Abstract
Individuals with spinal cord injury (SCI) have significant dysfunction in cardiovascular autonomic regulation. Although recent findings postulate that spinal cord stimulation improves autonomic regulation, limited scope of past methods have tested only above level sympathetic activation, leaving significant uncertainty. To identify whether transcutaneous spinal cord stimulation improves cardiovascular autonomic regulation, two pairs of well-matched individuals with and without high thoracic, complete SCI were recruited. Baseline autonomic regulation was characterized with multiple tests of sympathoinhibition and above/below injury level sympathoexcitation. At three subsequent visits, testing was repeated with the addition submotor threshold transcutaneous spinal cord stimulation at three previously advocated frequencies. Uninjured controls demonstrated no autonomic deficits at baseline and had no changes with any frequency of stimulation. As expected, individuals with SCI had baseline autonomic dysfunction. In a frequency-dependent manner, spinal cord stimulation enhanced sympathoexcitatory responses, normalizing previously impaired Valsalva’s maneuvers. However, stimulation exacerbated already impaired sympathoinhibitory responses, resulting in significantly greater mean arterial pressure increases with the same phenylephrine doses compared with baseline. Impaired sympathoexcitatory response below the level of injury were also further exacerbated with spinal cord stimulation. At baseline, neither individual with SCI demonstrated autonomic dysreflexia with the noxious foot cold pressor test; the addition of stimulation led to a dysreflexic response in every trial, with greater relative hypertension and bradycardia indicating no improvement in cardiovascular autonomic regulation. Collectively, transcutaneous spinal cord stimulation demonstrates no improvements in autonomic regulation after SCI, and instead likely generates tonic sympathoexcitation which may lower the threshold for dangerous autonomic dysreflexia.
NEW & NOTEWORTHY Spinal cord stimulation increases blood pressure after spinal cord injury, though it is unclear if this restores natural autonomic regulation or induces a potentially dangerous pathological reflex. We performed comprehensive autonomic testing batteries, with and without transcutaneous spinal cord stimulation at multiple frequencies. Across 96 independent tests, stimulation did not change uninjured control responses, though all frequencies facilitated pathological reflexes without improved autonomic regulation for those with spinal cord injuries.
Keywords: autonomic regulation, cardiovascular control, neuromodulation, spinal cord injury
INTRODUCTION
Individuals with spinal cord injury (SCI) commonly have blood pressure instability, manifesting as orthostatic hypotension (insufficient sympathetic activation through the injured cord to maintain adequate blood pressure; 1) and/or autonomic dysreflexia (AD, a potentially dangerous reflex hypertension triggered by noxious stimuli below the level of injury, causing regional sympathoexcitation that cannot be counterregulated; 2). After SCI, descending regulatory brainstem signals are interrupted, decoupling important spinal cord sympathetic circuitry and compromising blood pressure regulation (3). To address this, several recent small, high-impact studies have used spinal neuromodulation to stabilize blood pressure during orthostatic challenge (4–7). Though demonstrating proof of principle, differing stimulation frequencies and limited physiological monitoring/challenges provide a narrow view of autonomic nervous system responses, and it is unclear if autonomic regulation is improved through the injured spinal cord as claimed. If regulation is improved, both sympathoexcitatory and sympathoinhibitory responses should be uniformly enhanced. Orthostatic challenge requires sympathoexcitation alone; hence, improvements seen with stimulation may simply be a hypertensive response via local, unperceived noxious stimuli (AD) (8). Given ongoing industry-sponsored clinical trials and the current limited understanding, a more comprehensive characterization of the autonomic regulatory effects of spinal cord stimulation is critically needed.
MATERIALS AND METHODS
This study was conducted according to the guidelines of the Declaration of Helsinki, approved by the Institutional Review Board of Mass Gen Brigham, and preregistered at ClinicalTrials.gov (NCT04858178). Each participant provided written informed consent before study initiation. Individuals aged 18–30 yr with high thoracic, motor, and sensory complete SCI were recruited for this study as well as age, sex, body mass index, and exercise routine matched controls. Individuals with SCI had confirmatory testing of neurological level of injury and American Spinal Injury Association Impairment Scale by a trained physician assessor (9). Health histories and autonomic symptom surveys [COMPASS-31 (10) and Autonomic Dysfunction Following SCI (11)] were recorded at baseline for all participants. Testing was completed in our temperature-controlled academic physiology laboratory, initially with no intervention to establish baseline autonomic regulation, and then with repeated testing sessions and the addition of transcutaneous spinal cord stimulation.
Recordings to Quantify Autonomic Regulation
To develop a comprehensive assessment of cardiovascular autonomic regulation, individuals were instrumented with a five-lead electrocardiogram (Dinamap Dash 2000, GE) for continuous heart rate, respiratory inductance plethysmography (Respitrace, SensorMedics) for continuous breathing pattern, automated finger photoplethysmography (Finapres, Ohmeda Medical) for beat-to-beat arterial blood pressure in the left hand, and oscillometric cuff on the contralateral side (Dinamap Dash 2000, GE) for brachial arterial pressure and calibration with the finger cuff. Respiratory frequency was monitored throughout the experiment, and participants were alerted to any respiratory pattern irregularities that could confound results. Popliteal artery blood flow velocity was recorded from a 4-MHz Doppler ultrasound (Doppler-BoxX, DWL) at the left popliteal fossa and monitored throughout the experiments. Vascular resistance was calculated on a beat-to-beat basis using Ohm’s law as a measure of sympathetic engagement in the lower extremities. Supralesional cardiovascular sympathetic activation would not be expected to significantly increase this below level vascular resistance in an individual with an autonomically complete SCI. Galvanic skin response (Empatica E4) was monitored at the plantar surface of the foot throughout testing as an assessment of sublesional sympathetic sudomotor activation (Fig. 1A). If sudomotor sympathetic activation occurred in the foot (below level of injury for those with SCI), increases in skin surface sweat should decrease the electrical resistance between surface leads. Recordings were digitized at 1,000 Hz for offline analysis. Following instrumentation and supine, quiet rest for at least 10 min, 5-min baseline recordings were collected. All tests were completed in a supine position. Each test of cardiovascular sympathetic inhibition, sub/supralesional sympathetic activation was then performed to establish measures of autonomic regulation.
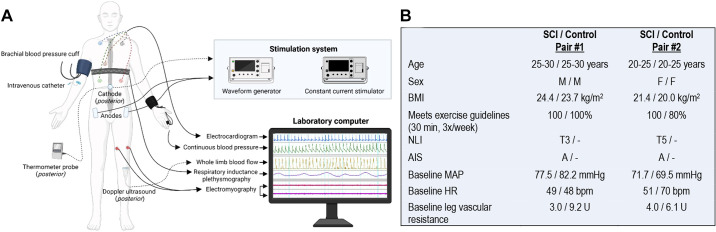
Figure 1.
A: study participant instrumentation. B: demographics and resting baseline characteristics for two matched spinal cord injury (SCI) and uninjured control pairs. Expectedly, lower extremity vascular resistance at baseline is decreased after SCI compared with controls (P = 0.003). AIS, ASIA impairment scale (9); BMI, body mass index; HR, heart rate; MAP, mean arterial pressure; NLI, neurological level of injury (9). Figure 1 was created with a licensed version of BioRender.com.
Tests of Supralesional Cardiovascular Sympathetic Activation
Each participant performed at least three Valsalva’s maneuvers, expiring for 15 s against a handheld mouthpiece to generate a goal expiratory pressure of 30 mmHg. Valsalva’s maneuver, a sustained forced exhalation against resistance generates characteristic responses across four phases. Phase IIa, the initial fall in blood pressure due to intrathoracic pressure restricting venous return to the heart requires whole body sympathetic activation to maintain blood pressure (phase IIb) (12). This is particularly relevant after SCI, where such sympathetic activation through the injured cord to facilitate below level vasoconstriction may not be possible. An air leak was placed in the mouthpiece to ensure that the glottis remained open, and pressure at the mouthpiece was indicative of intrathoracic pressure. Change in mean arterial pressure (MAP) from a 10-beat, premaneuver baseline to the nadir in phase IIb was calculated. Pressure recovery time, the time from release of the strain maneuver until return to baseline MAP is achieved, was further calculated for each maneuver as a measure of sympathetic engagement. Finally, Valsalva’s ratios were calculated as a measure of heart rate response to the strain maneuver.
Hand cold pressor test was additionally performed to quantify the degree of sympathetic activation which was able to be recruited. The mildly noxious stimuli from this test, arising above the level of injury in those with thoracic SCI, should result in increased blood pressure from tachycardia and global sympathoexcitation (which may be restricted to only above level vasoconstriction after SCI) (13). Following 3 min of resting baseline, the right hand was submersed in 0°C ice water for 3 min. Pain levels were assessed at 1-min intervals on a visual analog scale. MAP and R-R interval area under the curve, change in peak galvanic skin response, and change in lower extremity vascular resistance were calculated to quantify this sympathetic engagement.
Tests of Cardiovascular Sympathetic Inhibition
Bolus intravenous phenylephrine, an α1-adrenoreceptor agonist, using the Oxford technique was administered with three progressive doses (14) through an antecubital venous catheter. With a goal increase of >15 mmHg in systolic pressure, dosages were started at 75 μg for uninjured controls and 25 μg for those with SCI. A maximum of 2.5 μg/kg was used for controls and 2.0 μg/kg for those with SCI. The increased blood pressure from phenylephrine is normally buffered by both bradycardia and widespread sympathetic inhibition, the latter of which may be limited below the level of injury in those with SCI. Each bolus of phenylephrine was separated by at least 8 min to allow adequate metabolism and return to baseline. Change in MAP with each dose was calculated as the difference between a 30-s stable baseline immediately before each injection and a 20-s window centered on the initial nadir in heart rate. This approach was taken to standardize the time of maximal blood pressure effect and associated heart rate compensation. Furthermore, MAP area under the curve was calculated for the first 2 min after each dose administration and normalized to the dose of phenylephrine and participant body weight.
Heart rate and blood pressure variability were calculated using beat-to-beat time series of interval between R-waves and systolic blood pressure (SBP), identified using custom peak detection algorithms after visual inspection for artifacts (Matlab, Mathworks). Recordings were made during 5-min segments of paced breathing at 0.25 Hz, guided by an audio recording, to control for known confounders from respiration (15). The time series was then linearly detrended and spectral analysis was completed using Welch’s modified periodogram algorithm (16) as previously described (17). Spectral densities encompassing both low frequency (0.05–0.15 Hz) and high frequency (0.20–0.30 Hz) were integrated to produce average power.
Tests of Sublesional Cardiovascular Sympathetic Activation
Following 3 min of resting baseline, a 3-min foot cold pressor test was performed with the foot emersed in 0°C ice water. Normally, this would result in a typical pressor response with increased blood pressure and tachycardia. Following SCI, however, this noxious stimulus below the injury level in SCI may instead increase blood pressure because of AD, resulting in reflexive baroreflex-mediated bradycardia rather than tachycardia. Pain was assessed at 1-min intervals using a visual analog scale. MAP and R-R interval area under the curve for the duration of the cold pressor test were calculated as a measure of sympathetic engagement and autonomic regulation.
Transcutaneous Electrical Spinal Cord Stimulation
At three subsequent testing sessions separated by at least 7 days to allow for adequate washout of any carryover effects, this battery of tests was repeated with the addition of transcutaneous spinal cord stimulation at T10–T11 for all individuals. A 3.2-cm circular electrode was placed at T10–T11 (acting as a cathode), and two 5 × 9 cm rectangular electrodes were placed at the bilateral iliac crests (acting as anodes). A thermometer probe was positioned at the cathode to ensure safe temperatures ≤38.5°C were maintained. The electrodes were connected to the stimulation system, consisting of a biphasic constant current stimulator (DS8R, Digitimer) triggered by a waveform generator (DG1022, RIGOL, Fig. 1A). Spinal cord stimulation was administered at one of either 120 Hz (4), 30 Hz (5), or 30 Hz with 5 kHz carrier frequency (6, 7) during a single session. To promote replication of these results, the setting configurations are presented in Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.7910/DVN/N2MC2Q). For each of these sessions, motor threshold testing was first performed at the selected frequency, with confirmation of motor activation observed visually and via surface electromyogram (EMG). Stimulation amplitude was set at 80% of the motor threshold to prevent contraction-related hemodynamic contributions. One frequency was tested per session in a randomized order set by a sequence generator with participants blinded. For each individual test, stimulation was started 30 s before test initiation to control for potential confounders related to electrical signal activation. Stimulation remained on for 30 s following est initiation of Valsalva’s maneuver, for 30 s following completion of the cold pressor and bladder pressor tests, and for the first 120 s of the bolus phenylephrine test. Responses to each test were compared with individual baselines without spinal cord stimulation and to responses between matched pairs.
In addition, to gain understanding of the effects of spinal cord stimulation alone, following threshold testing, each session was started with 2 min of stimulation alone (without any provocative maneuver). Paired t tests were completed between matched pairs, with stimulation at all frequencies grouped when data trends were homogenous. Results are reported as means ± SE, with P < 0.05 treated as significant.
RESULTS
Four individuals composing two well-matched pairs of participants with SCI and uninjured controls completed all study sessions (Fig. 1B). Baseline motor and sensory thresholds for each frequency appear in Supplemental Table S1. Stimulation alone, in the absence of any provocative autonomic maneuvers, tended to increase both resting MAP and lower extremity vascular resistance only in individuals with SCI. This effect was most pronounced at 120-Hz stimulation, though did not reach statistical significance (Supplemental Fig. S1).
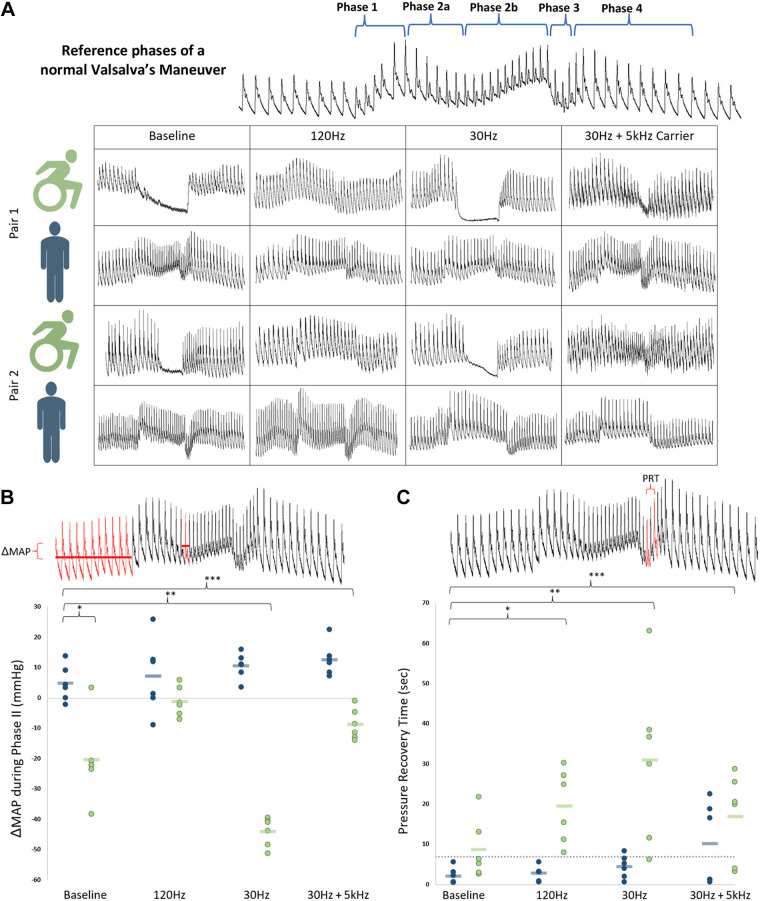
At baseline, phase IIb pressure stabilization of Valsalva’s maneuver was present in controls and reliably absent in both individuals with SCI across all trials, demonstrating impaired sympathoexcitation. Mean arterial pressure (MAP) decrease during phase II was significantly greater at baseline for individuals with SCI than controls (−20.3 ± 5.5 vs. +4.9 ± 2.4 mmHg, P = 0.01), demonstrating impaired baseline sympathoexcitation. Stimulation variably restored phase IIb pressure stabilization in a frequency-dependent manner (Fig. 2), with no such changes for matched controls. One hundred twenty hertz stimulation best-corrected blood pressure responses in those with SCI compared with controls (mean decrease of 1.1 ± 2.0 mmHg, P = 0.20), whereas 30 Hz and 30 Hz + 5 kHz stimulation were ineffective or less effective. Although pressure recovery time (18) was not significantly different at baseline between those with SCI and controls (8.7 ± 3.1 s for SCI vs. 2.2 ± 0.8 s for controls, P = 0.09), stimulation at all frequencies lengthened pressure recovery time for individuals with SCI. Valsalva’s ratios were not different at baseline and stimulation had no effect in either cohort. During hand cold pressor test, baseline MAP area under the curve was generally lower in those with SCI, though to a nonsignificant degree (P = 0.28). This MAP area increased with all frequencies of stimulation (P = 0.09), with a similar mirrored increased in lower extremity vascular resistance (P = 0.08) and less tachycardia. Galvanic skin response at the foot was low at baseline after SCI and was not significantly impacted by spinal cord stimulation (Supplemental Fig. S2).
Figure 2.
A: Valsalva’s maneuver at a goal sustained pressure of 30 mmHg for 15 s. Representational traces of beat-to-beat blood pressure at baseline and with each stimulation frequency for all individuals. Of note, both individuals with spinal cord injury (SCI) at baseline lack phase IIb stabilization of blood pressure (a marker of impaired baseline sympathoexcitation), which was subsequently restored with 120 Hz. B: mean arterial pressure (MAP) changes from baseline to nadir of phase IIb of Valsalva’s maneuver. Group means represented as blue and green bars. C: pressure recovery time from nadir MAP in phase III to return to baseline MAP in phase IV. Dashed line represents upper limits of normal (18). In SCI, this time is significantly lengthened at baseline—an effect which is accentuated with transcutaneous spinal cord stimulation at all frequencies (120 Hz, P = 0.005; 30 Hz, P = 0.02; 30 Hz + 5 kHz, P = 0.03). PRT, pressure recovery time. *,**,***, statistically significant.
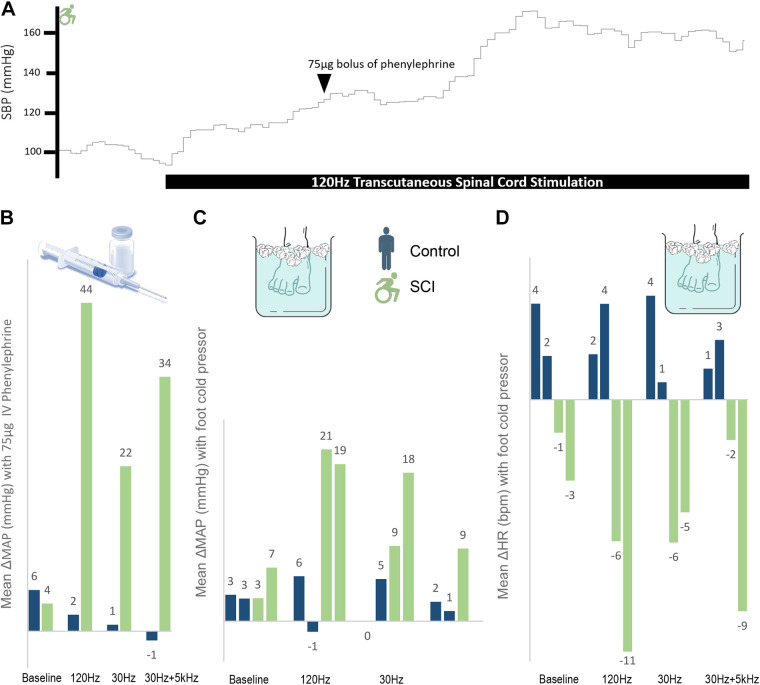
Bolus phenylephrine tended to produce greater ΔMAP and relative resistance in SCI than control (demonstrating impaired sympathoinhibition), though this did not reach significance (P = 0.21 and P = 0.16, respectively, Fig. 3). Across all settings (n = 18 trials) compared with baseline (n = 6 trials), stimulation significantly increased phenylephrine-induced ΔMAP in SCI (27.0 ± 2.4 vs. 6.8 ± 1.1 mmHg, P < 0.001) but not in control (4.5 ± 1.2 vs. 6.3 ± 0.6 mmHg, P = 0.36, Fig. 3). Similarly, vascular resistance was significantly increased with stimulation in SCI (+0.68 ± 0.03 vs. +1.6 ± 0.19 U, P = 0.001) but not in controls (+0.12 ± 0.31 vs. +0.01 ± 0.22 U, P = 0.79). This demonstrates that baseline impairments in sympathoinhibition after SCI are worsened by spinal stimulation. Heart rate and blood pressure variability and their cross-spectrum relationships were unchanged by stimulation in this sample (Supplemental Fig. S3).
Figure 3.
A: representational trace of systolic blood pressure (SBP) with bolus phenylephrine and transcutaneous spinal cord stimulation. B: mean arterial pressure (MAP) change with 75-μg dose of intravenous phenylephrine per frequency for matched pair 1 [uninjured control and individual with spinal cord injury (SCI)]. Change was calculated as 30 s of stable baseline recordings immediately before each successive dose compared with a 20-s window centered at initial nadir in heart rate (HR), when maximal effect was identified. The addition of stimulation caused significantly greater ΔMAP in SCI compared with control (33.5 ± 6.4 vs. 0.6 ± 1.0 mmHg, P = 0.03). C and D: MAP and HR changes with foot cold pressor test. Of note, missing 30 Hz + 5 kHz MAP due to equipment malfunction for second individual with SCI. Although controls exhibit the expected tachycardic response with foot cold pressor, individuals with SCI conversely have bradycardia, indicating a disengaged baroreflex pathway attempting to compensate for sympathetic tone generated below the level of injury. Analyses compared 3 min of stable baseline with 20-s window around the peak MAP.
The foot cold pressor resulted in small MAP increases and a slight tachycardia that were unaltered by stimulation in controls (Fig. 3, C and D). In contrast, foot cold pressor resulted in small MAP increases and a modest bradycardia that were enhanced by stimulation in those with SCI. Across all stimulation frequencies, SBP increased on average by 27.7 ± 4.5 mmHg accompanied by a bradycardia of 6.3 ± 1.3 beats. The greatest effect was seen at 120 Hz (Fig. 3). Hence, without stimulation in those with SCI, the foot cold pressor evidenced a modest noxious response that did reach threshold for AD, but stimulation augmented this such that every trial of the cold pressor test resulted in AD, a marker of ongoing lack of cardiovascular autonomic regulation.
DISCUSSION
Taken together, transcutaneous spinal cord stimulation consistently caused tonic sympathetic activation in a frequency-dependent manner after SCI and had no effect in matched, uninjured controls. There was no evidence that spinal stimulation at previously advocated frequencies improved cardiovascular autonomic regulation. Tonic sympathetic activation from stimulation agrees with previous results demonstrating restored blood pressure maintenance during head-up tilt when sympathoexcitation is needed. Our results further demonstrate that this tonic sympathetic activation can restore phase IIb of Valsalva’s maneuver when sympathetic activation is similarly needed. However, previous studies have largely used only head-up tilt (4–6, 19). Although these studies postulate that improvements in autonomic regulation are the source of increases in blood pressure, our expanded battery of testing shows that stimulation demonstrates no recorded beneficial effect on sympathoinhibitory responses. This lack of normalized sympathoinhibition signals that cardiovascular autonomic regulation is unlikely to be improved. Adding to this evidence, spinal stimulation may promote AD responses to noxious stimuli below the level of lesion. This last effect is of significant clinical importance. For example, during 120-Hz stimulation with foot cold pressor, one individual with SCI demonstrated an almost 20-mmHg rise in MAP despite a robust 11-beat bradycardia; this indicates that there was massive vasoconstriction below the level of lesion in response to the cold noxious stimulus and an ongoing decoupled HR response—opposite of what would be expected with improved autonomic regulation. As AD is a potentially dangerous condition, which is often asymptomatic (8) at initial stages, future studies should adequately monitor for this when stimulation is used.
Differing from most studies, Sachdeva et al. (20) used a test of below level sympathetic activation, digital rectal stimulation, in an individual with SCI, demonstrating that transcutaneous spinal cord stimulation may block and even abort AD episodes. This is counter to our results demonstrating tonic sympathetic activation. It is unclear if the differing location of the stimulation they used (T7–T8 vs. T10–T11 in this study) or potentially an order effect, where repeated stimuli elicit less afferent response as evidenced by lack EMG contraction (20), explains this difference. Further studies are needed to explore these potentials.
This is the first head-to-head study comparing previously advocated frequencies of spinal cord stimulation in a comprehensive manner. Stimulation at 120 Hz had the greatest degree of sympathetic activation, followed by 30 Hz with a 5 kHz carrier frequency, and then 30 Hz. This effect was mirrored across study tests. Although this sample size is typical of the growing field of spinal cord neuromodulation after SCI (4–7), generalizability may be limited with a small cohort.
However, across 96 independent autonomic tests to systematically characterize cardiovascular autonomic regulatory effects of stimulation, results were remarkably consistent. Transcutaneous spinal cord stimulation led to tonic sympathetic activation in a frequency-dependent manner, without evidence of improvements in cardiovascular autonomic regulation after SCI. This may lower the threshold for AD and place individuals with SCI at increased risk for serious complications.
DATA AVAILABILITY
Data are available via the Harvard Dataverse “Transcut stim autonomic, https://doi.org/10.7910/DVN/RDPHZJ” along with associated Data Dictionary “Transcut stim autonomic: data dictionary: https://doi.org/10.7910/DVN/CLYHYV.”
SUPPLEMENTAL DATA
Supplemental Figs. S1–S3 and Supplemental Table S1: https://doi.org/10.7910/DVN/N2MC2Q.
GRANTS
Research reported in this publication was supported by pilot funding from the National Institutes of Health (NIH) National Center of Neuromodulation for Rehabilitation, the National Center for Complementary and Integrative Health, the National Institute on Deafness and Other Communication Disorders, and the National Institute of Neurological Disorders and Stroke; and NIH Grant P2CHD086844, which was awarded to the Medical University of South Carolina. R. Solinsky’s time was protected by NIH Grant K23HD102663.
DISCLAIMERS
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or National Institute of Child Health and Human Development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.S. and J.A.T. conceived and designed research; R.S., K.B., C.T., and J.W.H. performed experiments; R.S., K.B., J.W.H., and J.A.T. analyzed data; R.S., J.W.H., and J.A.T. interpreted results of experiments; R.S., K.B., and J.W.H. prepared figures; R.S. drafted manuscript; R.S., K.B., C.T., J.W.H., and J.A.T. edited and revised manuscript; R.S., K.B., C.T., J.W.H., and J.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
Trial Registration: Transcutaneous Spinal Cord Neuromodulation to Normalize Autonomic Phenotypes; NCT04858178. https://clinicaltrials.gov/ct2/show/NCT04858178.
REFERENCES
- 1. Wecht JM, Bauman WA. Implication of altered autonomic control for orthostatic tolerance in SCI. Auton Neurosci 209: 51–58, 2018. doi: 10.1016/j.autneu.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 2. Solinsky R, Kirshblum SC, Burns SP. Exploring detailed characteristics of autonomic dysreflexia. J Spinal Cord Med 41: 549–555, 2018. doi: 10.1080/10790268.2017.1360434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 81: 506–516, 2000. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- 4. Squair JW, Gautier M, Mahe L, Soriano JE, Rowald A, Bichat A, , et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 590: 308–314, 2021. doi: 10.1038/s41586-020-03180-w. [DOI] [PubMed] [Google Scholar]
- 5. West CR, Phillips AA, Squair JW, Williams AM, Walter M, Lam T, Krassioukov AV. Association of epidural stimulation with cardiovascular function in an individual with spinal cord injury. JAMA Neurol 75: 630–632, 2018. [Erratum in JAMA Neurol 75: 1575, 2018]. doi: 10.1001/jamaneurol.2017.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harkema SJ, Wang S, Angeli CA, Chen Y, Boakye M, Ugiliweneza B, Hirsch GA. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front Hum Neurosci 12: 83, 2018. doi: 10.3389/fnhum.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhavkiran K, Narkeesh A. Effect of surface spinal stimulation on autonomic nervous system in the patients with spinal cord injury. Arch Med Health Sci 2: 126–130, 2014. doi: 10.4103/2321-4848.144297. [DOI] [Google Scholar]
- 8. Burns M, Solinsky R. Toward rebalancing blood pressure instability after spinal cord injury with spinal cord electrical stimulation: a mini review and critique of the evolving literature. Auton Neurosci 237: 102905, 2022. doi: 10.1016/j.autneu.2021.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burns SP, Tansey KE. The expedited international standards for neurological classification of spinal cord injury (E-ISNCSCI). Spinal Cord 58: 633–634, 2020. doi: 10.1038/s41393-020-0462-2. [DOI] [PubMed] [Google Scholar]
- 10. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 87: 1196–1201, 2012. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens 28: 173–181, 2015. doi: 10.1093/ajh/hpu122. [DOI] [PubMed] [Google Scholar]
- 12. Nishimura RA, Tajik AJ. The Valsalva maneuver and response revisited. Mayo Clin Proc 61: 211–217, 1986. doi: 10.1016/s0025-6196(12)61852-7. [DOI] [PubMed] [Google Scholar]
- 13. Victor RG, Leimbach WN Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- 14. La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation 78: 816–824, 1988. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 15. Solinsky R, Schleifer GD, Draghici AE, Hamner JW, Taylor JA. Methodologic implications for rehabilitation research: differences in heart rate variability introduced by respiration. PM R 14: 1483–1489, 2022. doi: 10.1002/pmrj.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 15: 70–73, 1967. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
- 17. Solinsky R, Vivodtzev I, Hamner JW, Taylor JA. The effect of heart rate variability on blood pressure is augmented in spinal cord injury and is unaltered by exercise training. Clin Auton Res 31: 293–301, 2021. doi: 10.1007/s10286-020-00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 65: 1533–1537, 2005. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]
- 19. Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma 35: 446–451, 2018. doi: 10.1089/neu.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sachdeva R, Nightingale TE, Pawar K, Kalimullina T, Mesa A, Marwaha A, Williams AMM, Lam T, Krassioukov AV. Noninvasive neuroprosthesis promotes cardiovascular recovery after spinal cord injury. Neurotherapeutics 18: 1244–1256, 2021. doi: 10.1007/s13311-021-01034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1–S3 and Supplemental Table S1: https://doi.org/10.7910/DVN/N2MC2Q.
Data Availability Statement
Data are available via the Harvard Dataverse “Transcut stim autonomic, https://doi.org/10.7910/DVN/RDPHZJ” along with associated Data Dictionary “Transcut stim autonomic: data dictionary: https://doi.org/10.7910/DVN/CLYHYV.”