Abstract
Objective:
White matter hyperintensities (WMH) on brain MRI images are the most common feature of cerebral small vessel disease (CSVD). Studies have yielded divergent findings on the modifiable risk factors for WMH and WMH’s impact on cognitive decline. Mounting evidence suggests sex differences in WMH burden and subsequent effects on cognition. Thus, we aimed to identify sex-specific modifiable risk factors for WMH. We then explored whether there were sex-specific associations of WMH to longitudinal clinical dementia outcomes.
Methods:
Participants aged 49–89 years were recruited at memory clinics and underwent a T2-weighted fluid-attenuated inversion recovery (FLAIR) 3T MRI scan to measure WMH volume. Participants were then recruited for two additional follow-up visits, 1–2 years apart, where clinical dementia rating sum of boxes (CDR-SB) scores were measured. We first explored which known modifiable risk factors for WMH were significant when tested for a sex-interaction effect. We additionally tested which risk factors were significant when stratified by sex. We then tested to see whether WMH is longitudinally associated with clinical dementia that is sex-specific.
Results:
The study utilized data from 713 participants (241 males, 472 females) with a mean age of 72.3 years and 72.8 years for males and females, respectively. 57.3% and 59.5% of participants were diagnosed with mild cognitive impairment (MCI) for males and females, respectively. 40.7% and 39.4% were diagnosed with dementia for males and females, respectively. Of the 713 participants, 181 participants had CDR-SB scores available for three longitudinal time points. Compared to males, females showed stronger association of age to WMH volume. Type 2 Diabetes was associated with greater WMH burden in females but not males. Finally, baseline WMH burden was associated with worse clinical dementia outcomes longitudinally in females but not in males.
Discussion:
Elderly females have an accelerated increase in cerebrovascular burden as they age, and subsequently are more vulnerable to clinical dementia decline due to CSVD. Additionally, females are more susceptible to the cerebrovascular consequences of diabetes. These findings emphasize the importance of considering sex when examining the consequences of CSVD. Future research should explore the underlying mechanisms driving these sex differences and personalized prevention and treatment strategies.
Clinical trial registration:
The BICWALZS is registered in the Korean National Clinical Trial Registry (Clinical Research Information Service; identifier, KCT0003391). Registration Date 2018/12/14.
Introduction
Cerebral small vessel disease (CSVD) is a common cause of stroke and cognitive impairment in older adults. White matter hyperintensities (WMH) observed on brain T2-weighted fluid-attenuated inversion recovery (T2-FLAIR) magnetic resonance imaging (MRI) are surrogate markers of CSVD. While there is clear evidence that WMH leads to cognitive decline, the magnitude of its relationship to cognition and to the rate of cognitive decline varies considerably across individuals1. Moreover, studies have yielded divergent findings on the risk factors for development and progression of WMH2. Sex appears to be an important moderator in how risk factors are related to WMH incidence and severity, yet few have reported sex-specific risk factor differences3. A comprehensive understanding of sex-specific modifiable risk factors for WMH can inform improved diagnostics and targeted treatment.
Studies reporting sex-specific differences for the consequences of WMH have been inconsistent. A majority of studies found postmenopausal, elder females to have higher WMHV burden compared to males4–10, yet some have observed no differences11 or that males have higher WMH burden12, 13. Previous studies have also reported that modifiable risk factors for WMH are sex-specific. Hypertension and higher body mass index (BMI) have been observed to have a stronger association with WMH burden in males compared to females14–17. Studies have reported that diabetes and smoking are risk factors for females but not males8, 18–20. It has also been reported that WMH is associated with worse cognitive and clinical outcomes in females compared to males16.
Thus, it is necessary to identify modifiable risk factors of CSVD have sex-specific associations with CSVD, which in turn can inform approaches to the prevention and treatment of dementia. In this study of 713 predominantly cognitively impaired participants, we tested whether associations of modifiable risk factors for WMH varied by sex. We then tested any sex differences in longitudinal associations of WMH to clinical dementia outcomes. We hypothesized that females would have a stronger association of age with WMH, the modifiable risk factors would be sex-specific, and that WMH would have a stronger effect on clinical dementia outcomes in females.
Methods
Participants
This study was a part of the ongoing Biobank Innovations for Chronic Cerebrovascular Disease With ALZheimer’s Disease Study (BICWALZS) and the Centre for Convergence Research of Neurological Disorders. The BICWALZS was planned and initiated in October 2016 by the Korea Disease Control and Prevention Agency for the Korea Biobank Project, a national innovative biobanking program that fosters biomedical and healthcare research and development infrastructure. The original goal was to facilitate, regulate, and ensure the optimal use of human biological specimens for research from real-world data in the fields of subjective cognitive decline (SCD), mild cognitive impairment (MCI), Alzheimer’s disease (AD), and subcortical vascular dementia (SVaD).
All participants underwent Clinical Dementia Rating (CDR) global score and sum of boxes of CDR (CDR-SB). The CDR is obtained by interviewing patients and their care givers and captures cognition and function. It assesses six domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care) and the score for each domain range from 0 to 3, with a higher score indicating greater impairment. Then, CDR-SB score range from 0 to 18 and is a validated outcome measure used in clinical trials of dementia21, 22.
The clinical diagnosis criteria used for this study were as follows: SCD criteria included self-and/or informant reports of cognitive decline but no objective impairment in cognitive tasks (no less than − 1.5 SD in each of the neurocognitive test domains and CDR = 0)23; patients with MCI were evaluated based on a CDR21 score of 0.5, the expanded Mayo Clinic criteria24, patients with AD dementia were evaluated using the National Institute on Aging-Alzheimer’s Association Core Clinical Probable AD Dementia Criteria25; and subcortical vascular dementia (SVaD) was evaluated based on above-moderate WMH and vascular dementia criteria in accordance with the Diagnostic Statistical Manual of Mental Disorders, fifth edition26. Patients with a history of neurological or medical conditions such as territorial cerebral infarction, intracranial hemorrhage, Parkinson’s disease, heart failure, renal failure, or others that could interfere with the study were excluded. The presence or absence of diabetes, hypertension, and hyperlipidemia was based on the clinical history of treatment with the diagnosis by a physician. Blood pressure, pulse pressure, body mass index and smoking status also were evaluated.
The BICWALZS is registered in the Korean National Clinical Trial Registry (Clinical Research Information Service; identifier, KCT0003391). The study was approved by the Institutional Review Board of Ajou University Hospital (AJOUIRB-SUR-2021–038), and written informed consent was obtained from all the participants and caregivers. Participants from the BICWALZS were recruited at the memory clinics of Ajou University Hospital and Suwon Community Geriatric Centers in South Korea. All the participants were Korean (Eastern Asian ethnicity). Among these individuals, we used data from 713 participants with brain MRI, amyloid PET, APOE, CDR, and blood laboratory assessments. Within this cohort, 181 participants had two additional follow-up visits where their CDR was measured.
Blood sampling and laboratory assessments
Blood samples were collected by venipuncture after an overnight fast in the morning. Blood laboratory tests included HbA1c, serum lipid, homocysteine, and thyroid function tests.
APOE genotyping
Informed consent was obtained from all participants regarding the collection and genotyping of blood genomic DNA. Genomic DNA was isolated from the blood samples, and single-nucleotide polymorphism (SNP) genotyping was performed by DNA Link, Inc. (Seoul, Korea) using the Affymetrix Axiom KORV1.0–96 Array (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. The APOE genotypes were derived from rs429358 and rs7412, which were included in the array.
Amyloid PET acquisition and measurement of amyloid deposition
18 F-flutemetamol PET scan was performed on a Discovery STE/690 PET/CT scanner (GE, Milwaukee, WI, USA), with the same protocol used on all participants. 18F-flutemetamol was injected into the antecubital vein as a bolus (mean dose, 185 MBq). After 90 min, a 20-min PET scan (4 × 5 min dynamic frames) was performed. The PET sequence parameters are listed in Supplementary Table 1. 18F-flutemetamol PET scans were co-registered to individual MRI scans, which were normalized to a T1-weighted MRI template using transformation parameters. To quantify 18F-flutemetamol retention, the standard uptake value ratio (SUVR) was obtained using the pons as a reference region. Global cortical 18F-flutemetamol retention was calculated using an automated anatomical labeling (AAL) atlas.
Image acquisition and MR data processing for white matter hyperintensities
Participants completed the baseline MRI scans on a GE Discovery MR750w 3T scanner or a Philips Achieva 3T Scanner, including the following two sequences: a three-dimensional (3D) magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted sequence and a T2-weighted (T2w) fluid-attenuated inversion recovery (FLAIR) sequence. The MRI sequence parameters are listed in Supplementary Table 1. To quantify the WMH on T2w FLAIR images, we leveraged a pretrained deep learning model described in our previous study27. Brie y, the deep learning segmentation model consists of a transformer-based encoder and a convolutional decoder to ensure a larger receptive field for lesion segmentation. The model was trained on an unparalleled dataset including FLAIR images acquired at 1.5T, 3T and 7T with significant data augmentation incorporating commonly seen MR artifacts, such as, noise, inhomogeneity, and minor ghosting. FreeSurfer (version 7.1.1, https://surfer.nmr.mgh.harvard.edu/) was used to calculate intracranial volume (ICV). The total WMH volume (WMHV) was normalized by the ICV [WMHV = WMH/ICV] and log-transformed for analysis.
Statistical analysis
We performed two analyses using multivariate linear regression: a sex-interaction analysis and a main effect analysis. Sex-specific risk factors for WMH were tested using multivariate linear regression models performed in R (version 4.3.1 https://www.R-project.org). Predictors of interest included age, diabetes status, hypertension status, body mass index (BMI), cardiovascular risks (pulse pressure, systolic and diastolic blood pressure, low-density and high-density lipid levels), thyroid stimulation hormone (TSH) levels, and amyloid β (Aβ) burden (global 18F-flutemetamol SUVR). TSH, HbA1c and homocysteine were log-transformed due to rightward skew. We first performed a sex-interaction analysis to test for individual risk factor’s interaction with sex on WMHV. Risk factors tested included age, hypertension status, diabetes status, HbA1c, pulse pressure, diastolic and systolic blood pressure, homocysteine, TSH, APOE4 status, HDL, and LDL. In the main effect analysis, we tested individual risk factors stratified by sex. The analyses controlled for scanner site and cognitive diagnosis (i.e., no cognitive decline, SCD/MCI, dementia). Multiple comparisons correction were performed for each analysis using the Benjamini-Hochberg false discovery rate method (FDR)28.
Of all the 713 participants with cross-sectional data available, there were 181 participants who completed two additional follow-up visits and obtained additional CDR sum of boxes (CDR-SB) scores. Follow-up visits were acquired approximately one year after the prior visit. To analyze the association between WMHV and clinical outcomes longitudinally, we utilized a Linear Mixed Effects model (LME) to test for associations of CDR-SB with the interaction effect of WMHV*visit time (baseline, follow-up, visit 3), Aβ burden*visit time, stratified by sex. The LME model controlled for fixed effects of age and scanner site, as well as a random slope for each participant. LME models were implemented with the lmer function from the lme4 package in R29. To visualize results, we used the function ‘plot_model’ in the R package sjPlot to generate plots of the marginal effects of WMHV on visit time30.
Results
Table 1 displays the characteristics of the entire cohort stratified by sex. Participants were 241 males and 472 females, with an average age 72.3 years and 72.8 years for males and females, respectively. 57.3% and 59.5% of participants were diagnosed with SCD or MCI for males and females, respectively. 40.7% and 39.4% were diagnosed with dementia for males and females, respectively. There were no significant sex differences in the proportion of those with SCD/MCI vs. dementia vs. no cognitive decline at baseline. Males had a significantly higher number of years of education, proportion with diabetes, and LDL and HDL levels. Females had significantly higher homocysteine levels. Supplementary Table 2 displays the characteristics of the participants in the cross-sectional cohort who had two additional follow-up visits with a Clinical Dementia Rating Sum of Boxes (CDR-SB) measured versus those who did not have longitudinal measures. No demographic characteristics varied significantly between the two subgroups. Additionally, CDR-SB scores did not significantly differ between male and female at any of the three longitudinal time points (not shown, p = 0.97, 0.66, and 0.91 for visit at baseline, visit 2, and visit 3, respectively).
Table 1.
Participant Characteristics
| Characteristic | Group; Mean (SD) | Statistical Test | p-value | |
|---|---|---|---|---|
| Male, n = 241 | Female, n = 472 | t-testa/chi- squared testb | ||
| Age | 72.3 (7.44) | 72.8 (7.59) | −0.93a | 0.35 |
| Years of Education | 10.4 (4.74) | 7.04 (4.54) | 9.04a | <0.001 |
| BMIa | 23.9 (3.03) | 24.0 (3.31) | −0.41a | .68 |
| HbA1cb, % mmol/mol | 6.12 (1.04) | 5.98 (0.89) | −1.80a | 0.073 |
| log(TSH)c, mIU/L | 0.51 (0.82) | 0.46 (0.97) | −0.78a | 0.43 |
| log(Homocysteine)d, umol/L | 2.69 (0.445) | 2.50 (0.36) | −5.57a | <0.001 |
| Global 18F-flutemetamol SUVRe | 0.68 (0.15) | 0.67 (0.15) | −0.93a | 0.35 |
| ‡APOE ɛ4 positive, N (%) | 80 (33.2%) | 135 (28.6%) | 1.39b | 0.24 |
| Hypertension, N (%) | 132 (54.8%) | 256 (54.2%) | 0.0031b | 0.96 |
| Diabetes, N (%) | 67 (27.8%) | 93 (19.7%) | 5.6b | 0.018 |
| Smoker, N (%) | 152 (63.1%) | 23 (4.9%) | 288.64b | <0.001 |
| Cognitive Diagnosis, n (%) | 1.38b | 0.50 | ||
| No cognitive decline | n = 5 (2.1%) | n = 5 (1.1%) | ||
| SCD or MCI | n = 138 (57.3%) | n = 281 (59.5%) | ||
| Dementia | n = 98 (40.7%) | n = 186 (39.4%) | ||
| Cardiovascular Risk Factors | ||||
| Pulse Pressure, mmHg | 75.8 (12.4) | 76.0 (11.6) | −0.24a | 0.81 |
| Systolic Blood Pressure, mmHg | 130.7 (17.3) | 134.6 (19.3) | −2.71a | 0.0070 |
| Diastolic Blood Pressure, mmHg | 76.0 (12.1) | 76.7 (11.5) | −0.70a | 0.48 |
| LDL-C, mg/dL | 92.7 (33.3) | 104 (38.8) | 4.10a | <0.001 |
| HDL-C, mg/dL, M (SD) | 51.6 (14.1) | 58.3 (14.8) | 5.89a | <0.001 |
Unless otherwise indicated
BMI information is available for all males and 471 out of 472 female participants.
HbA1c is available for 231 out of 241 male participants and 453 out of 472 female participants.
TSH is available for all male participants and 470 out of 472 female participants.
Homocysteine is available for 224 out of 241 male participants and 434 out of 472 femeale participants.
Global 18F-flutemetamol SUVR is available for 209 out of 241 male participants and 435 out of 472 female participants.
WMHV expressed as log(cm3/Intracranial volume)
APOE\##\ 4 positive: 2/4, 3/4, 4/4
BMI, body mass index, DBP, diastolic blood pressure, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure, WMHV, whit matter hyperintensity volume; TSH, thyroid-stimulating hormone; SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer’s Disease; SUVR, standard uptake value ratio; APOE, apolipoprotein
We tested each WMH risk factor for a sex interaction effect controlling for scanner site and cognitive diagnosis. Two interaction effects tested survived multiple comparisons: age*sex and diabetes*sex (Table 2). WMHV increased at a higher rate as females aged compared to males (Fig. 1A). Diabetic females had higher WMHV compared to non-diabetic females whereas there were no group differences for males (Fig. 1B). Notably, HbA1c*sex was close to surviving multiple comparison (FDR corrected p-value = 0.07). Higher HbA1c was associated with greater WMHV for females but not males (Fig. 1C).
Table 2. The interaction between sex with age, diabetes status is associated with WMHV.
Multiple linear regression analysis tested for interaction effect with sex and known risk factors in relation to normalized white matter hyperintensity volume. Analysis controlled for scanner site and cognitive diagnosis and FDR multiple comparisons p-value correction was performed.
| WMHV | |||
|---|---|---|---|
| β (Std. error) | β 95% Confidence Interval | Uncorrected p-value | |
| Age*Sex | −0.037 (0.011) | (−0.058, −0.016) | 0.00050 |
| Hypertension*Sex | −0.15 (0.17) | (−0.48, 0.17) | 0.36 |
| Diabetes*Sex | −0.52 (0.20) | (−0.91, −0.14) | 0.00079 |
| log(HbA1c)*Sex | −1.55 (0.63) | (−2.78, −0.32) | 0.014 |
| Pulse Pressure *Sex | −0.014 (0.0069) | (−0.028, −0.00068) | 0.040 |
| Systolic BP *Sex | −0.0062 (0.0047) | (−0.016, 0.0031) | 0.19 |
| log(Homocysteine) *Sex | −0.21 (0.21) | (−0.62, 0.21) | 0.33 |
| log(TSH) *Sex | 0.17 (0.097) | (−0.025, 0.36) | 0.089 |
| Diastolic BP *Sex | −0.0076 (0.0071) | (−0.022, 0.0065) | 0.29 |
| APOE4 Status *Sex | 0.27 (0.18) | (−0.089, 0.63) | 0.14 |
| HDL *Sex | 0.0064 (0.0060) | (−0.0054, 0.018) | 0.28 |
| LDL*Sex | 3.7E-4 (0.0024) | (−0.0043, 0.0051) | 0.88 |
| Global18F-flutemetamol SUVR *Sex | 0.30 (0.60) | (−0.88, 1.47) | 0.62 |
| BMI *Sex | −0.0041 (0.027) | (−0.058, 0.050) | 0.88 |
| Smoking Status *Sex | 0.10 (0.27) | (−0.42, 0.63) | 0.70 |
Bolded font indicates the risk factor survived multiple comparisons.
BMI, body mass index; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; SBP, systolic blood pressure; WMHV, white matter hyperintensity volume; TSH, thyroid-stimulating hormone; SUVR, standard uptake value ratio; APOE, apolipoprotein
Figure 1. Elderly females appear to have an accelerated increase in cerebrovascular burden as they age and are more susceptible to the cerebrovascular consequences of diabetes.
A.) Females are observed to have a higher increase in WMHV as they age compared to males. B.) Diabetic females were associated with larger WMHV compared to non-diabetic females. There were no differences observed for males. For visualization purposes, WMHV is plotted versus age and grouped by sex and Diabetes status. C.) Higher HbA1c levels were associated with larger WMHV for females but not males, but the interaction effect for HbA1c*sex did not survive multiple comparisons.
WMHV= white matter hyperintensity volume
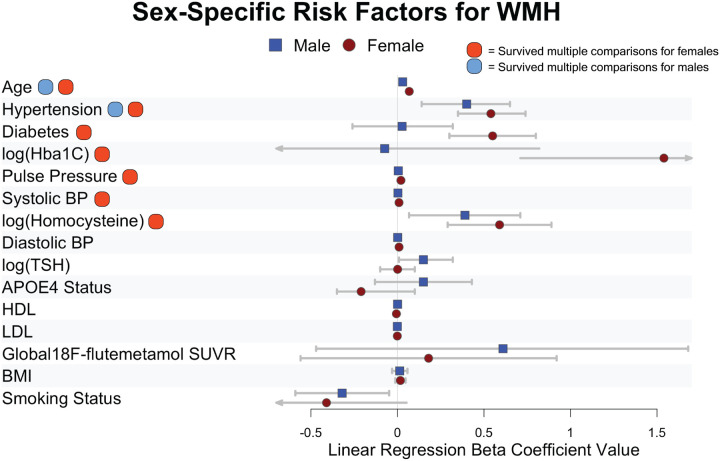
To visualize sex-specific risk architectures, we tested which risk factors were significantly associated with WMHV when stratified by sex and controlling for scanner site and cognitive diagnosis (Supplementary Table 3, Fig. 2). After correction for multiple comparisons, for males, age was the largest risk factor for WMHV followed by hypertension. For females, age was the largest risk factor for WMHV followed by hypertension, pulse pressure, diabetes, homocysteine, and HbA1c. All of the variables survived multiple comparisons correction for females.
Figure 2. Sex-specific risk factors for WMH.
Beta coefficient values from linear regression models for individual risk factors are displayed.
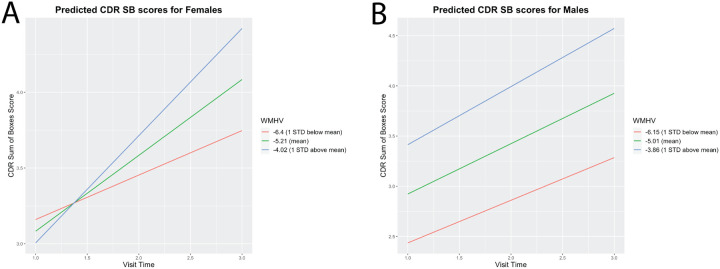
We then explored whether baseline WMHV is differentially related to longitudinal trajectories of clinical dementia outcomes by sex (Table 4). The interaction effect between Aβ burden and visit time on CDR-SB scores was observed to be significant for both males and females. We also observed a significant interaction effect between baseline WMHV and visit time on CDR sum of boxes score for females but not males. For females, larger baseline WMHV was associated with a greater CDR sum of boxes score increase over the three visits (Fig. 3).
Figure 3. Higher WMHV is associated with clinical dementia outcomes over two years among females but not males.
A.) A significant interaction effect between WMHV and visit time on CDR sum of boxes was observed for females in a linear mixed effect model. The predicted slopes for a participant with a mean and +− 1 standard deviation of WMHV for females is plotted. B.) The predicted slopes for a participant with a mean and +− 1 standard deviation of WMHV for males is plotted. There was no observed interaction effect between WMHV and visit time on CDR sum of boxes score for males.
WMHV= white matter hyperintensity volume
Discussion/Conclusions
In a large South Korean cohort of predominantly cognitively impaired participants, we tested sex differences in WMH, in associations of known risk factors to WMH, and in associations of WMH to longitudinal clinical outcomes. Our study has three main findings. First, females appear to be protected from white matter lesions at middle age but are observed to be more vulnerable at older age compared to males. Second, diabetes status was associated with greater WMH burden in females but not males. Finally, WMHV was associated with worse clinical dementia outcomes longitudinally in females only.
A significant number of studies in the last two decades have observed elder, predominantly postmenopausal females to have a higher prevalence for cerebrovascular burden and disease compared to males4–10, 31. There is limited understanding of the underlying mechanisms through which these sex differences arise, but the different trajectories of endogenous sex hormones has been proposed to play a role. In particular, during the premenopause, endogenous estrogen is thought to be protective for neuronal and cerebrovascular health32, 33. Recently, the Rhineland study reported that postmenopausal females had more WMH compared with premenopausal females and men of the same age range10. Moreover, a recent UK-Biobank study observed that females with a longer reproductive lifespan had significantly smaller WMH burden in late life independent of the history of oral contraceptive use or hormone replacement therapy34. Taken together, our findings contribute to the growing body of literature which displays an increased risk for CSVD in late life for postmenopausal females compared to males of the same age.
Diabetes status and its severity are risk factors for CSVD. Chronic hyperglycemia stimulates the overproduction of mitochondrial superoxide radicals in endothelial cells, resulting in oxidative stress, endothelial dysfunction, and inflammation. These events are associated with the pathogenesis of vascular damage, in both small and large blood vessels35. Numerous cross-sectional and longitudinal studies have displayed an association between diabetes status and HbA1c levels with WMH burden18, 36–39. Large observational studies observe type 2 diabetes confers a greater risk of incident cardiovascular disease in women compared with men40, and evidence also supports a more adverse effect of diabetes on CSVD in females compared to males41. For example, diabetes was reported to confer an increased risk of vascular dementia in females but not males in both a large community-based cohort study as well as a meta-analysis with over 2.3 million individuals42, 43. Some studies have also reported diabetes to be associated with greater WMH or lacune volume in females but not males18–20. Furthermore, animal models have supported the finding that diabetes is associated with worse cerebrovascular burden in females. A recent study observed that when mice are given a high fat diet causing hyperglycemia, white matter damage was only observed in females while neuroinflammatory activation was only observed in males44. Moreover, a study utilizing a genetic mouse model of diabetes reported that females had larger ischemic infarcts compared to males. These infarcts were exacerbated by ovariectomy and ameliorated by E2 treatment, which suggests estrogen directly influences diabetes effect on CSVD45. Collectively, our findings contribute to the idea that diabetes is differentially associated with CSVD burden between sexes. There is still limited understanding of the potential sex-specific mechanisms through which diabetes may incur sex differences in CSVD.
Marked sex differences have been reported for the trajectories of cognitive and clinical decline in dementia. In a pooled analysis of 26,000 participants, females were reported to have greater cognitive reserve but faster cognitive decline than men46. Data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study displayed that female MCI participants experienced cognitive deterioration faster than males with MCI47, 48. Sex-specific differences in the trajectories of cognitive decline have also been supported by fMRI studies looking at memory network alterations. In particular, in a longitudinal fMRI study, we recently reported that male memory network alterations were more associated with amyloid burden while female’s alterations were more correlated to WMH volume49. Our longitudinal findings on clinical dementia outcomes are similar in that baseline amyloid burden was associated with worse longitudinal CDR-SB scores in both sexes, but only females are associated with worse outcomes due to baseline WMH volume. Our findings also agree with a recent longitudinal study analyzing ADNI participants, where females had worse CDR-SB scores over time compared to males with the same level of WMH burden16. When examining other clinical manifestations of CSVD, some studies reported that females had worse functional outcomes and cognitive decline after stroke50, 51. Combined, our findings suggest that elder females may have less resilience to CSVD burden compared to males.
Our study has limitations. First, we do not have information available regarding participants’ medication use. Uncontrolled hypertension has been reported to be a sex-specific risk factor for WMH10, thus our lack of information on participants’ medication may have contributed to our lack of findings of sex differences in associations of hypertension to WMHV. We do not have information of diabetes medication, but our findings for diabetes status can be supported by our similar observations for HbA1c, which is an indicator for diabetic control. Additionally, we do not have information on menopausal status nor whether participants received hormone therapy. Finally, our hospital-based cohort may have recruitment or survival bias, which has been observed to be associated with higher effect sizes for sex differences in cerebrovascular disease3.
In conclusion, our study provides valuable insights into the complex interplay between sex, modifiable risk factors, and clinical dementia outcomes in the context of CSVD. Elderly females appear to have an accelerated increase in cerebrovascular burden as they age, and subsequently are more vulnerable to clinical dementia decline due to CSVD. Our findings also suggest that females are more susceptible to the cerebrovascular consequences of diabetes. These findings emphasize the importance of considering sex when examining the risk factors for and cognitive sequelae of CSVD. Future research should explore the underlying mechanisms driving these sex differences and personalized prevention and treatment strategies.
Table 3. WMHV is associated with longitudinal clinical dementia outcomes in females but not males.
A linear mixed effects model was utilized to test for interaction effects between WMHV*visit time, Global18Futemetamol SUVR*visit time on CDR-SB.
| CDR sum of boxes | |||
|---|---|---|---|
| β (Std. error) | β 95% Confidence Interval | Uncorrected p-value | |
| Females | |||
| Intercept | 4.69 (4.38) | (−3.93, 13.30) | 0.29 |
| Age (years) | −0.044 (0.044) | (−0.13, 0.04) | 0.32 |
| Visit Time | −0.73 (0.57) | (−1.85, 0.38) | 0.20 |
| Global18F-flutemetamol SUVR | 0.34 (2.17) | (−3.94, 4.61) | 0.88 |
| WMHV | −0.18 (0.88) | (−0.75, 0.41) | 0.56 |
| Scanner Site (Site 2) | 0.15 (0.87) | (−1.55, 1.86) | 0.86 |
| Scanner Site (Site 3) | −0.18 (0.88) | (−1.91, 1.55) | 0.84 |
| Scanner Site (Site 4) | 0.90 (1.92) | (−2.88, 4.69) | 0.64 |
| Scanner Site (Site 5) | 1.74 (2.01) | (−2.23, 5.70) | 0.39 |
| Scanner Site (Site 6) | −1.32 (0.73) | (−2.76, 0.12) | 0.073 |
| Visit Time * Global18F-flutemetamol SUVR | 3.11 (0.60) | (1.92, 4.30) | 5.50e-07 *** |
| Visit Time * WMHV | 0.18 (0.072) | (0.04, 0.32) | 0.014 * |
| Males | |||
| Intercept | 0.90 (4.60) | (−8.18, 9.98) | 0.85 |
| Age (years) | 0.0084 (0.053) | (−0.10, 0.11) | 0.88 |
| Visit Time | −1.48 (-0.71) | (−2.89, −.07) | 0.039 * |
| Global18F-flutemetamol SUVR | 2.68 (2.83) | (−2.90, 8.27) | 0.34 |
| WMHV | 0.20 (0.42) | (−0.63, 1.02) | 0.64 |
| Scanner Site (Site 2) | −0.83 (1.10) | (−3.01, 1.35) | 0.46 |
| Scanner Site (Site 3) | −1.00 (1.51) | (−3.99, 1.98) | 0.50 |
| Scanner Site (Site 4) | 0.39 (1.50) | (−2.58, 3.35) | 0.80 |
| Scanner Site (Site 5) | −0.30 (2.13) | (−4.51, 3.91) | 0.89 |
| CDR sum of boxes | |||
| Scanner Site (Site 6) | −0.31 (1.35) | (−2.99, 2.36) | 0.82 |
| Visit Time * Global18F-flutemetamol SUVR | 2.99 (0.72) | (1.56, 4.41) | 7.04e-05 *** |
| Visit Time * WMHV | 0.019 (0.097) | (−0.17, 0.21) | 0.84 |
p < 0.05,
p < 0.01,
p < 0.001
Acknowledgements
Personal thanks:
We would like to thank the staff of BICWALZS and the Suwon Geriatric Mental Health Centre for their involvement in this study.
Funding and assistance:
This work was supported by the National Institute on Aging (R01AG067018 to Wu). This study was conducted using biospecimens and data from the consortium of the Biobank Innovations for Chronic Cerebrovascular Disease With ALZheimer’s Disease Study (BICWALZS), which was funded by the Korea Disease Control and Prevention Agency for the Korea Biobank Project (#6637-303). This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2019R1A5A2026045). This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR21C1003, HI22C0724 and HR22C1734). Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Funding Statement
This work was supported by the National Institute on Aging (R01AG067018 to Wu). This study was conducted using biospecimens and data from the consortium of the Biobank Innovations for Chronic Cerebrovascular Disease With ALZheimer’s Disease Study (BICWALZS), which was funded by the Korea Disease Control and Prevention Agency for the Korea Biobank Project (#6637-303). This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2019R1A5A2026045). This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR21C1003, HI22C0724 and HR22C1734). Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Ethics approval and consent to participate
Written informed consent was obtained from all the participants and caregivers. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ajou University Hospital Institutional Review Board (Board Approval Number AJIRB-BMR-SUR-16–362, approval date 2016/11/11).
Competing interests: Thurston: Astellas, Bayer, Hello Therapeutics (Advisory Board). All other authors declare no competing interests.
Supplementary Files
Contributor Information
Noah Schweitzer, University of Pittsburgh.
Sang Joon Son, Ajou University School of Medicine.
Rebecca C. Thurston, University of Pittsburgh School of Medicine
Jinghang Li, University of Pittsburgh.
Chang-Le Chen, University of Pittsburgh.
Howard Aizenstein, University of Pittsburgh.
Shaolin Yang, University of Pittsburgh.
Bistra Iordanova, University of Pittsburgh.
Chang Hyung Hong, Ajou University School of Medicine.
Hyun Woong Roh, Ajou University School of Medicine.
Yong Hyuk Cho, Ajou University School of Medicine.
Sunhwa Hong, Ajou University School of Medicine.
You Jin Nam, Ajou University School of Medicine.
Dong Yun Lee, Ajou University School of Medicine.
Bumhee Park, Ajou University School of Medicine.
Na-Rae Kim, Ajou University School of Medicine.
Jin Wook Choi, Ajou University School of Medicine.
Jaeyoun Cheong, Ajou University School of Medicine.
Sang Woon Seo, Samsung Medical Centre, Sungkyunkwan University School of Medicine.
Young-Sil An, Ajou University School of Medicine.
So Young Moon, Ajou University School of Medicine.
Seung Jin Han, Ajou University School of Medicine.
Minjie Wu, University of Pittsburgh School of Medicine.
Data and resource availability
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
- 1.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–65. [DOI] [PubMed] [Google Scholar]
- 2.Jochems ACC, Arteaga C, Chappell F et al. Longitudinal Changes of White Matter Hyperintensities in Sporadic Small Vessel Disease: A Systematic Review and Meta-analysis. Neurology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiménez-Sánchez L, Hamilton OKL, Clancy U, et al. Sex Differences in Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Front Neurol. 2021;12:756887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Heuvel DM, Admiraal-Behloul F, ten Dam VH, et al. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology. 2004;63:1699–701. [DOI] [PubMed] [Google Scholar]
- 6.Fatemi F, Kantarci K, Graff-Radford J, et al. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology. 2018;90:e466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longstreth WT Jr., Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Cardiovasc Health Study Stroke. 1996;27:1274–82. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946–56. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–9. [DOI] [PubMed] [Google Scholar]
- 10.Lohner V, Pehlivan G, Sanroma G, et al. Relation Between Sex, Menopause, and White Matter Hyperintensities: The Rhineland Study. Neurology. 2022;99:e935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhuang FJ, Chen Y, He WB, Cai ZY. Prevalence of white matter hyperintensities increases with age. Neural Regen Res. 2018;13:2141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Than S, Moran C, Beare R, et al. Interactions Between Age, Sex, Menopause, and Brain Structure at Midlife: A UK Biobank Study. J Clin Endocrinol Metab. 2021;106:410–20. [DOI] [PubMed] [Google Scholar]
- 13.Geerlings MI, Appelman AP, Vincken KL, et al. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis. 2010;210:130–6. [DOI] [PubMed] [Google Scholar]
- 14.Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946–56. [DOI] [PubMed] [Google Scholar]
- 15.Assareh AA, Mather KA, Crawford JD, et al. Renin-angiotensin system genetic polymorphisms and brain white matter lesions in older Australians. Am J Hypertens. 2014;27:1191–8. [DOI] [PubMed] [Google Scholar]
- 16.Morrison C, Dadar M, Collins DL, Initiative ftAsDN. Sex differences in risk factors, burden, and outcomes of cerebrovascular disease in Alzheimer’s disease populations. Alzheimer’s Dement. 2024;20:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alqarni A, Jiang J, Crawford JD, et al. Sex differences in risk factors for white matter hyperintensities in non-demented older individuals. Neurobiol Aging. 2021;98:197–204. [DOI] [PubMed] [Google Scholar]
- 18.de Bresser J, Tiehuis AM, van den Berg E, et al. Progression of Cerebral Atrophy and White Matter Hyperintensities in Patients With Type 2 Diabetes. Diabetes Care. 2010;33:1309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jongen C, van der Grond J, Kappelle LJ, et al. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50:1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas EG, Rhodius-Meester H, Exalto L et al. Sex-Specific Associations of Diabetes With Brain Structure and Function in a Geriatric Population. Front Aging Neurosci 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- 22.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388:9–21. [DOI] [PubMed] [Google Scholar]
- 23.Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement. 2017;13:296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. [DOI] [PubMed] [Google Scholar]
- 25.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Association AP. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association Publishing; 2013. [Google Scholar]
- 27.Li J, Santini T, Huang Y et al. wmh_seg: Transformer based U-Net for Robust and Automatic White Matter Hyperintensity Segmentation across 1.5T, 3T and 7T. 2024;arXiv:2402.12701. https://ui.adsabs.harvard.edu/abs/2024arXiv240212701L. Accessed February 01, 2024. [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc: Ser B (Methodol). 1995;57:289–300. [Google Scholar]
- 29.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 30.Lüdecke MD. Package ‘sjPlot’. 2023.
- 31.Das AS, Regenhardt RW, Vernooij MW, Blacker D, Charidimou A, Viswanathan A. Asymptomatic Cerebral Small Vessel Disease: Insights from Population-Based Studies. J Stroke. 2019;21:121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol (1985) 2006;101:1252–1261. [DOI] [PubMed] [Google Scholar]
- 33.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cote S, Perron TL, Baillargeon JP, Bocti C, Lepage JF, Whittingstall K. Association of Cumulative Lifetime Exposure to Female Hormones With Cerebral Small Vessel Disease in Postmenopausal Women in the UK Biobank. Neurology. 2023;101:e1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. [DOI] [PubMed] [Google Scholar]
- 36.Sanahuja J, Alonso N, Diez J, et al. Increased Burden of Cerebral Small Vessel Disease in Patients With Type 2 Diabetes and Retinopathy. Diabetes Care. 2016;39:1614–20. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein G, Maillard P, Himali JJ, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology. 2015;84:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: A population-based cohort study. Alzheimer’s Dement. 2019;15:25–33. [DOI] [PubMed] [Google Scholar]
- 39.Schweitzer N, Son SJ, Aizenstein H et al. Higher HbA1c is Associated with Greater Two-Year Progression of White Matter Hyperintensities. Diabetes. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62:1761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int. 2019;127:38–55. [DOI] [PubMed] [Google Scholar]
- 42.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Disease Assoc Disorders. 2006;20:93–100. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee S, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abi-Ghanem C, Salinero AE, Kordit D, et al. Sex differences in the effects of high fat diet on underlying neuropathology in a mouse model of VCID. Biology Sex Differences. 2023;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakata A, Mogi M, Iwanami J, et al. Female type 2 diabetes mellitus mice exhibit severe ischemic brain damage. J Am Soc Hypertens. 2011;5:7–11. [DOI] [PubMed] [Google Scholar]
- 46.Levine DA, Gross AL, Briceño EM, et al. Sex Differences in Cognitive Decline Among US Adults. JAMA Netw Open. 2021;4:e210169–210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am J Neuroradiol. 2013;34:2287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). 2015;1:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweitzer N, Li J, Thurston RC et al. Sex-dependent alterations in hippocampal connectivity are linked to cerebrovascular and amyloid pathologies in normal aging. Alzheimer’s & Dementia;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhamoon MS, McClure LA, White CL, Lakshminarayan K, Benavente OR, Elkind MS. Long-term disability after lacunar stroke: secondary prevention of small subcortical strokes. Neurology. 2015;84:1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasquin SMC, Verhey FRJ, Lousberg R, Winkens I, Lodder J. Vascular cognitive disorders: Memory, mental speed and cognitive flexibility after stroke. J Neurol Sci. 2002;203–204:115–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.