Abstract
Wilms tumor is the most common kidney cancer in children, and diffusely anaplastic Wilms tumor is the most chemoresistant histological subtype. Here we explore how Wilms tumor cells evade the common chemotherapeutic drug actinomycin D, which inhibits ribosomal RNA biogenesis. Using ribosome profiling, protein arrays, and a genome-wide knockout screen, we describe how actinomycin D disrupts protein homeostasis and blocks cell cycle progression. We found that, when ribosomal capacity is limited by actinomycin D treatment, anaplastic Wilms tumor cells preferentially translate proteasome components and upregulate proteasome activity. Furthermore, the proteasome inhibitor bortezomib sensitizes cells to actinomycin D treatment by inducing apoptosis both in vitro and in vivo. Lastly, we show that increased levels of proteasome components are associated with anaplastic histology and with worse prognosis in non-anaplastic Wilms tumor. In sum, maintaining protein homeostasis is critical for Wilms tumor proliferation, and it can be therapeutically disrupted by blocking protein synthesis or turnover.
INTRODUCTION
Wilms tumors, or nephroblastomas, are the most common pediatric kidney cancer. Globally, Wilms tumor is diagnosed in 10.4 per 1 million children less than 15 years old each year 1. North American risk stratification criteria classify Wilms tumors as having favorable or anaplastic histology. The development of effective combinations of chemotherapy, radiation, and surgery has pushed the 5-year overall survival rate to over 90% for those with favorable histology Wilms tumor (FHWT). These strong cure rates have allowed us to de-intensify therapy for FHWT, where up to 24% of long-term survivors who were treated on historical regimens developed therapy-related chronic health conditions 2. On the other hand, patients with diffusely anaplastic Wilms tumor (DAWT), which accounts for ~10% of Wilms tumor patients, continue to have a relapse rate of over 40% and a 4-year overall survival rate of 66% despite being treated with more aggressive drugs such as doxorubicin, etoposide, cyclophosphamide, and carboplatin 1,3–5. Recent attempts to intensify chemotherapy for those with DAWT have increased short-term toxicity with minimal improvements in cure rate 6. Anaplastic histology is associated with loss or mutation of p53 7,8, but the molecular mechanisms underpinning chemoresistance in anaplasia are unknown, and there remain no targeted therapies effective in Wilms tumor.
Since the 1960s, chemotherapy regimens utilizing actinomycinD (actD), also called dactinomycin, have been routinely used to treat Wilms tumors 9,10, rhabdomyosarcoma, and other sarcomas 11,12. In DAWT, however, actD has been replaced by doxorubicin, which can provide higher rates of cure but carries significantly more short- and long-term toxicity 13. It is unknown why DAWT is less sensitive to actD. Understanding how to improve actD sensitivity and avoid the toxicities associated with anthracyclines like doxorubicin could improve outcomes for Wilms tumor as well as other malignancies.
ActD is a DNA-intercalating agent that can block transcription and DNA replication at high concentrations. However, at the low-nanomolar serum concentrations typically achieved in patients, it primarily inhibits transcription of ribosomal RNA (rRNA), which is transcribed by RNA polymerase I (Pol I) and transfer RNA (tRNA), which is transcribed by RNA polymerase III (Pol III) 14–18. Ribosomes are composed of rRNA and ribosomal proteins (RP), and impaired Pol I activity results in fewer fully formed ribosomes, which consequently reduces global translation. In ribosome-depleted settings, the remaining ribosomes are not uniformly distributed across remaining transcripts; instead, they favor certain transcripts, based on factors such as their intracellular localization, secondary structure, and presence of specific sequence motifs 18,19 (Supplementary Figure S1A). We thus reasoned that preferentially translated genes following actD exposure could be targetable vulnerabilities in anaplastic Wilms tumor cells. To date, however, no published findings have characterized how actD affects translation in Wilms tumors.
In this study, we used ribosome profiling to find that proteasome components are preferentially translated in anaplastic Wilms tumor cell lines following actD treatment. The proteasome inhibitor bortezomib (BTZ) increases sensitivity of anaplastic Wilms tumor cells to actD in vitro and in vivo. Lastly, DAWTs express higher levels of proteasome components than FHWTs, and higher levels of proteasome components are associated with worse prognosis.
MATERIALS AND METHODS
Tissue culture
The anaplastic, TP53-mutated Wilms tumor cell line WiT49 (RRID: CVCL_0583) was maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 1000 mg/L-glucose, 4 mM L-glutamine, 1 mM pyruvate (Gibco 11995065) supplemented with antibiotic-antimycotic (Gibco 15240062) and fetal bovine serum (Sigma F2442) to 10% final concentration in 37°C at 5% CO2. A second anaplastic, TP53-mutated Wilms tumor cell line 17.94 (Ximbio 153333, RRID:CVCL_D704) was grown in DMEM supplemented with antibiotic-antimycotic and heat inactivated fetal bovine serum (Gibco 16140063) to 20% final concentration in 37°C at 5% CO2. Cell line identity is annually verified by short tandem repeat genotyping (last verified February 2024 for WiT49 and April 2024 for 17.94). They are also tested biannually for mycoplasma contamination (Latest negative screen February 2024, Lonza LT07–318).
Ribosomal RNA quantification
To determine the effect of actD on rRNA expression, WiT49 or 17.94 cells were seeded at ~50% confluency in 3 different plates. The following day, cells were treated with either vehicle (DMSO) or 2nM actD for 6 hours or 24 hours. At experiment endpoint, total RNA was extracted using the miRNeasy kit with DNase I digestion (Qiagen 217004 and 79254), and cDNA synthesis was performed with iScript™ Reverse Transcription Supermix (Bio-Rad 1708841). Quantitative PCR (qPCR) was performed using iTaq™ Universal SYBR® Green Supermix (Bio-Rad 1725125) with primers listed: 18s rRNA (GTAACCCGTTGAACCCCATT, CCATCCAATCGGTAGTAGCG); 45s pre-rRNA (ACCCACCCTCGGTGAGA, CAAGGCACGCCTCTCAGAT); GAPDH (CGGAGTCAACGGATTTGGT, ACCAGAGTTAAAAGCAGCCC). Relative expression was calculated by the 2−ΔΔCt method by normalizing each rRNA species to GAPDH. Significance was determined by unpaired two-tailed Student’s T-test versus vehicle control.
Protein synthesis quantification
To determine the effect of actD on protein synthesis, WiT49 cells were seeded at ~30% confluency in cell culture chamber slides. The following day, cells were treated with either vehicle (DMSO) or 2nM actD for 48 hours or 72 hours. At the endpoint, protein synthesis was measured using a fluorescence-based O-propargyl-puromycin (OPP) incorporation kit (Invitrogen C10457) according to manufacturer protocols. Nuclei were counterstained with DAPI (4’,6-diamidino-2-phenylindole). Each condition was photographed at 5 non-overlapping regions at 20x magnification on a BZ-X810 Fluorescence microscope (Keyence Corporation) and quantified using Fiji 20. Necrotic regions were disregarded. Relative fluorescence units were calculated from the blue (DAPI) and red (OPP-Alexa Fluor 488) channels. The number of nuclei was counted from the DAPI channel using the Watershed and Analyze Particles functions in Fiji. Raw protein synthesis was calculated as the average red fluorescence intensity in pixels with any fluorescence using the Area and Integrated Density functions in Fiji. For each image, raw protein synthesis was then normalized to the number of nuclei in that field of view. Each field of view was treated as a replicate, and statistical analysis was performed by unpaired two-tailed Student’s t-test against untreated cells.
Reverse phase protein array
WiT49 cells at 20% density were treated with 1 nM actD or DMSO as vehicle control for 72 hours in duplicate. 1×106 cells were collected, washed, and frozen in liquid nitrogen, and sent to the MD Anderson Functional Proteomic Reverse Phase Protein Array Core 21–23. Normalized, log2-transformed, median-centered values from “validated” antibodies were used for analysis 24. The log2-fold-change for each antibody was calculated as the difference between the means of actD-treated and DMSO-treated samples, and the standard deviation for each antibody was calculated as the standard deviation of all four values.
Ribosome profiling
WiT49 cells were incubated in complete growth medium with 2nM actD or DMSO for 6 or 72 hours, in three replicates per condition. For 2-week long treatments, WiT49 were maintained and passaged in two 7-day cycles of drug/vehicle-supplemented media for 3 days, followed by drug-free complete growth media for 4 days, three replicates per condition.
1.5 × 107 cells were used for each replicate for ribosome sequencing. Ribosome footprints were isolated based on previous publications 25,26, and each RNA sample was spiked with 24 fmol of the synthetic 28-nt RNA oligo 5’-AUGUAACACGGAGUCGACCCGCAACGCGA-3’. rRNA depletion was performed using the Low Input RiboMinus™ Eukaryote System v2 (Thermo Fisher A15027), and sequencing libraries were generated using the NEBNext® Small RNA Library Prep Set for Illumina® (NEB E7330). Libraries were pooled and sequenced using NextSeq 500 High Output, single-end reads, 75 cycles. In parallel, RNA was extracted for total RNA sequencing using the Direct-zol RNA Miniprep Kit (ZymoResearch R2050) or the miRneasy Mini Kit (Qiagen 217004) according to manufacturer protocols, followed by rRNA depletion as above and DNase-treatment. Sequencing libraries were prepared with the TruSeq Stranded Total RNA LT or TruSeq Stranded Total RNA-seq Sample Prep Kit from Illumina and sequenced paired-end for 6-hour and 72-hr, and single-end for 72-hour time points.
Trimmed reads from ribosome profiling and RNA sequencing were aligned to the hg38 reference genome using HISAT2 27. For ribosome profiling, reads longer than 30 nucleotides were filtered out, and remaining reads were assembled to GENCODE v26 transcript annotations using StringTie 28. Ribosome profiling quantifications were normalized to mapped spike-in reads, and RNA-seq reads were normalized to million mapped reads. Translational efficiency (TE) for each gene was calculated by dividing the number of normalized ribosome footprint reads by the number of normalized RNA sequencing reads. Differential TE was calculated using t-tests on log2-transformed TE of three actD-treated replicates versus three DMSO-treated replicates. Geneset enrichment analysis (GSEA) was performed using the fgsea package (v1.26.0) on log2-fold change of TE using gene sets from MSigDB v7.117,29–31.
CRISPR screen
CRISPR knock-out screen was performed on WiT49 cells using the Human Brunello CRISPR knockout pooled library, a gift from David Root and John Doench (Addgene 73178). The plasmid library was propagated, verified for maintenance of representation, and transfected as recommended 32. The viral supernatant was transduced into 1.0 × 108 WiT49 cells, at multiplicity of infection of 0.3. Forty-eight hours after transduction, the cells were selected with 0.5 μg/ml puromycin for 7 days. After selection, 3 × 107 transduced cells were collected as transduction reference, and the rest were split into two groups. These remaining cells were expanded and treated with either 2nM actD or DMSO for 4 days. The media was then replaced in both conditions for a washout of 3 days without actD or DMSO. Following this, another 7-day cycle (4 days actD or DMSO treatment followed by 3 days washout) was carried out. Finally, genomic DNA was extracted using DNeasy Blood & Tissue Kit (Qiagen 69506), and a sequencing library was prepared from genomically-integrated single guide RNA (sgRNA) sequences 32. These were gel-size selected and sequenced at ~2.4 million single-end reads each, 100-bp length.
Computational analysis of CRISPR screens was performed using the MAGeCKFlute pipeline follow published guidelines 33. In brief, we mapped reads using the command ‘mageck count’, and we tested sgRNA knockout efficiency using the command ‘mageck mle’. Downstream analysis was performed using the FluteRRA function in the MAGeCKFlute R package, with the ‘mageck mle’ output file as the input. To perform KEGG pathway enrichment analysis, the ‘mageck pathway’ command was used with the KEGG gene set from the Molecular Signatures Database (v7.1) 29.
Western blot
For the actD timecourse, WiT49 or 17.94 was seeded at ~30% confluency overnight. The following day, without replacing the media, actD (or an equivalent volume of DMSO) was spiked into the media to a final concentration of 2 nM, for 72-hour treatment. The next day, another plate of WiT49 or 17.94 began 48-hour treatment with 2nM actD. We repeated this the following day for the 24-hour treatment and 18-hour treatments. Upon completion of the allotted incubation times, we collected cells for protein lysate extraction. For actD and BTZ in vitro drug treatments, we seeded WiT49 or 17.94 at 20–30% confluency overnight. We then treated cells with either 2nM actD alone, 8nM BTZ alone, 2nM actD + 8nM BTZ, or DMSO at equivalent volume without changing media. After 48 hours of drug exposure, cells were collected for protein lysate extraction.
Protein lysates were extracted from flash-frozen pellets with RIPA buffer (Sigma R0278), supplemented with protease and phosphatase inhibitors (Invitrogen A32961), sonicated, and quantified by BCA protein assay (Thermo Scientific 23227). Following denaturing SDS-PAGE and transfer to PVDF or nitrocellulose membranes, blots were blocked with 5% bovine serum albumin in tris-buffered saline with Tween-20. Primary antibodies used are as follows and were diluted to 1:3,000 unless otherwise stated: Tubulin (Cell Signaling Technology 3873, RRID:AB_1904178), GAPDH (Cell Signaling Technology 97166, RRID:AB_2756824), Caspase-3 (Cell Signaling Technology 9665, RRID:AB_2069872), cleaved Caspase-3 (Cell Signaling Technology 9664, RRID:AB_2070042; 1:1,000), Caspase-7 (Cell Signaling Technology 12827, RRID:AB_2687912), cleaved Caspase-7 (Cell Signaling Technology 8438, RRID:AB_11178377; 1:1,000), Caspase-9 (Cell Signaling Technology 9508, RRID:AB_2068620), cleaved Caspase-9 (Cell Signaling Technology 7237, RRID:AB_10895832; 1:1,000), Cyclin D2 (Cell Signaling Technology 3741; RRID:AB_2070685), CDT1 (Cell Signaling Technology 8064, RRID:AB_10896851), Cyclin E1 Cell Signaling Technology 20808, RRID:AB_2783554), Cyclin A2 (Cell Signaling Technology 91500RRID:AB_3096041), PARP (Cell Signaling Technology 9532, RRID:AB_659884), cleaved PARP (Cell Signaling Technology 5625, RRID:AB_10699459; 1:1,000), POMP (Cell Signaling Technology 15141, RRID:AB_2798726), PSMA6 (Cell Signaling Technology 2459, RRID:AB_2268879), PSMB1 (Invitrogen PA5–49648, RRID: AB_2635102; 1:5,000), PSMB2 (Proteintech 15154–1-AP, RRID:AB_2300322; 1:5,000), PSMB5 (Cell Signaling Technology 12919, RRID:AB_2798061; 1:5,000), PSMD1 (Sigma SAB2104781, RRID:AB_10668741), RPL5 (Cell Signaling Techonology 51345, RRID:AB_2799391), RPL7 (abcam ab72550, RRID:AB_1270391), RPL11 (Cell Signaling Technology 18163, RRID:AB_2798794), RPL26 (Cell Signaling Technology 2065, RRID:AB_2146242), Total AKT (Cell Signaling Technology 9272, RRID:AB_329827), Phospho-AKT (Ser473) (Cell Signaling Technology 4060, RRID:AB_2315049; 1:1,000), Total 4E-BP1 (Cell Signaling Technology 9452, RRID:AB_331692), Phospho-4E-BP1 (Thr37/46) (Cell Signaling Technology 2855, RRID:AB_560835; 1:1,000), Total P70-S6K1 (Cell Signaling Technology 34475, RRID:AB_2943679), and Phospho- P70-S6K1 (Thr389) (Invitrogen 710095, AB_2532559; 1:1,000). HRP-conjugated secondary antibodies used are Anti-Mouse IgG (Cell Signaling Technology 7076, RRID:AB_330924; 1:10,000) or Anti-Rabbit IgG (Cell Signaling Technology 7074, RRID:AB_2099233; 1:5,000).
In vitro drug inhibition
To quantify drug inhibitory activity, 1,000 cells per well were seeded in black-walled 96-well plates at 100 μl/well. The following day, serial dilutions of actD (Sigma A1410), BTZ (Sigma 5043140001), or Rapamycin (Selleckchem S1039) were added in 3 replicates per dose. Vehicle control was also included to yield equivalent final concentrations of DMSO (<0.1%) in all wells. For synergy determination experiments, each drug of interest was serially diluted alone and then mixed into a range of combinations. Each dose was then added onto the cells in triplicate. After 72 hours, cell density was assayed by adding 20 μL 0.15 mg/ml resazurin in phosphate-buffered saline in each well, including cell-free wells as background controls and drug-free wells as normalization controls. After 1–4 hours at 37°C, fluorescence was measured using a microplate reader with excitation set at 550 nm and emission at 590 nm. Combination treatment effects were determined using the Loewe synergy model dose-response calculation through Synergyfinder+ 34.
Proteasome activity assay
To measure proteasome enzymatic activity, we seeded 1,000 cells per well concurrently in duplicate 96-well plates: one black-walled and one white-walled. The following day, the cells were treated with each of the following conditions in quadruplicate with identical conditions in both plates: 2nM actD, 2 nM actD + 1 mM MG-132 (Selleckchem S2619), 8 nM BTZ, 8 nM + 1 mM MG-132, DMSO, or DMSO + 1 mM MG-132. Wells without cells were used as background controls. After 48 hours, the black-walled plate was used to determine cell viability as described above. The white-walled plate was used to quantify proteasome enzymatic activity. We used Proteasome-Glo™ Chymotrypsin-like Assay (Promega G8621) and calculated the proteasome enzymatic activity in accordance with manufacturer recommendations. Briefly, after subtracting background controls, we normalized each condition to viability based on the corresponding wells in the black-walled plate to account for differences in cell density. We then subtracted the normalized luminescence of each condition with MG-132 from the corresponding wells without MG-132 to yield the viability-normalized luminescence attributable to proteasome activity. Statistical significance was calculated using unpaired, two-tailed Student’s T-test.
Xenograft tumor models
All experiments involving animals were reviewed, approved, and monitored for compliance by the UT Southwestern Institutional Animal Care and Use Committee.
To generate xenografts, log growth-phase 17.94 cells or freshly-thawed cryopreserved TP53 mutated anaplastic Wilms tumor PDX line KT-53 35 were injected subcutaneously into the flanks of NOD scid gamma (NSG) mice in 1 part DPBS and 1 part Matrigel (Corning 354234) at 4 million 17.94 per 100 μl or 1.6 million KT-53 cells per 100 μl per mouse. Tumor growth was measured once a week with calipers in two dimensions. Tumor volume was calculated by dividing the product of the length and the square of the width by 2. Upon reaching the thresholds of 150 mm3 for 17.94 and 100 mm3 for KT-53, mice were entered into one of four treatment arms: actD only, BTZ only, both actD + BTZ and vehicle. Mice in the actD only and actD+BTZ arms were dosed with actD (in 2.5% DMSO, 40% PEG300, 5% Tween-80, 52.5% saline) once a week intraperitoneally (0.2 mg/kg for 17.94, 0.15 mg/kg for KT-53). Mice in the BTZ only or actD+BTZ arms were dosed with BTZ (in 0.5% DMSO, 30% PEG300, 69.5% distilled deionized water) once a week intravenously (0.2mg/kg). Mice in the vehicle arm received both solvents once a week. Treatment continued until tumor volumes reached 1,500 mm3. At the end of therapy, tumors were harvested for histological processing. Statistical analyses for tumor volume were performed by two-tailed Student’s T-test for each pairwise comparison between the four treatment arms. Kaplan-Meier survival curves were compared between each group by log-rank test.
IHC & TUNEL
Two representative samples from each treatment arm were formalin-fixed, paraffin-embedded, and sectioned at 4 μm thickness. These were used for immunohistochemistry (IHC) and TUNEL assay.
For IHC, antibodies used were Ki-67 (Abcam ab15580, RRID:AB_443209, 1:1,000 for primary) with anti-Rabbit HRP (Vector Laboratories Inc. MP-7451, RRID:AB_2631198 for secondary). Each slide was photographed in at least four distinct 40x magnified fields of view (Keyence Corporation). Numbers of total nuclei and DAB-positive nuclei in each field were quantified using Fiji 36. Non-tumor regions were omitted from quantification. Statistical analysis was performed by unpaired two-tailed Student’s t-test between each pair of treatment arms.
To measure apoptosis in tumor sections, we used Click-iT™ Plus TUNEL Assay kit (Invitrogen C10617) according to the manufacturer recommendations and counterstained nuclei with DAPI. Each tissue slide was photographed in at least four distinct 40x magnified fields of view (Keyence Corporation). Numbers of total nuclei and TUNEL-positive nuclei and quantified using Fiji 36. Non-tumor regions were omitted from quantification. Statistical analysis was performed by unpaired two-tailed Student’s t-test between each pair of treatment arms.
TARGET database reanalysis
Tables of Wilms tumor RNA-seq counts and clinical annotation were downloaded from the NCI TARGET8 website on May 21, 2019. Protein-coding genes were identified based on ENSEMBL v86 annotations. Differential expression analysis for DAWT versus FHWT was performed with DESeq2 (v1.40.2), followed by gene set enrichment analysis using fgsea (v1.26.0) based on MSigDB v7.1 gene sets 17,31,37.
We compared outcomes for these patients according to expression of proteasome enzymatic subunits in cBioportal 38,39. Specifically, we obtained RNA-seq RPKM z-scores for each gene of interest compared to tumors that are diploid for that gene. For patients with multiple samples, the primary tumor sample was used. Based on expression of PSMB5, PSMB6, and PSMB7, tumors with at least one gene z-score ≥ +1 were categorized as “PSMs high”, while those with at least one gene z-score ≤ −1 were categorized as “PSMs low”. Samples with z-scores between −1 and +1 for all three genes were categorized as “PSMs medium”. Those with one gene z-score ≥ +1 and another gene z-score ≤ −1 were omitted (n=8). Log-rank tests were used to compare survival curves.
Data availability
Ribosome profiling and accompanying RNA-seq data from WiT49 are deposited in the NCBI GEO database under accession GSE270330.
RESULTS
Actinomycin alters the translational landscape of anaplastic Wilms tumor cells
ActD blocks rRNA transcription at nanomolar doses and mRNA transcription at micromolar doses 18. To examine whether actD is effective at nanomolar doses in anaplastic Wilms tumor, we measured 72-hour actD sensitivity in two anaplastic Wilms tumor cell lines, WiT49 and 17.94 (Supplementary Figure S1B). For these cell lines, we measured IC50 to be 1.3 and 2.2 nM, respectively. Next, we confirmed that 2 nM actD reduces levels of 45s pre-rRNA and 18s mature rRNA in both cell lines (Supplementary Figure S1C). This results in decreased overall protein synthesis, consistent with specific impairment of Pol I activity (Supplementary Figure S1D and Figure 1A).
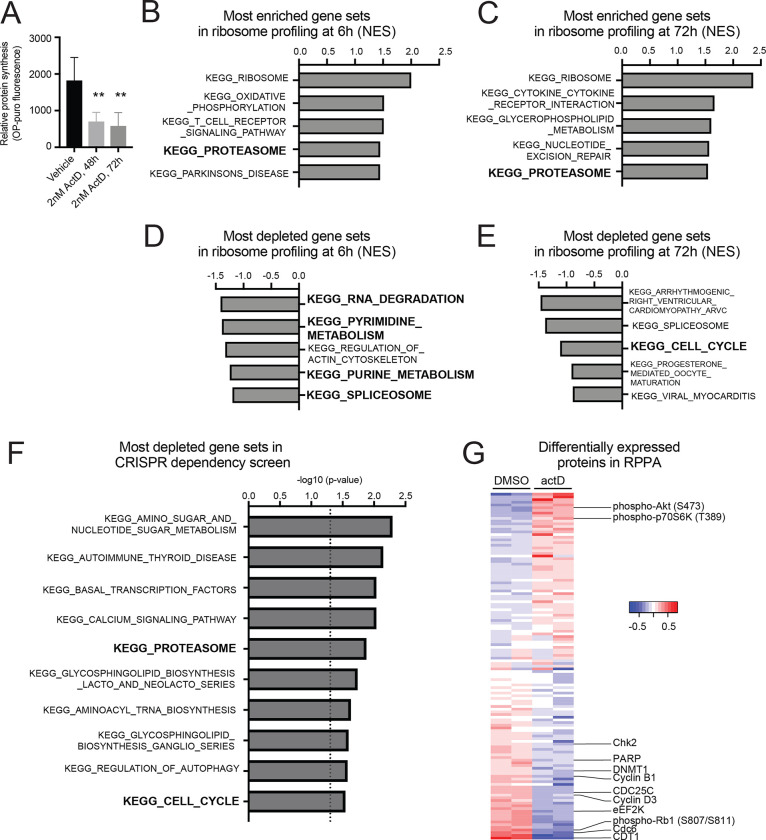
Figure 1. ActD disrupts protein homeostasis in anaplastic Wilms tumor cells.
(A) Quantification of protein synthesis assay (OPP incorporation) in WiT49 cells treated with 48 and 72 hours of 2nM actD or vehicle DMSO.
(B,C) Most enriched KEGG gene sets in ribosome profiling of actD- vs. DMSO-treated WiT49 after 6 hours (B) or 72 hours (C).
(D,E) Most depleted KEGG gene sets in ribosome profiling of actD- vs. DMSO-treated WiT49 after 6 hours (D) or 72 hours (E).
(F) GSEA of genome-wide CRISPR knockout library screen reveals top 10 most significant KEGG canonical pathways that sensitize WiT49 to actD pathways, ranked by p-value. Highlighted are the KEGG_PROTEASOME and KEGG_CELL_CYCLE, where the dotted line represents p-value at 0.05.
(G) Heatmap displaying results from RPPA of actD- versus DMSO-treated WiT49. Normalized, log2-transformed, median-centered values from validated antibodies with standard deviation over 0.1 are shown here.
Thus, to understand how actD affects protein levels and cellular functions in Wilms tumor, we next performed three assays in parallel: ribosome profiling, to identify preferentially translated transcripts; reverse-phase protein arrays (RPPA), to identify differences in protein levels and post-translational modifications; and a CRISPR dropout screen, to identify targetable vulnerabilities.
First, to ascertain preferentially translated transcripts, we performed ribosome profiling in WiT49 cells treated with actD or vehicle control at 6 hours, 72 hours, and two weeks. At each timepoint, we performed ribosome profiling to quantify gene-level differences in translational efficiency in actD- versus vehicle-treated cells. To confirm the expected effect of depleting ribosomes, we examined the locations of the ribosome footprint edges in metagene plots around translation start sites (Supplementary Figure S2A). As expected, in both DMSO- and actD-treated cells, there were essentially no detectable reads in 5’ untranslated regions (UTRs), while coding sequences exhibit a trinucleotide periodicity, reflecting the reading frame of elongating ribosomes. In DMSO-treated cells, the left edge of ribosome footprints accumulated just upstream of translation start sites, which reflects pausing of the ribosome at the initiation site, as expected for normal conditions. In actD-treated cells, however, the initiation site peak was blunted, suggesting that when ribosomes are depleted, the ribosomes that remain spend less time paused at initiation. For all durations of treatment, actD appeared to have minimal effect on the transcriptome (Supplementary Figure S2B). On the other hand, ribosome footprints revealed gross perturbation of translational landscapes by actD (Supplementary Figure S2C). After two weeks of intermittent actD dosing, cells appear to return to a new steady state with globally reduced translation.
We next compared the translational efficiency of each gene in actD-treated cells at 6 and 72 hours, and we connected preferentially translated genes (Supplementary Tables S1, S2) into Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways using GSEA. The most enriched KEGG pathway by translational efficiency at both timepoints was KEGG_RIBOSOME, which is composed of RPs and genes that regulate ribosome biosynthesis (Figures 1B, 1C; Supplementary Figures S3A, S3B; Supplementary Tables S3, S4). This is consistent with reports that RPs are unstable when actD depletes rRNA, and a decrease in ribosomes leads to feedback signaling to upregulate translation of RPs via the mTORC1 pathway 40,41. KEGG_PROTEASOME was the only other gene set in the top five most preferentially translated gene sets at both timepoints (Figure 1B, 1C; Supplementary Figure S3C, S3D; Supplementary Tables S3, S4). On the other hand, the most downregulated gene sets at 6 hours entailed nucleotide turnover, which led to a depletion of cell cycle genes at 72 hours (Figure 1D, 1E; Supplementary Tables S3, S4).
Next, we used a genome-wide CRISPR screen to identify therapeutic vulnerabilities in cells with intermittent actD or DMSO for 14 days. We again used KEGG pathways to categorize dependency genes (Figure 1F; Supplementary Table S5). Here, we again found enrichment for pathways related to protein turnover, including KEGG_PROTEASOME, as well as nucleic acid turnover and cell cycle.
Thirdly, since actD regulates protein synthesis, we also performed RPPA on WiT49 cells treated with actD or DMSO for 72 hours to understand how actD affects protein levels and post-translational modifications (Figure 1G; Supplementary Table S6). We found an increase in phosphorylation of some components of the mTORC1 signaling pathway, which mediates the feedback signaling to upregulate translation of RPs in the setting of rRNA depletion 41. On the other hand, cell cycle markers such as CDT1, CDC6, and phosphorylated RB1 were depleted in actD-treated cells. This is consistent with depletion of cell cycle gene sets in ribosome profiling at 72 hours.
mTORC1 inhibition does not sensitize Wilms tumor cells to actinomycin D
Based on phosphorylation of mTORC1 signaling intermediates in RPPA and prior interest in mTORC1 signaling in Wilms tumor 42,43, we next investigated how actD affected mTORC1 signaling in WiT49 and a second anaplastic Wilms tumor cell line, 17.94. Using Western blots, we confirmed that actD induced phosphorylation of AKT and 4E-BP1 in WiT49 and 17.94 cells (Supplementary Figure S3E). (The phosphorylation of another mTORC1 target, p70S6K, was not clearly upregulated.) We treated WiT49 and 17.94 with rapamycin at doses up to 100 μM and found that this drug confers a more cytostatic rather than cytotoxic effect (Supplemental Figure S3F). We also measured cell viability in combinations of actD and the mTORC1 inhibitor rapamycin using the Loewe independence model (Supplementary Figure S3G). However, the interaction did not show synergy at the serum concentrations of these drugs typically achieved in patients. In other words, mTORC1 inhibition with rapamycin did not appear to sensitize Wilms tumor cells to actD.
ActD induces proteotoxic stress and cell cycle arrest in anaplastic Wilms tumor cells
One effect of mTORC1 signaling is to upregulate translation of RPs 44, and KEGG_RIBOSOME was the most enriched gene set in actD-treated cells. Although actD induced preferential translation of RPs, we found that actD treatment in fact reduced total protein levels of multiple RPs in both WiT49 and 17.94 (Figure 2A). This is consistent with prior reports that excess RP subunits are unstable without rRNA and are degraded by the proteasome to maintain appropriate RP:rRNA stoichiometry 40,45,46. For some RPs, this effect was partially rescued by the proteasome inhibitor bortezomib (BTZ).
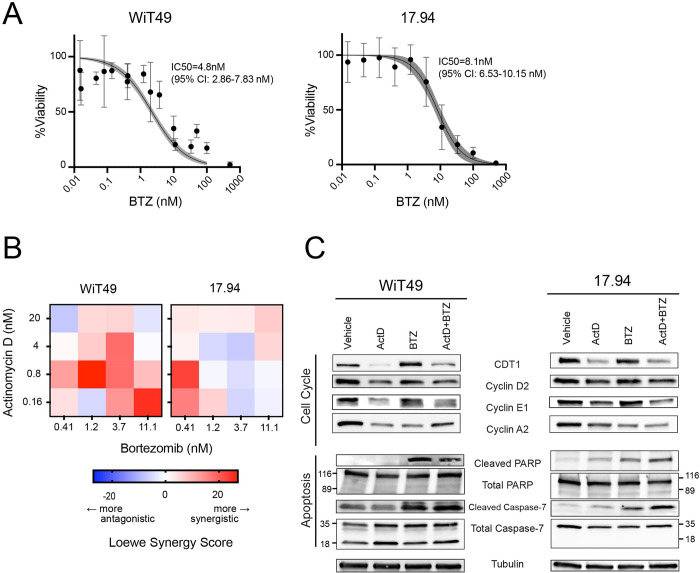
Figure 2. ActD promotes proteasome level and activity in anaplastic Wilms tumor cells.
(A) Western blots for ribosomal protein subunits RPL5, RPL7, RPL11, and RPL26 in WiT49 and 17.94 following 48-hour treatment of DMSO, 2nM actD, 8nM BTZ, or 2nM ActD + 8nM BTZ.
(B) Protein levels of proteasome subunits PSMA6 (α1), PSMB1 (β6), PSMB2 (β4), PSMB5 (β5), and PSMD1 (P112), and POMP in WiT49 and 17.94 following 18-, 24-, 48-, and 72-hour 2nM actD treatment versus vehicle (DMSO).
(C) Quantification of relative proteasome-specific chymotrypsin-like activity in WiT49 and 17.94 cells following 24 hours of DMSO versus 2nM actD, 8nM BTZ, or 2nM actD + 8nM BTZ treatments (Student’s t-test p-value versus vehicle: *<0.05, ** <0.01, ****<0.0001).
On the other hand, preferential translation of proteasome components led to their accumulation. When we exposed WiT49 and 17.94 to actD for up to 72 hours, both cell lines exhibited an appreciable increase in proteasome complex subunits PSMD1 (also known as P112), PSMA6 (subunit α1), PSMB1 (β6), PSMB2 (β4), and PSMB5 (β5), as well as the molecular chaperone for proteasome assembly, POMP, at one or more timepoints (Figure 2B). These increases in protein levels of proteasome components corresponded to increased proteasome chymotrypsin-like enzymatic activity in both cell lines, which was blunted by bortezomib (Figure 2C).
Because actD caused preferential translation of proteasome subunits, we next examined how WiT49 and 17.94 respond to proteasome inhibition. These two cell lines were sensitive to BTZ alone at nanomolar concentrations, in the range of serum levels typically achieved in patients 47,48 (Figure 3A). Furthermore, in this dose range, actD and BTZ synergistically inhibited both WiT49 and 17.94 (Figure 3B). In other words, BTZ could be a way to enhance or restore actD sensitivity in anaplastic Wilms tumors.
Figure 3. BTZ sensitizes anaplastic Wilms tumor cell lines to actD in vitro.
(A) BTZ kill curves for WiT49 and 17.94 with IC50 values indicated (four-point regression line with shaded region representing 95% confidence interval).
(B) Heat maps of Loewe synergy scores for combinations of actD and BTZ in WiT49 and 17.94 cells.
(C) Effect of 48-hour treatment WiT49 and 17.94 cells with DMSO, actD, BTZ, and combination actD + BTZ on cell cycle and apoptosis markers by Western blot.
BTZ has previously been shown to trigger cell death, cell cycle arrest, and autophagy in cancer cell lines 49–54. To understand whether actD and BTZ could cooperate in Wilms tumor, we treated WiT49 and 17.94 with actD and/or BTZ for 48 hours. In both cell lines, actD alone reduces cell cycle markers, including chromatin licensing and DNA replication factor 1 (CDT1), cyclin D2, cyclin E1, and cyclin A2 (Figure 3C) 55–57. These cell cycle regulators are synthesized and degraded in each turn of the cell cycle 58,59. These support our findings from CRISPR screen and protein arrays, which showed that actD induced a dependency on cell cycle genes and led to a fall in cell cycle markers (Figures 1F, 1G) Together, these results suggest that the proteotoxic stress caused by actD triggers cell cycle arrest.
In addition to impaired cell cycle progression, use of the proteasome inhibitor BTZ resulted in the accumulation of apoptotic markers in both cell lines (Figure 3C) 60–63. ActD alone induced cleaved caspase-7 and PARP in 17.94 cells, but even more so in BTZ alone and combination treatment for 17.94 and for WiT49. The combination of these effects supports the potential for using actD and BTZ to target anaplastic Wilms tumors.
Combined treatment of actinomycin D and bortezomib in Wilms tumor xenografts suppresses growth in vivo
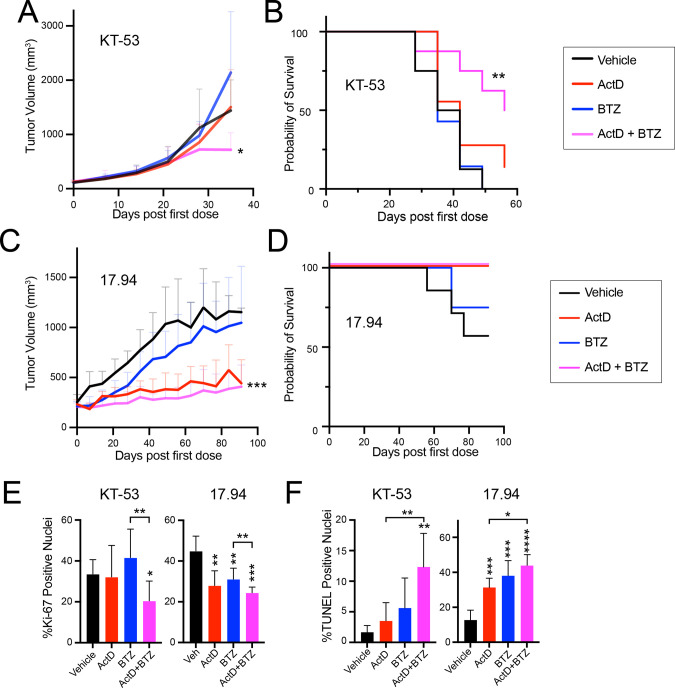
We next examined the effect of combining BTZ with actD against two anaplastic Wilms tumor lines in vivo: cell line-derived xenografts from 17.94 and the TP53 mutated patient-derived xenograft (PDX) line KT-53 35,64. We implanted both lines into NSG mice and treated them with vehicle only, actD, BTZ, or both. We found that combination treatment significantly reduced tumor volume and conferred a significant survival advantage for mice bearing KT-53 xenografts compared to the other three arms (Figure 4A, 4B, Supplementary Figure S4A). Similarly, 17.94 xenografts treated with the combination of actD and BTZ were significantly smaller than those who received vehicle control or BTZ alone (Figure 4C, 4D, Supplementary Figure S4B). (Although it did not reach statistical significance, tumor volume in the combination treatment cohort was also smaller than the actD-only arm.)
Figure 4. BTZ sensitizes subcutaneous anaplastic Wilms tumor xenografts to actD.
(A) Subcutaneous tumor volumes of NSG mice bearing KT-53 xenografts treated with vehicle, actD, BTZ, and combination actD + BTZ (Student’s t-test of endpoint volumes of combination versus vehicle or actD only: * <0.05).
(B) Kaplan-Meier survival curves for KT-53 tumor-bearing mice treated with vehicle, actD only, BTZ only, and combination (log-rank test versus vehicle ** <0.01).
(C) Subcutaneous tumor volumes of NSG mice bearing 17.94 xenografts treated with vehicle, actD only, BTZ only, and combination (Student’s t-test of endpoint volumes of combination versus vehicle: ***<0.001).
(D) Kaplan-Meier survival curves for 17.94 tumor-bearing mice treated with vehicle, actD only, BTZ only, and combination.
(E,F) Quantification of Ki-67 positive nuclei (E) and TUNEL positive nuclei (F) in in KT-53 and 17.94 subcutaneous tumors treated with vehicle, actD only, BTZ only, or combination actD + BTZ (Student’s t-test p-value versus vehicle unless otherwise indicated: * <0.05, **<0.01, ***<0.001, ****<0.0001).
Based on the effects of actD and BTZ on cell cycle and apoptosis we had observed in vitro, we next measured cell cycle and apoptosis markers in these subcutaneous tumors. We used immunohistochemistry for Ki-67 as a marker of proliferation and TUNEL assay as a marker of apoptosis. In both lines, tumors from combination-treated mice had a statistically significant reduction in proliferation compared to vehicle or BTZ alone (Figure 4E, Supplemental Figure S4C, S4D). Similarly, in both lines, combination treatment yielded significantly more apoptosis than vehicle and actD alone (Figure 4F, Supplemental Figure S4E, S4F). Taking these into consideration, we find that the compounding effects of adding BTZ to actD could be a powerful approach to targeting anaplastic Wilms tumor cells.
Proteasome subunit expression levels correlate with outcome
Lastly, we examined expression of proteasome genes in publicly available RNA-seq data from 42 DAWT and 83 relapsed FHWT samples generated by the NCI Therapeutically Applicable Research to Generate Effective Treatments (TARGET) project 8. Compared to FHWT, the single most enriched KEGG gene set in DAWT was KEGG_PROTEASOME (Figure 5A, Supplementary Figure S5A, Supplementary Table S7). Similarly, two of the three most enriched Reactome gene sets were related to APC/C-mediated degradation of cell cycle proteins, suggesting that proteasomal degradation promotes proliferation in DAWT by degrading proteins in each cell cycle (Figure 5B, Supplementary Figure S5B, Supplementary Table S8).
Figure 5. RNA expression of proteasome genes in TARGET favorable and anaplastic histology Wilms tumors.
(A) Top KEGG pathways enriched in RNA-seq of DAWT versus FHWT.
(B) Top reactome pathways enriched in RNA-seq of DAWT versus FHWT.
(C,D) Overall survival of tumors stratified according to expression of proteasome enzymatic subunit genes PSMB1, PSMB2, PSMB5 among patients with favorable histology tumors (C) or anaplastic histology (D). (Log Rank test p-value: *<0.05, ** 0.01)
Next, we examined whether expression of proteasome subunits correlated with outcome in Wilms tumor. We stratified FHWT and DAWT patients into three categories based on high, medium, or low expression of the enzymatic proteasome subunits PSMB5 (β5), PSMB6 (β1), and PSMB7 (β2). Among relapsed FHWT patients, who usually receive actD-based chemotherapy regimens at diagnosis, proteasome-high patients fared far worse than proteasome-low or proteasome-medium patients (Figure 5C). Among DAWT patients, who do not usually receive actD-based therapy regimens, the relationship between proteasome expression and outcome was less evident; proteasome-medium patients fared worse than proteasome-low patients, but proteasome-high patients were not significantly different from proteasome-medium or -low 8,38,39 (Figure 5D). In the entire Wilms tumor cohort, nevertheless, higher proteasome subunit expression correlated with worse survival (Supplementary Figure S5C). Together, these data suggest that proteasome subunit levels may underlie some of the clinical differences between DAWT and FHWT, and that FHWT with high proteasome subunit levels are as aggressive as DAWT. Proteasome inhibition could benefit both subgroups.
DISCUSSION
Developments in Wilms tumor research have illuminated the mutational, epigenetic, mRNA, and miRNA expression landscapes of these tumors, yet relevant targetable vulnerabilities remain elusive 8,65–69. Anaplastic Wilms tumors exhibit relative resistance to conventional chemotherapy, including actD, and little is known about why anaplastic Wilms tumors are resistant to actD or how to overcome such resistance. Furthermore, despite its widespread use, little is known about how actD influences the translational landscape of cancer. Understanding the mechanisms underlying these effects and their consequences could potentially uncover targetable vulnerabilities in relatively chemo-refractory anaplastic Wilms tumors, which could enhance outcomes while minimizing off-target toxicities in survivors. Through several orthogonal approaches, our work reveals that actD disrupts protein homeostasis in Wilms tumor and suggests proteasome inhibition as a potential targeted therapy for inducing actD sensitivity in anaplastic Wilms tumors.
Protein homeostasis involves a synchronized and responsive balance between protein synthesis and degradation, which are both affected by actD. Since protein synthesis is energy-intensive, particularly in rapidly proliferating cancers where proteins like cyclins are continuously synthesized and degraded, maintaining protein homeostasis is crucial to ensure optimal levels of amino acids and other nutrients for growth 70–72. Blocking protein synthesis with actD mimics amino acid starvation, leading cells to upregulate Akt/mTORC1 signaling, which in turn increases the translation of ribosomal protein (RP) genes 44,73–75. Although the mTOR pathway is a potential therapeutic target in Wilms tumor and other cancers 76–78, the combination of actD and rapamycin did not consistently show synergy in vitro for either cell line, and rapamycin alone only exhibited a cytostatic effect. Our cells were in nutrient-rich media which may be compounding to the variables that dictate the effect of rapamycin 77,79, and our results do not rule out the possibility that other mTOR signaling inhibitors could still have potential.
On the other hand, based on our finding that proteasome subunits were preferentially translated after actD exposure, we found that proteasome inhibition with BTZ sensitizes cells to actD in vitro and in vivo. The proteasome is upregulated in response to proteotoxic stresses 45,46,80, and Akt/mTORC1 activates proteasome subunit expression via Nrf1/NFE2L1 81–84 to enhance the intracellular amino acid pool and the unfolded protein response. BTZ was the first proteasome inhibitor approved by the United States Food and Drug Administration 85–87, and it has been tested in pediatric cancer patients 47,88,89. However, other than a study showing a lack of cross-resistance between actD and BTZ in gliobastoma 90, no published studies have explored combinatorial treatment of actD and BTZ to our knowledge. Compellingly, for both KT-53 and 17.94, combination treatment reduced proliferative cells and increased apoptosis. In particular, our study on KT-53 recapitulated a previously demonstrated insensitivity to actD 35, which we found could be overcome by BTZ. Thus, our findings indicate that the combined use of actD and BTZ can be a promising strategy for combating therapy resistance in anaplastic Wilms tumors.
We demonstrated that actD impairs cell cycle progression in vitro and in vivo, which we attribute to dysregulated proteostasis 15,91,92. Normally, D-type cyclin levels accumulate in response to mitogenic signaling, promoting the transcription of S-phase genes such as E-type cyclins and chromatin licensing and DNA replication factor 1 (CDT1) 58,93,94. Consistent with our RPPA in WIT49, we observed in both cell lines that actD reduces Cyclin D2, Cyclin E1, and CDT1 levels, suggesting that actD prevents transition to S-phase, when cyclin A is produced. On the other hand, these proteins are normally targeted for proteasomal destruction by the SKP1-CUL1-F-boc protein (SCF) ubiquitin ligase 95, and indeed, we found that BTZ caused them to accumulate. Moreover, BTZ treatment induced apoptosis in vitro and in vivo, consistent with studies on other cancer cell lines 49–54.
In the TARGET cohort 8, we found that higher levels of proteasome gene expression correlate with anaplastic histology and with poor outcome among favorable-histology Wilms tumors. Studies across other types of cancer show that the association of proteasome activity with prognosis is context-specific 96. Lower expression of proteasome subunit genes was associated with reduced survival in head and neck squamous cell carcinoma 97, while studies in breast cancer, glioma, and hepatocellular carcinoma found that higher expression of proteasome subunit genes is associated with worse survival 98–100. To our knowledge, this is the first study to correlate proteasome expression with worse outcome in Wilms tumor. Anaplastic Wilms tumors are strongly associated with P53 mutation 7,8, and P53 mutation is known to contribute to proteasome subunit overexpression 101. Taken together with our experimental data, these data provide a potential molecular basis to explain the divergent efficacy of actD in FHWT versus DAWT.
To our knowledge, this is the first study to explore the translational landscape of actD treatment and highlight the importance of the proteasome in Wilms tumor. Through in vitro and in vivo models, we propose a model of protein homeostasis-dependent actD sensitivity (Supplementary Figure S5D). Use of actD impairs ribosome biogenesis and cell cycle progression while upregulating proteasome activity. Higher proteasome capacity may help Wilms tumor cells escape chemotherapeutics like actD, and this effect may be reversed with proteasome inhibition. For these reasons, proteasome inhibition could improve outcomes in both favorable-histology and diffusely anaplastic Wilms tumor. Repurposing widely used drugs like BTZ could allow for an accelerated path to clinical translation. This strategy could improve treatment not only for Wilms tumor, but also for other tumors where actD is used, such as rhabdomyosarcoma and Ewing sarcoma.
Supplementary Material
Supplementary Table S1. Average log2-transformed translational efficiency for WiT49 in 6-hour actD versus DMSO treatments derived from ribosome profiling sequencing.
Supplementary Table S2. Average log2-transformed translational efficiency for WiT49 in 72- hour actD versus DMSO treatments derived from ribosome profiling sequencing.
Supplementary Table S3. GSEA of translationally enriched KEGG pathways for WiT49 6-hour actD versus DMSO treatments from ribosome profiling sequencing.
Supplementary Table S4. GSEA of translationally enriched KEGG pathways for WiT49 72-hour actD versus DMSO treatments from ribosome profiling sequencing.
Supplementary Table S5. KEGG pathway enrichment summary table from Mageck-Flute pathway output.
Supplementary Table S6. Normalized, log2-transformed, median-centered RPPA values of validated antibodies from WiT49 cells treated with actD or DMSO.
Supplementary Table S7. GSEA of enriched KEGG pathways in DAWT versus FHWT.
Supplementary Table S8. GSEA of enriched Reactome pathways in DAWT versus FHWT.
ACKNOWLEDGMENTS
This work was supported by funds from the Pablove Foundation (to P.D.B.T.); Alex’s Lemonade Stand Foundation (to K.S.C.); Cancer Prevention and Research Institute of Texas (RP180805 to L.X., RR180071 to K.S.C.); and National Cancer Institute (K08CA207849 to K.S.C., R21CA259771 and P30CA142543 to L.X., and Cancer Center Support Grant P30CA142543). This research used computational resources provided by the BioHPC supercomputing facility in the Lyda Hill Department of Bioinformatics at UT Southwestern, which is supported by Cancer Prevention and Research Institute of Texas (RP150596). The Functional Proteomics Reverse Phase Protein Array Core was supported in part by The University of Texas MD Anderson Cancer Center, P30CA016672 and R50CA221675.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Howlader N, N. A., Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2018, National Cancer Institute, <https://seer.cancer.gov/csr/1975_2018/,> (2018). [Google Scholar]
- 2.Termuhlen A. M. et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 57, 1210–1216 (2011). 10.1002/pbc.23090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrlich P. et al. Results of the First Prospective Multi-institutional Treatment Study in Children With Bilateral Wilms Tumor (AREN0534): A Report From the Children’s Oncology Group. Ann Surg 266, 470–478 (2017). 10.1097/SLA.0000000000002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenendijk A. et al. Prognostic Factors for Wilms Tumor Recurrence: A Review of the Literature. Cancers (Basel) 13 (2021). 10.3390/cancers13133142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brok J. et al. Unmet needs for relapsed or refractory Wilms tumour: Mapping the molecular features, exploring organoids and designing early phase trials - A collaborative SIOP-RTSG, COG and ITCC session at the first SIOPE meeting. Eur J Cancer 144, 113–122 (2021). 10.1016/j.ejca.2020.11.012 [DOI] [PubMed] [Google Scholar]
- 6.Daw N. C. et al. Activity of Vincristine and Irinotecan in Diffuse Anaplastic Wilms Tumor and Therapy Outcomes of Stage II to IV Disease: Results of the Children’s Oncology Group AREN0321 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 38, 1558–1568 (2020). 10.1200/JCO.19.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooms A. H. et al. Significance of TP53 Mutation in Wilms Tumors with Diffuse Anaplasia: A Report from the Children’s Oncology Group. Clinical cancer research : an official journal of the American Association for Cancer Research 22, 5582–5591 (2016). 10.1158/1078-0432.CCR-16-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadd S. et al. A Children’s Oncology Group and TARGET initiative exploring the genetic landscape of Wilms tumor. Nature genetics 49, 1487–1494 (2017). 10.1038/ng.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macmahon H. E., Bedizel M. & Ellis C. A. Vincristine (Leurocristine) Sulfate in the Treatment of Children with Metastatic Wilms’ Tumor. Pediatric Division, Southwest Cancer Chemotherapy Group. Pediatrics 32, 880–887 (1963). [PubMed] [Google Scholar]
- 10.Windmiller J., Berry D. H., Haddy T. B., Vietti T. J. & Sutow W. W. Vincristine sulfate in the treatment of neuroblastoma in children. Am J Dis Child 111, 75–78 (1966). 10.1001/archpedi.1966.02090040111014 [DOI] [PubMed] [Google Scholar]
- 11.Gaspar N. et al. Ewing Sarcoma: Current Management and Future Approaches Through Collaboration. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 33, 3036–3046 (2015). 10.1200/JCO.2014.59.5256 [DOI] [PubMed] [Google Scholar]
- 12.Miwa S. et al. Recent Advances and Challenges in the Treatment of Rhabdomyosarcoma. Cancers (Basel) 12 (2020). 10.3390/cancers12071758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Angio G. J. The National Wilms Tumor Study: a 40 year perspective. Lifetime Data Anal 13, 463–470 (2007). 10.1007/s10985-007-9062-0 [DOI] [PubMed] [Google Scholar]
- 14.Veal G. J. et al. Pharmacokinetics of dactinomycin in a pediatric patient population: a United Kingdom Children’s Cancer Study Group Study. Clinical cancer research : an official journal of the American Association for Cancer Research 11, 5893–5899 (2005). 10.1158/1078-0432.CCR-04-2546 [DOI] [PubMed] [Google Scholar]
- 15.Walsh C. et al. Development of a physiologically based pharmacokinetic model of actinomycin D in children with cancer. Br J Clin Pharmacol 81, 989–998 (2016). 10.1111/bcp.12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry R. P. Selective effects of actinomycin D on the intracellular distribution of RNA synthesis in tissue culture cells. Experimental Cell Research 29, 400–406 (1963). [Google Scholar]
- 17.Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550 (2005). 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schöfer C., Weipoltshammer K., Almeder M., Muller M. & Wachtler F. Redistribution of ribosomal DNA after blocking of transcription induced by actinomycin D. Chromosome Res 4, 384–391 (1996). 10.1007/BF02257274 [DOI] [PubMed] [Google Scholar]
- 19.Mills E. W. & Green R. Ribosomopathies: There’s strength in numbers. Science 358 (2017). 10.1126/science.aan2755 [DOI] [PubMed] [Google Scholar]
- 20.Schindelin J. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682 (2012). 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ju Z. et al. Development of a robust classifier for quality control of reverse-phase protein arrays. Bioinformatics (Oxford, England) 31, 912–918 (2015). 10.1093/bioinformatics/btu736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shehwana H. et al. RPPA SPACE: an R package for normalization and quantitation of Reverse-Phase Protein Array data. Bioinformatics (Oxford, England) 38, 5131–5133 (2022). 10.1093/bioinformatics/btac665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siwak D. R., Li J., Akbani R., Liang H. & Lu Y. Analytical Platforms 3: Processing Samples via the RPPA Pipeline to Generate Large-Scale Data for Clinical Studies. Adv Exp Med Biol 1188, 113–147 (2019). 10.1007/978-981-32-9755-5_7 [DOI] [PubMed] [Google Scholar]
- 24.Hoff F. W., Lu Y. & Kornblau S. M. Antibody Screening. Adv Exp Med Biol 1188, 149–163 (2019). 10.1007/978-981-32-9755-5_8 [DOI] [PubMed] [Google Scholar]
- 25.McGlincy N. J. & Ingolia N. T. Transcriptome-wide measurement of translation by ribosome profiling. Methods 126, 112–129 (2017). 10.1016/j.ymeth.2017.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingolia N. T., Brar G. A., Rouskin S., McGeachy A. M. & Weissman J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nature protocols 7, 1534 (2012). https://doi.org:10.1038/nprot.2012.086 https://www.nature.com/articles/nprot.2012.086#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D., Paggi J. M., Park C., Bennett C. & Salzberg S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37, 907–915 (2019). 10.1038/s41587-019-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pertea M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33, 290–295 (2015). 10.1038/nbt.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberzon A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425 (2015). 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mootha V. K. et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature genetics 34, 267–273 (2003). 10.1038/ng1180 [DOI] [PubMed] [Google Scholar]
- 31.Korotkevich G. et al. Fast gene set enrichment analysis. bioRxiv, 060012 (2021). 10.1101/060012 [DOI] [Google Scholar]
- 32.Doench J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol 34, 184–191 (2016). 10.1038/nbt.3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B. et al. Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nature protocols 14, 756–780 (2019). 10.1038/s41596-018-0113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng S. et al. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genomics Proteomics Bioinformatics 20, 587–596 (2022). 10.1016/j.gpb.2022.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy A. J. et al. Forty-five patient-derived xenografts capture the clinical and biological heterogeneity of Wilms tumor. Nature communications 10, 5806 (2019). 10.1038/s41467-019-13646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perry R. P. & Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol 76, 127–139 (1970). 10.1002/jcp.1040760202 [DOI] [PubMed] [Google Scholar]
- 37.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550 (2014). 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerami E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404 (2012). 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao J. J. et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci Signal 6 (2013). https://doi.org:ARTN pl1 DOI 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam Y. W., Lamond A. I., Mann M. & Andersen J. S. Analysis of nucleolar protein dynamics reveals the nuclear degradation of ribosomal proteins. Curr Biol 17, 749–760 (2007). 10.1016/j.cub.2007.03.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R. et al. Impairing the production of ribosomal RNA activates mammalian target of rapamycin complex 1 signalling and downstream translation factors. Nucleic Acids Res 42, 5083–5096 (2014). 10.1093/nar/gku130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C. C. et al. Predicting relapse in favorable histology Wilms tumor using gene expression analysis: a report from the Renal Tumor Committee of the Children’s Oncology Group. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 1770–1778 (2009). 10.1158/1078-0432.CCR-08-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flores L. G. 2nd, et al. Monitoring therapy with MEK inhibitor U0126 in a novel Wilms tumor model in Wt1 knockout Igf2 transgenic mice using 18F-FDG PET with dual-contrast enhanced CT and MRI: early metabolic response without inhibition of tumor growth. Mol Imaging Biol 15, 175–185 (2013). 10.1007/s11307-012-0588-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iadevaia V., Liu R. & Proud C. G. mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin Cell Dev Biol 36, 113–120 (2014). 10.1016/j.semcdb.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 45.Sung M. K. et al. A conserved quality-control pathway that mediates degradation of unassembled ribosomal proteins. Elife 5 (2016). 10.7554/eLife.19105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung M. K., Reitsma J. M., Sweredoski M. J., Hess S. & Deshaies R. J. Ribosomal proteins produced in excess are degraded by the ubiquitin-proteasome system. Mol Biol Cell 27, 2642–2652 (2016). 10.1091/mbc.E16-05-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanley M. J. et al. Population Pharmacokinetic Analysis of Bortezomib in Pediatric Leukemia Patients: Model-Based Support for Body Surface Area-Based Dosing Over the 2- to 16-Year Age Range. J Clin Pharmacol 57, 1183–1193 (2017). 10.1002/jcph.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreau P. et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin Pharmacokinet 51, 823–829 (2012). 10.1007/s40262-012-0010-0 [DOI] [PubMed] [Google Scholar]
- 49.Gupta I., Singh K., Varshney N. K. & Khan S. Delineating Crosstalk Mechanisms of the Ubiquitin Proteasome System That Regulate Apoptosis. Front Cell Dev Biol 6, 11 (2018). 10.3389/fcell.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ling Y. H. et al. Mechanisms of proteasome inhibitor PS-341-induced G(2)-M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clinical cancer research : an official journal of the American Association for Cancer Research 9, 1145–1154 (2003). [PubMed] [Google Scholar]
- 51.Nawrocki S. T. et al. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol Cancer Ther 1, 1243–1253 (2002). [PubMed] [Google Scholar]
- 52.Selimovic D. et al. Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells. Cell Signal 25, 308–318 (2013). 10.1016/j.cellsig.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 53.Bao X. et al. Bortezomib induces apoptosis and suppresses cell growth and metastasis by inactivation of Stat3 signaling in chondrosarcoma. Int J Oncol 50, 477–486 (2017). 10.3892/ijo.2016.3806 [DOI] [PubMed] [Google Scholar]
- 54.Lou Z. et al. Bortezomib induces apoptosis and autophagy in osteosarcoma cells through mitogen-activated protein kinase pathway in vitro. J Int Med Res 41, 1505–1519 (2013). 10.1177/0300060513490618 [DOI] [PubMed] [Google Scholar]
- 55.Hofmann J. F. & Beach D. cdt1 is an essential target of the Cdc10/Sct1 transcription factor: requirement for DNA replication and inhibition of mitosis. EMBO J 13, 425–434 (1994). 10.1002/j.1460-2075.1994.tb06277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fagundes R. & Teixeira L. K. Cyclin E/CDK2: DNA Replication, Replication Stress and Genomic Instability. Front Cell Dev Biol 9, 774845 (2021). 10.3389/fcell.2021.774845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caldon C. E. & Musgrove E. A. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div 5, 2 (2010). 10.1186/1747-1028-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pozo P. N. & Cook J. G. Regulation and Function of Cdt1; A Key Factor in Cell Proliferation and Genome Stability. Genes (Basel) 8 (2016). 10.3390/genes8010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez-Alonso D. & Malumbres M. Mammalian cell cycle cyclins. Semin Cell Dev Biol 107, 28–35 (2020). 10.1016/j.semcdb.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 60.Kaufmann S. H., Desnoyers S., Ottaviano Y., Davidson N. E. & Poirier G. G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 53, 3976–3985 (1993). [PubMed] [Google Scholar]
- 61.Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res 49, 5870–5878 (1989). [PubMed] [Google Scholar]
- 62.Boulares A. H. et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 274, 22932–22940 (1999). 10.1074/jbc.274.33.22932 [DOI] [PubMed] [Google Scholar]
- 63.Rager J. E. in Systems Biology in Toxicology and Environmental Health 187–205 (2015). [Google Scholar]
- 64.Stewart E. et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature 549, 96–100 (2017). 10.1038/nature23647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walz A. L. et al. Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer cell 27, 286–297 (2015). 10.1016/j.ccell.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wegert J. et al. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer cell 27, 298–311 (2015). 10.1016/j.ccell.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 67.Torrezan G. T. et al. Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nature communications 5, 4039 (2014). 10.1038/ncomms5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakheja D. et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nature communications 2, 4802 (2014). 10.1038/ncomms5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tiburcio P. D. B. et al. DROSHA regulates mesenchymal gene expression in Wilms tumor. Mol Cancer Res (2024). 10.1158/1541-7786.MCR-23-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthews H. K., Bertoli C. & de Bruin R. A. M. Cell cycle control in cancer. Nat Rev Mol Cell Biol 23, 74–88 (2022). 10.1038/s41580-021-00404-3 [DOI] [PubMed] [Google Scholar]
- 71.Zou T. & Lin Z. The Involvement of Ubiquitination Machinery in Cell Cycle Regulation and Cancer Progression. Int J Mol Sci 22 (2021). 10.3390/ijms22115754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alber A. B. & Suter D. M. Dynamics of protein synthesis and degradation through the cell cycle. Cell Cycle 18, 784–794 (2019). 10.1080/15384101.2019.1598725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayer C., Zhao J., Yuan X. & Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev 18, 423–434 (2004). 10.1101/gad.285504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayer C. & Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25, 6384–6391 (2006). 10.1038/sj.onc.1209883 [DOI] [PubMed] [Google Scholar]
- 75.Ni C. & Buszczak M. The homeostatic regulation of ribosome biogenesis. Semin Cell Dev Biol 136, 13–26 (2023). 10.1016/j.semcdb.2022.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hua H. et al. Targeting mTOR for cancer therapy. J Hematol Oncol 12, 71 (2019). 10.1186/s13045-019-0754-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saxton R. A. & Sabatini D. M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976 (2017). 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou Z., Tao T., Li H. & Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 10, 31 (2020). 10.1186/s13578-020-00396-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park S. H., Choi W. H. & Lee M. J. Effects of mTORC1 inhibition on proteasome activity and levels. BMB Rep 55, 161–165 (2022). 10.5483/BMBRep.2022.55.4.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tye B. W. et al. Proteotoxicity from aberrant ribosome biogenesis compromises cell fitness. Elife 8 (2019). 10.7554/eLife.43002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y. & Manning B. D. mTORC1 signaling activates NRF1 to increase cellular proteasome levels. Cell Cycle 14, 2011–2017 (2015). 10.1080/15384101.2015.1044188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y. et al. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 513, 440–443 (2014). 10.1038/nature13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao J., Zhai B., Gygi S. P. & Goldberg A. L. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci U S A 112, 15790–15797 (2015). 10.1073/pnas.1521919112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaiser M. S. et al. Dual roles of mTORC1-dependent activation of the ubiquitin-proteasome system in muscle proteostasis. Commun Biol 5, 1141 (2022). 10.1038/s42003-022-04097-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen D., Frezza M., Schmitt S., Kanwar J. & Dou Q. P. Bortezomib as the First Proteasome Inhibitor Anticancer Drug: Current Status and Future Perspectives. Current Cancer Drug Targets 11, 239–253 (2011). 10.2174/156800911794519752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leonardo-Sousa C. et al. Revisiting Proteasome Inhibitors: Molecular Underpinnings of Their Development, Mechanisms of Resistance and Strategies to Overcome Anti-Cancer Drug Resistance. Molecules 27 (2022). 10.3390/molecules27072201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu S. & Wang J. The resistance mechanisms of proteasome inhibitor bortezomib. Biomark Res 1, 13 (2013). 10.1186/2050-7771-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.August K. J., Guest E. M., Lewing K., Hays J. A. & Gamis A. S. Treatment of children with relapsed and refractory acute lymphoblastic leukemia with mitoxantrone, vincristine, pegaspargase, dexamethasone, and bortezomib. Pediatr Blood Cancer 67, e28062 (2020). 10.1002/pbc.28062 [DOI] [PubMed] [Google Scholar]
- 89.Teachey D. T. et al. Children’s Oncology Group Trial AALL1231: A Phase III Clinical Trial Testing Bortezomib in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia and Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 40, 2106–2118 (2022). 10.1200/JCO.21.02678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Styczynski J., Olszewska-Slonina D., Kolodziej B., Napieraj M. & Wysocki M. Activity of bortezomib in glioblastoma. Anticancer Res 26, 4499–4503 (2006). [PubMed] [Google Scholar]
- 91.Chang D., Chen F., Zhang F., McKay B. C. & Ljungman M. Dose-dependent effects of DNA-damaging agents on p53-mediated cell cycle arrest. Cell Growth Differ 10, 155–162 (1999). [PubMed] [Google Scholar]
- 92.Chen C. S. et al. AKT mediates actinomycin D-induced p53 expression. Oncotarget 5, 693–703 (2014). 10.18632/oncotarget.1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang V. W. in Physiology of the Gastrointestinal Tract 197–219 (2018). [Google Scholar]
- 94.Santo L., Siu K. T. & Raje N. Targeting Cyclin-Dependent Kinases and Cell Cycle Progression in Human Cancers. Semin Oncol 42, 788–800 (2015). 10.1053/j.seminoncol.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 95.Chu C., Geng Y., Zhou Y. & Sicinski P. Cyclin E in normal physiology and disease states. Trends Cell Biol 31, 732–746 (2021). 10.1016/j.tcb.2021.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voutsadakis I. A. Proteasome expression and activity in cancer and cancer stem cells. Tumour Biol 39, 1010428317692248 (2017). 10.1177/1010428317692248 [DOI] [PubMed] [Google Scholar]
- 97.Lagadec C. et al. Tumor cells with low proteasome subunit expression predict overall survival in head and neck cancer patients. BMC Cancer 14, 152 (2014). 10.1186/1471-2407-14-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Byers H. A. et al. Evaluation of the NRF1-proteasome axis as a therapeutic target in breast cancer. Sci Rep 13, 15843 (2023). 10.1038/s41598-023-43121-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He W. et al. PSMB2 plays an oncogenic role in glioma and correlates to the immune microenvironment. Sci Rep 14, 5861 (2024). 10.1038/s41598-024-56493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J., Mi J., Liu S., Chen H. & Jiang L. PSMB5 overexpression is correlated with tumor proliferation and poor prognosis in hepatocellular carcinoma. FEBS Open Bio 12, 2025–2041 (2022). 10.1002/2211-5463.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walerych D. et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat Cell Biol 18, 897–909 (2016). 10.1038/ncb3380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Average log2-transformed translational efficiency for WiT49 in 6-hour actD versus DMSO treatments derived from ribosome profiling sequencing.
Supplementary Table S2. Average log2-transformed translational efficiency for WiT49 in 72- hour actD versus DMSO treatments derived from ribosome profiling sequencing.
Supplementary Table S3. GSEA of translationally enriched KEGG pathways for WiT49 6-hour actD versus DMSO treatments from ribosome profiling sequencing.
Supplementary Table S4. GSEA of translationally enriched KEGG pathways for WiT49 72-hour actD versus DMSO treatments from ribosome profiling sequencing.
Supplementary Table S5. KEGG pathway enrichment summary table from Mageck-Flute pathway output.
Supplementary Table S6. Normalized, log2-transformed, median-centered RPPA values of validated antibodies from WiT49 cells treated with actD or DMSO.
Supplementary Table S7. GSEA of enriched KEGG pathways in DAWT versus FHWT.
Supplementary Table S8. GSEA of enriched Reactome pathways in DAWT versus FHWT.
Data Availability Statement
Ribosome profiling and accompanying RNA-seq data from WiT49 are deposited in the NCBI GEO database under accession GSE270330.