ORF50/BCBL-1(L) that were most active in induction of K8 mRNA (Fig. 1B) also led to higher levels of expression of ORF50 protein (not shown). Under the conditions of the experiment, it was not determined whether the ORF50 protein was expressed from the endogenous virus or the transfected vector. The ORF50 protein appeared as multiple species on an immunoblot consistent with the postulate that it is extensively modified after translation, perhaps by phosphorylation (19).
Sensitivity of late-gene expression to phosphonoacetic acid (PAA).
By use of a probe (Fig. 1A) that encompassed both K8, an early gene, and K8.1, a late gene, we found that the kinetics of expression of these two genes differed following transfection of an ORF50 expression plasmid (not shown). At 10 h following transfection, the 1.2-kb mRNA of K8, the early gene, was stimulated more than threefold by ORF50/Rta, but the 0.9-kb transcript of K8.1, the late gene, was not activated. However, at 20 h after transfection and thereafter, there was a progressive increase in the abundance of the 0.9-kb late transcript in cells transfected with ORF50/Rta. Similarly, the 0.9-kb mRNA from ORF65, another late gene encoding sVCA, was not stimulated at 10 h after transfection of ORF50 but was markedly induced at 20 and 40 h (15-fold and 11-fold, respectively) relative to transfection with KSHV gRta (mut). Thus, KSHV late-gene expression induced by ORF50 was delayed relative to early gene expression.
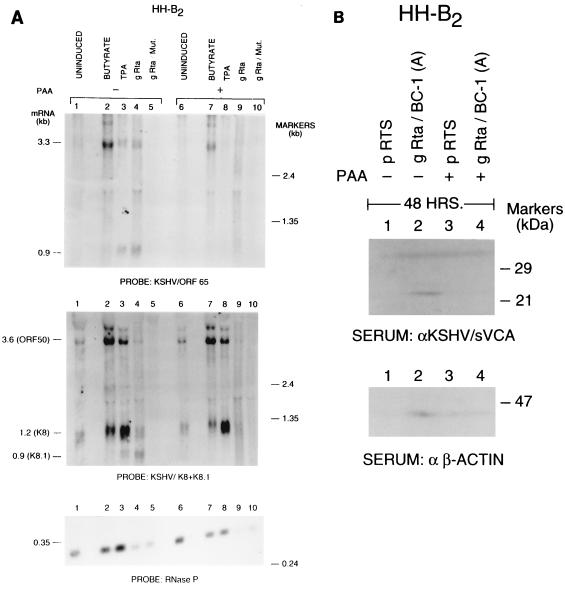
To determine whether late-gene expression downstream of ORF50 relied on viral DNA synthesis, we studied the effects of PAA, an inhibitor of lytic viral DNA synthesis mediated by the viral DNA polymerase (38), used in conjunction with transfection of ORF50 expression plasmids. PAA inhibited the expression of the 0.9-kb ORF65 late mRNA induced either by TPA or by gRta (Fig. 2A, top). By use of the dual probe for K8 and K8.1, it was shown that PAA inhibited the expression of the 0.9-kb K8.1 late mRNA, but had little effect on expression of the K8 early mRNA (Fig. 2A, bottom).
FIG. 2.
Inhibition of KSHV Rta-induced late-gene expression by PAA. (A) HH-B2 cells were untreated (lanes 1 and 6), exposed to chemical inducing stimuli (lanes 2, 3, 7, and 8), or transfected with a KSHV ORF50 expression plasmid, gRta (lanes 4 and 9), or gRta mutant plasmids (lane 5 and 10). One-half of the cultures were exposed to 500 μM PAA at time zero immediately after electroporation (lanes 6 to 10). RNA harvested 30 h after transfection was analyzed by Northern blotting with probes for KSHV ORF65 (top) and K8 and K8.1 (bottom). RNaseP was used to control for RNA loading. (B) HH-B2 cells were transfected with vector pRTS or with vector containing ORF50/Rta from the BC-1 strain, in the presence or absence of PAA. Cell extracts prepared 48 h after transfection were analyzed by immunoblotting with antibodies to ORF65.
A plasmid containing gRta/ORF50/BC-1(A) stimulated expression of sVCA (Fig. 2B) and a 30- to 33-kDa polypeptide complex reactive with an antibody to K8.1 (not shown). Neither empty vector nor a stop codon mutant of ORF50 stimulated late-protein expression. PAA blocked the capacity of ORF50 protein to stimulate late-polypeptide expression. These results indicated that late-viral-gene expression induced by ORF50 was dependent on lytic viral DNA replication.
ORF50 induces an increase in the content of extracellular DNase-resistant KSHV DNA.
We next attempted to obtain direct evidence that did not rely on use of an inhibitor for an increase in the amount of viral DNA following introduction of ORF50/Rta expression plasmids. The high content of latent viral DNA coupled with the low efficiency of transfection did not permit detection of an increase in total intracellular viral DNA in HH-B2 cells that had been transfected with ORF50/Rta. Therefore, we determined whether DNase-resistant viral DNA was enriched in the supernatants of ORF50/Rta-transfected HH-B2 cells.
To validate the assay for DNase resistance, we determined that DNase could eliminate unencapsidated plasmid DNA that was suspended in tissue culture medium with or without serum. Plasmid DNA resuspended in RPMI medium was completely digested by DNase even in the absence of added Mg++ and Ca++ ions, for the RPMI medium contains these ions. Similarly, DNase completely digested the plasmid DNA even in the presence of 15% fetal bovine serum and the absence of additional divalent cations (data not shown).
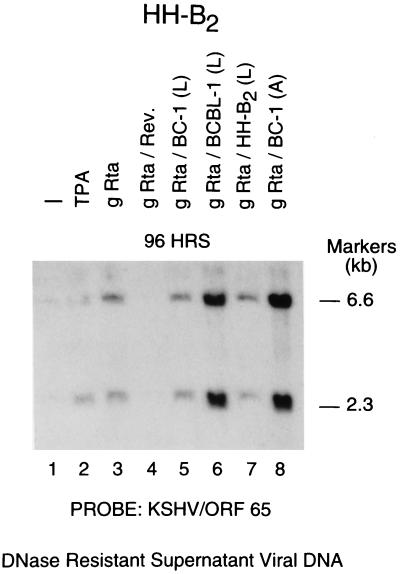
The addition of TPA or transfection of an ORF50 expression plasmid caused an increase in the amount of DNase-resistant viral DNA detectable in culture fluids of HH-B2 cells (Fig. 3). In kinetics experiments, we found that expression plasmids containing ORF50 from three KSHV strains caused an increase in DNase-resistant viral DNA in the supernatant fluid by 24 h after transfection. The maximum increase in extracellular viral DNA occurred 72 to 96 h after transfection. This increase in extracellular viral DNA was not observed following transfection of KSHV gRta(rev) (Fig. 3, lane 4) or empty vector, pRTS (not shown). The ability of transfected ORF50 expression plasmids to induce an increase in extracellular viral DNA was inhibited by treatment of transfected cells with PAA (data not shown).
FIG. 3.
Transfection of KSHV ORF50/Rta induces an increase in extracellular DNase-resistant viral DNA. Cells were untreated, treated with TPA, or transfected with KSHV gRta or KSHV gRta (rev) in reverse orientation or with a panel of ORF50 expression plasmids (see Fig. 1C). DNase-resistant viral DNA present in culture supernatant fluids harvested 96 h after transfection was analyzed by Southern blotting using a probe derived from KSHV ORF65.
The major impetus for the experiments described in this report was the unanswered question of whether KSHV ORF50/Rta was competent to initiate the entire viral lytic cascade leading to viral DNA replication, late-gene expression, and viral release. The answer to this question is affirmative. The experiments provide several novel observations about the biology of the ORF50 activator in the HH-B2 PEL cell line. (i) The abundance of the 3.6-kb ORF50 mRNA increased in the presence of CHX, a finding that indicates that ORF50 is a true immediate-early gene. (ii) Transfected ORF50 expression plasmids activate expression of the 3.6-kb ORF50 mRNA from the endogenous virus, a finding that suggests that ORF50/Rta is able to activate its own expression. (iii) Biochemical methods have been used to demonstrate that ORF50/Rta stimulates expression of late-gene mRNAs and late-gene polypeptides with appropriate kinetics. (iv) Late-gene expression induced by ORF50/Rta is inhibited by PAA, an inhibitor of the viral polymerase, a finding that indicates that ORF50-driven late-gene expression is dependent on viral DNA replication. (v) Introduction of ORF50/Rta expression plasmids leads to an increase in the amount of DNase-resistant viral DNA in culture supernatants. Since release of encapsidated viral DNA induced by ORF50 is inhibited by PAA, it must represent newly replicated DNA. The foregoing conclusions are based on unique features of the HH-B2 PEL cell line that were favorable for these experiments, namely, its susceptibility to gene transfer, its low background of spontaneous KSHV lytic gene expression, and its capacity to release DNase resistant viral DNA. A question for future study is whether ORF50/Rta also can cause an increase in the production of biologically active KSHV. Such experiments await the development of sensitive and quantitative infectivity assays for the virus. Since KSHV ORF50/Rta alone is sufficient to activate the entire viral lytic cycle, another question for future study is to define the functional role of the other immediate-early transcripts (45).
Acknowledgments
Supported by grants CA70036 and CA16038 from the NCI.
We are grateful to Jae Jung for gifts of antisera and to Ren Sun and Tobias Ragoczy for reading the manuscript critically.
Footnotes
This paper is dedicated to the memory of Elizabeth Grogan, our close colleague and collaborator for many years, who isolated the HH-B2 cell line.
REFERENCES
- 1.Andre S, Schatz O, Bogner J R, Zeichhardt H, Stoffler-Meilicke M, Jahn H U, Ullrich R, Sonntag A K, Kehm R, Haas J. Detection of antibodies against viral capsid proteins of human herpesvirus 8 in AIDS-associated Kaposi's sarcoma. J Mol Med. 1997;75:145–152. doi: 10.1007/s001090050099. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 3.Ballestas M E, Chatis P A, Kaye K M. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 4.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Sturzl M. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 7.Chan S R, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- 8.Chandran B, Bloomer C, Chan S R, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 9.Chandran B, Smith M S, Koelle D M, Corey L, Horvat R, Goldstein E. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology. 1998;243:208–217. doi: 10.1006/viro.1998.9055. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kedes D H, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J Clin Investig. 1997;100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowles D M, Inghirami G, Ubriaco A, Della-Favera R. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792–799. [PubMed] [Google Scholar]
- 13.Lee H, Guo J, Li M, Choi J K, DeMaria M, Rosenzweig M, Jung J U. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol Cell Biol. 1998;18:5219–5228. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers R C, Jung J U. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat Med. 1998;4:435–440. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 15.Li M, McKey J, Czajak S C, Desrosiers R C, Lackner A A, Jung J U. Identification and characterization of Kaposi's sarcoma-associated herpesvirus K8.1 glycoprotein. J Virol. 1999;73:1341–1349. doi: 10.1128/jvi.73.2.1341-1349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S-F, Robinson D R, Miller G, Kung H-J. Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to ZEBRA of Epstein-Barr virus. J Virol. 1999;73:1909–1917. doi: 10.1128/jvi.73.3.1909-1917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S-F, Sun R, Heston L, Gradoville L, Shedd D, Haglund K, Rigsby M, Miller G. Identification, expression, and immunogenicity of Kaposi's sarcoma-associated herpesvirus-encoded small viral capsid antigen. J Virol. 1997;71:3069–3076. doi: 10.1128/jvi.71.4.3069-3076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 19.Lukac D M, Kirshner J R, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348–9361. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matolcsy A, Nador R G, Cesarman E, Knowles D M. Immunoglobulin VH gene mutational analysis suggests that primary effusion lymphomas derive from different stages of B cell maturation. J Pathol. 1998;153:1609–1614. doi: 10.1016/S0002-9440(10)65749-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov G M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller G, Rigsby M O, Heston L, Grogan E, Sun R, Metroka C, Levy J A, Gao S J, Chang Y, Moore P. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N Engl J Med. 1996;334:1292–1297. doi: 10.1056/NEJM199605163342003. [DOI] [PubMed] [Google Scholar]
- 23.Muralidhar S, Pumfery A M, Hassani M, Sadaie M R, Azumi N, Kishishita M, Brady J N, Doniger J, Medveczky P, Rosenthal L J. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980–4988. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholas J, Ruvolo V, Zong J, Ciufo D, Guo H-G, Reitz M S, Hayward G S. A single 13-kilobase divergent locus in the Kaposi's sarcoma-associated herpesvirus genome contains nine open reading frames that are homologous to or related to cellular proteins. J Virol. 1997;71:1963–1974. doi: 10.1128/jvi.71.3.1963-1974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G, Reitz M S. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 26.Poole L J, Zong J C, Ciufo D M, Alcendor D J, Cannon J S, Ambinder R, Orenstein J M, Reitz M S, Hayward G S. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J Virol. 1999;73:6646–6660. doi: 10.1128/jvi.73.8.6646-6660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raab M S, Albrecht J C, Birkmann A, Yaguboglu S, Lang D, Fleckenstein B, Neipel F. The immunogenic glycoprotein gp35-37 of human herpesvirus 8 is encoded by open reading frame K8.1. J Virol. 1998;72:6725–6731. doi: 10.1128/jvi.72.8.6725-6731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabkin C S, Su I J, Powell K F, Croxson M, Foreman K E, Nickoloff B J, Alkan S, Hayward G S. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J Virol. 1999;73:4156–4170. doi: 10.1128/jvi.73.5.4156-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ragoczy T, Miller G. Role of the Epstein-Barr virus Rta protein in activation of distinct classes of viral lytic cycle genes. J Virol. 1999;73:9858–9866. doi: 10.1128/jvi.73.12.9858-9866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LANA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by ORF73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 32.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5722–5730. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson G R, Schulz T F, Whitby D, Cook P M, Boshoff C, Rainbow L, Howard M R, Gao S J, Bohenzky R A, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder R S, Weller I V, Weiss R A, Moore P S. Prevalence of Kaposi's sarcoma-associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 36.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staskus K A, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett-Smith H, Haase A T. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's Sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73:4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summers W C, Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976;18:151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun R, Lin S F, Gradoville L, Miller G. Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1996;93:11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun R, Lin S-F, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232–2242. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 43.Zhong W, Ganem D. Characterization of ribonucleoprotein complexes containing an abundant polyadenylated nuclear RNA encoded by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) J Virol. 1997;71:1207–1212. doi: 10.1128/jvi.71.2.1207-1212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu F X, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]