Abstract
Background
Intermittent preventive treatment for malaria in pregnancy (IPTp) can improve birth outcomes, but whether it confers benefits to postnatal growth is unclear. We investigated the effect of IPTp on infant growth in Uganda and its pathways of effects using causal mediation analyses.
Methods
We analyzed data from 633 infants born to mothers enrolled in a randomized trial of monthly IPTp with dihydroartemisinin-piperaquine (DP) vs sulfadoxine-pyrimethamine (SP) (NCT02793622). Weight and length were measured from 0–12 months of age. Using generalized linear models, we estimated effects of DP vs. SP on gravidity-stratified mean length-for-age (LAZ) and weight-for-length Z-scores (WLZ). We investigated mediation by placental malaria, gestational weight change, maternal anemia, maternal inflammation-related proteins, preterm birth, birth length, and birth weight. Mediation models adjusted for infant sex, gravidity, gestational age at enrollment, maternal age, maternal parasitemia at enrollment, education, and wealth.
Findings
SP increased LAZ by 0.18–0.28 Z from birth through age 4 months compared to DP, while DP increased WLZ by 0.11–0.28 Z from 2–8 months compared to SP among infants of multigravidae. We did not observe these differences among primigravida. Mediators of SP included increased birth weight and length and maternal stem cell factor at delivery. Mediators of DP included placental malaria and birth length, maternal IL-18, CDCP1, and CD6 at delivery.
Interpretation
In high malaria transmission settings, different IPTp regimens influenced infant growth among multigravidae through distinct pathways in the period of exclusive breastfeeding, when few other interventions are available.
Funding
Stanford Center for Innovation and Global Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bill & Melinda Gates Foundation
Introduction
Child growth faltering is associated with a large burden of disease, including increased risk of death and infections in childhood and lower productivity in adulthood.1–4 Children in low-and middle income countries often experience growth faltering before age 6 months, when complementary feeding and most child nutrition interventions are initiated.2,5,6 In malaria-endemic settings, prenatal malaria infection may be an important contributor to growth failure because of its effects on inflammation, anemia, and intrauterine growth restriction,7–9 which are linked to low birth weight, premature birth, stillbirth, and fetal death.10–12
In moderate-to-high Plasmodium falciparum (Pf) malaria transmission settings, the World Health Organization recommends intermittent preventive treatment of malaria in pregnancy (IPTp) with sulfadoxine-pyrimethamine (IPTp-SP).13 However, in eastern and Southern Africa, the antimalarial efficacy of SP has waned due to increasing parasite resistance to SP.14 Three trials in areas of high SP resistance of Kenya and Uganda found that IPTp with dihydroartemisinin-piperaquine (IPTp-DP) significantly reduced the risk of malaria during pregnancy, but were not associated with better birth outcomes relative to IPTp-SP.15–17 Ultimately, the purpose of IPTp is not only to improve outcomes at birth but also in infancy and beyond. Yet no prior studies have assessed effects of IPTp on child growth.
SP and DP likely influence child growth through distinct pathways given DP’s higher efficacy against malaria15–17 and SP’s antibiotic properties.18 Prior studies reported that effects of IPTp on birth weight were mediated by placental malaria for DP;19 given that maternal malaria infection is associated with impaired child height and weight gain in infancy, DP’s benefits may extend into childhood.20–23 Another study found that SP’s effect on birth weight was mediated by gestational weight gain,24 and maternal anthropometry is strongly associated with child growth.2 Maternal inflammation may be another important pathway: sequestration of Pf parasites in the placenta can result in inflammation, dysregulated development, and impaired nutrient transport in the placenta, which can negatively impact fetal development25 and child growth.26–30
Using data from a randomized trial of Ugandan mother-infant dyads, we assessed whether IPTp-DP improved infant growth from birth to 12 months compared to IPTp-SP. In addition, we investigated whether maternal inflammation, anemia, preterm birth, gestational weight gain, and birth weight and length mediated the effects of IPTp on child growth from birth to 12 months.
Methods
Study population
This is a secondary analysis of data collected in a double-blind randomized phase III trial in Busia district, a high malaria transmission area of southeastern Uganda (NCT02793622). Between September 6, 2016, and May 29, 2017, the study enrolled 782 HIV-uninfected women, at least 16 years of age, with a viable pregnancy between 12 and 20 weeks of gestation confirmed by ultrasound. The study followed their live births for 12 months from April 1, 2017 to October 31, 2018. Eligible women with a history of serious adverse events to SP or DP, early or active labor, chronic medical conditions or active medical problems requiring inpatient evaluation, or previous antimalarial therapy during the pregnancy were not enrolled in the study. Women were randomized at a 1:1 ratio to receive monthly IPTp with SP or monthly DP starting at 16 or 20 weeks of gestation. Both pharmacists administering medications and study participants were masked. We excluded multiple births (e.g., twins), spontaneous abortions, and stillbirths. The final analysis dataset included data from 633 children in the birth cohort.
Follow-up measurements
Women were scheduled for routine visits every 4 weeks. During each visit, blood was collected to detect the presence of malaria parasites by microscopy or quantitative PCR (qPCR). Women also underwent routine laboratory testing every 8 weeks. Children born to the study participants were scheduled for visits to the clinic at 1, 4, 6, and 8 weeks of age and then every 4 weeks until they reached 52 weeks of age. Women were encouraged to visit the study clinic for all medical care for themselves or their children; the clinic was open daily, and participants received a refund for transportation costs.
Study participants, both mothers and their children, had a standardized history and physical exam taken during clinic visits which included temperature, pulse, and blood pressure measurements. Those who were febrile (tympanic temperature > 38.0 °C) or reported a fever in the past 24 hours and had a positive blood smear were treated for malaria with artemether-lumefantrine for uncomplicated malaria and intravenous artesunate for complicated malaria.
Intervention
DP was given as 3 tablets taken once a day for 3 consecutive days every month (40 mg dihydroartemisinin and 320 mg piperaquine; Duo-Cotecxin, Holley-Cotec, Beijing). SP was given as a single dose consisting of 3 tablets every month (500 mg of sulfadoxine and 25 mg of pyrimethamine; Kamsidar, Kampala Pharmaceutical Industries, Uganda). To ensure participant blinding, participants in the DP arm received SP placebos, and participants in the SP arm received DP placebos each month. Study staff directly observed administration of the first dose of each intervention. Subsequent doses were self-administered at home, and adherence for those doses was assessed by self-report.
Mediators
Mediators included gestational weight change, prenatal anemia, placental malaria, preterm birth, low birth weight, and maternal inflammation. Since pre-pregnancy weight was not available, instead of measuring gestational weight gain, we calculated the change in gestational weight from 20 to 36 weeks gestation. We used weight at 36 weeks instead of delivery to increase consistency between women since the majority of women delivered after 36 weeks. Staff measured maternal hemoglobin at 28 weeks gestation and classified mothers as anemic if blood hemoglobin was < 11 g/dL. Placental malaria was assessed at delivery using histopathological assessment of placenta tissue or testing for parasitemia in the placental blood by loop-mediated isothermal amplification. In the analysis, we considered women who had placental malaria under either method to be positive. We classified low birth weight as birth weight ≤2500g and preterm birth as deliveries prior to 37 weeks gestation.
Using maternal plasma samples collected at delivery in a random, arm-stratified subsample of 264 mothers, we measured inflammation-related proteins using the Olink Target 96 inflammation panel. This high-multiplex immunoassay panel identifies 92 proteins associated with immune response. Inflammation biomarker data were log2 transformed and normalized to a Normalized Protein eXpression (NPX) relative quantification unit such that a one-unit change in NPX is equivalent to a two-fold increase in protein concentration. Twenty-five proteins were excluded from analysis because they failed to quantify in >50% of samples or the NPX value was below its limit of detection in >50% of samples (Table S1). We used principal components analysis and established pathway analysis and term enrichment databases (Blood Transcriptional Modules, Gene Ontology, KEGG) to reduce the dimensionality and identify clusters of proteins. However, we did not observe meaningful clusters, so statistical analyses used individual proteins. We corrected p-values for Olink analyses using Benjamini-Hochberg correction (false discovery rate p-value < 0.05).31
Outcomes
Study staff measured length and weight in infants each month from birth to 12 months. We calculated Z-scores using the World Health Organization (WHO) Child Growth Reference Standard.32 We calculated mean length-for-age (LAZ), weight-for-length (WLZ), and weight-for-age (WAZ) Z-scores in quarterly intervals. In a sensitivity analysis, we corrected Z-scores for gestational age: for infants with gestational age < 37 weeks, we calculated age as the postnatal age subtracted by difference between gestational age at birth and 280 days.
We defined stunting as LAZ < 2 standard deviations (SD) below the median of the WHO standard and wasting as WLZ < 2 SDs below the median. For binary outcomes, we calculated incidence in quarterly intervals as the number of children who became stunted or wasted during the interval divided by the number who were not previously stunted or wasted at the start of the interval. We assumed children were at risk of each outcome at birth or their first measurement.
Statistical analysis
The analysis plan was pre-specified at https://osf.io/f8wy4/. See deviations in Supplement 1.
To understand the mechanisms through which IPTp affects child growth faltering, we used causal mediation analyses (Figure S1).33–35 We estimated total effects as the effect of IPTp on child growth faltering through all pathways, including through measured and unmeasured mediators. The total effect can be decomposed into the direct effect (i.e., “natural direct effect”) and the mediated effect (i.e., “natural indirect effect”).36 The direct effect measures the effect of IPTp on child growth if we disabled pathways through mediators, while the mediated effect measures the effect of IPTp on child growth that operates through mediators.
We estimated total effects of IPTp DP vs. SP using generalized linear models with a Gaussian family and identity link for continuous outcomes (Z-scores) and generalized linear models with a Poisson family and log link for dichotomous outcomes (stunting and wasting).37 We fit unadjusted models because characteristics were balanced at baseline between study groups.17
We fit models to estimate effects of IPTp (DP vs. SP) on mediators and associations between mediators and each outcome using generalized linear models as defined above. We report crude intervention-mediator effects since the intervention was randomized. For mediation analyses, mediator and outcome models adjusted for mediator-outcome confounders by choosing nodes sufficient to block backdoor pathways in Figure S1. Potential confounders included maternal gravidity, infant sex, gestational age at enrollment, maternal age, maternal parasitaemia status at enrollment, education, and household wealth. We estimated mediation parameters and obtained 95% confidence intervals using a quasi-Bayesian Monte Carlo approach with 1,000 simulations.38 We compared outcome models with and without intervention-mediator interaction terms; because results were similar, we report estimates from models without interaction terms. We performed a complete case analysis. The percentage of live births with anthropometric measurements was 98% at birth, 89% at 6 months, and 78% at 12 months; follow-up was similar between arms.
Identification assumptions of causal mediation analyses include no unmeasured confounding of the intervention-outcome relationship, mediator-outcome relationship, or intervention-mediator relationship; temporal ordering intervention, mediators, and outcomes; and no mediator-outcome confounder that is itself affected by the intervention.34 The first assumption is met because intervention was randomized. We adjusted for confounders in intervention-outcome and mediator-outcome models to minimize confounding. Intervention, mediators, and outcomes were temporally sequenced.
Ethics
This study was approved by the Institutional Review Board at Stanford University (#40857); the original trial was approved by ethics committees of Makerere University School of Biomedical Sciences (Kampala, Uganda, approval number SBS-342), the Uganda National Council for Science and Technology (Kampala, Uganda; HS 2052).
Role of funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Characteristics of the study population
The study initially enrolled 782 women. This analysis excluded 149 individuals due to study withdrawal, spontaneous abortions, stillbirth, non-singleton birth, and missing placental malaria measurements. The analysis sample included 633 singleton mother-infant dyads (Figure S2), 152 (24%) of which were primigravidae. Mothers’ mean age was 24 years. The average gestational weight change between week 20 and week 36 was 3.4 kg. We documented 296 cases of anemia and 34 (5%) preterm deliveries. The prevalence of placental malaria at delivery was 80% among primigravidae and 33% among multigravidae.
Child growth faltering
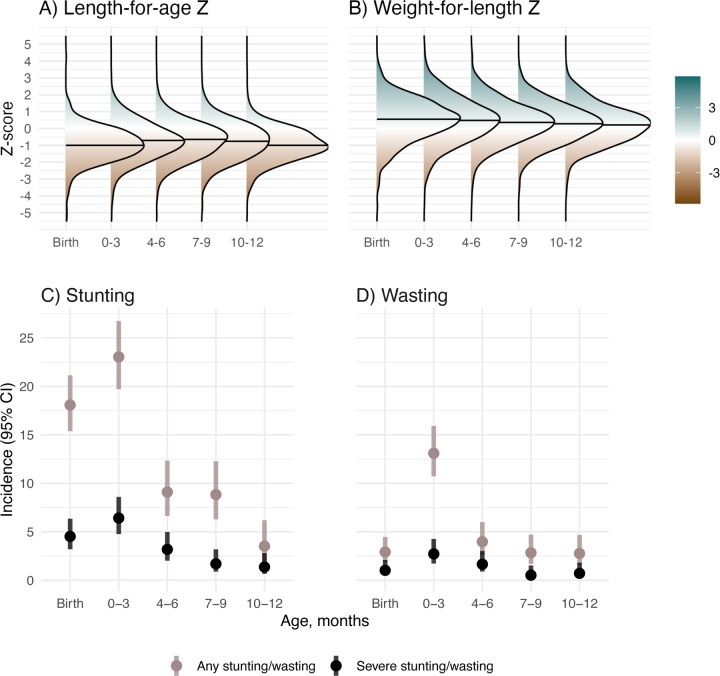
At birth, median LAZ was −1.0 (IQR=1.05) and median WLZ was 0.55 (IQR=1.63); mean LAZ was −0.99 (95% CI −1.07, −0.91) and mean WLZ was 0.49 (95% CI 0.40, 0.59) (Figure 1a–b). The proportion of children experiencing incident stunting onset was 18% at birth and 23% from age 1 day to 3 months, and <10% at subsequent ages (Figure 1c). At each age, the incidence of severe stunting was 6% or lower, with higher incidence before age 3 months. Wasting incidence was 13% from 1 day to 3 months and was <4% at all other ages (Figure 1d). Severe wasting incidence was <3% at all ages.
Figure 1. Child growth from birth through 12 months.
0–3 includes children aged 1 day to 3 months, 4–6 includes children aged >3 to 6 months, 7–9 includes children aged >6 to 9 months, and 10–12 includes children aged >9 to 12 months. In A) and B) horizontal lines in each density plot indicate the median Z-score for each age category.
Effects of IPTp on child growth
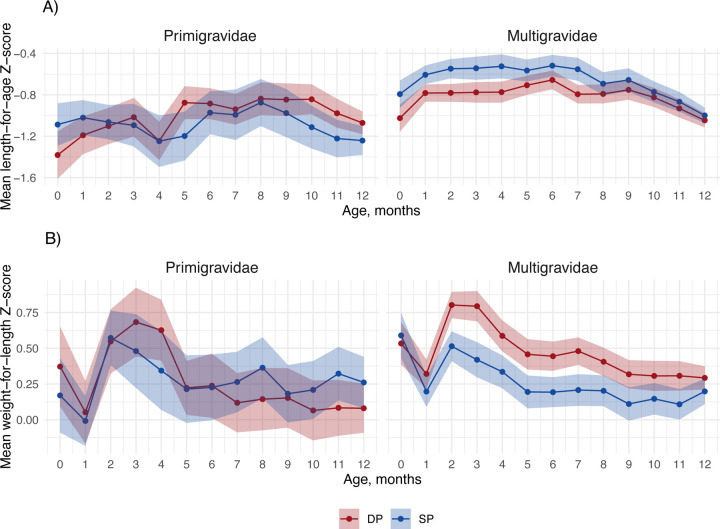
Among primigravidae, there were minimal differences in LAZ and WLZ by age between study arms (Figure 2). Among multigravidae, SP increased mean LAZ by 0.19–0.27 Z from birth to 4 months compared to DP, with mean LAZ around −0.55 in the SP arm and −0.75 in the DP arm at these ages. Compared to SP, DP increased mean WLZ from 2–8 months by 0.11–0.28 Z. Gestational age correction increased LAZ by approximately 0.2 Z and WLZ by approximately 0.5 Z and did not change patterns by arm (Figure S3). The incidence of stunting and wasting by age was similar between study arms (Table S2).
Figure 2. Total effect of IPTp DP vs. SP on mean child growth Z-scores by child age and gravidity.
Includes 633 children measured from birth to twelve months. The shaded region represents the 95% confidence interval, colored by IPTp group.
Effects of IPTp on mediators
SP improved mean birth length by 0.47 cm (95% CI 0.11, 0.82) and mean birth weight by 60 g (95% CI 0, 130) compared to DP (Figure 3). For birth length, the effect was larger among primigravidae, but for birth weight, the effect was only present among multigravidae. Placental malaria was lower for DP compared to SP (incidence ratio = 0.45, 95% CI 0.35, 0.58), and the effect was stronger among multigravidae.
Figure 3. Effect of IPTp-DP vs. IPTp-SP on potential mediators.
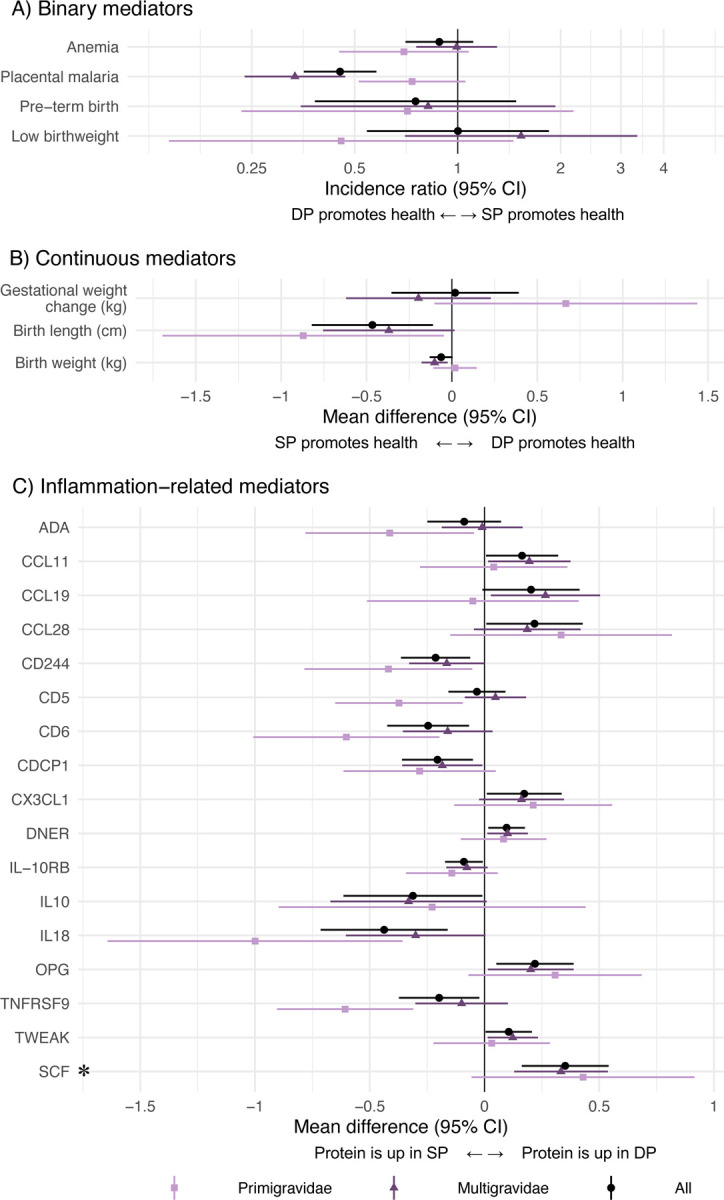
Unadjusted incidence ratios and mean differences between IPTp-DP and IPTp-SP. The asterisk indicates a statistically significant effect in Olink inflammation analyses among all gravidae after Benjamini-Hochberg correction (false discovery rate p-value < 0.05). C) only includes Olink results with statistically significant differences between arms; results for all Olink proteins are shown in Figure S4.
We identified 17 biomarkers that were either upregulated in DP relative to SP or vice versa. DP increased certain inflammation-related proteins at delivery, including Fractalkine (CX3CL1), Delta and Notch-like epidermal growth factor-related receptor (DNER), Osteoprotegerin (OPG), and SCF relative to SP. SP increased CD244, T cell surface glycoprotein CD6 isoform (CD6), CUB domain-containing protein 1 (CDCP1), interleukin-18 (IL-18), and tumor necrosis factor receptor superfamily member 9 (TNFRSF9) compared to DP. The largest effects were on OPG, IL-18, and SCF. Compared to SP, DP increased SCF by 0.35 NPX (95% CI 0.16, 0.54), increased OPG by 0.24 NPX (95% CI 0.08, 0.41), and decreased IL-18 by 0.48 NPX (95% CI 0.20, 0.76). These are equivalent to 70%, 48%, and −96% percent changes, respectively. After applying the Benjamini-Hochberg correction, only stem cell factor (SCF) was upregulated in DP (Figure 3, Figure S4). We did not observe differences in anemia, pre-term birth, low birth weight, gestational weight change, or other inflammation-related proteins between arms.
Association between mediators and child growth
Mediators strongly associated with LAZ and WLZ included pre-term birth, birth weight, and birth length (Figure S5). Placental malaria was associated with 0.30 (95% CI 0.03, 0.57) lower mean LAZ at birth among multigravidae, but there was no association at other ages or among primigravidae. Preterm birth was associated with increased stunting incidence at birth and increased stunting and wasting incidence from 1 day to 3 months (Table S3). Birth length was associated with lower stunting incidence from birth through 9 months and higher wasting incidence at birth. Birth weight was associated with lower stunting and wasting incidence at certain ages. Multiple inflammation-related proteins were associated with LAZ, WLZ, stunting, and wasting (Figure S6–S7). After multiple testing correction, only two protein associations with WLZ at certain ages were statistically significant (Figure S6).
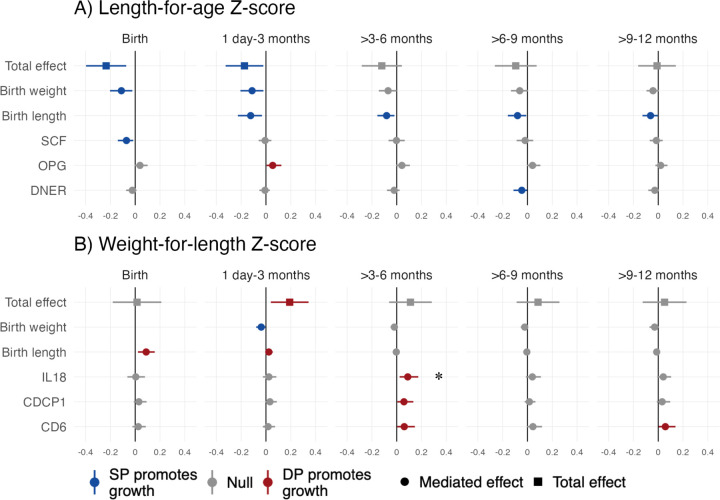
Mediators of the effect of IPTp on linear growth
Mediators of the effect of SP compared to DP on LAZ included birth weight and birth length and inflammation-related proteins SCF and DNER. Birth weight was a mediator from birth through 3 months, and birth length was a mediator at all ages (Figure 4a, Figure 5). Reductions in SCF mediated SP’s effects on LAZ at birth (Benjamini-Hochberg (BH) p=0.072), and reductions in DNER mediated effects at 6–9 months (BH p=0.504). Compared to SP, DP increased LAZ from 1 day-3 months by increasing OPG (BH p=0.396). The effect of IPTp on stunting at birth was mediated by pre-term birth, low birth weight, and SCF (BH p=0.543) (Figure S8). For linear growth outcomes, there was no evidence of mediation by placental malaria, maternal anemia, preterm birth, gestational weight change, or other inflammation-related proteins (Table S4, Figures S9, S10, S11).
Figure 4. Total effects and mediated effects on child growth Z-scores.
The total effects compare mean Z-scores between IPTp-DP and IPTp-SP using unadjusted models. The mediated effects were adjusted by infant sex, maternal age, maternal baseline parasitemia, gestational age at enrollment, maternal education, household wealth, and gravidity. The reference group was SP. Includes all gravidae.
*Statistically significant among all gravidae after Benjamini-Hochberg correction (false discovery rate p-value < 0.05).
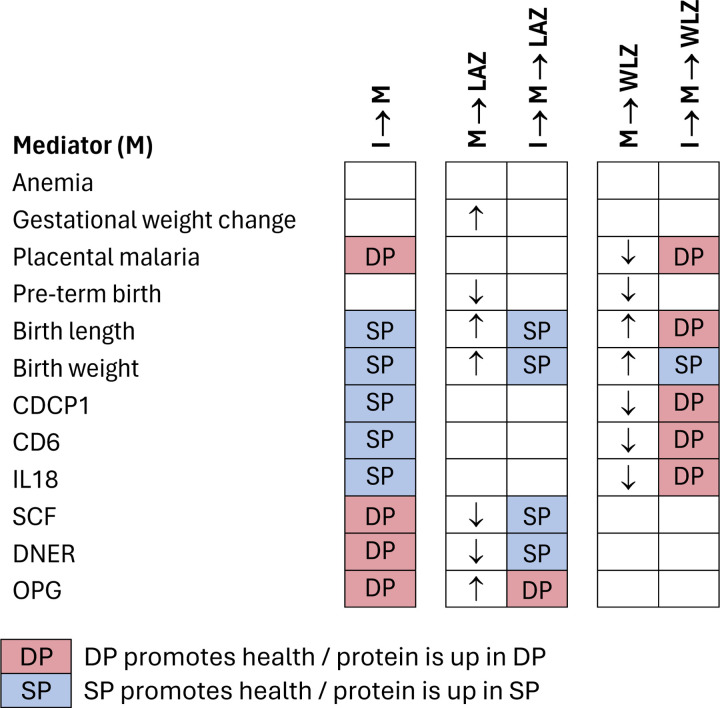
Figure 5. Summary of results for each mediating pathway.
I: Intervention (IPTp DP vs. SP); LAZ: length-for-age Z; WLZ: weight-for-length Z
I → M indicate results of intervention-mediator models. M → LAZ indicate results of models of the association between each mediator and LAZ (and analogously for WLZ). I → M→ LAZ indicate results of mediation models for LAZ (and analogously for WLZ). Cells with DP indicate that IPTp-DP improved the outcome compared to IPT-SP or that DP increased inflammation-related proteins relative to SP. We considered improvements to be reductions in anemia, placental malaria, pre-term birth, and inflammation-related proteins and increases in gestational weight change and birth length or weight. Arrows indicate whether the mediator was associated with higher(↑) or lower (↓) mean Z-scores.
Mediators of the effect of IPTp on ponderal growth
Mediators of the effect of DP compared to SP on WLZ included birth size up to 3 months and certain inflammation-related proteins later in infancy. Relative to DP, SP increased WLZ from birth through 3 months by increasing birth weight (Figure 4b, Figure 5). DP’s effect on WLZ from birth to 3 months was mediated by birth length; this reflects lower birth lengths and greater weight gain in DP vs. SP. The effect of DP on increased WLZ at birth was also mediated by reduced placenta malaria relative to SP, however, the confidence interval included the null (Table S4). Relative to SP, DP improved WLZ by decreasing IL-18, CDCP1, and CD6 from 4–6 months; CD6 was also a mediator from 9–12 months. After multiple testing correction, the only BH p-value <0.05 was for IL-18 at 3–6 months. The effect of IPTp on wasting was mediated by low birth weight and birth weight up to age 3 months (Figure S8). There was no evidence of mediation of the effect of DP or SP on WLZ or wasting by placental malaria, maternal anemia, preterm birth, gestational weight change, or other inflammation-related proteins (Table S4, Figures S9, S12, S13).
Discussion
In this secondary analysis of a randomized trial of Ugandan mother-infant dyads, we found that IPTp-SP improved linear growth from birth to age 4 months compared to DP, and IPTp-DP improved ponderal growth from ages 2 to 8 months compared to SP among infants born to multigravidae. IPTp regimens affected growth through distinct pathways: SP increased linear and ponderal growth via increased birth size. DP increased ponderal growth through reduced inflammation and reduced placental malaria, consistent with a prior analysis,19 though evidence was weak for the latter. SP’s effects on linear growth and DP’s effects on ponderal growth were mediated by reductions in different inflammation-related proteins, with the strongest evidence for mediation of DP’s effect on ponderal growth by reduced IL-18. We did not observe differences between IPTp regimens on child growth in primigravidae, possibly because the adverse consequences of placental malaria are more severe in this subgroup, which may have offset the ‘non-malarial’ benefits of SP.19
Overall, the effects on child growth among multigravidae were similar in size or larger than effect sizes of preventive nutritional interventions. IPTp-SP increased mean LAZ by up to 0.27 Z compared to DP, and DP increased mean WLZ by up to 0.28 Z compared to SP. These differences in Z are similar to effect sizes for infant multiple micronutrient supplements in the exclusive breastfeeding stage.39 Many other early life nutritional interventions, such as nutrition education and counseling,40 complementary feeding,40 maternal micronutrient supplements,39 and small quantity lipid-based nutrient supplements, have had smaller effects.41 Importantly, we observed benefits of IPTp to child growth during the exclusive breastfeeding stage, when growth faltering onset is high and few nutritional interventions are delivered.5,6
Certain inflammation-related proteins mediated the effects of IPTp on growth, with the strongest evidence for IL-18 as a mediator of DP and WLZ. IL-18 is a proinflammatory cytokine that is up-regulated during Pf malaria infection;42,43 it is present throughout pregnancy in the placenta44 and in the blood, where levels become elevated in labor.45 Increased levels of IL-18 are associated with early pregnancy loss, recurrent miscarriages and other pregnancy complications.44–46 Some studies suggest that maternal inflammation may impact infant growth outcomes.27,47 Taken together, our findings support the growing body of research pointing to maternal inflammation in pregnancy as a mediator of fetal and infant growth.
Potential mediating pathways not investigated in this study could also explain the observed differences in child growth between SP and DP. A separate analysis of this trial suggested that some of SP’s benefit may result from reduced febrile respiratory infections.48 Other studies point to SP’s effects on maternal nutrition: a trial found that SP increased maternal mid-upper arm circumference relative to DP,49 and an in vitro study found that SP improved nutrient absorption.50 Another trial identified gestational weight change as a mediator of the effect of IPTp-SP compared to DP.24 We did not observe mediation by gestational weight change, possibly because weight changes were relatively small in our study. In future studies, it would also be valuable to investigate whether SP’s antibiotic properties influence the vaginal or gut microbiome and whether SP modulates maternal immunity.19
Limitations include the relatively low incidence of stunting and wasting, particularly at older ages, which may have limited statistical power, particularly in primigravid women. Second, we only had data on inflammation-related proteins in a subsample of mothers at delivery, which could have confounded our findings due to the extensive inflammation that occurs during labor and delivery. Inflammation earlier in pregnancy may also be important. Third, it is possible that mediator-outcome models were subject to residual unmeasured confounding. Finally, we investigated mediators individually, which implicitly assumed that mediators operated independently; it is possible that some mediators operated jointly.
Our findings suggest that in high malaria transmission settings, different IPTp regimens influence infant growth through distinct pathways from birth through 6 months, when there is a dearth of other effective nutritional interventions. WHO IPTp policies have focused on low birth weight, but our findings suggest that different IPTp regimens could improve ponderal and linear growth beyond birth. In areas of high parasite resistance to SP, SP and DP likely improve infant growth through different mechanisms, warranting research on their combined use for enhanced public health impact compared to either regimen alone.
Supplementary Material
Research in context.
Evidence before this study
Intermittent Preventive Treatment in Pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP) is recommended by the WHO for regions with moderate-to-high malaria transmission. While SP is effective in reducing neonatal mortality and low birth weight, its efficacy has diminished in some areas of sub-Saharan Africa due to widespread parasite resistance to SP. Although IPTp with dihydroartemisinin-piperaquine (IPTp-DP) has demonstrated superior efficacy in reducing malaria in pregnancy, its impact on birth outcomes has not significantly surpassed that of SP. The ultimate goal of IPTp extends beyond enhancing birth outcomes to include benefits during infancy and later stages. Yet, the effects of SP vs. DP in relation to infant growth post-birth and the underlying mechanisms remain unknown. Prior studies also found that different IPTp regimens worked through different pathways, with DP influencing birth outcomes by reducing placental malaria and SP influencing them through non-malarial pathways such as maternal weight gain. Here, we re-analyzed data from of a randomized trial in Uganda to explore the impacts of these two IPTp regimens on infant growth and to understand potential mechanisms underlying its impacts on infant growth.
Added value of this study
This study quantified how IPTp with SP compared to DP influenced infants’ growth trajectories, both ponderal and linear, during the first year of life. We found that SP improved linear growth of infants up to age 4 months compared to DP, and DP improved ponderal growth of infants from 2–8 months compared to SP among babies who were born to multigravidae. In addition, we identified birth size, placental malaria, and certain markers of maternal inflammation measured at delivery using the Olink Target 96 inflammation panel as pathways through which IPTp influenced infant growth. Our approach provides new insights into effects of IPTp beyond birth and the mechanisms by which IPTp impacts infant growth.
Implications of all the available evidence
Our study provides evidence that different IPTp regimens can influence infant postnatal growth through distinct pathways. Our findings highlight the potential of combined SP and DP IPTp regimens and bolster the evidence base for continued delivery of IPTp to improve maternal and child health outcomes, particularly in malaria-endemic regions.
Acknowledgements
This study was supported by the Stanford Center for Innovation in Global Health (270519). The original trial was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01 HD059454) and the Bill & Melinda Gates Foundation. Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers K01AI141616 (PI: Benjamin-Chung), U01 AI155325 (PI: Jagannathan), and the National Heart, Lung, And Blood Institute of the National Institutes of Health under award number T32HL151323 (Nguyen). MER is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Pathway to Independence Award (K99HD111572). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jade Benjamin-Chung is a Chan Zuckerberg Biohub Investigator.
Data and Code Availability
Data from the original trial is available at https://clinepidb.org/ce/app/workspace/analyses/DS_8786631aaf/new/variables/EUPATH_0000096/EUPATH_0015457. Replication scripts are available at https://github.com/YanweiTong-Iris/IPTp-BC3-mediation/tree/main
References
- 1.McDonald CM, Olofin I, Flaxman S, et al. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr 2013; 97: 896–901. [DOI] [PubMed] [Google Scholar]
- 2.Mertens A, Benjamin-Chung J, Colford JM, et al. Causes and consequences of child growth faltering in low-resource settings. Nature 2023; 621: 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet 2013; 382: 427–51. [DOI] [PubMed] [Google Scholar]
- 4.Adair LS, Fall CH, Osmond C, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet 2013; 382: 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin-Chung J, Mertens A, Colford JM, et al. Early-childhood linear growth faltering in low-and middle-income countries. Nature 2023; 621: 550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens A, Benjamin-Chung J, Colford JM, et al. Child wasting and concurrent stunting in low-and middle-income countries. Nature 2023; 621: 558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakama AK, Ozarslan N, Gaw SL. Placental Malaria. Curr Trop Med Rep 2020; 7: 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. The Lancet Infectious Diseases 2007; 7: 93–104. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ. Management of malaria in pregnancy. Indian J Med Res 2017; 146: 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Ouf NM, Jan MM. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med J 2015; 36: 146–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colella M, Frérot A, Novais ARB, Baud O. Neonatal and Long-Term Consequences of Fetal Growth Restriction. Curr Pediatr Rev 2018; 14: 212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menendez C, Ordi J, Ismail MR, et al. The Impact of Placental Malaria on Gestational Age and Birth Weight. The Journal of Infectious Diseases 2000; 181: 1740–5. [DOI] [PubMed] [Google Scholar]
- 13.WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). https://www.who.int/publications-detail-redirect/WHO-HTM-GMP-2014.4 (accessed Oct 5, 2023).
- 14.Eijk AM van, Larsen DA, Kayentao K, et al. Effect of Plasmodium falciparum sulfadoxine-pyrimethamine resistance on the effectiveness of intermittent preventive therapy for malaria in pregnancy in Africa: a systematic review and meta-analysis. The Lancet Infectious Diseases 2019; 19: 546–56. [DOI] [PubMed] [Google Scholar]
- 15.Desai M, Gutman J, L’lanziva A, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin–piperaquine versus intermittent preventive treatment with sulfadoxine–pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. The Lancet 2015; 386: 2507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakuru A, Jagannathan P, Muhindo MK, et al. Dihydroartemisinin-Piperaquine for the Prevention of Malaria in Pregnancy. N Engl J Med 2016; 374: 928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajubi R, Ochieng T, Kakuru A, et al. Monthly sulfadoxine–pyrimethamine versus dihydroartemisinin–piperaquine for intermittent preventive treatment of malaria in pregnancy: a double-blind, randomised, controlled, superiority trial. The Lancet 2019; 393: 1428–39. [DOI] [PubMed] [Google Scholar]
- 18.Capan M, Mombo-Ngoma G, Makristathis A, Ramharter M. Anti-bacterial activity of intermittent preventive treatment of malaria in pregnancy: comparative in vitro study of sulphadoxine-pyrimethamine, mefloquine, and azithromycin. Malar J 2010; 9: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roh ME, Kuile FO ter, Rerolle F, et al. Overall, anti-malarial, and non-malarial effect of intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine on birthweight: a mediation analysis. The Lancet Global Health 2020; 8: e942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Beaudrap P, Turyakira E, Nabasumba C, et al. Timing of malaria in pregnancy and impact on infant growth and morbidity: a cohort study in Uganda. Malaria Journal 2016; 15: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalanda BF, van Buuren S, Verhoeff FH, Brabin BJ. Catch-up growth in Malawian babies, a longitudinal study of normal and low birthweight babies born in a malarious endemic area. Early Human Development 2005; 81: 841–50. [DOI] [PubMed] [Google Scholar]
- 22.Walther B, Miles DJ, Crozier S, et al. Placental Malaria is associated with reduced early life weight development of affected children independent of low birth weight. Malar J 2010; 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holding PA, Kitsao-Wekulo PK. Describing the burden of malaria on child development: What should we be measuring and how should we be measuring it? The American Journal of Tropical Medicine and Hygiene 2004; 71: 71–9. [PubMed] [Google Scholar]
- 24.Waltmann A, McQuade ETR, Chinkhumba J, et al. The positive effect of malaria IPTp-SP on birthweight is mediated by gestational weight gain but modifiable by maternal carriage of enteric pathogens. eBioMedicine 2022; 77: 103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chua CLL, Hasang W, Rogerson SJ, Teo A. Poor Birth Outcomes in Malaria in Pregnancy: Recent Insights Into Mechanisms and Prevention Approaches. Frontiers in Immunology 2021; 12. 10.3389/fimmu.2021.621382 (accessed Jan 15, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. American Journal of Reproductive Immunology 2018; 80: e13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafiq M, Mathad JS, Naik S, et al. Association of Maternal Inflammation During Pregnancy With Birth Outcomes and Infant Growth Among Women With or Without HIV in India. JAMA Network Open 2021; 4: e2140584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeates AJ, McSorley EM, Mulhern MS, et al. Associations between maternal inflammation during pregnancy and infant birth outcomes in the Seychelles Child Development Study. Journal of Reproductive Immunology 2020; 137: 102623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadaret CN, Merrick EM, Barnes TL, et al. Sustained maternal inflammation during the early third-trimester yields intrauterine growth restriction, impaired skeletal muscle glucose metabolism, and diminished β-cell function in fetal sheep,. J Anim Sci 2019; 97: 4822–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimasuay KG, Aitken EH, Rosario F, et al. Inhibition of placental mTOR signaling provides a link between placental malaria and reduced birthweight. BMC Med 2017; 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society: Series B (Methodological) 1995; 57: 289–300. [Google Scholar]
- 32.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006; : 312 pages. [Google Scholar]
- 33.Pearl J. Direct and indirect effects. In: Proceedings of the Seventeenth conference on Uncertainty in artificial intelligence. San Francisco, CA, USA: Morgan Kaufmann Publishers Inc., 2001: 411–20. [Google Scholar]
- 34.VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford University Press, 2015. [Google Scholar]
- 35.Robins JM, Greenland S. Identifiability and Exchangeability for Direct and Indirect Effects. Epidemiology 1992; 3: 143–55. [DOI] [PubMed] [Google Scholar]
- 36.Pearl J. The Causal Mediation Formula—A Guide to the Assessment of Pathways and Mechanisms. Prev Sci 2012; 13: 426–36. [DOI] [PubMed] [Google Scholar]
- 37.Zou G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol 2004; 159: 702–6. [DOI] [PubMed] [Google Scholar]
- 38.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation : R Package for Causal Mediation Analysis. J Stat Soft 2014; 59. DOI: 10.18637/jss.v059.i05. [DOI] [Google Scholar]
- 39.Park JJH, Fang ML, Harari O, et al. Association of Early Interventions With Birth Outcomes and Child Linear Growth in Low-Income and Middle-Income Countries: Bayesian Network Meta-analyses of Randomized Clinical Trials. JAMA Netw Open 2019; 2: e197871–e197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panjwani A, Heidkamp R. Complementary Feeding Interventions Have a Small but Significant Impact on Linear and Ponderal Growth of Children in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. The Journal of Nutrition 2017; 147: 2169S–2178S. [DOI] [PubMed] [Google Scholar]
- 41.Dewey KG, Wessells KR, Arnold CD, et al. Characteristics that modify the effect of small-quantity lipid-based nutrient supplementation on child growth: an individual participant data meta-analysis of randomized controlled trials. Am J Clin Nutr 2021; : nqab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kojima S, Nagamine Y, Hayano M, Looareesuwan S, Nakanishi K. A potential role of interleukin 18 in severe falciparum malaria. Acta Tropica 2004; 89: 279–84. [DOI] [PubMed] [Google Scholar]
- 43.Otterdal K, Berg A, Michelsen AE, et al. IL-18 and IL-18 binding protein are related to disease severity and parasitemia during falciparum malaria. BMC Infect Dis 2021; 21: 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Löb S, Vilsmaier T, Schmoeckel E, Mahner S, Wöckel A, Jeschke U. The cytokines IL-1b and IL-18 are upregulated in the placenta of recurrent miscarriage patients. Journal of Reproductive Immunology 2023; 158: 103583. [Google Scholar]
- 45.Ida A, Tsuji Y, Muranaka J, et al. IL-18 in pregnancy; the elevation of IL-18 in maternal peripheral blood during labour and complicated pregnancies. Journal of Reproductive Immunology 2000; 47: 65–74. [DOI] [PubMed] [Google Scholar]
- 46.Wilson R, Moor J, Jenkins C, et al. Abnormal First Trimester Serum Interleukin 18 Levels are Associated with a Poor Outcome in Women with a History of Recurrent Miscarriage. American Journal of Reproductive Immunology 2004; 51: 156–9. [DOI] [PubMed] [Google Scholar]
- 47.Omoni AO, Ntozini R, Evans C, et al. Child Growth According to Maternal and Child HIV Status in Zimbabwe. The Pediatric Infectious Disease Journal 2017; 36: 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JJ, Kakuru A, Jacobson KB, et al. Monthly sulfadoxine-pyrimethamine during pregnancy prevents febrile respiratory illnesses: A secondary analysis of a malaria chemoprevention trial in Uganda. Open Forum Infectious Diseases 2024; : ofae143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madanitsa M, Barsosio HC, Minja DTR, et al. Effect of monthly intermittent preventive treatment with dihydroartemisinin–piperaquine with and without azithromycin versus monthly sulfadoxine–pyrimethamine on adverse pregnancy outcomes in Africa: a double-blind randomised, partly placebo-controlled trial. Lancet 2023; 401: 1020–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S, Naziripour A, Prabhala P, et al. Direct therapeutic effect of sulfadoxine-pyrimethamine on nutritional deficiency-induced enteric dysfunction in a human Intestine Chip. EBioMedicine 2024; 99: 104921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the original trial is available at https://clinepidb.org/ce/app/workspace/analyses/DS_8786631aaf/new/variables/EUPATH_0000096/EUPATH_0015457. Replication scripts are available at https://github.com/YanweiTong-Iris/IPTp-BC3-mediation/tree/main