Abstract
Chemokine mRNA expression by pulmonary leukocytes following infection of BALB/c mice with two strains of respiratory syncytial virus (RSV) and one strain of parainfluenza virus type 3 (PIV-3) was determined. The results suggest that RSV G and/or SH proteins inhibit early MIP-1α, MIP-1β, MIP-2, MCP-1, and IP-10 mRNA expression. TCA-3 mRNA expression was found to be increased during PIV-3 infection.
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract disease in infants and young children and is associated with bronchiolitis (3, 4). Bronchiolitis is manifested by obstruction of the airways and is associated with the inflammatory response to infection (7–9, 17, 31). The viral and host factors contributing to the inflammatory response are not well understood but likely involve the production of cytokines and chemokines by immune and respiratory epithelial cells. For example, in mice, the enhanced disease that occurs following formalin-inactivated RSV vaccination is associated with a Th2 cytokine response (6, 10, 11, 30), while live RSV infection does not induce enhanced disease and is associated with a Th1 cytokine response (15, 27, 29). The link between cytokine production and enhanced disease is supported by abrogation of enhanced disease when interleukin 4 (IL-4) and IL-10 are neutralized with antibodies (6). Recent studies suggest that the RSV G glycoprotein is an important determinant of the cytokine response associated with enhanced disease (12, 18, 19). For example, G and/or SH glycoproteins alter Th1 cytokine, particularly gamma interferon (IFN-γ), expression as well as decrease polymorphonuclear leukocyte (PMN) and NK cell trafficking to the lung (27, 29). It has been proposed that lack of IFN-γ and CD8+ T-cell regulation of the CD4+ T-cell response to RSV infection may contribute to enhanced disease (14, 26).
Antigen nonspecific granular cells that produce chemokines govern the earliest stages of the inflammatory response. Chemokines promote an influx of immune cells to the site of infection, which in turn express chemokines that help refine the inflammatory response. Several groups have shown in vitro that epithelial cells respond to RSV infection by expressing IL-8, RANTES, MIP-1α, and MIP-1β (13, 22), suggesting that these chemokines are important during RSV infection; however, the characteristics of the chemokine response to in vivo RSV infection have not been well studied.
In this study, we examined the kinetics of chemokine mRNA expression by pulmonary leukocytes following primary infection of 4- to 6-week-old female BALB/c mice (Harlan Sprague Dawley Laboratories, Indianapolis, Ind.) with two strains of RSV, one which has the G and SH genes (B1) and one which lacks them (CP52), or with the JS strain of parainfluenza virus type 3 (PIV-3), all of which were propagated as described previously (28, 29). Mice were intranasally infected with 104 PFU of B1, CP52, or PIV-3 diluted in phosphate-buffered saline (PBS) or with uninfected Vero cell-free lysate (VCL) (GIBCO, Grand Island, N.Y.). At various times postinfection (p.i.); bronchoalveolar lavage (BAL) cells from 4 to 6 mice/time point were collected by washing the lungs three times with 1 ml of PBS (GIBCO) containing 1% bovine serum albumin (Sigma, St. Louis, Mo.). Total cell numbers in the BAL cells of B1-infected mice ranged from 3.4 × 105 to 9 × 105 cells/ml, in CP52-infected mice they ranged from 5 × 105 to 8.5 × 105 cells/ml and in PIV-3-infected mice they ranged from 5 × 105 to 12 × 105 cells/ml. RNA isolation and multiprobe RNase protection analysis were performed according to the instructions of the probe manufacturer (PharMingen, San Diego, Calif.). BAL cells were used for RNA extraction. Total RNA was extracted using RNA STAT-50 LS (TEL-TEST Inc., Friendswood, Tex.) as described by the manufacturer. Chemokine mRNA was detected by RNase protection analysis using the RiboQuant Multi-Probe RNase Protection Assay System (PharMingen). 32P-labeled antisense RNA probes specific for eight chemokine mRNA sequences and two housekeeping mRNA sequences were used to detect CC chemokines (RANTES, Eotaxin, MIP-1α, MIP-1β, MIP-2, MCP-1 and TCA-3), CXC chemokine (IP-10), and the L32 and GAPDH housekeeping genes.
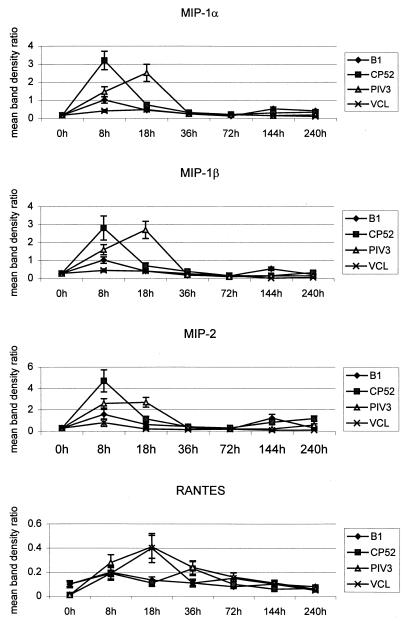
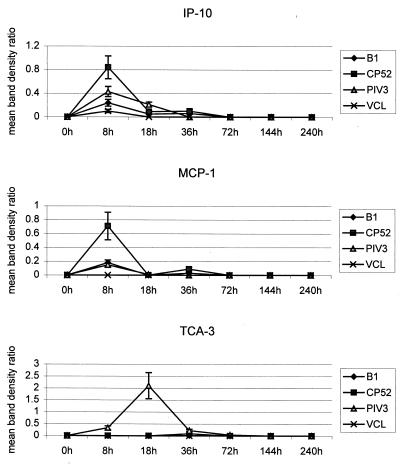
The mean results from experiments (n ≥ 3) examining chemokine mRNA expression at 0, 8, 18, 36, 72, 144, and 240 h p.i. with B1, CP52, and PIV-3 are shown in Fig. 1 and 2. Overall, there was low constitutive expression of the macrophage inflammatory proteins MIP-1β, MIP-1α, and MIP-2, as well as of MCP-1 and IP-10, followed by a marked early chemokine response to infection and a return to constitutive levels, followed by a small, second increase in chemokine expression later in the infection. At 8 h p.i., MIP, IP-10, and MCP-1 expression peaked for the two RSV strains, whereas at 18 h p.i. MIP and TCA-3 expression peaked for PIV-3 (Fig. 1 and 2). By 36 h p.i., except for that of RANTES, chemokine expression had returned to near constitutive levels (Fig. 1). The magnitude of RANTES mRNA expression varied following virus infection or VCL treatment, and no virus-specific increases were detected (Fig. 1). Overall, treatment of mice with uninfected VCL induced a chemokine expression pattern similar to that for B1 infection, but having a much lower magnitude.
FIG. 1.
Mean band density ratios for RANTES, MIP-1β, MIP-1α, and MIP-2 mRNA expression. mRNA was isolated from BAL cells collected from 4 to 6 mice/time point during three or more separate experiments with CP52-, B1-, or PIV-3-infected or naïve mice. Chemokine mRNA expression was quantified using a PhosphorImager to determine band densities. The band density ratio was determined by dividing the mean band density of the L32 housekeeping gene by the mean band density of the chemokine. Error bars, standard errors of the means.
FIG. 2.
Mean band density ratios for IP-10, MCP-1, and TCA-3 mRNA expression. mRNA was isolated from BAL cells collected from 4 to 6 mice/time point during three or more separate experiments with CP52-, B1-, or PIV-3-infected or naïve mice. Chemokine mRNA expression was quantified using a PhosphorImager to determine band densities. The band density ratio was determined by dividing the mean band density of the L32 housekeeping gene by the mean band density of the chemokine. Error bars, standard errors of the means.
There was consistently higher expression of MIP, MCP-1, and IP-10 mRNAs following CP52 infection than there was following B1 infection (Fig. 1 and 2). To control for by-products in the virus inoculum that might affect chemokine induction, mice were infected with sucrose gradient-purified (5) B1 or CP52 virus (data not shown). Both purified viruses induced MIP, RANTES, IP-10, and MCP-1 mRNA expression between 8 h and 18 h p.i.; however, CP52 induced higher chemokine expression than B1 (e.g., 34% greater for MIP-1α, 37% greater for MIP-1β, 22% greater for MIP-2, and 67% greater for IP-10). TCA-3 mRNA was also induced by purified CP52 but was not observed following infection with CP52-infected VCL; however, the magnitude of expression was significantly lower than that observed following PIV-3 infection. Since the titers of B1 and CP52 in the lung are comparable at day 3 p.i. (29), the differences in MIP, MCP-1, and IP-10 expression are likely due to the absence of G and/or SH proteins.
As observed previously (29), the absence of the G and SH genes (as in strain CP52) consistently resulted in more PMN and NK cells in the lung of mice than did the presence of the G and SH genes (as in strain B1), whereas PIV-3-infected mice had intermediate levels of cells positive for these surface markers (data not shown).
The present study suggests that G and/or SH gene expression reduces MIP, MCP-1, and IP-10 mRNA expression. Chemokines interact with receptors expressed by Th1 cells (CCR5, CXCR3, and CXCR5) (23; P. Loetscher, M. Uguccioni, L. Bordoli, M. Baggiolini, B. Moser, C. Chizzolini, and J. M. Dayer, Letter, Nature 391:344–345, 1998) and with those preferentially expressed by Th2 cells (CCR3, CCR4, and CCR8) (16, 20, 21, 25, 32). MIPs interact with CCR1 and CCR5, MCP-1 interacts with the CCR2 receptor, and IP-10 interacts with the CXCR3 receptor on Th1 cells (1, 2, 24, 33). In earlier studies we showed decreased Th1 cytokine (IFN-γ) expression after infection with B1 compared to after infection with CB52, which lacks these genes (29). The decreased MIP, MCP-1, and IP-10 expression associated with G and/or SH glycoproteins likely impairs the Th1 response; thus, these chemokines may be important in RSV immunity or disease pathogenesis.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Balashov K E, Rottman J B, Weiner H L, Hancock W W. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanock R M, Parrott R H, Connors M, Collins P L, Murphy B R. Serious respiratory tract disease caused by respiratory syncytial virus: prospects for improved therapy and effective immunization. Pediatrics. 1992;90:137–143. [PubMed] [Google Scholar]
- 4.Chin J, Magoffin R L, Shearer L A, Schieble J H, Lennette E H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 5.Collins P L, Mottet G. Membrane orientation and oligomerization of the small hydrophobic protein of human respiratory syncytial virus. J Gen Virol. 1993;74:1445–1450. doi: 10.1099/0022-1317-74-7-1445. [DOI] [PubMed] [Google Scholar]
- 6.Connors M, Giese N A, Kulkarni A B, Firestone C Y, Morse III H C, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dezateux C, Fletcher M E, Dundas I, Stocks J. Infant respiratory function after RSV-proven bronchiolitis. Am J Respir Crit Care Med. 1997;155:1349–1355. doi: 10.1164/ajrccm.155.4.9105078. . (Erratum, 156:675, 1997.) [DOI] [PubMed] [Google Scholar]
- 8.Domachowske J B, Rosenberg H F. Respiratory syncytial virus infection: immune response, immunopathogenesis, and treatment. Clin Microbiol Rev. 1999;12:298–309. doi: 10.1128/cmr.12.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everard M L, Milner A D. The respiratory syncytial virus and its role in acute bronchiolitis. Eur J Pediatr. 1992;151:638–651. doi: 10.1007/BF01957564. [DOI] [PubMed] [Google Scholar]
- 10.Graham B S. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am J Respir Crit Care Med. 1995;152:S63–S66. doi: 10.1164/ajrccm/152.4_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 11.Graham B S, Henderson G S, Tang Y W, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 12.Hancock G E, Speelman D J, Heers K, Bortell E, Smith J, Cosco C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J Virol. 1996;70:7783–7791. doi: 10.1128/jvi.70.11.7783-7791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison A M, Bonville C A, Rosenberg H F, Domachowske J B. Respiratory syncytial virus-induced chemokine expression in the lower airways: eosinophil recruitment and degranulation. Am J Respir Crit Care Med. 1999;159:1918–1924. doi: 10.1164/ajrccm.159.6.9805083. [DOI] [PubMed] [Google Scholar]
- 14.Hussell T, Baldwin C J, A. O G, Openshaw P J. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- 15.Hussell T, Spender L C, Georgiou A, O'Garra A, Openshaw P J. Th1 and Th2 cytokine induction in pulmonary T cells during infection with respiratory syncytial virus. J Gen Virol. 1996;77:2447–2455. doi: 10.1099/0022-1317-77-10-2447. [DOI] [PubMed] [Google Scholar]
- 16.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray P W, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Jeng M J, Lemen R J. Respiratory syncytial virus bronchiolitis. Am Fam Physician. 1997;55:1139–1146. [PubMed] [Google Scholar]
- 18.Johnson T, Graham B. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J Virol. 1999;73:8485–8495. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson T R, Johnson J E, Roberts S R, Wertz G W, Parker R A, Graham B S. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochi H, Hirani W M, Yuan Q, Friend D S, Austen K F, Boyce J A. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Garra A, McEvoy L M, Zlotnik A. T-cell subsets: chemokine receptors guide the way. Curr Biol. 1998;8:R646–R649. doi: 10.1016/s0960-9822(07)00413-7. [DOI] [PubMed] [Google Scholar]
- 22.Olszewska-Pazdrak B, Casola A, Saito T, Alam R, Crowe S E, Mei F, Ogra P L, Garofalo R P. Cell-specific expression of RANTES, MCP-1, and MIP-1α by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. J Virol. 1998;72:4756–4764. doi: 10.1128/jvi.72.6.4756-4764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson B K, Czerniewski M, Andersson J, Sullivan Y, Su F, Jiyamapa D, Burki Z, Landay A. Regulation of CCR5 and CXCR4 expression by type 1 and type 2 cytokines: CCR5 expression is downregulated by IL-10 in CD4-positive lymphocytes. J Appl Biomater (Orlando) 1999;91:254–262. doi: 10.1006/clim.1999.4713. [DOI] [PubMed] [Google Scholar]
- 24.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 25.Sallusto F, Lanzavecchia A, Mackay C R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 26.Srikiatkhachorn A, Braciale T J. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srikiatkhachorn A, Braciale T J. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripp R A, Anderson Larry J. Cytotoxic T-lymphocyte precursor frequencies in BALB/c mice after acute respiratory syncytial virus (RSV) infection or immunization with a formalin-inactivated RSV vaccine. J Virol. 1998;72:8971–8975. doi: 10.1128/jvi.72.11.8971-8975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripp R A, Moore D, Jones L, Sullender W, Winter J, Anderson L J. Respiratory syncytial virus (RSV) G and/or SH proteins alter Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol. 1999;73:7099–7107. doi: 10.1128/jvi.73.9.7099-7107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waris M E, Tsou C, Erdman D D, Zaki S R, Anderson L J. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welliver R C, Kaul A, Ogra P L. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr. 1979;94:370–375. doi: 10.1016/s0022-3476(79)80573-9. [DOI] [PubMed] [Google Scholar]
- 32.Zingoni A, Soto H, Hedrick J A, Stoppacciaro A, Storlazzi C T, Sinigaglia F, D'Ambrosio D, O'Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]
- 33.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]