Abstract
Internal ribosomal entry sites (IRESs) can function in foreign viral genomes or in artificial dicistronic mRNAs. We describe an interaction between the wild-type hepatitis C virus (HCV)-specific sequence and the poliovirus (PV) 5′-terminal cloverleaf in a PV/HCV chimeric virus (containing the HCV IRES), resulting in a replication phenotype. Either a point mutation at nucleotide (nt) 29 or a deletion up to nt 40 in the HCV 5′ nontranslated region relieved the replication block, yielding PV/HCV variants replicating to high titers. Fortuitous yet crippling interactions between an IRES and surrounding heterologous RNA must be considered when IRES-based dicistronic expression vectors are being constructed.
All picornavirus genomes contain a genetic element named the internal ribosomal entry site (IRES) that allows translation of viral genomic mRNAs in a 5′- and cap-independent manner (5, 12, 13, 16, 20, 25). Similar genetic elements have also been discovered in genomes of Hepatitis C virus (HCV) (24), a Hepacivirus, and Bovine viral diarrhea virus (21), a Pestivirus, of the Flaviviridae family. IRESs are located in the 5′-terminal segment of the genomes, where they control initiation of polyprotein synthesis. Certain insect RNA viruses, e.g., Plautia stali intestine virus, however, are exceptional in that their cognate IRES maps several thousand nucleotides downstream of the 5′ end of the genome, separating two cistrons encoding two different polyproteins (23). It is of interest that we have previously engineered a dicistronic poliovirus (PV) by inserting the IRES of encephalomyocarditis virus into the coding region of the PV polyprotein (16). The genetic organization of this dicistronic PV, which encodes two polyproteins separated by an IRES element, closely resembles that of P. stali intestine virus.
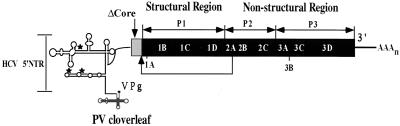
IRES elements are defined by function and not by nucleotide sequence. Indeed, the IRESs of PV, a picornavirus, and HCV, a flavivirus, have little if any sequence in common, yet both function as promoters of internal ribosomal entry for initiation of translation. This phenomenon is most apparent in chimeric PV/HCV viruses in which the cognate PV IRES has been exchanged with that of HCV (15, 28). Unexpectedly, the HCV IRES includes a sequence downstream of the AUG codon initiating its polyprotein (15, 22). In constructing a viable PV/HCV chimeric virus, a portion of the core protein-encoding sequence was necessary to obtain a viable virus (Fig. 1, ΔCore) (15). The ΔCore sequence leads to the formation of a ΔCore/PV fusion polyprotein which must be processed through cleavage by the PV viral proteinase 2Apro at the ΔCore*1A junction (Fig. 1) (28). However, the production of core sequence-encoded peptides resulting from translation of the ΔCore sequence is not necessary for the function of the HCV IRES in P/H710-d17* (28). (In previous studies, P/H710-d17 was designated P/H701-2A because the chimeric genome contained the HCV-specific sequence from nucleotides [nt] 18 to 710 of the HCV genome [28]. The numbering of the HCV 5′ nontranslated region [5′NTR] in this paper conforms to the numbering of the full-length HCV 5′NTR [see, for example, reference 11].)
FIG. 1.
Schematic diagram of the genomic organization of P/H710-d17. The cloverleaf-like RNA structure of PV, an essential cis-acting replication signal terminated with the genome-linked protein VPg, is located at the 5′ end of the genome. Noninitiating AUG codons found in the HCV 5′NTR are denoted by stars. The shaded (HCV) and filled (PV) boxes depict open reading frames encoding viral polypeptides; the position of the HCV core protein gene is marked ΔCore. Overall, the HCV-specific sequence in P/H710-d17 spans from nt 18 to 710 (15, 28). PV-encoded polypeptides within the polyprotein are indicated by 1A, 2B, etc. The PV-encoded proteinase 2Apro is responsible for the cleavage between ΔCore and capsid precursor P1.
In general, IRES elements were discovered and studied by constructing artificial dicistronic mRNAs (13), by exchanging IRES elements in viral genomes (1, 6, 8, 15, 28), for example, as in P/H710-d17, or by inserting an extra IRES into a viral genome (1, 16). In all of these cases, the IRESs must function in the context of RNA sequences foreign to them. Since IRESs consist of remarkably large, continuous RNA segments (300 to 400 nt long) that undoubtedly form complex higher-order structures, the possibility exists that elements of the IRES and surrounding sequences may interact by base pairing. Such fortuitous contacts may have consequences either for the function of the heterologous, adjacent RNA elements or for the IRES. Here we describe an interesting observation made with chimera P/H710-d17, in which the poliovirus cloverleaf forms base pairs with nucleotides of the HCV 5′NTR, resulting in a replication phenotype.
We previously noticed that chimera P/H710-d17 yielded plaques of different sizes when passaged on HeLa cells (28). This observation can be an indicator of the emergence of new genotypes with different replication properties. We therefore performed a genetic analysis of the passaged virus, starting with infectious transcripts derived from a plasmid harboring P/H710-d17 (for experimental details, see reference 28). When P/H710-d17 was passaged several times on HeLa cell R19 monolayers, the average plaque size expanded (Fig. 2A). Similarly, the virus titer per unit of transfecting RNA increased (Fig. 2A). It was therefore likely that a variant(s) with improved growth properties, relative to P/H710-d17, was selected in each passage that eventually outcompeted the parental virus.
FIG. 2.
Plaque phenotypes and genetic variation of P/H710-d17 (A) and P/H710-d40 (B) viruses on HeLa R19 cell monolayers, assayed after different passages. Each passage is indicated with a G and the passage number; G0 indicates virus produced after RNA transfection. Details of the plaque assays are as described before (14). Dilution factors for an isolate obtained after a given passage are included in the parentheses. (C) Nucleotide sequence of a genetic variant of P/H710-d17 isolated after the first passage (A at nt 29 was mutated to G in P/H710-d17 G1). (D) Plaque phenotype of mutant P/H710-d17-A29G, derived from P/H710-d17, in which the base at nt 29 was changed from A to G by site-directed mutagenesis.
A mutation(s) resulting in a larger-plaque phenotype and improved replication properties could reside in the HCV 5′NTR (which in our constructs begins at nt 18 of the HCV genome) (15), in the HCV core sequence, or in PV-specific sequences. For further studies, we selected a large-plaque variant that had emerged after the first passage of P/H710-d17. The variant (P/H710-d17 G1) was expanded once in HeLa cells, and its genotype in the 5′NTR was determined. Sequence analyses revealed a single nucleotide change (A→G) in position 29 of the HCV-specific sequence (Fig. 2C). To test whether this mutation alone was responsible for both plaque phenotype and increased virus yield, we introduced a single A→G change into the P/H710-d17 genome at nt 29. This was done by PCR-based mutagenesis using primers A29G (5′-CT TAGAATTCGGCGACACTCCgCCATAGATCACTCCCC- 3′) and PVVP4 (5′-CGTTACTAGCTGAATCTCTATAATAATTAATGG-3′). The PCR fragments were gel purified, digested with EcoRI and SacI, and ligated with cloning vector P/H710dES (28), lacking HCV IRES and core sequences, to yield construct P/H710-d17-A29G, which was selected by restriction mapping and verified by sequencing. As can be seen in Fig. 2D, the plaque size of the genetically engineered variant P/H710-d17-A29G was similar to those obtained after multiple passages of P/H710-d17.
Cell-free translations in a HeLa cell extract (17) were carried out with RNA of PV type 1, Mahoney, and with transcript RNAs of P/H710-d17 and P/H710-d17-A29G. Regardless of whether the translations were carried out at 30°C for 16 h or at 34°C for 7 h, the pattern obtained with P/H710-d17-A29G RNA revealed no significant difference from those obtained with P/H710-d17 RNA (data not shown). Although only circumstantial, this result suggests that the increase in plaque size and virus yield is not the result of increased translation efficiency of the IRES in P/H710-d17-A29G.
All PV/HCV chimeric viruses described by us carry a PV-specific structure, the cloverleaf, at the 5′ end of the genome (Fig. 1) (15, 28). The cloverleaf (Fig. 3A) serves as a cis-acting signal in PV RNA replication (reviewed in reference 27). It forms an RNP of unknown structure with the PV proteinase 3CDpro (2, 3) and either PV polypeptide 3AB (10, 26, 27) or cellular RNA binding poly(rC) binding protein (4, 7, 18). Genetic analysis has identified stem-loop D as the binding domain of 3CDpro (3; E. Rieder, W. Xiang, and E. Wimmer, unpublished data).
FIG. 3.
Interaction of the PV cloverleaf with HCV-specific 5′-terminal nucleotides. (A) Structure of the PV cloverleaf at the 5′ end of P/H710-d17. The covalently linked protein VPg is shown as a closed circle. The nucleotides involved in the putative interaction with the HCV sequences are in boldface. (B) A putative interaction between the 5′-terminal nucleotides of HCV and stem-loop D of the PV cloverleaf. Nucleotides possibly involved in the interaction are highlighted, and the putative base pairing is indicated by lines. The adenosine residue mutated to guanosine after the passage of P/H710-d17 is indicated by an arrow. The location of the HCV sequences is boxed in the diagram. (C) A stem-loop structure likely to form after mutation A29G.
Inspection of stem-loop D and the HCV-specific 5′-terminal sequences revealed the possibility of an interaction between these regions by base pairing (Fig. 3B). If so, the single mutation (A→G) in P/H710-d17 G1 at nt 29 would destabilize this interaction. It seemed unlikely to us, however, that the change from an A-U to a G-U would be sufficient to relieve P/H710-d17 G1 from its growth restriction. We favor the hypothesis that the A→G change at nt 29 leads to the formation of a new stem-loop structure in the HCV 5′NTR (Fig. 3C). This new stem-loop structure would resist base pairing with the cloverleaf and thus explain the replication phenotype of P/H710-d17-A29G.
The possibility of base pairing between the PV cloverleaf and HCV sequence (Fig. 3B) was eliminated altogether by changing the HCV sequence between nt 17 and 31 from GGCGACACUCCACC to CCGCTGTCUCCTGG (nucleotides changed are in boldface), thereby generating variant P/H710-d17-IM. This was done by PCR-based mutagenesis, using primers IM (5′-ACTTAGGAATTCCCGCTGTCTCCTGGATAGATCACTCCCCTGTGAGGAACTACTGTC-3′) and PVVP4 (5′-CGTTACTAGCTGAATCTCTATAATAATTAATGG-3′). The cloning strategy was the same as for P/H710-d17-A29G. This variant expressed plaque and replication phenotypes matching, but not surpassing, that of P/H710-d17-A29G (data not shown).
If a single A29G mutation at nt 29 or the drastic change of the sequence between nt 17 and 31 relieves virus P/H710-d17 from replication restriction, deletions within this region of the HCV 5′NTR are likely to yield a virus with a phenotype matching that of P/H710-d17-A29G. Accordingly, we have deleted the HCV-specific sequence up to nt 40 (W. D. Zhao and E. Wimmer, unpublished data). As can be seen in Fig. 2B, construct P/H710-d40 produced large plaques upon transfection, yielding 105 PFU of virus per ml. On further passage, the plaque phenotype produced by this virus did not change. The growth properties of variant P/H710-d40 will be described elsewhere (Zhao and Wimmer, unpublished data).
IRES elements have been used to construct artificial dicistronic mRNAs (12, 13, 20) in a variety of different eukaryotic expression vectors, either for the study of IRES function per se or for the expression of reporter genes or delivery of gene products for therapeutic or commercial reasons. IRESs have been exchanged between different virus genomes for the studies of viral replication (1, 6, 16, 19), host range (8, 9), or IRES function (15, 28). By necessity, the IRES elements in these constructs are surrounded by foreign RNA sequences. A fortuitous interaction between sequences of surrounding heterologous RNA segments with the IRES RNA could influence the function of either RNA, resulting in either reduced reporter gene expression or genome replication. The evidence presented in this report can be interpreted to mean that a fortuitous interaction between an essential cis-acting element of the PV genome and a sequence in the HCV 5′NTR resulted in impaired viral proliferation. No differences were detected in the translation of the chimeric RNAs, regardless of whether the predicted interaction between cloverleaf and HCV 5′NTR was interrupted. We suggest, therefore, that the chimera P/H710-d17 is impaired in the ability to replicate its genome.
Interactions between the IRES and surrounding heterologous sequences, resulting in an expression phenotype, may be common. They may escape detection because the engineered hybrid RNAs in most expression vectors are not responsive to genetic analysis.
Acknowledgments
We are indebted to A. Nomoto, Tokyo University, for his generous gift of HCV cDNA subclones of HCV. Critical reading of the manuscript by A. Paul is highly appreciated. Special thanks go to R. Duggal, S. Mueller, X. Z. Peng, T. Pfister, and E. Rieder for suggestions and to F. Maggiore for excellent technical assistance.
This work was supported in part by NIH grants NIAID 2R01AI15122-25 and 5R01AI32100-07. F.C.L. was supported in part by a fellowship from the Schering-Plough Research Institute.
REFERENCES
- 1.Alexander L, Lu H H, Wimmer E. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc Natl Acad Sci USA. 1994;91:1406–1410. doi: 10.1073/pnas.91.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Achacoso P L, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andino R, Rieckhof G E, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell. 1990;63:369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- 4.Blyn L B, Swiderek K M, Richards O, Stahl D C, Semler B L, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc Natl Acad Sci USA. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C Y, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 6.Frolov I, McBride M S, Rice C M. cis-acting RNA elements required for replication of bovine viral diarrhea virus-hepatitis C virus 5′ nontranslated region chimeras. RNA. 1998;4:1418–1435. doi: 10.1017/s1355838298981031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamarnik A V, Andino R. Two functional complexes formed by KH domain containing proteins with the 5′ noncoding region of poliovirus RNA. RNA. 1997;3:882–892. [PMC free article] [PubMed] [Google Scholar]
- 8.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier M, Bossert B, Arita M, Nomoto A, Wimmer E. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J Virol. 1999;73:958–964. doi: 10.1128/jvi.73.2.958-964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris K S, Xiang W, Alexander L, Lane W S, Paul A V, Wimmer E. Interaction of poliovirus polypeptide 3CDpro with the 5′ and 3′ termini of the poliovirus genome. Identification of viral and cellular cofactors needed for efficient binding. J Biol Chem. 1994;269:27004–27014. [PubMed] [Google Scholar]
- 11.Honda M, Brown E A, Lemon S M. Stability of a stem-loop involving the initiator AUG controls the efficiency of internal initiation of translation on hepatitis C virus RNA. RNA. 1996;2:955–968. [PMC free article] [PubMed] [Google Scholar]
- 12.Jang S K, Davies M V, Kaufman R J, Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5′ nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989;63:1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang S K, Krausslich H G, Nicklin M J, Duke G M, Palmenberg A C, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu H H, Alexander L, Wimmer E. Construction and genetic analysis of dicistronic polioviruses containing open reading frames for epitopes of human immunodeficiency virus type 1 gp120. J Virol. 1995;69:4797–4806. doi: 10.1128/jvi.69.8.4797-4806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H H, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molla A, Jang S K, Paul A V, Reuer Q, Wimmer E. Cardioviral internal ribosomal entry site is functional in a genetically engineered dicistronic poliovirus. Nature. 1992;356:255–257. doi: 10.1038/356255a0. [DOI] [PubMed] [Google Scholar]
- 17.Molla A, Paul A V, Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 18.Parsley T B, Towner J S, Blyn L B, Ehrenfeld E, Semler B L. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA. 1997;3:1124–1134. [PMC free article] [PubMed] [Google Scholar]
- 19.Paul A V, Mugavero J, Molla A, Wimmer E. Internal ribosomal entry site scanning of the poliovirus polyprotein: implications for proteolytic processing. Virology. 1998;250:241–253. doi: 10.1006/viro.1998.9376. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 21.Poole T L, Wang C, Popp R A, Potgieter L N, Siddiqui A, Collett M S. Pestivirus translation initiation occurs by internal ribosome entry. Virology. 1995;206:750–754. doi: 10.1016/s0042-6822(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds J E, Kaminski A, Kettinen H J, Grace K, Clarke B E, Carroll A R, Rowlands D J, Jackson R J. Unique features of internal initiation of hepatitis C virus RNA translation. EMBO J. 1995;14:6010–6020. doi: 10.1002/j.1460-2075.1995.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki J, Nakashima N. Translation initiation at the CUU codon is mediated by the internal ribosome entry site of an insect picorna-like virus in vitro. J Virol. 1999;73:1219–1226. doi: 10.1128/jvi.73.2.1219-1226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wimmer E, Hellen C U, Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 26.Xiang W, Harris K S, Alexander L, Wimmer E. Interaction between the 5′-terminal cloverleaf and 3AB/3CDpro of poliovirus is essential for RNA replication. J Virol. 1995;69:3658–3667. doi: 10.1128/jvi.69.6.3658-3667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang W, Paul A, Wimmer E. RNA signals in entero- and rhinovirus genome replication. Semin Virol. 1997;8:256–273. [Google Scholar]
- 28.Zhao W D, Wimmer E, Lahser F C. Poliovirus/hepatitis C virus (internal ribosomal entry site-core) chimeric viruses: improved growth properties through modification of a proteolytic cleavage site and requirement for core RNA sequences but not for core-related polypeptides. J Virol. 1999;73:1546–1554. doi: 10.1128/jvi.73.2.1546-1554.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]