Abstract
Hepatitis C virus translation is initiated on a ∼330-nucleotide (nt)-long internal ribosomal entry site (IRES) at the 5′ end of the genome. In this process, a 43S preinitiation complex (comprising a 40S ribosomal subunit, eukaryotic initiation factor 3 (eIF3), and a ternary [eIF2-GTP-initiator tRNA] complex) binds the IRES in a precise manner so that the initiation codon is placed at the ribosomal P site. This binding step involves specific interactions between the IRES and different components of the 43S complex. The 40S subunit and eIF3 can bind to the IRES independently; previous analyses revealed that eIF3 binds specifically to an apical half of IRES domain III. Nucleotides in the IRES that are involved in the interaction with the 40S subunit were identified by RNase footprinting and mapped to the basal half of domain III and in domain IV. Interaction sites were identified in locations that have been found to be essential for IRES function, including (i) the apical loop residues GGG266-268 in subdomain IIId and (ii) the pseudoknot. Extensive protection from RNase cleavage also occurred downstream of the pseudoknot in domain IV, flanking both sides of the initiation codon and corresponding in length to that of the mRNA-binding cleft of the 40S subunit. These results indicate that the 40S subunit makes multiple interactions with the IRES and suggest that only nucleotides in domain IV are inserted into the mRNA-binding cleft of the 40S subunit.
Hepatitis C virus (HCV), the main causative agent of non-A, non-B viral hepatitis in human populations, is classified as one of the genera of the family Flaviviridae (31). The HCV genome consist of a positive-stranded RNA molecule of ∼9,600 bases that contains a large open reading frame preceded by a ∼342-nucleotide (nt) 5′ nontranslated region (5′ NTR) (5). Clinical HCV isolates exhibit considerable genetic diversity, and there is extensive quasispecies sequence variation among isolates from individual infected patients (37). Sequence heterogeneity is limited to the coding region, whereas the sequence of the 5′ NTR is strongly conserved among different genotypes (39). The conservation of primary structure reflects requirements for higher-order structures that control replication and translation of the genome.
HCV translation initiation occurs on an internal ribosomal entry site (IRES) rather than by the 5′ cap-mediated mechanism that is used by most eukaryotic mRNAs (40, 42). IRESs also occur in members of the Pestivirus genus of the Flaviviridae such as bovine viral diarrhea virus (BVDV) and classical swine fever virus (CSFV) (30, 36). BVDV and CSFV IRESs are structurally similar to the HCV IRES (2, 11, 12, 19, 21, 41). The proposed secondary structure of the HCV 5′ NTR is highly conserved between genotypes and is characterized by four major domains (designated I to IV). Domain I is dispensable; otherwise nearly the entire HCV 5′ NTR (nt 40 to 372) and ∼30 nt of the adjacent coding region are required for full IRES activity (8, 11, 33, 35). Some reports indicate that deletion of domain II causes near-total loss of IRES function (8, 35, 42), whereas others indicate only partial consequent reduction in activity (13, 32, 40). Several structural elements, such as a pseudoknot upstream of the initiation codon (41, 43), are required for IRES function. Domain IV negatively regulates internal initiation by sequestering the initiation codon (12).
Mutational analyses have shown that ribosomes bind HCV and pestivirus IRESs directly at the initiation codon without scanning from an upstream position (32, 34, 36). We have begun to investigate the mechanism of this process by reconstituting it in vitro using purified translation components (27, 28). HCV-like IRESs use an initiation mechanism that differs fundamentally from the cap-mediated scanning ribosome mode of initiation used by most mRNAs. First, a ribosomal 43S complex comprising Met-tRNA; Met, GTP, eukaryotic initiation factor 2 (eIF2), eIF3, and a 40S ribosomal subunit is assembled. On capped mRNAs, eIF1, eIF1A, eIF4A, eIF4B, and eIF4F are required for its attachment to a cap-proximal region of the mRNA and for it to scan downstream to the initiation codon (26). On HCV-like IRESs, 43S complexes bind directly to the initiation codon independently of these five factors (26–28). Attachment of 43S complexes to these IRESs is very precise, such that the initiation codon is positioned in the immediate vicinity of the ribosomal P site. Ribosomal attachment results from specific interactions between elements in the IRES and components of the 43S complex (27, 28). Two such interactions have been identified in addition to codon-anticodon base pairing. HCV-like IRESs all contain determinants in the apical half of domain III that interact with eIF3 (4, 27, 28, 38). In addition, these IRESs contain as yet undefined determinants that mediate direct and precise factor-independent binding of 40S subunits (27, 28). This ability is a unique property of HCV-like IRESs that differentiates initiation on them from initiation by the cap-mediated scanning ribosome mode that is used by most eukaryotic mRNAs and from internal initiation on picornaviral mRNAs (26, 29).
Toeprinting and deletion analyses indicate that binary IRES-40S subunit complexes are stabilized by multiple interactions. Primer extension on the HCV IRES is arrested by bound ribosomes in the pseudoknot and downstream of the initiation codon. This observation suggests that the initiation codon and flanking residues are fixed in the mRNA-binding cleft of the 40S subunit, and that the 40S subunit binds the IRES at one or more additional positions. The ability of binary ribosomal complexes to assemble on an IRES fragment lacking domain IV is consistent with this hypothesis. The only contact on the 40S subunit that has been identified to date involves ribosomal protein S9, which can be specifically UV cross-linked to HCV and CSFV IRESs (28). We report here that we have used enzymatic footprinting to identify nucleotides that are involved in the interaction with 40S subunits. Interaction sites were identified in domain IIId, in the pseudoknot, and flanking the initiation codon. These observations are consistent with a model for IRES function in which ribosomal binding involves multiple interactions between the 40S subunit and the IRES.
MATERIALS AND METHODS
Plasmids.
pHCV(40-372).NS′ contains HCV nt 40 to 372 linked to a truncated influenza virus nonstructural protein (NS′) reporter (33). pΔF-CAT contains the complete HCV 5′ NTR (except for nt 229 to 238) linked to a chloramphenicol acetyltransferase reporter (35). PCR was used to generate pHCV(118-372).NS′ and pHCV(118-228/239-372).NS′. Dicistronic vector pXL.CSFV(1-442).NS′ contains CSFV nt 1 to 442 flanked upstream by a cyclin B2 reporter and downstream by the influenza virus NS′ reporter (28). The complete coding sequence of eukaryotic ribosomal protein S9 (7) was cloned by PCR from a human cDNA library (Clontech, Palo Alto, Calif.) and cloned between the BamHI and SacI sites in pET28b (Novagen, Madison, Wis.) to yield pET28-S9.
Purification of 40S ribosomal subunits and recombinant proteins.
40S ribosomal subunits were purified from rabbit reticulocyte lysate (RRL; Green Hectares, Oregon, Wis.) as described previously (28). Recombinant eIF4A and eIF4A(R362Q) mutant proteins were expressed in Escherichia coli BL21(DE3) and purified as described previously (25). Recombinant S9 protein was expressed in E. coli BL21(DE3) and was purified by chromatography using a Ni2+-nitrilotriacetic acid matrix (QIAGEN, Valencia, Calif.).
Transcription and translation.
HCV and dicistronic CSFV mRNAs were transcribed in vitro with T7 polymerase with or without [32P]UTP (∼3,000 Ci/mmol; ICN Radiochemicals) from plasmids that had been linearized at appropriate sites (28). Radiolabeled RNAs were purified using Nuc-Trap columns (Stratagene, La Jolla, Calif.) as described previously (29) and had specific activities of ∼300,000 to 500,000 cpm/μg. Monocistronic HCV mRNA (0.5 μg) and dicistronic CSFV mRNA (0.2 μg) were translated in RRL (Roche Molecular Biochemicals, Indianapolis, Ind.) in the presence of [35S]methionine, with or without recombinant eIF4A(R362Q) or ribosomal protein S9 as indicated, in 15-μl total reaction volumes. Translation products were resolved by electrophoresis using 12% polyacrylamide gels. Gels were dried and exposed to X-ray film.
Assembly and analysis of ribosomal complexes.
Ribosomal complexes were assembled on 1 μg (∼2.1 pmol) of HCV mRNA in 40-μl reaction volumes in binding buffer (20 mM Tris-HCl [pH 7.5], 2.5 mM magnesium acetate, 100 mM KCl, 2 mM dithiothreitol) with a threefold molar excess of ribosomal 40S subunits. These ribosomal complexes were analyzed by primer extension (29) using the primer 5′-CTCGTTTGCGGACATGCC-3′ (complementary to part of the NS′ coding sequence). cDNA products were ethanol precipitated, resuspended, and compared with appropriate dideoxynucleotide sequence ladders by electrophoresis through 6% polyacrylamide–7 M urea gels. For analysis by enzymatic footprinting (Fig. 1), IRES-40S subunit complexes were assembled in 20-μl reaction volumes essentially as described above. Free or ribosome-bound IRES-specific RNAs in binding buffer were digested by incubation for 10 min at 37°C with either RNase V1 or RNase T1 (Amersham Pharmacia Biotech) at a final RNase V1 concentration of 0.0007 U/μl (in the absence of 40S subunits) or 0.00105 U/μl (in their presence) and a final RNase T1 concentration of 0.015 U/μl (in the absence of 40S subunits) or 0.025 U/μl (in their presence). RNAs were extracted with phenol-chloroform and precipitated with 3 volumes of ethanol. The end-labeled primer 5′-CTCGTTTGCGGACATGCC-3′ (complementary to the NS′ coding sequence) or 5′-CGCAAGCACCCTATC-3′ (complementary to HCV nt 295 to 309) was annealed to RNA and extended (15, 16). cDNA products were analyzed by electrophoresis on 6% polyacrylamide–7 M urea gels.
FIG. 1.
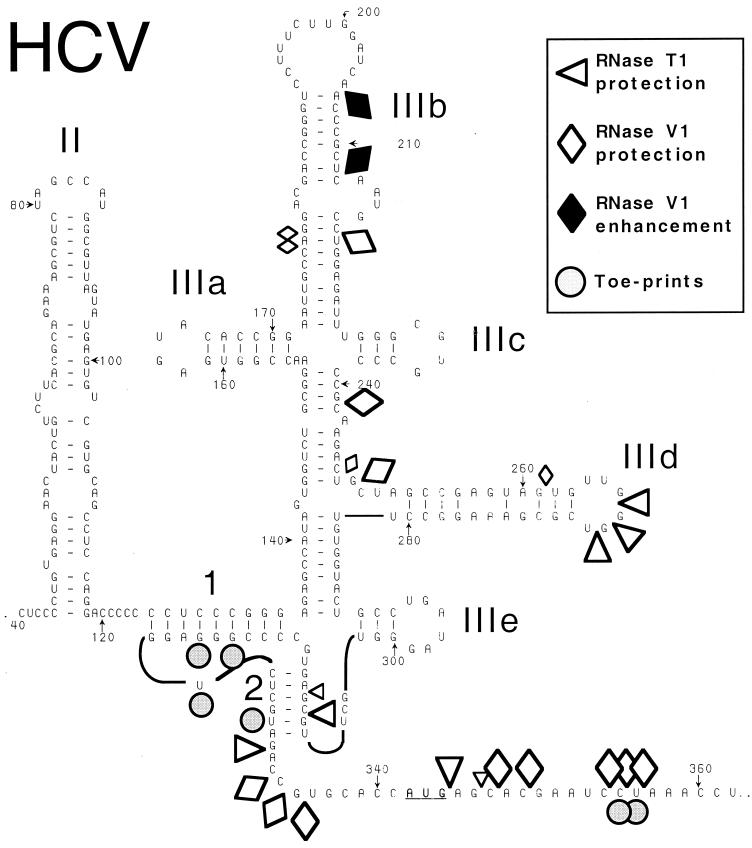
Schematic representation of the secondary structure of the HCV IRES (based on references 11 and 41), showing sites in nt 40-372.NS′ mRNA that are protected from cleavage by RNases T1 and V1 or at which cleavage is enhanced following binding of a 40S ribosomal subunit. These sites and the position of toeprints caused by bound 40S subunits are indicated by symbols shown at the upper right. Smaller symbols indicate weaker protection from cleavage. Domain IV (nt 331 to 354) is shown as an unstructured linear sequence for greater clarity. The initiation codon (AUG342-344) is underlined. The nomenclature used to describe subdomains of the IRES is from reference 12; the helices that constitute the pseudoknot are labeled 1 and 2.
UV cross-linking.
UV cross-linking of binary IRES-40S subunit complexes was done essentially as described previously (28). Ribosomal proteins were resolved by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were dried and exposed to X-ray film.
RESULTS
HCV IRES domain II enhances ribosomal binding at the initiation codon.
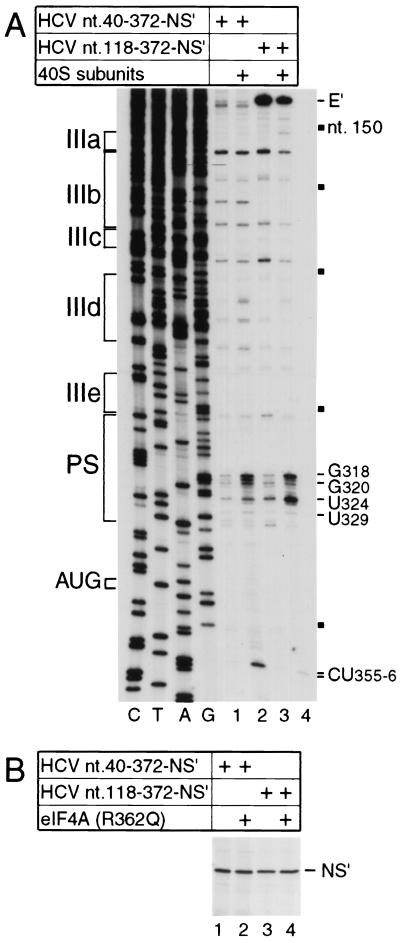
Binding of ribosomal 40S subunits to the HCV IRES is stabilized by multiple IRES-40S subunit contacts. For example, when primer extension inhibition (toeprinting) is used to assay the interaction of 40S subunits with HCV nt 40-372.NS′ mRNA, toeprints are detected at CU355-356 downstream of the initiation codon AUG340-342 and at G318, G320, U324, and U329 in the pseudoknot (Fig. 2A, lanes 1 and 2), consistent with previous reports (28). In this mRNA, domain I is absent, and the influenza virus NS′ reporter gene is fused to 30 nt of the HCV coding region, which are important for IRES function (22, 33). To determine the influence of domain II on these interactions, we next used nt 118-372.NS′ mRNA from which domains I and II had been deleted completely. The intensity of toeprints downstream of the initiation codon caused by the bound 40S subunit was significantly lower on this mRNA, whereas the intensities of toeprints in the pseudoknot of this RNA were similar (Fig. 2A, lanes 3 and 4). Comparison between nt 40-372.NS′ and nt 118-372.NS′ mRNAs showed that deletion of domain II reduced the translation activity of the IRES to 67% (Fig. 2B, lanes 1 to 4). To verify that the significant level of residual activity detected on translation of this mutant RNA was not in fact due to end-dependent translation of fragmented RNA, eIF4A(R362Q) was included in parallel translation reactions. This protein is a trans-dominant inhibitor of end-dependent initiation of translation, but it has no effect on HCV-like IRESs because initiation on them does not involve eIF4A (25, 27, 28). Inclusion of eIF4A(R362Q) had no effect on residual translation activity. In parallel control experiments, inclusion of wild-type eIF4A in translation reactions also had no effect on translation of these HCV mRNAs (data not shown). Domain II is thus not essential for but contributes to IRES function, consistent with previous reports (13, 32, 40).
FIG. 2.
Influence of domain II on IRES function. (A) Toeprint analysis of binary ribosomal complex formation on the HCV IRES. Ribosomal 40S subunits were incubated with HCV nt 40-372.NS′ or nt 118-372.NS′ mRNA under standard reaction conditions and then analyzed by primer extension. Full-length nt 118-372.NS′ cDNA is marked E′; other cDNA products terminated at the sites indicated on the right. Reference lanes C, T, A, and G depict HCV sequences; IRES subdomains IIIa, IIIb, IIIc, IIId, IIIe, and PS (pseudoknot) are indicated on the left; HCV nucleotides are indicated by black squares at 50-nt intervals from nt 150 to 350 on the right. (B) Translation in RRL of HCV nt 40-372.NS′ mRNA (lanes 1 and 2) or nt 118-372.NS′ mRNA (lane 3 and 4) in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of a 10-fold molar excess of mutant eIF4A(R362Q). Translation products were analyzed by autoradiography after electrophoresis on a 12% polyacrylamide gel. The position of the NS′ translation product is indicated.
Localization of 40S subunit binding sites on the IRES by enzymatic footprinting.
Toeprinting assays such as that described above and sucrose density gradient centrifugation analysis (28) indicated that the HCV IRES is able to bind a 40S subunit in the absence of initiation factors to form a stable binary complex. We used enzymatic footprinting to determine the sites at which 40S subunits bind the IRES, using a ∼3-fold molar excess of 40S subunits and buffer conditions appropriate for HCV translation. Footprinting was done using RNase V1 (which cleaves base-paired or stacked RNA) and RNase T1 (which cleaves RNA specifically after unpaired G residues) (6). Enzymatic cleavage of RNA is detected by arrest of primer extension at the nucleotide at the 3′ side of the cleaved bond, and numbering of residues therefore refers to this nucleotide. Some sites at which cleavage appears to be altered coincide with strong stops formed during primer extension on this highly structured RNA. Protection of such residues is therefore equivocal and is not discussed. Results of this analysis are summarized in Fig. 1.
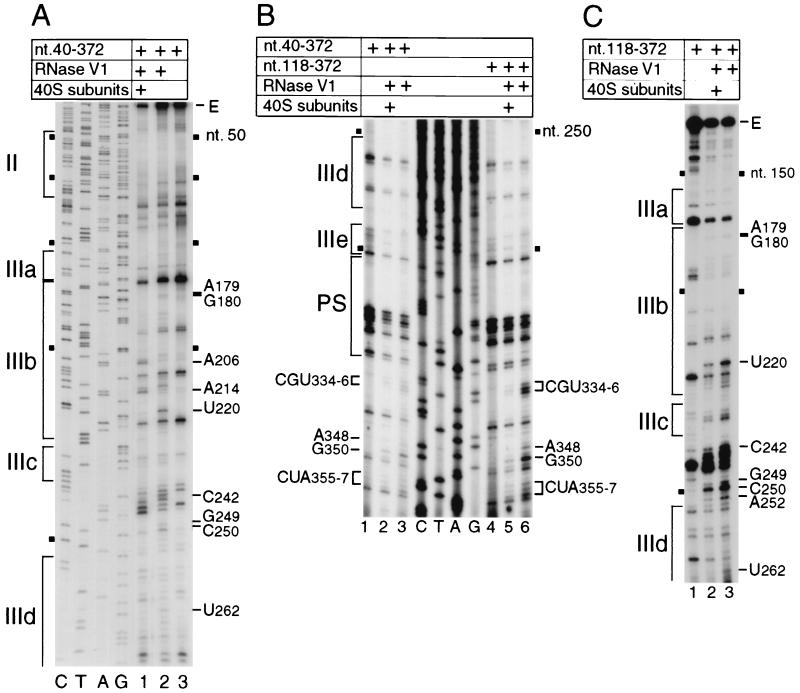
Ribosomal 40S subunits protected the HCV IRES (nt 40 to 372) from RNase V1 cleavage at CGU334-336, A348, G350, and CUA355-357 downstream of the pseudoknot (Fig. 3B, lanes 1 to 3), weakly at U262 in subdomain IIId, at GC249-250 and C242 between subdomains IIIc and IIId, and at U220 and weakly at AG179-180 in IIIb (Fig. 3A, lanes 1 to 3). Residues CUA355-357 are downstream of the initiation codon AUG342-344 and overlap the toeprints at CU355-356 caused by 40S subunits bound to the IRES. Binding of 40S subunits to the IRES enhanced cleavage at A206 and A214 at the apex of IIIb (Fig. 3A, lanes 1 and 2). No additional sites were detected at which RNase V1 cleavage was consistently altered as a result of ribosomal binding.
FIG. 3.
RNase V1 footprinting of the binary 40S subunit-HCV IRES complex. The gels show polyacrylamide-urea gel fractionation of cDNA products obtained after primer extension. (A) Sensitivity of HCV nt 40-372 RNA upstream of nt 272 to cleavage (lanes 1 and 2) either alone (lane 2) or bound by a 40S subunit (lane 1); (B) sensitivity of HCV nt 40-372 RNA (lanes 1 to 3) or nt 118-372 RNA (lanes 4 to 6) upstream of nt 360 to cleavage (lanes 2, 3, 5, and 6) either alone (lanes 3 and 6) or bound by a 40S subunit (lanes 2 and 5); (C) sensitivity of HCV nt 118-372 RNA upstream of nt 265 to cleavage (lanes 2 and 3) either alone (lane 3) or bound by a 40S subunit (lane 2). cDNA products obtained after primer extension of untreated HCV RNA are shown in lane 3 of panel A, lanes 1 and 4 of panel B, and lane 1 of panel C. A dideoxynucleotide sequence generated with the same primer (shown in lanes C, T, A, and G in panels A and B) was run in parallel on each gel. IRES subdomains II, IIIa, IIIb, IIIc, IIId, IIIe, and PS (pseudoknot), as appropriate, are indicated on the left of each panel; HCV nucleotides are indicated by black squares at 50-nt intervals, and the positions of protected residues are indicated on the sides of each panel.
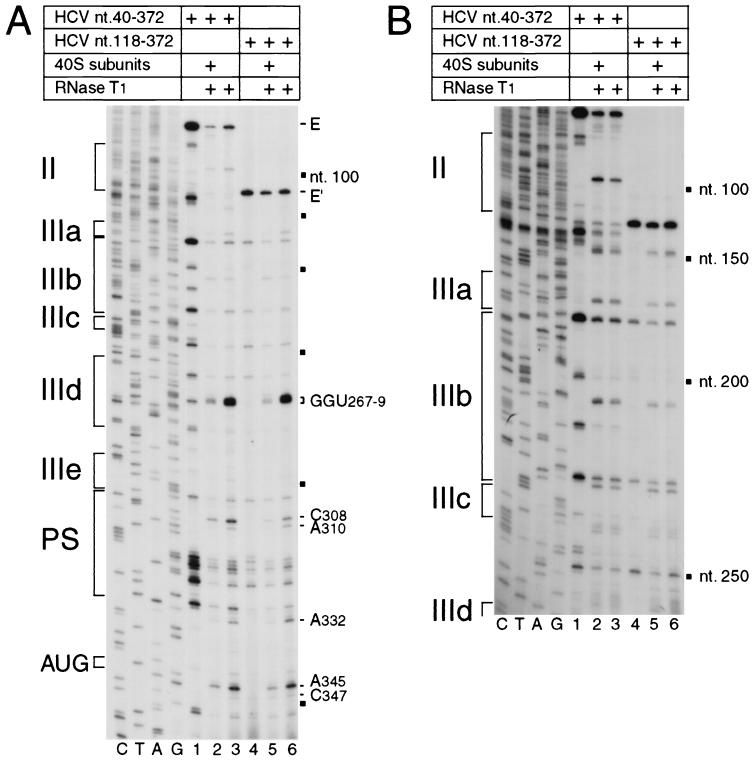
40S subunits bound to the IRES protected it from RNase T1 cleavage at GGU267-269 in the apical loop of subdomain IIId, at C308 and weakly at A310 in the pseudoknot, and at A332, A345, and weakly at C347 downstream of the pseudoknot (Fig. 3A, lanes 1 to 3). No additional sites of altered RNase T1 cleavage were identified anywhere in domain II or in the 5′ half of domain III (Fig. 4B, lanes 1 to 3). The positions of these sites of altered RNase T1 cleavage correlate well with the sites of altered RNase V1 cleavage. The residues downstream of the pseudoknot that are protected by 40S subunits from cleavage by RNases T1 and V1 flank the initiation codon AUG342-344. The 40S subunit likely covers about 25 nt flanking the initiation codon of a eukaryotic mRNA (3, 17, 18, 20), and the leading edge of a 40S subunit bound to the HCV IRES has been mapped to CU355-356 by toeprinting (28). Taken together, these observations suggest that protection by a 40S subunit of residues A332-U356 downstream of the HCV pseudoknot from RNase cleavage (Fig. 1) is consistent with these residues having been inserted into the mRNA-binding cleft of the 40S subunit. The cleavage of residues in helix 2 of the pseudoknot in naked RNA by RNase T1 is consistent with a previous report (41) and strongly suggests that helix 2 is not static but may instead be in equilibrium with alternate higher-order structures. RNase V1 cleavage in helix 2 was not enhanced by binding of a 40S subunit to the IRES; therefore, we suggest that the 40S subunit protects helix 2 from cleavage directly, rather than simply stabilizing the pseudoknot so that helix 2 is base paired and thus cannot be cleaved by RNase T1 (which cleaves only unpaired RNA). The protected residues at the apex of subdomain IIId are absolutely conserved in all HCV genotypes and isolates (39) and in related pestivirus IRESs (10, 21) and are essential for the function of HCV and CSFV IRESs (14; V. G. Kolupaeva, C. U. T. Hellen, and T. V. Pestova, unpublished data).
FIG. 4.
RNase T1 footprinting of the binary 40S subunit-HCV IRES complex. Polyacrylamide-urea gel fractionation of cDNA products obtained after primer extension shows the sensitivity of HCV nt 40-372 RNA (lanes 1 to 3) or nt 118-372 RNA (lanes 4 to 6) upstream of nt 360 (A) or of nt 250 (B) to cleavage (lanes 1, 2, 5, and 6) either alone (lanes 3 and 6) or bound by a 40S subunit (lanes 2 and 5). cDNA products obtained after primer extension of untreated HCV RNA are shown in lanes 1 and 4 of both panels. A dideoxynucleotide sequence generated with the same primer and run in parallel is shown to the left of both panels. IRES subdomains II, IIIa, IIIb, IIIc, IIId, IIIe, and PS (pseudoknot) are indicated on the left of each panel; HCV nucleotides are indicated by black squares at 50-nt intervals, and the positions of protected residues are marked on the right of each panel. The position of the initiation codon AUG is indicated to the left of panel A.
Enzymatic footprinting analysis of the interaction of 40S subunits with mutant HSV IRESs.
Domain II was not protected from RNase cleavage by a 40S subunit bound to the IRES (Fig. 3 and 4). We therefore investigated whether domain II is necessary for a 40S subunit to interact with all of the binding sites that we identified in domains III and IV of the IRES. Binary ribosomal complexes were assembled on HCV nt 118-272.NS′ mRNA and probed enzymatically. There were no major differences in the patterns of cleavage in domains III and IV by RNase T1 of transcripts lacking either nt 1 to 40 or nt 1 to 117 (Fig. 4A, lanes 3 and 6; Fig. 4B, lanes 3 and 6). Similarly, there was no significant difference in the pattern of RNase V1 cleavage in domain III of these two RNAs, except that sequences at the base of IIId and downstream of the pseudoknot were more susceptible to RNase V1 cleavage in the RNA lacking domain II (compare Fig. 3A, lane 2, and Fig. 3C, lane 3; Fig. 3B, lanes 3 and 6). These results indicate that deletion of domains I and II did not cause major structural alterations in domain III or in the pseudoknot.
Ribosomal 40S subunits protected nt 118-272.NS′ mRNA from RNase V1 cleavage in IIIb (U220 and weakly at AG179-180), in and adjacent to IIId (C242, GC249-250, A252, and U262), and downstream of the pseudoknot (CGU334-336, A348, G350, and CUA355-357) in a similar manner to the protection of the nt 40-372.NS′ RNA that contained domain II (Fig. 3A and C). Protection of nucleotides in and adjacent to IIId was slightly greater in the RNA that lacked domain II. The only other difference in the pattern of RNase V1 cleavage of these two RNAs bound to 40S subunits is that no enhancement of cleavage was noted at A206 or A214 on the RNA lacking domain II (compare Fig. 3A, lane 1, with Fig. 3C, lane 2). IRES transcripts with or without domain II were otherwise protected in an equivalent manner by bound 40S subunits from cleavage by RNase T1 in IIId (GGU267-269), in the pseudoknot (C308 and weakly at A310), and flanking the initiation codon (A332, A345, and weakly at C347) (Fig. 4). We conclude that a 40S subunit binds to essentially the same sites on the HCV IRES whether or not domain II is present. Deletion of domain II caused minor changes in the sensitivity to nuclease cleavage of domains III and IV and in their interactions with 40S subunits (Fig. 3 and 4). Domain II may therefore interact weakly with one or both of these domains, as has been suggested previously on the basis of the unanticipated resistance to RNase T1 cleavage of some residues in these regions of the IRES (14). An interdomain interaction of this type could indirectly influence the precise interaction between a 40S ribosomal subunit and the essential domain III/domain IV core of the IRES. A change in the interaction of 40S subunits with the IRES in the absence of the additional stabilization due to codon-anticodon base pairing was detected by toeprinting (Fig. 2A, lanes 2 and 4).
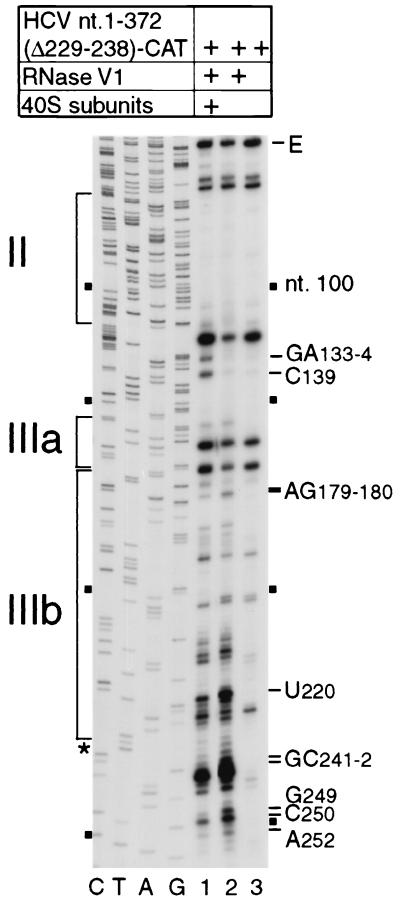
Subdomain IIIc is essential for binding of eIF3 to the IRES but is not required for a 40S subunit to bind to form a stable binary complex (28, 38). It is also not protected from RNase cleavage by a 40S subunit bound to the IRES. Deletion of IIIc (35) caused only few alterations in the pattern of RNase V1 cleavage. We used RNase V1 footprinting to examine the effect of this deletion on ribosomal protection of residues in and upstream of IIId (Fig. 5, lanes 1 and 2). Sites at AG179-180, U220, GC241-242, GC249-250, and A252 were protected by bound 40S subunits from cleavage in a similar manner to ribosomal protection of the intact IRES. Overall, the pattern of ribosomal protection of this mutant RNA closely resembled the pattern of protection of nt 118-372 and nt 40-372 RNAs. This result indicates that the sites on the IRES that are protected by a bound 40S subunit are therefore not significantly altered by deletion of IIIc and/or domain II. However, some differences in the changes induced by ribosomal binding to these three RNAs was noted. Ribosomal binding to the mutant lacking IIIc resulted in strong enhancement of cleavage at GA133-134 and C139 at the base of domain III, adjacent to the pseudoknot; this effect was not observed for the other two RNAs. Deletion of IIIc therefore increases conformational changes at sites close to the pseudoknot caused by ribosomal binding. Enhancement of cleavage in the presence of ribosomes was noted at A205 or A214 only on nt 40-372 RNA and not on RNAs that lacked either IIIc or domains I and II (compare Fig. 5, lane 1, with Fig. 3C, lane 2).
FIG. 5.
RNase V1 footprinting of the binary ribosomal complex formed on HCV nt 40-372 RNA lacking subdomain IIIc (nt 229 to 238). Polyacrylamide-urea gel fractionation of cDNA products obtained after primer extension shows the sensitivity of the HCV RNA upstream of nt 255 to cleavage (lanes 1 and 2) either alone (lane 2) or bound by a 40S subunit (lane 1). cDNA products obtained after primer extension of untreated HCV RNA are shown in lane 3. A dideoxynucleotide sequence generated with the same primer and run in parallel is shown to the left; HCV nucleotides are indicated by black squares at 50-nt intervals from nt 100 to 250. IRES subdomains II, IIIa, and IIIb are indicated on the left, and the positions of protected residues are indicated to the right. The position of the nt 229-238 deletion is indicated by an asterisk.
Interaction of the HCV IRES with ribosomal protein S9.
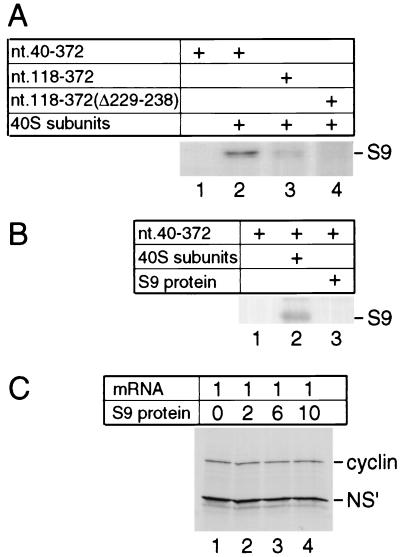
UV cross-linking of binary 40S subunit-HCV IRES complexes assembled on HCV nt 40 to 372 or a corresponding fragment of the CSFV IRES results in specific radiolabeling of ribosomal protein S9 (Fig. 6A, lane 2), as reported previously (28). This ribosomal protein also became radiolabeled after UV cross-linking of binary complexes assembled on HCV nt 118-372.NS′ mRNA, albeit somewhat more weakly than on the transcript containing nt 40 to 373 (Fig. 6A, lane 3). Domain II is therefore not necessary for this interaction to occur and does not contain the site on the IRES to which S9 binds. However, an additional deletion of nt 229 to 238 (i.e., of IIIc) made within nt 118-372.NS′ mRNA resulted in an almost total loss of the ability of this IRES fragment to be UV cross-linked to S9 (Fig. 6A, lane 4). We have previously suggested that S9 binds to the IRES downstream of the initiation codon (28), and we note that these two deletions both weaken the interaction of the ribosome with this region (Fig. 2; reference 28).
FIG. 6.
Ribosomal protein S9 requires the context of the ribosomal 40S subunit to bind to the HCV IRES. (A and B) UV cross-linking of native S9 as a constituent of 40S subunits (A, lanes 2 and 3; B, lane 2) and (B) UV cross-linking of recombinant S9 (B, lane 3) to [32P]UTP-labeled HCV nt 40-372 RNA (A, lanes 1 and 2; B, lanes 1 to 3), nt 118 to 372 RNA (A, lane 3) or nt 118-372(Δ229-238) RNA (panel A, lane 4). Samples were treated with RNases after irradiation, and proteins were resolved by gel electrophoresis. The position of S9 is indicated to the right of both panels. (C) Effect of recombinant S9 added with dicistronic cyclin-CSFV IRES-NS′ mRNA at the indicated molar ratios to translation reaction mixtures on CSFV IRES-mediated NS′ translation.
Ribosomal protein S9 either may be a primary determinant of the HCV IRES's interaction with the 40S subunit or could bind to the IRES as a secondary consequence of another interaction. To investigate whether S9 alone is able to bind to the HCV IRES specifically, we expressed and purified it as a recombinant protein and then assayed its binding to the HCV IRES by UV cross-linking. Cross-linking of the IRES to S9 was not detected (Fig. 6B, lane 3). This observation suggests that S9 is unable to bind to the HCV IRES except in the context of a 40S ribosomal subunit. If S9 were to bind directly to the HCV or CSFV IRES, we reasoned that it would inhibit translation directed by the IRES because it would act as a competitive inhibitor of ribosomal binding. However, no inhibition of CSFV IRES-mediated translation of an NS′ reporter was observed when purified recombinant S9 protein was added in up to 10-fold molar excess over mRNA to translation reactions programmed with dicistronic mRNA containing the CSFV IRES (Fig. 6C). In this experiment, translation of the upstream cistron of the dicistronic mRNA served as a control that the S9 protein did not have general inhibitory effects on translation. Taken together, these results suggest that S9 does not act as a primary determinant of the IRES-40S subunit interaction by itself, but they do not exclude the possibility that it does so in the context of the 40S ribosomal subunit.
DISCUSSION
The ability of the HCV IRES to bind specifically and precisely to a ribosomal 40S subunit in the absence of initiation factors is an important characteristic of the mechanism of HCV translation initiation (10). It is also a step that differentiates initiation on HCV-like IRESs from initiation by the cap-mediated ribosome-scanning mode that is used by the majority of eukaryotic mRNAs (24).
Toeprinting and deletion analyses indicated that a 40S subunit makes multiple contacts with the HCV IRES. By using enzymatic footprinting, we have now identified the principal sites in the IRES that are protected by a bound 40S subunit. These sites include the helix between IIIc and IIId, the apex of IIId, the pseudoknot, and nucleotides flanking both sides of the initiation codon (Fig. 1). These results confirm that the 40S subunit makes multiple interactions with the IRES and indicate that these interactions sites are centered in the basal half of the essential core (nt 120 to 360) of the IRES. The binding site for eIF3 is located in the apical part of this structure (38), and these two components of the 43S preinitiation complex therefore bind to distinct recognition domains in the IRES. These ribosomal interaction sites are located in regions of very high sequence conservation in the HCV IRES (39). More generally, analogous structural regions in pestivirus IRESs also have highly conserved sequences (1, 9) that are closely related to that of HCV.
Significantly, the importance of these interaction sites for HCV IRES function is supported by mutational analyses. Substitution of the apical GGG266-268 nucleotides in IIId abrogated HCV IRES function (14). Corresponding nucleotides in the CSFV IRES are also essential for its function, and are also protected from RNase T1 cleavage by a bound 40S subunit (Kolupaeva et al., unpublished data). Taken together, the available data lead us to conclude that they are therefore likely to be one of the primary determinants of ribosomal binding that are recognized in a base-specific manner by a component of the 40S subunit. Substitutions that disrupted base pairing in helix 2 of the HCV pseudoknot reduced IRES-mediated translation 50- to 100-fold, but significantly, translation was restored by compensatory “pseudo-revertant” substitutions in the opposite strand of the helix that restored the potential for base pairing (41). Precisely analogous observations have also been made regarding the pseudoknot in the CSFV IRES (36). The third principal area of the IRES that is protected by a bound 40S subunit comprises nucleotides flanking the initiation codon, extending over 26 nt from A332 at the 3′ border of the pseudoknot to A357, 12 nt downstream of the initiation codon and overlapping the positions of toeprints at the leading edge of the 40S subunit. The sequence of the coding region is important for HCV IRES function (22, 33); it is strongly conserved (33, 37, 39), and toeprinting has shown that substitutions within it alter the interaction of this region with the 40S subunit (28). Mutations that enhance the propensity of this region to form base-paired structures also reduce the efficiency of translation (12); on the basis of results presented here, we suggest that these mutations impair the ability of the initiation codon and flanking residues to enter the mRNA-binding cleft of the 40S subunit.
The 26-nt sequence protected by a 40S subunit bound to the HCV initiation codon corresponds closely to the length of sequences protected by ribosomes on other eukaryotic cellular and viral mRNAs, which have led to estimates that the eukaryotic ribosomal mRNA-binding cleft covers 10 to 11 nt upstream and 11 to 14 nt downstream of the initiation codon (3, 18, 20). This region of the IRES is therefore likely to be in a nearly fully extended conformation and in fact undergoes only a minor structural rearrangement when base pairing between the initiation codon and the anticodon of initiator tRNA is established (28). This observation suggests that additional contacts between the 40S subunit and elements of the IRES such as IIId and the pseudoknot involve regions of the 40S subunit outside the mRNA-binding cleft. One of these contacts involves ribosomal protein S9, which is located on the 40S subunit at the other end of the mRNA-binding cleft from the eIF3-binding site (23). Domain II of the IRES is not required for this interaction (Fig. 6A) and therefore does not contain the site on the IRES to which S9 binds. We have previously suggested that this interaction involves nucleotides immediately downstream of the pseudoknot (28). The experiments reported here indicate that S9 does not by itself act as a determinant of ribosomal attachment to the IRES, although we cannot rule out that it may do so in the context of the 40S subunit. How ribosomal binding occurs has not yet been established, but is clear from the experiments reported here that this process is more complicated than simple docking of the 40S subunit onto a wholly preformed IRES structure. Instead, ribosomal binding to the IRES requires recognition of specific structural elements, and in addition induces conformational changes in several regions of the IRES, including IIIb, the pseudoknot, and domain IV.
ACKNOWLEDGMENTS
This work was supported by grant AI44108-01 from the NIH to T.V.P. and C.U.T.H.
We thank R. Romain for technical assistance.
REFERENCES
- 1.Becher P, Orlich M, Shannon A D, Horner G, König M, Thiel J-J. Phylogenetic analysis of pestiviruses from domestic and wild ruminants. J Gen Virol. 1997;78:1357–1366. doi: 10.1099/0022-1317-78-6-1357. [DOI] [PubMed] [Google Scholar]
- 2.Brown E A, Zhang H, Ping L-H, Lemon S M. Secondary structure of the 5′ nontranslated regions of hepatitis C virus and pestivirus genomic RNAs. Nucleic Acids Res. 1992;20:5041–5045. doi: 10.1093/nar/20.19.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning K S, Leung D W, Clark J M. Protection of satellite tobacco necrosis virus ribonucleic acid by wheat germ 40S and 80S ribosomes. Biochemistry. 1980;19:2276–2283. doi: 10.1021/bi00551a044. [DOI] [PubMed] [Google Scholar]
- 4.Buratti E, Tisminetzky S, Zotti M, Baralle F E. Functional analysis of the interaction between HCV 5′ UTR and putative subunits of eukaryotic translation initiation factor eIF3. Nucleic Acids Res. 1998;26:3179–3187. doi: 10.1093/nar/26.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke B. Molecular virology of hepatitis C virus. J Gen Virol. 1997;78:2397–2410. doi: 10.1099/0022-1317-78-10-2397. [DOI] [PubMed] [Google Scholar]
- 6.Ehresmann C, Baudin F, Mougel M, Romby P, Ebel J-P, Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987;15:9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frigerio J-M, Dagorn J-C, Iovanna J L. Complete sequencing and expression of the L5, L21, L27A, L28, S5, S9, S10 and S29 human ribosomal protein RNAs. Biochim Biophys Acta. 1995;1262:64–68. doi: 10.1016/0167-4781(95)00045-i. [DOI] [PubMed] [Google Scholar]
- 8.Fukushi S, Katayama K, Kurihara C, Ishiyama N, Hoshino F B, Ando T, Oya A. Complete 5′ noncoding region is necessary for the efficient internal initiation of hepatitis C virus RNA. Biochem Biophys Res Commun. 1994;199:425–432. doi: 10.1006/bbrc.1994.1246. [DOI] [PubMed] [Google Scholar]
- 9.Harasawa R. Phylogenetic analysis of pestivirus based on the 5′-untranslated region. Acta Virol. 1996;40:49–54. [PubMed] [Google Scholar]
- 10.Hellen C U T, Pestova T V. Translation of hepatitis C virus RNA. J Viral Hepatitis. 1999;6:79–88. doi: 10.1046/j.1365-2893.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 11.Honda M, Beard M R, Ping L-H, Lemon S M. A phylogenetically conserved stem-loop structure a the 5′ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J Virol. 1999;73:1165–1174. doi: 10.1128/jvi.73.2.1165-1174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda M, Brown E A, Lemon S M. Stability of a stem-loop involving the initiator AUG controls the efficiency of internal initiation of translation on hepatitis C virus RNA. RNA. 1996;2:955–968. [PMC free article] [PubMed] [Google Scholar]
- 13.Kamoshita N, Tsukiyama-Kohara K, Kohara M, Nomoto A. Genetic analysis of internal ribosome entry site on hepatitis C virus RNA: implication for involvement of the highly ordered structure and cell type-specific trans-acting factors. Virology. 1997;233:9–18. doi: 10.1006/viro.1997.8600. [DOI] [PubMed] [Google Scholar]
- 14.Kieft J S, Zhou K, Jubin R, Murray M G, Lau J Y N, Doudna J A. The hepatitis C virus internal ribosomal entry site adopts an ion-dependent tertiary fold. J Mol Biol. 1999;292:513–529. doi: 10.1006/jmbi.1999.3095. [DOI] [PubMed] [Google Scholar]
- 15.Kolupaeva V G, Hellen C U T, Shatsky I N. Structural analysis of the interaction of the pyrimidine tract-binding protein with the internal ribosomal entry site of encephalomyocarditis virus and foot-and-mouth disease virus RNAs. RNA. 1996;2:1199–1212. [PMC free article] [PubMed] [Google Scholar]
- 16.Kolupaeva V G, Pestova T V, Hellen C U T, Shatsky I N. Translation initiation factor eIF4G recognizes a specific structural element within the internal ribosomal entry site of encephalomyocarditis virus RNA. J Biol Chem. 1998;273:18599–18604. doi: 10.1074/jbc.273.29.18599. [DOI] [PubMed] [Google Scholar]
- 17.Kozak M. Nucleotide sequences of 5′-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977;269:390–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- 18.Kozak M, Shatkin A J. Sequences and properties of two ribosome binding sites from the small size class of reovirus messenger RNA. J Biol Chem. 1977;252:6895–6908. [PubMed] [Google Scholar]
- 19.Le S-Y, Liu W M, Maizel J V. Phylogenetic evidence for the improved RNA higher-order structure in internal ribosome entry sequences of HCV and pestiviruses. Virus Genes. 1998;17:279–295. doi: 10.1023/a:1008073905920. [DOI] [PubMed] [Google Scholar]
- 20.Legon S. Characterization of the ribosome-protected regions of 125I-labelled rabbit globin messenger RNA. J Mol Biol. 1976;106:37–53. doi: 10.1016/0022-2836(76)90299-0. [DOI] [PubMed] [Google Scholar]
- 21.Lemon S M, Honda M. Internal ribosome entry sites within the RNA genomes of hepatitis C virus and other flaviviruses. Semin Virol. 1997;8:274–288. [Google Scholar]
- 22.Lu H H, Wimmer E. Poliovirus chimeras replicating under the translational control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lütsch G, Bielka H, Enzmann G, Noll F. Electron microscopic investigations on the location of rat liver ribosomal proteins S3a, 5, S6, S7, and S9 by means of antibody labeling. Biomed Biochim Acta. 1983;42:705–723. [PubMed] [Google Scholar]
- 24.Merrick W C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pause A, Méthot N, Svitkin Y, Merrick W C, Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994;13:1205–1215. doi: 10.1002/j.1460-2075.1994.tb06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pestova T V, Borukhov S I, Hellen C U T. Eukaryotic ribosomes require eIFs 1 and 1A to locate initiation codons. Nature. 1998;394:854–859. doi: 10.1038/29703. [DOI] [PubMed] [Google Scholar]
- 27.Pestova T V, Hellen C U T. Internal initiation of translation of bovine viral diarrhea virus RNA. Virology. 1999;258:249–256. doi: 10.1006/viro.1999.9741. [DOI] [PubMed] [Google Scholar]
- 28.Pestova T V, Shatsky I N, Fletcher S P, Jackson R J, Hellen C U T. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal initiation of translation of hepatitis C virus and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pestova T V, Hellen C U T, Shatsky I N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol Cell Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole T L, Wang C, Popp R A, Potgieter L N D, Siddiqui A, Collett M S. Pestivirus translation initiation occurs by internal ribosome entry. Virology. 1995;206:750–754. doi: 10.1016/s0042-6822(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 31.Pringle C R. The universal system of virus taxonomy, updated to include the new proposals ratified by the International Taxonomy of Viruses during 1998. Arch Virol. 1999;144:421–429. doi: 10.1007/s007050050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds J E, Kaminski A, Carroll A R, Clarke B E, Rowlands D J, Jackson R J. Internal initiation of translation of hepatitis C virus RNA: the ribosome entry site is at the authentic initiation codon. RNA. 1996;2:867–878. [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds J E, Kaminski A, Kettinen H-J, Carroll A R, Rowlands D J, Jackson R J. Unique features of internal initiation on hepatitis C virus. EMBO J. 1995;14:6010–6020. doi: 10.1002/j.1460-2075.1995.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rijnbrand R C, Abbink T E, Haasnoot P C, Spaan W J, Bredenbeek P J. The influence of AUG codons in the hepatitis C virus 5′ nontranslated region on translation and mapping of the translation initiation window. Virology. 1996;226:47–56. doi: 10.1006/viro.1996.0626. [DOI] [PubMed] [Google Scholar]
- 35.Rijnbrand R, Bredenbeek P J, van der Straaten T, Whetter L, Inchauspé G, Lemon S, Spaan W. Almost the entire 5′ non-translated region of hepatitis C virus is required for cap-independent translation. FEBS Lett. 1995;365:115–119. doi: 10.1016/0014-5793(95)00458-l. [DOI] [PubMed] [Google Scholar]
- 36.Rijnbrand R, Van der Straaten T, van Rijn P A, Spaan W J, Bredenbeek P J. Internal entry of ribosomes is directed by the 5′ noncoding region of classical swine fever virus and is dependent on the presence of an RNA pseudoknot upstream of the initiation codon. J Virol. 1997;71:451–457. doi: 10.1128/jvi.71.1.451-457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmonds P. Variability of hepatitis C virus. Hepatology. 1995;21:570–583. doi: 10.1002/hep.1840210243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sizova D V, Kolupaeva V G, Pestova T V, Shatsky I N, Hellen C U T. Specific interaction of eukaryotic translation initiation factor 3 with the 5′ nontranslated regions of hepatitis C virus and classical swine fever virus RNAs. J Virol. 1998;72:4775–4782. doi: 10.1128/jvi.72.6.4775-4782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith D B, Mellor J, Jarvis L M, Davidson F, Kolberg J, Urdea M, Yap P-L, Simmonds P The International HCV Collaborative Study Group. Variation of the hepatitis C virus 5′ non-coding region: implications for secondary structure, virus detection and typing. J Gen Virol. 1995;76:1749–1761. doi: 10.1099/0022-1317-76-7-1749. [DOI] [PubMed] [Google Scholar]
- 40.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosomal entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Le S, Ali N, Siddiqui A. An RNA pseudoknot is an essential structural element of the internal ribosome entry site located within the hepatitis C virus 5′ noncoding region. RNA. 1995;1:526–537. [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Sarnow P, Siddiqui A. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J Virol. 1993;67:3338–3344. doi: 10.1128/jvi.67.6.3338-3344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C, Sarnow P, Siddiqui A. A conserved helical element is essential for internal initiation of translation of hepatitis C virus RNA. J Virol. 1994;68:7301–7307. doi: 10.1128/jvi.68.11.7301-7307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]