Abstract
Over the past several decades, there has been a notable increase in the total number of spinal fusion procedures worldwide. Advanced spinal fusion techniques, surgical approaches, and new alternatives in grafting materials and implants, as well as autologous cellular therapies, have been widely employed for treating spinal diseases. While the potential of cellular therapies to yield better clinical results is appealing, supportive data are needed to confirm this claim. This meta‐analysis aims to compare the radiographic and clinical outcomes between graft substitutes with autologous cell therapies and graft substitutes alone. PubMed, Scopus, Web of Science, ClinicalTrials.gov, and the Cochrane Central Register of Controlled Trials were searched for studies comparing graft substitutes with autologous cell therapies and graft substitutes alone up to February 2024. The risk of bias of the included studies was evaluated using the Downs and Black checklist. The following outcomes were extracted for comparison: fusion success, complications/adverse events, Visual Analog Scale (VAS) score, and Oswestry Disability Index (ODI) score. Thirteen studies involving 836 patients were included, with 7 studies considered for the meta‐analysis. Results indicated that the use of graft substitutes with autologous cell therapies demonstrated higher fusion success rates at 3, 6, and 12 months, lower VAS score at 6 months, and lower ODI score at 3, 6, and 12 months. The complication rate was similar between graft substitutes with autologous cell therapies and graft substitutes alone. Although the current literature remains limited, this meta‐analysis suggests that the incorporation of cellular therapies such as bone marrow and platelet derivatives with graft substitutes is associated with a higher fusion rate and significant improvements in functional status and pain following spinal fusion. Future well‐designed randomized clinical trials are needed to definitively assess the clinical effectiveness of cellular therapies in spinal fusion.
Keywords: autologous cell therapies, graft substitutes, meta‐analysis, spinal fusion
This meta‐analysis aims to compare radiographic and clinical outcomes between graft substitutes with autologous cell therapies and graft substitutes alone. Thirteen studies involving 836 patients were included, with 7 considered for meta‐analysis. Meta‐analysis results showed higher fusion success with graft substitutes using autologous cell therapies at 3, 6, and 12 months, along with lower Visual Analog Scale (VAS) scores at 6 months and lower Oswestry Disability Index (ODI) scores at 3, 6, and 12 months. Complication rates were similar between the two approaches. Although current literature is limited, this meta‐analysis suggests that incorporating cellular therapies with graft substitutes leads to a higher fusion rate and significant functional and pain improvements post‐spinal fusion. Further randomized trials are needed to definitively assess cellular therapy's clinical effectiveness in spinal fusion.
1. INTRODUCTION
Spine fusion has emerged as a common treatment modality for various spinal pathologies, including degenerative disorders, fractures, spinal tumors, and deformities such as scoliosis and kyphosis. 1 Rajaee et al. reported a substantial increase in spinal fusion rates from 64.5 cases per 100 000 adults in 1998 to 135.5 cases per 100 000 in 2008. 2
The attainment of solid bony arthrodesis stands as a primary objective in spine fusion surgery, entailing the formation of new bone between two or more adjacent vertebrae to restore stability to the affected spinal segment. 3 A diverse array of spinal fusion techniques exists, with the specific anatomical location and pathology guiding the choice of surgical approach, stabilizing instrumentation, and procedure to optimize stability and prompt healing while minimizing surgical trauma. 4 Nonetheless, achieving robust arthrodesis in complex spine surgeries can pose a difficult challenge. The incidence of pseudarthrosis, or nonunion, in spinal fusion surgery can be as high as approximately 25%–35%, significantly influenced by factors such as the type of procedure, surgical approach, and patient‐specific variables including bone quality, overall health, and comorbidities. 5 , 6 , 7 , 8 , 9 When bone formation fails, unsuccessful fusion can result in pain, instability, implant failure, the need for reoperation, patient distress, and a substantial increase in healthcare costs. 6 This represents a notably high occurrence rate for a procedure that is both widely performed and costly. Methods aimed at preventing pseudarthrosis have emerged as focal points of research and investment within contemporary spine surgery. The traditional gold standard for bone grafting remains the iliac crest autograft and local autograft (spinous processes, laminae), harvested from either a donor site or the surgical site. 1 , 10 Autologous bone possesses the three essential properties of osteogenesis, oste‐oinductivity, and osteoconductivity, all of which are crucial for optimizing spine arthrodesis. 1 , 10 However, the availability and quality of autologous bone may be limited, contingent upon factors such as patient age and biology. 11 Consequently, a variety of alternatives have been developed and investigated. These options include allografts, synthetics grafts, and growth factors. 12 , 13 , 14 Furthermore, autologous cell therapies with osteoinductive potential, such as bone marrow aspirate (BMA), mesenchymal stem cells (MSCs), as well as platelet products, have been incorporated into regenerative regimens to enhance spinal fusion rates. 12 , 13 , 14 Specifically, autologous therapy is a therapeutic intervention that involves utilizing an individual's own cells or tissues. These cells or tis‐sues are processed outside the body and then reintroduced into the same individual, serving as a personalized treatment approach.
The use of BMA is a simple, safe, clean, and inexpensive procedure that allows immediate transplantation of various cell populations, including osteoprogenitors and hematoprogenitors, into the fusion site. 13 It is easily obtained from the posterior iliac bone while lying supine, although vertebral bodies have also been used to collect marrow. 15 Concerning the use MSCs, the most common source for their isolation in spinal fusion is bone marrow, followed by adipose tissue. 16 , 17 , 18 , 19 Bone marrow MSCs have demonstrated their capacity to differentiate into osteogenic lineage cells under appropriate conditions. 16 , 17 Adipose‐derived MSCs can be obtained via liposuction, a procedure generally less painful than bone marrow aspiration. 18 , 19 , 20 MSCs constitute a minor fraction of the total population of nucleated cells, necessitating an in vitro expansion phase to obtain adequate stem cell numbers before implantation. 21 Various expansion techniques exist, yet challenges such as sterility techniques, culture duration, medium selection, and MSCs quantity required remain unresolved. 21 , 22 , 23 Additionally, the reliability of this cell source may diminish in the elderly population due to a decline in MSCs potency. 22 Although benefit has been suggested in animal models of spinal fusion, 23 information on BMA and MSCs clinical application is scarce and clinical literature investigating their use in spine surgery consists mainly of small observational studies with not defined results. 24
In addition to BMA and MSCs also platelets cells may contribute to tissue regeneration by stimulating progenitors, by dampening local inflammatory responses, and by promoting angiogenesis. 25 Platelet products, such as platelet concentrates, platelet rich plasma (PRP), platelet gel and platelet glue, are in fact considered as autologous cell therapy products containing an array of growth factors such as platelet‐derived growth factor (PDGF), transforming growth factor (TGF), insulin‐like growth factor (IGF), epidermal growth factor (EGF), epithelial cell growth factor (EGR), and hepatocyte growth factor (HGF) with osteoinductive properties. 26 As BMA and MSCs also the platelet products demonstrated the ability to facilitate spinal fusion in animal experiments, however their effectiveness in enhancing spinal fusion in humans are limited and remains contentious. 27 , 28
Although autologous cellular therapies have the potential to yield better clinical out‐comes than bone substitutes alone in spine fusion, it is important to confirm this assertion with supportive data. Therefore, it remains essential to establish the clinical efficacy and safety of these therapies through higher level evidence to gain widespread acceptance. To clarify the available evidence, we conducted a systematic review and meta‐analysis of clinical studies to investigate whether the adjunctive use of autologous cell therapies with graft substitutes promotes spinal fusion and influences clinical outcomes, and affects the rate of complications.
1.1. Key questions
Regarding the use of graft substitutes with autologous cell therapies versus graft substitutes alone in spinal fusion surgery:
Key Question 1: Is the use of autologous cell therapies in conjunction with graft substitutes more effective for spinal fusion compared to using graft substitutes alone?
Key Question 2: Does the integration of autologous cell therapies with graft substitutes lead to improved clinical patient‐reported outcome compared to using graft substitutes alone?
Key Question 3: What are the complications associated with using autologous cell therapies in spinal fusion, and is their use safer than spinal fusion with graft substitutes alone?
2. METHODS
2.1. Eligibility criteria
The PICOS model (population, intervention, comparison, outcomes, study design) was used to project this review: the studies that evaluated the clinical effectiveness of graft substitutes with autologous cells therapies in patients (Population), submitted to spinal fusion surgery (Intervention), with bone substitutes alone as comparison group (Comparison), and that described spinal fusion outcomes (Outcomes) in clinical studies (Study design) will be included. 29 , 30 The primary outcomes considered were spinal fusion success evaluated through imaging techniques, patient‐centered clinical evaluations before and after surgery, such as Oswestry Disability Index (ODI) and Visual Analog Scale (VAS), and complications/adverse events (rate). 31 , 32
Studies from February 15, 1994, to February 15, 2024, were comprised in this review if they met the PICOS criteria. Excluded from the review were those studies that lacked a control group or did not use the same graft substitutes as the treatment group, did not involve the use of autologous cellular therapies, had incomplete data, or had a drop‐out rate of over 30% at the first follow‐up. In addition, reviews, letters, comment to Editor, meta‐analysis, case‐report, protocols and recommendations, editorials, guidelines, and articles not written in English were excluded.
2.2. Search strategy
The literature review involved a systematic search conducted in February 2024 according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 33 The search was conducted on five data bases: PubMed, Scopus, Web of Science, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials.
The resulting combination of terms was used (spinal fusion OR spinal arthrodesis OR vertebral fusion OR vertebral arthrodesis) AND (graft OR grafting OR substitutes OR transplant) AND (autologous cell therapy OR autologous cellular therapy) and for each of these terms, free words, and managed vocabulary specific to each bibliographic database were merged using the operator “OR.” The combination of free‐vocabulary and/or Medical Subject Headings (MeSH) terms for the recognition of studies in all databases were reported in Table S1. Reference lists of studies were also reviewed to identify studies potentially eligible for our systematic review and meta‐analysis.
2.3. Selection process
After the duplicate elimination by a public reference manager (Mendeley Desktop v.1.19.8) the potential pertinent articles were screened using title and abstract by two re‐viewers (FS and DC). Studies that did not meet the inclusion criteria were eliminated and any disagreement was resolved through debate until a consensus was reached, or with the involvement of a third reviewer (GG). Finally, the remaining studies were comprised in the final stage of data extraction.
2.4. Data collection process and synthesis methods
The data extraction and synthesis started with cataloguing the detail of the studies. The following data were abstracted from all included studies by two authors (FS and DC): study type, patient demographics (gender and age), study design, spinal disease and surgery, preoperative assessment, postoperative assessment, follow‐up, complications (graft‐related, infections, and neurological, etc.), main results, and reference. To increase validity and avoid omitting potentially findings for the synthesis all extracted data were reported tabulated (Table 1).
TABLE 1.
Main characteristics of clinical studies included in the review.
| Study type | Patient demographics | Study design | Spinal disease and surgery | Preoperative assessment | Postoperative assessment | Follow‐ups | Complications | Main results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Prospective |
M: n = 53 F: n = 54 Mean age: 58 yrs |
Study group: local autograft + TCP/HA + APC (n = 67) Control group: local autograft + TCP/HA (n = 40) |
Lumbar degenerative diseases PLF (L3, L4, L5, S1) |
X‐ray | X‐ray | 3, 6, 12, 24 mos |
Study group: 13.4% (lack or irregular fusion mass) Control group: 5% (lack or irregular fusion mass) |
Study group: fusion success = 24 mos 74.6% Control group: fusion success = 24 mos 92.5% |
Acebal‐Cortina et al. 42 |
| Retrospective |
M: n = 80 F: n = 72 Mean age: 50 yrs |
Study group: autologous ICBG + AGF platelet gel (n = 76) Control group: autologous ICBG (n = 76) |
Lumbar degenerative diseases (stenosis, degenerative disc disease, spondylolisthesis) PLF (1, 2, 3‐level) |
NR | CT | 24 mos | NR |
Study group: fusion success = 75% Control group: fusion success = 83% |
Carreon et al. 47 |
| Retrospective |
M: n = 27 F: n = 57 Mean age: 48 yrs |
Study group: autologous ICBG + AGF platelet gel (n = 22) Control group: autologous ICBG (n = 62) |
Degenerative disc disease, stenosis, pseudoarthrosis, spondylolisthesis TLIF (1, 2‐level) | Hemoglobin, hematocrit | X‐ray, drain output, transfusion, LOS | NR |
Study group: 58% (stroke and meningitis, pseudoarthrosis, revision) Control group: 66% (pseudoarthrosis, adjacent segment degeneration, arachnoiditis, instrumentation malposition or removal) |
Study group: fusion success = 36% drain output = 436 mL transfusions = 23% LOS = 5.3 Control group: fusion success = 55% drain output = 567 mL transfusions = 37% LOS = 5.1 |
Castro et al. 48 |
| RCT |
M: n = 5 F: n = 7 Mean age: 59 yrs |
Study group: local autograft + BMC (n = 6) Control group: local autograft (n = 6) |
Low‐grade Degenerative Spondylolisthesis PLF (L4‐L5) |
ODI | ODI, CT | 3, 6 mos |
Study group: 16.7% (inflammation) Control group: 0% |
Study group: fusion success = 6 mos 58.3% ODI = pre‐op 48 ± 20.1; 3 mos 35 ± 12; 6 mos 24 ± 19 Control group: fusion success = 6 mos 100% ODI = pre‐op 44 ± 15; 3 mos 29 ± 19; 6 mos 21 ± 18 |
Chotivichit et al. 38 |
| Retrospective |
M: n = 39 F: n = 22 Mean age: 50 yrs |
Study group: allograft + BMA (n = 30) Control group: allograft (n = 31) |
Lumbar degenerative diseases (instability, scoliosis, intervertebral space stenosis, spinal canal stenosis) XLIF (1, 2‐level) |
ODI, VAS | X‐ray, CT, ODI, VAS | 3, 6, 12 mos |
Study group: 0% Control group: 3.22% (cage displacement) |
Study group: fusion success = 3 mos 40%; 6 mos 66.7%; 12 mos 86.7% VAS = pre‐op 6.85 ± 0.81; 3 mos 4.08 ± 0.72; 6 mos 1.97 ± 0.82; 12 mos 1.85 ± 0.64 ODI = pre‐op 52.16 ± 5.91; 3 mos 29.10 ± 5.04; 6 mos 22.07 ± 6.32; 12 mos 7.23 ± 2.66 Control group: fusion success = 3 mos 16.1%; 6 mos 45.2%; 12 mos 71% VAS = pre‐op 6.81 ± 0.87; 3 mos 4.52 ± 0.65; 6 mos 2.39 ± 0.90; 12 mos 2.19 ± 0.83 ODI = pre‐op 53.03 ± 5.96; 3 mos 31.45 ± 4.05; 6 mos 25.23 ± 3.98; 12 mos 19.42 ± 3.26 |
Gao et al. 46 |
| RCT |
M: n = 23 F: n = 46 Mean age: 61 yrs |
Study group: autologous ICBG + BM‐MSCs (n = 34) Control group: autologous ICBG (n = 35) |
Degenerative spondylolisthesis and/or degenerative disc disease TLIF (1, 2‐level; L4‐L5) |
VAS, ODI, SF‐36 | X‐ray, VAS, ODI, SF‐36, CT | 3, 6, 12 mos |
Study group: 11.8% (urinary infection) Control group: 2.9% (urinary infection) |
↑SF‐36 in both groups Study group: fusion success = 3 mos 78.6%; 6 mos 75%; 12 mos 85.7% VAS = pre‐op 6.68 ± 2.38; 3 mos 3.58 ± 2.66; 6 mos 3.57 ± 2.61; 12 mos 3.20 ± 2.95 ODI = pre‐op 40.63 ± 13.50; 3 mos 29.20 ± 16.67; 6 mos 25.87 ± 19.83; 12 mos 21.43 ± 17.68 Control group: fusion success = 3 mos 65.6%; 6 mos 43.8%; 12 mos 56.3% VAS = pre‐op 6.77 ± 2.20; 3 mos 2.76 ± 2.35; 6 mos 3.43 ± 2.53; 12 mos 3.09 ± 2.73 ODI = pre‐op 43.41 ± 15.56; 3 mos 27.01 ± 18.31; 6 mos 25.85 ± 16.97; 12 mos 23.01 ± 14.68 |
Garcìa de Frutos et al. 39 |
| RCT |
M: n = 22 F: n = 58 Mean age: 61 yrs |
Study group: allograft chips + BMC (n = 40) Control group: allograft chips (n = 40) |
Lumbar degenerative disease (spondylodesis) PLF (1, 2, 3‐level) |
NR | X‐ray, CT, LOS | 12, 24 mos |
Study group: 5% (hematoma) Control group: 5% (hematoma) |
Study group: fusion success = 12 mos 15%; 24 mos 80%; LOS = 12 days Control group: fusion success = 12 mos 0%; 24 mos 40% LOS = 11.8 days |
Hart et al. 40 |
| Retrospective |
M: n = 23 F: n = 12 Mean age: 42 yrs |
Study group: local autograft + PRP (n = 15) Control group: local autograft (n = 20) |
Traumatic fractures Anterior thoracic or lumbar fusion (1, 2‐level) |
VAS | CT, VAS | 12 mos |
Study group: 6.7% (neurological deficit) Control group: 0% |
Study group: fusion success = 40% VAS = pre‐op 85.1; 12 mos 69.3 Control group: fusion success = 40% VAS = pre‐op 93.2; 12 mos 71.3 |
Hartmann et al. 49 |
| Prospective |
M: n = 27 F: n = 3 Mean age: 29 yrs |
Study group: autologous ICBG + BM‐MNC (n = 15) Control group: autologous ICBG (n = 15) |
Cervical anomalies NR |
NR | X‐ray, CT, LOS | 3, 6 mos |
Study group: 46.7% (CSF, wound infection, skin breakdown, pneumonia, chronic pain) Control group: 66.7% (CSF, wound infection, meningitis, chronic pain, seroma) |
Study group: fusion success = 3 mos 60%; 6 mos 78% LOS = 10.9 Control group: fusion success = 3 mos 20%; 6 mos 47% LOS = 10.3 days |
Lakshmi et al. 43 |
| Prospective |
M: n = 7 F: n = 14 Mean age: 58 yrs |
Study group: autologous ICBG + BMA (n = 21) Control group: autologous ICBG (n = 21) |
Instability, stenosis, spondylolisthesis PLF (1‐level; L3‐L4 or L4‐L5) |
NR | X‐ray, CT | 12 mos | NR |
Study group: fusion success = 85.7% Control group: fusion success = 90.5% |
Niu et al. 44 |
| RCT |
M: n = 24 F: n = 14 Mean age: NR |
Study group: autologous ICBG + PRP (n = 19) Control group: autologous ICBG (n = 19) |
Spondylolisthesis, disc degeneration, disc Herniation PLF (1‐level; L3‐L4, L4‐L5, L5‐S1) |
X‐ray, VAS, ODI, SF‐36 | CT, VAS, ODI, SF‐36 | 3, 6, 12, 24 mos |
Study group: 15.8% (dural tear, transient radiculopathy, hardware removed) Control group: 26.3% (transient radiculopathy, Instrumentation removed) |
↑SF‐36 in both groups Study group: VAS = pre‐op 7.4 ± 1.9; 3 mos 3.6 ± 0.7; 6 mos 2.9 ± 0.8; 12 mos 2.4 ± 0.8; 24 mos 2.4 ± 0.9 ODI = pre‐op 42.6 ± 17.4; 3 mos 20 ± 4; 6 mos 15 ± 5; 12 mos 14 ± 5; 24 mos 13 ± 4 Control group: VAS = pre‐op 7.4 ± 1.4; 3 mos 3.6 ± 0.6; 6 mos 3.9 ± 1.2; 12 mos 3.5 ± 1.3; 24 mos 3.6 ± 1.4 ODI = pre‐op 49.4 ± 11.1; 3 mos 23 ± 7; 6 mos 22 ± 6.5; 12 mos 16 ± 7; 24 mos 19 ± 7 |
Sys et al. 41 |
| Prospective |
M: n = 17 F: n = 50 Mean age: 61 yrs |
Study group: local autograft + platelet glue (n = 34) Control group: local autograft (n = 33) |
Spondylolisthesis, segmental instability PLF (1‐level; L4‐L5, L5‐S1) | NR | X‐ray, CT, drain output | 24 mos |
Study group: 14.7% (pseudoarthrosis) Control group: 9.1% (pseudoarthrosis) |
Study group: fusion success = 85% drain output = 395 mL Control group: fusion success = 90% drain output = 362 mL |
Tsai et al. 45 |
| Retrospective |
M: n = 24 F: n = 35 Mean age: 58 yrs |
Study group: autologous ICBG + AGF (n = 32) Control group: autologous ICBG (n = 27) |
Degenerative disk disease or degenerative spondylolisthesis ILIF (1‐level; L3‐L4, L4‐L5, L5‐S1) | NR | X‐ray | 6, 12, 24 mos |
Study group: 37.5% (pseudoarthrosis) Control group: 3.7% (pseudoarthrosis) |
Study group: fusion success = 24 mos 62% Control group: fusion success = 24 mos 91% |
Weiner et al. 50 |
Abbreviations: 3D‐CT, three‐dimensional computed tomography; AGF, autologous growth factor; APC, autologous platelet concentrate; BM, bone marrow; BMA, bone marrow aspirate; BMC, bone marrow concentrate; CSF, cerebrospinal fluid; CT, computerized tomography; F, female; HA, hidroxiapatite; ICBG, iliac crest bone graft; ILIF, intertransverse lumbar interbody fusion; LOS, length of stay; M, male; MNC, mononuclear cells; mos, months; MSCs, mesenchymal stem cells; n, number; NR, not reported; ODI, Oswestry Disability Index; PLF, posterolateral lumbar fusion; pre‐op, preoperative; PRP, platelet‐rich plasma; RCT, randomized clinical trial; SF‐36, short form 36; TCP, tricalcium phosphate; TLIF, transforaminal lumbar interbody fusion; VAS, Visual Analogue Scale; XLIF, extreme lateral interbody fusion; yrs, years.
2.5. Risk of bias assessment (RoB)
Two reviewers (FS and DC) individually analyzed the methodological quality of the included studies. In case of disagreement, they tried to reach consensus; if this failed, a third reviewer (GG) made the definitive decision. The methodological quality of included clinical studies was evaluated by modified Downs and Black checklist, 34 a quality index with high internal consistency, high retest reliability, and good interrater reliability. This checklist consists of 27 items that are distributed over the five subscales of reporting, external validity, internal validity: bias, internal validity: confounding, and power. The Downs and Black checklist may be used to assess the methodological quality of randomized controlled trials (RCTs) and nonrandomized studies, with scores greater than or equal to 20 considered good, between 15 and 19 considered fair, and 14 or below considered poor. 35 Studies with good and fair methodological quality were considered for meta‐analysis.
2.6. Quantitative synthesis and statistical analysis
A level I meta‐analysis was performed on RCTs and nonrandomized studies with good or fair quality based on the modified Downs and Black checklist results and that analyze the outcome of graft substitutes with autologous cell therapies (treatment group) in comparison to graft substitutes alone (control group). The statistical analysis and the forest plot were carried out according to Neyeloff et al. 36 using the Meta XL tool for Microsoft Excel. The analysis was carried out using Random effects 37 for weighted mean difference of the continuous variables. The analysis of binary variables was based on the Odds Ratio (OR) between the two groups; a statistical test for heterogeneity was first conducted with the Cochran Q statistic and I 2 metric and was considered the presence of significant heterogeneity with I 2 values ≥25%. When no heterogeneity was found with I 2 < 25%, a fixed effect model was used to estimate the expected values and 95% CIs; otherwise, a random‐effect model was applied, and an I 2 metric was evaluated for the random effect to check the correction of heterogeneity. The studies rate confidence intervals were carried out using the continuity‐corrected Wilson interval. All statistical analysis was carried out with Microsoft Excel 2010.
3. RESULTS
3.1. Study selection
As shown in Figure 1, the database search identified 264 records (62 from PubMed, 78 from Scopus, 94 from Web of Science, 4 from ClinicalTrials.gov, and 26 from the Cochrane Central Register of Controlled Trials). Following duplicate removal, the remaining articles (n = 210) underwent title and abstract review based on the inclusion/exclusion criteria, resulting in 54 full‐text articles being evaluated for eligibility. Six additional publications were identified from the reference lists of the selected articles. Among these 60 articles, 47 were excluded for various reasons, including lack of a control group or use of different graft substitutes in the treatment group, absence of autologous cell therapy, unavailability of results, non‐English language, abstracts from conferences, or originating from symposiums. Consequently, a total of 13 studies were included in the qualitative analysis, comprising 4 RCTs, 38 , 39 , 40 , 41 4 prospective studies, 42 , 43 , 44 , 45 and 5 retrospective studies. 46 , 47 , 48 , 49 , 50 The search strategy and study inclusion and exclusion criteria are detailed in Figure 1.
FIGURE 1.
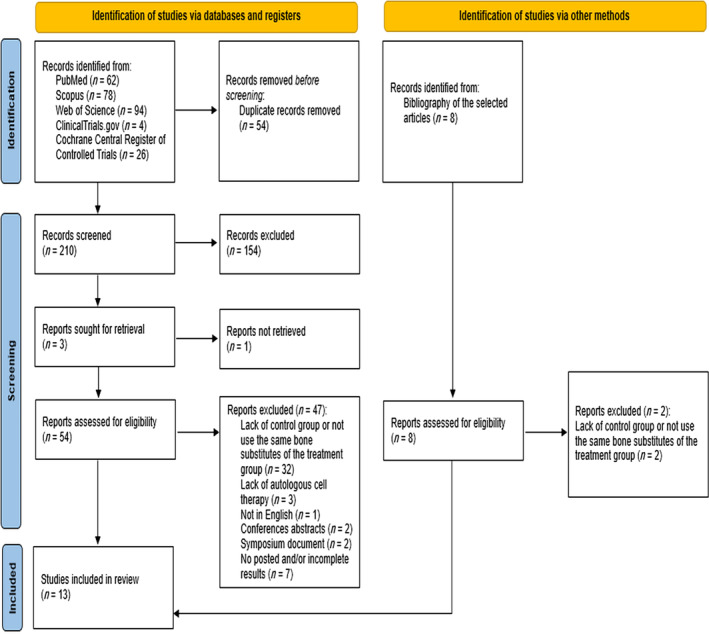
The PRISMA flow diagram for the systematic review detailing the database searches, the number of abstracts screened, and the full texts retrieved.
3.2. Study type
The included studies spanned publication years from 2003 to 2020. 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 Among them, 4 studies were RCTs, 38 , 39 , 40 , 41 while the remaining were prospective (n = 4) 42 , 43 , 44 , 45 and retrospective (n = 5) studies. 46 , 47 , 48 , 49 , 50 Most of the studies were not blinded (n = 7). 38 , 43 , 45 , 46 , 47 , 48 , 49 Six studies were single blinding, 39 , 40 , 41 , 42 , 44 , 50 with blinding applied to the clinical assessor in one study 44 and to radiologists in five studies. 39 , 40 , 41 , 42 , 50
3.3. Risk of bias assessment (RoB)
The RoB assessment for the 13 clinical studies included in this review is presented in Figure 2. Among these, 6 clinical studies were rated as having poor quality, with total scores ranging from 9 to 11. 44 , 45 , 47 , 48 , 49 , 50 Conversely, the remaining 7 studies were categorized as fair or good, with total scores ranging from 15 to 27. 38 , 39 , 40 , 41 , 42 , 43 , 46 Studies with good and fair quality were considered for meta‐analysis. In the reporting section, all included studies clearly described the interventions of interest (n = 13), with 92% of the studies (n = 12) clearly stating the hypothesis/aim/objective, patient characteristics, and main findings. Main outcomes in the introduction or methods were described in 85% of the studies (n = 11), while principal confounders were clearly outlined in 61% (n = 8). Estimates of random variability were provided for main outcomes and all adverse events of the intervention in approximately 54% of the studies (n = 7). Characteristics of patients lost to follow‐up were described in about 46% of the studies (n = 6), and probability values were reported for main outcomes in approximately 38% of the studies (n = 5).
FIGURE 2.

Quality assessment risk of bias using the modified Downs and Black checklist.
Regarding external validity, subjects asked to participate were representative of the source population in only one study, while subjects prepared to participate were representative of the source population in three studies. The location and delivery of study treatment were representative of the source population in approximately 69% of the studies (n = 9).
In the internal validity—bias section, study participants were blinded to treatment in 23% of the studies, and blinded outcome assessment was conducted in approximately 38% of the studies. Data dredging was clearly described in about 46% of the studies (n = 6), and analyses were adjusted for differing lengths of follow‐up in 61% of the studies (n = 8). Appropriate statistical tests were performed in about 69% of the studies (n = 9), and compliance with interventions was deemed reliable in 92% of the studies (n = 12). Outcome measures were considered reliable and valid across all studies (n = 13).
Concerning internal validity—confounding (selection bias), all participants were recruited from the same source population in 77% of the studies (n = 10), and all participants were recruited over the same time in 85% of the studies (n = 11). Participants were randomized to treatments in approximately 31% of the studies (n = 4), and allocation of treatment was concealed from investigators and participants in about 46% of the studies (n = 6). Adequate adjustment for confounding was noted in about 38% of the studies (n = 5), and losses to follow‐up were considered in 61% of the studies (n = 8).
In the power section, sufficient power to detect treatment effects at a significance level of 0.05 was reported in approximately 31% of the studies (n = 4).
3.4. Qualitative analysis
3.4.1. Patient characteristics
The 13 studies included in the systematic review involved 836 patients, comprising 371 males and 444 females, with a mean age of 53 years, ranging from 29 to 61 years for both genders. 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 Patients across all studies presented various degenerative pathologies, including steno‐sis, disc degeneration, disc herniation, spondylolisthesis, pseudoarthrosis, instability, scoliosis, traumatic fractures and cervical anomalies. According to three studies, the mean body mass index (BMI, kg/m2) ranged from 19.84 to 32.9 for the study groups and from 19.94 to 32.1 for the control groups.
3.4.2. Spinal fusion surgery characteristics
Various fusion techniques were employed in the 13 studies included. These techniques encompassed posterolateral lumbar fusion (PLF) in nine studies, intertransverse lumbar interbody fusion (ILIF) in one study, transforaminal lumbar interbody fusion (TLIF) in two studies, 39 , 48 and extremely lateral interbody fusion (XLIF) in one study. 46 Alongside the different fusion methods, associations with specific pedicle screw systems, rod systems, cages, and instrumentation were described in most studies. 39 , 40 , 41 , 42 , 43 , 46 , 50 Most studies conducted one‐, two‐, or three‐level instrumented fusion, focusing predominantly on lumbar or lumbosacral vertebrae (e.g., L3‐L4, L4‐L5, and L5‐S1), 38 , 39 , 40 , 41 , 42 , 44 , 45 , 46 , 47 , 48 , 50 with one study considering the first two cervical vertebrae 43 and another study focusing on thoracic vertebrae. 49
3.4.3. Bone substitutes and autologous cell therapies characteristics
A total of 411 patients underwent treatment involving graft substitutes combined with autologous cell therapies, while 425 patients were part of control groups treated solely with graft substitutes. Concerning cell therapies, 54% of the studies (n = 7) used platelet products, 41 , 42 , 45 , 47 , 48 , 49 , 50 while in 46% of the studies (n = 6), patients received treatment with bone marrow or derived cells. 38 , 39 , 40 , 43 , 44 , 46
For patients treated with platelet products, before surgery, 50–500 mL of blood were drawn to prepare the platelet derivatives. 41 , 42 , 45 , 46 , 47 , 48 , 49 PRP was combined with thrombin to activate platelets, resulting in increased growth factor levels. For platelet gel, a two‐stage pheresis process was conducted to collect three fractions. 45 , 47 , 48 , 50 Fractions containing platelet‐poor plasma and red blood cells were immediately returned to the patient, while the buffy coat, comprising platelets and white cells, was collected. This buffy coat was processed to obtain an autologous growth factor (AGF) concentrate, which was then combined with thrombin and calcium chloride to form a platelet gel or with cryoprecipitate. 45 , 47 , 48 , 50
For patients treated with BMA, a variable bone marrow volume was collected from posterior iliac crest before surgery. 39 , 40 , 43 , 44 , 46 In some studies, whole BMA 44 , 46 or concentrated bone marrow 38 , 40 was used directly in the clinical scenario. In the remaining two studies, BMA was processed in the laboratory to yield concentrated mononuclear cells (MNCs) or to isolate multipotent MSCs. 39 , 43 In the case of MSCs, they were expanded in vitro for 21 days before their use. 39
Regarding the graft substitutes employed in the different studies, in 54% of them (n = 7), autologous iliac crest bone graft (ICBG) was used. 39 , 41 , 43 , 44 , 47 , 48 , 50 In 23% of the studies (n = 3), local autologous bone graft was used. 38 , 45 , 49 Conversely, two studies employed allogeneic bone grafts. 40 , 46 Finally, only one study applied local autologous bone graft associated with tricalcium phosphate/hydroxyapatite (TCP/HA).
3.4.4. Clinical outcomes and imaging features
Spinal fusion success was assessed using x‐ray and/or computerized tomography (CT) scans. X‐ray imaging was used in three studies, 42 , 48 , 50 while CT scans were employed in four studies, 38 , 41 , 47 , 49 and a combination of both x‐ray and CT scans in six studies. 39 , 40 , 43 , 44 , 45 , 46 The evaluation of spinal fusion success occurred at various intervals of follow‐up: at 3 months in three studies, 39 , 43 , 46 at 6 months in four studies, 38 , 39 , 40 , 43 , 46 at 12 months in five studies, 39 , 40 , 44 , 46 , 49 and at 24 months in six studies. 40 , 41 , 42 , 45 , 47 , 50
Concerning complications, they were reported in 11 out of the 13 studies reviewed. Two studies did not report any complications. 44 , 47 Among the reported complications, the most frequently included pseudoarthrosis, 42 , 45 , 48 , 50 wound infections, 39 , 43 chronic pain, and local inflammation. 38 , 43
Clinical outcomes used to assess fusion surgery outcomes were prevalently ODI and VAS, while Short Form Health Survey 36 (SF‐36) was used only in two studies at 3‐, 6‐, 12‐, and 24‐month of follow‐ups. 39 , 41 ODI scores were measured at baseline, 3, and 6 months of follow‐up in four studies, 38 , 39 , 41 , 46 at 12 months of follow‐up in three of these studies, 39 , 41 , 46 and at 24 months of follow‐up only in one study. 41 Concerning VAS score, it was measured at baseline and at 12 months of follow‐up in four studies 39 , 41 , 46 , 49 ; at 3 and 6 months of follow‐up in three studies 39 , 41 , 46 ; at 24 months of follow‐up in one study. 41
Forty‐six percent of the studies (n = 6) utilized autologous cell therapies involving treatment with bone marrow or derived cells. Six‐month postoperative the study by Chotivichit et al. revealed complete PLF bridging in 58.3% of patients treated with local autologous bone associated with bone marrow (BM) concentrate and in 100% of patients treated with local autologous bone alone. 38 Meanwhile, ODI scores did not show differences between groups preoperatively and at various follow‐up intervals. 38 In contrast, Hart et al. observed complete PLF bridging at both 12 and 24 months when BM concentrate was added to allograft. 40 When BMA was combined with autologous bone, no significant differences in fusion rate were observed compared to autologous bone alone 12 months after surgery. 44 Similarly, Lakshmi et al. found higher fusion rates at 3 and 6 months when MNCs were added to autologous bone, though without statistical significance. 43 Likewise, Garcia de Frutos et al. noted significantly higher posterior spinal fusion rates at 6 and 12 months of follow‐up when expanded MSCs were added to the graft substitute. 39 Additionally, they demonstrated post‐surgery clinical improvements in ODI, VAS, and SF‐36 without significant differences between groups. 39
Regarding autologous cell therapies based on platelet derivatives, 54% of the studies (n = 7) utilized them. Acebal‐Cortina et al. demonstrated a lower fusion rate (74.6% vs. 92.5%) when autologous platelet concentrate (APC) was added to a mixture of local autograft plus TCP/HA. 42 In contrast, 12 months after surgery, Hartmann et al. did not observe significant differences in spinal fusion rates and VAS scores when autologous bone with PRP was compared to autologous bone alone. 40 Similarly, Carreon et al., Castro et al., and Tsai et al., using autologous bone mixed with platelet gel or glue, did not find significant differences in spinal fusion rates com‐pared to autologous bone alone. 45 , 47 , 48 Weiner et al. reported a lower fusion rate in autologous bone with platelet gel compared to autologous bone alone at 24 months post‐surgery. 50 Finally, Sys et al., evaluating patients treated with autologous bone with PRP versus autologous bone alone, noted a higher improvement in VAS, ODI, and SF‐36 from baseline to 24 months of follow‐up in patients who received autologous bone with PRP, though without statistical significance. 41
3.5. Quantitative analysis
Six studies were excluded from the meta‐analysis due to poor quality, 44 , 45 , 47 , 48 , 49 , 50 as determined by the modified Downs and Black checklist. Consequently, the meta‐analysis was conducted using data from seven studies. 38 , 39 , 40 , 41 , 42 , 43 , 46
3.5.1. Fusion success
Three studies reported spinal fusion success at 3 months, 39 , 43 , 46 four studies at 6 months, 38 , 39 , 43 , 46 and three studies at 12 months. 39 , 40 , 46 No studies reporting spinal fusion success at 24 months of follow‐up. Frequency analysis based on OR between the groups receiving graft substitutes alone and those receiving graft substitutes with autologous cell therapies revealed a significantly higher spinal fusion success rate in the graft substitutes with autologous cell therapies group compared to the graft substitutes alone group at 3‐, 6‐, and 12‐month follow‐ups (3 months: p = 0.003, 6 months: p = 0.048, 12 months: p = 0.001) (Figure 3).
FIGURE 3.
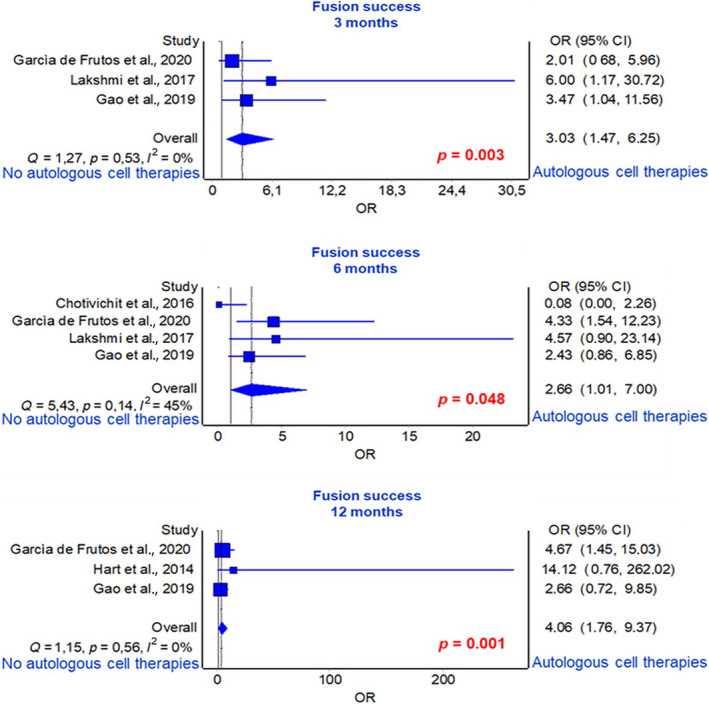
Forest plot of fusion success at 3‐, 6‐, and 12‐month of follow‐ups.
3.5.2. Complications
Frequency analysis based on odds ratio from seven studies did not reveal significant differences between the groups receiving graft substitutes alone and those receiving graft substitutes with autologous cell therapies (p = 0.342) (Figure 4).
FIGURE 4.
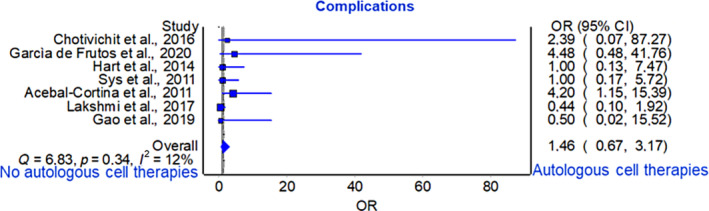
Forest plot for complications.
3.5.3. Oswestry Dysfunction Index (ODI)
Four studies reported ODI scores preoperatively, 4 at 3 months, 38 , 39 , 41 , 46 4 at 6 months, 38 , 39 , 41 , 46 and 3 at 12 months. 39 , 41 , 46 However, ODI scores at 24 months of follow‐up were reported only in one study, 41 so no meta‐analysis was conducted for this time point. Average analysis based on Weighted Mean Difference (WMD) of ODI scores revealed a significantly greater improvement in the group receiving graft substitutes with autologous cell therapies, with the lowest score values, compared to the group receiving graft substitutes alone at 3‐, 6‐, and 12‐month of follow‐ups (3 months: p = 0.022, 6 months: p = 0.006, 12 months: p = 0.002) (Figure 5).
FIGURE 5.
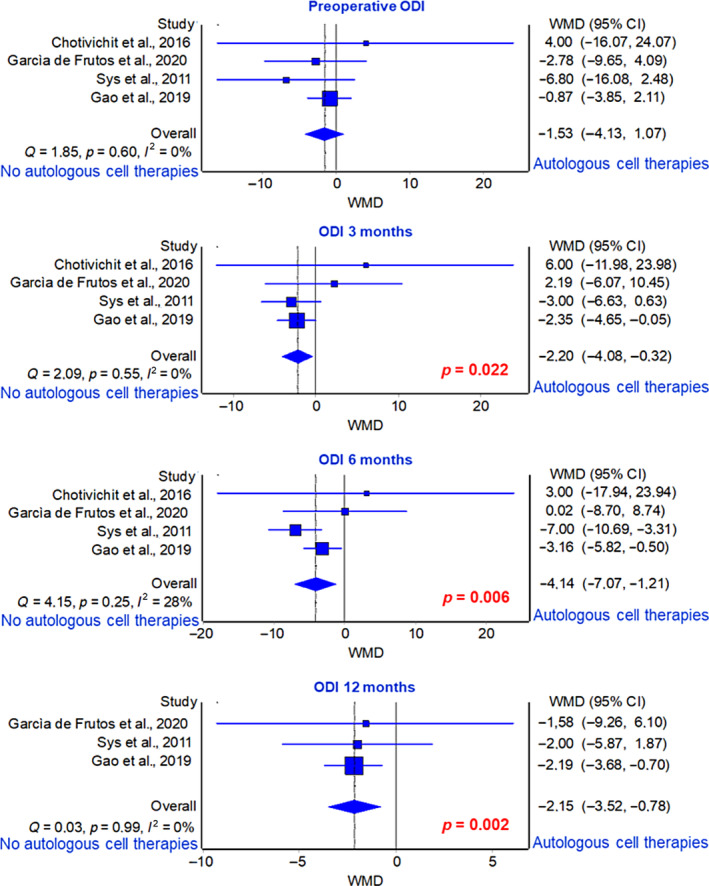
Forest plot of ODI score at 3‐, 6‐, and 12‐month of follow‐ups.
3.5.4. Visual Analogue Scale (VAS)
Three studies reported VAS score pre‐operatively, at 3‐, 6‐, and 12‐months of follow‐up. 39 , 41 , 46 Since the VAS score at 24 months of follow‐up was reported only in one study, 41 no meta‐analysis was conducted. Average analysis based on WMD of VAS scores revealed a significantly greater improvement in the group receiving graft substitutes with autologous cell therapies, with the lowest score values, compared to the group receiving graft substitutes alone at 6 months of follow‐up (p = 0.039) (Figure 6).
FIGURE 6.
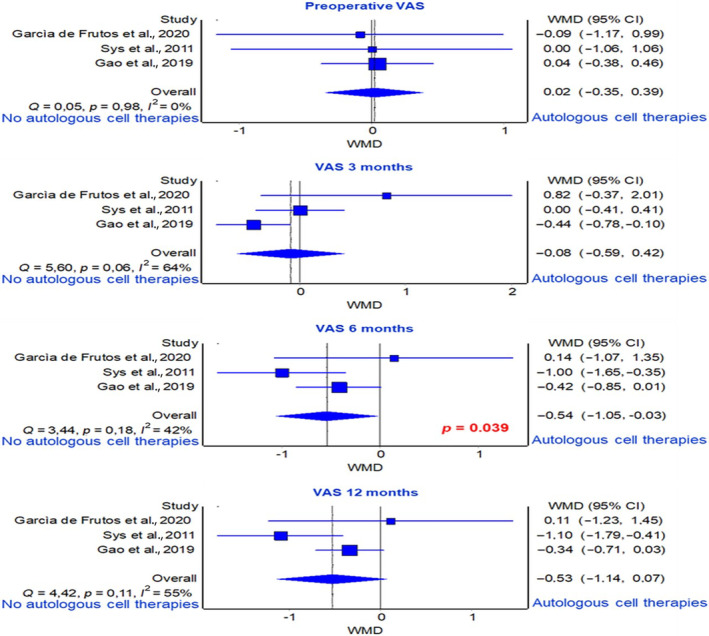
Forest plot of VAS score at 3‐, 6‐, and 12‐month of follow‐ups.
4. DISCUSSION
This systematic review and meta‐analysis demonstrated that in the context of spinal fusion surgery the use of autologous cellular therapies associated to a graft substitute were linked to a better fusion rate over the use of graft substitute alone. Another benefit of cellular therapies associated to a graft substitute over graft substitute alone was linked to clinical patient‐reported outcome, while no differences were found in complications rate.
Spinal fusion is one of the most common surgical procedures used to treat various spinal pathologies. Since 1990, spinal fusion procedures have increased by more than 220%, surpassing the combined increase for knee and hip arthroplasty. 51 The primary goal of spinal fusion surgery is to fuse two or more vertebrae between them, achieving a complete mobilization. Insufficient bony fusion or pseudoarthrosis/non‐union after spinal arthrodesis can lead to loss of correction, instrumentation failure, or deterioration in patients' quality of life. 52 , 53 , 54 , 55 Recently, interest in using cellular therapies associated to different graft substitutes for spinal fusion has increased. 25 , 55 , 56 Although numerous pre‐clinical in vivo data on specific cellular therapies, that is, BMA and derived cells or platelet product, associated to biological or synthetic graft substitutes in spinal fusion has been extensively documented reporting encouraging results, the clinical data are still controversial. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 57 , 58 , 59
This meta‐analysis is the first that analyzed and compare autologous cellular therapies associated to a graft substitute and graft substitute alone for spinal fusion procedures. The autologous cellular therapies used in this review were bone marrow or derived cells and platelet derivatives. The analysis between graft substitutes alone and graft substitutes with autologous cell therapies groups demonstrated a significant higher spinal fusion rate in graft substitutes with autologous cell therapies in comparison to graft substitutes alone at all follow‐ups (3 months: 59.5% vs. 33.9%; 6 months: 69.5% vs. 59.0%; 12 months: 62.5% vs. 42.4%). The higher fusion rate underlined a superior rigidity and limited range of motion in graft substitutes with autologous cell therapies in comparison to graft substitutes alone. 60 A prospective study 42 and an RCT 40 also reported spinal fusion at 24 months of follow‐up. One of this study showed that the addition of BM concentrates to allograft evidenced unilateral continuous bridging bone in 80% of patients in comparison to a 40% observed in patients treated with allograft alone. 40 Differently, Acebal‐Cortina et al. showed that the adding of APC to a mixture of autologous bone graft plus TCP/HA decreased the rates of PLF. 42 Although there are no epidemiological differences between groups, a key aspect to underline in this study is the individual variability, which prevents precise determination of the amount and quality of AGFs in the platelet concentration harvest. 61
With respect to clinical outcomes used to assess fusion surgery outcomes and to provide an estimate average of improvement following surgical treatment, the most used among the analyzed studies were ODI and VAS. ODI score demonstrated a significant improvement in graft substitutes with autologous cell therapies in comparison to graft substitutes alone at 3‐, 6‐, and 12‐month of follow‐ups. In other words, these results showed that patient symptoms improve in a clinically meaningful way at all studied time‐points following surgery. Therefore, on average, a patient undergoing spinal fusion surgery is likely to experience significant improvement in functional status and pain when treated with a graft substitute associated to autologous cell therapies. Different from ODI, VAS demonstrated a significant improvement in graft substitutes with autologous cell therapies in comparison to graft substitutes alone groups only at 6 months of follow‐up. 62 A key reason for the difference between ODI and VAS values at different follow‐ups may be that, unlike the ODI, the VAS is used to assess pain rather than functional impairment and each study evaluating it used different calculation methods. 63 , 64 As such, because it varies more with baseline patient characteristics and indications for surgery, each method can come up with a different minimum clinically important difference. In addition, it was detected that clinical outcomes were highly interrelated with spinal alignment and spinopelvic parameters (pelvic index, pelvic tilt, sacral slope, sagittal vertical axis), as well as to spinopelvic‐femoral parameters such as femoral obliquity angle and T1 pelvic angle. 65 , 66 Thus, these parameters should be considered.
Concerning complications our analyses did not demonstrate significant differences between graft substitutes alone and graft substitutes with autologous cell therapies groups. Complications arising from spinal fusion are of paramount importance, as they can undermine the best outcomes, leading to patient morbidity and mortality. Therefore, the avoidance of complications is a top priority for spine surgeons. 67
There are several limitations in this meta‐analysis. First, there are few (n = 4) RCTs included in this study. Second, the number of patients included in the meta‐analysis is relatively small and heterogeneity was observed among these patients. This heterogeneity stemmed from clinical diversity in both treatment groups, supported by differences in assessing patients' baseline and outcomes, and the absence of systematic reports (e.g., the use of tobacco or drugs could have led to a misinterpretation of fusion rates). Third, potential clinical heterogeneity, related to different pathological conditions and fusion techniques, number of segments fused, use of internal fixation instrumentation, and the amounts of grafts provided, should also be considered. Finally, our analysis demonstrated a substantial variety in grafts substitute and cellular therapies used.
In summary, based on this meta‐analysis, the enrichment with an autologous cellular source combined with graft substitute peri‐operatively seem to be an effective strategy for spinal fusion surgery. However, future research in spinal fusion surgery should prioritize investigating the long‐term efficacy and safety of autologous cellular therapies combined with graft substitutes. Well‐designed randomized controlled trials with extended follow‐up periods beyond 2 years could offer insights into fusion outcomes' durability and late complications associated with cellular therapies. Additionally, research should address the lack of high‐level evidence in specific patient populations such as those with osteoporosis, diabetes, and smokers, aiming to tailor treatment approaches and improve outcomes in individuals with comorbidities. Efforts should also target understanding treatment response variability among different demographic groups, including age, gender, and ethnicity, for more personalized insights into cellular therapies' effectiveness across diverse patient populations. Establishing large multicenter prospective registries could facilitate collecting real‐world data on cellular therapies' utilization and outcomes, enhancing our understanding of treatment patterns, long‐term outcomes, and potential adverse events on a broader scale. Overall, future research should prioritize rigorous study designs, longer follow‐up periods, inclusion of diverse patient populations, and collaboration among research institutions to advance knowledge in spinal fusion surgery and improve patient outcomes.
AUTHOR CONTRIBUTIONS
Conceptualization: F.S. Methodology: F.S. and D.C. Validation: G.T. and A.R. Formal analysis: F.S., D.C., M.M., G.V., and M.T. Investigation: F.S. and D.C. Data curation: F.S., D.C., M.M., G.V., and M.T. Writing‐original draft preparation: F.S., D.C., G.T., and A.R. Writing‐review and editing: F.S., D.C., G.G., G.T., and A.R. Visualization: F.S., D.C., G.T., A.R., M.M., G.V., M.T., C.F., and G.G. Supervision: C.F. and G.G. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Table S1. Combination of free‐vocabulary and/or MeSH terms for the identification of studies in PubMed, Scopus, Web of Science, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials.
ACKNOWLEDGMENTS
The authors thank Elettra Pignotti for her contribution to the statistical analysis. Open access funding provided by BIBLIOSAN.
Salamanna F, Contartese D, Tedesco G, et al. Efficacy of using autologous cells with graft substitutes for spinal fusion surgery: A systematic review and meta‐analysis of clinical outcomes and imaging features. JOR Spine. 2024;7(3):e1347. doi: 10.1002/jsp2.1347
REFERENCES
- 1. Katsuura Y, Shafi K, Jacques C, Virk S, Iyer S, Cunningham M. New strategies in enhancing spinal fusion. HSS J. 2020;16(2):177‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37(1):67‐76. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Jiang Y, Zou D, Yuan B, Ke HZ, Li W. Therapeutics for enhancement of spinal fusion: a mini review. J Orthop Translat. 2021;31:73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stephan SR, Kanim LE, Bae HW. Stem cells and spinal fusion. Int J Spine Surg. 2021;15(s1):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthrosis in long adult spinal deformity instrumentation and fusion to the sacrum: prevalence and risk factor analysis of 144 cases. Spine (Phila Pa 1976). 2006;31(20):2329‐2336. [DOI] [PubMed] [Google Scholar]
- 6. Dickson DD, Lenke LG, Bridwell KH, Koester LA. Risk factors for and assessment of symptomatic pseudarthrosis af‐ter lumbar pedicle subtraction osteotomy in adult spinal deformity. Spine (Phila Pa 1976). 2014;39(15):1190‐1195. [DOI] [PubMed] [Google Scholar]
- 7. Hermann PC, Webler M, Bornemann R, et al. Influence of smoking on spinal fusion after spondylodesis surgery: a comparative clinical study. Technol Health Care. 2016;24(5):737‐744. [DOI] [PubMed] [Google Scholar]
- 8. Buchlak QD, Yanamadala V, Leveque JC, Sethi R. Complication avoidance with pre‐operative screening: insights from the Seattle spine team. Curr Rev Musculoskelet Med. 2016;9(3):316‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim YJ, Bridwell KH, Lenke LG, Rinella AS, Edwards C 2nd. Pseudarthrosis in primary fusions for adult idiopathic scoliosis: incidence, risk factors, and outcome analysis. Spine (Phila Pa 1976). 2005;30(4):468‐474. [DOI] [PubMed] [Google Scholar]
- 10. Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976). 2002;27(16 suppl 1):S26‐S31. [DOI] [PubMed] [Google Scholar]
- 11. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single‐level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2003;28(2):134‐139. [DOI] [PubMed] [Google Scholar]
- 12. Liu Z, Zhu Y, Ge R, et al. Combination of bone marrow mesenchymal stem cells sheet and platelet rich plasma for posterolateral lumbar fusion. Oncotarget. 2017;8(37):62298‐62311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salamanna F, Tedesco G, Sartori M, et al. Safety and efficacy of autologous bone marrow clot as a multifunctional bioscaffold for in‐strumental posterior lumbar fusion: a 1‐year follow‐up pilot study. Front Endocrinol (Lausanne). 2024;14:1245344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salamanna F, Cepollaro S, Contartese D, et al. Bio‐logical rationale for the use of vertebral whole bone marrow in spinal surgery. Spine (Phila Pa 1976). 2018;43(20):1401‐1410. [DOI] [PubMed] [Google Scholar]
- 15. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone marrow aspirate concentrate harvest‐ing and processing technique. Arthrosc Tech. 2017;6(2):e441‐e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7(4):568‐578. [DOI] [PubMed] [Google Scholar]
- 17. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lad SP, Bagley JH, Karikari IO, et al. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73(3):440‐449. [DOI] [PubMed] [Google Scholar]
- 19. Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP‐2) in spine surgery. Spine J. 2014;14(3):552‐559. [DOI] [PubMed] [Google Scholar]
- 20. Crevensten G, Walsh AJ, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32(3):430‐434. [DOI] [PubMed] [Google Scholar]
- 21. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143‐147. [DOI] [PubMed] [Google Scholar]
- 22. Jing W, Smith AA, Liu B, et al. Reengineering autologous bone grafts with the stem cell activator WNT3A. Biomaterials. 2015;47:29‐40. [DOI] [PubMed] [Google Scholar]
- 23. Salamanna F, Sartori M, Barbanti Brodano G, et al. Mesenchymal stem cells for the treatment of spinal arthrodesis: from preclinical research to clinical scenario. Stem Cells Int. 2017;2017:3537094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitchel SH. A preliminary comparative study of radiographic results using mineralized collagen and bone marrow aspi‐rate versus autologous bone in the same patients undergoing posterior lumbar interbody fusion with instrumented poster‐olateral lumbar fusion. Spine J. 2006;6(4):405‐412. [DOI] [PubMed] [Google Scholar]
- 25. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet‐rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602‐608. [DOI] [PubMed] [Google Scholar]
- 26. Marx RE. Platelet‐rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225‐228. [DOI] [PubMed] [Google Scholar]
- 27. Manini DR, Shega FD, Guo C, Wang Y. Role of platelet‐rich plasma in spinal fusion surgery: systematic review and meta‐analysis. Adv Orthop. 2020;2020:8361798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoo JS, Ahn J, Patel DS, Hrynewycz NM, Brundage TS, Singh K. An evaluation of biomaterials and osteobiologics for arthrodesis achievement in spine surgery. Ann Transl Med. 2019;7(Suppl 5):S168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106:420‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saaiq M, Ashraf B. Modifying "Pico" question into "Picos" model for more robust and reproducible presentation of the methodology employed in a scientific study. World J Plast Surg. 2017;6:390‐392. [PMC free article] [PubMed] [Google Scholar]
- 31. Delgado DA, Lambert BS, Boutris N, et al. Validation of digital visual analog scale pain scoring with a traditional paper‐based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2(3):e088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Page SJ, Shawaryn MA, Cernich AN, Linacre JM. Scaling of the revised Oswestry low back pain questionnaire. Arch Phys Med Rehabil. 2002;83(11):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 33. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of ran‐domised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kennelly J. Methodological approach to assessing the evidence. In: Handler A, Kennelly J, Peacock N, eds. Reducing Racial/Ethnic Disparities in Reproductive and Perinatal Outcomes. Springer; 2011:7‐19. [Google Scholar]
- 36. Neyeloff JL, Fuchs SC, Moreira LB. Meta‐analyses and Forest plots using a microsoft excel spreadsheet: step‐by‐step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 38. Chotivichit A, Ruangchainikom M, Tongdee T, Wongkajornsilp A, Permpikul P, Korwutthikulrangsri E. A Prospec‐tive randomized controlled trial comparing posterolateral lumbar fusion with and without bone marrow concentrate augmentation in single‐level lumbar spondylolisthesis. J Med Assoc Thai. 2016;99(10):1073‐1079. [PubMed] [Google Scholar]
- 39. García de Frutos A, González‐Tartière P, Coll Bonet R, et al. Randomized clinical trial: expanded autologous bone marrow mesenchymal cells com‐bined with allogeneic bone tissue, compared with autologous iliac crest graft in lumbar fusion surgery. Spine J. 2020;20(12):1899‐1910. [DOI] [PubMed] [Google Scholar]
- 40. Hart R, Komzák M, Okál F, Náhlík D, Jajtner P, Puskeiler M. Allograft alone versus allograft with bone marrow con‐centrate for the healing of the instrumented posterolateral lumbar fusion. Spine J. 2014;14(7):1318‐1324. [DOI] [PubMed] [Google Scholar]
- 41. Sys J, Weyler J, Van Der Zijden T, Parizel P, Michielsen J. Platelet‐rich plasma in mono‐segmental posterior lumbar interbody fusion. Eur Spine J. 2011;20(10):1650‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Acebal‐Cortina G, Suárez‐Suárez MA, García‐Menéndez C, Moro‐Barrero L, Iglesias‐Colao R, Torres‐Pérez A. Evalu‐ation of autologous platelet concentrate for intertransverse lumbar fusion. Eur Spine J. 2011;20(suppl 3):361‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lakshmi Prasad G, Kale SS, Mohanty SP, Sinha S, Chandra PS. Autologous iliac crest bone marrow mononuclear cells in bone fusion. J Clin Diagn Res. 2017;11(12):PC11‐PC15. [Google Scholar]
- 44. Niu CC, Tsai TT, Fu TS, Lai PL, Chen LH, Chen WJ. A comparison of posterolateral lumbar fusion comparing au‐tograft, autogenous laminectomy bone with bone marrow aspirate, and calcium sulphate with bone marrow aspirate: a prospective randomized study. Spine (Phila Pa 1976). 2009;34(25):2715‐2719. [DOI] [PubMed] [Google Scholar]
- 45. Tsai CH, Hsu HC, Chen YJ, Lin MJ, Chen HT. Using the growth factors‐enriched platelet glue in spinal fusion and its efficiency. J Spinal Disord Tech. 2009;22(4):246‐250. [DOI] [PubMed] [Google Scholar]
- 46. Gao Y, Li J, Cui H, et al. Comparison of intervertebral fusion rates of different bone graft materials in extreme lateral interbody fusion. Medicine (Baltimore). 2019;98(44):e17685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carreon LY, Glassman SD, Anekstein Y, Puno RM. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine (Phila Pa 1976). 2005;30(9):E243‐E247. [DOI] [PubMed] [Google Scholar]
- 48. Castro FPJ. Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17(5):380‐384. [DOI] [PubMed] [Google Scholar]
- 49. Hartmann EK, Heintel T, Morrison RH, Weckbach A. Influence of platelet‐rich plasma on the anterior fusion in spinal injuries: a qualitative and quantitative analysis using computer tomography. Arch Orthop Trauma Surg. 2010;130(7):909‐914. [DOI] [PubMed] [Google Scholar]
- 50. Weiner BK, Walker M. Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine (Phila Pa 1976). 2003;28(17):1968‐1971. [DOI] [PubMed] [Google Scholar]
- 51. Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976). 2005;30(12):1441‐1447. [DOI] [PubMed] [Google Scholar]
- 52. Cannada LK, Scherping SC, Yoo JU, Jones PK, Emery SE. Pseudoarthrosis of the cervical spine: a comparison of ra‐diographic diagnostic measures. Spine (Phila Pa 1976). 2003;28(1):46‐51. [DOI] [PubMed] [Google Scholar]
- 53. Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long‐term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004;29(7):726‐734. [DOI] [PubMed] [Google Scholar]
- 54. Makino T, Kaito T, Fujiwara H, et al. Risk factors for poor patient‐reported quality of life outcomes after posterior lumbar Interbody fusion: An analysis of 2‐year follow‐up. Spine (Phila Pa 1976). 2017;42(19):1502‐1510. [DOI] [PubMed] [Google Scholar]
- 55. Makino T, Kaito T, Fujiwara H, et al. Does fusion status after posterior lumbar interbody fusion affect patient‐based QOL outcomes? An evaluation performed using a patient‐based outcome measure. J Orthop Sci. 2014;19(5):707‐712. [DOI] [PubMed] [Google Scholar]
- 56. Salamanna F, Veronesi F, Maglio M, Della Bella E, Sartori M, Fini M. New and emerging strategies in platelet‐rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed Res Int. 2015;2015:846045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blanco JF, Villarón EM, Pescador D, et al. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: results of a prospective phase I/II clinical trial with long‐term follow‐up. Stem Cell Res Ther. 2019;10(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with po‐rous beta‐tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29(29):3973‐3982. [DOI] [PubMed] [Google Scholar]
- 59. Mazziotta C, Badiale G, Cervellera CF, Tognon M, Martini F, Rotondo JC. Regulatory mechanisms of circular RNAs during human mesenchymal stem cell osteogenic differentiation. Theranostics. 2024;14(1):143‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fogel GR, Turner AW, Dooley ZA, Cornwall GB. Biomechanical stability of lateral interbody implants and supple‐mental fixation in a cadaveric degenerative spondylolisthesis model. Spine (Phila Pa 1976). 2014;39(19):E1138‐E1146. [DOI] [PubMed] [Google Scholar]
- 61. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet‐rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97‐102. [DOI] [PubMed] [Google Scholar]
- 62. Brodke DS, Goz V, Lawrence BD, Spiker WR, Neese A, Hung M. Oswestry Disability Index: a psychometric analysis with 1,610 patients. Spine J. 2017;17(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 63. Nayak NR, Coats JM, Abdullah KG, Stein SC, Malhotra NR. Tracking patient‐reported outcomes in spinal disorders. Surg Neurol Int. 2015;6(Suppl 19):S490‐S499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ravishankar P, Winkleman R, Rabah N, Steinmetz M, Mroz T. Analysis of patient‐reported outcomes measures used in lumbar fusion surgery research for degenerative spondylolisthesis. Clin Spine Surg. 2022;35(6):287‐294. [DOI] [PubMed] [Google Scholar]
- 65. Yoshihara H, Hasegawa K, Okamoto M, Hatsushikano S, Watanabe K. Relationship between sagittal radiographic pa‐rameters and disability in patients with spinal disease using 3D standing analysis. Orthop Traumatol Surg Res. 2018;104(7):1017‐1023. [DOI] [PubMed] [Google Scholar]
- 66. Perna A, Proietti L, Smakaj A, et al. The role of femoral obliquity angle and T1 pelvic angle in predicting quality of life after spinal surgery in adult spinal deformities. BMC Musculoskelet Disord. 2021;22(Suppl 2):999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brown CA, Eismont FJ. Complications in spinal fusion. Orthop Clin North Am. 1998;29(4):679‐699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Combination of free‐vocabulary and/or MeSH terms for the identification of studies in PubMed, Scopus, Web of Science, ClinicalTrials.gov, and Cochrane Central Register of Controlled Trials.


