Abstract
Post-Acute Sequelae of SARS-CoV-2 infection (PASC), also known as Long-COVID, encompasses a variety of complex and varied outcomes following COVID-19 infection that are still poorly understood. We clustered over 600 million condition diagnoses from 14 million patients available through the National COVID Cohort Collaborative (N3C), generating hundreds of highly detailed clinical phenotypes. Assessing patient clinical trajectories using these clusters allowed us to identify individual conditions and phenotypes strongly increased after acute infection. We found many conditions increased in COVID-19 patients compared to controls, and using a novel method to associate patients with clusters over time, we additionally found phenotypes specific to patient sex, age, wave of infection, and PASC diagnosis status. While many of these results reflect known PASC symptoms, the resolution provided by this unprecedented data scale suggests avenues for improved diagnostics and mechanistic understanding of this multifaceted disease.
Introduction
The long-term health consequences of SARS-CoV-2 are not fully understood.1 Research suggests that many patients experience persistent symptoms,2–4 known as Post-Acute Sequelae of SARS-CoV-2 infection (PASC; also known as Long-COVID), affecting multiple organ systems, including pulmonary, cardiovascular, hematological, neurological, and renal systems.5 The disruption of the host immune response is suspected to play a role in various PASC-associated conditions, including reactivation of dormant persistent infections,6 autoimmune responses,7 and multi-inflammatory syndrome of children (MIS-C).8 Emerging evidence indicates that PASC may present with one or more sub-phenotypes, comprising potentially overlapping clusters of frequently co-occurring symptoms, and prevalence of these may be influenced by patient demographics or infection severity.4,9–12
Various clustering algorithms have been applied to identify sub-phenotypes of PASC from patient data, including k-means clustering with disease ontology data, multiple correspondence analysis, hierarchical ascendant classification, association rule mining, and latent class analysis.9,10,13–15 Topic models, a class of natural-language clustering techniques suitable for electronic health record (EHR) data, are also widely used. Broadly, topic models identify ‘topics’ as sets of frequently co-occurring terms in a document corpus. In EHR contexts, a patient’s clinical history is treated as a document, and their conditions or other events serve as terms, often encoded in medical vocabularies such as ICD-10-CM codes. Topic modeling methods employed for PASC and COVID-19 include Poisson factor analysis, non-negative matrix factorization, and Latent Dirichlet Allocation (LDA).16–18 LDA is particularly well studied in the context of EHR data,19,20 characterizing each topic (interpreted as a phenotype or sub-phenotype depending on context) as a distinct probability distribution over terms (conditions or other medical events), and each document (patient) as a distinct distribution over topics.
While these studies have identified PASC sub-phenotypes in unique ways, common themes have emerged, including clusters representing cardiovascular,9,10,16,18,21 pulmonary,10,14,16,21, neurocognitive,10,14, musculoskeletal,9,10,14,16,21,22, and fatigue-related symptoms.10,14,21 In many cases these are combined and there exist one or more multi-system clusters.9,10,13,15 Despite the diverse methods applied, a variety of limitations prevent understanding these sub-phenotypes in a broader clinical context. For example, many studies cluster symptoms and diseases from the post-infection period only,9,10,13–17,22 and do not consider the presence of pre-existing sub-phenotypes beyond predetermined risk factors (though see Humpherys et al. who investigate mortality risk via independent pre- and post-infection clusterings18). Though some studies focus only on patients suspected or diagnosed with PASC,9,10,13,16 or differentiate between COVID-19 patients with and without PASC symptoms,14,21 few include individuals lacking any indicators of COVID-19.15,22 Without this broader comparison, identifying sub-phenotypes unique to these diseases is challenging. Finally, while clustering methods are naturally data driven, some research limits scope to conditions already presumed linked to PASC,9,13,15,16 potentially missing rare or otherwise unknown associations.
To better understand the long-term effects of COVID-19, we employed LDA topic modeling on EHR data from the National COVID Cohort Collaborative (N3C), encompassing over 12 million patients across 63 clinical sites and more than 230 healthcare locations.23 The resulting model identified hundreds of clinically-consistent condition clusters as topics representing potential sub-phenotypes of interest. New-onset rates for a diverse set of top-weighted conditions were significantly higher in held-out PASC and COVID-19 patients compared to Controls with no COVID-19 indication. Further, by analyzing whole-topic associations temporally (pre- and post-infection) among these cohorts, we identified several sub-phenotypes associated with COVID-19 or PASC, specific to patient age, sex, or pandemic wave. Many of our findings confirm established features of PASC, while others present new insights or more detailed perspectives, prompting further investigation into this complex and heterogeneous condition.
Methods
Study design
The LDA implementation we use aims to represent a document corpus as a set of topics, where each topic is characterized by a multinomial distribution over possible words or terms, and each document is characterized by a multinomial distribution over topics. It assumes a hierarchical generative model, where for any given term in a given document, a topic is first selected according to the document’s topic distribution, and then a term is selected according to that topic’s term distribution.24 In our application patient medical histories represent documents and conditions recorded for those patients represent terms, a common approach in topic modeling for EHR data.19,20
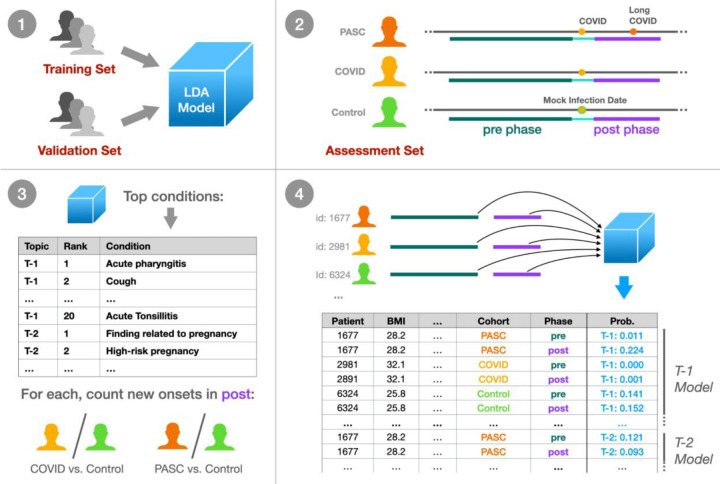
Figure 1 illustrates our approach. Initially, we trained a model on a diverse set of patient histories, including those with and without COVID-19, using independent training and validation patient sets to guide model development. The resulting topics represent a comprehensive set of phenotypes across the N3C cohort; to understand how these topics relate to patients over time and with respect to infection status, we held out an additional independent assessment patient set. Within this set we identified three cohorts: PASC, patients with a clinical PASC diagnosis, COVID, those with an indicated COVID-19 infection but no PASC diagnosis, and Control, those with neither. These patients’ histories were divided into pre- and post-infection clinical phases, assigning Control patients a mock infection date for the purpose. In a first set of statistical tests we individually assessed the top 20 high-relevance conditions from each topic, comparing new-onset rates post-infection for PASC and COVID patients compared to Controls. Next we assessed each topic as a whole, by modeling topic probability estimates derived separately from pre- and post-infection data. Including patient demographics such as age and sex in these models reveals which patient cohorts and groups experience significant changes in which topics post-infection, as compared to Controls.
Figure 1: Experimental design summary.
(1) We trained an LDA topic model on a broad set of N3C patient data, tuning and evaluating the model with a held-out validation set using the UCI coherence metric. (2) Within a separate held-out assessment patient set, we defined three cohorts: PASC (patients with Long COVID), COVID (COVID-19 only), and Control (neither). For these patients we defined a 1-year pre-infection phase 6-month post-infection phase, utilizing a mock infection date for Control patients. (3) For the top 20 conditions per topic, we assessed new onset rates for COVID and PASC patients compared to Controls in the post-infection phase. (4) Finally, we defined per-topic logistic models, with outcome variables as the topic model’s assigned probabilities to individual patient phase data. Model coefficients then relate patient demographics, cohort, infection phase, and combinations of these factors to topic assignment for further study.
Data pre-processing
N3C multi-site EHR data are harmonized to the OMOP common data model. We analyzed condition records from OMOP’s condition_era table, which merges repeated, identical condition records into single condition timeframes via a 30-day sliding window.25 We used N3C release v87, representing data as of Aug. 2, 2022, using only records from a subset of sites passing minimal quality filters (see Suppl. Methods). The COVID-19 diagnostic code (U07.1), being a major criterion for N3C data selection and present in 22% of patients, was excluded, along with early pandemic alternatives such as unspecified viral disease (10%) and disease due to coronaviridae (0.5%). Additional clinically-uninformative terms such as Clinical finding and Findings of sexual activity were removed, as were all entries not expected in the OMOP condition domain (Suppl. Methods, Suppl. Table 1). Records with implausible dates, either starting before January 1, 2018 (N3C’s earliest inclusion date) or extending beyond a site’s data contribution date, were also excluded. Patients were randomly assigned to sets, with 20% allocated to the assessment set and the remaining patients split 80% for training and 20% for validation (Suppl. Figure 1).
Cohort selection and clinical phases
Before conducting statistical analyses, we filtered patients in the assessment set to ensure data quality and consistency. Some N3C-contributing sites reported no U09.9 PASC diagnosis codes, possibly due to lack of implementation in their EHR software,26 leading us to exclude all patients from these sites to prevent misclassification of PASC patients. For individual-condition tests, we required patients to have at least two weeks of active condition history in both pre- and post-infection phases. For per-topic tests, we assessed patient features requiring complete data across all covariates and excluded Omicron patients due to incomplete data (see Statistical analyses below).
Each patient in the assessment cohorts was assigned an index date, representing their estimated first COVID-19 infection, or a mock infection date for Control patients. From these dates we identified two clinical phases per patient: a 1-year, pre-infection phase ending 15 days before the index (to account for acute symptoms prior to first diagnosis or test), and a 6-month, post-infection phase beginning 45 days after the index to capture possible PASC conditions.27 Conditions were counted in any phase they overlapped, allowing a single condition to be represented in both phases if applicable. Cohorts were restricted to patients supporting these three contiguous phases within a single observation period, as recorded in the OMOP observation_period table.
PASC:
Patients with a U09.9 PASC diagnosis code on or after Oct. 1, 2021 when this code was released, or the CDC-recommended alternative B94.8 Sequelae of other specified infectious and parasitic diseases prior to this date.28 For patients with a strong primary infection indicator, a positive SARS-CoV-2 Polymerase Chain Reaction (PCR) or Antigen (Ag) test (Suppl. Table 2) or U07.1 COVID-19 diagnosis, we chose the first of any of these as the infection index date. For patients without these we used the first PASC indicator as the index date. In cases where the primary infection indicator occurs within 45 days of PASC we considered the test or diagnosis unreliable and used the PASC indicator as the index date.
COVID:
Patients with a confirmed primary COVID-19 infection as indicated by a positive PCR test, antigen test, or U07.1 COVID-19 diagnosis, and who are not in the PASC cohort. Their infection index date was the first of any of these indicators.
Control:
Patients with no indication of COVID-19 in N3C data, including positive PCR, antigen, or antibody test (Suppl. Table 3), COVID-19 (U07.1) or PASC diagnosis (U09.9 or B94.8), or a visit to a PASC specialty clinic (unique information provided by six N3C-contributing sites).
Patients diagnosed with Multisystem inflammatory syndrome (M35.81) were excluded as potential confounders. Control patients were assigned a mock infection date, chosen uniformly at random to simulate pre-infection, acute, and post-infection phases of the correct lengths contained entirely within their longest continuous observation period. Mock infection dates were constrained to be after March 1, 2020 to align temporally with pandemic trends.
Machine learning methods
To train our topic model, we used full pre-processed N3C patient histories from the training set as documents, employing per-patient counts of OMOP condition_concept_id entries from the condition_era table. While many LDA variations exist, most are computationally prohibitive for the scale of data considered. We therefore adopted the online LDA method described by Hoffman et al.,24 implemented in Apache Spark version 3.2.1,29 with a 5% batch size and 10 iterations over the data (maxIter = 200, subsamplingRate = 0.05).
Using the held-out validation patient set we computed UCI Coherence30 to measure model quality and choose the final number of topics. For each topic, this unitless metric assesses how frequently top-weighted conditions co-occur in patients relative to random chance (see Suppl. Methods), with higher values indicating higher-quality topics. As coherence scores are normally distributed (see Results), we report coherence as a z-score C, with positive values indicating higher-than-average topic coherence and quality.
We further defined a usage value U (range 0–100%) as the average assigned probability across patients. Given that topic usage varied across N3C-contributing sites, we calculated a usage uniformity metric H, expressed as the normalized information entropy (range from 0–1) of site usage, with values approaching 1.0 indicating more uniform usage across sites. We also conducted topic similarity analysis via Jensen-Shannon distance (range 0–1), a symmetrical metric with values closer to 0.0 suggesting more similar topics.19,31,32
Common conditions such as Essential Hypertension may be highly weighted by many topics. A condition’s relevance to a topic is the log-ratio of the topic-specific probability to the condition’s global probability (as defined by the LDAVis package33 when γ = 0), with positive values indicating conditions more specific to a given topic. Highly-weighted terms with low relevance thus indicate those heavily used by multiple topics. Topic usage and term relevance were computed using both the validation and training sets for completeness.
Statistical analyses
As discussed above, we used assessment cohort data to determine how individual conditions, and whole topics, uniquely manifest post-infection in PASC and COVID patients as compared to Controls.
Across topics, we selected the top 20 conditions with positive relevance scores for individual testing of new-onset rates, a count chosen to balance the total tests required with depth of topic exploration, and roughly aligned with our topic visualizations (see Results). For each condition we considered patients with no incidence in the pre-infection phase, counting those who did and did not go on to experience the condition in the post-infection phase per cohort. 2×2 Fisher’s exact tests assessed these counts for PASC versus Control and COVID versus Control patients separately. Tests were multiply-corrected (Bonferroni) and used fisher.test in R (v3.5.1) with alternative = “two.sided” and simulate.p.value = TRUE to allow for large and small counts.34
For whole-topic assessment, we used the trained topic model to independently estimate posterior topic distributions for pre- and post-infection data. Given the generative model assumed by LDA, a probability of p for topic T in phase i suggests that p% of newly sampled conditions in i would be sourced from T.24 More formally, single-topic probability estimates follow a Beta distribution as a result of the Dirichlet prior.35 For each topic we model these as success rates in binomial trials (Figure 1), resulting in a potentially overdispersed Beta-binomial distribution.36 We thus use Generalized Estimating Equations (GEEs; geeglm in geepack v1.3.9), both to capture within patient pre- and post-infection correlation structure (with id = person_id, corstr = “exchangeable”),37 and to employ robust error estimation while allowing for overdispersion (with scale.fix = FALSE).38,39 Outcomes were equally weighted, as opposed to weighted by the number of conditions represented, to avoid high-utilization patients dominating results.
In addition to phase (pre- or post-infection) and cohort (PASC, COVID, Control), each topic’s logistic model included patient demographic covariates: sex (Male, Female), race (White, Black or African American, Asian or Pacific Islander, Native Hawaiian or Other Pacific Islander, Other or Unknown), BMI, life stage (Pediatric 0–10, Adolescent 11–18, Adult 19–65, Senior 66+), Quan-based Charleson comorbidity index (Suppl. Methods),40 and date-based “wave” of infection. We defined infection waves based on CDC surveillance data,41 categorizing them as Early (prior to March 1, 2021), Alpha (March 1, 2021 to June 30, 2021), or Delta (July 1, 2021 to Dec. 31, 2021). Patients with index during the Omicron wave (Jan. 1, 2022 and later) were excluded due to limited data across covariates, as were all patients without complete information. Models included site-level covariates as potential sources of heterogeneity,28,42 including source common data model (PCORnet, ACT, OMOP, TrinetX, OMOP-PedsNet), percentage of PASC patients, and site-specific topic usage (Suppl. Methods). We also developed models without site-level covariates for a subset of topics to assess their importance.
After fitting these models, we applied a difference-in-differences approach using estimated marginal means contrasts to look for changes in topic rates pre-to-post infection, for PASC patients versus Controls, within specific groups defined by sex, life stage, and wave of infection. The same tests were run for COVID versus Control patients. To validate this approach, we conducted additional ‘baseline’ contrasts for expected differences in females versus males, and pediatric, adolescent, and senior patients versus adults. In total we conducted 22 contrasts for each topic, multiply-correcting the complete set across all topics (Holm). Estimated marginal means contrasts were provided by emmeans (v1.8.9) in R (v3.5.1).
Results
Topic usage and coherence across sites
Of 75 available sites, 63 passed initial quality filtering, representing 12,486,133 patients with at least one condition recorded between 1/1/2018 and 8/2/2022. The topic model training set contained 7,992,339 patients and 387,401,304 conditions, while the validation set contained 1,996,380 patients and 96,738,753 conditions, representing a corpus of 48,372 unique condition identifiers. Mean topic coherence improved as the number of generated topics increased from 150 to 300, but not beyond (Suppl. Figure 2), so we selected the model with 300 topics for final analysis.
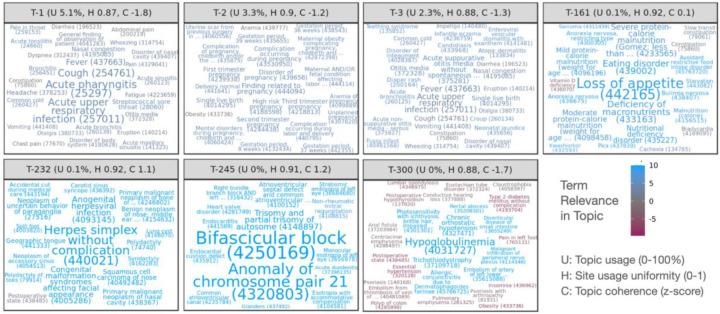
Figure 2 illustrates selected topics as word clouds, displaying the top conditions of each by weight. Topics are named T-1 to T-300 in order of their usage U (rounded to nearest 0.1%, see Methods), font size is proportional to condition weight in each topic, and color indicates condition relevance to the topic. Supplementary materials include word clouds for all topics (Suppl. Figure 3). Jensen-Shannon distance indicates that topics have little overlap (Suppl. Figure 4), with a median distance of 0.82 (range 0.39–0.83). The last 10 topics however, T-290 to T-300, form a group with increased co-similarity and many generic, low-relevance conditions mixed with a small number of high-relevance conditions.
Figure 2: Word clouds illustrating top-weighted conditions for selected topics.
Conditions are sized according to probability within each topic and colored according to relevance, with positive relevance indicating conditions more probable in the topic than overall. Each condition displays the numeric OMOP concept ID encoding the relevant medical code used for clustering, as well as the first few words of the condition name. Per-topic statistics in panel headers show usage of each of each topic across sites (U, rounded to nearest 0.1%), topic uniformity across sites (H, 0–1, higher values being more uniform), and relative topic quality as a normalized coherence score (C, z-score, higher values being more coherent).
Coherence scores follow a roughly normal distribution across topics (Suppl. Figure 5), and overall coherence tends to increase with rarer, more specific topics except for the last 10. Topic coherence varies by site, moreso for rarer topics (Suppl. Figure 6). All sites exhibit low coherence for the final 10 topics, and most of the final ~35 are low coherence for most sites except for one. Two sites report low coherence for most topics. Topic usage also varies by site, though most sites and topics follow similar patterns of usage (Suppl. Figure 7). T-4 was used almost exclusively by a single site and has very low coherence with only a few high-relevance terms, although this site uses other topics similarly to other sites.
N3C sites contribute data from one of several source common data models (CDMs). The source CDM used by sites is not strongly correlated with coherence or usage (Suppl. Figures 6 and 7), except for two sites in the PEDSnet network specializing in pediatric care and another using TriNetX. These three sites exhibit distinctive patterns, including lower coherence and usage for T-153 pertaining to Gout (not typically associated with pediatric patients) and higher usage for T-127 pertaining to male pediatric conditions such as Phimosis and Undescended testicle.
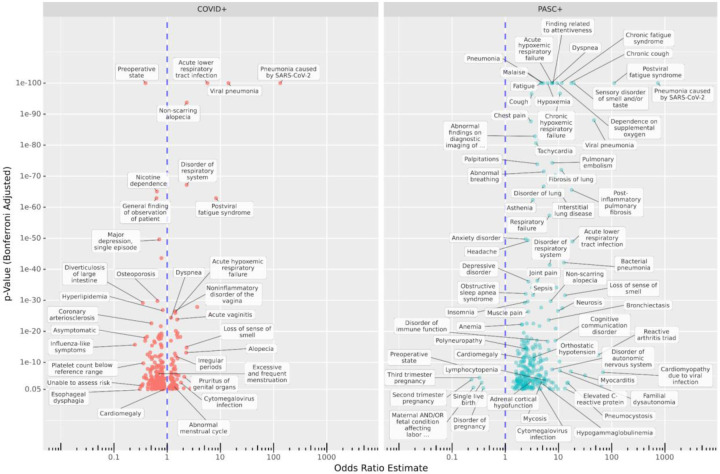
Individual conditions significant for PASC and COVID
From the 2,495,414-patient assessment set, 4,386 PASC, 105,967 COVID, and 335,841 Control patients met cohort eligibility requirements for individual-condition tests. Amongst PASC patients, 36% had a strong primary infection indicator at least 45 days prior to their PASC indication. After removing duplicates from the top entries for each topic, we tested 4,794 individual conditions for new onset post-infection. Of these, 213 are significant for the PASC cohort, 208 for COVID, and 89 for both with p < 0.05 after multiple correction. The complete list of significant results is available in Suppl. Table 4, and Figure 3 labels a subset of these. The PASC cohort shows larger rates for most significant conditions, although several conditions are represented in the COVID cohort as well, such as Pneumonia caused by SARS-CoV-2, Viral pneumonia, Postviral fatigue syndrome, Loss of sense of smell, and Abnormal menstrual cycle. Additionally, the following conditions have significant estimated odds ratios (ORs) greater than 2 in both cohorts: Loss of sense of smell, Disorder of respiratory system, Acute lower respiratory tract infection, Upper respiratory tract infection due to Influenza, Telogen effluvium, and Non-scarring alopecia.
Figure 3: Increased and decreased new-onset conditions in PASC and COVID patients compared to Controls post-infection.
The x-axis shows estimated odds ratios and the y-axis shows the adjusted p-values for new incidence of top-weighted, positive-relevance terms from all topics amongst COVID (left) and PASC (right) cohorts compared to Controls, in the six-month post-acute period compared to the previous year. Many known PASC-associated conditions increased in both cohorts, while some conditions are cohort-specific. Additionally, in the COVID cohort, incidence of many conditions associated with regular care or screening is reduced compared to controls.
Several conditions are strongly increased in the PASC cohort, including Chronic fatigue syndrome, Malaise, Finding related to attentiveness, Headache, Migraine (with and without aura), and Anxiety disorder. Neurosis is also present, but it should be noted that site-labeled source codes for this are almost entirely ICD-10-CM F48.9, Non-psychotic mental disorder, unspecified or similar (F48.8 and ICD-9 300.9). Notably, Impaired cognition is more common in PASC patients (OR 4.26) but less common in COVID patients (OR 0.53) compared to Controls. Other neurological conditions increased in PASC include Inflammatory disease of the central nervous system, Disorder of autonomic nervous system, Polyneuropathy, Orthostatic hypotension, and Familial dysautonomia (a genetic condition–see Discussion).
The significant results for PASC also highlight a variety of symptoms related to the cardiovascular, pulmonary, and immune systems. Cardiac conditions such as Tachycardia, Palpitations, Congestive heart failure, Myocarditis, Cardiomyopathy, and Cardiomegaly are observed. Pulmonary issues are well represented with Pulmonary embolism, Bronchiectasis, Fibrosis of lung, and various generic labels for respiratory failure or disorder. Amongst immunological conditions are Reactive arthritis triad, Elevated C-reactive protein, Lymphocytopenia, Hypogammaglobulinemia, Systemic mast cell disease, and generic Immunodeficiency disorder. In addition, bacterial, viral, and fungal infections are increased, including Bacterial infection due to Pseudomonas, Aspergillosis, and Pneumocystosis. Other common themes include musculoskeletal issues (Fibromyalgia, Muscle weakness, various types of pain) and hematological issues (Blood coagulation disorder, Anemia, Hypocalcemia, Hypokalemia).
The analysis also reveals estimated odds ratios less than 1, indicating decreased incidence post-infection compared to Controls, for 219 conditions in one or both cohorts. Most of these (174) were significant only for the larger COVID cohort, and several are related to routine screening or elective procedures potentially disrupted by a COVID-19 infection or lack of care access during the pandemic, such as Pre-operative state, Nicotine dependence, Radiological finding, Gonarthrosis, and Hypertensive disorder.43 Preoperative state was largely coded as SNOMED CT 72077002 or ICD-10-CM Z01.818, both widely used across sites and indicative of pre-surgical examination. Unable to Assess Risk appears to be a custom code used by a single site, mapped to OMOP concept ID 42690761 by N3C. Other conditions may be more difficult to identify in the six months after a COVID-19 infection due to symptom masking or altered care-seeking behavior. Examples include Diverticulosis of large intestine and Esophageal dysphagia.44,45 In addition to Pre-operative state, five conditions are significantly decreased for PASC patients, all related to late-term pregnancy, while Third trimester pregnancy is increased in COVID patients (see Discussion).
Topics significant for PASC and COVID by demographic
From the assessment set, 2,859 PASC patients, 89,374 COVID patients, and 303,017 Control patients met cohort eligibility criteria for per-topic logistic models; Suppl. Table 5 provides per-group patient counts. Baseline contrasts broadly reflected expected trends by life stage and sex (Suppl. Figure 3). T-2 for example pertains to pregnancy, with an estimated female/male OR of 45, pediatric/adult OR 0.06, adolescent/adult 0.2, and senior/adult 0.03. Similarly, T-3 highly weights neonatal conditions and generates a pediatric/adult OR of 43, but no significant female/male trend.
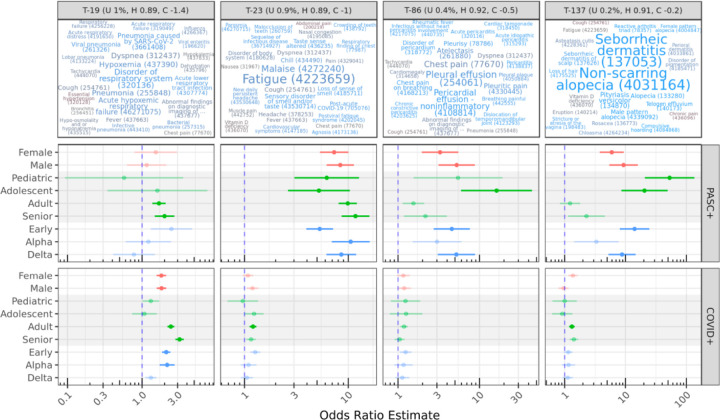
Our primary contrasts considered life stage, sex, and infection-wave demographic groups, evaluating post-vs-pre topic odds radios for PASC or COVID patients compared to corresponding odds ratios for Controls. For example, the contrast ((PASC adult post) / (PASC adult pre)) / ((Control adult post) / (Control adult pre)) results in an OR estimate of 9.89 for T-23, suggesting that post-infection, adult PASC patients increase their odds of generating conditions from this topic nearly 10 times more than Controls do over a similar timeframe. Figure 4 illustrates this result and others for the subset of topics with significant OR estimates >2 for more than one demographic group. All effectiveness and contrast results are listed in Suppl. Table 6 and visualized in Suppl. Figure 4.
Figure 4: Topics with significant OR estimates >2 for at least two demographic groups.
The top row illustrates topics using the same color and size scales as Figure 2; OR estimates are shown for demographic-specific contrasts of PASC or COVID pre-vs-post odds ratios compared to similar Control odds ratios. For example, adult PASC patients increase odds of generating conditions from T-23 post-infection nearly 10 times more than Controls do over a similar timeframe (see Results). Lines show 95% confidence intervals for estimates; semi-transparent estimates are shown for context but were not significant after multiple-test correction.
Amongst the 5,400 sex, life-stage, and wave-specific contrasts, 314 are significant after multiple correction, representing 68 distinct topics. Of these, 130 are represented by the final 10 low quality topics with OR ~0.6 for all patient groups, potentially reflecting broad healthcare access patterns driven given their shared similarity and few high-relevance terms. Most contrasts have small ORs, with only 30 contrasts across 9 topics having an OR of 2 or higher. The majority of strong effects are seen for the PASC cohort, and while topic coherence was largely uncorrelated with PASC or COVID association, topics with the strongest significant increases in the PASC cohort were less coherent than average (Suppl. Figure 8). PASC confidence intervals were larger due to this cohort’s much smaller size, a trend also seen across relative group sizes.
T-23 stands out as a topic with strong migration among PASC patients, with all subgroups having significant estimated ORs of 5–10. High-weight, high-relevance conditions in T-23 include Fatigue, Malaise, Loss of sense of smell, and other well-known PASC symptoms, as well as the diagnosis code for PASC itself (Post-acute COVID-19). By contrast, COVID patients do not show statistically significant migration to this topic, with the exception of Adults with a small OR of 1.2.
T-19 shows significant OR estimates for several PASC and COVID groups with similar magnitudes. This topic includes several variants of pneumonia and acute respiratory infection symptoms (Disorder of respiratory system, Dyspnea, Hypoxemia, Cough), suggesting significant long-term COVID-19 or secondary infections at least 45 days post-primary-infection. For both PASC and COVID cohorts, these increases are most associated with early-wave infections.
Topics 86 and 137 show increases for several PASC groups, especially pediatric and adolescent patients. While T-86 is characterized by Pleural and Pericardial effusion and related pain, T-137 describes skin conditions, particularly hair loss, including Non-scarring alopecia and Telogen effluvium, both identified individually above. While effusion is a known factor for severe COVID-19 pneumonia, especially in older patients,46 these results highlight similar outcomes in young patients. A systematic review of alopecia in COVID-19 patients by Nguyen and Tosti found that Anagen effluvium was associated with younger patients compared to other types of alopecia, but few of the reviewed studies included young patients.47
Figure 5 displays additional results for selected topics with cohort or demographic-specific patterns. T-8 represents cardiovascular conditions, and shows a mild but significant increase for adult COVID patients compared to controls. T-43 (not shown) is also significant for PASC adult patients, and encompasses pulmonary conditions. Several of the top-weighted conditions within these topics were individually significant, such as Palpitations, Cardiac arrhythmia, Chronic obstructive lung disease, and Pulmonary emphysema for both cohorts, and for PASC Dizziness and giddiness and Tachycardia. While all of these were individually increased in the PASC cohort, Cardiac arrhythmia, Chronic obstructive lung disease, and Pulmonary emphysema were decreased in the COVID cohort relative to controls.
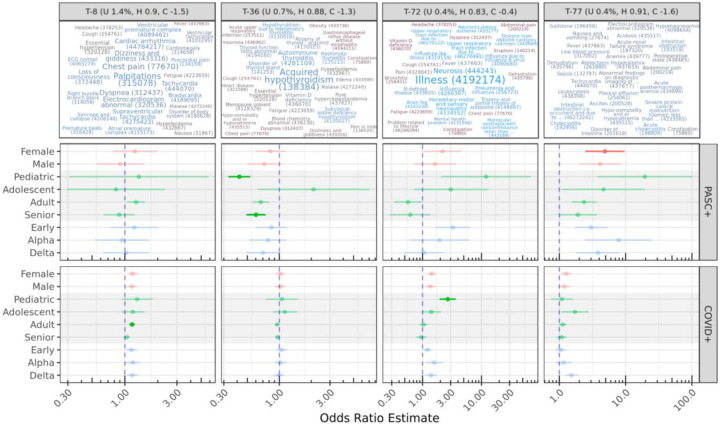
Figure 5: Other select topics with demographic or cohort-specific trends.
T-8 is statistically significant only for COVID adults compared to controls. Topics 72 and 77 include diffuse sets of conditions, while T-36 is reduced for PASC pediatric and senior patients, despite representing known PASC outcomes (see Discussion).
T-72 is increased for both COVID and PASC pediatric patients compared to Controls, though this is only statistically significant for the larger COVID cohort. It covers a range of non-specific PASC-like conditions, including Illness, Neurosis (also discussed above), Ill-defined disease, Mental health problem, and Disease type and/or category unknown. Brain fog and Neurocirculatory asthenia are additionally found in this topic.
T-77 is increased in female PASC patients compared to controls. This topic is diffuse and has no particularly highly weighted conditions, although many had high relevance scores to the topic. Several of these are laboratory-based, such as Hypokalemia, Anemia, and Hyponatremia. Tachycardia, Pleural effusion, Deficiency of macronutrients, and Adult failure to thrive syndrome are also present. The low specificity and coherence of T-77 make it difficult to interpret, although many of these conditions were individually significant above. T-20 (not shown) was increased for COVID adults and COVID delta-wave patients, and also has few high-weight terms, but relevant conditions include Acute renal failure syndrome, Sepsis, and Acidosis.
T-36 strongly decreased for both pediatric and senior PASC patients, and covers only a few conditions with high weights and relevance scores, including Acquired hypothyroidism and Autoimmune thyroiditis. This result is paradoxical, as these conditions are common long-term outcomes of COVID-19 infection.48 Another paradoxical result is a strong (OR 11.7) increase in T-92 for adolescent PASC patients, which covers a variety of physical contusions, lacerations, and abrasions. The highest-weighted condition in this topic however is Traumatic and/or non-traumatic injury, all of which were originally coded as ICD-10 T14.8 Other injury of unspecified body region for these patients.
Adolescent PASC patients are increased in four topics: T-23, T-86, and T-137 already discussed, and T-174 which highly weights Thyrotoxicosis, C-reactive protein abnormal, and Polymyalgia rheumatica. PASC pediatric patients increase significantly in T-23 and T-137 already discussed, as well as T-57 covering a variety of pulmonary issues such as Chronic cough, Bronchiectasis, and Hemoptysis. On the other hand, PASC adolescent patients were reduced in seven topics and PASC pediatric patients showed a reduction in sixteen, covering a broad range of conditions. These assessment cohorts are small, with 49 pediatric and 66 adolescent patients. Chart reviews revealed that they were distributed across 18 and 20 sites, respectively, and had a similar mean number of conditions recorded in the year prior to infection as other cohorts in the same life stages. However, mean condition counts for these PASC patients were nearly 50% higher in the 6-month post-infection phase (Suppl. Table 5).
These models included covariates to account for site-level differences in topic usage, percentage of PASC patients, and source common data model. To assess the importance of these, we also ran models without them for the subset of topics shown in Figures 4 and 5. Results are highly similar (Suppl. Figure 9), with models without site-level covariates showing slightly higher (< 6%) odds ratios for topics 23, 36, and 72.
Discussion
While an ICD-10-CM diagnosis code (U09.9) and specialty clinics exist to treat Long COVID, there is still work to be done identifying PASC conditions and how these new diagnoses and referrals are used in practice.28,49 Our model, trained on 387 million condition records from 8.9 million patients in the N3C, is one of the most extensive applications of topic modeling to EHR data to date, generating hundreds of diverse and clinically-relevant topics. Only a handful of topics were of low quality, and those in the middle by usage tended to have the highest coherence scores. We hypothesize that common topics are encumbered by a variety of coding options and practices, while rare topics support only a few relevant conditions on top of more common and unrelated background conditions. We found these trends across models with different topic counts, potentially driven by the use of Dirichlet distributions initialized with sparse uniform priors. Topic usage and coherence varied across contributing sites, with notable patterns of usage at PEDsnet sites in particular. Topic modeling may provide insights into site differences in coding practices or data quality, which are concerns in federated and centralized data repositories.42
Investigating top-weighted topic terms revealed many conditions associated with increased new-onset rates in PASC and COVID cohorts compared to Controls, including neurocognitive, cardiovascular, pulmonary, and immune-related. Most of these were significant for both cohorts or only the PASC cohort, despite its smaller size. A number of conditions showed lower new incidence in COVID patients compared to Controls, possibly due to decreased access to routine care (e.g. breast cancer50) or behavioral changes (e.g. diverticulosis44) through the pandemic.
Modeling patient-topic assignment supports queries across patient demographics at a topic level. This approach identified several topics increasing in PASC and COVID patient groups relative to Controls. T-23 stands out as the clearest PASC-related topic across demographics, and includes many conditions commonly associated with Long COVID such as fatigue, malaise, new daily headache, and dyspnea. Other topics are demographic-specific, such as T-86 covering Pleural and Pericardial effusion, T-137 with Non-scarring alopecia and Seborrheic dermatitis, and T-57 covering other pulmonary issues for younger PASC patients.
While most effects are larger for PASC patients, T-19 shows similar effect sizes for COVID adults and seniors. This topic largely represents secondary pneumonias and related symptoms, suggesting that while these are not used as indicators for PASC, they are nevertheless long-term issues for COVID-19 patients. The association is strongest with the early waves of the pandemic, reflecting severity of illness and lack of effective treatment protocols during this period.51 Few such wave effects were significant overall; T-20 with Acute renal failure syndrome, Acidosis, and Sepsis is an exception showing increases for COVID delta-wave patients. Despite the few PASC pediatric patients and wide confidence interval ranges, several topics were increased for this group indicating a unique cohort with significant long-term COVID-19 health outcomes. On the other hand, estimates for COVID-only pediatric patients for most topics, including T-23, T-57, and T-137, are non-significant despite a larger sample size.
While this study reaffirms many known PASC trends, several results merit further investigation. Female PASC patients increased in T-77, which is diffuse, multisystem, and covers many conditions identified in other tests. More targeted analyses of this set may reveal a unique sub-phenotype or mix of sub-phenotypes experienced by a unique population. Additionally, T-72 represents a cluster of ill-defined conditions; its increase for COVID pediatric patients may reflect difficulties in PASC identification for this group. For example, the highest-weighted term, Illness, was originally coded as ICD-10 R69 Illness, unspecified in the vast majority of cases. Amongst individual conditions, Impaired cognition increased in PASC patients but decreased in COVID patients. Many of these were originally coded as R41.844, Frontal lobe and executive function disorder. Executive dysfunction has been linked to COVID-19, particularly for patients with acute respiratory distress syndrome.52 This diagnosis is distinct from those typical for ADHD (F90), so it is unclear whether the reduction observed in COVID patients was a result of reduced healthcare access.
In contrast to other studies,53,54 we found few gastrointestinal conditions increased in PASC or COVID patients, though Abdominal pain, Viral gastroenteritis, and Dysphagia were increased in PASC patients (Suppl. Table 4). Neither did we find statistically significant sex differences, despite a known increased risk for PASC in female patients. Our experiments, however, evaluate cohorts defined by PASC diagnosis. While female patients are more likely to develop PASC, our results suggest minimal sex differences amongst patients who have been positively identified. Still, other work has suggested sex differences,55 and similar non-significant trends in our results may be worthy of followup. The apparent reduction in late-term pregnancy conditions for PASC patients and simultaneous increase for COVID patients (both in comparison to Controls) is notable. We hypothesize that pregnant patients are less likely to be diagnosed with PASC given the similarity of presentation, but more likely to be monitored if infected during pregnancy.
A high incidence of postural orthostatic tachycardia syndrome (POTS) has been identified in PASC clinical research,56 but a POTS-specific ICD-10 code did not exist prior to October 1, 2022, and therefore POTS is not present in our dataset. The closest available term in the SNOMED hierarchy, Orthostatic hypotension, was found to be significantly elevated in PASC, as were Disorder of the autonomic nervous system and Familial dysautonomia. Many symptoms significant for the PASC cohort, such as Tachycardia, Palpitations, Dizziness and giddiness, Fatigue, and Finding related to attentiveness are suggestive of POTS or similar forms of dysautonomia. The presence of Familial dysautonomia (ICD-10-CM G90.1), a rare genetic disorder, is unlikely to be due to increased screening given that we saw no corresponding uptake in genetic testing. Rather, we suspect that frequent mis-coding may occur because the ICD-10-CM catalog has only one match for the term “dysautonomia” (G90.1 Familial dysautonomia), which when used alone encompasses multiple PASC-related conditions.57 Such errors are not uncommon when using medical record software.58
Many of our results are immune-related, including conditions (Lymphocytopenia, Hypogammaglobulinemia, Systemic mast cell disease) and infections more common in immunocompromised patients (Aspergillosis, Pneumocystosis). Topic 36 highly weighting Hypothyroidism and Thyroiditis shows reductions for PASC pediatric and senior patients, a paradoxical result given that these are known post-acute sequelae.48 It may be that patients with pre-existing thyroid disorders are underdiagnosed for PASC, while new thyroid disorders after COVID-19 infection are identified as PASC and related symptoms alone. Together these results suggest an important role for thyroid-mediated dysfunction in PASC patients, and we recommend investigation into how these related diseases are diagnosed and treated.
Design choices and limitations of this study should be considered when interpreting results. To maximize the number and specificity of testable topics, we trained our model on a diverse set of patients with and without COVID-19 and PASC, using complete patient histories to maximize effective document size. LDA does not model temporal relationships between terms when generating topics, and topics may thus highly weigh both risk factors and outcomes. Like many clustering methods, LDA and its online variant are subject to suboptimal convergence resulting in possible variation in topics across runs.59,60 To mitigate these risks we employed hyperparameter tuning, increased training iterations, and model evaluation via coherence on an independent validation set. In addition to data filtering for quality and fitness of use, we removed diagnoses for COVID-19 itself prior to topic modeling. Because these conditions largely define inclusion criteria for both N3C and our COVID cohort, they are broadly correlated and their inclusion would likely influence topic composition significantly.
While many results are shared between the COVID and PASC cohorts, it’s important to note that these are computed against a common Control cohort rather than between PASC and COVID directly, and the larger size of the COVID cohort results in increased sensitivity. Overall trends in healthcare utilization and access during the pandemic should be considered, and these may be influenced by COVID-19 infection itself. N3C’s observational EHR data, although extensive, are not a random sample and represent a diversity of specialties, coding practices, and other factors, as evidenced by variation in topic usage and coherence across sites. We excluded patients from sites without any U09.9 PASC diagnoses to minimize misclassification of PASC patients, but these sites may serve unique populations or use topics in distinct ways. Topic-level models included several site-level covariates, including per-site topic usage and percentage of PASC patients. Models excluding these covariates yielded highly similar results, supporting cross-site generalizability, and the use of a held-out assessment set for these tests provides a level of independence from topic generation. Finally, we’ve focused on group-level inferential analyses to broadly understand PASC sub-phenotypes. Although topic models provide per-patient topic associations, the probabilistic nature of LDA limits its utility for individual patients and further research is needed prior to patient-level predictive applications.
With this context in mind, topic modeling applied to a large EHR dataset has proven highly effective for assessing the progression of post-acute sequelae of SARS-CoV-2 infection. Our LDA model identified hundreds of fine-grain potential sub-phenotypes in the data, and interpreting the probabilistic assignment of patients to them through GEE-based logistic regression is a novel and flexible approach, supported by properties of both methods and empirically by expected demographic baselines. Future investigations may assess other factors such as acute disease severity, contrast different cohorts, analyze inter-topic patterns to uncover sub-phenotype-specific risk factors, or employ time-series techniques to examine topic distributions across multiple time windows.
Ultimately, a finer understanding of presentations across populations can inform research, diagnostics, treatment, and health equity for multi-faceted diseases such as PASC. Tracking patient clinical trajectories over time in light of model-derived sub-phenotypes revealed post-acute sequelae of SARS-CoV-2 infection, several of which were associated with patient sex, age, wave of infection, or presence of a PASC diagnosis. Some results, such as those highlighting immune dysfunction, thyroid involvement, and secondary infections improve our understanding of potential mechanisms for PASC. Others, such as those highlighting non-specific phenotypes in the COVID cohort, may lead to improved diagnostics and support for patients suffering from Long-COVID but yet to receive a PASC diagnosis.
Supplementary Material
| Site | IRB Name | Exempted vs Approved | Protocol Number |
|---|---|---|---|
| University of Colorado | Colorado Multiple Institutional Review Board | approved | 21–2759 |
| Johns Hopkins University | Johns Hopkins Office of Human Subjects Research - Institutional Review Board | approved | IRB00249128 |
| University of North Carolina | University of North Carolina Chapel Hill Institutional Review Board | exempt | 21–0309 |
| Stony Brook University | Office of Research Compliance, Division of Human Subject Protections, Stony Brook University | exempt | IRB2021–00098 |
| OCHIN | Advarra IRB | approved | Pro00060719 |
| RTI International | RTI Office of Research Protection | exempt | MOD10001700 |
Acknowledgements
We sincerely thank the reviewers for their insightful comments, which significantly enhanced the generalizability and readability of this manuscript.
The analyses described in this publication were conducted with data and tools accessed through the NCATS N3C Data Enclave https://covid.cd2h.org and N3C Attribution & Publication Policy v 1.2–2020-08–25b supported by NCATS U24 TR002306, Axle Informatics Subcontract: NCATS-P00438-B, National Institutes of Health grant NHLBI RECOVER Agreement OT2HL161847–01, and CTSA award No. UM1TR004360 from the National Center for Advancing Translational Sciences. This research was possible because of the patients whose information is included within the data and the organizations (https://ncats.nih.gov/n3c/resources/data-contribution/data-transfer-agreement-signatories) and scientists who have contributed to the on-going development of this community resource [https://doi.org/10.1093/jamia/ocaa196]. This study is part of the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative (https://recovercovid.org/), which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC), and was conducted under the N3C DUR RP-5677B5. We would like to thank the National Community Engagement Group (NCEG), all patient, caregiver and community Representatives, and all the participants enrolled in the RECOVER Initiative. Authorship was determined using ICMJE recommendations.
The N3C Publication committee confirmed that this manuscript is in accordance with N3C data use and attribution policies; however, this content is solely the responsibility of the authors and does not necessarily represent the official views of the RECOVER or N3C programs or the National Institutes of Health.
Use of the N3C data for this study is authorized under the following IRB Protocols:
We gratefully acknowledge the following core contributors to N3C: Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O’Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O’Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors
The following institutions whose data is released or pending: Available: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Aurora Health Care Inc — UL1TR002373: Wisconsin Network For Health Research • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Case Western Reserve University — UL1TR002548: The Clinical & Translational Science Collaborative of Cleveland (CTSC) • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Dartmouth College — None (Voluntary) Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Harvard Medical School — UL1TR002541: Harvard Catalyst • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Louisiana Public Health Institute — None (Voluntary) • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Mary Hitchcock Memorial Hospital & Dartmouth Hitchcock Clinic — None (Voluntary) • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • MITRE Corporation — None (Voluntary) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Alliance at the University of Puerto Rico, Medical Sciences Campus — U54GM133807: Hispanic Alliance for Clinical and Translational Research (The Alliance) • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • The Queens Medical Center — None (Voluntary) • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University Medical Center of Southern Nevada — None (voluntary) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Pittsburgh — UL1TR001857: The Clinical and Translational Science Institute (CTSI) • University of Pennsylvania — UL1TR001878: Institute for Translational Medicine and Therapeutics • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — None (Voluntary) • Georgetown University — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation
Footnotes
Competing Interests
The authors declare no competing interests.
Code Availability
Analysis code is available at https://github.com/oneilsh/lda_pasc.
Data Availability
The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources. The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. Enclave data is protected, and can be accessed for COVID-related research with an approved (1) IRB protocol and (2) Data Use Request (DUR). Enclave and data access instructions can be found at https://covid.cd2h.org/for-researchers.
References
- 1.Brüssow H. & Timmis K. COVID-19: long covid and its societal consequences. Environ. Microbiol. 23, 4077–4091 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Reardon S. Long COVID risk falls only slightly after vaccination, huge study shows. Nature Publishing Group UK; 10.1038/d41586-022-01453-0 (2022) doi: 10.1038/d41586-022-01453-0. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-de-Las-Peñas C. et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Intern. Med. 92, 55–70 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Q., Zheng B., Daines L. & Sheikh A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalbandian A. et al. Post-acute COVID-19 syndrome. Nat. Med. 27, 601–615 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proal A. D. & VanElzakker M. B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Frontiers in Microbiology vol. 12 Preprint at 10.3389/fmicb.2021.698169 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight J. S. et al. The intersection of COVID-19 and autoimmunity. J. Clin. Invest. 131, (12 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hageman J. R. Long COVID-19 or post-acute sequelae of SARS-CoV-2 infection in children, adolescents, and young adults. Pediatr. Ann. (2021). [DOI] [PubMed] [Google Scholar]
- 9.Kenny G. et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect Dis 9, ofac060 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reese J. T. et al. Generalizable Long COVID Subtypes: Findings from the NIH N3C and RECOVER Programs. medRxiv (2022) doi: 10.1101/2022.05.24.22275398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ståhlberg M. et al. Post-COVID-19 Tachycardia Syndrome: A Distinct Phenotype of Post-Acute COVID-19 Syndrome. Am. J. Med. 134, 1451–1456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durstenfeld M. S., Hsue P. Y., Peluso M. J. & Deeks S. G. Findings from mayo clinic’s post-COVID clinic: PASC phenotypes vary by sex and degree of IL-6 elevation. Mayo Clin. Proc. 97, 430–432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer A. et al. Long COVID Classification: Findings from a Clustering Analysis in the Predi-COVID Cohort Study. Int. J. Environ. Res. Public Health 19, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagliati A. et al. Characterization of long COVID temporal sub-phenotypes by distributed representation learning from electronic health record data: a cohort study. EClinicalMedicine 64, 102210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowyer R. C. E. et al. Characterising patterns of COVID-19 and long COVID symptoms: evidence from nine UK longitudinal studies. Eur. J. Epidemiol. 38, 199–210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H. et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat. Med. (2022) doi: 10.1038/s41591-022-02116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y. et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler Looking for Clarity in the Haze of the Pandemic. Clin. Nurs. Res. 31, 1390–1398 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humpherys J. et al. Topic-to-Topic Modeling for COVID-19 Mortality. in 2021 IEEE 9th International Conference on Healthcare Informatics (ICHI) 258–264 (2021). [Google Scholar]
- 19.Pivovarov R. et al. Learning probabilistic phenotypes from heterogeneous EHR data. J. Biomed. Inform. 58, 156–165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustakim M., Wardoyo R., Mustofa K., Rahayu G. R. & Rosyidah I. Latent Dirichlet Allocation for Medical Records Topic Modeling: Systematic Literature Review. in 2021 Sixth International Conference on Informatics and Computing (ICIC) 1–7 (2021). [Google Scholar]
- 21.Huang C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397, 220–232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarpino I., Zucco C., Vallelunga R., Luzza F. & Cannataro M. Investigating Topic Modeling Techniques to Extract Meaningful Insights in Italian Long COVID Narration. BioTech (Basel) 11, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haendel M. A. et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J. Am. Med. Inform. Assoc. 28, 427–443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman M. D. & Blei D. M. Online learning for latent Dirichlet allocation. https://papers.nips.cc/paper/2010/file/71f6278d140af599e06ad9bf1ba03cb0-Paper.pdf (2010). [Google Scholar]
- 25.OMOP CDM v5.3. https://ohdsi.github.io/CommonDataModel/cdm53.html. [Google Scholar]
- 26.Pfaff E. R. et al. Coding Long COVID: Characterizing a new disease through an ICD-10 lens. medRxiv (2022) doi: 10.1101/2022.04.18.22273968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-de-Las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Cuadrado M. L. & Florencio L. L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 18, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaff E. R. et al. Coding long COVID: characterizing a new disease through an ICD-10 lens. BMC Med. 21, 58 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng X. et al. MLlib: Machine Learning in Apache Spark. arXiv [cs.LG] (2015). [Google Scholar]
- 30.Newman D., Lau J. H., Grieser K. & Baldwin T. Automatic Evaluation of Topic Coherence. in Human Language Technologies: The 2010 Annual Conference of the North American Chapter of the Association for Computational Linguistics 100–108 (Association for Computational Linguistics, Los Angeles, California, 2010). [Google Scholar]
- 31.Bhattacharya M., Jurkovitz C. & Shatkay H. Co-occurrence of medical conditions: Exposing patterns through probabilistic topic modeling of snomed codes. J. Biomed. Inform. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen R., Aviram I., Elhadad M. & Elhadad N. Redundancy-aware topic modeling for patient record notes. PLoS One 9, e87555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei Q., Shen X. & Zhai C. Automatic labeling of multinomial topic models. in Proceedings of the 13th ACM SIGKDD international conference on Knowledge discovery and data mining 490–499 (Association for Computing Machinery, New York, NY, USA, 2007). [Google Scholar]
- 34.Patefield W. M. Algorithm AS 159: An Efficient Method of Generating Random R × C Tables with Given Row and Column Totals. J. R. Stat. Soc. Ser. C Appl. Stat. 30, 91–97 (1981). [Google Scholar]
- 35.Ng K. W., Tian G.-L. & Tang M.-L. Dirichlet and Related Distributions: Theory, Methods and Applications. (John Wiley & Sons, 2011). [Google Scholar]
- 36.Wilcox R. R. A Review of the Beta-Binomial Model and its Extensions. J. Educ. Behav. Stat. 6, 3–32 (1981). [Google Scholar]
- 37.Hanley J. A., Negassa A., Edwardes M. D. D. & Forrester J. E. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am. J. Epidemiol. 157, 364–375 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Højsgaard S., Halekoh U. & Yan J. The R Package geepack for Generalized Estimating Equations. J. Stat. Softw. 15, 1–11 (2006). [Google Scholar]
- 39.Yan J. & Fine J. Estimating equations for association structures. Stat. Med. 23, 859–74; discussion 875–7,879–80 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Quan H. et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 173, 676–682 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Lambrou A. S. et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants - United States, June 2021-January 2022. MMWR Morb. Mortal. Wkly. Rep. 71, 206–211 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaff E. R. et al. Synergies between centralized and federated approaches to data quality: a report from the national COVID cohort collaborative. J. Am. Med. Inform. Assoc. 29, 609–618 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisó-Almirall A., Kostov B., Sánchez E., Benavent-Àreu J. & González de Paz L. Impact of the COVID-19 Pandemic on Primary Health Care Disease Incidence Rates: 2017 to 2020. Ann. Fam. Med. 20, 63–68 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pj W., Tv V. & Whiley P. J. The impact of delayed acute diverticulitis presentations during the COVID-19 pandemic on acuity and surgical complexity in the long-term. Glob. Surg. (2022) doi: 10.15761/GOS.1000239. [DOI] [Google Scholar]
- 45.Miles A. et al. An International Commentary on Dysphagia and Dysphonia During the COVID-19 Pandemic. Dysphagia 37, 1349–1374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li K. et al. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest. Radiol. 55, 327–331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen B. & Tosti A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int 7, 67–77 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naguib R. Potential relationships between COVID-19 and the thyroid gland: an update. J. Int. Med. Res. 50, 3000605221082898 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaff E. R. et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health 4, e532–e541 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowry K. P. et al. Breast Biopsy Recommendations and Breast Cancers Diagnosed during the COVID-19 Pandemic. Radiology 303, 287–294 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuriakose S. et al. Developing Treatment Guidelines During a Pandemic Health Crisis: Lessons Learned From COVID-19. Ann. Intern. Med. 174, 1151–1158 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali Awan H. et al. SARS-CoV-2 and the Brain: What Do We Know about the Causality of ‘Cognitive COVID? J. Clin. Med. Res. 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norouzi Masir M. & Shirvaliloo M. Symptomatology and microbiology of the gastrointestinal tract in post-COVID conditions. JGH Open 6, 667–676 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Sylvester S. V. et al. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Curr. Med. Res. Opin. 38, 1391–1399 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Seeley M.-C. et al. High Incidence of Autonomic Dysfunction and Postural Orthostatic Tachycardia Syndrome in Patients with Long COVID: Implications for Management and Health Care Planning. Am. J. Med. (2023) doi: 10.1016/j.amjmed.2023.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fedorowski A. & Sutton R. Autonomic dysfunction and postural orthostatic tachycardia syndrome in post-acute COVID-19 syndrome. Nat. Rev. Cardiol. 20, 281–282 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bologva E. V., Prokusheva D. I., Krikunov A. V., Zvartau N. E. & Kovalchuk S. V. Human-Computer Interaction in Electronic Medical Records: From the Perspectives of Physicians and Data Scientists. Procedia Comput. Sci. 100, 915–920 (2016). [Google Scholar]
- 59.Roberts M. E., Stewart B. M. & Tingley D. Navigating the local modes of big data: The case of topic models. in Computational Social Science: Discovery and Prediction 51–97 (Cambridge University Press, 2016). [Google Scholar]
- 60.Syed S. & Spruit M. Selecting Priors for Latent Dirichlet Allocation. in 2018 IEEE 12th International Conference on Semantic Computing (ICSC) 194–202 (IEEE, 2018). [Google Scholar]
- 61.R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2020). [Google Scholar]
- 62.Lenth R., Singmann H., Love J., Buerkner P. & Herve M. Emmeans: Estimated marginal means, aka least-squares means. R package version (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources. The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. Enclave data is protected, and can be accessed for COVID-related research with an approved (1) IRB protocol and (2) Data Use Request (DUR). Enclave and data access instructions can be found at https://covid.cd2h.org/for-researchers.