Key Points
PAD enzymes and citrullinated proteins are present in COPD airways.
Citrullinated elastin is susceptible to proteolytic degradation.
Abstract
In chronic obstructive pulmonary disease (COPD), inflammation gives rise to protease-mediated degradation of the key extracellular matrix protein, elastin, which causes irreversible loss of pulmonary function. Intervention against proteolysis has met with limited success in COPD, due in part to our incomplete understanding of the mechanisms that underlie disease pathogenesis. Peptidyl arginine deiminase (PAD) enzymes are a known modifier of proteolytic susceptibility, but their involvement in COPD in the lungs of affected individuals is underexplored. In this study, we showed that enzyme isotypes PAD2 and PAD4 are present in primary granules of neutrophils and that cells from people with COPD release increased levels of PADs when compared with neutrophils of healthy control subjects. By examining bronchoalveolar lavage and lung tissue samples of patients with COPD or matched smoking and nonsmoking counterparts with normal lung function, we reveal that COPD presents with markedly increased airway concentrations of PADs. Ex vivo, we established citrullinated elastin in the peripheral airways of people with COPD, and in vitro, elastin citrullination significantly enhanced its proteolytic degradation by serine and matrix metalloproteinases, including neutrophil elastase and matrix metalloprotease-12, respectively. These results provide a mechanism by which neutrophil-released PADs affect lung function decline, indicating promise for the future development of PAD-based therapeutics for preserving lung function in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally (1). COPD is progressive and incurable, characterized by chronic shortness of breath, inflammation, and infective pulmonary exacerbations. Emphysema is prevalent in COPD, typified by air space enlargement, caused by degradation of the extracellular matrix (ECM) (2), with diminished gas exchange causing morbidity due to reduced oxygen perfusion. Risk factors for COPD development include smoke exposure, through either cigarette smoking or airborne pollutants, or deficiency in the antiprotease α-1 antitrypsin (AAT) (3). The degradation of lung parenchyma is mediated by proteases derived from infiltrating neutrophils, drawn to the inflamed airway (4, 5). Elastin is arguably the most important target for ECM destruction in COPD, because its polymers comprise the core of the elastic fibers that afford the lungs their durable elasticity (6). Intriguingly, the effect of nonprotease molecules released by neutrophils on the progression of lung tissue damage in COPD is underexplored.
Of interest are the peptidyl arginine deiminase family of enzymes (PADs), which catalyze the calcium-dependent, irreversible conversion of positively charged peptidyl arginine residues to uncharged citrulline. Citrullination can cause significant protein conformational change and unfolding, leading to increased susceptibility to protease-mediated cleavage, as previously reported for profilaggrin (7) or for myelin basic protein (8), with implications for multiple sclerosis (9). A further consequence of PAD activity involves generation of anti-cyclic citrullinated peptide autoantibodies (10), a specific serologic marker for rheumatoid arthritis (RA), with PAD levels markedly elevated in the RA synovium (11). Relevant to airway disease, anti-PAD autoantibodies correlate with airway obstruction in cystic fibrosis (12), and anti-cyclic citrullinated peptide autoantibodies are elevated in COPD (13). Of relevance, while PADs are expressed by a range of immune cells (14–16) and implicated in diverse cellular activities including neutrophil extracellular traps (NETs) (17) and NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation (18), the consequence of increased PAD expression in airway samples of smokers (19) with respect to ECM integrity has not, to our knowledge, been explored. Therefore, with the aim of clarifying the role of neutrophil-released PADs in COPD-related disease, we investigated the impact of PAD-induced citrullination on elastin integrity. This study identified a pathogenic mechanism associated with COPD and illustrates PAD2 and PAD4 involvement in the susceptibility of citrullinated elastin to proteolysis by major emphysema-associated proteases (20, 21). Ultimately, this study highlights the need for development and exploration of new PAD inhibition–based therapies for treatment of COPD.
Materials and Methods
Study design
Subjects with COPD were recruited if they had postbronchodilator (forced expiratory volume in 1 s)/(forced vital capacity) < 0.7 according to global initiative for COPD (GOLD) guidelines and had a radiographic finding of emphysema by CT imaging, had normal AAT levels by nephelometry, and had not experienced an exacerbation in the preceding 6 wk (n = 24). Control subjects with normal lung function and without recognized lung disease were recruited (n = 18), as well as individuals with normal lung function and without recognized lung disease who were active smokers at the time of broncho alveolar lavage (n = 15). Control smokers and nonsmokers were outpatients who attended the hospital for investigation of symptoms such as unexplained cough, and they were fully evaluated with clinical review, computed tomography of thorax, pulmonary function testing, and bronchoscopy, which excluded pulmonary disease or other confounding factors in these participants. Participants were recruited from Beaumont Hospital and St Vincent’s Hospital (Dublin, Ireland) and Royal Victoria Hospital (Belfast, U.K.). All participants signed informed consent prior to inclusion in the study, and the samples were anonymized. The study was approved by the Beaumont Hospital Research Ethics Committee (reference 18/52). The recruitment of patients from Northern Ireland was approved by the East of England–Cambridge East Research Ethics Committee (reference 18/EE/0048) with samples managed as required under the Human Tissue Act (2004) legislation until cell-free. Patient demographics are reported in Table I.
Table I. Demographics of study participants.
| Characteristic | Control (n = 18) | Smoker (n = 15) | COPD (n = 24) |
|---|---|---|---|
| Age (SD), y | 54.1 (13)a,b | 50 (12)b,c | 62.7(8)a,c |
| Male, no. (%) | 12 (66) | 6 (40) | 12 (50) |
| FEV1pp | 102.8 (16)d,e | 96.8 (13)d,f | 67.9 (20)e,f |
| FEV1/FVC | 77.7 (6)g,h | 76.2 (6)g,i | 61 (16)h,i |
| Smoking history (SD), pack-yr | 0* (0) | 29.1 (16)j | 37.9§ (11)j |
| BAL macrophages (SD), % | 95.1 (3) | 92.5 (4) | 92 (7) |
| BAL, Neutrophils (SD), % | 1.5 (1) | 2.2 (1) | 2.7 (4) |
Statistical significance was tested by ANOVA with Tukey post hoc comparison. Pairwise comparisons are denoted by superscript letters, with significant differences denoted in bold type. Some people in the nonsmoker groups were former smokers (three in the control group and ten in the COPD group).
p = 0.0636.
p = 0.6899.
p = 0.0099.
p = 0.684.
p < 0.0001.
p < 0.0001.
p = 0.8872.
p < 0.0001.
p < 0.0001.
p = 0.0178.
BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; FEV1pp, forced expiratory volume in 1 s, percent predicted; FVC, forced vital capacity.
Human lung sampling
Bronchoscopy was performed under conscious sedation. Bronchoalveolar lavage (BAL) was performed by instillation of 100 ml of saline into the right upper lobe with retrieval by manual suction. Peripheral airway tissue was collected by video-assisted thoracic surgical biopsy. BAL cells were separated by centrifugation at 450g at 4°C for 5 min, and the supernatants stored at −80°C; supernatants intended for ELISA or Western blot analysis of BAL proteins were treated with protease inhibitor mixture (Thermo Scientific) prior to storage at −80°C. PAD protein levels in BAL fluid were quantified directly in 50-µl duplicate volumes using PAD2- or PAD4-specific ELISA kits (Cayman Chemical) as per the manufacturers’ recommendations.
Neutrophil isolation, degranulation, and subcellular fraction
Circulating neutrophils were isolated using dextran sedimentation and Ficoll (LymphoPrep; Axis-Shield) density centrifugation. Neutrophils were gently resuspended in Dulbecco’s PBS, pH 7.4, containing 5 mM glucose (PBS-G) with 1 mM CaCl2 and 1 mM MgCl2 and used immediately. Cell purity was assessed as previously described (22), and cell viability was assessed by trypan blue exclusion and exceeded 98%. For degranulation assays, neutrophils (1.5 × 107/ml) were stimulated with TNF-α (10 ng/ml) and fMLF (1 µM) for up to 20 min in PBS-G containing 10 mg/ml Pefabloc, leupeptin, and tosyl-l-lysyl-chloromethane hydrochloride before recovery of supernatants for detection of PADs. Protease inhibitors were omitted for assays of PAD activity.
In a subset of experiments, neutrophils were subcellular fractionated (23). Isolated neutrophils (8 × 107 cells) were treated with diisopropyl fluorophosphate and then lysed by N2 cavitation (400 psi for 20 min) at 4°C in break buffer (10 mM KCl, 3 mM NaCl, 2 mM MgCl2, 1 mM EDTA, 1 mM ATP, 20 mM PIPES, pH 7.2) containing protease inhibitors (10 mg/ml leupeptin, PMSF, tosyl-l-lysyl-chloromethane hydrochloride, pepstatin A, and aprotinin). Lysates (2 ml) were centrifuged at 40,000g at 4°C for 1 h through 17.5% (w/w, 2 ml), 35% (w/w, 2 ml), and 60% (w/w, 7 ml) discontinuous sucrose gradients. Using a pipette, the cytosolic fraction was recovered above the 17.5% sucrose layer, and the plasma membrane fraction was recovered from the interface between the 17.5 and 35% layers. Granules were recovered from the interface between the 35 and 60% sucrose layers. The membrane fraction was pelleted by centrifugation at 14,000g for 30 min at 4°C. Sucrose concentration in the granule fraction was diluted below 30% and verified by refractometer, and then granules were segregated by centrifugation at 40,000g for 1 h over 30, 43, and 55% (w/w) discontinuous sucrose gradients. Primary granules were recovered from the interface between 43 and 55% layers, whereas secondary and tertiary granules were recovered as a mixed suspension between the 30 and 43% sucrose layers. Fractions were stored at −80°C following isolation. Granule identity was confirmed by Western blot of fractionated material for myeloperoxidase and human cathelicidin protein-18 (hCAP-18), which have peak abundance in neutrophil primary and secondary granules, respectively.
Western blot analysis
All samples were denatured in Laemmli buffer with DTT at 95°C for 5 min before separation through a 4–20% SDS-PAGE gel at 130 V for 90 min. Protein was then transferred to polyvinylidene difluoride membrane at 4°C and 30 V overnight. The membranes were blocked using 5% (w/v) nonfat milk in PBS with 0.05% (w/v) Tween 20 for 1 h at ambient temperature and incubated with a 1:1,000 dilution of an anti-elastin polyclonal Ab (ab23747, Abcam), anti-PAD2 (ROI002, CosmoBio), or anti-PAD4 (ab96758, Abcam) Ab for 4 h or a 1:2,000 dilution of anti-MPO Ab (anti-myeloperoxidase, NB600-923; Novus) or anti-hCAP18 (anti-human cathelicidin protein, NBP1-76864; Novus) for 2 h. The membranes were then washed and incubated with 1:2,000 dilution of an HRP-conjugated secondary Ab prior to chemiluminescent visualization. Citrullinated proteins were detected using a 1:1,250 dilution of a mouse anti-modified citrulline Ab (MQR1.602, ImmunoPrecise) following modification of citrulline by treatment with butanedione monoxime and antipyrine at 37°C by the method of Senshu et al. (24).
Confocal microscopy
Human lung tissue sections were deparaffinized by immersion in xylene followed by repeated ethanol washes. Citrulline residues were modified as per Western blot. The sections were blocked with 4% (v/v) normal goat serum for 30 min and probed overnight with mouse-raised anti-modified citrulline (1:500; ImmunoPrecise) and rabbit anti-elastin (1:500). The sections were rinsed and probed with biotinylated anti-mouse IgG Ab (1:1000, 1 h) followed by Alexa Fluor 594–conjugated streptavidin (1:2,000) and Alexa Fluor 488–conjugated anti-rabbit IgG (1:2,000). Coverslips were mounted with Prolong Gold antifade mountant with Sytox deep red DNA stain (Thermo Scientific). The coverslips were mounted with Prolong Gold Antifade Mountant (Thermo Scientific). Imaging was carried out using a 63× oil immersion objective mounted to an LSM710 confocal microscope (Zeiss). Colocalization of signals was determined using Coloc2 v3.0.5 plugin for ImageJ software.
Protein citrullination
AAT (1 µM; Athens Research), histone H3 (1 μM, Cayman Chemical), or TIMP-1 (0.5 µM; BioLegend) was incubated with PAD2 or PAD4 (1 nM, Cayman Chemical) in 100 mM Tris, 125 mM NaCl, 0.05% Igepal CA-630, 1 mM DTT, pH 7.4, at 37°C for 60 min in the absence or presence of 1 mM CaCl2 (25) or following pretreatment with the irreversible PAD inhibitor BB-Cl-amidine (100 μM, Cayman Chemical) by incubation of PAD2 or PAD4 (125 nM) with BB-Cl-amidine at 37°C for 10 min at pH 7.6. To assess citrullination of elastin by PADs in neutrophil degranulated material or in cell-free BAL fluid, human lung-derived polymeric elastin (5 μg; HS51, Elastin Products Company) was incubated in 100 μl of neutrophil supernatant, prepared as described above, or BAL fluid, in the presence of final concentrations of 1 mM CaCl2 and 400 μM GSH for 2 h at 37°C. The citrullination of respective proteins (1 μg/lane) was determined by Western blot as described above.
Assessment of elastin proteolysis following citrullination
Assays of elastin were conducted using a soluble, polymeric form of lung-derived elastin (HS51, Elastin Products Co.). Elastin (15 µg) was incubated with PAD2 or PAD4 (62.5 nM) at 37°C for 60 min in the absence or presence of CaCl2 (1 mM) for subsequent treatment with neutrophil elastase (NE). For matrix metalloprotease (MMP)-12, PAD activity was controlled by pretreatment with the inhibitor BB-Cl-amidine (100 µM) for 10 min at 37°C (26). NE or MMP-12 (62.5 nM) was then added, and the reactions were incubated at 37°C for 0, 15, 60, or 180 min.
Protease activity assays
NE (1 nM, Elastin Products Co.) was incubated with PAD2 or PAD4 (1 nM, Cayman Chemical) in 100 mM Tris, 125 mM NaCl, 0.05% Igepal CA-630, 1 mM DTT, pH 7.4, at 37°C for 60 min in the absence or presence of 1 mM CaCl2. Reaction products were distributed to a final concentration of 0.5 nM NE into fresh buffer with NE substrates, Me-O-Suc-AAPV-paranitroanilide (pNA) for PAD2 (10 mM stock in DMSO; Sigma) or 2-aminobenzoyl (Abz)-APEEIMRRQ-EDDnp for PAD4 (Peptides Int’l), at a range of substrate concentrations. The reaction product pNA was measured at 37°C for up to 30 min at 405 nm using a SpectraMax M3 plate reader (Molecular Devices). Fluorescent Abz was measured by excitation at 320 nm and emission detection at 420 nm. NE activity was determined to exceed 90% by active site titration using AAT standard. ProMMP-12 was activated by treatment for 1 h at 37°C with 4-aminophenylmercuric acetate (1 mM) in MMP assay buffer (100 mM Tris, 125 mM NaCl, 5 mM CaCl2, 0.05% Igepal CA-630). Because MMPs require calcium for activity, direct inhibition of PADs by BB-Cl-amidine was performed; PAD2 or PAD4 (125 nM) were incubated with BB-Cl-amidine (100 µM) at 37°C for 10 min at pH 7.6 before incubation with an equal volume of active MMP-12 (125 nM) for 60 min. Using the MMP FRET substrate, Mca-PLGL-Dnp-AR-NH2 (Anaspec), product formation was monitored at 37°C for 30 min at 420 nm. The reaction rates were derived from the initial burst phase of product formation and used to determine Km values for proteases incubated with active or inactive PADs.
Protease inhibition assay
AAT (120 µM) or TIMP-1 (0.5 µM; BioLegend) was incubated with PAD2 or PAD4 (67.5 nM) for 60 min in the absence or presence of 1 mM CaCl2. Treated antiprotease was then diluted to a range of concentrations in 100 mM Tris, 125 mM NaCl, 0.05% Igepal CA-630, pH 7.1, and incubated with 10 nM NE for 30 min at 37°C in wells of a black 96-well plate. An equal volume of protease FRET substrate, diluted in the same medium, was then added at a final concentration of 10 µM. Product formation was monitored over 30 min at 37°C using a SpectraMax M3 plate reader. Reaction rates were derived from the initial burst phase of product formation and used to determine IC50 values for proteases pretreated with AAT or citrullinated AAT.
Statistical analysis
The data are reported as means and ranges or SD. The data were analyzed using Prism software version 9.5 (GraphPad). Significant differences were tested for using one-way or two-way ANOVA, including repeated measures for within-subject variance as appropriate, followed by Tukey or Šídák post hoc tests for groupwise comparisons. The strength of associations between data was tested by linear regression. A value of p < 0.05 was considered statistically significant.
Results
PAD2 and PAD4 are present and active in COPD airways
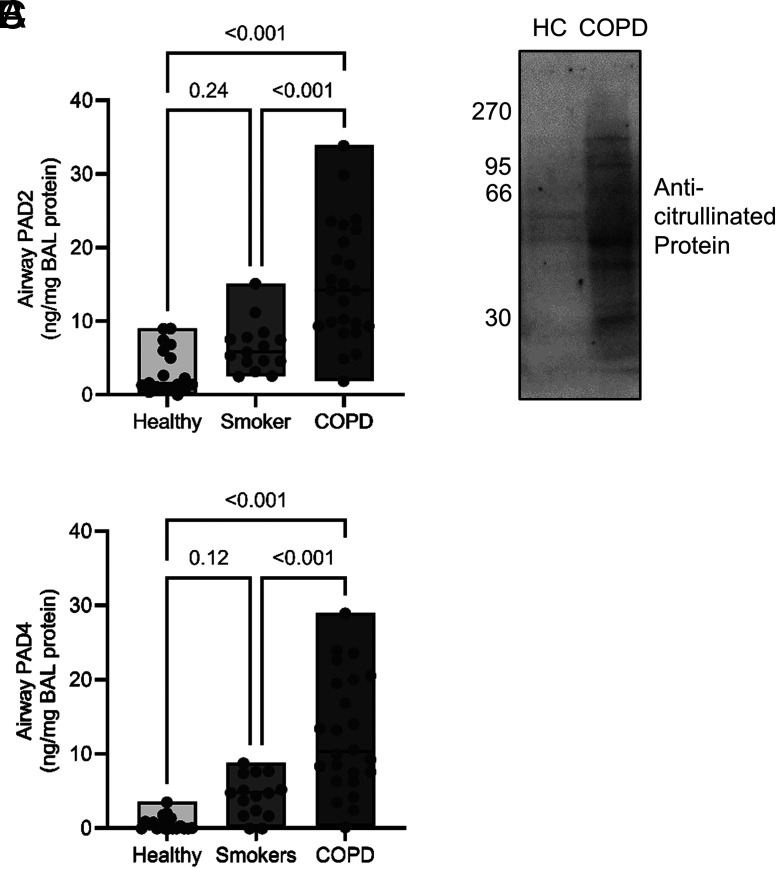
To study PADs as potential disease modifiers in emphysematous lung disease, we investigated their levels in airway samples of patients with COPD. BAL was performed on people with COPD in whom emphysema was the dominant presentation on computed tomography scan and were not experiencing infective exacerbation (n = 24) or who were free from obstructive lung disease and were current smokers (n = 15) or nonsmokers (n = 18) (Table I). By ELISA, PAD isotypes 2 and 4 were markedly elevated in COPD BAL samples over unobstructed controls (PAD2: 5-fold, p < 0.0001; PAD4: 24-fold, p < 0.0001; Fig. 1A, 1B). Ex vivo immunoblotting analysis revealed a range of citrullinated proteins in patient BAL fluid samples (Fig. 1C). Collectively, these data indicate increased levels of PAD enzymes and citrullinated proteins in COPD airways.
FIGURE 1.
PAD2 and PAD4 and citrullinated proteins are present in the COPD airway. BAL was performed on people with COPD (n = 24) as well as smokers (n = 15) and nonsmokers (n = 18) who were free of obstructive pulmonary disease. (A and B) BAL fluid levels of PAD2 (A) or PAD4 (B) were measured by ELISA. The data are expressed as means and ranges. Statistical significance was set at p < 0.05 and tested for by one-way ANOVA, with the Tukey post hoc test. (C) Western blot for citrullinated proteins of BAL fluid from healthy control (HC) or COPD (50 μg of total protein loaded per well). Shown are representative images from n = 4 biological repeats.
Neutrophils harbor PAD2 and PAD4 in their primary granules
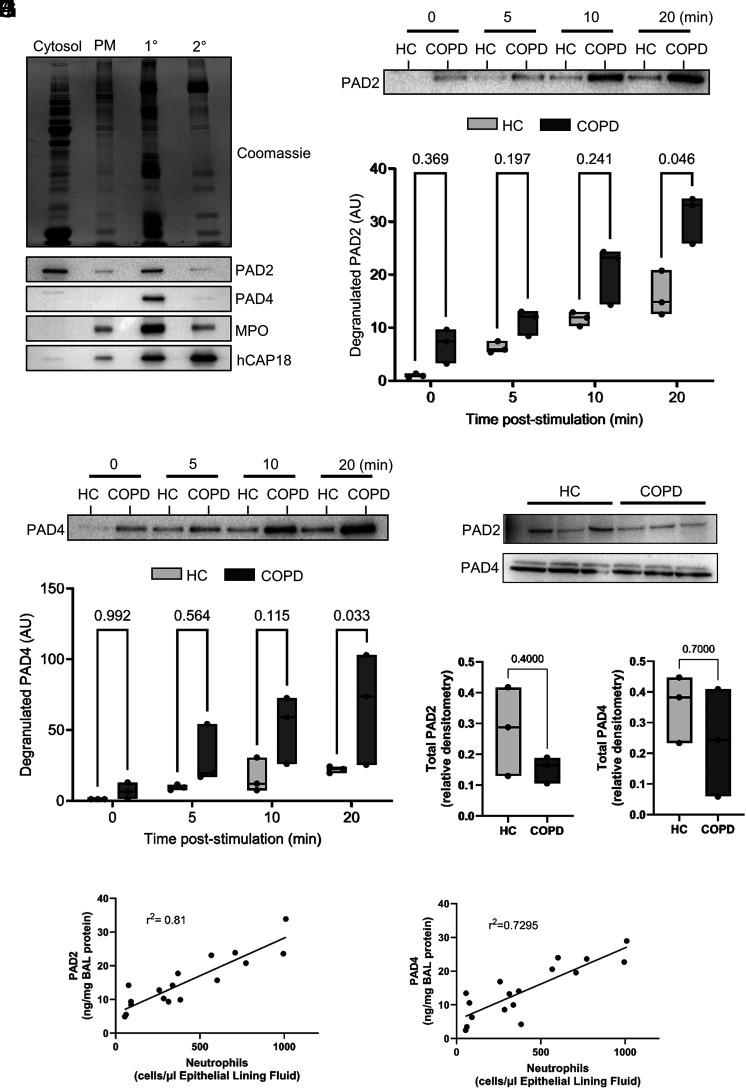
To investigate a source of PADs, we focused on neutrophils, previously shown to contain PADs in cytosol and cytoplasmic granules (27). By use of sucrose gradient ultracentrifugation, subcellular fractionation of neutrophils was carried out. Immunoblotting of cellular fractions identified PAD2 and PAD4 in primary granules of neutrophils (Fig. 2A). Dual stimulation of neutrophils ex vivo with TNF-α and fMLF (10 ng/ml) caused the cells to degranulate PAD2 and PAD4 into the extracellular milieu (Fig. 2B–E). Of note, neutrophils of patients with COPD degranulated 2-fold greater levels of PAD2 and 3-fold greater PAD4 than healthy control cells (Fig. 2C, 2E), possibly due to the reported primed state of these cells (28, 29). Moreover, the increased release of PADs was not due to altered total protein, because intracellular levels of PADs did not differ significantly between neutrophils isolated from people with or without COPD (Fig. 2F–H). Finally, PAD levels associated with the percentage of neutrophils in COPD airway samples (r2 = 0.81, p = 0.0003 for PAD2; r2 = 0.7295, p = 0.0051 for PAD4) (Fig. 2I, 2J).
FIGURE 2.
PADs are located in neutrophil primary granules and are released upon cell activation. Subcellular fractionation of neutrophils (8 × 107) from healthy donors was performed and subjected to SDS-PAGE. (A) Total protein by Coomassie Blue staining (upper panel). PADs were detected in respective fractions by Western blot, along with the primary granule marker myeloperoxidase (MPO) and secondary granule marker, human cathelicidin antimicrobial protein (hCAP-18). Shown are representative images from n = 4 biological repeats. (B–E) Neutrophils (2 × 107) of healthy donors or people with COPD were incubated with TNF-α (10 ng) and fMLF (1 µM) at the indicated time points. Extracellular degranulation of PAD2 and PAD4 from neutrophils was determined by Western blot. (B) Representative Western blot showing PAD2 staining. (C) Densitometry of PAD2 staining in supernatants of stimulated neutrophils. (D) Representative Western blot showing PAD4 staining. (E) Densitometry of PAD4 staining in supernatants of stimulated neutrophils. The data are expressed as means and ranges (n = 3 biological repeats). Statistical significance was set at p < 0.05 and tested for by two-way repeated measures ANOVA, with the Šídák post hoc test. (F) Lysates were prepared from peripheral blood neutrophils (107) and probed for PAD2 or PAD4 by Western blot (n = 3 subjects per group). (G) Densitometry of PAD2 staining in lysates of neutrophils. (H) Densitometry of PAD4 staining in lysates of neutrophils. (I and J) An association between BAL PAD2 (I) and PAD4 (J) levels and the proportion of BAL neutrophils in people with COPD (n = 18) was tested for by linear regression (p = 0.0003 for PAD2 and p = 0.0051 for PAD4). HC, healthy control; PM, plasma membrane; 1°, primary granule; 2°, secondary granule.
Peptidyl arginine deiminase activity does not influence the activity of proteases or antiproteases central to COPD
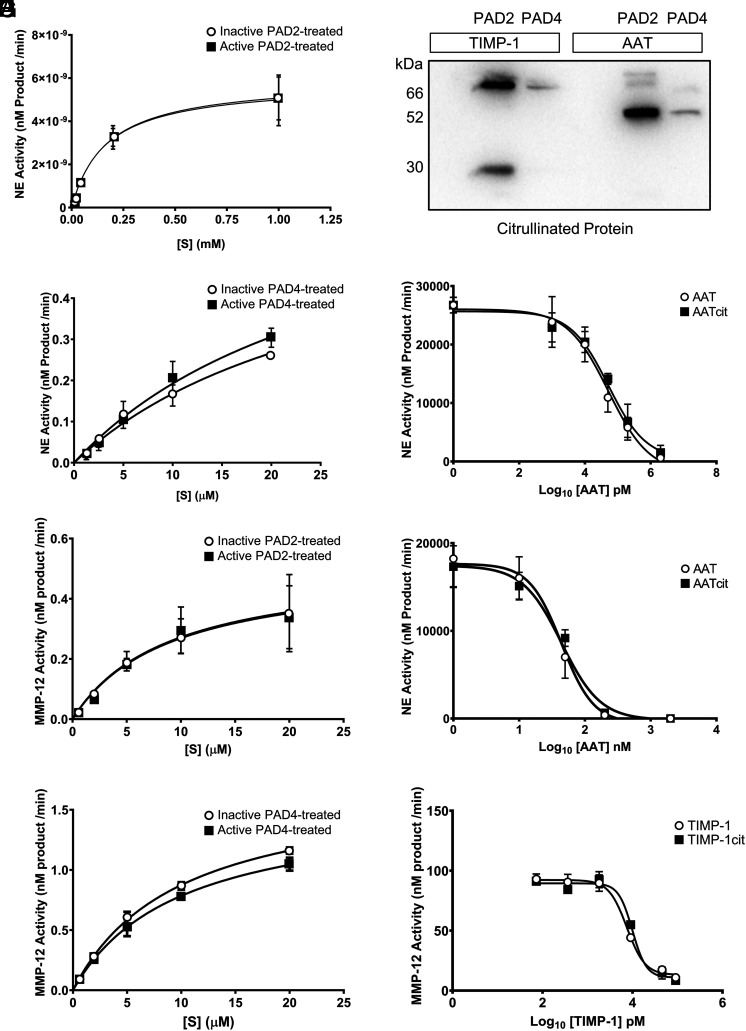
To address the intersection of PAD activity with target proteins relevant to COPD pathogenesis, we examined the effect of PAD2 and PAD4 on the key proteases and antiproteases implicated in emphysema development. First, we focused on NE (20) and MMP-12 (21), because airway levels of both proteases are predictive of disease progression and severity in COPD (30, 31). We verified that PADs did not influence either NE or MMP-12 protease activity by incubating PAD2 or PAD4 with each protease and also by repeating experiments under conditions that negatively affected PAD activity including omission of Ca2+ or inclusion of the arginine-based peptidomimetic irreversible PAD inhibitor BB-Cl-amidine (26). The affinity (Km) of each protease for its respective oligopeptide substrate remained unchanged following incubation with PAD-2 (Fig. 3A, 3C) or PAD-4 (Fig. 3B, 3D). Affinity of NE for fluorescently labeled AAPV was 164 ± 77 μM in the presence of inactive PAD2 and 170 ± 66 μM with active PAD2 (Fig. 3A; p = 0.9915). Similarly, using the fluorescently labeled oligopeptide APEEIMRRQ, substrate affinity of NE was unaffected by the presence of active PAD4 (21.9 ± 16 μM with inactive PAD4 versus 33.5 ± 21 μM with active PAD4; Fig. 3B; p = 0.6591). MMP-12 affinity for fluorescently labeled PLGL-Dnp-AR was 8.93 ± 6.5 μM in the presence of inhibited PAD2 and 8.96 ± 6.2 μM in the presence of active PAD2 (Fig. 3C; p = 0.9924). The same outcome was observed for PAD4 inclusion, with comparable affinity evident regardless of PAD4 inhibition (9.63 ± 0.8 μM with inhibited PAD4 and 9.95 ± 1.3 μM with active PAD4; Fig. 3D; p = 0.826). Although the endogenous inhibitor of NE, AAT, can be readily citrullinated by PAD2 and PAD4 and tissue inhibitor of metalloproteinase-1 (TIMP-1) can be citrullinated by PAD2 (Fig. 3E), this did not affect their ability to inhibit these proteases (Fig. 3F–H). Citrullinated AAT demonstrated an IC50 for NE of 66.3 ± 53 nM compared with 39.8 ± 33 nM for unmodified AAT (Fig. 3F; p = 0.5476), when PAD2 was the enzyme used, whereas PAD4 citrullination of AAT yielded a similar outcome (37.8 ± 16 nM for inactive PAD4-treated AAT versus 52.1 ± 12 nM for PAD4-citrulinated AAT; Fig. 3G; p = 0.6591). Moreover, IC50 values were 3.11 ± 1.4 and 3.45 ± 1.3 nM for TIMP-1 and PAD2-citrullinated TIMP-1, respectively (Fig. 3H; p = 0.9942). To summarize, these data establish that citrullination of proteases relevant to the pathogenesis of COPD and their cognate inhibitors has no impact on their associated activity.
FIGURE 3.
Protease activity of NE and MMP-12 is unchanged by direct interaction with PADs. (A) Rate kinetics of product formation from the FRET substrate Me-O-Suc-AAPV-pNA by neutrophil elastase (NE) (1 nM) that had been incubated with PAD2 (1 nM) in the absence or presence of calcium (1 mM) for 30 min before substrate addition. (B) Rate kinetics of product formation from the FRET substrate Abz-APEEIMRRQ-EDDnp by NE (65 nM) that had been incubated with PAD4 (65 nM) in the absence or presence of calcium (1 mM) for 30 min before substrate addition. (C and D) PAD2 (125 nM) or PAD4 (125 nM) was incubated with or without the PAD inhibitor BB-Cl-amidine (100 µM) at 37°C for 10 min before incubation with MMP-12 (125 nM). (C) Rate kinetics of product formation from the FRET substrate, Mca-PLGL-Dnp-AR-NH2 by active MMP-12 in the presence of PAD2. (D) Rate kinetics of product formation by active MMP-12 in the presence of PAD4 (n = 3; mean ± SEM; sum-of-squares F test). (E) Western blot for peptidyl citrulline showing that AAT (1 μM; 52 kDa) was citrullinated by PAD2 and PAD4 (1 nM) and tissue inhibitor of metalloproteinase (TIMP)-1 (0.5 μM; 28 kDa) was citrullinated by PAD2 (1 nM), each following 1 h of incubation. (F) Equivalent inhibitory capacity of untreated or PAD2-citrullinated AAT (AATcit) against NE. (G) Equivalent inhibitory capacity of untreated or PAD4-citrullinated AAT (AATcit) against NE. (H) Comparable inhibitory capacity of untreated or PAD2-citrullinated TIMP-1 (TIMP-1cit) against MMP-12 (n = 3; mean ± SEM; significance was tested for by sum-of-squares F test).
Citrullinated elastin is more susceptible to degradation by neutrophil elastase or MMP-12
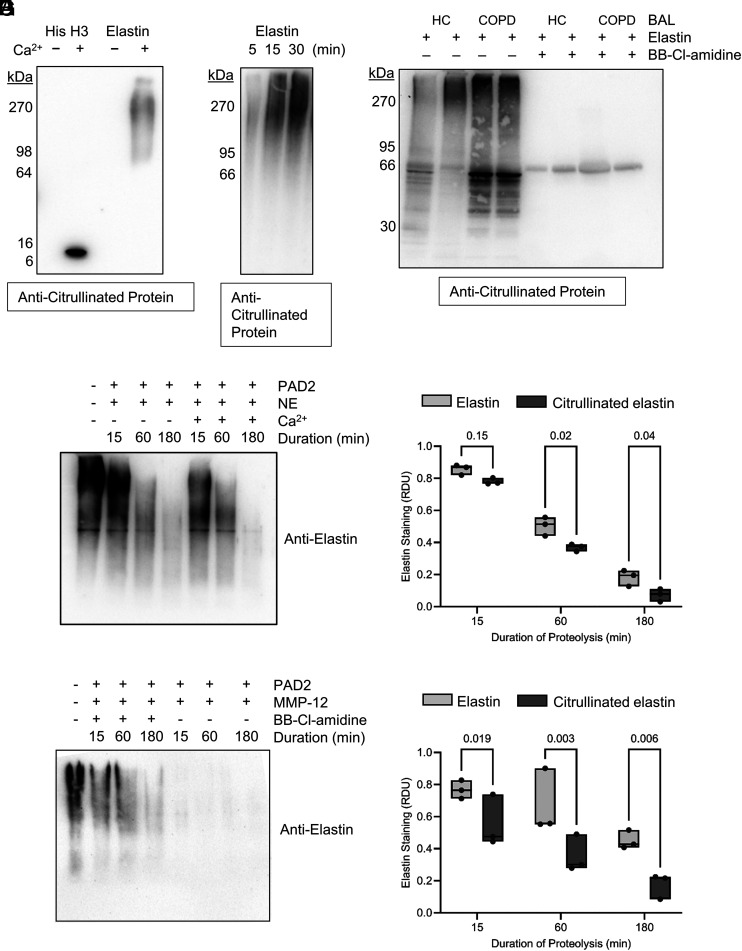
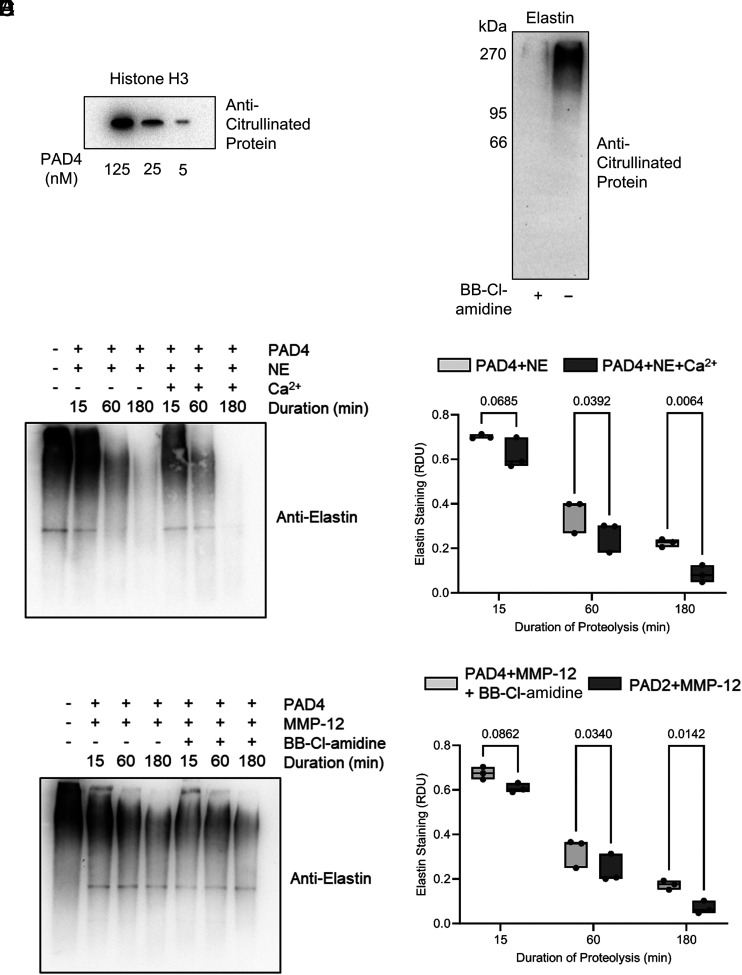
To investigate the potential for pathogenic involvement of PADs in COPD, given prior studies showing that citrullinated proteins can become more susceptible to proteolysis (7, 8), we examined the effect of PADs on proteolysis of the key structural protein of the lung, elastin, by major emphysema-linked proteases. Notably, by Western blot analysis, citrullination of polymeric human airway-derived elastin (15 μg) was readily achieved in vitro, with citrullination detectable from 60 min of incubation with purified PAD2 (62.5 nM), and could be prevented by Ca2+ omission (Fig. 4A). Positive controls for this experiment included the ability of PADs to citrullinate histone H3 (Fig. 4A) (32, 33). Moreover, incubation of elastin (5 μg) with degranulated proteins from neutrophils (2 × 107) that had been stimulated with fMLF (1 μM) and TNFɑ (10 ng) caused elastin citrullination within 5 min, confirming the release of active PADs from degranulating neutrophils (Fig. 4B). To address whether PADs were active in the airway, human lung-derived polymeric elastin was incubated in control and COPD BAL fluid ex vivo. This showed a marked citrullination of high m.w. elastin (Fig. 4C). Notably, citrullination was prevented by inclusion of the PAD inhibitor BB-Cl-amidine (100 μM).
FIGURE 4.
PAD2 citrullinates elastin and enhances elastin proteolysis by serine- and metallo-proteases. (A) Western blots probed for peptidyl citrulline show elastin citrullination following 1 h of coincubation in the presence of Ca2+ (1 mM) by PAD2 (62.5 nM) (histone H3 served as positive control). (B) Representative Western blot showing progressive citrullination of elastin during incubation with degranulated neutrophil proteins. Neutrophils (2 × 107) from healthy donors were stimulated for 5, 15, and 30 min with TNF-α (10 ng) and fMLF (1 µM). Polymeric elastin (5 μg) was added to extracellular supernatants of stimulated neutrophils at the different time points and subsequently probed for protein citrullination (n = 4 biological repeats). (C) Western blot demonstrating exogenous elastin (5 μg) citrullination following incubation with BAL proteins from unobstructed control donors (HC) or COPD individuals that could be prevented by inclusion of BB-Cl-amidine (100 μM). Shown is a representative image from n = 3 biological repeats. (D–G) Unmodified or citrullinated polymeric elastin (15 μg) was subjected to proteolysis. (D) Representative Western blot for polymeric human lung elastin showing its proteolysis (lanes 2–4) over 3 h by NE and enhanced proteolysis of PAD2-treated elastin (lanes 5–7). (E) Densitometry for residual elastin or PAD2 citrullinated elastin following its proteolysis by NE (n = 3 technical repeats). (F) Digestion of elastin by MMP-12 with or without PAD2 pretreatment. (G) Densitometry for residual elastin or PAD2 citrullinated elastin following its proteolysis by MMP-12 (n = 3 technical repeats). The data are presented as means and ranges. Statistical significance was set at p < 0.05 and tested for by two-way ANOVA with the Šídák post hoc test. HC, healthy control.
For elastin proteolysis, we focused on NE (20) and MMP-12 (21), both involved in progression of COPD (30, 31). Upon addition of NE (62.5 nM) to PAD2-citrullinated, polymeric elastin (15 μg), degradation was evident within 15 min, progressing over 3 h (Fig. 4D). Notably, citrullination of elastin by PAD2 rendered it more susceptible to degradation by protease activity (92.7 ± 0.04% NE-mediated digestion of PAD2-citrullinated elastin, 81.8 ± 0.05% digestion of unmodified elastin after 3 h; p = 0.039; Fig. 4E), an effect prevented by omission of the PAD cofactor Ca2+ or by inclusion of BB-Cl-amidine (100 μM). MMP-12 showed markedly more effective proteolysis of citrullinated elastin, degrading 82.4 ± 0.1% of citrullinated starting material compared with only 55 ± 0.1% of unmodified elastin (p = 0.006; Fig. 4G). Moreover, PAD4 (62.5 nM) successfully citrullinated both histone H3 (in control experiments; Fig. 5A) and polymeric human airway-derived elastin (15 μg) in vitro, an effect prevented by inclusion of BB-Cl-amidine (100 μM) (Fig. 5B). Similarly, elastin that had been citrullinated by PAD4 showed enhanced susceptibility to proteolysis by either NE or MMP-12, with proteolysis by NE enhanced by 15 and 11% for MMP-12 after 3 h when acting on citrullinated elastin (Fig. 5C–F). These data confirm PAD2 and PAD4 involvement in the susceptibility of citrullinated elastin to proteolysis.
FIGURE 5.
PAD4 citrullinates elastin and enhances elastin proteolysis by serine- and metallo-proteases. (A and B) Western blots probed for peptidyl citrulline show histone H3 citrullination following 1 h of coincubation in the presence of Ca2+ (1 mM) by PAD4 (A) and elastin citrullination by PAD4 (62.5 nM) in the presence or absence of BB-Cl-amidine (100 μM) (B). (C–F) Unmodified or citrullinated polymeric elastin (15 μg) was subjected to proteolysis. (C) Representative Western blot for polymeric human lung elastin showing its proteolysis (lanes 2–4) over 3 h by NE and enhanced proteolysis of PAD4-treated elastin (lanes 5–7). (D) Densitometry for residual elastin or PAD4 citrullinated elastin following its proteolysis by NE (n = 3 technical repeats). (E) Digestion of elastin by MMP-12 with or without PAD4 pretreatment. (F) Densitometry for residual elastin or PAD4 citrullinated elastin following its proteolysis by MMP-12 (n = 3 technical repeats). The data are presented as means and ranges. Statistical significance was set at p < 0.05 and tested for by two-way ANOVA with the Šídák post hoc test.
Airway elastin is a target for citrullination
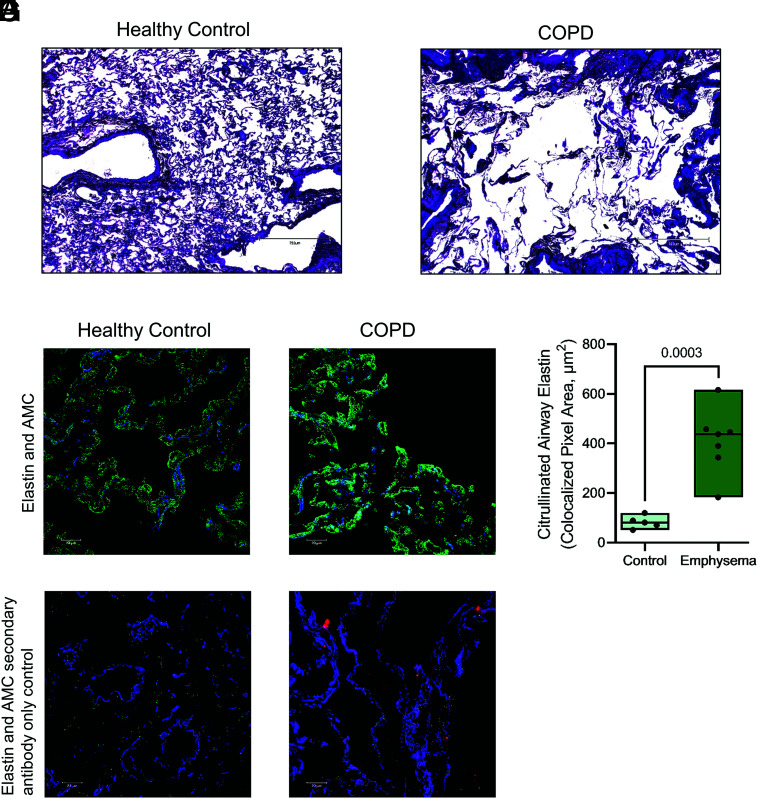
Our results show that elastin can undergo citrullination in vitro and that citrullination enhances the susceptibility of elastin to proteolysis by major proteases associated with COPD. We therefore investigated the citrullination of elastin in the airway. To confirm elastin citrullination in COPD, surgical biopsies were obtained from patients with COPD and emphysema or from people without obstructive lung disease matched for age and smoking history. Sections stained with hematoxylin and eosin demonstrated emphysematous tissue destruction in COPD stained tissue (Fig. 6A, 6B). Moreover, immunofluorescent staining for elastin and for peptidyl citrulline residues revealed considerable citrullination of elastin in emphysematous tissue, compared with nonobstructed control samples (Fig. 6C, 6E), which was not evident in control samples when anti-citrullinated protein Ab was omitted from staining (Fig. 6D, 6F). Elastin citrullination was 5-fold higher in COPD small airways than controls (p = 0.0003; Fig. 6G). Collectively, these data show that PADs are present and active in the COPD airway, where they citrullinate elastin in the small airways and thereby endanger parenchymal elastic fibers for proteolytic degradation.
FIGURE 6.
Citrullinated elastin in the lung of patients with COPD. Lung tissue sections were obtained via surgical biopsy from donors free from obstructive lung disease (n = 5) or people with COPD emphysema (n = 7). (A–C and E) Sections were stained with hematoxylin and eosin showing normal alveolar tissue in a healthy control (A) and emphysematous tissue destruction in COPD (B). Fluorescent immunostaining was performed and imaged by confocal laser scanning microscopy for healthy (C) and COPD (E) lung tissue. Tissue was stained for elastin (blue) and citrullinated protein (green), with DNA stained (red). (D and F) In control experiments, primary Ab against citrullinated protein was omitted. (G) Colocalized staining for elastin and citrullination in control or COPD emphysema peripheral airways. The data are presented as means and ranges. Statistical significance was set at p < 0.05 and tested for by one-way ANOVA, with the Tukey post hoc test. AMC, Anti-modified citrulline.
Discussion
In the current study, we report citrullination of elastin in the COPD airway and present evidence that this creates a vulnerability to enhanced degradation of the lung extracellular matrix. We observed that elastin citrullination is extensive in the peripheral airways of people with COPD, coinciding with highly elevated levels of PAD2 and PAD4. Neutrophils may be one source of these PADs, because both PAD2 and PAD4 are degranulated by activated neutrophils, consistent with their presence in neutrophil primary granules as we have shown and which corroborates existing proteomic data (27).
Neutrophils are abundant in the inflamed airways of individuals with progressive lung disease (34–36) and exhibit heightened degranulation including protease release in COPD (29, 37, 38) and PAD release, as demonstrated here. Elevated PAD2 has previously been noted in the epithelial lining fluid of smokers without diagnosed lung disease, indicating potential early involvement of PADs in disease development (39). Moreover, levels of both PAD2 and PAD4 are increased in lung tissue from people with COPD, coinciding with increased presence of citrullinated proteins (40). We show here for the first time, to our knowledge, that this includes the key structural protein of the lung, elastin. PADs released into the airway lumen encounter conditions suitable for maximal activity, including a reducing environment and sufficient calcium (41). PADs benefit from high levels of reduced glutathione in the airway surface liquid, having an active site cysteine that requires reducing conditions. Although oxidation may diminish PAD activity (42), compensatory secretion of GSH mitigates against oxidation engendered by inflammation (43). We have shown that PADs remain active following degranulation from neutrophils ex vivo. Consequently, elastin becomes highly citrullinated in the lungs of people with COPD. Notably, in RA, there is a strong association between PAD activity and levels of neutrophilic inflammation in the synovial joint (44), and inhibition of PAD activity alleviates symptoms of arthritis in murine models (45), supporting our proposed role for neutrophil-derived, PAD-mediated citrullination in the COPD airway.
Citrullinated proteins frequently display altered susceptibility to proteolysis (7, 8), and it is proteolysis of elastic fibers that drives emphysema. Citrullination is used by neutrophils in certain contexts to facilitate proteolysis, such as dissolution of the nuclear lamina that precedes NET release, wherein PAD4 citrullinates HMGB1 prior to its cleavage by calpain 1 (46, 47). Protein citrullination is well described in RA (44), but citrullination of elastin is a novel finding of central importance to COPD. Hence, to explore the pathogenic mechanism of PADs in emphysema, we examined the susceptibility of citrullinated elastin to proteolysis by NE or MMP-12. In COPD, airway levels of NE correlate with disease severity and are predictive of disease progression (32). In mice, knockout of NE protects against cigarette smoke–induced emphysema (20), and it is accepted that NE causes emphysema by degradation of elastin (48). MMP-12 is also implicated in elastin degradation. MMP-12 levels are elevated in COPD, and its activity correlates with severity of COPD in smokers (21, 33, 49). In mice, knockout of MMP-12 also prevents cigarette smoke-induced COPD (50). In this context, we examined the ability of NE and MMP-12 to degrade citrullinated elastin. By citrullinating polymeric, lung-derived elastin, increased susceptibility to proteolysis by either protease was demonstrated. Considering the inability of PADs to directly influence either NE or MMP-12 protease activity, results strongly suggest that the increased degradation of citrullinated elastin is due to its increased susceptibility to proteolysis by either serine- or matrix metallo-proteases. Moreover, neither PAD2 nor PAD4 affected the antiprotease activity of cognate inhibitors AAT and TIMP-1. Hence, PAD activity would not interfere with AAT augmentation therapy in AAT-deficient patients with COPD (51).
The reduction in local cationic charge of elastin, mediated by conversion of arginine to citrulline, may have improved the association of elastin with protease, as has been demonstrated in other contexts with NE (52), as well as for calpain 1 (46). Peptidyl arginine is itself an unfavorable target for NE (53); consequently, degranulated PADs may promote elastic fiber degradation in the airway. Citrullination of airway tissue may explain why COPD symptoms continue to progress following cessation of smoking or treatment of inflammation. Because the turnover of pulmonary elastin is extremely slow (54), this irreversible post-translational modification may serve as a neoepitope to stimulate on-going leukocyte activity and endanger citrullinated elastin for subsequent injury (55, 56).
In summary, airflow obstruction in COPD occurs through a process that is yet to be fully elucidated, and emphysematous airspace enlargement continues to progress after smoking cessation. Collectively, our data show that PAD activity can contribute to emphysematous tissue degradation. Our study has several limitations, including low numbers of tissue samples and a lack of longitudinal samples over the course of airway disease. The study focused on neutrophils as one source of PADs and their extracellular release following degranulation but did not explore PADs release by macrophages, with PAD2 expressed in M1 macrophages (57). Moreover, NET-mediated consequences (58) and broader implications of PAD activity for autoimmunity or antimicrobial immunity require investigation (59, 60). Nevertheless, the results of our study expand on the longstanding dogma that disruption of the ECM in COPD occurs mainly due to protease activity and introduces PADs as key players that work in synergy with proteolytic enzymes for ECM degradation. The current standard of care for COPD focuses on alleviation of symptoms by relaxing airway obstruction with β2 agonists or anticholinergic agents, with limited utility for inhaled corticosteroids to mitigate inflammation (61). Due to the strong tissue-damaging potential of PADs, PAD2 and PAD4 are attractive pharmaceutical candidates to dampen PAD-driven COPD disease. Of great interest are initial findings regarding PAD inhibition as a treatment strategy in diverse conditions including cancers (62, 63), multiple sclerosis (64), and ulcerative colitis (65), as well as in inflammatory arthritis (45), in which PAD2 and PAD4 have well recognized pathogenic roles (66, 67). Although PAD2 or PAD4 specific inhibitors have been designed that may have fewer off-target effects (68), for our findings to be applied in the clinic, ensuing steps would include the generation of novel potent PAD inhibitors for the treatment and delay of COPD progression.
Acknowledgments
We thank all the patients with COPD and healthy donors who participated in this study.
This article is featured in Top Reads, p. 1
Footnotes
This work was supported by the U.S. Alpha-1 Foundation (EPR). Health Research Board of Ireland and Medical Research Charities Group (EPR). Borders and Regions Airways Training Hub project (BREATH; INT-VA/045) funded by the European Union (EU), under the INTERREG VA Program, managed by the Special EU Programs Body (SLM). α-1 Laurells Training Award, Grifols (MPM).
- AAT
- α1 antitrypsin
- Abz
- 2-aminobenzoyl
- BAL
- bronchoalveolar lavage
- COPD
- chronic obstructive pulmonary disease
- ECM
- extracellular matrix
- MMP
- matrix metalloprotease
- NE
- neutrophil elastase
- NET
- neutrophil extracellular trap
- PAD
- peptidyl arginine deiminase
- pNA
- paranitroanilide
- RA
- rheumatoid arthritis
Disclosures
The authors have no financial conflicts of interest.
References
- 1. WHO . 2012. Global Health Estimates 2020: deaths by cause, age, sex, by country and region, 2000–2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2. Tuder, R. M., Petrache I.. 2012. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Invest. 122: 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greene, C. M., Marciniak S. J., Teckman J., Ferrarotti I., Brantly M. L., Lomas D. A., Stoller J. K., McElvaney N. G.. 2016. α1-Antitrypsin deficiency. Nat. Rev. Dis. Primers 2: 16051. [DOI] [PubMed] [Google Scholar]
- 4. Stockley, R. A. 2002. Neutrophils and the pathogenesis of COPD. Chest 121: 151S–155S. [DOI] [PubMed] [Google Scholar]
- 5. Turato, G., Zuin R., Miniati M., Baraldo S., Rea F., Beghé B., Monti S., Formichi B., Boschetto P., Harari S., et al. 2002. Airway inflammation in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 166: 105–110. [DOI] [PubMed] [Google Scholar]
- 6. Kielty, C. M., Sherratt M. J., Shuttleworth C. A.. 2002. Elastic fibres. J. Cell Sci. 115: 2817–2828. [DOI] [PubMed] [Google Scholar]
- 7. Hsu, C.-Y., Henry J., Raymond A.-A., Méchin M.-C., Pendaries V., Nassar D., Hansmann B., Balica S., Burlet-Schiltz O., Schmitt A.-M., et al. 2011. Deimination of human filaggrin-2 promotes its proteolysis by calpain 1. J. Biol. Chem. 286: 23222–23233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pritzker, L. B., Joshi S., Gowan J. J., Harauz G., Moscarello M. A.. 2000. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry 39: 5374–5381. [DOI] [PubMed] [Google Scholar]
- 9. Carrillo-Vico, A., Leech M. D., Anderton S. M.. 2010. Contribution of myelin autoantigen citrullination to T cell autoaggression in the central nervous system. J. Immunol. 184: 2839–2846. [DOI] [PubMed] [Google Scholar]
- 10. Ireland, J., Herzog J., Unanue E. R.. 2006. Unique T cells that recognize citrullinated peptides are a feature of protein immunization. J. Immunol. 177: 1421–1425. [DOI] [PubMed] [Google Scholar]
- 11. Spengler, J., Lugonja B., Ytterberg A. J., Zubarev R. A., Creese A. J., Pearson M. J., Grant M. M., Milward M., Lundberg K., Buckley C. D., et al. 2015. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 67: 3135–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yadav, R., Yoo D.-G., Kahlenberg J. M., Bridges S. L., Oni O., Huang H., Stecenko A., Rada B.. 2019. Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis. J. Cyst. Fibros. 18: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood, A. M., de Pablo P., Buckley C. D., Ahmad A., Stockley R. A.. 2011. Smoke exposure as a determinant of autoantibody titre in alpha(1)-antitrypsin deficiency and COPD. Eur. Respir. J. 37: 32–38. [DOI] [PubMed] [Google Scholar]
- 14. Arandjelovic, S., McKenney K. R., Leming S. S., Mowen K. A.. 2012. ATP induces protein arginine deiminase 2-dependent citrullination in mast cells through the P2X7 purinergic receptor. J. Immunol. 189: 4112–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou, Y., Chen B., Mittereder N., Chaerkady R., Strain M., Rahman S., Ma W., Low C. P., Chan D., Neal F., et al. 2017. Spontaneous secretion of peptidyl-arginine deiminase 2 and cell surface exposure of peptidyl-arginine deiminase 4 by neutrophils. J. Immunol. 198: 207.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishnamurthy, A., Ytterberg A. J., Sun M., Sakuraba K., Steen J., Joshua V., Tarasova N. K., Malmström V., Wähämaa H., Réthi B., et al. 2019. Citrullination controls dendritic cell transdifferentiation into osteoclasts. J. Immunol. 202: 3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thålin, C., Aguilera K., Hall N. W., Marunde M. R., Burg J. M., Rosell A., Daleskog M., Månsson M., Hisada Y., Meiners M. J., et al. 2020. Quantification of citrullinated histones: development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J. Thromb. Haemost. 18: 2732–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mishra, N., Schwerdtner L., Sams K., Mondal S., Ahmad F., Schmidt R. E., Coonrod S. A., Thompson P. R., Lerch M. M., Bossaller L., et al. 2019. Protein arginine deiminase 2 and 4 regulate NLRP3 inflammasome-dependent IL-1β maturation and ASC speck formation in macrophages. J. Immunol. 203: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Makrygiannakis, D., Hermansson M., Ulfgren A.-K., Nicholas A. P., Zendman A. J. W., Eklund A., Grunewald J., Skold C. M., Klareskog L., Catrina A. I., et al. 2008. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 67: 1488–1492. [DOI] [PubMed] [Google Scholar]
- 20. Shapiro, S. D., Goldstein N. M., Houghton A. M., Kobayashi D. K., Kelley D., Belaaouaj A.. 2003. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am. J. Pathol. 163: 2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demedts, I. K., Morel-Montero A., Lebecque S., Pacheco Y., Cataldo D., Joos G. F., Pauwels R. A., Brusselle G. G.. 2006. Elevated MMP-12 protein levels in induced sputum from patients with COPD. Thorax 61: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayes, E., Murphy M. P., Pohl K., Browne N., McQuillan K., Saw L. E., Foley C., Gargoum F., McElvaney O. J., Hawkins P., et al. 2020. Altered degranulation and pH of neutrophil phagosomes impacts antimicrobial efficiency in cystic fibrosis. Front. Immunol. 11: 600033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergin, D. A., Reeves E. P., Meleady P., Henry M., McElvaney O. J., Carroll T. P., Condron C., Chotirmall S. H., Clynes M., O’Neill S. J., et al. 2010. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J. Clin. Invest. 120: 4236–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Senshu, T., Akiyama K., Kan S., Asaga H., Ishigami A., Manabe M.. 1995. Detection of deiminated proteins in rat skin: probing with a monospecific antibody after modification of citrulline residues. J. Invest. Dermatol. 105: 163–169. [DOI] [PubMed] [Google Scholar]
- 25. Damgaard, D., Bjørn M. E., Steffensen M. A., Pruijn G. J. M., Nielsen C. H.. 2016. Reduced glutathione as a physiological co-activator in the activation of peptidylarginine deiminase. Arthritis Res. Ther. 18: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knight, J. S., Subramanian V., O’Dell A. A., Yalavarthi S., Zhao W., Smith C. K., Hodgin J. B., Thompson P. R., Kaplan M. J.. 2015. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL lpr mice. Ann. Rheum. Dis. 74: 2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lominadze, G., Powell D. W., Luerman G. C., Link A. J., Ward R. A., McLeish K. R.. 2005. Proteomic analysis of human neutrophil granules. Mol. Cell. Proteomics 4: 1503–1521. [DOI] [PubMed] [Google Scholar]
- 28. Murphy, M. P., McEnery T., McQuillan K., McElvaney O. F., McElvaney O. J., Landers S., Coleman O., Bussayajirapong A., Hawkins P., Henry M., et al. 2020. Alpha-1 antitrypsin therapy modulates the neutrophil membrane proteome and secretome. Eur. Respir. J. 55: 1901678. [DOI] [PubMed] [Google Scholar]
- 29. Bergin, D. A., Reeves E. P., Hurley K., Wolfe R., Jameel R., Fitzgerald S., McElvaney N. G.. 2014. The circulating proteinase inhibitor α-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci. Transl. Med. 6: 217ra1. [DOI] [PubMed] [Google Scholar]
- 30. Paone, G., Conti V., Vestri A., Leone A., Puglisi G., Benassi F., Brunetti G., Schmid G., Cammarella I., Terzano C., et al. 2011. Analysis of sputum markers in the evaluation of lung inflammation and functional impairment in symptomatic smokers and COPD patients. Dis. Markers 31: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chaudhuri, R., McSharry C., Brady J., Donnelly I., Grierson C., McGuinness S., Jolly L., Weir C. J., Messow C. M., Spears M., et al. 2012. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J. Allergy Clin. Immunol. 129: 655–663.e8. [DOI] [PubMed] [Google Scholar]
- 32. Leshner, M., Wang S., Lewis C., Zheng H., Chen X. A., Santy L., Wang Y.. 2012. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biron, B. M., Chung C.-S., Chen Y., Wilson Z., Fallon E. A., Reichner J. S., Ayala A.. 2018. PAD4 deficiency leads to decreased organ dysfunction and improved survival in a dual insult model of hemorrhagic shock and sepsis. J. Immunol. 200: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keatings, V. M., Collins P. D., Scott D. M., Barnes P. J.. 1996. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 153: 530–534. [DOI] [PubMed] [Google Scholar]
- 35. Tanabe, N., Vasilescu D. M., Hague C. J., Ikezoe K., Murphy D. T., Kirby M., Stevenson C. S., Verleden S. E., Vanaudenaerde B. M., Gayan-Ramirez G., et al. 2020. Pathological comparisons of paraseptal and centrilobular emphysema in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 202: 803–811. [DOI] [PubMed] [Google Scholar]
- 36. Peleman, R. A., Rytila P. H., Kips J. C., Joos G. F., Pauwels R. A.. 1999. The cellular composition of induced sputum in chronic obstructive pulmonary disease. Eur. Respir. J. 13: 839–843. [DOI] [PubMed] [Google Scholar]
- 37. Burnett, D., Hill S., Chamba A., Stockley R.. 1987. Neutrophils from subjects with chronic obstructive lung disease show enhanced chemotaxis and extracellular proteolysis. Lancet 2: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 38. Noguera, A., Batle S., Miralles C., Iglesias J., Busquets X., MacNee W., Agustí A. G.. 2001. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 56: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Damgaard, D., Friberg Bruun Nielsen M., Quisgaard Gaunsbaek M., Palarasah Y., Svane-Knudsen V., Nielsen C. H.. 2015. Smoking is associated with increased levels of extracellular peptidylarginine deiminase 2 (PAD2) in the lungs. Clin. Exp. Rheumatol. 33: 405–408. [PubMed] [Google Scholar]
- 40. Lugli, E. B., Correia R. E. S. M., Fischer R., Lundberg K., Bracke K. R., Montgomery A. B., Kessler B. M., Brusselle G. G., Venables P. J.. 2015. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res. Ther. 17: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Effros, R. M., Biller J., Foss B., Hoagland K., Dunning M. B., Castillo D., Bosbous M., Sun F., Shaker R.. 2003. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am. J. Respir. Crit. Care Med. 168: 1500–1505. [DOI] [PubMed] [Google Scholar]
- 42. Damgaard, D., Bjørn M. E., Jensen P. O., Nielsen C. H.. 2017. Reactive oxygen species inhibit catalytic activity of peptidylarginine deiminase. J. Enzyme Inhib. Med. Chem. 32: 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Drost, E. M., Skwarski K. M., Sauleda J., Soler N., Roca J., Agusti A., MacNee W.. 2005. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 60: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sipilä, K. H., Ranga V., Rappu P., Mali M., Pirilä L., Heino I., Jokinen J., Käpylä J., Johnson M. S., Heino J., et al. 2017. Joint inflammation related citrullination of functional arginines in extracellular proteins. Sci. Rep. 7: 8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willis, V. C., Gizinski A. M., Banda N. K., Causey C. P., Knuckley B., Cordova K. N., Luo Y., Levitt B., Glogowska M., Chandra P., et al. 2011. N-α-Benzoyl-N5-(2-chloro-1-iminoethyl)-l-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J. Immunol. 186: 4396–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gößwein, S., Lindemann A., Mahajan A., Maueröder C., Martini E., Patankar J., Schett G., Becker C., Wirtz S., Naumann-Bartsch N., et al. 2019. Citrullination licenses calpain to decondense nuclei in neutrophil extracellular trap formation. Front. Immunol. 10: 2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thiam, H. R., Wong S. L., Qiu R., Kittisopikul M., Vahabikashi A., Goldman A. E., Goldman R. D., Wagner D. D., Waterman C. M.. 2020. NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc. Natl. Acad. Sci. U. S. A. 117: 7326–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sandhaus, R. A., Turino G.. 2013. Neutrophil elastase-mediated lung disease. COPD 10(Suppl. 1): 60–63. [DOI] [PubMed] [Google Scholar]
- 49. Haq, I., Lowrey G. E., Kalsheker N., Johnson S. R.. 2011. Matrix metalloproteinase-12 (MMP-12) SNP affects MMP activity, lung macrophage infiltration and protects against emphysema in COPD. Thorax 66: 970–976. [DOI] [PubMed] [Google Scholar]
- 50. Hautamaki, R. D., Kobayashi D. K., Senior R. M., Shapiro S. D.. 1997. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 277: 2002–2004. [DOI] [PubMed] [Google Scholar]
- 51. Chapman, K. R., Burdon J. G. W., Piitulainen E., Sandhaus R. A., Seersholm N., Stocks J. M., Stoel B. C., Huang L., Yao Z., Edelman J. M., et al. RAPID Trial Study Group . 2015. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 386: 360–368. [DOI] [PubMed] [Google Scholar]
- 52. Kagan, H. M., Crombie G. D., Jordan R. E., Lewis W., Franzblau C.. 1972. Proteolysis of elastin-ligand complexes. Stimulation of elastase digestion of insoluble elastin by sodium dodecyl sulfate. Biochemistry 11: 3412–3418. [DOI] [PubMed] [Google Scholar]
- 53. Korkmaz, B., Moreau T., Gauthier F.. 2008. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie 90: 227–242. [DOI] [PubMed] [Google Scholar]
- 54. Shapiro, S. D., Endicott S. K., Province M. A., Pierce J. A., Campbell E. J.. 1991. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of d-aspartate sand nuclear weapons-related radiocarbon. J. Clin. Invest. 87: 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Polverino, F., Seys L. J. M., Bracke K. R., Owen C. A.. 2016. B cells in chronic obstructive pulmonary disease: moving to center stage. Am. J. Physiol. Lung Cell. Mol. Physiol. 311: L687–L695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kirkham, P. A., Caramori G., Casolari P., Papi A. A., Edwards M., Shamji B., Triantaphyllopoulos K., Hussain F., Pinart M., Khan Y., et al. 2011. Oxidative stress–induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 184: 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stachowicz, A., Pandey R., Sundararaman N., Venkatraman V., Van Eyk J. E., Fert-Bober J.. 2022. Protein arginine deiminase 2 (PAD2) modulates the polarization of THP-1 macrophages to the anti-inflammatory M2 phenotype. J. Inflamm. (Lond.) 19: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khandpur, R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J. S., Friday S., Li S., Patel R. M., Subramanian V., et al. 2013. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5: 178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Koziel, J., Bryzek D., Sroka A., Maresz K., Glowczyk I., Bielecka E., Kantyka T., Pyrć K., Svoboda P., Pohl J., et al. 2014. Citrullination alters immunomodulatory function of LL-37 essential for prevention of endotoxin-induced sepsis. J. Immunol. 192: 5363–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wong, A., Bryzek D., Dobosz E., Scavenius C., Svoboda P., Rapala-Kozik M., Lesner A., Frydrych I., Enghild J., Mydel P., et al. 2018. A novel biological role for peptidyl-arginine deiminases: citrullination of cathelicidin LL-37 controls the immunostimulatory potential of cell-free DNA. J. Immunol. 200: 2327–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nici, L., Mammen M. J., Charbek E., Alexander P. E., Au D. H., Boyd C. M., Criner G. J., Donaldson G. C., Dreher M., Fan V. S., et al. 2020. Pharmacologic management of chronic obstructive pulmonary disease: an official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 201: e56–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Teng, Y., Chen Y., Tang X., Wang S., Yin K.. 2023. PAD2: a potential target for tumor therapy. Biochim. Biophys. Acta Rev. Cancer 1878: 188931. [DOI] [PubMed] [Google Scholar]
- 63. Zhu, D., Lu Y., Wang Y., Wang Y.. 2022. PAD4 and its inhibitors in cancer progression and prognosis. Pharmaceutics 14: 2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei, L., Wasilewski E., Chakka S. K., Bello A. M., Moscarello M. A., Kotra L. P.. 2013. Novel inhibitors of protein arginine deiminase with potential activity in multiple sclerosis animal model. J. Med. Chem. 56: 1715–1722. [DOI] [PubMed] [Google Scholar]
- 65. Chumanevich, A. A., Causey C. P., Knuckley B. A., Jones J. E., Poudyal D., Chumanevich A. P., Davis T., Matesic L. E., Thompson P. R., Hofseth L. J., et al. 2011. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am. J. Physiol. Gastrointest. Liver Physiol. 300: G929–G938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Darrah, E., Giles J. T., Ols M. L., Bull H. G., Andrade F., Rosen A.. 2013. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci. Transl. Med. 5: 186ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Romero, V., Fert-Bober J., Nigrovic P. A., Darrah E., Haque U. J., Lee D. M., van Eyk J., Rosen A., Andrade F.. 2013. Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med. 5: 209ra150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martín Monreal, M. T., Rebak A. S., Massarenti L., Mondal S., Šenolt L., Ødum N., Nielsen M. L., Thompson P. R., Nielsen C. H., Damgaard D., et al. 2021. Applicability of small-molecule inhibitors in the study of peptidyl arginine deiminase 2 (PAD2) and PAD4. Front. Immunol. 12: 716250. [DOI] [PMC free article] [PubMed] [Google Scholar]