Abstract
Aortic aneurysm (AA) refers to the persistent dilatation of the aorta, exceeding three centimeters. Investigating the pathophysiology of this condition is important for its prevention and management, given its responsibility for more than 25000 deaths in the United States. AAs are classified based on their location or morphology. various pathophysiologic pathways including inflammation, the immune system and atherosclerosis have been implicated in its development. Inflammatory markers such as transforming growth factor β, interleukin-1β, tumor necrosis factor-α, matrix metalloproteinase-2 and many more may contribute to this phenomenon. Several genetic disorders such as Marfan syndrome, Ehler-Danlos syndrome and Loeys-Dietz syndrome have also been associated with this disease. Recent years has seen the investigation of novel management of AA, exploring the implication of different immune suppressors, the role of radiation in shrinkage and prevention, as well as minimally invasive and newly hypothesized surgical methods. In this narrative review, we aim to present the new contributing factors involved in pathophysiology of AA. We also highlighted the novel management methods that have demonstrated promising benefits in clinical outcomes of the AA.
Keywords: Aortic aneurysm, Abdominal aneurysm, Thoracic aneurysm, Immunotherapy, Surgical management, Pathophysiology, Inflammation and molecular pathways
Core Tip: This manuscript explores diverse pathophysiologic pathways (inflammation, atherosclerosis and immune system), varied treatment methods (pharmacological, radiation and surgical), and associated factors like inflammatory markers [transforming growth factor-β, interleukin (IL)-1β, tumor necrosis factor-, matrix metalloproteinase-2, IL-6, IL-8]. Genetic disorders linked to aortic aneurysms (AA) include Marfan syndrome, Ehler-Danlos syndrome, Loeys-Dietz syndrome, Cantu syndrome, and JAK-2 mutation. Approaches such as Low laser irradiation, photobiomodulation, UV-B irradiation may impact AA prevention and shrinkage. Medications like Canakinumab, Paricalcitol, peroxisome proliferator-activated receptor-γ agonist and mesenchymal stem cell transplantation are currently under investigation. Additionally, Different minimally invasive, endovascular surgical methods are highlighted.
INTRODUCTION
A persistent dilatation of the aorta with a diameter of three centimeters or more is termed as an aortic aneurysm (AA)[1]. In a given year, AA is responsible for more than 25000 deaths in the United States[2]. According to reports, the prevalence of AAA ranges from 2% to 12% and affects 8% of males over the age of 65[3]. Aneurysms often remain asymptomatic until a rupture, which is frequently fatal with a mortality rate between 85% and 90%. For those seeking medical treatment post-rupture, survival rates vary between 50% and 70%. Hence, the principal aim in the management of aneurysms is the timely identification and implementation of interventions to mitigate the risk of rupture[4]. In contrast to the observed occurrences in thoracic aneurysms, abdominal variations of these aneurysms are substantially more common. Aortic diseases continue to have a significant global impact despite notable improvements in both diagnostic techniques and treatments[5]. The United States Preventive Services Task Force’s most recent guidelines state that male smokers between the ages of 65 and 75 ought to undergo a single ultrasonography examination[6]. Aortic aneurysms can be categorized according to their location distinguishing between thoracic aortic aneurysm (TAA), abdominal aortic aneurysms (AAA) or a combination of both. Morphologically, aneurysms may present as saccular, fusiform, or pseudoaneurysm. Etiological classifications encompass those associated with atherosclerosis, inflammation, genetic disorders, trauma, infection, and autoimmune conditions[7]. This review aims to explore various aspects of the literature on aortic aneurysms. It includes a detailed examination of the underlying pathology, the molecular pathways, trials related to immunotherapeutic interventions, the role of radiation therapy, and surgical methods within the field of aortic aneurysm. This review is driven by two main goals: firstly, to comprehensively understand and analyze the common causes and pathophysiology of aortic aneurysms, and secondly, to offer insights into the latest treatment modalities. This endeavor aims to inform and guide future research practices in the field.
DIFFERENCE BETWEEN ABDOMINAL VS THORACIC AORTA
AA encompasses two main types, TAA and AAA, with distinct risk factors and pathophysiological mechanisms. Notably, the origin of vascular smooth muscle cells (VSMCs) in TAA is from the neural crest[8]. whereas in AAA, they originate from the endothelium and mesoderm, emphasizing the fundamental difference in their pathogenesis[9]. The diameters of the aorta, in both the thoracic and abdominal regions, were significantly impacted by age, gender, body surface area, and modifiable risk factors such as diastolic blood pressure and cigarette smoking. These findings highlight the multifactorial nature of aortic aneurysm development, demonstrating the combined influence of both intrinsic and modifiable factors[10]. TAA typically exhibit a more robust genetic basis, often linked to autosomal gene mutations in conditions like Marfan and Loeys-Dietz syndrome. In contrast, AAA is primarily associated with atherosclerosis, indicating a divergence in the underlying pathophysiological mechanisms between the two types of aneurysms[11]. AAA tends to expand more rapidly (0.3-0.45 cm annually) compared to TAA, which expands slower (up to 0.3 cm annually in non-bicuspid aortic valve patients). The pathogenesis of AAA is associated with elevated levels of matrix metallopeptidases, contributing to degradation of the extracellular matrix. Meanwhile, an overactive transforming growth factor-beta is a major factor in the development of TAA[12]. While TAA and AAA exhibit distinct genetic backgrounds and etiologies, they share several common pathological characteristics. Both types display a pathologically dilated aortic phenotype, often characterized by the loss of smooth muscle cells, an inflammatory response within the arterial wall, and alterations in the extracellular matrix composition[2]. This study identified CX3CR1 and HBB as shared biomarkers for TAA and AAA while highlighting the significant infiltration of innate and adaptive immune cells in aortic aneurysm development and progression. The close correlation of CX3CR1 and HBB with immune cell infiltration suggests their potential as targets for immunotherapeutic interventions[13]. AAAs exhibited downregulation of the blood coagulation pathway and upregulation of the integrin signaling pathway. In contrast, TAAs showed the opposite trends, suggesting these differences contribute to the distinct pathogenesis of AAAs and TAAs[14]. Findings revealed that 22.5% of TAA patients also had AAAs, with notable associations between AAAs and age ≥ 65, smoking history, hypertension, and specific TAA locations, suggesting the importance of AAA screening in TAA patients, especially those over 55 years old with systemic hypertension, a smoking history, or TAAs in the descending thoracic aorta[15]. This study induced TAA and AAA in mice by combining hypertension and elastic lamina degeneration. Hypertension was a crucial factor in aneurysm formation. At the same time, the direct effects of angiotensin II on the vascular wall were not the primary cause, indicating distinct pathophysiological mechanisms for AAA and TAA[16].
PATHOPHYSIOLOGY; ROLE OF ATHEROSCLEROSIS AND INFLAMMATION
The relationship between atherosclerosis and AA)has been debated, given their shared risk factors and the belief that atherosclerotic lesions may contribute to some aneurysms. Recent studies, however, suggest that while they share some similarities, there are distinct local responses to atherosclerosis in humans, with plaque deposition potentially leading to aneurysm formation in one scenario and lumen narrowing in another, indicating that the vascular response to atherosclerotic lesions can vary widely[17]. Despite these differences, AAA and atherosclerosis share many similar biomarkers, such as fibrinogen, C-reactive protein, and low high-density lipoprotein (HDL) levels[18]. Additionally, a locus on chromosome 9p21 is associated with both conditions, suggesting shared genetic risk factors[19]. These findings are further supported by the observation that some mice (e.g., Apolipoprotein E deficient) prone to atherosclerosis are also more susceptible to AAA induction[20]. AAA and atherosclerosis are distinct diseases despite their shared risk factors and pathophysiological aspects. However interventions that protect against AAA also frequently reduce atherosclerosis, suggesting that these two conditions are interconnected[21]. Inflammatory processes are evident in both conditions, with inflammatory cells in advanced AAAs and inflammatory pathways implicated in atherosclerosis progression. Matrix degradation occurs in the arterial wall of both AAA and atherosclerosis, contributing to aneurysm expansion and plaque formation. Thrombosis is a critical factor, leading to complications in AAAs and plaque rupture in atherosclerosis. Hemodynamic forces like shear stress drive arterial remodeling in both conditions. Signaling molecules influence inflammation, matrix remodeling, and vascular cell behavior in both AAA and atherosclerosis. Both conditions are associated with coronary heart disease, suggesting shared risk factors or mechanisms. While the precise relationship between AAA and atherosclerosis is intricate and influenced by common factors, their interplay underscores the need for comprehensive understanding to develop effective therapies[22]. The involvement of dysregulations in key angiogenesis and inflammation-related factors is potentially identified in AAA formation. Expression levels of ANGPT1, CXCL8, PDGFA, TGFB1, VEGFB, and VEGFC and plasma levels of transforming growth factor (TGF)-alpha, TGF-β1, VEGF-A, and VEGF-C were found to be significantly altered in the AAA group compared to the control subjects without AAA. These findings have implications for identifying diagnostic biomarkers and therapeutic targets. Elevated production of pro-inflammatory cytokines hastens atherosclerosis. Similarly, in AAA, inflammatory cytokines are expressed in affected tissues of symptomatic patients and mouse models. However, direct proof of cytokine involvement in AAA through knockout animal studies is lacking and needs further investigation. Comparisons of atherosclerosis and AAA advancement in available animal models and human studies propose that certain cytokines, like interferon (IFN)-γ, have conflicting roles in these diseases, suggesting differing origins and mechanisms between atherosclerosis and AAA. Conversely, other cytokines yield analogous effects in both diseases, as seen with interleukin (IL)-1β (encouraging disease) and TGF-β (mitigating disease). This underscores the intricate interplay of cytokines in these vascular conditions[23]. Most macrophages found in AAAs come from circulating monocytes, but some are aortic tissue-resident macrophages. Throughout AAA development and progression, macrophages have destructive and reparative roles in extracellular matrix remodeling and promotion and resolution of inflammation, depending on the microenvironmental conditions and the chemokines and cytokines present[24]. The presence of TNF-alpha and IL-1β in tissue extracts of AAA is indicative of that infiltrative inflammatory process[25]. IL-1β plays a central role in the inflammatory process of aneurysm formation via cascading of signaling pathways and various mechanisms of action[26]. Endothelin-1 (ET-1) contributes to atherosclerosis and AAA development by reducing protective HDL levels, increasing oxidative stress, and prompting inflammation through immune cell infiltration. ET-1 enhances matrix metalloproteinase-2 (MMP-2) activity, leading to structural protein degradation in perivascular fat, vascular walls, and atherosclerotic lesions. This weakening of tissues fosters aneurysm formation and plaque instability. Overall, ET-1’s involvement encompasses diminished HDL, heightened oxidative stress, inflammation, and MMP-2 activation, significantly impacting atherosclerosis and AAA progression[27]. The NLRP3 inflammasome, a cytosolic multiprotein complex, serves as a pivotal regulator in the activation of caspase-1, orchestrating the controlled release of pro-inflammatory cytokines IL-1β and IL-18. Additionally, it plays a role in instigating apoptosis, thereby contributing to the inflammatory processes observed in both atherosclerotic disease and AA[28]. Metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection[29].
The general inflammatory conditions contributing to AAA formation also involve activating the following pro-inflammatory transcription factors, NF-κB, AP-1 and markedly increased expression of IL-6 and IL-8 and the involvement of neutrophils[30]. Inflammatory cytokines promote the degeneration of the vascular wall by inducing increased MMP-9 expression in macrophages, which degrade the components of the extracellular matrix (ECM) of the arterial wall, contributing to the expansion process[31]. This process also involves an immune response, notably CD4+ T cells, which aggregate within the AAA lesion and secrete Th1 cytokines like IL-4, which continue to stimulate MMP secretion by macrophages[32]. The release of neutrophil extracellular traps by activated neutrophils in the presence of IL-1β is also believed to play a central role in AAA formation, as their suppression in mice models stopped aneurysm formation[33]. AAA formation results from inflammatory injury that leads to an imbalance in proteolysis. Experimental models that try to stop this vicious cycle have shown promise, but preclinical studies have also shown worsened disease progression, This raises doubts on the accuracy of mice models to portray human disease[34]. The ECM is a dynamic structural component of the aortic wall that plays a crucial role in AAA formation, notably through modification by MMPs. Various MMPs have different ECM collagen substrates. A set of processes involving individual risk factors, molecular mechanisms, and triggers leads to degradation of the ECM in the tunica intima and media, all of which contribute to inflammatory infiltration[35]. Loss of vascular smooth muscle cells (vSMCs), predominant in the middle aortic wall layer, causes weakening and contributes to AAA formation and progression. This condition occurs due to inflammatory cell infiltration that induces apoptosis, mechanical wall stress, and detachment of the ECM[36].
IMMUNE SYSTEM IMPLICATIONS
The development and progression of AAAs heavily rely on the immune system’s responses, involving both cell-mediated and antibody-based reactions[37]. Numerous studies have demonstrated a robust association between immune factors and AAA. IgG antibodies extracted from the AAA wall exhibited significantly elevated levels compared to normal aortas, with IgG1, IgG2, IgG3 and IgG4 showing marked increases of 193, 160, 389, and 627 times, respectively. Moreover, IgG antibodies targeting HDL were more prevalent in both tissue and plasma of AAA patients, showing a correlation between aortic size and AAA presence. This suggests a potential contribution of these autoantibodies to AAA formation[38]. Research indicated a substantial increase in IL-17 and IL-23 cytokine production in AAA patients compared to controls. These cytokines, associated with inflammatory autoimmune disease, were proposed as significant players in AAA pathophysiology[39]. Studies linking bioactive peptides and AAA revealed higher concentrations of IL-1B, IL-6 and IL-17 in individuals with AAA, with an observed association between bioactive peptides and aortic diameter. IL-1α levels are typically detectable in healthy individuals, primarily concentrated in the cell membrane and nucleus. However, in AAA patients, IL-1α was detected in serum, correlating with affected vessel diameter in vitro. This indicated a potential role of IL-1a in AAA enlargement via neutrophil mobilization. Conversely, other research found no statistically significant association between serum IL-1a levels and AAA size or growth rate[40-42]. A comprehensive 2018 investigation delved into genome-wide expression patterns to understand perivascular adipose tissue (PVAT) involvement and immunological aspects in AAA. Comparison between dilated and non-dilated areas of the aortic areas revealed an overrepresentation of innate and peripheral immune tolerance-breaking factors, leading to clonal T cell proliferation in dilated PVAT compared to non-dilated PVAT. These findings suggest an immunological aspect in AAA, potentially involving an autoimmune component, especially triggers like a high-fat diet or smoking[43].
MOLECULAR PATHWAYS
The onset of AAA stems from vascular inflammation, marked by infiltration of inflammatory cells from adventitia into the intima, gradually upregulating MMPs. MMPs, a group of extracellular enzymes, play a pivotal role in various physiological processes, including tissue remodeling and resorption. Elevated MMP9 Levels, responsible for serving elastin, collagen Type 1 and IV, and fibrinogen, have been observed in both plasma and aortic aneurysmal tissue. Persistent elevation of MMP9 Levels post-endovascular repair might indicate an increased risk of developing leaks[44,45]. Studies in humans and mice highlight high levels of cytokines released by Th2 cells in aneurysm tissues, contributing significantly to AAA formation. The absence of IFN-γ intensifies AAA development in mice, while IL-4 deficiency prevents AAA development. In contrast, in human fibroblasts, IL-4 influences ECM protein production differently. Two major groups of macrophages, M1 (proinflammatory) and M2 (repair of ECM) play significant role in AAA formation. GM-CSF, a crucial cytokine moderating macrophage infiltration, regulates MMP9 secretion, reducing it when the GM-CSF pathway is blocked[35]. Dysregulated TGF-Beta signaling and mast cells contribute to AAA formation and inflammatory processes within the adventitia[46,47]. Distinct immunological characteristics exist between small AAAs (< 55 mm) and large (> 55 mm) AAAs. Notably, the upregulation of the key T-cell regulatory gene, cytotoxic T-lymphocyte-associated protein 4 (CTLA4 or CD152) is exclusive to small AAAs. Biomarker identification remains crucial for understanding pathogenesis and developing targeted therapeutics. G0S2 has been identified as a highly accurate biomarker for early AAA diagnosis[48,49]. Macrophage ADAR1 has been implicated in aneurysm formation through Drosha protein degradation, eliciting macrophage-mediated vascular inflammation in AAA by inhibiting microRNAs targeting NF-kB signaling[50]. Osteoprotegerin (OPG), found in elevated concentrations in AAA, holds promise as a diagnostic marker and potential treatment target. Inactivation of OPG in mice induced AAA development, suggesting its crucial role[51].
GENETIC DISORDERS
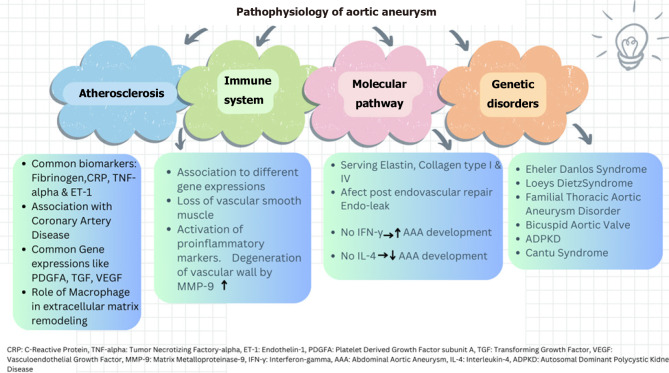
TAA correlates with syndromes linked to connective tissue defects. Mutations in molecular pathways, such as activation of TGF-, notch ligands, and angiotensin II, contribute to AA[52]. Genetic syndromes associated with aortic aneurysm include Marfan syndrome, Ehler-Danlos syndrome, Loeys-Dietz syndrome, familial thoracic aortic aneurysm and dissection, bicuspid aortic valve disease, autosomal dominant polycystic kidney disease and Turner syndrome[53]. In Marfan syndrome, mutation in Fibrillin-1 disrupts TGF-B, leading to tissue integrity impairment. Prophylactic beta blockers are administered to slow aortic aneurysm progression[54]. Loeys-Dietz-syndrome exhibits a triad of hypertelorism, bifid uvula, AA with tortuosity. Genetic causes involve pathways enhancing TGF-β activity, including TGFBR1, TGFBR2, TGFB2, TGFB3, SMAD2, SMAD3[55]. Meester-Loer-syndrome represents an x-linked form of TAA with multiorgan involvement including craniofacial malformation, cardiovascular, neurological and cutaneous system issues. It is caused by BGN loss of function, encoding small leucine-rich proteoglycan biglycan which increases TGF-B pathway activity[56]. Autosomal recessive cutis laxa type 1B, involving aortopathy, vascular tortuosity and aneurysm formation, results from a mutation in EFEMP2, that encodes fibrillin-4[57]. Cantu syndrome, an autosomal dominant overgrowth syndrome, manifests congenital hypertrichosis, facial dysmorphism, cardiomegaly, and AA. This syndrome is caused by the ABCC2 gene, a regulatory subunit for ATP-sensitive potassium channels in the cardiac, vascular smooth muscle and skeletal system[58]. The presence of JAK-2 mutation in bone marrow-transplanted mice has shown an increased incidence of AA by accelerating the degradation of aortic elastic lamina and activation of matrix metalloproteinase[59]. Elevated homocysteine levels have been associated with aortic aneurysm progression, though it remains a weak risk factor given the multifactorial causes of aortic aneurysm[60]. A brief summary of pathophysiologic etiologies involved in AA is illustrated in Figure 1.
Figure 1.
Pathophysiologic etiologies of aortic aneurysm.
TREATMENTS; IMMUNOLOGICAL IMPLICATIONS
Numerous therapies targeting the immune system have been developed in recent years. In a study spanning 12 months, administration of Canakinumab, a monoclonal antibody neutralizing IL-1B, showed similar progression to AAA compared to the placebo group[61]. Another approach involving selective suppression of inflammation in the aneurysm wall used perioperative treatment of paricalcitol, a specific vitamin D receptor agonist, and demonstrated selective disruption of nuclear factor of activated T cells-2-mediated inflammation[62]. Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist is recognized for suppressing atherosclerosis progression. Perioperative administration of the PPAR-γ agonist, pioglitazone, significantly reduced macrophage infiltration and adiponectin expression in retroperitoneal periaortic fat and aortic wall. This suggests a potential avenue for preventing or delaying aortic aneurysm progression[63]. However, in the FAME-2 trial, administration of fenofibrate did not impact AAA growth rate or inflammatory markers such as osteopontin and kallistatin[64]. Tyrosine kinase inhibitor, Imatinib, may inhibit AAA formation and growth by preventing pathological vascular formation[65]. A Mendelian randomization (MR) analysis was aimed to identify therapeutic targets associated with AAA. Four drug targets (BTN3A1, FASN, PLAU, and PSMA4) exhibited significant MR results across two independent datasets. Proteasome 20S subunit alpha 4 (PSMA4) and plasminogen activator, urokinase (PLAU), showed compelling evidence of colocalization with aortic aneurysms, particularly in AAAs. The study suggests that targeting PLAU and inhibiting PSMA4 through drug interventions may potentially lower the risk of developing AAs[66]. Nanotherapy could be an ideal method of delivering medications to target cells. A Rapamycin-loaded nanotherapy, involving macrophage cell membrane implication by creating reactive oxygen species to AAA cells, demonstrated efficacy in preventing aneurysm expansion[67]. Despite numerous promising interventions identified in preclinical studies, this substantial investment has not yielded any clinical applications, and currently, there is no medical therapy available for stabilizing growing AAAs. Nevertheless, ongoing clinical studies like IMAGEN trial to evaluate effect of myo-inositol and ARREST trial to investigate the effect of mesenchymal stem cells, are underway to explore potential treatments[68,69].
TREATMENTS; IMPLICATION OF RADIATION
Certainly, investigating the potential positive effects of radiation on regressing AA is an intriguing avenue. The use of Radiation is commonly employed during the endovascular intervention for aortic repair, impacting both patients and medical staff[70]. However, exploring whether radiation might exhibit positive effects on AA regression warrants careful investigation. In an in vivo study involving simulated aortic dilatation in apolipoprotein-E deficient mice, low level laser irradiation exhibited a noteworthy outcome. It showed a significant reduction in the maximum diameter of suprarenal aorta. Moreover, it effectively prevented further dilation, restraining it to less than 50% of cross sectional diameter of aorta compared to the non-treated control group[71]. A recently hypothesized method involves photobiomodulation of the abdominal aorta. This approach entails delivering treatment through percutaneous insertion of LED-studded patch onto the posterior abdominal wall. It is intended for small aneurysms ( 3-4.5 cm in anteroposterior diameters) that have proven refractory to pharmacological intervention. Both in vivo and in vitro studies have indicated that this method can effectively prevent progression to urgent surgical intervention or impending rupture[72]. In an interventional study on angiotensin II infused hypercholesterolemic mice, it was discovered that irradiation using Ultraviolet B (UVB) had the potential to prevent the progression and lower the mortality rate associated with aortic aneurysm. The effect was attributed to a significant reduction in CD4 T cells and macrophage, along with a systemic expansion of CD4+Foxp3+ regulatory T-cells, effectively restricting the growth of AA[73]. Additionally, experiments employing irradiation in combination with bone marrow transplantation demonstrated a reduction in the inflammatory process linked to atherosclerosis and the development of aortic aneurysm in angiotensin II infused mouse models[74].
TREATMENTS; SURGICAL IMPLICATIONS
The surgical approach to aortic aneurysms has a dynamic landscape of treatment strategies, with exploration of open thoracoabdominal repair, endovascular techniques, and the new innovative integration of 3D printing technology. The surgical process involved in open thoracoabdominal repair, characterized by midline transabdominal or retroperitoneal incisions, aortic, and iliac artery clamping, is considered one of the most invasive procedures. But contrary to belief, an open approach is not inferior and is effective, and durable in terms of the graft integrity with preservation of the renal function, even in patients with increased cardiovascular risk[75-77]. An approach to prevent complications during the surgery using a left heart bypass (LHB) which is a circulatory support system used to perfuse the distal aorta during TAAA operation. The advantages of LHB ensure distal perfusion, decreasing the use of heparin, and mitigating the risk of bleeding and postoperative neurological deficits, yielding favorable outcomes[78]. Some of the main side effects of surgical aortic aneurysmal repair include hemorrhage, acute renal failure, ischemic colitis, distal emboli, graft thrombosis, infection, pseudoaneurysm formation, aorto-cava and aorto-enteric fistula, neurologic deficit, ureteral obstruction, sexual dysfunction, chylous ascited and prigraft seroma[79]. The endovascular aneurysm repair (EVAR) has revolutionized the management of AAA. However, later studies showed lower long term benefit compared to open surgery, yet it remains the most commonly utilized approach. It is the go-to option for patients unfit for an open approach, offering a survival benefit and proving to be cost-effective[80]. The primary reason for EVAR’s reduced survival benefit is attributed to secondary aneurysm ruptures caused by endo-leaks and a high rate of secondary intervention. Advancements aim to mitigate these complications by limiting the leaks and focusing on shrinkage of aneurysms. One approach involves pre-emptive embolization, such as embolization of aneurysm sac side branches (ASSB) and using aneurysm sac coil embolization (ASCE), which has shown promise in decreasing the development of endovascular leaks[81]. A comprehensive meta-analysis by Wu et al[82] showcased enhanced clinical outcomes in EVAR clinical through implementation of prophylactic pre-emptive embolization targeting the inferior mesenteric artery (IMA-ASSB). This approach notably curbed aneurysm sac expansion and minimized the requirement for re-intervention. Additionally, it was noted that non-selective embolization of ASSB (NS-ASSB) proved more effective in decreasing the incidence of leaks[82]. Doumenc et al[83] examined novel surgical approaches for resolving Type IA endoscopic leaks during EVAR, with Custom-made fenestrated endovascular aortic aneurysm repair and open explanation. Many physician modified endografts including fenestrated-branch EVAR for complex AAAs showed acceptable long term outcomes however, demonstrated low survival rates due to underlying comorbidities[84]. Thoracoabdominal branch endoprosthesis (TAMBE) device is also used to offer endovascular treatment for all types of TAAAs and pararenal aneurysms. TAMBE is a four-branch device that can be used for a range of visceral anatomic configurations and showed promising results with more ongoing clinical trials[85]. There is another similar graft called the Valiant Navion stent graft showing promising results with rare complications such as endo-leak and access/deployment failures[86]. Advancement in making 3D models of visceral artery aneurysms with more accurate visualization and analysis of vascular anatomy could assist operators in attempting minimally invasive treatment with good results. The imaging studies using 3D printing models that allow for the assessment of the position, morphology and geometry of the aneurysm sac, particularly of vessel branches, could encourage surgeons to perform endovascular procedures[87]. 3D printing technology has been collaborating utilizing artificial intelligence to update the map throughout the patient journey, and has been attempted in one center so far with promising results[88].
Studies showed EVAR has better long term outcomes on shrinking AAAs rather than stable AAAs. Factors such as older age and larger infra-renal angle were found to be associated with this finding. However, underlying factors which are contributing to this shrinkage, are still under investigation[89]. Additionally, Studies have shown that shrinkage of > 10 mm are less prone to developing subsequent aneurysm growth and have significantly lower risk of requiring surgery for endo-leaks[90]. On the other hand, smaller population studies didn’t show any significant association between aneurysm shrinkage ≥ 5 mm and survival or reintervention rate within one year[91]. Inflammatory markers such as neutrophil to lymphocyte ratio and platelet to lymphocyte ratio emerged as negative predictors of post-EVAR shrinkage of AAAs[92]. Some of the major side effects of endovascular repair are Endo-leaks, stent migration, endograft infection, limb kinking or occlusion, endograft collapse, and systematic complications like ischemia of major branches[93]. There are some promising studies focused on minimally invasive approaches, to be non-inferior compared to gold standard approaches. However, more trials are required to determine the benefit of both the device and technology of less-invasive EVAR[94].
CONCLUSION
The development of aortic aneurysms, regardless of their location in the thorax or abdomen, has been shown to be associated with inflammatory process, atherosclerosis, immune system dysregulation, and genetic disorders. The potential implications of immune-suppressive therapies, such as canakinumab and pioglitazone, as well as radiation therapies such as photbiomodulation, may play a role in both prevention and regression of aortic aneurysms. Several newer surgical methods, including open thoracoabdominal aortic repair and endovascular aortic repair techniques have demonstrated promising results in recent years. However, these techniques may carry certain risks such as endo-leaks and endograft infection or collapse. Nevertheless, the overall benefits compare to previous methods highlights the innovated approach to the prevention and treatment of aortic aneurysm.
Footnotes
Conflict-of-interest statement: No potential conflict of interest was reported by the authors.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 28, 2023
First decision: January 16, 2024
Article in press: March 18, 2024
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng C, China S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
Contributor Information
Maryam Barkhordarian, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States. marybarkhordar@gmail.com.
Hadrian Hoang-Vu Tran, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Aiswarya Menon, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Sai Priyanka Pulipaka, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Izage Kianifar Aguilar, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Axel Fuertes, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Shraboni Dey, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Angel Ann Chacko, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Tanni Sethi, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Ayrton Bangolo, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
Simcha Weissman, Department of Internal Medicine, Palisades Medical Center, North Bergen, NJ 07047, United States.
References
- 1.Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2018;15:2805. doi: 10.3390/ijerph15122805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintana RA, Taylor WR. Cellular Mechanisms of Aortic Aneurysm Formation. Circ Res. 2019;124:607–618. doi: 10.1161/CIRCRESAHA.118.313187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostakos J, Lal BK. Abdominal aortic aneurysms. Prog Cardiovasc Dis. 2021;65:34–43. doi: 10.1016/j.pcad.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371:2101–2108. doi: 10.1056/NEJMcp1401430. [DOI] [PubMed] [Google Scholar]
- 5.Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331–348. doi: 10.1038/s41569-020-00472-6. [DOI] [PubMed] [Google Scholar]
- 6.Chang TW, Gracon AS, Murphy MP, Wilkes DS. Exploring autoimmunity in the pathogenesis of abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2015;309:H719–H727. doi: 10.1152/ajpheart.00273.2015. [DOI] [PubMed] [Google Scholar]
- 7.Cornelia A, Irina-Draga Cr. Etiology and Pathogenesis of Aortic Aneurysm. In: Cornelia A, editor. Aortic Aneurysm - Recent Advances. Rijeka: IntechOpen; 2013. [Google Scholar]
- 8.Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circ Res. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- 9.Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999;19:1589–1594. doi: 10.1161/01.atv.19.7.1589. [DOI] [PubMed] [Google Scholar]
- 10.Rogers IS, Massaro JM, Truong QA, Mahabadi AA, Kriegel MF, Fox CS, Thanassoulis G, Isselbacher EM, Hoffmann U, O'Donnell CJ. Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study) Am J Cardiol. 2013;111:1510–1516. doi: 10.1016/j.amjcard.2013.01.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinard A, Jones GT, Milewicz DM. Genetics of Thoracic and Abdominal Aortic Diseases. Circ Res. 2019;124:588–606. doi: 10.1161/CIRCRESAHA.118.312436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap ZJ, Sharif M, Bashir M. Is there an immunogenomic difference between thoracic and abdominal aortic aneurysms? J Card Surg. 2021;36:1520–1530. doi: 10.1111/jocs.15440. [DOI] [PubMed] [Google Scholar]
- 13.He B, Zhan Y, Cai C, Yu D, Wei Q, Quan L, Huang D, Liu Y, Li Z, Liu L, Pan X. Common molecular mechanism and immune infiltration patterns of thoracic and abdominal aortic aneurysms. Front Immunol. 2022;13:1030976. doi: 10.3389/fimmu.2022.1030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto K, Satoh K, Maniwa T, Tanaka T, Okunishi H, Oda T. Proteomic comparison between abdominal and thoracic aortic aneurysms. Int J Mol Med. 2014;33:1035–1047. doi: 10.3892/ijmm.2014.1627. [DOI] [PubMed] [Google Scholar]
- 15.DeFreitas MR, Quint LE, Watcharotone K, Nan B, Ranella MJ, Hider JR, Liu PS, Williams DM, Eliason JL, Patel HJ. Evaluation for abdominal aortic aneurysms is justified in patients with thoracic aortic aneurysms. Int J Cardiovasc Imaging. 2016;32:647–653. doi: 10.1007/s10554-015-0807-7. [DOI] [PubMed] [Google Scholar]
- 16.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Cao H, Hu G, Wu Y, Xu Y, Cui H, Lu HS, Zheng L. The mechanism and therapy of aortic aneurysms. Signal Transduct Target Ther. 2023;8:55. doi: 10.1038/s41392-023-01325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118:2382–2392. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helgadottir A, Thorleifsson G, Magnusson KP, Grétarsdottir S, Steinthorsdottir V, Manolescu A, Jones GT, Rinkel GJ, Blankensteijn JD, Ronkainen A, Jääskeläinen JE, Kyo Y, Lenk GM, Sakalihasan N, Kostulas K, Gottsäter A, Flex A, Stefansson H, Hansen T, Andersen G, Weinsheimer S, Borch-Johnsen K, Jorgensen T, Shah SH, Quyyumi AA, Granger CB, Reilly MP, Austin H, Levey AI, Vaccarino V, Palsdottir E, Walters GB, Jonsdottir T, Snorradottir S, Magnusdottir D, Gudmundsson G, Ferrell RE, Sveinbjornsdottir S, Hernesniemi J, Niemelä M, Limet R, Andersen K, Sigurdsson G, Benediktsson R, Verhoeven EL, Teijink JA, Grobbee DE, Rader DJ, Collier DA, Pedersen O, Pola R, Hillert J, Lindblad B, Valdimarsson EM, Magnadottir HB, Wijmenga C, Tromp G, Baas AF, Ruigrok YM, van Rij AM, Kuivaniemi H, Powell JT, Matthiasson SE, Gulcher JR, Thorgeirsson G, Kong A, Thorsteinsdottir U, Stefansson K. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–224. doi: 10.1038/ng.72. [DOI] [PubMed] [Google Scholar]
- 20.Deng GG, Martin-McNulty B, Sukovich DA, Freay A, Halks-Miller M, Thinnes T, Loskutoff DJ, Carmeliet P, Dole WP, Wang YX. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 21.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golledge J, Norman PE. Atherosclerosis and abdominal aortic aneurysm: cause, response, or common risk factors? Arterioscler Thromb Vasc Biol. 2010;30:1075–1077. doi: 10.1161/ATVBAHA.110.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peshkova IO, Schaefer G, Koltsova EK. Atherosclerosis and aortic aneurysm - is inflammation a common denominator? FEBS J. 2016;283:1636–1652. doi: 10.1111/febs.13634. [DOI] [PubMed] [Google Scholar]
- 24.Raffort J, Lareyre F, Clément M, Hassen-Khodja R, Chinetti G, Mallat Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat Rev Cardiol. 2017;14:457–471. doi: 10.1038/nrcardio.2017.52. [DOI] [PubMed] [Google Scholar]
- 25.Newman KM, Jean-Claude J, Li H, Ramey WG, Tilson MD. Cytokines that activate proteolysis are increased in abdominal aortic aneurysms. Circulation. 1994;90:II224–II227. [PubMed] [Google Scholar]
- 26.Wenjing F, Tingting T, Qian Z, Hengquan W, Simin Z, Agyare OK, Zhisheng J, Shunlin Q. The role of IL-1β in aortic aneurysm. Clin Chim Acta. 2020;504:7–14. doi: 10.1016/j.cca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2013;33:2306–2315. doi: 10.1161/ATVBAHA.113.302028. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M. NLRP3 Inflammasome as a Common Denominator of Atherosclerosis and Abdominal Aortic Aneurysm. Circ J. 2021;85:2129–2136. doi: 10.1253/circj.CJ-21-0258. [DOI] [PubMed] [Google Scholar]
- 29.Cui H, Chen Y, Li K, Zhan R, Zhao M, Xu Y, Lin Z, Fu Y, He Q, Tang PC, Lei I, Zhang J, Li C, Sun Y, Zhang X, Horng T, Lu HS, Chen YE, Daugherty A, Wang D, Zheng L. Untargeted metabolomics identifies succinate as a biomarker and therapeutic target in aortic aneurysm and dissection. Eur Heart J. 2021;42:4373–4385. doi: 10.1093/eurheartj/ehab605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdul-Hussien H, Hanemaaijer R, Kleemann R, Verhaaren BF, van Bockel JH, Lindeman JH. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J Vasc Surg. 2010;51:1479–1487. doi: 10.1016/j.jvs.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 31.Shah PK. Inflammation, metalloproteinases, and increased proteolysis: an emerging pathophysiological paradigm in aortic aneurysm. Circulation. 1997;96:2115–2117. doi: 10.1161/01.cir.96.7.2115. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 33.Klopf J, Brostjan C, Eilenberg W, Neumayer C. Neutrophil Extracellular Traps and Their Implications in Cardiovascular and Inflammatory Disease. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22020559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindeman JH. The pathophysiologic basis of abdominal aortic aneurysm progression: a critical appraisal. Expert Rev Cardiovasc Ther. 2015;13:839–851. doi: 10.1586/14779072.2015.1052408. [DOI] [PubMed] [Google Scholar]
- 35.Stepien KL, Bajdak-Rusinek K, Fus-Kujawa A, Kuczmik W, Gawron K. Role of Extracellular Matrix and Inflammation in Abdominal Aortic Aneurysm. Int J Mol Sci. 2022;23 doi: 10.3390/ijms231911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rombouts KB, van Merrienboer TAR, Ket JCF, Bogunovic N, van der Velden J, Yeung KK. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. Eur J Clin Invest. 2022;52:e13697. doi: 10.1111/eci.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirose H, Tilson MD. Abdominal aortic aneurysm as an autoimmune disease. Ann N Y Acad Sci. 2001;947:416–418. doi: 10.1111/j.1749-6632.2001.tb03976.x. [DOI] [PubMed] [Google Scholar]
- 38.Lu S, White JV, Nwaneshiudu I, Nwaneshiudu A, Monos DS, Solomides CC, Oleszak EL, Platsoucas CD. Human abdominal aortic aneurysm (AAA): Evidence for an autoimmune antigen-driven disease. Autoimmun Rev. 2022;21:103164. doi: 10.1016/j.autrev.2022.103164. [DOI] [PubMed] [Google Scholar]
- 39.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, Saadatzadeh MR, Su G, Bhamidipati CM, Mehta GS, Kron IL, Laubach VE, Murphy MP, Ailawadi G, Upchurch GR Jr. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126:S38–S45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad M, Kuravi S, Hodson J, Rainger GE, Nash GB, Vohra RK, Bradbury AW. The Relationship Between Serum Interleukin-1α and Asymptomatic Infrarenal Abdominal Aortic Aneurysm Size, Morphology, and Growth Rates. Eur J Vasc Endovasc Surg. 2018;56:130–135. doi: 10.1016/j.ejvs.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Huanggu H, Yang D, Zheng Y. Blood immunological profile of abdominal aortic aneurysm based on autoimmune injury. Autoimmun Rev. 2023;22:103258. doi: 10.1016/j.autrev.2022.103258. [DOI] [PubMed] [Google Scholar]
- 42.Yates CM, Abdelhamid M, Adam DJ, Nash GB, Bradbury AW, Rainger GE. Endovascular aneurysm repair reverses the increased titer and the inflammatory activity of interleukin-1α in the serum of patients with abdominal aortic aneurysm. J Vasc Surg. 2011;54:497–503. doi: 10.1016/j.jvs.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 43.Piacentini L, Werba JP, Bono E, Saccu C, Tremoli E, Spirito R, Colombo GI. Genome-Wide Expression Profiling Unveils Autoimmune Response Signatures in the Perivascular Adipose Tissue of Abdominal Aortic Aneurysm. Arterioscler Thromb Vasc Biol. 2019;39:237–249. doi: 10.1161/ATVBAHA.118.311803. [DOI] [PubMed] [Google Scholar]
- 44.Joviliano EE, Ribeiro MS, Tenorio EJR. MicroRNAs and Current Concepts on the Pathogenesis of Abdominal Aortic Aneurysm. Braz J Cardiovasc Surg. 2017;32:215–224. doi: 10.21470/1678-9741-2016-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Bai S, Ao Q, Wang X, Tian X, Li X, Tong H, Hou W, Fan J. Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets. J Immunol Res. 2018;2018:7213760. doi: 10.1155/2018/7213760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HW, Stansfield BK. Genetic and Epigenetic Regulation of Aortic Aneurysms. Biomed Res Int. 2017;2017:7268521. doi: 10.1155/2017/7268521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102:1368–1377. doi: 10.1161/CIRCRESAHA.108.173682. [DOI] [PubMed] [Google Scholar]
- 48.Biros E, Gäbel G, Moran CS, Schreurs C, Lindeman JH, Walker PJ, Nataatmadja M, West M, Holdt LM, Hinterseher I, Pilarsky C, Golledge J. Differential gene expression in human abdominal aortic aneurysm and aortic occlusive disease. Oncotarget. 2015;6:12984–12996. doi: 10.18632/oncotarget.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong T, Lv XS, Wu GJ, Guo YX, Liu C, Hou FX, Wang JK, Fu YF, Liu FQ. Single-Cell Sequencing Analysis and Multiple Machine Learning Methods Identified G0S2 and HPSE as Novel Biomarkers for Abdominal Aortic Aneurysm. Front Immunol. 2022;13:907309. doi: 10.3389/fimmu.2022.907309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai D, Sun C, Murashita T, Que X, Chen SY. ADAR1 Non-Editing Function in Macrophage Activation and Abdominal Aortic Aneurysm. Circ Res. 2023;132:e78–e93. doi: 10.1161/CIRCRESAHA.122.321722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migacz M, Janoska-Gawrońska A, Holecki M, Chudek J. The role of osteoprotegerin in the development, progression and management of abdominal aortic aneurysms. Open Med (Wars) 2020;15:457–463. doi: 10.1515/med-2020-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson NK, Gould RA, Gallo MacFarlane E Consortium ML. Pathophysiology of aortic aneurysm: insights from human genetics and mouse models. Pharmacogenomics. 2016;17:2071–2080. doi: 10.2217/pgs-2016-0127. [DOI] [PubMed] [Google Scholar]
- 53.Cury M, Zeidan F, Lobato AC. Aortic disease in the young: genetic aneurysm syndromes, connective tissue disorders, and familial aortic aneurysms and dissections. Int J Vasc Med. 2013;2013:267215. doi: 10.1155/2013/267215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coelho SG, Almeida AG. Marfan syndrome revisited: From genetics to the clinic. Rev Port Cardiol (Engl Ed) 2020;39:215–226. doi: 10.1016/j.repc.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Velchev JD, Van Laer L, Luyckx I, Dietz H, Loeys B. Loeys-Dietz Syndrome. Adv Exp Med Biol. 2021;1348:251–264. doi: 10.1007/978-3-030-80614-9_11. [DOI] [PubMed] [Google Scholar]
- 56.Meester JAN, De Kinderen P, Verstraeten A, Loeys B. Meester-Loeys Syndrome. Adv Exp Med Biol. 2021;1348:265–272. doi: 10.1007/978-3-030-80614-9_12. [DOI] [PubMed] [Google Scholar]
- 57.Hibino M, Sakai Y, Kato W, Tanaka K, Tajima K, Yokoyama T, Iwasa M, Morisaki H, Tsuzuki T, Usui A. Ascending Aortic Aneurysm in a Child With Fibulin-4 Deficiency. Ann Thorac Surg. 2018;105:e59–e61. doi: 10.1016/j.athoracsur.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 58.Hiraki Y, Miyatake S, Hayashidani M, Nishimura Y, Matsuura H, Kamada M, Kawagoe T, Yunoki K, Okamoto N, Yofune H, Nakashima M, Tsurusaki Y, Satisu H, Murakami A, Miyake N, Nishimura G, Matsumoto N. Aortic aneurysm and craniosynostosis in a family with Cantu syndrome. Am J Med Genet A. 2014;164A:231–236. doi: 10.1002/ajmg.a.36228. [DOI] [PubMed] [Google Scholar]
- 59.Yokokawa T, Misaka T, Kimishima Y, Wada K, Minakawa K, Sugimoto K, Ishida T, Morishita S, Komatsu N, Ikeda K, Takeishi Y. Crucial role of hematopoietic JAK2 V617F in the development of aortic aneurysms. Haematologica. 2021;106:1910–1922. doi: 10.3324/haematol.2020.264085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halazun KJ, Bofkin KA, Asthana S, Evans C, Henderson M, Spark JI. Hyperhomocysteinaemia is associated with the rate of abdominal aortic aneurysm expansion. Eur J Vasc Endovasc Surg. 2007;33:391–4; discussion 395. doi: 10.1016/j.ejvs.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Lindeman JH, Matsumura JS. Pharmacologic Management of Aneurysms. Circ Res. 2019;124:631–646. doi: 10.1161/CIRCRESAHA.118.312439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nieuwland AJ, Kokje VB, Koning OH, Hamming JF, Szuhai K, Claas FH, Lindeman JH. Activation of the vitamin D receptor selectively interferes with calcineurin-mediated inflammation: a clinical evaluation in the abdominal aortic aneurysm. Lab Invest. 2016;96:784–790. doi: 10.1038/labinvest.2016.55. [DOI] [PubMed] [Google Scholar]
- 63.Motoki T, Kurobe H, Hirata Y, Nakayama T, Kinoshita H, Rocco KA, Sogabe H, Hori T, Sata M, Kitagawa T. PPAR-γ agonist attenuates inflammation in aortic aneurysm patients. Gen Thorac Cardiovasc Surg. 2015;63:565–571. doi: 10.1007/s11748-015-0576-1. [DOI] [PubMed] [Google Scholar]
- 64.Pinchbeck JL, Moxon JV, Rowbotham SE, Bourke M, Lazzaroni S, Morton SK, Matthews EO, Hendy K, Jones RE, Bourke B, Jaeggi R, Favot D, Quigley F, Jenkins JS, Reid CM, Velu R, Golledge J. Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME -2 Trial. J Am Heart Assoc. 2018;7:e009866. doi: 10.1161/JAHA.118.009866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vorkapic E, Dugic E, Vikingsson S, Roy J, Mäyränpää MI, Eriksson P, Wågsäter D. Imatinib treatment attenuates growth and inflammation of angiotensin II induced abdominal aortic aneurysm. Atherosclerosis. 2016;249:101–109. doi: 10.1016/j.atherosclerosis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Xu X, Wang L, Li K, Sun Y, Xiao L, Dai J, Huang M, Wang Y, Wang DW. Genetic insights into therapeutic targets for aortic aneurysms: A Mendelian randomization study. EBioMedicine. 2022;83:104199. doi: 10.1016/j.ebiom.2022.104199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng J, Zhang R, Li C, Tao H, Dou Y, Wang Y, Hu H, Zhang J. A Targeting Nanotherapy for Abdominal Aortic Aneurysms. J Am Coll Cardiol. 2018;72:2591–2605. doi: 10.1016/j.jacc.2018.08.2188. [DOI] [PubMed] [Google Scholar]
- 68.Rowbotham SE, Pinchbeck JL, Anderson G, Bourke B, Bourke M, Gasser TC, Jaeggi R, Jenkins JS, Moran CS, Morton SK, Reid CM, Velu R, Yip L, Moxon JV, Golledge J. Inositol in the MAnaGemENt of abdominal aortic aneurysm (IMAGEN): study protocol for a randomised controlled trial. Trials. 2017;18:547. doi: 10.1186/s13063-017-2304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang SK, Green LA, Gutwein AR, Drucker NA, Motaganahalli RL, Fajardo A, Babbey CM, Murphy MP. Rationale and Design of the ARREST Trial Investigating Mesenchymal Stem Cells in the Treatment of Small Abdominal Aortic Aneurysm. Ann Vasc Surg. 2018;47:230–237. doi: 10.1016/j.avsg.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 70.Rohlffs F, Spanos K, Debus ES, Heidemann F, Tsilimparis N, Kölbel T. Modern Image Acquisition System Reduces Radiation Exposure to Patients and Staff During Complex Endovascular Aortic Repair. Eur J Vasc Endovasc Surg. 2020;59:295–300. doi: 10.1016/j.ejvs.2019.07.044. [DOI] [PubMed] [Google Scholar]
- 71.Gavish L, Rubinstein C, Bulut A, Berlatzky Y, Beeri R, Gilon D, Gavish L, Harlev M, Reissman P, Gertz SD. Low-level laser irradiation inhibits abdominal aortic aneurysm progression in apolipoprotein E-deficient mice. Cardiovasc Res. 2009;83:785–792. doi: 10.1093/cvr/cvp149. [DOI] [PubMed] [Google Scholar]
- 72.Gavish L, Gilon D, Beeri R, Nachman D, Gertz SD. Photobiomodulation for Abdominal Aortic Aneurysm: Can It Work? Photobiomodul Photomed Laser Surg. 2022;40:519–521. doi: 10.1089/photob.2022.0046. [DOI] [PubMed] [Google Scholar]
- 73.Hayashi T, Sasaki N, Yamashita T, Mizoguchi T, Emoto T, Amin HZ, Yodoi K, Matsumoto T, Kasahara K, Yoshida N, Tabata T, Kitano N, Fukunaga A, Nishigori C, Rikitake Y, Hirata KI. Ultraviolet B Exposure Inhibits Angiotensin II-Induced Abdominal Aortic Aneurysm Formation in Mice by Expanding CD4(+)Foxp3(+) Regulatory T Cells. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel J, Douglas G, Kerr AG, Hale AB, Channon KM. Effect of irradiation and bone marrow transplantation on angiotensin II-induced aortic inflammation in ApoE knockout mice. Atherosclerosis. 2018;276:74–82. doi: 10.1016/j.atherosclerosis.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrena-Blázquez S, Díez-Alonso M, Riera Del Moral LF, Sanchez Coll S, Alvarez-Mon M, Ortega MA, Ruiz Grande F. Quality of Life of Patients Treated for Abdominal Aortic Aneurysm: Open Surgery and Endoprosthesis. J Clin Med. 2022;11 doi: 10.3390/jcm11082195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ibrahim A, Yordanov MD, Hasso M, Heine B, Oberhuber A. Open Treatment of Abdominal Aortic Aneurysm in the Endovascular Era. J Clin Med. 2022;11 doi: 10.3390/jcm11113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lederle FA, Kyriakides TC, Stroupe KT, Freischlag JA, Padberg FT Jr, Matsumura JS, Huo Z, Johnson GR OVER Veterans Affairs Cooperative Study Group. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N Engl J Med. 2019;380:2126–2135. doi: 10.1056/NEJMoa1715955. [DOI] [PubMed] [Google Scholar]
- 78.Huang L, Chen X, Hu Q, Luo F, Hu J, Duan L, Wang E, Ye Z, Zhang C. The application of modular multifunctional left heart bypass circuit system integrated with ultrafiltration in thoracoabdominal aortic aneurysm repair. Front Cardiovasc Med. 2022;9:944287. doi: 10.3389/fcvm.2022.944287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wittenstein GJ. Complications of aortic aneurysm surgery: prevention and treatment. Thorac Cardiovasc Surg. 1987;35 Spec No 2:136–139. doi: 10.1055/s-2007-1020275. [DOI] [PubMed] [Google Scholar]
- 80.Shahin Y, Dixon S, Kerr K, Cleveland T, Goode SD. Endovascular aneurysm repair offers a survival advantage and is cost-effective compared with conservative management in patients physiologically unfit for open repair. J Vasc Surg. 2023;77:386–395.e3. doi: 10.1016/j.jvs.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Dosluoglu HH, Rivero M, Khan SZ, Cherr GS, Harris LM, Dryjski ML. Pre-emptive nonselective perigraft aortic sac embolization with coils to prevent type II endoleak after endovascular aneurysm repair. J Vasc Surg. 2019;69:1736–1746. doi: 10.1016/j.jvs.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 82.Wu J, Wu Y, Li F, Zhuang D, Cheng Y, Chen Z, Yang J, Liu J, Li X, Fan R, Sun T. Natural history of isolated abdominal aortic dissection: A prospective cohort study. Front Cardiovasc Med. 2023;10:1002832. doi: 10.3389/fcvm.2023.1002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doumenc B, Mesnard T, Patterson BO, Azzaoui R, De Préville A, Haulon S, Sobocinski J. Management of Type IA Endoleak After EVAR by Explantation or Custom Made Fenestrated Endovascular Aortic Aneurysm Repair. Eur J Vasc Endovasc Surg. 2021;61:571–578. doi: 10.1016/j.ejvs.2020.10.033. [DOI] [PubMed] [Google Scholar]
- 84.Chait J, Tenorio ER, Hofer JM, DeMartino RR, Oderich GS, Mendes BC. Five-year outcomes of physician-modified endografts for repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2023;77:374–385.e4. doi: 10.1016/j.jvs.2022.09.019. [DOI] [PubMed] [Google Scholar]
- 85.Oderich GS, Farber MA, Silveira PG, Tadros R, Marin M, Fillinger M, Makaroun M, Hemmer J, Madden M. Technical aspects and 30-day outcomes of the prospective early feasibility study of the GORE EXCLUDER Thoracoabdominal Branched Endoprosthesis (TAMBE) to treat pararenal and extent IV thoracoabdominal aortic aneurysms. J Vasc Surg. 2019;70:358–368.e6. doi: 10.1016/j.jvs.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 86.Azizzadeh A, Desai N, Arko FR 3rd, Panneton JM, Thaveau F, Hayes P, Dagenais F, Lei L, Verzini F. Pivotal results for the Valiant Navion stent graft system in the Valiant EVO global clinical trial. J Vasc Surg. 2019;70:1399–1408.e1. doi: 10.1016/j.jvs.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 87.Soliński DG, Celer M, Dyś K, Witkiewicz W, Wiewióra M. 3D printing in the endovascular treatment of visceral artery aneurysms. Medicine (Baltimore) 2023;102:e35844. doi: 10.1097/MD.0000000000035844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whooley S. Cydar Medical, Medtronic treat first patient in AI mapping collaboration. 2023 [Cited 23 August 2023]. Available from: https://www.massdevice.com/cydar-medical-medtronic-first-patient-ai-mapping/
- 89.van Rijswijk RE, Groot Jebbink E, Holewijn S, Stoop N, van Sterkenburg SM, Reijnen MMPJ. Predictors of Abdominal Aortic Aneurysm Shrinkage after Endovascular Repair. J Clin Med. 2022;11 doi: 10.3390/jcm11051394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Langenberg JC, Roijers J, Ho GH, Veen EJ, Vos D, Buimer T, De Groot HG, van der Laan L. Post-EVAR aneurysm sac shrinkage is prognostically favorable, but does not justify withholding follow-up. J Cardiovasc Surg (Torino) 2020;61:317–322. doi: 10.23736/S0021-9509.18.10584-2. [DOI] [PubMed] [Google Scholar]
- 91.Vedani SM, Petitprez S, Weinz E, Corpataux JM, Déglise S, Deslarzes-Dubuis C, Côté E, Ricco JB, Saucy F. Predictors and Consequences of Sac Shrinkage after Endovascular Infrarenal Aortic Aneurysm Repair. J Clin Med. 2022;11 doi: 10.3390/jcm11113232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pasqui E, de Donato G, Molino C, Abu Leil M, Anzaldi MG, Galzerano G, Palasciano G. Residual Aneurysmal Sac Shrinkage Post-Endovascular Aneurysm Repair: The Role of Preoperative Inflammatory Markers. Biomedicines. 2023;11 doi: 10.3390/biomedicines11071920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daye D, Walker TG. Complications of endovascular aneurysm repair of the thoracic and abdominal aorta: evaluation and management. Cardiovasc Diagn Ther. 2018;8:S138–S156. doi: 10.21037/cdt.2017.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berretta P, Galeazzi M, Cefarelli M, Alfonsi J, De Angelis V, Pierri MD, Matteucci SML, Alessandroni E, Zingaro C, Capestro F, D'Alfonso A, Di Eusanio M. Minimally invasive approach: is this the future of aortic surgery? Indian J Thorac Cardiovasc Surg. 2022;38:171–182. doi: 10.1007/s12055-021-01258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]