Abstract
The physiological response of a cell to stimulation depends on its proteome configuration. Therefore, the abundance variation of regulatory proteins across unstimulated single cells can be associatively linked with their response to stimulation. Here we developed an approach that leverages this association across individual cells and nuclei to systematically identify potential regulators of biological processes, followed by targeted validation. Specifically, we applied this approach to identify regulators of nucleocytoplasmic protein transport in macrophages stimulated with lipopolysaccharide (LPS). To this end, we quantified the proteomes of 3,412 individual nuclei, sampling the dynamic response to LPS treatment, and linking functional variability to proteomic variability. Minutes after the stimulation, the protein transport in individual nuclei correlated strongly with the abundance of known protein transport regulators, thus revealing the impact of natural protein variability on functional cellular response. We found that simple biophysical constraints, such as the quantity of nuclear pores, partially explain the variability in LPS-induced nucleocytoplasmic transport. Among the many proteins newly identified to be associated with the response, we selected 16 for targeted validation by knockdown. The knockdown phenotypes confirmed the inferences derived from natural protein and functional variation of single nuclei, thus demonstrating the potential of (sub-)single-cell proteomics to infer functional regulation. We expect this approach to generalize to broad applications and enhance the functional interpretability of single-cell omics data.
Introduction
Single-cell omics methods have rapidly scaled up1,2 and enabled the investigation of cellular heterogeneity and the creation of cell atlases3. However, the functional interpretation of such omics data has lagged behind4–7. To investigate the link between proteomic and functional variability across single-cells, we sought to develop an approach that associates the pre-existing differences in proteome configurations to their correspondingly variable cellular responses; such an approach may enable the inference of novel regulatory associations8. In particular, we aimed to investigate how pre-existing proteomic variability influences, and therefore explains the variability of, LPS-induced transport of proteins to and from the nucleus.
To quantify this variation between individual cells and individual nuclei, we built upon the conceptual and technological advances in single-cell mass spectrometry proteomics9–21. Specifically, we used the framework of multiplexed data-independent acquisition (plexDIA) to implement the isotopologous carrier suggestion22,23, which generalizes the concept of isobaric carrier24,25 to plex-DIA. Isotopologous carriers have already shown promise in some applications18,26,27, and here we used them to enable the first proteomic analysis of individual organelles isolated from human cells. These methodological advances enabled global exploration of protein transport within individual cells, which for decades has been studied by imaging fluorescent proteins28–32. Our approach allows for more comprehensive initial discovery, which can expand previous observations that pro-inflammatory stimulation leads to heterogeneous nuclear import of transcription factors33,34. These discoveries can later be examined with much higher temporal resolution using fluorescent imaging35,36.
Here, we demonstrate a generalizable approach that enables the inference of functional regulators from (sub-)single-cell proteomics data. Specifically, we identified proteins regulating subcellular transport, including the differential contributions of the subunits of the nuclear pore complex. Our inferences were derived from natural protein variation across single cells and nuclei. Subsequent validation of these results by targeted knockdown experiments provide direct evidence for the functional relevance of our inferential approach.
Results
Protein transport in bulk macrophage populations
To first study protein transport between the nucleus and the rest of the cell in bulk populations, six biological replicates were generated using a model system of macrophage-like cells derived from THP-1 monocytes treated with phorbol 12-myristate 13-acetate (PMA). Their nuclei were extracted with mild detergent using methods adapted from an established workflow37. This physical isolation produced sufficient nuclear enrichment as demonstrated by a 5 – 25-fold depletion of proteins from non-nuclear cellular compartments from the nuclei, Extended Data Fig. 1a.
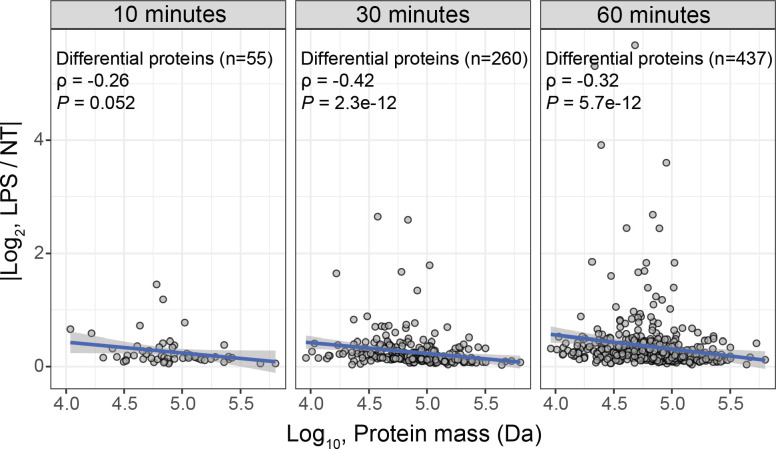
Having validated the enrichment of the isolated nuclear fractions, we next sought to quantify the LPS-induced dynamics of protein transport. To this end, we quantified differential protein abundances for nuclei and whole-cells using MS-EmpiRe38. The abundance changes in the whole cells reflect protein synthesis, degradation, and secretion; changes at the nuclear level also include nucleocytoplasmic transport. As expected, changes mediated by protein synthesis and degradation are slow compared to the kinetics of nucleocytoplasmic transport. Indeed, 60 minutes after LPS-stimulation, the changes are dominated by transport with 15.7% of nuclear proteome exhibiting differential abundance compared to only 2.3% of the whole-cell proteome, Fig. 1a. This difference is even more pronounced for earlier time points where changes affect less than 0.1% of the whole-cell proteome 30 minutes after LPS stimulation, but 9.3% of the nuclear proteome.
Figure 1 |. Protein dynamics in whole cells and nuclei reveal coordinated spatiotemporal control of biological processes in bulk populations of macrophage-like cells.
a Percent of differentially abundant proteins (5% FDR) in nuclear (grey) and whole-cell (black) proteomes after 10, 30, or 60 minute LPS treatments. b Differentially abundant proteins in the nucleus were analyzed to investigate the relationship between protein mass and transport kinetics. Transport dynamics for each protein were interpolated across the four time points, and ordered with respect to protein mass. The heatmap is colored according to each protein’s absolute-valued magnitude of protein transport. Using a moving median approach to average the inherent biological variability, the data suggest smaller proteins achieve half of their total transport at earlier time points than larger proteins. The dashed black line marks a commonly recognized theoretical limit of passive diffusion (40 kDa). c Time-series data over the course of the LPS-treatment for differentially abundant proteins in the nucleus; pro-inflammatory transcription factors are labeled and highlighted in red, and other proteins with >4-fold change are highlighted in blue. d Protein set enrichment analysis of nuclei and whole-cell bulk samples for NT, 10 minute, 30 minute, and 60 minute LPS treated samples. Brackets correspond to the number of proteins included in computing the enrichment of Gene Ontology terms. e Gene Ontology terms associated with mediating gene-expression, which significantly change in nuclear abundance in response to LPS, are plotted. The 95% confidence intervals are derived from the change of all proteins which correspond to the respective Gene Ontology term. f Proteins were grouped thematically and plotted to show their change in nuclear abundance; all proteins were differentially abundant at 5% FDR in at least one time point. g Nucleoporins which were differentially abundant in the nucleus in at least one time point are plotted to display their change in nuclear and whole-cell abundance; TPR is highlighted in red.
Given that hundreds of proteins without innate immunity associations significantly changed their nuclear abundance in response to LPS, we aimed to evaluate the temporal continuity of each protein to further assess the confidence in these findings. As a quantifiable continuity metric, we calculated a ranked version of von Neumann’s ratio (RVN)39 for each protein as shown in Extended Data Fig. 2a. Indeed, proteins found to be differentially abundant at 5% FDR for nuclei and whole-cells were mostly monotonic and generally continuous, beyond the monotonicity of a simulated null model where the same number of proteins were assigned random fold-changes and RVN ratios were calculated, Extended Data Fig. 2b,c. Therefore, the large proportion (~16%) of nuclear proteins changing significantly in response to LPS exhibit mostly continuous dynamics, which supports the validity of the findings.
Mass dependence of nucleocytoplasmic transport
The kinetics of nucleocytoplasmic transport is mass-dependent, and we sought to investigate this dependence in our data. There are two mechanisms by which transport occurs: passive diffusion and active transport40,41. Molecules with masses less than 40 kDa have been reported to passively diffuse through nuclear pores with kinetics that are mass-dependent42. Kinetics of active transport are also dependent on the size of the cargo, but to a lesser degree, as the energy barrier is reduced through interactions with chaperones40,43. We investigated this relationship in our data and found that protein transport was indeed negatively correlated with protein mass, as shown in Extended Data Fig. 3. To increase the time resolution of this analysis, we interpolated protein transport across the 4 time points and found that smaller proteins achieved half their total transport at an earlier time than larger proteins, as shown as a heatmap and scatter plot in Fig. 1b. While this analysis cannot conclude whether these trends reflect passive diffusion or simply mass-dependence in active transport, the results are consistent with more recent findings of passive diffusion occurring without a precise size threshold44. Irrespective of the mechanism, these data corroborate the mass-dependence of protein transport kinetics globally in the natural response of macrophages to LPS.
Spatiotemporal control of inflammatory response in bulk cell populations
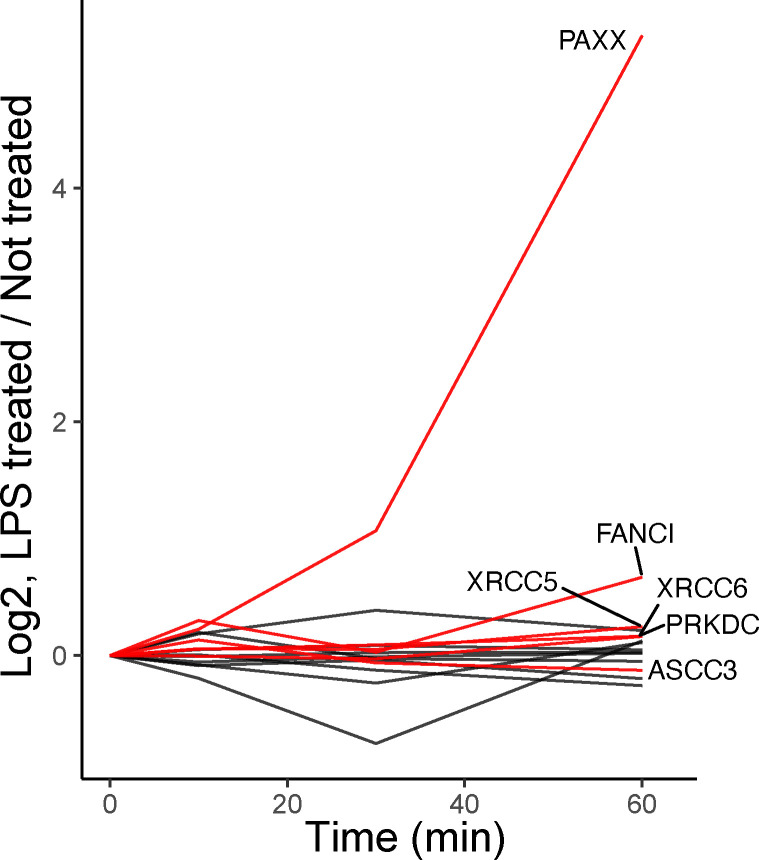
While pro-inflammatory transcription factors are known to be imported to the nucleus in response to LPS, we identify hundreds of additional proteins significantly changing in nuclear abundance, many of which have no prior association with LPS-response. As expected, transcription factors NF-κB1, REL, RELA, and FOS increase in nuclear abundance by approximately 4-fold within 10–60 minutes of LPS-treatment29,32,33,45, as shown in Fig. 1c. Interestingly, hundreds of additional proteins experience monotonic or generally continuous changes in nuclear abundance; one of these proteins, PAXX––a protein required for Non-Homologous End-Joining (NHEJ) DNA repair46,47, reached a 40-fold increase within 60 minutes of LPS-stimulation. This protein acts as a scaffold for the accumulation of XRCC5 and XRCC6 to facilitate NHEJ DNA repair48. Likewise, XRCC5 and XRCC6 also increased significantly in the nucleus by 60 minutes, Extended Data Fig. 4.
To characterize the effects of protein synthesis, degradation, and secretion, we computed protein set enrichment on whole-cell proteomes; similar analysis of nuclear proteomes would additionally reflect nucleocytoplasmic transport, Fig. 1d. LPS-stimulation induced whole-cell proteome enrichment of Gene Ontology (GO) terms associated with gene-expression, such as “translation,” “spliceosomal complex,” and “ribosome biogenesis.” Interestingly, changes to the nuclear proteome revealed spatiotemporal changes consistent with this whole-cell enrichment; specifically GO terms whose sites of action are in the nucleus, such as “general transcription initiation factor activity” and “ribosome biogenesis” increased in nuclear abundance, while GO terms whose sites of action are outside of the nucleus, such as “cytosolic ribosome” decreased in nuclear abundance. These changes which reflect coordinated spatiotemporal rearragment of proteins involved in mediating gene-expression are shown for five GO terms in Fig. 1e. Together, the whole-cell and nuclear proteomic data provide complementary evidence which suggests macrophages increase their capacity to mediate gene-expression in response to LPS, as observed through increases in the absolute abundances of and the spatiotemporal rearrangement of proteins involved in this process.
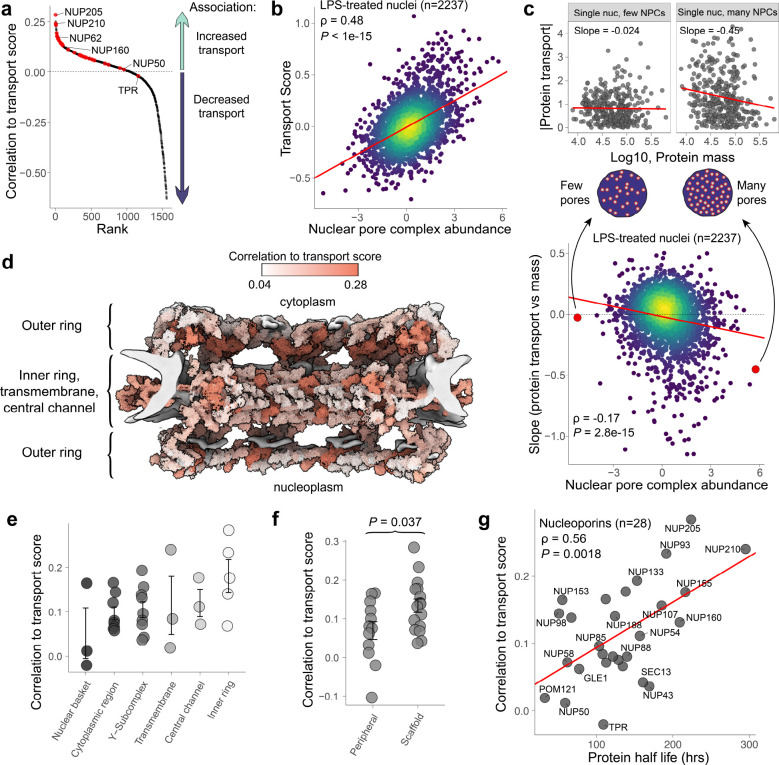
To more closely investigate the concordance of changes in nuclear biological processes, differentially abundant proteins were grouped thematically by function and plotted in Fig. 1f. We found concerted dynamics for proteasomal subunits and proteins associated with stress granule assembly, which decreased in nuclear abundance in response to LPS. Proteins associated with DNA repair, RNA processing, and nucleoporins were generally found to increase in nuclear abundance following LPS stimulation. Intriguingly, of all nucleoporins which changed significantly in nuclear abundance, only TPR––a negative regulator of NPC assembly49––decreased, Fig. 1g. Whole-cell abundances of the same nucleoporins were generally found to increase, including TPR. Taken together, these findings are consistent with LPS-induced upregulation of NPC assembly.
Benchmarking protein quantification of single nuclei
Using natural variation across single-cells to identify regulators of protein transport requires accurate quantification of many proteins in single nuclei. To accomplish this, we used highly parallel sample preparation by nPOP19,50 and the plexDIA framework combined with an isotopologous carrier. This methodology is analogous to isobaric SCoPE-MS15,24,51, thus we term it SCoPE-DIA (Single Cell ProtEomics by Data-Independent Acquisition). As we previously suggested and demonstrated with plexDIA, this framework uses the chromatographic coelution of peptides from different samples to increase data-completeness22,23. Thus, protein identification across all samples can be enhanced by including a highly abundant sample, e.g., an isotopologous carrier, in parallel to single-cells or single-organelles. This benefit was evident with the introduction of plexDIA22 and has since been reproduced for single-cell analysis18. However, complex isotopologous carriers might increase interferences and thus decrease quantitative accuracy. To evaluate these trade-offs and find an optimal carrier level for acquiring single-nucleus data, we created and acquired data from a mixed-species spike-in of H. sapiens nuclei and S. cerevisiae run with 0x, 1x, 5x, 10x, 25x, or 50x carrier amounts, as shown in Extended Data Fig. 5a. We found that intersected protein-level quantitation was comparable across all carrier-levels, Extended Data Fig. 5b. Thus, the potential for interference by larger carriers has little effect on quantitative accuracy in our experiments, which is likely due to the lower proteomic complexity of nuclei as compared to whole cells.
To improve quantitative accuracy, the carrier channel was used as a reference to estimate quantitative compression and remove poorly quantified precursors similar to previous filters15. We applied this filter at various levels to converge upon an optimal balance between coverage and accuracy, Extended Data Fig. 5c–e. Using this strategy, we compared the coverage and quantitative accuracy and for all proteins identified at various carrier levels and found reduced quantitative accuracy at higher carrier levels, Extended Data Fig. 5f. This likely reflects the increasing proportion of lowly abundant proteins, which naturally suffer from noisier quantitation. Indeed, the quantitative accuracy for the subset of proteins quantified across all carrier levels is high even for the 50x carrier, Extended Data Fig. 5b. To summarize, large nuclear carriers were found to not worsen quantitative accuracy, but rather enable quantification of additional lowly abundant proteins that are naturally less well-quantified. Therefore, we chose to acquire single-nucleus data with 25x and 50x nuclear proteome carriers.
Variability in LPS-induced nucleocytoplasmic transport across single nuclei
Next, we sought to infer potential regulators of nucleocytoplasmic transport from the natural variability of macrophage proteomes prior to the LPS stimulation and its regulatory impact on the LPS response. Using SCoPE-DIA, we acquired a dataset consisting of 3,412 nuclei across four biological replicates, with a median of 1,366 proteins quantified per nucleus, Fig. 2a. After filtering to remove nuclei and precursors with poor quantitation (Extended Data Fig. 6a–b), and nuclei with insufficient nuclear enrichment (Extended Data Fig. 6c–d), we retained 2,997 nuclei with a median of 1,287 proteins per nucleus to be used in downstream analyses, Fig. 2a.
Figure 2 |. Associating single-nucleus proteomic variability to heterogeneous LPS-induced nucleocytoplasmic protein transport.
a LC-MS/MS single-nucleus proteomics data was acquired with SCoPE-DIA for 3412 nuclei, quantifying a median of 1366 proteins per nucleus. Single nuclei which were insufficiently prepared, acquired, or depleted of non-nuclear proteins were removed, yielding 2997 nuclei with a median of 1287 proteins per nucleus post-filtering; these nuclei were used for downstream analyses. b Weighted PCA produced partial separation between LPS-treated (10, 30, and 60 minute) nuclei and not-treated (NT) nuclei. c Distribution of transport scores from single nuclei. Macrophages treated with LPS for longer durations yielded nuclei which had undergone more protein transport. d Rank sorted correlations between the transport score of each nucleus and its proteome. The underlying data used to compute these associations is shown for two proteins, NUP205 and VIM. e Distributions of relative protein abundances of NUP205 and VIM in the NT and LPS-treated populations of single nuclei. The overlap quantifies the proportion of commonality between the untreated and the treated populations. High values suggest pre-existing variability is much larger than LPS-induced changes. f Distribution of overlaps for all proteins between NT and LPS-treated populations of single nuclei.
Projecting the single-nuclei proteomes onto their principal components produced a partial separation between NT and LPS-treated populations, Fig. 2b. Aside from measurement error, three compounding sources of variability may result in this observed partial separation: pre-existing variability of protein abundances at the whole-cell level, pre-existing variability in subcellular protein localization, and variability in nucleocytoplasmic transport in response to LPS. We sought to overcome the first two sources of variability and investigate the latter, heterogeneity of nucleocytoplasmic transport. This presents a challenge as the pre-existing protein variability is large relative to the magnitude of protein transport induced by a short LPS exposure.
To explore the variability of LPS-induced nucleocytoplasmic transport, we derived a metric aiming to quantify the amount of protein transport experienced by each nucleus; however, given that we did not measure proteomes of the same nuclei before and after stimulation, our estimate cannot directly quantify protein transport. As a substitute, we derived a ‘transport score’ metric, which quantifies the deviation of single LPS-treated nuclear proteomes from the proteome distribution of untreated single nuclei. To mitigate noise, we used our bulk data to differentially apply weights based on transporting proteins, as illustrated in Extended Data Fig. 7. As expected, the resulting distributions of transport scores for single nuclei indicate increased nucleocytoplasmic transport for longer time-periods of LPS treatment, Fig. 2c.
To link proteomic variability to functional variability in protein transport, we computed correlations of relative protein abundances to transport scores for all LPS-treated single nuclei, Fig. 2d; two proteins, NUP205 (ρ = 0.28) and VIM (ρ = −0.63), which are among the most (anti-)correlated to protein transport, are highlighted. Protein-set-enrichment results on this ordered vector indicate associations to cell division, cell adhesion, and other processes shown in Extended Data Fig. 8. To explore whether these associations are driven by pre-existing proteomic variability or LPS-induced changes, we compared the protein abundance distributions before and after LPS treatment, Fig. 2e. The results indicate that LPS-induced changes are small relative to the natural variation across untreated nuclei, Fig. 2e,f. Therefore, our associations are dominated by the pre-exisiting variation across proteomic configurations; this initial variability likely influences the amount LPS-induced protein transport each cell undergoes. Thus, associations between single-nucleus protein abundances and transport scores may be used to identify proteins whose abundance affected nucleocytoplasmic protein transport.
More nuclear pore complexes, more transport
Nearly all nucleoporins were positively correlated to nucleocytoplasmic protein transport, Fig. 3a, suggesting nuclei with more nuclear pore complexes (NPCs) experience more transport. The only exception was TPR, which has been reported to negatively regulate NPC assembly, as validated through knockdowns of TPR yielding cells with more assembled NPCs49, as well as in patient fibroblasts with reduced TPR abundances52. Interestingly, our data support these previous results, as TPR is the only nucleoporin whose abundance was negatively associated with protein transport. To more clearly investigate this relationship, we collapsed all nucleoporin abundances to the complex-level, and correlated these NPC abundances to transport scores in LPS-treated nuclei (n = 2,237) and found a relatively strong and highly significant association (ρ = 0.48, P < 10−15), as shown in Fig. 3b. Indeed, this suggests a highly significant association between NPC abundance and nucleocytoplasmic transport.
Figure 3 |. Nucleoporin abundances and their associations to LPS-induced nucleocytoplasmic transport.
a Nucleoporins are variably associated with LPS-induced nucleocytoplasmic protein transport. b Single nuclei with more nuclear pore complexes experience more LPS-induced nucleocytoplasmic protein transport (P< 10−15, ρ = 0.48). c For each single LPS-treated nucleus, a slope of the absolute value of differentially abundant proteins vs protein mass was computed to quantify mass-dependence of transport for each single nucleus; single nuclei which had the least and greatest abundance of nuclear pore complexes are shown at the top of the panel. The resulting slopes were plotted against their respective nuclear pore complex abundances, as shown at the bottom of the panel. The data suggest protein transport becomes increasingly mass-dependent, favoring transport of smaller proteins, when nuclear pore complex abundances are high (P< 10−14, ρ = −0.17). d Predicted human nuclear pore complex from Mosalaganti et al., with nucleoporins colored by their correlations to transport score. e Distribution of each nucleoporin’s correlation to transport score grouped by their localization inside the NPC. f Distribution of each nucleoporin’s correlation to transport score grouped by whether it is a peripheral or scaffold protein in the NPC (P = 0.037). g Nucleoporins’ correlations to transport are associated with their respective half lifes (half-life data from Mathieson et al.).
Having uncovered this association between transport and NPC abundance in single nuclei, we sought to additionally investigate whether the mass-dependence of nucleocytoplasmic transport also changes as the number of NPCs varies across single nuclei; specifically, the nuclear envelope should become more permeable to passive diffusion at higher pore densities. To test this hypothesis, we first quantified the mass dependence of protein transport for each nucleus by regressing the molecular masses of proteins on their absolute deviations from the NT-population of single nuclei––a substitute for measuring protein-specific transport. The slopes of these regressions were plotted against corresponding NPC abundances in single nuclei, Fig. 3c. The results revealed a modest association across all 2,237 LPS-treated nuclei (ρ = −0.17) with high statistical significance (P < 10−14). This association indicates that nucleocytoplasmic transport of smaller proteins increasingly outpaced transport of larger proteins, at least in part as a function of NPC abundance, Fig. 3c. Given that passive diffusion is highly mass-dependent, it may suggest that single nuclei with more NPCs experience more passive diffusion, thus providing empirical support for this theoretical expectation.
While the abundances of nearly all nucleoporin proteins correlate positively with transport scores, the magnitude of this correlation varies across nucleoporin proteins, as shown in Fig. 3a. To explore this variability, we investigated what might be associated with these differences. To visually inspect the structural dependence of the correlations, the predicted structure of the human nuclear pore complex from Mosalaganti et al.53 was colored according to each proteins’ correlation to the transport score, Fig. 3d. This was investigated more directly by grouping nucleoporins based on their localizations inside the nuclear pore complex, as shown in Fig. 3e, and according to whether the nucleoporins were peripheral or scaffold proteins of the NPC54, as shown in Fig. 3f. The abundances of scaffold proteins were significantly more associated with nucleocytoplasmic transport than peripheral proteins of the NPC (P = 0.037). This variation across nucleoporins is further supported by a significant correlation with protein half-lives derived from Mathieson et al.’s SILAC turnover data54 (ρ = 0.56, P = 0.0018), as shown in Fig. 3g, as scaffold nucleoporins have been reported to have longer half-lives than peripheral nucleoporins54,55. These results suggest the observed variability in each nucleoporins’ correlation to transport is significantly associated with the nucleoporin’s localization inside the NPC and their respective half-lives.
Targeted perturbations validate the single-nucleus derived associations
Having used single-nucleus proteomics data to infer novel regulators of LPS-induced nucleocytoplasmic protein transport, we aimed to directly test these potential enhancers and suppressors of nucleocytoplasmic transport through targeted perturbations. The highest ranking hypotheses from the single-nucleus proteomics analysis include proteins directly involved in transport such as nucleoporins and ribosomal export proteins such as MDN156. However, many other proteins without functional annotations for protein transport or innate immunity are also highly (anti-)correlated to transport. Perhaps some of these proteins influence LPS-induced protein transport, and these functional associations present discovery opportunities. We sought to test these associations experimentally through directed perturbations using siRNA mediated knock downs.
To test potential transport regulators, we chose to knock down 16 proteins spanning the continuum shown in Fig. 4a and quantified the change in LPS-induced nucleocytoplasmic protein transport. The knockdowns were performed using siRNAs as shown in Fig. 4b, and the decrease of the corresponding gene products was validated in whole-cell and nuclear bulk lysates, as shown in Supplementary Fig. 1 and Supplementary Fig. 2. To quantify how each knockdown affected LPS-induced nucleocytoplasmic protein transport, slopes were calculated from fold-changes (LPS/NT) between the original bulk data and each siRNA condition, using the negative control (non-targeting) siRNA as a reference for each biological replicate, as shown in Fig. 4b. Results for individual biological replicates are shown in Supplementary Fig. 3. With this strategy, we aimed to quantify the global change in nucleocytoplasmic transport as a result of each knockdown.
Figure 4 |. Genetic perturbations validate single-nucleus-derived protein associations.
a Correlations of protein abundances to transport score from single-nucleus data. 16 proteins were selected to be knocked-down and colored with green or purple depending on whether it is associated with increased or decreased transport. b Workflow for siRNA-mediated knockdowns in THP-1 monocytes, followed by differentiation to macrophages. A negative control (non-targeting) siRNA was included in each biological replicate, and used to compute a change in transport as the result of each siRNA knockdown (KD); change in transport was calculated as the difference in slopes between the negative control siRNA and a gene-targeting siRNA. c Here we evaluate the predictive power of the single-nucleus findings through experimental genetic perturbations in bulk data. The biological replicates from the single-nucleus data were used to compute significance of correlations to transport scores, shown on the y-axis; the x-axis measures the significance of how the knockdown affected nucleocytoplasmic protein transport. The single-nucleus associations were highly predictive of the functional effects in genetic perturbations (P = 0.00046, ρ = −0.77). d Significance of how individual gene knockdowns affect nucleocytoplasmic transport. Knockdowns of IMMT, RPSA, HSD17B4, SND1, DHX15, and VIM were found to globally increase LPS-induced nucleocytoplasmic protein transport; knockdowns of RBM6 and MYBBP1A were found to decrease transport.
Associating relative protein abundances to function in single cells may serve as a means of inferring regulatory potential. We sought to evaluate the predictive power of these associations by computing the significance of protein correlations to transport from the single-nuclei data and the significance of the change in transport from corresponding knockdowns, Fig. 4c. Indeed, as a result of the knockdowns, protein transport for 13/16 of the conditions was affected in the expected direction. Specifically, knockdowns of potential transport suppressors increased transport in 7/7 cases; knockdowns of potential transport enhancers decreased transport in 6/9 cases. The protein correlations to transport scores from the single-nucleus data were highly predictive of the functional effects of the knockdowns (ρ = −0.77, P < 5×10−4). The slopes, which were used for determining the change in LPS-induced nucleocytoplasmic transport, were computed from the 100 most differentially abundant proteins from the original bulk data. Various filtering levels were applied to examine the robustness of the findings, as shown in Extended Data Fig. 9, and all three levels showed similar trends.
Knockdowns of IMMT, RPSA, HSD17B4, SND1, DHX15, and VIM were found to significantly increase transport, whereas knockdowns of RBM6 and MYBBP1A were found to significantly reduce transport. Therefore, 8/16 knockdowns significantly changed LPS-induced nucleocytoplasmic transport, 7/8 of which changed nucleocytoplasmic transport in the expected direction. The knockdown which most significantly affected transport was IMMT, a mitochondrial protein without prior association to innate immunity or nucleocytoplasmic protein transport. Whole-cell lysates of these knockdowns complementarily indicate a gradient of unpreparedness and primedness to respond to LPS and facilitate nucleocytoplasmic transport, as shown in Extended Data Fig. 10. These findings validate the potential of (sub-)single-cell proteomics to identify novel regulators of biological functions.
Discussion
Identifying functional regulators of complex biological processes is an ongoing challenge in proteomics data interpretation. Here we demonstrate an interpretation approach that exploits the natural variability inherent in a population of single cells to empower inference of functional regulation. It is conceptually similar to the identification of functional regulators through perturbation-induced variability in bulk samples57 but instead utilizes the natural variability across individual cells. In our approach, we assumed that the initial proteomic states of single macrophages would explain the variability in LPS-induced nucleocytoplasmic protein transport. Operating under this principle, we quantified a metric (the transport score) from which we could evaluate the influence of resting proteomic variability on this response. We found that simple biophysical constraints, such as the quantity of nuclear pores, partially explain the variance in nucleocytoplasmic transport. Interestingly, the abundance of scaffold subunits is more strongly associated with transport rates than the abundance of peripheral subunits, which is consistent with modular and specialised structures of the nuclear pore complex58.
Beyond the role of nuclear pores, our analysis identified hundreds of additional proteins, many of which without annotated associations to innate immunity or protein transport, yet whose abundances were strongly correlated to the magnitude of nucleocytoplasmic protein transport experienced in single cells. We tested these protein-associations through genetic perturbations and found that the associations derived from single-nucleus data were, as a whole, highly predictive of their respective effects on LPS-induced nucleocytoplasmic transport. This work demonstrates an approach which uses (sub-)single cell proteomics to infer functional regulators of complex biological processes, such as LPS-induced nucleocytoplasmic transport. We expect approaches such as this will generalize to broad applications and support the functional interpretation of single-cell proteomics data.
Methods
Cell culture
THP-1 monocytes (TIB-202, ATCC) were cultured in RPMI-1640 Medium (R8758, Sigma-Aldrich) supplemented with 10% fetal bovine serum (10439016, Gibco) and 1% penicillin-streptomycin (15140122, Gibco) and grown at 37°C and 5% CO2. Cells were transferred to 100×15mm Nunclon dishes (150350, Thermo Scientific) at a density of approximately 200,000 cells/mL and volume of 10 mL. Differentiation proceeded following an established protocol by Lund et al.59; THP-1 cells were incubated in the presence of 25 nM phorbol 12-myristate 13-acetate (PMA) for 48 hours, then cells were detached with accutase, mixed to prevent any batch-effects resulting from differentiation occurring in isolated plates, washed 2-times with PBS, centrifuging at 300g for 5 min to pellet for washes. The cells were then allowed to incubate in growth media without PMA for 24 hours, and in this time reattach to fresh 100×15mm Nunclon dishes. The resulting M0 macrophages were either left not-treated (NT), or treated with 1 μg/mL lipopolysaccharides from Escherichia coli O111:B4 (L4391, MilliporeSigma) for 10 minutes, 30 minutes, or 60 minutes.
Protein knockdowns
Proteins of interest were knocked down via reverse transfection of Silencer siRNAs (Ambion) and Silencer Select siRNAs (Ambion) into THP-1 monocytes using methodology adapted from manufacturer instructions for RNAi transfection. Silencer siRNAs were transfected in 12-well Nunclon plates and Silencer Select siRNAs were transfected in individual 60 mm dishes. For Silencer siRNAs, 10 pmol of siRNA was complexed with 3 μL of RNAiMAX. For Silencer Select siRNAs, 55 pmol of siRNA was complexed with 17 μL of RNAiMAX (Invitrogen) in 552 μL of serum-free RPMI (Sigma) in a 60 mm Nunclon dish (Thermo). siRNAs were complexed with the RNAiMAX lipid reagent for 5 minutes at room temperature. THP-1 monocytes were added in RPMI supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin to reach a final density of 300,000 cells per mL. Samples were cultured at 37°C and 5% CO2 for 24 hours before adding PMA, at which point the same aforementioned cell culture process was followed.
Nuclear isolation
Macrophages were detached from Nunclon dishes with accutase on ice for approximately 20 minutes. The cells were washed twice with 1x PBS to remove media, centrifuging at 300g for 5 min each time. The cell concentration was measured with a hemocytometer. 15% of cells were retained as whole-cells for downstream analysis by resuspending the whole-cell pellet in LC-MS-grade water to a concentration of 3,000 cells/μL. These whole-cells were then stored at −80°C. The remaining 85% of cells were resuspended in 1 mL of ice-cold Lysis Buffer (0.1% NP-40, 250 mM sucrose, 25 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH = 7.4)) in a 2 mL Eppendorf tube, and left to lyse on ice for 20 min. At this point in the protocol, the cells are lysed but the nuclei are unlikely to be free from cellular debris and other organelles. To purify the nuclei, the 1 mL nuclei suspension was sheared through a 25G needle with even pressure, avoiding bubbling and foaming as much as possible. The shearing was repeated for a total of 5 times. Nuclei were visually inspected under a microscope with Trypan Blue staining to ensure sufficient shearing of subcellular organelles from nuclei.
After nuclei are sufficiently sheared, the suspension was centrifuged at 1000g for 8 min at 4 °C. The nuclear pellet was resuspended in 500 μL of 250 mM sucrose, 25 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH = 7.4). The mixture was underlayed with 500 μL of 350 mM sucrose, 25 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH = 7.4) to form a sucrose cushion, which was then centrifuged at 4,000g for 10 min (4 °C). The supernatant was removed, and the nuclear pellet was resuspended in 200 μL of LC-MS water, gently flicking to mix. 4 μL of nuclei suspension was mixed with 4 μL of Trypan Blue, then counted with a hemocytometer to find the final concentration. 150 μL of the bulk nuclei were stored at −80°C. The remaining 50 μL was diluted to a concentration of roughly 400 nuclei/μL and used for single-nuclei sorting and sample preparation by nPOP19,50.
Sample preparation for proteomic analysis
Bulk samples
Nuclei and whole-cell fractions, which were frozen at −80°C at a concentration of 3,000 cells/μL, were heated to 90°C for 10 minutes as part of the mPOP protocol60. Trypsin Gold (V5280, Promega) was added to a final concentration of 20 ng/μL, in addition to final concentrations of 100 mM TEAB and 0.25 U/μL benzonase nuclease (E1014, Millipore Sigma). The cell lysates were digested at 37°C for 18 hours in a thermocycler. Samples were labeled with mTRAQ following manufacturer’s instructions. In short, the labels, which arrived suspended in isopropyl alcohol at a concentration of 0.05 U/μL (where 1 unit labels 100 μg), were added in a 1:2 proportion of label:peptide where the peptides were concentrated to 500 ng/μL in 100 mM TEAB. The labeling reaction was left at room temperature for 2 hours, and then quenched with 0.25% hydroxylamine for 1 hour.
Creating a mixed species standard to benchmark SCoPE-DIA
Digested protein lysate from THP-1 macrophage nuclei were mixed with digested S. cerevisiae protein lysate (V7461, Promega) to generate three samples. The proportions of Sample A (Δ0):Sample B (Δ4) was 4:1 for S. cerevisiae and 1:1 for H. sapiens. The unlabeled versions of these samples were mixed in a 1:1 ratio to generate a carrier sample which was then labeled with mTRAQ Δ8. Samples A and B were diluted such that the H. sapiens amount was 1 nucleus worth of protein (approximately 45 pg). The carrier sample was pooled with Samples A and B at 0x, 1x, 5x, 10x, 25x, and 50x concentrations to generate the SCoPE-DIA benchmarks.
Single nuclei
Single nuclei were prepared for proteomic analysis by nPOP as previously described by Leduc et al.19,50. In short, single-nuclei were sorted by CellenOne into 8 nL droplets of 100% DMSO on a fluorocarbon-coated glass slide to lyse. The lysed nuclei were digested with Trypsin Gold at a concentration of 120 ng/μL and 5 mM HEPES (pH 8.5) and 200 mM TEAB buffer (pH 8.5). Peptides were then chemically labeled with mTRAQ Δ0 or Δ4. Single nuclei were pooled into sets of Δ0 and Δ4, and dispensed into individual wells of a 384 well-plate. The pooled samples in the 384 well-plate were then dried in a speed-vacuum then stored at −80°C until use. Before sample loading for LC-MS/MS, a 25x or 50x nuclei carrier labeled with mTRAQ Δ8 was pooled into each set to create the final Δ0, Δ4, and Δ8 SCoPE-DIA set. Four single-nucleus biological replicates were prepared and acquired, for a total of 3412 single nuclei.
Data acquisition
All single nuclei and bulk samples were resuspended in 0.01% n-Dodecyl β-d-maltoside (DDM)61 with 0.1% formic acid. Sample pickup occurred out of a 384 well-plate for single-nucleus samples, or from glass vials for bulk samples. Peptides were separated for LC-MS acquistion using a Neo Vanquish UHPLC and 25 cm × 75 μm IonOpticks Aurora Series UHPLC columns (AUR2–25075C18A) at a flow-rate of 200 nL/min. LC was run with the following settings: Direct Injection, nano/cap flow, maximum pressure = 1500 bar, maximum pressure change = 1000 bar/min. Sample loading had the following settings: 1μL injection with 1.2 μL loading volume, ”Pressure Control” mode with maximum pressure set to 1450 bar, and fast loading enabled. Wash and equilibration settings: ”Pressure Control” mode with maximum pressure set to 1450 bar, equilibration factor = 4.0, and fast equilibration was enabled.
The LC gradient proceeded as follows: Ramp from 2.5%B to 6.5%B over 0.2 minutes, to 11.5%B over 0.9 minutes, to 21.0%B over 3.1 minutes, to 31.5%B over 6.2 minutes, to 40%B over 2.8 minutes, to 55%B over 1.7 minutes, to 95%B over 0.65 minutes and then hold at 95%B for 4 minutes. This method allows for approximately 15 minutes of active chromatography, at a throughput of approximately 45 runs/day (90 single nuclei/day with SCoPE-DIA or 135 bulk samples/day with plexDIA) after accounting for sample loading and column equilibration overheads.
All data were acquired on a Bruker timsTOF SCP using captive spray, dia-PASEF scan mode62,63, and positive polarity. The duty cycle consisted of 8 total PASEF frames, 26 Th MS2 windows with 1 Th overlaps. To improve temporal sampling of the MS1-elution profiles which are used for quantification, an MS1 scan was taken every 2 PASEF frames, for a total of 4 MS1 scans per duty cycle. MS2 scan range: 300 m/z-1000 m/z, MS1 scan range: 100 m/z-1700 m/z, 1/K0 start: 0.64, 1/K0 end: 1.20, ramp and accumulation times: 100 ms. The estimated duty cycle time is 1.28 seconds. Collision energy settings were 20 eV at 1/K0 = 0.60 and 59 eV at 1/K0 = 1.60. Collision RF was set to 2000 Vpp.
Raw data searching with DIA-NN
Empirical spectral libraries of mTRAQ-labeled nuclei and whole-cells were generated from dia-PASEF runs on the timsTOF SCP, searching with a DIA-NN in-silico-predicited Swiss-Prot H. sapiens FASTA (canonical & isoform). Similarly, a combined H. sapiens and S. cerevisiae library was generated for searching SCoPE-DIA benchmarks.
DIA-NN (version 1.8.1) was used to search raw data as previously implemented22,64. The following search settings were used: {–fixed-mod mTRAQ 140.0949630177, nK}, {–channels mTRAQ, 0, nK, 0:0; mTRAQ , 4, nK, 4.0070994:4.0070994; mTRAQ, 8, nK, 8.0141988132:8.0141988132}, {–original-mods}, {–peak-translation}, {–report-lib-info}, {–qvalue 0.01}, {–mass-acc-ms1 5.0}, {–mass-acc 15.0}, {–mass-acc-quant 5.0}, {–reanalyse}, {–rt-profiling}, and {–peak-height}.
Computational data analysis
Nuclear enrichment
To assess nuclear enrichment, the first two bulk biological replicates were acquired in a plexDIA set of whole-cell and nuclear fractions. The fractions were subset for histones, which should only be present in the nucleus, and both fractions were normalized by a scalar such that histone proteins between whole-cells and nuclear fractions were in a 1:1 ratio. Ratios of MaxLFQ protein abundances for protein markers from The Human Protein Atlas for endoplasmic reticulum (ER), nuclear compartments, mitochondria, plasma membrane, cytosol, and Golgi were calculated between nuclear and whole-cell fractions to assess nuclear protein enrichment65–67.
Differential protein abundance analysis
Precursor quantities acquired by plexDIA and analyzed by DIA-NN were corrected for isotopic envelope carryover, as previously performed22. These precursor quantities were filtered for Lib.PG.Q.Value < 0.01 and Q.Value < 0.01 for bulk data from all six biological replicates and their corresponding nested technical replicates. The bulk data used to generate weights for single-nuclei analyses were taken from cell-batches that corresponded to those single nuclei, in this case, biological replicates 1, 2, 3, and 6; the bulk data used for single-nucleus weighting were further filtered for Translated Q-value and Channel Q-value < 0.05.
Differential protein abundance was calculated through the MS-Empire workflow38 for each biological replicate. Precursors used for analysis were required to be quantified in at least two replicates per condition. Data normalization was performed using operations from the MS-Empire workflow, as described by Ammar et al.38. Differential abundance analysis collapsed precursors to gene-level annotations; this produced p-values calculated after outlier correction for each protein for each biological replicate; Stouffer’s method was applied to collapse multiple p-values from several biological replicates to a single combined p-value. Briefly, this involved converting p-values for each protein from each biological replicate to z-scores, multiplying by the sign of the fold-change, summing the signed z-scores, then dividing by the square root of the number of observations for that protein, and finally converting that z-score back to a single p-value for each protein. The resulting p-values were corrected for multiple hypothesis testing using the Benjamini-Hochberg (BH) correction68.
Protein set enrichment analysis
Protein set enrichment analysis was performed using the g:GOSt R package69,70. An ordered vector of gene names was used as the input. Results were filtered at 1% FDR, and the relative abundance for the Gene Ontology terms was computed across all samples from the intersect of protein abundances to represent the relative abundance of a given Gene Ontology term. Calculating the relative abundance of a Gene Ontology term from the subset of intersected proteins allowed for fairer comparisons of enrichment between samples. Specifically regarding the analysis of whole-cell bulk knockdowns shown in Extended Data Fig. 10, in some cases this was performed on data in which NT and 60 minute LPS-treated cell-lysates were combined. These samples were experimentally combined for ease of labor and due to the minimal impact of 60 minute LPS-treatment on the whole-cell proteome.
Evaluating relationship of protein mass and transport
Protein masses were downloaded from Uniprot71, and matched to proteins which we found significantly increased or decreased in response to LPS (Q-value < 0.05) in nuclear fractions. The absolute value of the log2 fold-change was plotted with the mass of the protein to statistically evaluate the relationship between protein mass and the amount of transport that occurred as a result of LPS treatment. Because the masses are purely based on gene-encoded sequences, this analysis does not account for changes in mass as the result of proteolytic cleavage or the transport of protein complexes.
Additionally, the dynamics of protein transport were interpolated from the four data points (NT, 10 min, 30 min, and 60 min LPS) using a 3rd degree polynomial for each of the proteins that was found to significantly change in at least one of the conditions relative to NT. A total of 300 time points were predicted from the fit, ranging from 0 to 60 minutes. The proteins were arranged from smallest to largest and a moving median of the absolute value of the log2 fold-change from NT was calculated for each time point from that protein’s adjacent 40 smaller and 40 larger proteins. In this way, biological differences between proteins could be averaged to generally assess the influence of protein mass on transport kinetics. For each protein’s averaged kinetics, the time when half of the total absolute value log2 fold-change had occurred was marked. This was plotted against the protein mass and a Spearman correlation was calculated.
Benchmarking SCoPE-DIA quantification
To benchmark how different levels of carrier affect protein-level quantitation of SCoPE-DIA as shown in Extended Data Fig. 5f, precursors were intersected across all carrier levels, and thus proteins as well; precursor quantities were collapsed to protein-level quantities using MaxLFQ, and then ratio values between Δ0 and Δ4 were computed, normalizing H. sapiens to a be median-centered at a 1:1 ratio to account for systematic differences in loading amounts, if any exist. To compare quantitation at different carrier levels for all proteins, as shown in Extended Data Fig. 5f, the same process was performed without intersecting.
In regards to compression filtering, acquiring single cell (or nucleus) data with a constant, known amount of carrier is useful to identify precursors which have systematically interfered quantification. Precursors which have systematically compressed quantification across all samples relative to the expected carrier amount, are unlikely to be well-quantified and can be removed. To identify which precursors to remove, a ratio for each precursor from the single-nucleus channels to its respective carrier channel was calculated. Precursors with median ratios > 6-fold greater than the theoretical ratio (e.g. 1:50 is expected for a 50x carrier) were considered to be systematically poorly quantified and were excluded from downstream analyses.
Single-nucleus quality control
To identify which single nuclei were successfully prepared and acquired, precursors were filtered for Channel and Translated Q-values < 0.1 and the number of remaining high quality precursors was counted. Single nuclei with ≥ 50 high quality precursors were retained for downstream analysis as this threshold was sufficiently separable from negative controls. Precursors were required to be quantified in ≥ 1% of single nuclei, and have < 6x ratio compression to be retained for downstream analysis.
Single nuclei were further filtered to ensure sufficient depletion of non-nuclear proteins. To quantify the nuclear purity of each single-nucleus, the nuclear proteomes were scaled to a reference bulk nuclear fraction, and compared to a ratio of whole-cell to nuclear fraction. A 3rd degree polynomial was used to fit a curve for each single-nucleus, and an absolute value of the AUC was computed. This value was used to identify single-nuclei which were insufficiently pure. Only single nuclei with AUCs < 5 were used in downstream analyses.
Single-nucleus protein quantification, imputation, and batch correction
Precursor abundances from single nuclei were normalized to their respective carrier channel, which serves as a reference similar to the isobaric SCoPE-MS workflow15,24. For each precursor across all carrier samples, the mean abundance was calculated and used to scale the data of the single nuclei, to be compatible for MaxLFQ protein quantification65. The resulting protein-level data of each nucleus was normalized by its respective median protein abundance to account for differences in absolute abundances, then each protein was normalized by the mean abundance across single nuclei. The log2-transformed data was imputed using a weighted kNN approach (k=5), where the weights were proportional to the similarity to its nearest neighbors. Finally, the data was batch-corrected using ComBat72,73 to account for mTRAQ label-biases, LC-batches, and biological replicates.
Weighted Principal Component Analysis (PCA)
The variance of single-nucleus protein data was weighted based on protein correlations, bulk differential abundance, and bulk continuity of spatiotemporal trends. Effectively these weights assign higher variance based on: 1) how correlated a protein’s abundance is to other proteins abundances, 2) how differentially abundant the protein was in the bulk data, and 3) how continuous the trend was in its spatiotemporal response to LPS.
Specifically, the bulk differential abundance data computed from MS-Empire38 analysis had p-values which where converted to signed Z-scores. Using Stouffer’s method they were combined for all the bulk biological replicates that correspond to the single-nucleus replicates, in this case, biological replicates 1, 2, 3, and 6. The resulting Z-score for each protein was squared, then used to weight the variance of that protein in the single-nucleus data. Additionally, the inverse of the ranked version of the von-Neumman Ratio (RVN) calculated from bulk data was used to weight the variance of each respective protein in the single-nucleus data.
Computing single-nucleus transport scores
Directly measuring protein transport in a cell using LC-MS/MS was not possible with this experimental design as we did not measure the same nucleus before and after LPS stimulation. However, we derived a ‘transport score’ metric to serve as a substitute which serves to quantify the deviation of single LPS-treated nuclei from the population of single NT nuclei. The transport score accounts for pre-existing variability in the not-treated (NT) population of single nuclei for each protein by converting protein abundances for each LPS-treated nucleus to a Z-score in reference to the NT-population. For each nucleus, the resulting vector of Z-scores is weighted based on the signed-Z-scores of the differential protein abundances in the bulk data. Therefore, a protein’s contribution to the transport score is weighted by its significance in the bulk data, where more differential proteins carry greater weights. The mean component of the resulting vector of each nucleus is taken as that nucleus’ transport score.
Computing protein overlap coefficients between populations of single nuclei
The overlap between the distributions of relative protein abundances between populations of single nuclei was computed to quantify the commonality of protein abundances pre-stimulation and post-LPS stimulation. This overlap coefficient was computed by the overlap function of bayestestR74. It is computed by modeling the distribution of relative protein abundances as densities for two populations and then computing the proportion that is shared. For example, a protein that has high-overlap between abundances in NT and LPS-treated populations, is one that is minimally transported relative to its natural variability.
Quantifying nuclear pore complex abundances and dependence on mass
To quantify nuclear pore complex abundances in each single-nucleus, relative abundances of nucleoporins were converted to Z-scores and combined for all nucleoporins using Stouffer’s method. Mass dependence as a function of NPC abundance was computed for each LPS-treated single-nucleus based on the slopes (protein Z-scores vs log10-transformed protein mass) of differentially abundant proteins in the single-nucleus data.
Investigating the association between nucleoporin half-lives and their correlations to transport score
Half-lives were derived from SILAC turnover data of primary monocytes, NK cells, neurons, hepatocytes, and B-cells from Mathieson et al.54. Specifically, the half-lives of all replicates for each cell-type was collapsed to the mean, then the mean of all cell-types was collapsed to a final value, which was used as the half-life in our analysis.
Visualizing the nuclear pore complex
The predicted structure of the human nuclear pore complex (PDB Entry: 7r5k)53 from Mosalaganti et al. was used visualize the NPC. The nucleoporins were colored by their respective correlations to transport score using ChimeraX (v1.6.1)75.
Quantifying the effect of protein knockdowns on transport
To quantify how each protein knockdown affected LPS-induced nucleocytoplasmic transport, each biological replicate included a matched negative (non-targeting) siRNA control to account for how much transport would have occurred without the knockdown. A slope was calculated from the 100 most differentially abundant proteins in the original (no knockdown) bulk data to the data for each knockdown. The fold-changes for proteins used for computing these slopes were weighted by their associated Z-score significance of differential abundances from the original bulk data; this serves to weight each protein’s contribution to the slope-calculation based on its significance. The difference in slopes between the negative control (non-targeting) siRNA and the knockdown was computed. This difference in slope is interpreted as the extent to which nucleocytoplasmic transport changed as a result of the knockdown. Additionally, the associated standard error of each slope was used between the negative control and experimental knockdown to derive a Z-score for the significance of the difference in transport. Significance from additional biological replicates were combined using Stouffer’s method to arrive at a cumulative Z-score. To assess the significance of the effect on transport of individual knockdowns, this Z-score was converted to a p-value and adjusted for multiple testing hypotheses using a BH-correction.
Calculating significance of correlation to transport score
To derive a metric for the significance of protein correlations to the transport score, a rank-based normalization approach (Rankit) was used to transform the correlations into a standard normal distribution. The resulting Z-scores were combined using Stouffer’s method to arrive at a final Z-score for each protein, as shown on the y-axis of Fig. 4c. This approach was chosen as an alternative to a traditional Z-score operation given that the distribution of correlations to transport scores were non-Gaussian and have a skewed tail.
Extended Data
Extended Data Fig. 1 |. Evaluating nuclear enrichment of bulk samples.
Proteins markers from The Human Protein Atlas were used to benchmark the enrichment of nuclear proteins, and the depletion of non-nuclear proteins in nuclear lysate relative to whole-cell lysate.
Extended Data Fig. 2 |. Assessing the continuity of time-series data for LPS-induced differentially abundant proteins.
a Calculation for the ranked version of von Neumann’s ratio (RVN). b Distribution of RVN ratios for differentially abundant proteins (5% FDR) in nuclei (“Sig. proteins”) compared to a simulated null model. c Same as b, but for whole-cells.
Extended Data Fig. 3 |. Fold-change of LPS-induced protein transport is mass-dependent.
Absolute value fold-changes between LPS-treated and NT nuclear proteomes, and molecular masses are plotted for differentially abundant proteins (5% FDR). Smaller proteins tend to have greater fold-changes than larger proteins. Spearman correlations and associated statistical significance are noted.
Extended Data Fig. 4 |. Change in the nuclear abundance of proteins associated with DNA repair complex in response to 1μg/mL LPS.
Time series trends of DNA repair complex proteins computed from up to 6 bulk biological replicates. Proteins shown in red are differentially abundant at 5% FDR, otherwise they are shown in black.
Extended Data Fig. 5 |. Benchmarking SCoPE-DIA for single nucleus proteomics.
a We sought to test whether acquiring single nucleus data in parallel with a more abundant carrier sample may improve protein coverage with minimal effect to quantitative accuracy. To test this, a mixed-species spike-in of 4:1 S. cerevisiae and 1:1 H. sapiens was created at 0x, 1x, 5x, 10x, 25x, and 50x carrier amounts. b Quantitative accuracy of proteins quantified across all carrier-levels (n=658) remains accurate despite the potential for increased interference. Dashed lines correspond to the theoretical expectation of the spike-in ratio. c Median ratios of precursor abundances to the carrier were computed and plotted as a histogram. Precursors with systematically compressed ratios from the theoretical expectation of the carrier level (e.g. 1:50) are likely poorly quantified, and were removed from downstream analysis. d Quantitative accuracy for intersected proteins at 4 filtering thresholds with the 50x carrier. Filtering precursors based on observed compression relative to the carrier improves quantitative accuracy. e Number of protein ratios quantified between Δ0 and Δ4 with 50x carrier after various filtering thresholds. f After filtering to remove poorly quantified precursors, the 50x carrier still enables nearly 4-fold greater proteomic coverage. The protein-level quantitative accuracy for all proteins is shown in the bottom panel. The data indicate that the overall quantitative accuracy decreases as the carrier enables identification of otherwise unidentifiable proteins; naturally, these proteins are lowly abundant and thus poorly quantified.
Extended Data Fig. 6 |. Single nucleus quality control.
a Single nuclei and negative controls were filtered for Translated and Channel Q-values < 0.1, and the number of precursors quantified was counted and plotted as a histogram. Successfully prepared and acquired nuclei, which were separable from negative controls, were retained for downstream analysis (highlighted in green). b Single nuclei were acquired with either a 25x or 50x carrier, and the median ratio of precursor intensities from single nuclei to normalized carrier was computed for each precursor. Only precursors with <6-fold compression were retained for downstream analysis (highlighted in green). c Nuclear purity was assessed for each single nucleus to a reference bulk nuclear fraction, shown in the y-axis, and a 3rd degree polynomial was fit for expected protein enrichment in the x-axis. Each line denotes the 3rd degree polynomial fit for a given single nucleus, and is colored by the computed area under the curve (AUC). Single nuclei with greater AUC reflect nuclei which are generally less pure (more whole-cell like). Only nuclei with AUC < 5 were retained for down-stream analysis. d Similar to the previous plot, this shows the distribution of AUCs for single nuclei. Only relatively pure nuclei (AUC < 5) were retained for downstream analysis.
Extended Data Fig. 7 |. Computing the transport score of single nuclei.
Single-nucleus protein transport cannot be directly quantified in our analysis due to the lack of measurements before and after LPS stimulation for the same nucleus. Here, we derive a metric, we term ‘transport score,’ that serves as an approximation. Deviations of protein abundances from the NT-population of single-nuclei are calculated globally, for all proteins, for each LPS-treated nucleus. The resulting vectors are weighted according to the differential protein abundances derived from the original bulk data presented in Figure 1. The mean component of the resulting vector is the weighted deviation of a single-nucleus from the NT-population of single-nuclei; we use this metric (transport score) to infer the magnitude of protein transport.
Extended Data Fig. 8 |. Protein set enrichment analysis on protein correlations to transport score.
The vector of ordered protein correlations to transport score were analyzed for enriched protein sets (Gene-Ontology terms). The results for which are shown at 1% FDR, ordered by their relative enrichment, some of which are highlighted.
Extended Data Fig. 9 |. Predictive power of correlations to transport remain similar at different filtering levels.
To assess the robustness of our findings, changes in transport were either calculated based on a the 25 most differentially abundant proteins in the original bulk data, or b all differentially abundant proteins at 1% FDR (308 proteins). The trends are similar, but with decreasing predictive power as proteins with less biological signal are included for computing the slopes.
Extended Data Fig. 10 |. Protein-set enrichment analysis of whole-cells as a result of each siRNA knockdown.
The abundances of differentially enriched Gene-Ontology terms are plotted (y-axis) for each siRNA condition (x-axis); the x-axis is ordered based on each knocked-down protein’s correlation to transport score. knockdowns of potential transport suppressors are generally enriched for macrophage-like processes and nucleocytoplasmic transport compared to knockdowns of potential transport enhancers.
Supplementary Material
Acknowledgments:
We thank H. Specht, R. Huffman, A. Petelski, D. Perlman, and E. Emmott for discussions pertaining to experimental methodology for single-nucleus proteomics. We thank M. Rout and T. Eeuwen for thoughtful discussions regarding nuclear pore complexes. And finally, we thank I. Zanoni and M. Di Gioia for discussions regarding LPS-response in macrophages and early experimental work. The work was funded by an Allen Distinguished Investigator award through The Paul G. Allen Frontiers Group to N.S., a Bits to Bytes award from MLSC to N.S., an NIGMS award R01GM144967 to N.S., and a MIRA award from the NIGMS of the NIH (R35GM148218) to N.S.
Footnotes
Competing interests: N.S. is a founding director and CEO of Parallel Squared Technology Institute, which is a nonprofit research institute.
Availability
All data are reported and deposited in accordance with the community guidelines13. Raw data, spectral libraries, FASTAs, and DIA-NN search outputs are available at MassIVE: MSV000094829. Code, processed data, supporting files, supplementary figures, and interactive supplementary analyses (e.g. volcano plots and time-series data) are available at scp.slavovlab.net/Derks et al 2024.
References
- 1.Macosko E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slavov N. Scaling Up Single-Cell Proteomics. Molecular & Cellular Proteomics 21, 100179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quake S. R. A decade of molecular cell atlases. [DOI] [PubMed]
- 4.Symmons O. & Raj A. Whats Luck Got to Do with It: Single Cells, Multiple Fates, and Biological Nondeterminism. Molecular cell 62, 788–802 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slavov N. Unpicking the proteome in single cells. Science 367, 512–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb C., Wolock S., Tusi B. K., Socolovsky M. & Klein A. M. Fundamental limits on dynamic inference from single-cell snapshots. Proceedings of the National Academy of Sciences 115, E2467–E2476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leduc A., Harens H. & Slavov N. Modeling and interpretation of single-cell proteogenomic data. arXiv. eprint: 2308.07465 (2023). [Google Scholar]
- 8.Slavov N. Learning from natural variation across the proteomes of single cells. PLOS Biology 20, 1–4 (Jan. 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly R. T. Single-Cell Proteomics: Progress and Prospects. Molecular & Cellular Proteomics (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters-Clarke T. M. et al. Boosting the Sensitivity of Quantitative Single-Cell Proteomics with Infrared-Tandem Mass Tags. J. Proteome Res. (May 2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters-Clarke T. M., Coon J. J. & Riley N. M. Instrumentation at the Leading Edge of Proteomics. Analytical Chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slavov N. Driving Single Cell Proteomics Forward with Innovation. Journal of Proteome Research 20, 4915–4918 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatto L. et al. Initial recommendations for performing, benchmarking, and reporting single-cell proteomics experiments. Nat. Methods 20, 375–386 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slavov N. Single-cell protein analysis by mass spectrometry. Current Opinion in Chemical Biology 60, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Specht H. et al. Single-cell proteomic and transcriptomic analysis of macrophage heterogeneity using SCoPE2. Genome Biology 22, 50. (2021) (Jan. 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cong Y. et al. Ultrasensitive single-cell proteomics workflow identifies > 1000 protein groups per mammalian cell. Chemical Science (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huffman R. G. et al. Prioritized mass spectrometry increases the depth, sensitivity and data completeness of single-cell proteomics. en. Nature Methods 20, 714–722. (2024) (May 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thielert M. et al. Robust dimethyl-based multiplex-DIA doubles single-cell proteome depth via a reference channel. Mol. Syst. Biol. 19, e11503 (Sept. 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leduc A., Huffman R. G., Cantlon J., Khan S. & Slavov N. Exploring functional protein covariation across single cells using nPOP. Genome Biology 23, 261. (2023) (Dec. 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orsburn B. C., Yuan Y. & Bumpus N. N. Insights into protein post-translational modification landscapes of individual human cells by trapped ion mobility time-of-flight mass spectrometry. en. Nature Communications 13, 7246. (2024) (Nov. 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papalexi E. & Satija R. Single-cell RNA sequencing to explore immune cell heterogeneity. en. Nature Reviews Immunology 18, 35–45. (2024) (Jan. 2018). [DOI] [PubMed] [Google Scholar]
- 22.Derks J. et al. Increasing the throughput of sensitive proteomics by plexDIA. en. Nat. Biotechnol. (July 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derks J. & Slavov N. Strategies for increasing the depth and throughput of protein analysis by plexDIA. Journal of Proteome Research 22, 697–705 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budnik B., Levy E., Harmange G. & Slavov N. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biology 19, 161. (2024) (Oct. 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Specht H. & Slavov N. Optimizing Accuracy and Depth of Protein Quantification in Experiments Using Isobaric Carriers. Journal of Proteome Research 20, 880–887. (2021) (Jan. 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welter A. S. et al. Combining data independent acquisition with spike-in SILAC (DIA-SiS) improves proteome coverage and quantification. bioRxiv (2024). [DOI] [PubMed] [Google Scholar]
- 27.Van Bentum M. et al. Spike-in enhanced phosphoproteomics uncovers synergistic signaling responses to MEK inhibition in colon cancer cells en. May 2024. (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay S. et al. Single-cell NF-κB dynamics reveal digital activation and analogue information processing. Nature 466, 267–271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son M. et al. Spatiotemporal NF-κB dynamics encodes the position, amplitude, and duration of local immune inputs. Sci Adv 8, eabn6240 (Sept. 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy E. & Slavov N. Single cell protein analysis for systems biology. Essays In Biochemistry 62 (April 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang A. G., Son M., Kenna E., Thom N. & Tay S. NF-κB memory coordinates transcriptional responses to dynamic inflammatory stimuli. Cell reports 40 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q. et al. NF-κB dynamics discriminate between TNF doses in single cells. Cell systems 5, 638–645 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee R. E. C., Walker S. R., Savery K., Frank D. A. & Gaudet S. Fold change of nuclear NF-B determines TNF-induced transcription in single cells. eng. Molecular Cell 53, 867–879 (Mar. 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noursadeghi M. et al. Quantitative imaging assay for NF-kappaB nuclear translocation in primary human macrophages. eng. Journal of Immunological Methods 329, 194–200 (Jan. 2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai N. et al. Direct Visualization of the Translocation of the -Subspecies of Protein Kinase C in Living Cells Using Fusion Proteins with Green Fluorescent Protein. Journal of Cell Biology 139, 1465–1476. (2024) (Dec. 1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Shyy J. Y.-J. & Chien S. Fluorescence Proteins, Live-Cell Imaging, and Mechanobiology: Seeing Is Believing. en. Annual Review of Biomedical Engineering 10, 1–38. (2024) (Aug. 2008). [DOI] [PubMed] [Google Scholar]
- 37.Krishnaswami S. R. et al. Using single nuclei for RNA-seq to capture the transcriptome of postmortem neurons. en. Nature Protocols 11, 499–524. (2024) (Mar. 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ammar C., Gruber M., Csaba G. & Zimmer R. MS-EmpiRe Utilizes Peptide-level Noise Distributions for Ultra-sensitive Detection of Differentially Expressed Proteins[S]. English. Molecular & Cellular Proteomics 18, 1880–1892. (2024) (Sept. 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartels R. The Rank Version of von Neumann’s Ratio Test for Randomness. Journal of the American Statistical Association 77, 40–46. (2024) (1982). [Google Scholar]
- 40.Ghavami A., van der Giessen E. & Onck P. R. Energetics of Transport through the Nuclear Pore Complex. PLoS ONE 11, e0148876. (2024) (Feb. 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing C. E., Fung H. Y. J. & Chook Y. M. Karyopherin-mediated nucleocytoplasmic transport. en. Nature Reviews Molecular Cell Biology 23, 307–328. (2024) (May 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keminer O. & Peters R. Permeability of single nuclear pores. Biophysical Journal 77, 217–228. (2024) (July 1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paci G., Zheng T., Caria J., Zilman A. & Lemke E. A. Molecular determinants of large cargo transport into the nucleus. eLife 9 (eds Singer R. H.& Pfeffer S. R.) e55963. (2024) (July 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timney B. L. et al. Simple rules for passive diffusion through the nuclear pore complex. Journal of Cell Biology 215, 57–76. (2023) (Oct. 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou H.-R., Islam Z. & Pestka J. J. Kinetics of lipopolysaccharide-induced transcription factor activation/inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicology and Applied Pharmacology 187, 147–161. (2024) (Mar. 2003). [DOI] [PubMed] [Google Scholar]
- 46.Ochi T. et al. PAXX, a paralog of XRCC4 and XLF, interacts with Ku to promote DNA double-strand break repair. Science (New York, N.Y.) 347, 185–188. (2024) (Jan. 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xing M. et al. Interactome analysis identifies a new paralogue of XRCC4 in non-homologous end joining DNA repair pathway. en. Nature Communications 6, 6233. (2024) (Feb. 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Shao Z., Jiang W., Lee B. J. & Zha S. PAXX promotes KU accumulation at DNA breaks and is essential for end-joining in XLF-deficient mice. Nature Communications 8, 13816. (2024) (Jan. 2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCloskey A., Ibarra A. & Hetzer M. W. Tpr regulates the total number of nuclear pore complexes per cell nucleus. Genes & Development 32, 1321–1331. (2023) (Oct. 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leduc A., Koury L., Cantlon J. & Slavov N. Massively parallel sample preparation for multiplexed single-cell proteomics using nPOP en. Nov. 2023. (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petelski A. A. et al. Multiplexed single-cell proteomics using SCoPE2. Nature Protocols 16, 5398–5425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Bergen N. J. et al. Pathogenic variants in nucleoporin TPR (translocated promoter region, nuclear basket protein) cause severe intellectual disability in humans. Human Molecular Genetics 31, 362–375. (2024) (Feb. 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosalaganti S. et al. AI-based structure prediction empowers integrative structural analysis of human nuclear pores. Science 376, eabm9506. (2024) (June 2022). [DOI] [PubMed] [Google Scholar]
- 54.Mathieson T. et al. Systematic analysis of protein turnover in primary cells. Nature Communications 9, 689. (2024) (Feb. 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savas J. N., Toyama B. H., Xu T., Yates J. R. & Hetzer M. W. Extremely Long-lived Nuclear Pore Proteins in the Rat Brain. Science (New York, N.y.) 335, 942. (2024) (Feb. 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raman N., Weir E. & Muller S. The AAA ATPase MDN1 Acts as a SUMO-Targeted Regülator in Mammalian Pre-ribosome Remodeling. English. Molecular Cell 64, 607–615. (2024) (Nov. 2016). [DOI] [PubMed] [Google Scholar]
- 57.Schink S., Ammar C., Chang Y.-F., Zimmer R. & Basan M. Analysis of proteome adaptation reveals a key role of the bacterial envelope in starvation survival. Molecular Systems Biology 18, e11160. (2024) (Dec. 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ori A. et al. Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines. Molecular systems biology 9, 648 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lund M. E., To J., O’Brien B. A. & Donnelly S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a proinflammatory stimulus. Journal of Immunological Methods 430, 64–70. (2023) (Mar. 2016). [DOI] [PubMed] [Google Scholar]
- 60.Specht H. et al. Automated sample preparation for high-throughput single-cell proteomics en. Aug. 2018. (2022). [Google Scholar]
- 61.Dou M. et al. Automated Nanoflow Two-Dimensional Reversed-Phase Liquid Chromatography System Enables In-Depth Proteome and Phosphoproteome Profiling of Nanoscale Samples. Analytical Chemistry 91, 9707–9715. (2024) (Aug. 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meier F. et al. diaPASEF: parallel accumulation–serial fragmentation combined with data-independent acquisition. en. Nature Methods 17, 1229–1236. (2024) (Dec. 2020). [DOI] [PubMed] [Google Scholar]
- 63.Demichev V. et al. dia-PASEF data analysis using FragPipe and DIA-NN for deep proteomics of low sample amounts. en. Nature Communications 13, 3944. (2024) (July 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demichev V., Messner C. B., Vernardis S. I., Lilley K. S. & Ralser M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nature methods 17, 41–44 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cox J. et al. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Molecular & Cellular Proteomics : MCP 13, 2513–2526. (2022) (Sept. 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thul P. J. & Lindskog C. The human protein atlas: A spatial map of the human proteome. Protein Science : A Publication of the Protein Society 27, 233–244. (2021) (Jan. 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pontén F., Jirström K. & Uhlen M. The Human Protein Atlas—a tool for pathology. en. The Journal of Pathology 216, 387–393. (2024) (2008). [DOI] [PubMed] [Google Scholar]
- 68.Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. (2024) (1995). [Google Scholar]
- 69.Reimand J., Kull M., Peterson H., Hansen J. & Vilo J. g:Profiler—a web-based toolset for functional profiling of gene lists from large-scale experiments. en. Nucleic Acids Research 35, W193–W200. (2024) (July 2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kolberg L. et al. g:Profiler—interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). en. Nucleic Acids Research 51, W207–W212. (2024) (July 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The UniProt Consortium. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Research 51, D523–D531. (2024) (Jan. 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson W. E., Li C. & Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. eng. Biostatistics (Oxford, England) 8, 118–127 (Jan 2007). [DOI] [PubMed] [Google Scholar]
- 73.Leek J. T., Johnson W. E., Parker H. S., Jaffe A. E. & Storey J. D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882–883. (2024) (Mar. 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makowski D., Ben-Shachar M. S. & Lüdecke D. bayestestR: Describing Effects and their Uncertainty, Existence and Significance within the Bayesian Framework. en. Journal of Open Source Software 4, 1541. (2024) (Aug. 2019). [Google Scholar]
- 75.Goddard T. D. et al. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. en. Protein Science 27, 14–25. (2024) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are reported and deposited in accordance with the community guidelines13. Raw data, spectral libraries, FASTAs, and DIA-NN search outputs are available at MassIVE: MSV000094829. Code, processed data, supporting files, supplementary figures, and interactive supplementary analyses (e.g. volcano plots and time-series data) are available at scp.slavovlab.net/Derks et al 2024.