Abstract
While endoscopic retrograde cholangiopancreatography (ERCP) remains the primary treatment modality for common bile duct stones (CBDS) or choledocholithiasis due to advancements in instruments, surgical intervention, known as common bile duct exploration (CBDE), is still necessary in cases of difficult CBDS, failed endoscopic treatment, or altered anatomy. Recent evidence also supports CBDE in patients requesting single-step cholecystectomy and bile duct stone removal with comparable outcomes. This review elucidates relevant clinical anatomy, selection indications, and outcomes to enhance surgical understanding. The selection between trans-cystic (TC) vs trans-choledochal (TD) approaches is described, along with stone removal techniques and ductal closure. Detailed surgical techniques and strategies for both the TC and TD approaches, including instrument selection, is also provided. Additionally, this review comprehensively addresses operation-specific complications such as bile leakage, stricture, and entrapment, and focuses on preventive measures and treatment strategies. This review aims to optimize the management of CBDS through laparoscopic CBDE, with the goal of improving patient outcomes and minimizing risks.
Keywords: Choledocholithiasis, Common bile duct stone, Difficult common bile duct stone, Common bile duct exploration, Laparoscopic common bile duct exploration
Core Tip: Although the endoscopic-first approach is the most common for common bile duct stones (CBDS) treatment, surgical management remains crucial for subsequent cholecystectomy and additional CBDS removal in cases of failed endoscopy or surgical altered anatomy. Single-step common bile duct exploration offers non-inferior outcomes and may even advantageously reduce the number of hospital admissions. Proper selection between the trans-cystic and trans-choledochal approach is essential, and mastering both techniques through practice and rehearsal is necessary. Diligent attention to surgical anatomy and every step of the procedure can help prevent and reduce specific postoperative complications.
INTRODUCTION
Synchronous common bile duct stone (CBDS) or choledocholithiasis affects approximately 10%-20% of patients with gallstone (GS), a condition known as cholecystolithiasis or cholelithiasis[1,2]. This prevalence is attributed to advanced diagnostic modalities such as endoscopic ultrasonography (EUS) and magnetic resonance cholangiopancreatography[2,3]. CBDS can lead to various serious sequelae, including obstructive jaundice, ascending cholangitis, hepatic abscess, or GS pancreatitis. Although up to 50% of CBDS cases may remain asymptomatic, addressing and treating them in appropriate patients is crucial[2,4,5]. According to current recommendations, endoscopic retrograde cholangiopancreatography (ERCP) with advance adjuncts is the primary treatment approach[6,7]. In the field of ERCP, innovative endoscopic techniques for stone removal have significantly increased, including endoscopic large balloon dilatation, mechanical lithotripsy, cholangioscopy-assisted lithotripsy, electrohydraulic lithotripsy, extracorporeal shock wave lithotripsy, and laser lithotripsy, all of which have gained acceptance[6,8]. Additionally, the integration of artificial intelligence (AI) in CBDS management represents a significant advancement in the field of endoscopy. AI-based models offer the potential to enhance predictive accuracy and streamline decision-making of cannulation, as well as improve difficulty scoring systems for endoscopic stone removal[9-12]. Nonetheless, surgical management continues to play a crucial role in subsequent prophylactic cholecystectomy to prevent biliary-related sequelae. It is also essential in cases of difficult CBDS, particularly when dealing with large impacted CBDS or altered upper gastrointestinal tract anatomy[6,13,14]. This review aims to provide contemporary surgical concepts in CBDS treatment, with the ultimate objective of optimizing overall therapeutic outcomes.
BRIEF REVIEW OF RELEVANT CLINICAL ANATOMY
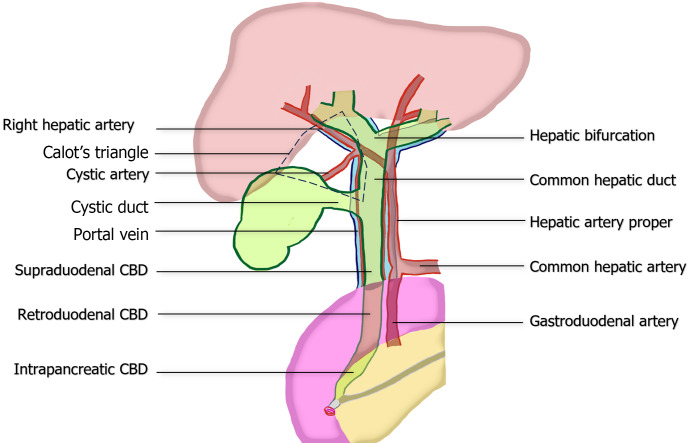
Despite a thorough preoperative evaluation, surgeons encounter difficulty in determining the anatomic structures surrounding the portal triad and the hepatocystic triangle of Calot because of the marked variations in these structures and the adhesions caused by prior inflammation. The extrahepatic bile duct (EHBD) descends from the hepatic bifurcation (porta hepatis) to the Ampulla of Vater in the second part of duodenum. It consists of the common hepatic duct (CHD) and the CBD, with the CHD located superior to the cystic duct junction and the CBD in the lower portion. The CBD can be further classified into three levels: Supraduodenal, retroduodenal, and intrapancreatic, based on its anatomical relationship to the duodenum. In its normal course, CHD/CBD runs anterior to portal vein and to the right of hepatic artery proper[15]. These three structures are collectively referred as the portal triad, and are enveloped by a connective tissue known as the hepatoduodenal ligament (HDL).
The boundaries of Calot’s triangle include the lower liver border, cystic duct, and CHD. The main arterial blood supply to the CHD/CBD typically arises from the right hepatic artery (RHA), which approaches CBD at the 3 and 9 o’clock positions[16-18]. Therefore, preserving blood supply in these areas is essential to ensure adequate tissue healing and reduce the risk of future strictures. Within Calot's triangle, several significant variations of the biliary tree or arteries may be encountered, such as the right posterior bile duct draining into the CHD, right posterior bile duct draining into the cystic duct, or aberrant RHA passing through the triangle. These variations can lead to complications if not handled with caution[19,20]. The critical anatomy is summarized in Figure 1.
Figure 1.
Common anatomy of portal triad, illustrating the relationship between the extrahepatic bile duct, hepatic artery proper, and portal vein. The figure also demonstrates the boundaries of the hepatocystic triangle of Calot and vascular supply to common bile duct at the 3 and 9 o’clock position. CBD: Common bile duct
Regarding the cystic artery, it mostly branches off from RHA (79%) within in the Calot’s triangle (82%) and traverse to the gallbladder. However, aberrant RHA and replaced RHA are observed in 15%-35% and 5%-21% of cases, respectively[21,22]. Approximately one-fourth of cystic arteries originate outside the triangle, either superficially or deeply to CHD/CBD[19,22-24]. During laparoscopic identification, the most common position of cystic artery in relation to cystic duct is superomedial (90.6%), followed by posterolateral, anterior, and absence positions, respectively[25].
In addition to the preoperative bile duct imaging, the anatomy EHBD can be evaluated intraoperatively through real-time cholangiography using fluoroscopy or indocyanine green (ICG) with a fluorescence imaging system[26,27]. However, a comprehensive understanding of basic EHBD anatomy and careful imaging evaluation is essential to minimize the risk of complications.
ENDOSCOPY APPROACH VERSUS SURGERY
CBDS management involves not only the CBD clearance, but also potential gallbladder removal. While recent advancements in endoscopic technologies mostly allow for successful CBD clearance, surgical cholecystectomy is still recommended in all surgically-fit candidates, either following endoscopy or simultaneously, as it remains the standard approach in numerous guidelines[6,7,28]. After endoscopic sphincterotomy (EST) and CBDS removal, a wait-and-see policy is associated with a significant increase in recurrent biliary symptoms, ranging from 5.1 to 22.4 times, and a 2.56-fold increase in mortality compared to prophylactic cholecystectomy. Interestingly, 26% of patients in the wait-and-see group required subsequent cholecystectomy, and the rate of open conversion increased by 2.67 times[29,30]. Therefore, subsequent cholecystectomy is encouraged in all patients with CBDS in medically-fit conditions. Several approaches for CBDS treatment have been discussed, including two-step ERCP with stone extraction followed by laparoscopic cholecystectomy (LC), LC with intraoperative ERCP, same-day approach of ERCP followed by LC or LC followed by postoperative ERCP, one-step LC with laparoscopic common bile duct exploration (LC-LCBDE), and one-step open surgery[2,31-33].
The surgical approach to remove CBDS, known as CBDE, can be carried out using minimally invasive techniques, such as laparoscopic surgery or robotic surgery, or through an open approach. If the minimally invasive approach is available, it is the preferred first line surgical treatment, particularly LC-LCBDE in high-volume institutions. Although robotic surgeries are on the rise, available publications of robot-assisted CBDE only exist in the form of case series and reports, all of them claimed it to be safe and feasible, with minimally invasive benefits[34,35]. In a randomized controlled trial comparing laparoscopic vs open CBDE, no significant differences were observed in terms of stone clearance rate (94.2% vs 96.6%, P > 0.2), operative time (82 min vs 90 min, P > 0.1), and overall morbidity (6.5% vs 12.7%, P > 0.05). However, the laparoscopic group demonstrated significantly less intraoperative blood loss (20 mL vs 285 mL, P < 0.01) and shorter length of stay (4.2 d vs 12.6 d, P < 0.01)[36].
Recent evidence highlights the advantages of the single-step LC-LCBDE approach over the conventional ERCP followed by LC. Numerous studies have shown that the one-step LC-LCBDE reduces hospital admissions, shortens length of stay, and lowers hospital costs compared to ERCP followed by LC[7,31,37-39]. Regarding treatment outcomes, meta-analyses conducted in 2019, 2021, and 2023 consistently demonstrated no significant difference in success rate, CBDS clearance, major morbidity, mortality, or total procedural time between LC-LCBDE and ERCP followed by LC. However, LC-LCBDE has shown a higher rate of bile leakage, and a lower rate of postoperative pancreatitis and cholangitis[40,41]. Conversely, a meta-analysis in 2018 revealed a higher CBDS clearance rate (94.1% vs 90.1%, P = 0.012), lower retained stones rate (1.2% vs 7.9%, P = 0.004), and lower stone recurrence (1.8% vs 5.6%, P = 0.005) with LC-LCBDE compared to ERCP then LC[42]. Another approach advocated by some specialists to shorten hospital stays and reduce admissions is LC with intraoperative ERCP. A 2021 meta-analysis demonstrated comparable rates of technical success, morbidity, and conversion between LC-LCBDE and intraoperative ERCP with LC. However, LC-LCBDE was associated with higher rates of biliary leaks, lower rates of postoperative pancreatitis, and an increased rate of retained stones[43].
On the other hand, LCBDE potentially reduces post-ERCP/EST complications such as pancreatitis, bleeding, duodenal perforation, and permanent Oddi’s sphincter injury, which can lead to papillary stenosis, duodeno-biliary reflux, and sine-materia cholangitis[44]. Limited endoscopy services in some countries, including Thailand, due to a shortage of trained endoscopists further emphasize the significance of LC-LCBDE as a potential first line treatment option for CBDS[45]. If evidence supporting LC-LCBDE in CBDS treatment becomes clearer, including from trials like the Gallstep trial, single-step LC-LCBDE can be considered another first line treatment in the near future.
Learning how to perform safe LCBDE using several techniques is essential. Surgeons have been able to reduce the operative time and postoperative complications of LCBDE after gaining experience handling 54 cases and implementing primary ductal suture[46]. Centers performing LCBDE should be high-volume institutes with experienced surgeons in minimally invasive or hepatobiliary surgery, who have comprehensive knowledge of biliary anatomy, skills to perform and interpret intraoperative cholangiogram (IOC) and intraoperative ultrasonography (IOUS) results, proficiency in intracorporeal suturing, and competence in various techniques for CBDS extraction, such as the Chopstick technique, balloon extraction, Dormia basket, and choledochoscopy-assisted extraction[47,48].
In conclusion, although the current standard for CBDS management involves ERCP followed by LC; the single-step LC-LCBDE offers numerous advantages and may become the new standard. Minimally invasive approaches of CBDE are preferred over open surgery, but they require skilled practitioners and high-volume centers.
INDICATIONS OF LCBDE
While there is currently no clear protocol for selecting LCBDE, experts often recommend surgical CBDE in cases defined as “difficult CBDS”, because it serves as a rescue treatment when endoscopic attempts fail. The European Society of Gastrointestinal Endoscopy guideline 2019 suggest that cases of CBDS with a diameter > 1.5 cm, multiple numbers, barrel-shaped, intrahepatic or cystic duct location, impaction, or altered surgical anatomy e.g. postgastrectomy, perivaterian diverticulum, distal bile duct stricture or angulation < 135 degree, sigmoid-shaped, and short length CBD, be considered as “difficult CBDS” that may require advanced endoscopic techniques or surgery[6]. Some studies also included the difficulty in endoscopic cannulation and the presence of ERCP-related complications in difficult CBDS[49]. Although there is no current recommendation of LCBDE as the first line CBDS treatment, in our point of view, LCBDE should be favored over ERCP in the expert centers for CBDS with: Large (> 1.5 cm) or impacted CBDSs; surgically altered gastric and duodenal anatomy; unavailable advanced endoscopic approaches; patients who preferred a single-step operation.
In previous ERCP cases, LCBDE was considered when CBD clearance was unsuccessful after 2 episodes of advanced endoscopic techniques. However, LC-LCBDE may not be preferred due to some limitations such as unavailable experienced surgeons and teams, suspicious choledochal cyst, concurrent distal CBD stricture, and malignancy-related CBDS. Alternative surgical approaches, such as resection or surgical bypass, should be considered in these groups.
TRANSCYSTIC VERSUS TRANSCHOLEDOCHAL (TRANSDUCTAL) APPROACH
Two conventional bile duct methods can be used in the surgical removal of CBDS: The trans-cystic (TC) and the trans-choledochal [or transductal (TD)] approaches. Factors influencing the success of LCBDE approaches have been outlined by the Society of American Gastrointestinal and Endoscopic Surgeons since 2018. In the TC approach, factors of success include stone diameter < 6 mm, cystic duct diameter > 4 mm, and the lateral entrance of cystic duct to CBD. In contrast, larger stones (> 6 mm), small cystic ducts (< 4 mm), intrahepatic stones, and distal or posterior entrances of cystic duct to CBD were factors associated with poor success rates for TC LCBDE, and the TD approach is favored in these situations. However, the TC approach is preferred for CBDS with small CBD (< 6 mm), markedly inflamed HDL, and poor suturing ability, which were the limitations of the TD approach[47].
LCBDE using the TD and TC approaches had success rates of over 84%, with open conversion reported in 5%-8% of cases. Although outcomes slightly favored the TC route in terms of bile leak rates, procedural time, duration of hospital stay, and morbidity, both approaches showed comparable success rates[50]. Two meta-analyses with large sample sizes in 2019 compared CBDS removal by TC vs TD. The first found no significant difference in CBD clearance rate (91.1% vs 94.1%, P = 0.77) or open conversion rate (3.2% vs 2.4%, P = 0.86) between the TC and TD groups. However, the TC approach significantly reduced overall complications (8.4% vs 13.7%, P = 0.001) and biliary complications (1.6% vs 7.0%, P = 0.0003), along with blood loss (23.6 mL vs 40.0 mL, P = 0.02), operative time (113.8 min vs 126.3 min, P = 0.005), and length of hospital stay (5.2 d vs 7.8 d, P < 0.0001), compared to TD approach[51]. The second meta-analysis demonstrated that TC LCBDE had a lower stone clearance rate [odds ratio (OR) = 0.38, P < 0.05)] but no significant difference in open conversion rate or need for reintervention compared to the TD group. TC LCBDE also reduced operative time (129 min vs 175 min, P < 0.05) and length of hospital stay compared to the TD approach. Regarding complications, TC LCBDE lowered the bile leakage rate (OR = 0.46, P < 0.05) but showed no difference in the stricture rate[52].
Based on the reviews mentioned, LCBDE via the cystic duct is the preferred approach when stone characteristics and biliary anatomy are favorable. Unfortunately, most CBD stones requiring surgical treatment, as many failed previous endoscopic attempts, are large and impacted. Consequently, in challenging situations or when facing limitations, stone removal via choledochotomy is considered a safe alternative by experienced surgeons. Surgeons performing LCBDE should possess knowledge and skills in both TC and TD approaches.
DIFFICULT LCBDE
There is no widely accepted definition of difficult LCBDE, although operative time, blood loss, open conversion, or incomplete stone removal can be used to define the difficulty in other operations. As mentioned, there is slightly lowered stone clearance in TC LCBDE compared to TD LCBDE; however, additional intraoperative TD LCBDE is considered in experienced centers[52]. The risk of incomplete ductal clearance after TC LCBDE increased in patients with bile duct diameter > 6 mm (6.9-folds) and CBDS size > 5 mm (3-folds)[53]. The conversion of LCBDE to open surgery varied in rate from 1.5%-43.1% depending on several factors[36,51,54,55]. Our institute reported a conversion rate of around 28%, with large impacted CBDS and tight intraabdominal adhesions, resulting in Calot’s triangle anatomical distortion, being the most common causes. This adhesion is significantly associated with previous upper abdominal surgery [relative risk (RR) = 4.32][48]. Patients with previous abdominal surgery had longer operative times, higher hospital costs, and a higher incidence of wound complications after LCBDE[56]. Another multivariate analysis revealed conversion to open CBDE was linked to maximal CBDS diameter (OR = 2.23), edema of CBD (OR = 12.50), and presence of > 1 CBDS (OR = 3.44)[55]. In addition, prior antibiotic use (OR = 2.98), previous ERCP (OR = 4.99), and abnormal biliary anatomy (OR = 9.37) were predictors of failed laparoscopy[54]. Also, 17% of LCBDE cases were converted to open CBDE in patients who underwent previous biliary tract surgery, while intraoperative blood loss, hospital stay, and time to oral diet were significant better in LCBDE than in open CBDE[57]. The study on grading the difficulty of CBDE, Nassar et al[58] found that difficult cases often present emergently, with symptoms like obstructive jaundice and prior sphincterotomy. Easy cases typically undergo TC exploration, whereas difficult cases were more likely to undergo TD exploration. Choledochoscopy was more commonly utilized in difficult cases. As difficulty increased, there was a rise in the use of biliary drains, open conversions, operative time, complications, hospital stay, readmissions, and retained stones[58].
Factors increasing the difficulty of LCBDE include large bile duct diameter, CBD edema, large CBDS size, multiple CBDS, previous upper abdominal surgery, prior antibiotic use, previous ERCP/EST, emergency CBDE, TD approach, and abnormal biliary anatomy. These factors contribute to increased operative complexity, prolonged operative times, higher hospital costs, and elevated risk of conversion to open surgery. Despite these challenges, LCBDE remains a viable option, with conversion to open surgery warranted when necessary for patient safety.
SURGICAL TECHNIQUES OF LCBDE
Currently, LCBDE is predominantly performed by specialists in minimally invasive surgery or hepatobiliary surgeons due to the declining number of CBDE procedures resulting from the emergence of endoscopic treatments, and the increased complexity of cases requiring CBDE. Our review intensively provided comprehensive intraoperative procedures covering basic patient positioning, port placement, stone removal techniques tailored to CBDS for the TC and TD approaches, and ductal closure techniques. Paying attention to every single step may improve patient outcomes and minimize surgical-related complications, emphasizing the necessity of rehearsing each step.
The key steps
The key steps of LCBDE consists of: (1) Positioning and port placement; (2) Calot’s triangle dissection; (3) stone characterization by laparoscopic IOUS (LIOUS) or IOC; (4) cystic duct dissection for TC LCBDE vs choledochotomy for TD LCBDE; (5) stone extraction; (6) confirmation of complete stone removal; and (7) choledochotomy closure.
General surgical principles and stone characterization
In our approach, patients were positioned either supine or in the French position on a tiltable operative table to facilitate optimal access for C-Arm fluoroscopy. Port placement typically included four ports, with the 12-mm trocar placed in the epigastrium area for easy access of instruments such as gauzes, suture needles, and inner plastic bag. Additional 5th port placement may be considered at the left subcostal area in the left midclavicular line for intracorporeal suturing. While there are limited reports on reduced port LCBDE, the single-incision LCBDE via a 25-mm vertical left paraumbilical incision showed promising outcomes, with a stone clearance rate of 100% and a conversion to standard ports in 17.8% of cases, without report of postoperative pain and incisional hernia rate[59].
Tilting the operative table to a right-sided-up reverse Trendelenburg position, followed by opening the peritoneum covering Calot’s triangle using infundibular approach was performed until achieving the critical view of safety as usual. The “three lines and one plane” concept, comprising of the duodenal edge, inferior border of right posterior hepatic pedicle or Rouviere’s sulcus, midline of CHD/CBD, and the plane of hilar plate, can help during dissection. In cases of severe inflammation or adhesion, inferior mobilization of duodenum enhances visibility of CBD for a clearer view[60].
In cases involving suspected bile duct anatomical variations or challenging scenarios, such as previous upper abdominal operations, real-time ICG imaging is a favorable option for ductal mapping. It enhances bile duct visibility and reduces postoperative complications and bile leaks[61]. Although there is no absolute consensus, recent research suggests that the optimal dose and timing for fluorescence cholangiography with ICG were intravenous administration of 10 mg of ICG around 10–12 h before the operation, respectively[62].
Characterization of the CBD and CBDS was based on stone size, shape, number, and location and was crucial for selecting between the TC and TD approaches. While LIOUS is the preferred technique when instruments and experienced surgeons are available, TC IOC serves as a viable alternative in cases of instrumental limitations. During TC real-time IOC, the cystic artery is controlled and divided, followed by distal control using a clip placed as close as possible to the gallbladder. A two-by-two gauze is placed near the Calot’s triangle, and then half of the cystic duct circumference is sharply incised by endoscissors just proximal to the clip. The cholangiocatheter or 5-Fr feeding tube is then prepared by flushing with saline solution to reduce air artifact.
The catheter is inserted via the 12-mm epigastric trocar, then advanced into the distal opening of the cystic duct, and bile is aspirated to confirm the proper position. Afterward, the operative table is repositioned into a neutral or slight Trendelenburg position, and C-Arm fluoroscopy is prepared for dynamic IOC. Around 10 to 15 mL of diluted 1:1 water-soluble contrast is injected via the catheter until a complete cholangiogram is achieved. Following this, a careful inspection of the cholangiogram is conducted, and the appropriate choice between TC and TD is made.
CBDS suitable for TC removal
To extract CBDS using the TC approach, the operative table is positioned into a right-sided-up reverse Trendelenburg posture. The cystic duct incision is then extended proximally towards the cystic duct-CBD junction through sharp dissection. Glucagon is injected intravenously at a dose of 1–2 mg to relax the Oddi’s sphincter, if available. Approximately 2 min are allowed for maximal effect. The CBD is then flushed with around 30 mL of saline solution via the catheter to clear the small CBDS.
If IOC reveals a retained filling defect, a guidewire is placed via the cystic duct into the duodenum. The cystic orifice is dilated using an angioplasty balloon catheter over the wire, up to 5–6 mm, without exceeding the CBD diameter. A collapsed 5–6 Fr Fogarty balloon catheter is then inserted through the cystic duct beyond the CBDS into the duodenum under fluoroscopy. The balloon catheter is inflated with diluted 1:1 contrast and continuously pulled back with steady force to extract the stone through the dilated cystic opening. The previously placed two-by-two gauze reduces the degree of bile and stone spillage. Care should be taken because the CBDS may be displaced upwards into the CHD if the cystic duct opening is too small.
TC cholangioscopy is performed using a 3.2-mm choledochoscope with a 1.2-mm channel. The guidewire is advanced to the opening of the cystic duct using the Seldinger technique. If manipulation is necessary, the choledochoscope is gently handled with an atraumatic endograsper. Continuous rinsing of the CBD through the choledochoscope using saline solution clears small CBDS and sludge. If ductal clearance is achieved, the cystic duct stump is closed with conventional clips. If retained CBDSs are identified, the retrieval wire basket is inserted via the working channel of the choledochoscope to catch and remove the stones under scope vision. The recent implementation of leveraging access to technology and enhanced surgical technique principles in TC-LCBDE includes four factors: Ultrathin choledochoscope, Lithotripsy assisted bile duct exploration by laparoendoscopy, correction of cystic duct-CBD junction, and trans-infundibular approach linked to higher TC exploration rates, improved stone clearance rates, reduced post-operative morbidity, and shorter hospital stays[63].
However, if CBDSs are grossly larger than the cystic duct orifice post-dilatation, basket use is avoided as it may lead to complication, such as irretrievable basket and stone. If complete ductal clearance is not achieved, conversion to TD CBDS removal is considered.
CBDS suitable for TD removal
The dissection proceeds distally along the cystic duct to the CBD junction. The anterior peritoneum covering the portal triad is then opened, followed by identification of the CBD. To confirm the presence of bile, a 25 G needle is used to puncture the CBD, and bile is aspirated. In cases of severe inflammation, the CBD can be located using a line from the hepatic duct bifurcation to the midpoint of the duodenal bulb[60]. Adequate placement of gauzes and an endobag is essential to minimize contamination and facilitate stone collection. Typically, the anterior wall of the CBD is opened vertically using a sharp dissection with endoscissors, ensuring avoidance of injury to vasculatures around the 3 and 9 o’clock positions. The choledochotomy is then made around the midpoint of the inferior surface of the quadrate lobe or approximately 1.5–2 cm superior to the duodenum. The size of the choledochotomy should be sufficient to accommodate the diameter of the largest stone and choledochoscope.
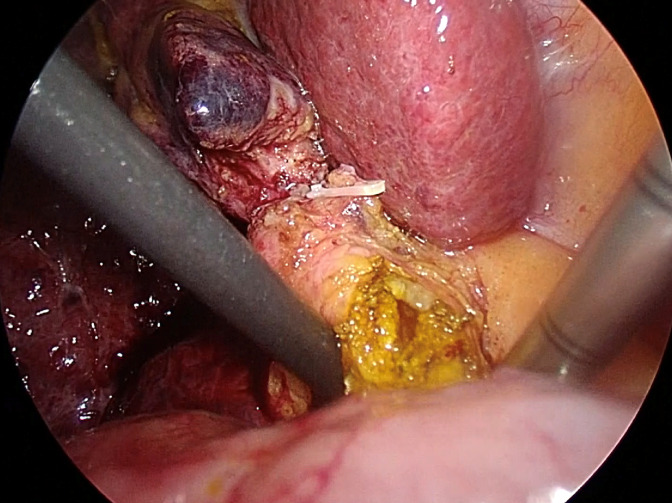
The preferred method for extracting CBDS involves several techniques, including the Chopstick technique, saline flushing via catheter or choledochoscope, Fogarty balloon catheter, and Dormia basket. At our institution, we prioritize the Chopstick technique via choledochotomy as the primary approach due to its simplicity and cost-effectiveness. This technique allows for the complete removal of two-thirds of CBDS, with the option to utilize additional extraction tools as needed without any restriction. Following the creation of an adequate choledochotomy, gentle milking of the CBDS from the distal to proximal direction is performed using a pair of atraumatic graspers, suction, or a Maryland dissector. One instrument is positioned on the right-posterior side avoiding injury to the portal vein, while the other is placed on the left-anterior side of the CBD, resembling the use of chopsticks, as shown in Figure 2. Subsequently, CBDS is safely extracted through the choledochotomy site[48].
Figure 2.
Transductal bile duct stone removal using the chopstick technique. Two atraumatic instruments are placed on the right-posterior and the left-anterior side of the common bile duct.
If the Chopstick technique proves unsuccessful, the subsequent alternatives include the use of either Fogarty balloon or basket extraction. The 5-6 Fr Fogarty balloon catheter can be directly inserted through the choledochotomy distal to CBDS under fluoroscopy without the need for a wire guide. The balloon catheter is inflated with diluted contrast, and then withdrawn to remove the stone through the choledochotomy site. A choledochotomy site that is too small may result in upward migration of the CBDS. In the TD approach, a 3.2-mm choledochoscope with a 1.2-mm channel is typically inserted through the epigastrium port to the choledochotomy site using the free drive technique, where gentle manipulation of the scope with an atraumatic endograsper is essential. Choledochoscopy with continuous saline infusion can aid in clearing stone fragments and sludge; however, relying solely on irrigation for stone clearance in choledochoscopy is often unsuccessful due to challenging stone characteristics. Stone retrieval using the through-the-scope wire basket follows a similar procedure to the TC approach but with a reduced risk of basket and stone entrapment in the TD approach.
Confirmation of complete stone removal
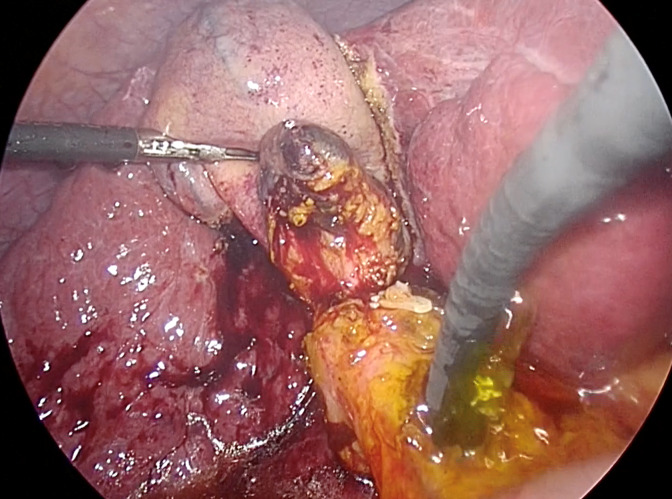
To ensure complete removal of stones, two available options include IOC and intraoperative choledochoscopy. According to a randomized trial by Vindal et al[64], choledochoscopy was found to be superior to IOC. They reported that IOC required more operative time (170 vs 140 min, P < 0.001) compared to the choledochoscopy group, and IOC may lead to false positives due to non-passage of contrast into the duodenum caused by sphincter of Oddi spasm[64,65]. It is crucial to thoroughly examine the ducts. Therefore, the scope should be advanced proximally to the right and left intrahepatic ducts, and distally into the duodenum, as demonstrated in Figure 3. However, if instrument availability is a concern, complete IOC can be applied after LCBDE, along with glucagon administration for sphincter relaxation.
Figure 3.
Confirmation of complete ductal clearance by choledochoscopy. The choledochoscope should pass proximally to visualize the left and right intrahepatic ducts, as well as distally to the duodenum, to ensure complete ductal examination. Additionally, irrigation with normal saline solution via the choledochoscope proves beneficial for flushing out small stones and sludge.
Primary ductal closure, Biliary Stent, and T-tube
To mitigate the risk of postoperative bile leakage following supraduodenal choledochotomy after TD LCBDE, it is advisable to alleviate intraductal pressure through biliary drainage, particularly in cases without sphincterotomy. Studies have shown that primary ductal closure (PDC) without stent placement after CBDE resulted in slight bile leakage in 16.7% of patients without prior EST[66]. While T-tube insertion was previously common practice after CBDE, current evidence largely discourages routine T-tube placement due to associated complications, such as patient discomfort, tube dislodgement, infection, fluid and electrolyte imbalance, bile peritonitis upon removal, unhealed fistula, delayed ductal stricture, etc[67]. Recent meta-analyses have indicated that no T-tube drainage post-LCBDE is preferred due to lower rates of bile peritonitis, shorter operative time, and reduced length of hospital stay, with no significant differences in retained stones, recurrent stones, or ductal stricture observed between groups[68-70]. Antegrade CBD stent placement has been found to significantly decrease postoperative stay (1.0 d vs 3.4 d) and complications (0% vs 11%) compared to T-tube placement[71]. A recent study comparing outcomes among the PDC (no-stent) group, closure-over-stent group, and T-tube group found no significant differences in operative time and biliary-specific complications, while hospital stay and recovery of liver function tests were significantly faster in the closure-over-stent group[72]. Internal stent placement not only provides pressure relief, but also offers advantages in cases with retained CBDS. The CBD stent can theoretically exert frictional force on the retained stones, leading to fragmentation. Additionally, the internal stent aids endoscopists during postoperative CBD cannulation in ERCP, thereby improving the stone extraction rate (82%-100%)[71]. Consequently, in TD LCBDE, it is customary to advance a 7-10 Fr biliary stent via laparoscopic approach through the choledochotomy site into the duodenum, regardless of whether sphincterotomy has been performed. T–Tube placement is generally avoided, except in cases of incomplete ductal clearance or unhealthy tissue.
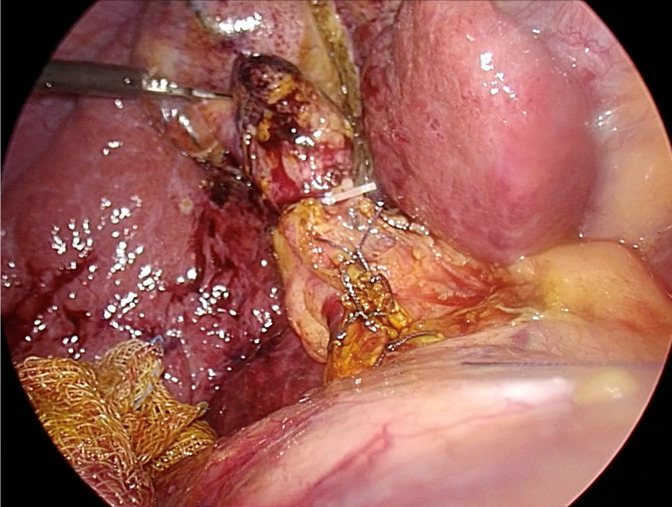
In the TC approach, closure of the cystic stump typically involves the use of plastic/metallic clips or intracorporeal ligation with absorbable sutures. Closure of the choledochotomy opening is commonly achieved with intracorporeal interrupted absorbable sutures, typically 4-0 or 5-0, perpendicular to the choledochotomy line, if possible, to reduce the risk of future ductal stricture, as shown in Figure 4. Surgeons performing choledochotomy closure should possess proficient intracorporeal suturing skills, as an inexperienced surgeon is an independent risk factor for bile leakage[67]. If the suturing angle is limited, placement of an additional 5-mm trocar in the left subcostal area may facilitate suturing. There is no significant difference in leakage rates between interrupted and continuous sutures[67].
Figure 4.
Ductal closure following complete transductal common bile duct stone removal. Typically, intracorporeal suturing with interrupted 4-0 or 5-0 absorbable sutures is utilized. Based on current evidence, the closure-over-stent technique, which involves inserting a 7-10 French biliary stent followed by primary suture, is recommended.
Following successful duct exploration, cholecystectomy is completed. Gross contamination is thoroughly cleaned through aspiration and saline irrigation. An endobag is inserted through the 12-mm port to collect visible stones, gauzes, and the gallbladder specimen for retrieval. Placement of a closed suction drainage system in the subhepatic area is preferred to enable early detection of bile leakage. Complete suction of carbon dioxide pneumoperitoneum before abdominal closure significantly reduces postoperative pain and discomfort[73].
CBDS IN SURGICALLY ALTERED ANATOMY
CBDS in patients with surgically altered anatomy, following procedures such as gastrectomy with Billroth I or II reconstruction, esophagectomy with gastric conduit, pancreaticoduodenectomy, hepaticojejunostomy, or Roux-en-Y gastric bypass for obesity, present significant challenges for endoscopic intervention. Nevertheless, experienced endoscopists have developed specialized techniques to address these challenges. These include balloon enteroscopy-assisted ERCP (BE-ERCP), EUS-guided biliary drainage, and laparoscopic-assisted transgastric ERCP. These innovative techniques hold promise in overcoming variations in anastomosis direction or dealing with very long afferent limb. Successful cholangiography rates achieved with these procedures typically ranging from 70% to 90%[74]. In patients with previous abdominal surgery, LCBDE does not significantly increase the open conversion rate or hospital stay compared to patients with no prior abdominal surgery, although operative time may slightly increase. Open conversion rates in this population range from 6.7% to 14.9%, with hospital stays ranging from 10.3 to 14.7 d[56,75]. Residual CBDS, bile leakage, and biliary stricture rates in patients with previous abdominal surgery were reported as 5.0%, 0.0%, and 1.7%, respectively[56]. Specifically, in the previous gastrectomy group, the mean operative time was 124.4 minutes, the open conversion rate was 6.7%, and the hospital stay averaged 10.3 d[75].
SPECIFIC COMPLICATIONS OF LCBDE
Retained and recurrent stone
The overall incidence of retained CBDS after LCBDE ranged from 0 to 5.58%[47,56]. Comparatively, the stone clearance rate in TC LCBDE was slightly lower at 91.1% compared to 94.1% in TD LCBDE, with an odds ratio of 0.38[51-52]. When comparing LC-LCBDE with ERCP followed by LC, LC-LCBDE showed a significantly lower rate of retained stones (1.2% vs 7.9%, P = 0.004) and stone recurrence (1.8% vs 5.6%, P = 0.005)[42]. There was no significant difference in the incidence of retained and recurrent stones between the T-tube and no T-tube groups after LCBDE[68,69]. In open CBDE, the prediction of residual stones at 4 to 8 wk postoperatively was classified based on three factors: Primary disease (GS = 0, CBDS with or without GS = 1, intrahepatic-CBDS = 2), stone diameter (less than 1 cm = 0, 1 cm or larger = 1), and choledochoscopy confirmation (yes = 0, no = 1). The residual stone rate was 5.6% for a total score of 0 to 1, 27.4% for a score of 2 to 3, and 80% for a score of 4, respectively[76]. Intraoperative confirmation of ductal clearance by choledochoscopy was strongly recommended in every case, with IOC as an alternative option. In cases of incomplete ductal clearance, the preferred approach is to employ an internal plastic ductal stent rather than resorting to T-tube placement, which is considered for instances involving unhealthy tissue. Both methods are designed to facilitate future endoscopic treatment and offer the possibility of subsequent trans-T-tube intervention if needed.
Bile leakage
The incidence of bile leakage following various techniques of LCBDE ranges from 1.3% to 16.7%[47,56,67]. Recent meta-analyses indicated that TC LCBDE was associated with lower rates of biliary complications (1.6% vs 7.0%) and reduced bile leakage compared to TD LCBDE[51,52]. PDC without stent placement after LCBDE resulted in a bile leakage rate of 11.3%. Narrow CBD diameter (< 1 cm) and surgeons with fewer than 70 LCBDE cases were identified as significant risk factors for leakage in cases with PDC, while the mode of closure and choledochotomy length were not associated with bile leakage[67]. In patients without previous sphincterotomy, PDC without stent led to slight bile leakage (less than 150 mL/d) in 16.7% of cases, which typically resolved spontaneously with the use of abdominal drain[66]. Therefore, preference is given to TC LCBDE, when feasible, and internal plastic ductal stent placement is routinely performed in TD LCBDE prior to well-trained ductal closure techniques to release intraductal pressure. Additionally, closed-system intraabdominal drainage is routinely employed for early detection and therapeutic management of bile leakage.
Stricture
Postoperative stricture occurrence following CBDE is rare, with reported rates ranging from 0% to 0.89%[47,56]. In the TD approach, effective prevention hinges on protecting the blood supply of the CBD, employing meticulous intracorporeal suturing techniques, and ensuring closure along a transverse line. Additionally, the TD approach is not recommended for CBDs measuring less than 7 mm in diameter. Notably, there is no significant statistical difference in the incidence of ductal stricture between groups that underwent T-tube placement and those that did not. Consequently, T-tubes are reserved for cases involving unhealthy tissue or incomplete ductal clearance[68,69]. In instances of postoperative CBD stricture, the primary rescue intervention typically involves endoscopic dilatation and stenting.
Wire basket and stone entrapment
This complication arises due to the inadequate diameter of the exit site for stone extraction, which is more common in the TC approach compared to the TD approach. To minimize this complication, alternative stone extraction procedures are considered. Proper selection between the TC and TD approaches, based on the characteristics of the stone and CBD, is the primary preventive measure. In cases where additional dilatation of the cystic duct opening is necessary in the TC approach, it is performed before extraction with a basket. If entrapment occurs, it can be addressed by either extending the cystic duct opening to the CBD or by performing a separate, sufficient ductotomy of the CBD, as is customary in the TD approach. Once an adequate exit site size is achieved, the stone-containing basket is gently removed after wire cutting in the case of a separated ductotomy.
CONCLUSION
Surgical management remains crucial in CBDS treatment despite the emergence of advanced endoscopic CBDS removal and AI technology. Fit patients with CBDS typically require further cholecystectomy to prevent recurrent biliary symptoms. In challenging cases or failed endoscopic procedures, CBDE serves as a rescue alternative and may even become the first line treatment of CBDS in the near future due to its efficacy and cost-effectiveness. Surgeons should possess a thorough understanding of CBDE techniques, with minimally invasive approaches now considered standard practice over open surgery. While TC stone removal is preferred over TD, familiarity with both methods is essential. Given that many CBDS cases undergoing CBDE have previously undergone ERCP, indicating potential difficulties with stone removal, most require the TD approach. The Chopstick technique is commonly recommended in TD stone removal due to its simplicity. Confirming stone removal completion with choledochoscopy is preferable, and duct closure with primary suture and internal stent placement typically favored over the use of T-tubes. Complications such as retained stones, bile leakage, duct strictures, and instrument entrapment must be anticipated and promptly addressed to optimize patient outcomes.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Stoyanova R, Austria S-Editor: Liu H L-Editor: A P-Editor: Cai YX
Contributor Information
Tharathorn Suwatthanarak, Minimally Invasive Surgery Unit, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok Noi 10700, Bangkok, Thailand; Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Bunkyo 113-8519, Tokyo, Japan.
Vitoon Chinswangwatanakul, Minimally Invasive Surgery Unit, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok Noi 10700, Bangkok, Thailand.
Asada Methasate, Minimally Invasive Surgery Unit, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok Noi 10700, Bangkok, Thailand.
Chainarong Phalanusitthepha, Minimally Invasive Surgery Unit, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok Noi 10700, Bangkok, Thailand.
Minoru Tanabe, Department of Hepatobiliary and Pancreatic Surgery, Tokyo Medical and Dental University, Bunkyo 113-8519, Tokyo, Japan.
Keiichi Akita, Department of Clinical Anatomy, Tokyo Medical and Dental University, Bunkyo 113-8519, Tokyo, Japan.
Thawatchai Akaraviputh, Minimally Invasive Surgery Unit, Department of Surgery, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok Noi 10700, Bangkok, Thailand. thawatchai.aka@mahidol.ac.th.
References
- 1.Yousaf MN, Mahmud Y, Sarwar S, Ahmad MN, Ahmad M, Abbas G. Predicting common bile duct stones: Comparison of SAGES, ASGE and ESGE criteria for accuracy. Pak J Med Sci. 2022;38:2095–2100. doi: 10.12669/pjms.38.8.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costi R, Gnocchi A, Di Mario F, Sarli L. Diagnosis and management of choledocholithiasis in the golden age of imaging, endoscopy and laparoscopy. World J Gastroenterol. 2014;20:13382–13401. doi: 10.3748/wjg.v20.i37.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tunruttanakul S, Chareonsil B, Verasmith K, Patumanond J, Mingmalairak C. Evaluation of the American Society of Gastrointestinal Endoscopy 2019 and the European Society of Gastrointestinal Endoscopy guidelines' performances for choledocholithiasis prediction in clinically suspected patients: A retrospective cohort study. JGH Open. 2022;6:434–440. doi: 10.1002/jgh3.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verbesey JE, Birkett DH. Common bile duct exploration for choledocholithiasis. Surg Clin North Am. 2008;88:1315–1328, ix. doi: 10.1016/j.suc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Kamalaporn P, Chirnaksorn S, Rattanasiri S, Kitiyakara T. Comparison of Radial Echoendoscopy and Predictive Factors in the Evaluation of Patients with Suspected Choledocholithiasis. Siriraj Med J. 2021;73:380–385. [Google Scholar]
- 6.Manes G, Paspatis G, Aabakken L, Anderloni A, Arvanitakis M, Ah-Soune P, Barthet M, Domagk D, Dumonceau JM, Gigot JF, Hritz I, Karamanolis G, Laghi A, Mariani A, Paraskeva K, Pohl J, Ponchon T, Swahn F, Ter Steege RWF, Tringali A, Vezakis A, Williams EJ, van Hooft JE. Endoscopic management of common bile duct stones: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2019;51:472–491. doi: 10.1055/a-0862-0346. [DOI] [PubMed] [Google Scholar]
- 7.ASGE Standards of Practice Committee. Buxbaum JL, Abbas Fehmi SM, Sultan S, Fishman DS, Qumseya BJ, Cortessis VK, Schilperoort H, Kysh L, Matsuoka L, Yachimski P, Agrawal D, Gurudu SR, Jamil LH, Jue TL, Khashab MA, Law JK, Lee JK, Naveed M, Sawhney MS, Thosani N, Yang J, Wani SB. ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. 2019;89:1075–1105.e15. doi: 10.1016/j.gie.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phalanusitthepha C, Augkurawaranon C, Sriprayoon T, Hokierti C, Akaraviputh T. Outcomes of endoscopic sphincteroplasty using large balloon dilatation for difficult common bile duct stone removal: a single endoscopist experience. J Med Assoc Thai. 2014;97:699–704. [PubMed] [Google Scholar]
- 9.Tranter-Entwistle I, Wang H, Daly K, Maxwell S, Connor S. The Challenges of Implementing Artificial Intelligence into Surgical Practice. World J Surg. 2021;45:420–428. doi: 10.1007/s00268-020-05820-8. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Xu Y, Chen J, Liu F, Wu D, Zhou W, Wu L, Pang T, Huang X, Zhang K, Yu H. An artificial intelligence difficulty scoring system for stone removal during ERCP: a prospective validation. Endoscopy. 2023;55:4–11. doi: 10.1055/a-1850-6717. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Lu X, Huang X, Zou X, Wu L, Zhou Z, Wu D, Tang D, Chen D, Wan X, Zhu Z, Deng T, Shen L, Liu J, Zhu Y, Gong D, Zhong Y, Liu F, Yu H. Intelligent difficulty scoring and assistance system for endoscopic extraction of common bile duct stones based on deep learning: multicenter study. Endoscopy. 2021;53:491–498. doi: 10.1055/a-1244-5698. [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Kim J, Choi HS, Kim ES, Keum B, Jeen YT, Lee HS, Chun HJ, Han SY, Kim DU, Kwon S, Choo J, Lee JM. Artificial intelligence-assisted analysis of endoscopic retrograde cholangiopancreatography image for identifying ampulla and difficulty of selective cannulation. Sci Rep. 2021;11:8381. doi: 10.1038/s41598-021-87737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanisaka Y, Ryozawa S, Mizuide M, Kobayashi M, Fujita A, Minami K, Kobatake T, Omiya K, Iwano H, Araki R. Usefulness of the "newly designed" short-type single-balloon enteroscope for ERCP in patients with Roux-en-Y gastrectomy: a pilot study. Endosc Int Open. 2018;6:E1417–E1422. doi: 10.1055/a-0754-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veld JV, van Huijgevoort NCM, Boermeester MA, Besselink MG, van Delden OM, Fockens P, van Hooft JE. A systematic review of advanced endoscopy-assisted lithotripsy for retained biliary tract stones: laser, electrohydraulic or extracorporeal shock wave. Endoscopy. 2018;50:896–909. doi: 10.1055/a-0637-8806. [DOI] [PubMed] [Google Scholar]
- 15.Sharma M, Rameshbabu CS, Dietrich CF, Rai P, Bansal R. Endoscopic ultrasound of the hepatoduodenal ligament and liver hilum. Endosc Ultrasound. 2018;7:168–174. doi: 10.4103/2303-9027.193584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro AL, ROBILLARD GL. The arterial blood supply of the common and hepatic bile ducts with reference to the problems of common duct injury and repair; based on a series of 23 dissections. Surgery. 1948;23:1–11. [PubMed] [Google Scholar]
- 17.Northover J, Terblanche J. Bile duct blood supply. Its importance in human liver transplantation. Transplantation. 1978;26:67–69. [PubMed] [Google Scholar]
- 18.Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. doi: 10.1002/bjs.1800660603. [DOI] [PubMed] [Google Scholar]
- 19.Andall RG, Matusz P, du Plessis M, Ward R, Tubbs RS, Loukas M. The clinical anatomy of cystic artery variations: a review of over 9800 cases. Surg Radiol Anat. 2016;38:529–539. doi: 10.1007/s00276-015-1600-y. [DOI] [PubMed] [Google Scholar]
- 20.Mariolis-Sapsakos T, Kalles V, Papatheodorou K, Goutas N, Papapanagiotou I, Flessas I, Kaklamanos I, Arvanitis DL, Konstantinou E, Sgantzos MN. Anatomic variations of the right hepatic duct: results and surgical implications from a cadaveric study. Anat Res Int. 2012;2012:838179. doi: 10.1155/2012/838179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malviya KK, Verma A. Importance of Anatomical Variation of the Hepatic Artery for Complicated Liver and Pancreatic Surgeries: A Review Emphasizing Origin and Branching. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding YM, Wang B, Wang WX, Wang P, Yan JS. New classification of the anatomic variations of cystic artery during laparoscopic cholecystectomy. World J Gastroenterol. 2007;13:5629–5634. doi: 10.3748/wjg.v13.i42.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memar SA, VanSickle C, Funk S, Houser JJ, Markand S. Variations of the Cystohepatic Blood Supply in American Midwestern Donor Cadavers. Cureus. 2022;14:e32260. doi: 10.7759/cureus.32260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki M, Akaishi S, Rikiyama T, Naitoh T, Rahman MM, Matsuno S. Laparoscopic cholecystectomy, Calot's triangle, and variations in cystic arterial supply. Surg Endosc. 2000;14:141–144. doi: 10.1007/s004649900086. [DOI] [PubMed] [Google Scholar]
- 25.Fateh O, Wasi MSI, Bukhari SA. Anaotmical variability in the position of cystic artery during laparoscopic visualization. BMC Surg. 2021;21:263. doi: 10.1186/s12893-021-01270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlek SL, van Dam DA, Rubinstein SM, de Lange-de Klerk ESM, Schoonmade LJ, Tuynman JB, Meijerink WJHJ, Ankersmit M. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc. 2017;31:2731–2742. doi: 10.1007/s00464-016-5318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Peng Y, Su S, Fang C, Qin S, Wang X, Xia X, Li B, He P. A meta-analysis of indocyanine green fluorescence image-guided laparoscopic cholecystectomy for benign gallbladder disease. Photodiagnosis Photodyn Ther. 2020;32:101948. doi: 10.1016/j.pdpdt.2020.101948. [DOI] [PubMed] [Google Scholar]
- 28.Williams E, Beckingham I, El Sayed G, Gurusamy K, Sturgess R, Webster G, Young T. Updated guideline on the management of common bile duct stones (CBDS) Gut. 2017;66:765–782. doi: 10.1136/gutjnl-2016-312317. [DOI] [PubMed] [Google Scholar]
- 29.Boerma D, Rauws EA, Keulemans YC, Janssen IM, Bolwerk CJ, Timmer R, Boerma EJ, Obertop H, Huibregtse K, Gouma DJ. Wait-and-see policy or laparoscopic cholecystectomy after endoscopic sphincterotomy for bile-duct stones: a randomised trial. Lancet. 2002;360:761–765. doi: 10.1016/S0140-6736(02)09896-3. [DOI] [PubMed] [Google Scholar]
- 30.McCarty TR, Farrelly J, Njei B, Jamidar P, Muniraj T. Role of Prophylactic Cholecystectomy After Endoscopic Sphincterotomy for Biliary Stone Disease: A Systematic Review and Meta-analysis. Ann Surg. 2021;273:667–675. doi: 10.1097/SLA.0000000000003977. [DOI] [PubMed] [Google Scholar]
- 31.Singh AN, Kilambi R. Single-stage laparoscopic common bile duct exploration and cholecystectomy versus two-stage endoscopic stone extraction followed by laparoscopic cholecystectomy for patients with gallbladder stones with common bile duct stones: systematic review and meta-analysis of randomized trials with trial sequential analysis. Surg Endosc. 2018;32:3763–3776. doi: 10.1007/s00464-018-6170-8. [DOI] [PubMed] [Google Scholar]
- 32.Akaraviputh T, Rattanapan T, Lohsiriwat V, Methasate A, Aroonpruksakul S, Lohsiriwat D. A same day approach for choledocholithiasis using endoscopic stone removal followed by laparoscopic cholecystectomy: a retrospective study. J Med Assoc Thai. 2009;92:8–11. [PubMed] [Google Scholar]
- 33.Nie S, Fu S, Fang K. Comparison of one-stage treatment versus two-stage treatment for the management of patients with common bile duct stones: A meta-analysis. Front Surg. 2023;10:1124955. doi: 10.3389/fsurg.2023.1124955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alkhamesi NA, Davies WT, Pinto RF, Schlachta CM. Robot-assisted common bile duct exploration as an option for complex choledocholithiasis. Surg Endosc. 2013;27:263–266. doi: 10.1007/s00464-012-2431-0. [DOI] [PubMed] [Google Scholar]
- 35.Jayaraman S, Davies W, Schlachta CM. Robot-assisted minimally invasive common bile duct exploration: a Canadian first. Can J Surg. 2008;51:E93–E94. [PMC free article] [PubMed] [Google Scholar]
- 36.Grubnik VV, Tkachenko AI, Ilyashenko VV, Vorotyntseva KO. Laparoscopic common bile duct exploration versus open surgery: comparative prospective randomized trial. Surg Endosc. 2012;26:2165–2171. doi: 10.1007/s00464-012-2194-7. [DOI] [PubMed] [Google Scholar]
- 37.Yang T, Ma Z, Xu B, Sun W, Meng H, Liu D, Zhou B, Song Z. Clinical role of frequency-doubled double-pulse neodymium YAG laser lithotripsy for removal of difficult biliary stones in laparoscopic common bile duct exploration. ANZ J Surg. 2019;89:E358–E362. doi: 10.1111/ans.15364. [DOI] [PubMed] [Google Scholar]
- 38.Boonmee C, Summart U, Arayajarernwong N, Pareesri W, Srigulawong T, Boonharn S, Naowarungsri S, Watayajinda W. Synchronous Approach vs the Sequential Approach in Laparoscopic Cholecystectomy with Endoscopic Retrograde Cholangiopancreatography in Patients with Gallstones and Suspected Common Bile Duct Stones. J Clin Diagn Res. 2019;13:PC08–PC11. [Google Scholar]
- 39.Rogers SJ, Cello JP, Horn JK, Siperstein AE, Schecter WP, Campbell AR, Mackersie RC, Rodas A, Kreuwel HT, Harris HW. Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Arch Surg. 2010;145:28–33. doi: 10.1001/archsurg.2009.226. [DOI] [PubMed] [Google Scholar]
- 40.Li ZQ, Sun JX, Li B, Dai XQ, Yu AX, Li ZF. Meta-analysis of single-stage versus two-staged management for concomitant gallstones and common bile duct stones. J Minim Access Surg. 2020;16:206–214. doi: 10.4103/jmas.JMAS_146_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lan WF, Li JH, Wang QB, Zhan XP, Yang WL, Wang LT, Tang KZ. Comparison of laparoscopic common bile duct exploration and endoscopic retrograde cholangiopancreatography combined with laparoscopic cholecystectomy for patients with gallbladder and common bile duct stones a meta-analysis of randomized controlled trials. Eur Rev Med Pharmacol Sci. 2023;27:4656–4669. doi: 10.26355/eurrev_202305_32477. [DOI] [PubMed] [Google Scholar]
- 42.Pan L, Chen M, Ji L, Zheng L, Yan P, Fang J, Zhang B, Cai X. The Safety and Efficacy of Laparoscopic Common Bile Duct Exploration Combined with Cholecystectomy for the Management of Cholecysto-choledocholithiasis: An Up-to-date Meta-analysis. Ann Surg. 2018;268:247–253. doi: 10.1097/SLA.0000000000002731. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Li G, Du P, Zhou X, Xiao W, Li Y. Laparoscopic common bile duct exploration versus intraoperative endoscopic retrograde cholangiopancreatography in patients with gallbladder and common bile duct stones: a meta-analysis. Surg Endosc. 2021;35:997–1005. doi: 10.1007/s00464-020-08052-y. [DOI] [PubMed] [Google Scholar]
- 44.Freeman ML. Adverse outcomes of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am. 2003;13:775–798, xi. doi: 10.1016/s1052-5157(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 45.Rerknimitr R, Akaraviputh T, Ratanachu-Ek T, Kongkam P, Pausawasdi N, Pisespongsa P. Current Status of GI Endoscopy in Thailand and Thai Association of Gastrointestinal Endoscopy (TAGE) Siriraj Med J. 2018;70:476–478. [Google Scholar]
- 46.Zhu H, Wu L, Yuan R, Wang Y, Liao W, Lei J, Shao J. Learning curve for performing choledochotomy bile duct exploration with primary closure after laparoscopic cholecystectomy. Surg Endosc. 2018;32:4263–4270. doi: 10.1007/s00464-018-6175-3. [DOI] [PubMed] [Google Scholar]
- 47.Zerey M, Haggerty S, Richardson W, Santos B, Fanelli R, Brunt LM, Stefanidis D. Laparoscopic common bile duct exploration. Surg Endosc. 2018;32:2603–2612. doi: 10.1007/s00464-017-5991-1. [DOI] [PubMed] [Google Scholar]
- 48.Suwatthanarak T, Akaraviputh T, Phalanusitthepha C, Chinswangwatanakul V, Methasate A, Swangsri J, Trakarnsanga A, Parakonthun T, Taweerutchana V, Srisuworanan N. Outcomes of Laparoscopic Common Bile Duct Exploration by Chopstick Technique in Choledocholithiasis. JSLS. 2021;25 doi: 10.4293/JSLS.2021.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tai CK, Tang CN, Ha JP, Chau CH, Siu WT, Li MK. Laparoscopic exploration of common bile duct in difficult choledocholithiasis. Surg Endosc. 2004;18:910–914. doi: 10.1007/s00464-003-8216-8. [DOI] [PubMed] [Google Scholar]
- 50.Marks B, Al Samaraee A. Laparoscopic Exploration of the Common Bile Duct: A Systematic Review of the Published Evidence Over the Last 10 Years. Am Surg. 2021;87:404–418. doi: 10.1177/0003134820949527. [DOI] [PubMed] [Google Scholar]
- 51.Hajibandeh S, Hajibandeh S, Sarma DR, Balakrishnan S, Eltair M, Mankotia R, Budhoo M, Kumar Y. Laparoscopic Transcystic Versus Transductal Common Bile Duct Exploration: A Systematic Review and Meta-analysis. World J Surg. 2019;43:1935–1948. doi: 10.1007/s00268-019-05005-y. [DOI] [PubMed] [Google Scholar]
- 52.Bekheit M, Smith R, Ramsay G, Soggiu F, Ghazanfar M, Ahmed I. Meta-analysis of laparoscopic transcystic versus transcholedochal common bile duct exploration for choledocholithiasis. BJS Open. 2019;3:242–251. doi: 10.1002/bjs5.50132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strömberg C, Nilsson M, Leijonmarck CE. Stone clearance and risk factors for failure in laparoscopic transcystic exploration of the common bile duct. Surg Endosc. 2008;22:1194–1199. doi: 10.1007/s00464-007-9448-9. [DOI] [PubMed] [Google Scholar]
- 54.Chue KM, Aw JWB, Chua SHM, Chen Z, Iyer SG, Madhavan K, Kow AWC. A predictive nomogram to identify factors influencing the success of a concomitant laparoscopic cholecystectomy with common bile duct exploration for choledocholithiasis. HPB (Oxford) 2018;20:313–320. doi: 10.1016/j.hpb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Xu B, Wang YX, Qiu YX, Meng HB, Gong J, Sun W, Zhou B, He J, Zhang T, Zheng WY, Song ZS. Risk factors and consequences of conversion to open surgery in laparoscopic common bile duct exploration. Surg Endosc. 2018;32:4990–4998. doi: 10.1007/s00464-018-6263-4. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, Feng Q, Wang K, Xiong X, Zou S. The safety and feasibility of laparoscopic common bile duct exploration for treatment patients with previous abdominal surgery. Sci Rep. 2017;7:15372. doi: 10.1038/s41598-017-15782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M, Tao Y, Shen S, Song L, Suo T, Liu H, Wang Y, Zhang D, Ni X. Laparoscopic common bile duct exploration in patients with previous abdominal biliary tract operations. Surg Endosc. 2020;34:1551–1560. doi: 10.1007/s00464-020-07429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nassar AHM, Sallam M, Khan KS, Kilpatrick R, Zino S, Katbeh TZ. A proposed difficulty grading system for laparoscopic bile duct exploration: benefits to clinical practice, training and research. Surg Endosc. 2023;37:7012–7023. doi: 10.1007/s00464-023-10169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuang SH, Hung MC, Huang SW, Chou DA, Wu HS. Single-incision laparoscopic common bile duct exploration in 101 consecutive patients: choledochotomy, transcystic, and transfistulous approaches. Surg Endosc. 2018;32:485–497. doi: 10.1007/s00464-017-5658-y. [DOI] [PubMed] [Google Scholar]
- 60.Wang L, Zhou D, Hou H, Wu C, Geng X. Application of "three lines and one plane" as anatomic landmarks in laparoscopic surgery for bile duct stones. Medicine (Baltimore) 2018;97:e0155. doi: 10.1097/MD.0000000000010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma C, Zhang L, Wen J, Zhang W, Chen H. Indocyanine Green Imaging in Laparoscopic Cholecystectomy Plus Laparoscopic Common Bile Duct Exploration: A Suitable Option for Patients With Difficult Exploration (With Videos) Surg Laparosc Endosc Percutan Tech. 2023;33:235–240. doi: 10.1097/SLE.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 62.Chen Q, Zhou R, Weng J, Lai Y, Liu H, Kuang J, Zhang S, Wu Z, Wang W, Gu W. Extrahepatic biliary tract visualization using near-infrared fluorescence imaging with indocyanine green: optimization of dose and dosing time. Surg Endosc. 2021;35:5573–5582. doi: 10.1007/s00464-020-08058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Navaratne L, Al-Musawi J, Vutipongsatorn K, Isla AM. Leveraging access to technology and enhanced surgical technique (LATEST) in laparoscopic bile duct exploration (LBDE) Surg Endosc. 2023;37:2367–2378. doi: 10.1007/s00464-022-09667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vindal A, Chander J, Lal P, Mahendra B. Comparison between intraoperative cholangiography and choledochoscopy for ductal clearance in laparoscopic CBD exploration: a prospective randomized study. Surg Endosc. 2015;29:1030–1038. doi: 10.1007/s00464-014-3766-5. [DOI] [PubMed] [Google Scholar]
- 65.Gupta N. Role of laparoscopic common bile duct exploration in the management of choledocholithiasis. World J Gastrointest Surg. 2016;8:376–381. doi: 10.4240/wjgs.v8.i5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Wu XD, Fan RG, Zhou GJ, Mu XM, Zha WZ, Jia J. Laparoscopic common bile duct exploration and primary closure of choledochotomy after failed endoscopic sphincterotomy. Int J Surg. 2014;12:645–648. doi: 10.1016/j.ijsu.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 67.Liu D, Cao F, Liu J, Xu D, Wang Y, Li F. Risk factors for bile leakage after primary closure following laparoscopic common bile duct exploration: a retrospective cohort study. BMC Surg. 2017;17:1. doi: 10.1186/s12893-016-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Podda M, Polignano FM, Luhmann A, Wilson MS, Kulli C, Tait IS. Systematic review with meta-analysis of studies comparing primary duct closure and T-tube drainage after laparoscopic common bile duct exploration for choledocholithiasis. Surg Endosc. 2016;30:845–861. doi: 10.1007/s00464-015-4303-x. [DOI] [PubMed] [Google Scholar]
- 69.Jiang C, Zhao X, Cheng S. T-Tube Use After Laparoscopic Common Bile Duct Exploration. JSLS. 2019;23 doi: 10.4293/JSLS.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Huang Y, Shi C, Wang L, Liu S, Zhang J, Wang W. Efficacy and safety of laparoscopic common bile duct exploration via choledochotomy with primary closure for the management of acute cholangitis caused by common bile duct stones. Surg Endosc. 2022;36:4869–4877. doi: 10.1007/s00464-021-08838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyon M, Menon S, Jain A, Kumar H. Use of biliary stent in laparoscopic common bile duct exploration. Surg Endosc. 2015;29:1094–1098. doi: 10.1007/s00464-014-3797-y. [DOI] [PubMed] [Google Scholar]
- 72.Xiao LK, Xiang JF, Wu K, Fu X, Zheng MY, Song XX, Xie W. The reasonable drainage option after laparoscopic common bile duct exploration for the treatment of choledocholithiasis. Clin Res Hepatol Gastroenterol. 2018;42:564–569. doi: 10.1016/j.clinre.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 73.Tuvayanon W, Silchai P, Sirivatanauksorn Y, Visavajarn P, Pungdok J, Tonklai S, Akaraviputh T. Randomized controlled trial comparing the effects of usual gas release, active aspiration, and passive-valve release on abdominal distension in patients who have undergone laparoscopic cholecystectomy. Asian J Endosc Surg. 2018;11:212–219. doi: 10.1111/ases.12451. [DOI] [PubMed] [Google Scholar]
- 74.Krutsri C, Kida M, Yamauchi H, Iwai T, Imaizumi H, Koizumi W. Current status of endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy. World J Gastroenterol. 2019;25:3313–3333. doi: 10.3748/wjg.v25.i26.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi HR, Kim JD, Choi DL. Laparoscopic Common Bile Duct Exploration: A Feasible Option for Choledocholithiasis in Patients with Previous Gastrectomy. J Minim Invasive Surg. 2016;19:130–134. [Google Scholar]
- 76.Zhang JF, Du ZQ, Lu Q, Liu XM, Lv Y, Zhang XF. Risk Factors Associated With Residual Stones in Common Bile Duct Via T Tube Cholangiography After Common Bile Duct Exploration. Medicine (Baltimore) 2015;94:e1043. doi: 10.1097/MD.0000000000001043. [DOI] [PMC free article] [PubMed] [Google Scholar]