Abstract
Biological sex is an important modifier of physiology and influences pathobiology in many diseases. While heart disease is the number one cause of death worldwide in both men and women, sex differences exist at the organ and cellular scales, affecting clinical presentation, diagnosis, and treatment. In this Review, we highlight baseline sex differences in cardiac structure, function, and cellular signaling and discuss the contribution of sex hormones and chromosomes to these characteristics. The heart is a remarkably plastic organ and rapidly responds to physiological and pathological cues by modifying form and function. The nature and extent of cardiac remodeling in response to these stimuli are often dependent on biological sex. We discuss organ- and molecular-level sex differences in adaptive physiological remodeling and pathological cardiac remodeling from pressure and volume overload, ischemia, and genetic heart disease. Finally, we offer a perspective on key future directions for research into cardiac sex differences.
Introduction
Biological sex influences nearly every aspect of human physiology and has important implications for many diseases. Heart disease is the leading cause of death for both men and women, but sexual dimorphisms exist in disease development and clinical outcomes (1). Male and female cells and animals are not identical; however, prior to the mandated inclusion of women in NIH-funded studies in 1993, clinical trials predominantly included men (2). This contributed to the development of therapies and recommended dosages that often favor positive outcomes in men (3, 4). In animal models, females were infrequently studied due to the incorrect assumption that males were less variable. It has since been demonstrated that female mice introduce variability equal to or less than that of males, irrespective of estrous cycle stage (5, 6). Federal funding agencies now require studying both the male and female sexes when possible, and research into sex differences in cardiac biology has become increasingly prevalent over the last two decades.
The heart is a dynamic organ and constantly interprets and responds to internal and external cues. Examples include cardiac adaptation occurring with endurance exercise training, where increased circulatory volume triggers reversible cardiac growth that is associated with improved function (7). Remodeling is also common in pathological settings, including aortic stenosis (AS) and aortic regurgitation, where increased left ventricular (LV) pressure and volume, respectively, trigger maladaptive growth that causes dysfunction (7). Sex differences are well documented in both physiological and pathological cardiac remodeling. In pathological settings, premenopausal women demonstrate protection against adverse remodeling, maintain better cardiac function, and have reduced mortality compared with men. This is often attributed to distinct cell signaling elicited by female and male sex hormones, although additional factors also influence cardioprotection in females.
In this Review, we first define baseline structural, functional, and molecular differences between male and female hearts to provide context. Additionally, we highlight factors influencing sex-biased cellular signaling, including circulating hormones and sex chromosomes. We then discuss sex differences in physiological and pathological remodeling. Sex differences in genetic heart disease are also prevalent, and we review the literature on these conditions. Finally, we offer our perspective on future directions for research into cardiac sex differences. In this study, we discuss only the role of chromosomal sex, which is a biological construct. The influence of gender — a nonbinary social construction — on cardiovascular disease risk and outcomes has been reviewed previously (2).
Baseline differences between male and female hearts
Cardiac sex differences begin in utero, are enhanced during puberty, and continue throughout adulthood. The purpose of this section is to highlight baseline differences between male and female hearts and thus establish context to inform the following discussion of sex biases in cardiac remodeling.
Sex differences in cardiac structure and function.
Cardiac sex differences begin in utero, where male fetuses experience increased LV preload (stretch induced by ventricular blood volume at the end of diastole) and reduced afterload (impedance against which the LV works to eject blood) (8). In children, normalized heart mass is 6% greater in males, but after puberty there is a divergence leading to the adult male heart being at least 25% larger (9–12). Postpubertal cardiac growth in males is particularly pronounced in young endurance athletes who continue to train throughout adolescence (13). Sex differences in adult heart mass were initially attributed to differences in lean body mass between men and women, which led to the oversimplification that the female heart was a small version of the male heart. In contrast, the female heart has unique geometry, and its mass does not directly correlate with body mass (14). The male heart is larger in every dimension, but sex differences in cardiac geometry do not scale linearly (14, 15). For example, an echocardiography study of 734 healthy men and women found that while LV mass was 30% greater in men, LV septum and posterior wall thicknesses were just 11% and 9% larger, respectively (16). LV chamber diameter in both sexes scales with heart mass (14). Cardiac mass increases with age in both sexes; however, the sex difference in normalized cardiac mass is diminished in the elderly, among whom the male heart has been reported as being 4%–9% larger on average (17, 18). This is likely due to greater aging-associated LV hypertrophy in females (11, 18, 19). In adults, total blood volume is lower in women, even when normalized to body size (20). It is therefore unsurprising that cardiac output and stroke volume are also reduced (21, 22). Interestingly, women have higher resting heart rates — due to distinct autonomic nervous system regulation of the sinoatrial node (23) — and some studies report they also have 2%- to 3%-higher baseline LV ejection fractions, which culminates in higher cardiac output in women when normalized to lean body mass (14, 23–26). Sex differences also exist in cardiac electrophysiology; however, this topic is outside the scope of the present Review and has been extensively reviewed previously (27).
Cellular and molecular sex differences.
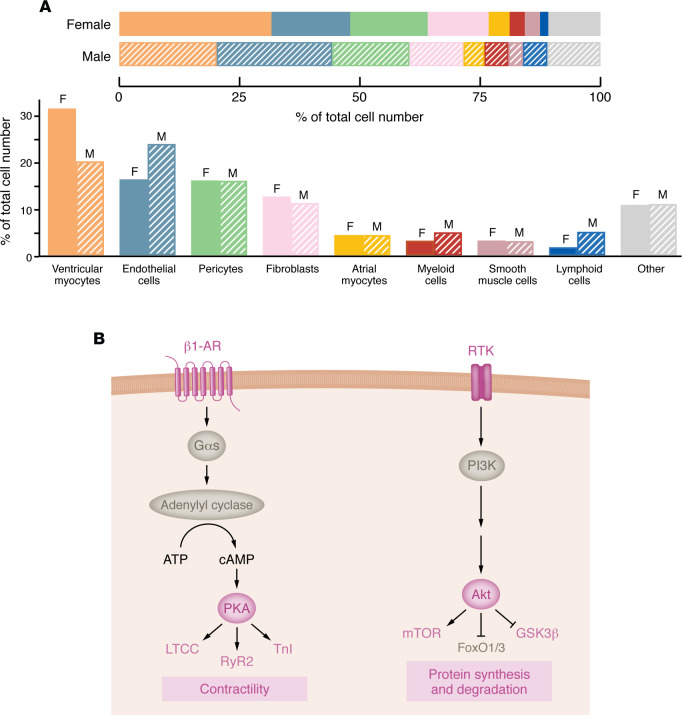
Cardiac cell type composition differs between sexes (Figure 1A). Male and female humans are born with the same number of cardiomyocytes — the predominant cardiac cell type by mass; however, in adulthood, males have a significantly smaller proportion of cardiomyocytes (28). This is thought to be due to testosterone-induced apoptosis in the male heart (29, 30), although differences in cardiomyocyte-regenerative capacity may also contribute (31). Not all cellular differences can be ascribed to hormones, however, as sex-biases exist prior to gonad development. In mice, male fetal hearts favor cardiac conduction and muscle cell action potential gene ontologies, while female fetal hearts are enriched with genes involved in RNA metabolism (32). In a study of age-matched adult mice, 223 genes and 95 proteins were differentially expressed (32). Examination of healthy human hearts revealed 178 genes that were significantly differentially expressed between sexes (33). In both studies, female hearts were characterized as having increased expression of immune factors. When cardiomyocytes were enriched prior to sequencing, 611 genes were found to be differentially expressed, with female cells displaying increased expression of factors involved in hormone receptor activation and PKA signaling (34). PKA acts downstream of β-adrenergic stimulation and modifies cardiomyocyte excitation-contraction coupling, in which sex differences have been identified in calcium signaling and myofilament function (Figure 1B) (35–37). Sex differences have also been observed for fatty acid metabolism and Akt signaling (Figure 1B) (38, 39). Cellular sex differences contribute to the general resistance to heart disease development in premenopausal women, who display less fibrosis, apoptosis, maladaptive hypertrophy, and inflammation (40–42). These favorable features are generally attributed to estrogen-dependent signaling, since cardioprotection is lost following menopause. The effect of changing hormone levels on cardiac remodeling throughout life is discussed in the following section.
Figure 1. Baseline cellular sex differences in the heart.
(A) Sex differences in cardiac cell type composition. The adult human heart is approximately 80% cardiomyocytes by mass, but also contains a plethora of other cell types, including fibroblasts, endothelial cells, and immune cells. Sex differences exist most notably in the relative proportion of cardiomyocytes, endothelial cells, and lymphoid cells. Proportions are based on data from single-cell RNA sequencing study performed by Litviňuková et al. (28). Cell types are ordered according to decreasing relative proportions in the female heart from left to right. Adapted with permission from Walker et al. (29). (B) Enriched cardiomyocyte signaling pathways in female hearts. PKA and Akt activity are enhanced in females at baseline. Proteins in pink have been directly shown to be enhanced in female hearts at baseline; proteins in gray are implicated based on the pathways activated. PKA phosphorylates proteins involved in contractility, while Akt regulates protein homeostasis. β1-AR, β1-adrenergic receptor; Gαs, Gα stimulatory protein; LTCC, L-type calcium channel; RyR2, ryanodine receptor 2; TnI, troponin I; RTK, receptor tyrosine kinase; FoxO, forkhead box protein O.
Contribution of hormones and chromosomes to cardiac sex differences
Steroid sex hormones.
The primary circulating form of estrogen is 17β-estradiol (E2). Both male and female children have circulating E2 concentrations of 5–15 pg/mL, which is secreted by extragonadal tissues (43). However, following menarche, the ovaries become the primary source of E2 in females, who then exhibit circulating levels ranging from 100 to 800 pg/mL depending on menstrual cycle stage (44). Circulating levels drop precipitously following menopause to levels matching those in men of similar ages (Figure 2A) (45). Higher circulating E2 concentrations are inversely correlated with heart disease incidence, and earlier age at menopause increases heart failure risk (46), indicating that E2 contributes to cardioprotection. Notably, women who experienced menopause earlier were also found to have accelerated postmenopausal LV remodeling, including a significant reduction in LV end-diastolic volume (47). Hormone replacement therapy did not mitigate remodeling, suggesting the structural phenotype is not solely the result of decreased cumulative E2 exposure (47). Nevertheless, postmenopausal women with higher circulating E2 levels have reduced disease risk (30). Menopause is also associated with increased myocardial fibrosis, ventricular stiffening, and diastolic dysfunction (48, 49). The receptiveness of the heart to circulating estrogen may also decline with age, as clinical and preclinical studies of E2 replacement in aging females found mixed results and highlight the importance of timing (30, 50). A study in rats found that the antihypertrophic effects of E2 are lost with aging (51), and the cellular effects of E2 in a mouse model of perimenopause were profoundly different from those in reproductively intact animals (52). Thus, it is not clear whether the mechanism of declining cardioprotection in aging females is due to reduced circulating E2, reduced cellular responsiveness to E2, or both. Progesterone is another hormone enriched in females that is known to contribute to cardiac remodeling during pregnancy through activation of calcineurin and protein synthesis (53, 54). However, the effect of progesterone on cardiac remodeling in other settings remains poorly understood.
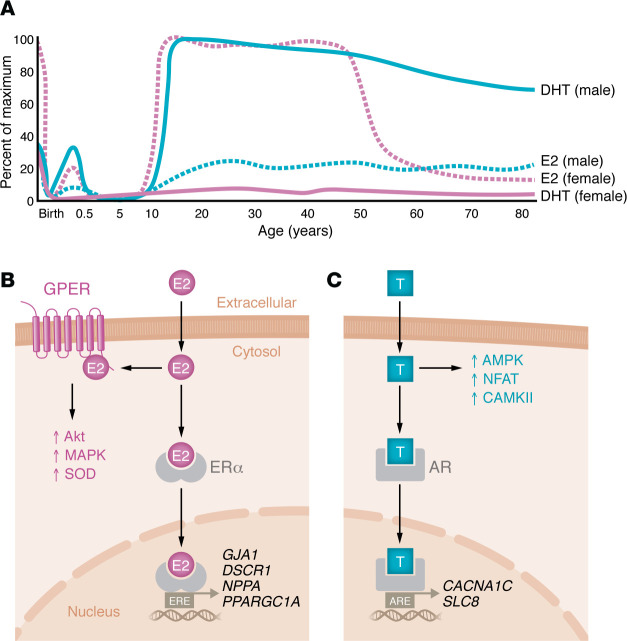
Figure 2. Circulating sex hormones throughout life and their effect on cellular signaling.
(A) Circulating estrogen (7β-estradiol [E2]) and testosterone (DHT) throughout life in male and female humans. Data are based on those Ober et al. (180) and are represented here with permission from the publisher and copyright holder. (B) E2 passively crosses the cardiomyocyte cell membrane and can interact with two different receptors: the G protein–coupled estrogen receptor (GPER) and estrogen receptor α (ERα). GPER signaling likely mediates the nongenomic functions, such as activation of Akt, MAPK, and SOD. ERα translocates to the nucleus and binds to estrogen response elements (ERE) in transcriptional promoters. ERα can also regulate transcription indirectly through interactions with other transcription factors. (C) Dihydroxy-testosterone (T) passively crosses cell membranes and interacts with the androgen receptor (AR) in the cytosol. The activated AR displays both genomic and nongenomic functions, which are associated with increased gene expression of calcium-handling genes and activation of AMPK, nuclear factor of activated T cells (NFAT), and calcium-calmodulin-dependent protein kinase II (CaMKII). ARE, androgen response element.
Androgens, including testosterone, are present in both male and female humans (Figure 2A). Circulating dihydroxytestosterone (DHT) concentrations are similar in male and female children and increase from age 6 through the teenage years (55). However, the increase is much more pronounced in males, who display divergence from females beginning around age 11 with the onset of testosterone production by Leydig cells (55). In most men, circulating DHT levels begin to decline after age 40, but — unlike the rapid reduction in circulating E2 in postmenopausal women — DHT levels fall gradually (Figure 2A) (56). Clinical studies have shown that both high and low testosterone levels are associated with increased risk of developing heart disease (57). Mechanistic studies in mice found reduced cardiomyocyte size and diastolic dysfunction with testosterone deficiency (58), supporting the conclusion that testosterone plays important roles for cardiac structure and function. However, in disease contexts, testosterone is associated with maladaptive hypertrophy and fibrotic remodeling of the LV, which leads to heart failure (59, 60). Compared with that of estrogen, the structural and functional effect of testosterone is relatively less understood and represents an important area for future research.
Cellular signaling initiated by DHT and E2 is mediated by cytosolic androgen receptors (ARs) and estrogen receptors (ERα, ERβ), which have both genomic and nongenomic targets. Genomic functions are mediated by receptor binding to DNA hormone response elements or indirectly through interactions with other transcription factors (61). E2 can also bind to the G protein–coupled estrogen receptor (GPER), a membrane receptor with strictly nongenomic functions (62). In cardiomyocytes, E2 regulates gene expression of the gap junction protein connexin-43 (GJA1); metabolic regulatory protein PGC1-α (PPARGC1A); atrial natriuretic factor (NPPA); and MCIP1 (DSCR1), a negative regulator of calcineurin (61). Notably, only ERα is expressed in rat cardiomyocytes, suggesting that the protective benefits of ERβ in cardiac remodeling stem from its effect on other cardiac cell types (63). Established nongenomic actions of E2 include modulation of PI3K/Akt, MAPKs, and the cellular antioxidant response through superoxide dismutase (SOD) (64). The modulation of PI3K and MAPK signaling likely arises through GPER-dependent regulation, as ERα is not competent to activate these pathways (63). AR-mediated cell signaling is not well understood; however, DHT exposure is known to activate pathology-associated factors in cardiomyocytes, including CaMKII and nuclear factor of activated T cells (NFAT) (65). ARs also directly regulate gene expression of the L-type calcium channel (CACNA1C) and sodium-calcium exchanger (SLC8), which contributes to alterations in calcium signaling between sexes (66). The effects of E2 and DHT on cardiomyocyte biology are summarized in Figure 2B.
Sex chromosomes.
Hormones play important roles in growth and remodeling; however, the heart develops prior to the gonads, and sex differences already exist at these early time points, suggesting involvement of sex chromosomes. Compared with that of hormones, the role of sex chromosomes in cardiac remodeling is poorly understood. The X and Y chromosomes contain approximately 800 and 78 protein-coding genes, respectively, and their expression in nongonadal tissues contributes to sex differences (67). The X chromosome also contains 118 miRNAs, which represents approximately 10% of the total known miRNAs (68). The involvement of miRNAs in cardiac remodeling is well established and has been reviewed previously (69). To account for X chromosome dosage differences, female somatic cells employ X inactivation — a mechanism whereby one X chromosome is functionally silenced by the RNA XIST (70). However, 15%–25% of X chromosome genes escape inactivation in humans, leading to increased relative expression of these genes in women (71, 72). X-linked genes account for approximately 30% of differentially expressed proteins between male and female mouse hearts at baseline (32). In mice, having two X chromosomes is associated with worse remodeling following ischemia/reperfusion injury, irrespective of hormone status (73). This coincides with increased expression of genes that escape X inactivation, including the elongation initiation factor Eif2s3x and lysine methyltransferases Kdm6a and Kdm5c (73). Genes that escape X inactivation have also been linked to activation of aortic valve myofibroblasts, which may underly sex differences observed in AS (discussed below) (74). Genes that escape X inactivation are tissue dependent, differ between species, and remain to be comprehensively investigated in cardiomyocytes. The role of X and Y chromosome genes in cardiac remodeling is largely unknown and represents an important future direction for research into cardiac sex differences.
Sex differences in physiological cardiac remodeling
Human studies.
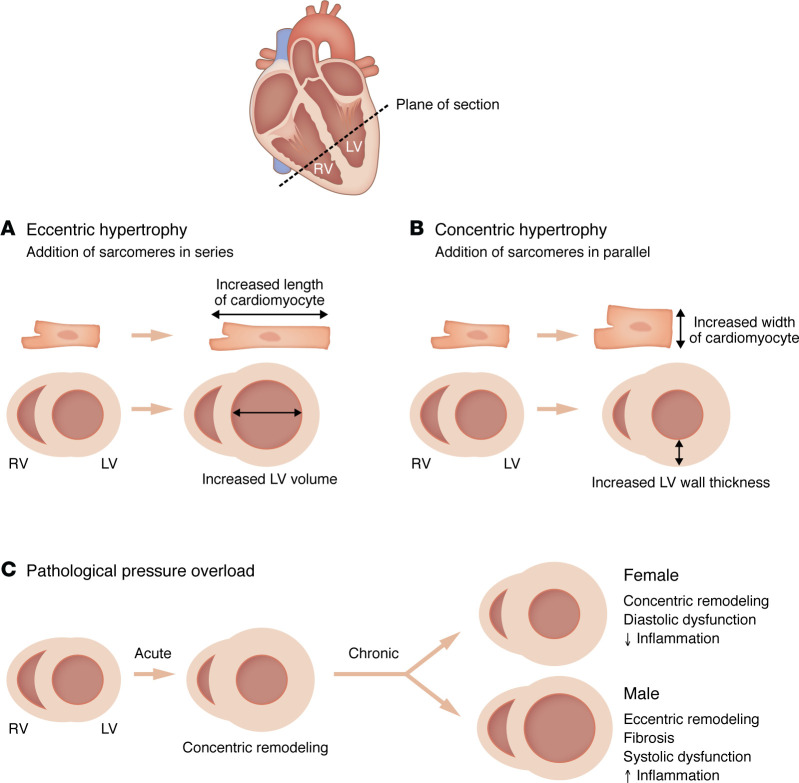
Volume overload, which increases LV preload, is the primary stimulus for adaptive cardiac growth with endurance exercise (75). Blood volume increases by 20%–25% with endurance training in both sexes (76), and, to reduce wall strain, the LV compensates by increasing chamber diameter and wall thickness through eccentric cardiomyocyte hypertrophy (Figure 3A) (7). Adaptive physiological cardiac growth is most common in rowers, but also occurs frequently in runners, swimmers, cross-country skiers, and cyclists (77). This hypertrophy develops rapidly and is associated with improved cardiac function (7). Across sporting disciplines, female athletes display smaller indexed LV mass and wall thickness, and these differences are sustained when normalized by training volume (Figure 3C) (78–82). Sex differences in LV chamber diameter are less pronounced, with some studies reporting no difference or even larger relative chamber dimensions in women (78–81, 83, 84). This suggests that structural remodeling in the female athlete’s heart especially favors increasing chamber diameter over wall thickness, which is supported by studies showing that female endurance athletes almost exclusively develop eccentric hypertrophy (78, 79). Exercise-induced LV remodeling in both sexes favors eccentric remodeling, although a study of 947 elite athletes (22% female) found that LV wall thicknesses in some men (2.2%) entered the diagnostic range for hypertrophic cardiomyopathy (77). Another study, of 1,083 athletes (41% female), found that 4% of highly-trained female athletes experienced concentric hypertrophy (Figure 3B), compared with 15% of men (78). These collective studies support the conclusion that the type of LV remodeling that occurs with exercise is influenced by biological sex. Women also display unique biventricular adaptations to exercise, with higher LV/RV mass ratio compared with men (79). Unlike most instances of pathological hypertrophy, exercise-induced LV remodeling is rapidly reversible (7). Detraining in athletes for 40–240 days following the 1988 Olympic Games caused an average 31% reduction in LV wall thickness (77); and an acute, 32% increase in LV mass that occurred during a 6-day, 622-kilometer ultramarathon was completely reversed 3 days after the race (85). Sex differences in regression have been described for both physiological and pathological hypertrophy; however, regulation of reverse remodeling is outside the scope of this Review and has recently been reviewed elsewhere (7).
Figure 3. Cell- and organ-level remodeling in eccentric and concentric hypertrophy.
(A) Eccentric remodeling in cardiomyocytes is associated with increased cardiomyocyte length-to-width ratio, stemming from the serial addition of contractile units known as sarcomeres. At the organ level, this manifests as increased LV chamber diameter. Eccentric cardiac hypertrophy is most common in conditions of volume overload, such as with endurance exercise training or aortic regurgitation. (B) Concentric remodeling arises from the addition of sarcomeres in parallel, which reduces the cardiomyocyte length-to-width ratio. The result is increased LV septum and free wall thickness and, in healthy conditions, this does not coincide with reduction in LV chamber volume. In pathological settings of pressure overload, such as AS or chronic hypertension, concentric remodeling is associated with reduced LV chamber diameter. (C) Sex differences in cardiac remodeling with pathological pressure overload.
Rodent models.
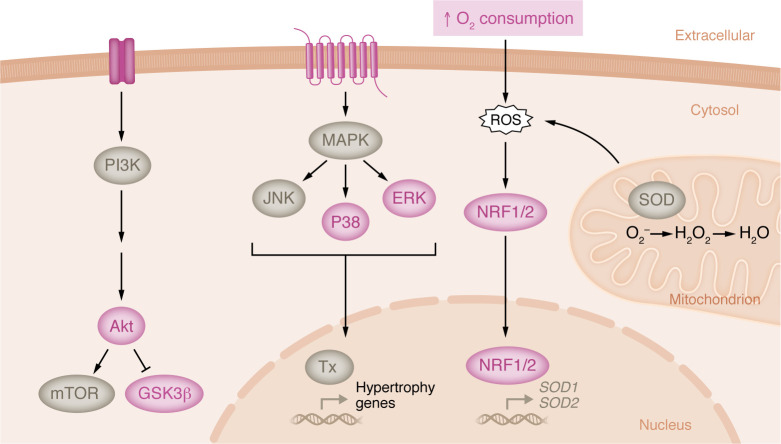
Opposite to what occurs in humans, female mice display greater exercise-induced hypertrophy than males, irrespective of exercise type or training volume (86–89). Swimming induces more remodeling than running but is also associated with an increase in stress-induced catecholamine release (90, 91). Voluntary wheel running, which does not cause stress, is therefore a more appropriate model; it induces a 10%–25% LV mass increase that peaks after 2–4 weeks of training (86, 92, 93). Hypertrophy is influenced by genetic background, as FVB/NJ mice display a more potent sex difference than C57BL/6J mice (86). Female mice run more daily distance than males, yet hypertrophy normalized to distance run remains higher than in males (86, 88, 92, 94). Exercise also increases plasma free fatty acid and triglyceride levels to a greater extent in females (89), suggesting that circulating factors may contribute to hypertrophy development as in other species (95). At the molecular level, trophic signaling pathway activation is enriched in females. Female mice display a higher proportional increase in CaMKII activity and sustained suppression of GSK3β with exercise (86). Activation of Akt has been observed in both sexes but is enhanced in females (86, 89), while AMPK activation is unique to males (92). MAPK signaling is also activated in females, which have increased phosphorylation of ERK and P38 (Figure 4) (94). The female heart is also enriched with the antioxidant factors NRF1 and NRF2 following exercise (Figure 4) (94). It is not known whether these same factors are induced with exercise in the human female heart; however, studies of human skeletal muscle adaptation with exercise identified sex differences in fatty acid metabolism, mitochondrial biogenesis, and Akt signaling, all of which were increased in women (96, 97). The sex difference in exercise-induced hypertrophy in mice is dependent on estrogen receptor β (ERβ) signaling, as ERβ-knockout females display reduced hypertrophy with the same running volume (94). ERβ knockout also prevents the exercise-induced activation of Akt, MAPKs, and protein synthesis in females (94). Notably, estrogens appear to have a suppressive effect on hypertrophy in males, as switching mice to a casein-based diet from traditional soy-based mouse chow, which is rich in phytoestrogens, resulted in greater hypertrophy (92). Female mice displayed equivalent hypertrophy with soy and casein (92). In adult men, high circulating estrogen levels are associated with increased myocardial fibrosis (98). The high phytoestrogen content in traditional mouse chow likely plays a major role in the observed discrepancy between the human and mouse data in exercise-induced cardiac hypertrophy between males and females.
Figure 4. Cellular signaling associated with exercise-induced cardiac hypertrophy in female mice.
Cardiac hypertrophy with exercise is pronounced in female mice compared with males. At the cellular level, female mice display increased Akt activation, which regulates protein synthesis through modulation of mTOR and GSK3β. Female hearts also display activation of factors in MAPK signaling cascade, including P38 and ERK, which indirectly control transcription of a hypertrophic gene program via direct modulation of various transcription factors (Tx). The female mouse heart also has increased expression of nuclear factor erythroid 2–related factors 1 and 2 (NRF1 and NRF2) after exercise. NRFs translocate to the nucleus in response to oxidative stress from ROS and upregulate expression of antioxidant factors, including SOD1 and SOD2. Factors in pink have been directly shown to be induced with exercise; factors in gray are implicated based on the pathways activated.
Sex differences in pathological cardiac remodeling
Pressure overload.
AS is a calcific valve disease that increases LV afterload and is present in more than 5% of the population over 65 years of age (99, 100). The frequency of AS is similar between males and females, although the disease is likely underdiagnosed in women (100, 101). AS progresses at a similar rate in both sexes (102); however, women maintain better systolic and diastolic function, regardless of AS severity (102–109). All-cause mortality with AS has been reported as not being different between sexes, although some studies found reduced mortality in women (100, 102, 110). The sex difference in systolic function stems from maintenance of higher peak LV pressures in women, which is due to differing LV geometry (106, 107, 109). LV mass index is typically lower in women, and they develop a more concentric form of hypertrophy, while maladaptive LV dilation is more prevalent in men with chronic pressure overload (Figure 3C) (103, 104, 108, 111). This leads to female hearts having greater relative LV wall thickness (107, 109). Male hearts have higher extracellular volume, suggestive of more fibrosis (106), which is supported by the finding that LVs from male AS patients have higher collagen gene expression (111). Female sex is associated with suppression of extracellular matrix (ECM) and inflammatory gene expression (111). AS severity in men directly correlates with circulating natriuretic factor levels, whereas these biomarkers do not track with disease severity in women (106, 108).
Preclinical studies of pressure overload use the transverse aortic constriction (TAC) rodent model (112). Cardiac hypertrophy and induction of pathological molecular factors are observable within 1 week after TAC (113). Most studies show that the extent of hypertrophy 2–4 weeks after TAC is similar in males and females (114–116); however, males develop greater LV hypertrophy with longer-term pressure overload that is associated with increased fibrosis (115, 117, 118). As in humans, female rodents develop a more concentric form of hypertrophy and maintain higher peak LV pressures, while males transition into heart failure earlier, experience greater systolic dysfunction, and display LV dilation (114, 118). Reduced systolic function in males arises from suppression of contractile reserve (116). At the cellular level, dysfunction is linked to reduced sarcoplasmic reticulum calcium ATPase (SERCA2A) and increased β-myosin heavy chain (MYH7) expression (116). Male hearts also have increased expression of ECM genes, suppression of mitochondrial antioxidant factors, and increased apoptosis after TAC (115, 117–120). Fibrotic remodeling in males stems from androgen-dependent TGF-β activation, and inhibiting this pathway through TGF-β neutralization or gonadectomy prevents fibrosis (119). Sex differences with regard to pressure overload are also linked to ERβ signaling. Mouse studies found that protection against fibrosis and apoptosis in females was abolished with ERβ knockout, which also led to greater hypertrophy in females after TAC (117, 118, 121). Mechanistically, ERβ helps maintain cardiac function after TAC through inhibition of inflammatory pathways and maintenance of mitochondrial metabolism (117, 120). ERα ablation has no effect on hypertrophy development with pressure overload (121). Together, the human and rodent data support the conclusion that estrogen is protective against maladaptive pressure overload–induced remodeling through modulation of ECM deposition and cardiac inflammation, while testosterone enhances pathology in disease contexts.
Volume overload.
Pathological cardiac remodeling can also arise in conditions of chronic volume overload — although this is a less-common cause than pressure overload — as occurs with aortic regurgitation and mitral regurgitation. Volume overload induces eccentric remodeling due to the increased pressure against the LV walls resulting from higher chamber volume. Sex differences in responses to chronic volume overload are consistent with those observed for pressure overload, and females generally experience less adverse remodeling. Clinical studies found reduced LV volume, reduced LV mass, and increased LV ejection fraction in women compared with men across the spectrum of aortic regurgitation severity (122–126). However, the female heart exhibits greater expansion of extracellular volume relative to the male heart, suggesting an association between chronic volume overload and higher levels of fibrosis (122). A study of skinned cardiac fibers from male and female patients with mitral regurgitation found that female fibers had higher developed force at maximum calcium concentrations, indicating that sex differences in the response to volume overload extend to the myofilament level (127). In a rat model of volume overload, hearts from males displayed increased LV hypertrophy and chamber dilation compared with female hearts (128). Males also had 10-fold-higher mortality, further supporting that females are protected against pathology involving volume overload (128). In mice with aortic regurgitation, LV remodeling in males was associated with activation of CaMKII and Akt, increased fetal gene expression, and induction of apoptosis factors, including Bax and cleaved caspase-3 (129). Another study showed that volume overload from atrioventricular shunt caused apoptosis and increased Bax and caspase-3 and -9 expression in the male rat heart, while hearts from females did not exhibit increased apoptosis, which was estrogen dependent (41). Despite evidence of reduced adverse remodeling in the female heart in response to pathological volume overload, women are more likely to experience symptoms with aortic regurgitation, are older at the time of diagnosis, and have worse prognosis (122, 124, 125), suggesting that the condition may be underdiagnosed.
Ischemia.
Coronary artery disease (CAD) contributes to increased risk of myocardial infarction (MI), the number one cause of death and morbidity in the United States, and manifests differently in men and women (130, 131). MI occurs a decade earlier on average in men due to earlier development of CAD (132); however, despite maintaining higher LV ejection fractions and stroke volumes than men after MI (131), women have an increased risk of developing heart failure and display higher mortality, especially at younger ages (133–136). Adverse remodeling occurs in half of all patients after MI, irrespective of sex, and is denoted by LV chamber dilation, wall thinning, and systolic dysfunction (137). However, men display significantly larger normalized LV chamber size and mass (134, 138, 139). Differences in remodeling are reflected at the cellular level, where myocyte volume and length are also greater in males (138). Transcriptional responses to MI also differ, with one study identifying 271 differentially expressed genes between sexes in LV biopsy samples from patients with ischemic heart disease (140). Pathway analysis of these genes identified oxytocin- and estrogen-dependent signaling as the top two significantly enriched pathways in the female heart (140).
In rodent models of MI from coronary artery ligation, female sex is associated with protection against adverse structural and functional outcomes (59, 141–143). Males have higher rates of cardiac rupture and neutrophil infiltration after MI, while female hearts display greater recruitment of macrophages and reparative monocytes (142, 144, 145). The increased proinflammatory response and activation of MMPs in males contribute to scar thinning (144, 146); however, one study found that MMP inhibition reduced rupture incidence by half (146). Both male and female mouse hearts display increased activation of MAPK signaling, denoted by phosphorylation of P38 and ERK1/2, while females additionally show activation of STAT3, which is estrogen dependent (147). In ovariectomized females, estrogen replacement is associated with worse outcomes in the acute phase after MI but better LV structure and function with chronic exposure (148), which may explain the increased risk of mortality in young women. Estrogen-dependent signaling is linked to improved long-term remodeling and reduced apoptosis and inflammation (144). These benefits are conferred by ERβ, and knockout of the receptor ablates protection from ischemic heart disease in female mice (143). Meanwhile, testosterone causes greater hypertrophy development and increased cardiac rupture risk in males (59, 142, 149). Interestingly, E2 administration in male mice suppresses the post-MI decline in systolic function and mitigates some of the adverse LV remodeling (59, 144). E2 administration in males is also associated with a reduction in proinflammatory cytokines and suppression of P38-dependent apoptosis (144). These collective findings support the conclusion that testosterone-dependent signaling in the heart worsens pathology in the context of ischemia, while the effects of estrogen are timing dependent.
Sex differences in remodeling with genetic cardiomyopathies
Genetic heart disease is estimated to account for approximately 25%–30% of all heart failure cases (150) and is broadly categorized into four subclassifications: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM), and arrhythmogenic right ventricular cardiomyopathy (ARVC). Biological sex can affect disease penetrance, onset, and pathogenesis in each of these conditions (151). In this section, we discuss the leading monogenic causes of HCM and DCM, the most common genetic heart diseases, and highlight the sex differences in disease development and structural remodeling.
HCM.
HCM is the most common form of inherited heart disease, occurring at a frequency of 1:250 to 1:500, and is also the leading cause of sudden death in adolescence (152). It is defined morphologically as LV hypertrophy in the absence of abnormal loading conditions, such as AS or hypertension (153). Missense and truncating mutations, respectively, in the genes encoding β-myosin heavy chain (MYH7) and cardiac myosin binding protein C (MYBPC3) are the most common causes of HCM (152). HCM has an autosomal dominant inheritance pattern and so is expected to occur at equal rates in men and women (152); however, women are more likely to present with pathogenic sarcomere variants (51% vs. 43% for men) (154). Disease onset in women occurs 3–7 years later on average, although they typically present with more-advanced disease and experience worse outcomes (154–157). Disease onset with MYH7 mutations is similar in the two sexes but is delayed in women with MYBPC3 mutations (154, 158, 159). HCM disease penetrance is typically higher in men, and the disease is found more commonly in men until age 60 years, suggesting that premenopausal women are partially protected (158, 160). Women with HCM have smaller LV diameters and increased relative septal and posterior wall thicknesses, and experience more LV outflow tract obstruction and diastolic dysfunction (154, 155, 161, 162). Adverse remodeling is also more prevalent at the cellular level in the female heart, which displays more fibrosis and lower capillary density than the male heart (161). Female sex is also associated with increased mortality, regardless of age or comorbidities (155). In mice, a missense mutation in myosin heavy chain (R403Q) — the first causative mutation identified for HCM — recapitulates human disease phenotypes (163). Studies of this model suggest that disease onset and progression are dependent on biological sex. At 3–4 months, males and females exhibit a similar degree of hypertrophy (164); however, 10-month-old females display concentric hypertrophy, while males progress into LV dilation with systolic dysfunction at this time point (163–165). These data support the conclusion that females are partially protected, which aligns with older age of diagnosis in female patients.
Dilated cardiomyopathy.
DCM is characterized by LV dilation and reduced systolic function (166). Unlike that occurring with endurance training, chamber dilation with DCM is associated with LV wall thinning. Approximately 40% of all DCM cases have a known monogenic cause (151). DCM is nearly as prevalent as HCM and is characterized by autosomal dominant inheritance for all but one known gene: DMD, encoded by the X chromosome (166, 167). Sex biases are not observed with pediatric DCM; however, in adults, women account for just 30% of total cases (151). Women with DCM maintain higher LV contractility, exhibit less dilation, and have reduced fibrosis (168). Despite these favorable characteristics, adverse outcome risk is higher in women (168). The leading genetic causes of DCM are truncating mutations in the gene encoding the sarcomeric protein titin (TTN) and missense mutations in lamin A (LMNA), a component of the nuclear membrane (169). Women with DCM from TTN or LMNA mutations typically fare better than men with respect to clinical outcomes (170, 171). Titin-truncating variants are the most common cause of DCM, accounting for approximately 20% of all cases (172, 173). In these patients, DCM typically presents in midlife and occurs earlier in men (170, 174). Titin mutations show less disease penetrance in women, who are also less likely to experience adverse remodeling and more often experience reverse remodeling with guideline-directed medical therapies (7, 170, 172, 174). Risk of ventricular arrhythmias is also lower in women with DCM due to TTN mutations (174). LMNA mutations account for approximately 5% of all DCM cases, and disease penetrance in adulthood is over 90% (151, 169). Men with LMNA mutations have increased risk of ventricular arrhythmias, heart failure, and mortality (171, 175). They also more frequently develop systolic dysfunction (175). In mouse models of genetic DCM, disease onset is earlier in males, which exhibit enhanced cardiac remodeling and disease progression that is linked to androgen signaling (176).
These collective studies suggest that remodeling develops differently in men and women with genetic heart diseases. Women also tend to have delayed disease onset but experience worse outcomes, which may be partially due to lesser awareness of the disease in women and/or to disease diagnosis occurring at later stages.
Important future directions
Modeling cardiac sex differences in in vitro systems.
Thus far, mechanistic investigations into cardiac sex differences have employed rodent models. These models, while valuable, fail to replicate human sex differences in cardiac remodeling in some cases. Moreover, while over 15% of human X chromosome–encoded genes escape inactivation, only 3% escape in mice (177). Thus, there is a need for new models to investigate mechanistic sex differences in humans. Human induced pluripotent stem cell–derived cardiomyocytes (iPSC-CMs) could represent such a model and have the added benefit of greater throughput compared with animal models; however, they are limited by their immaturity and failure to replicate metabolic and functional features of adult cardiomyocytes. Culturing iPSC-CMs in three-dimensional systems (e.g., engineered heart tissues [EHTs]) with fatty acid–rich maturation media improves cell maturity and enables replication of many features of adult cardiomyocytes (178). To our knowledge, there have been no studies investigating cellular sex differences in iPSC-CMs with or without the addition of relevant hormones. Future studies using EHTs should investigate whether these platforms recapitulate human sex differences in cardiomyocytes differentiated from male and female iPSC lines with physiological concentrations of sex hormones. Additionally, the role of X inactivation in iPSC-CMs should be investigated.
Consideration of biological sex with disease diagnosis and treatment.
One takeaway from clinical studies is that there is a clear need for sex-specific diagnostic criteria in both structural and functional characterizations of heart disease to avoid underestimation of disease in women (151, 179). Relative LV wall thickness in the male heart is greater at baseline, so reaching the diagnostic range for pathological cardiac hypertrophy (≥13 mm) requires less remodeling in men compared with women. Thus, if traditional diagnostic guidelines are followed, the degree of remodeling at diagnosis is expected to be substantially greater for women. This leads to risk of disease diagnosis at later heart failure stages in women, which may contribute to their observed greater heart disease mortality. Sex-specific criteria for cardiac dysfunction may also be valuable, since higher baseline LV ejection fractions have been reported in women (25). Increasing awareness around the high rates of heart disease mortality in women, and how the disease often manifests differently than in men, will surely bring additional benefits with respect to clinical outcomes.
Summary
There are profound sex differences in cardiac remodeling in response to physiological and pathological stimuli. These are borne out at the cellular level through biased cellular signaling mediated by sex hormones and chromosomes, which leads to the premenopausal female heart being partially protected against heart disease. Cardiac remodeling in all contexts may be further modified by age, genetic background, and diet, which can act to enhance or mitigate sex differences. Future studies should emphasize further mechanistic investigations into cardiac sex differences, particularly the role of sex chromosomes, which has been relatively understudied to date. Clarifying these sex-specific mechanisms of remodeling is essential to inform future therapeutic development for the benefit of both men and women with heart disease.
Acknowledgments
The authors acknowledge support from the NIH (F32HL170637 to TGM; R01GM029090 to LAL).
Version 1. 07/01/2024
Electronic publication
Footnotes
Conflict of interest: LAL is a co-founder of MyoKardia, acquired by Bristol-Myers Squibb.
Copyright: © 2024, Martin et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2024;134(13):e180074. https://doi.org/10.1172/JCI180074.
Contributor Information
Thomas G. Martin, Email: Thomas.Martin-2@colorado.edu.
Leslie A. Leinwand, Email: leslie.leinwand@colorado.edu.
References
- 1.Regitz-Zagrosek V, Gebhard C. Gender medicine: effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat Rev Cardiol. 2023;20(4):236–247. doi: 10.1038/s41569-022-00797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauvais-Jarvis F, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legato MJ, et al. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016;316(18):1865–1866. doi: 10.1001/jama.2016.13995. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg JR, et al. Analysis of female enrollment and participant sex by burden of disease in US clinical trials between 2000 and 2020. JAMA Netw Open. 2021;4(6):e2113749. doi: 10.1001/jamanetworkopen.2021.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smarr B, Kriegsfeld LJ. Female mice exhibit less overall variance, with a higher proportion of structured variance, than males at multiple timescales of continuous body temperature and locomotive activity records. Biol Sex Differ. 2022;13(1):41. doi: 10.1186/s13293-022-00451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy DR, et al. Mouse spontaneous behavior reflects individual variation rather than estrous state. Curr Biol. 2023;33(7):1358–1364. doi: 10.1016/j.cub.2023.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin TG, et al. Regression of cardiac hypertrophy in health and disease: mechanisms and therapeutic potential. Nat Rev Cardiol. 2023;20(5):347–363. doi: 10.1038/s41569-022-00806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schalekamp-Timmermans S, et al. In utero origin of sex-related differences in future cardiovascular disease. Biol Sex Differ. 2016;7(1):55. doi: 10.1186/s13293-016-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Simone G, et al. Gender differences in left ventricular growth. Hypertension. 1995;26(6 pt 1):979–983. doi: 10.1161/01.HYP.26.6.979. [DOI] [PubMed] [Google Scholar]
- 10.Molina DK, DiMaio VJM. Normal organ weights in women: Part II - the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2015;36(3):182. doi: 10.1097/PAF.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 11.Gebhard C, et al. Age- and gender-dependent left ventricular remodeling. Echocardiography. 2013;30(10):1143–1150. doi: 10.1111/echo.12264. [DOI] [PubMed] [Google Scholar]
- 12.Forså MI, et al. Young athlete’s growing heart: sex differences in cardiac adaptation to exercise training during adolescence. Open Heart. 2023;10(1):e002155. doi: 10.1136/openhrt-2022-002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjerring AW, et al. From talented child to elite athlete: The development of cardiac morphology and function in a cohort of endurance athletes from age 12 to 18. Eur J Prev Cardiol. 2021;28(10):1061. doi: 10.1177/2047487320921317. [DOI] [PubMed] [Google Scholar]
- 14.St. Pierre SR, et al. Sex matters: a comprehensive comparison of female and male hearts. Front Physiol. 2022;13:831179. doi: 10.3389/fphys.2022.831179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prajapati C, et al. Sex differences in heart: from basics to clinics. Eur J Med Res. 2022;27(1):241. doi: 10.1186/s40001-022-00880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kou S, et al. Echocardiographic reference ranges for normal cardiac chamber size: Results from the NORRE study. Eur Heart J Cardiovasc Imaging. 2014;15(6):680. doi: 10.1093/ehjci/jet284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikhazadi A, et al. Study of the normal internal organ weights in Tehran’s population. J Forensic Leg Med. 2010;17(2):78–83. doi: 10.1016/j.jflm.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz D, et al. High prevalence of left ventricular hypertrophy in octogenarian women: The Jerusalem Longitudinal Cohort Study. Blood Press. 2010;19(2):86–91. doi: 10.3109/08037050903516292. [DOI] [PubMed] [Google Scholar]
- 19.Ji H, et al. Sex differences in myocardial and vascular aging. Circ Res. 2022;130(4):566–577. doi: 10.1161/CIRCRESAHA.121.319902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Canestro C, et al. Sex differences in cardiorespiratory fitness are explained by blood volume and oxygen carrying capacity. Cardiovasc Res. 2022;118(1):334–343. doi: 10.1093/cvr/cvab028. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski DR, et al. Sex differences in cardiac flow dynamics of healthy volunteers. Radiol Cardiothorac Imaging. 2020;2(1):e190058. doi: 10.1148/ryct.2020190058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argiento P, et al. Exercise stress echocardiography of the pulmonary circulation: limits of normal and sex differences. Chest. 2012;142(5):1158–1165. doi: 10.1378/chest.12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenig J, Thayer JF. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci Biobehav Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Buonanno C, et al. Left ventricular function in men and women. Another difference between sexes. Eur Heart J. 1982;3(6):525–528. doi: 10.1093/oxfordjournals.eurheartj.a061347. [DOI] [PubMed] [Google Scholar]
- 25.Chung AK, et al. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: the Dallas Heart Study. Circulation. 2006;113(12):1597–1604. doi: 10.1161/CIRCULATIONAHA.105.574400. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Yin L. Sex differences in left ventricular stroke work and cardiac power output per unit myocardium relate to blood pressure in apparently healthy adults. PLoS One. 2023;18(1):e0280143. doi: 10.1371/journal.pone.0280143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadros R, et al. Sex differences in cardiac electrophysiology and clinical arrhythmias: epidemiology, therapeutics, and mechanisms. Can J Cardiol. 2014;30(7):783–792. doi: 10.1016/j.cjca.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Litviňuková M, et al. Cells of the adult human heart. Nature. 2020;588(7838):466–472. doi: 10.1038/s41586-020-2797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker CJ, et al. Matters of the heart: cellular sex differences. J Mol Cell Cardiol. 2021;160:42–55. doi: 10.1016/j.yjmcc.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yusifov A, et al. Mechanisms and implications of sex differences in cardiac aging. J Cardiovasc Aging. 2022;2(20):20517. doi: 10.20517/jca.2022.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hariharan N, Sussman MA. Cardiac aging — Getting to the stem of the problem. J Mol Cell Cardiol. 2015;83:32. doi: 10.1016/j.yjmcc.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi W, et al. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev Cell. 2021;56(21):3019–3034. doi: 10.1016/j.devcel.2021.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inanloorahatloo K, et al. Sex-based differences in myocardial gene expression in recently deceased organ donors with no prior cardiovascular disease. PLoS One. 2017;12(8):e0183874. doi: 10.1371/journal.pone.0183874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trexler CL, et al. Transcriptome and functional profile of cardiac myocytes is influenced by biological sex. Circ Cardiovasc Genet. 2017;10(5):e001770. doi: 10.1161/CIRCGENETICS.117.001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parks RJ, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch. 2013;465(5):747–763. doi: 10.1007/s00424-013-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vizgirda VM, et al. Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol. 2002;282(1):H256–H263. doi: 10.1152/ajpheart.2002.282.1.H256. [DOI] [PubMed] [Google Scholar]
- 37.Parks RJ, et al. Sex differences in SR Ca(2+) release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J Mol Cell Cardiol. 2014;75:162–173. doi: 10.1016/j.yjmcc.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97(1):1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 39.Camper-Kirby D, et al. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88(10):1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 40.Peter AK, et al. Cardiac fibroblasts mediate a sexually dimorphic fibrotic response to β-adrenergic stimulation. J Am Heart Assoc. 2021;10(11):e018876. doi: 10.1161/JAHA.120.018876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dent MR, et al. Gender differences in apoptotic signaling in heart failure due to volume overload. Apoptosis. 2010;15(4):499–510. doi: 10.1007/s10495-009-0441-8. [DOI] [PubMed] [Google Scholar]
- 42.Beale AL, et al. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138(2):198–205. doi: 10.1161/CIRCULATIONAHA.118.034271. [DOI] [PubMed] [Google Scholar]
- 43.Bidlingmaier F, et al. Plasma estrogens in childhood and puberty under physiologic and pathologic conditions. Pediatr Res. 1973;7(11):901–907. doi: 10.1203/00006450-197311000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Frederiksen H, et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J Clin Endocrinol Metab. 2020;105(3):754–768. doi: 10.1210/clinem/dgz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson H, et al. Baseline estrogen levels in postmenopausal women participating in the MAP.3 breast cancer chemoprevention trial. Menopause. 2020;27(6):693–700. doi: 10.1097/GME.0000000000001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appiah D, et al. Association of age at menopause with incident heart failure: a prospective cohort study and meta-analysis. J Am Heart Assoc. 2016;5(8):e003769. doi: 10.1161/JAHA.116.003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honigberg MC, et al. Menopausal age and left ventricular remodeling by cardiac magnetic resonance imaging among 14,550 women. Am Heart J. 2020;229:138–143. doi: 10.1016/j.ahj.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CY, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62(14):1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae S, et al. Early menopause is associated with abnormal diastolic function and poor clinical outcomes in women with suspected angina. Sci Rep. 2024;14(1):6306. doi: 10.1038/s41598-024-57058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. 2011;20(2):133–138. doi: 10.1097/MNH.0b013e3283431921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jazbutyte V, et al. Aging reduces the efficacy of estrogen substitution to attenuate cardiac hypertrophy in female spontaneously hypertensive rats. Hypertension. 2006;48(4):579–586. doi: 10.1161/01.HYP.0000240053.48517.c7. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes R Del, et al. Cardiac changes during the peri-menopausal period in a VCD-induced murine model of ovarian failure. Acta Physiol (Oxf) 2019;227(1):e13290. doi: 10.1111/apha.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung E, et al. Calcineurin activity is required for cardiac remodelling in pregnancy. Cardiovasc Res. 2013;100(3):402–410. doi: 10.1093/cvr/cvt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstein J, et al. Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertil Steril. 2004;82(2):430–436. doi: 10.1016/j.fertnstert.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 55.Senefeld JW, et al. Divergence in timing and magnitude of testosterone levels between male and female youths. JAMA. 2020;324(1):99–101. doi: 10.1001/jama.2020.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldman HA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda Y, et al. Effects of androgens on cardiovascular remodeling. J Endocrinol. 2012;214(1):1–10. doi: 10.1530/JOE-12-0126. [DOI] [PubMed] [Google Scholar]
- 58.Ayaz O, et al. Long-term testosterone deficiency modifies myofilament and calcium-handling proteins and promotes diastolic dysfunction in the aging mouse heart. Am J Physiol Heart Circ Physiol. 2019;316(4):H768–H780. doi: 10.1152/ajpheart.00471.2018. [DOI] [PubMed] [Google Scholar]
- 59.Cavasin MA, et al. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284(5):H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, et al. Androgen contributes to gender-related cardiac hypertrophy and fibrosis in mice lacking the gene encoding guanylyl cyclase-A. Endocrinology. 2004;145(2):951–958. doi: 10.1210/en.2003-0816. [DOI] [PubMed] [Google Scholar]
- 61.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109(6):687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimmerman MA, et al. GPER — Novel membrane oestrogen receptor. Clin Sci. 2016;130(12):1005. doi: 10.1042/CS20160114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pugach EK, et al. Estrogen receptor profiling and activity in cardiac myocytes. Mol Cell Endocrinol. 2016;431:62–70. doi: 10.1016/j.mce.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puglisi R, et al. Non-genomic effects of estrogen on cell homeostasis and remodeling with special focus on cardiac ischemia/reperfusion injury. Front Endocrinol (Lausanne) 2019;10:733. doi: 10.3389/fendo.2019.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willemars MMA, et al. Evaluation of the interaction of sex hormones and cardiovascular function and health. Curr Heart Fail Rep. 2022;19(4):200–212. doi: 10.1007/s11897-022-00555-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira C, et al. Sexual dimorphism in cardiac remodeling: the molecular mechanisms ruled by sex hormones in the heart. J Mol Med (Berl) 2022;100(2):245–267. doi: 10.1007/s00109-021-02169-w. [DOI] [PubMed] [Google Scholar]
- 67.Bachtrog D. Y-chromosome evolution: emerging insights into processes of Y-chromosome degeneration. Nat Rev Genet. 2013;14(2):113–124. doi: 10.1038/nrg3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Palo A, et al. What microRNAs could tell us about the human X chromosome. Cell Mol Life Sci. 2020;77(20):4069–4080. doi: 10.1007/s00018-020-03526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C, et al. MicroRNA as a therapeutic target in cardiac remodeling. Biomed Res Int. 2017;2017:1278436. doi: 10.1155/2017/1278436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang H, et al. X Inactivation and escape: epigenetic and structural features. Front Cell Dev Biol. 2019;7:219. doi: 10.3389/fcell.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wainer Katsir K, Linial M. Human genes escaping X-inactivation revealed by single cell expression data. BMC Genomics. 2019;20(201):201. doi: 10.1186/s12864-019-5507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tukiainen T, et al. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550(7675):244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, et al. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res. 2014;102(3):375–384. doi: 10.1093/cvr/cvu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aguado BA, et al. Genes that escape X chromosome inactivation modulate sex differences in valve myofibroblasts. Circulation. 2022;145(7):513–530. doi: 10.1161/CIRCULATIONAHA.121.054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weeks KL, McMullen JR. The athlete’s heart vs. the failing heart: can signaling explain the two distinct outcomes? Physiology (Bethesda) 2011;26(2):97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- 76.Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc. 1991;23(12):1338–1348. doi: 10.1249/00005768-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Pelliccia A, et al. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324(5):295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 78.Finocchiaro G, et al. Effect of sex and sporting discipline on LV adaptation to exercise. JACC Cardiovasc Imaging. 2017;10(9):965–972. doi: 10.1016/j.jcmg.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 79.D’Ascenzi F, et al. Female athlete’s heart: sex effects on electrical and structural remodeling. Circ Cardiovasc Imaging. 2020;13(12):E011587. doi: 10.1161/CIRCIMAGING.120.011587. [DOI] [PubMed] [Google Scholar]
- 80.Rowland T, Roti M. Influence of sex on the “Athlete’s Heart” in trained cyclists. J Sci Med Sport. 2010;13(5):475–478. doi: 10.1016/j.jsams.2009.10.488. [DOI] [PubMed] [Google Scholar]
- 81.Howden EJ, et al. Females have a blunted cardiovascular response to one year of intensive supervised endurance training. J Appl Physiol (1985) 2015;119(1):37–46. doi: 10.1152/japplphysiol.00092.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mumford M, Prakash R. Electrocardiographic and echocardiographic characteristics of long distance runners. Comparison of left ventricular function with age- and sex-matched controls. Am J Sports Med. 1981;9(1):23–28. doi: 10.1177/036354658100900105. [DOI] [PubMed] [Google Scholar]
- 83.George KP, et al. Electrocardiographic and echocardiographic characteristics of female athletes. Med Sci Sports Exerc. 1995;27(10):1362. doi: 10.1249/00005768-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Pelliccia A, et al. Athlete’s heart in women. Echocardiographic characterization of highly trained elite female athletes. JAMA. 1996;276(3):211–215. doi: 10.1001/jama.1996.03540030045030. [DOI] [PubMed] [Google Scholar]
- 85.Kim YJ, Park KM. Effects of super-ultramarathon running on cardiac structure and function in middle-aged men. J Cardiovasc Imaging. 2020;28(3):202–210. doi: 10.4250/jcvi.2020.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Konhilas JP, et al. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287(6):H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mole PA. Increased contractile potential of papillary muscles from exercise-trained rat hearts. Am J Physiol. 1978;234(4):H421–H425. doi: 10.1152/ajpheart.1978.234.4.H421. [DOI] [PubMed] [Google Scholar]
- 88.De Bono JP, et al. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R926–R934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- 89.Foryst-Ludwig A, et al. Sex differences in physiological cardiac hypertrophy are associated withexercise-mediated changes in energy substrate availability. Am J Physiol Hear Circ Physiol. 2011;301(1):H115. doi: 10.1152/ajpheart.01222.2010. [DOI] [PubMed] [Google Scholar]
- 90.Greenen D, et al. Cardiovascular and hormonal responses to swimming and running in the rat. J Appl Physiol (1985) 1988;65(1):116–123. doi: 10.1152/jappl.1988.65.1.116. [DOI] [PubMed] [Google Scholar]
- 91.Soares D dos S, et al. Cardiac hypertrophy in mice submitted to a swimming protocol: influence of training volume and intensity on myocardial renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2019;316(6):R776–R782. doi: 10.1152/ajpregu.00205.2018. [DOI] [PubMed] [Google Scholar]
- 92.Konhilas JP, et al. Diet and sex modify exercise and cardiac adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2015;308(2):H135–H145. doi: 10.1152/ajpheart.00532.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Allen DL, et al. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol (1985) 2001;90(5):1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 94.Dworatzek E, et al. Sex differences in exercise-induced physiological myocardial hypertrophy are modulated by oestrogen receptor beta. Cardiovasc Res. 2014;102(3):418–428. doi: 10.1093/cvr/cvu065. [DOI] [PubMed] [Google Scholar]
- 95.Tan Y, et al. Utility of the burmese Python as a model for studying plasticity of extreme physiological systems. J Muscle Res Cell Motil. 2023;44(2):95–106. doi: 10.1007/s10974-022-09632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Landen S, et al. Physiological and molecular sex differences in human skeletal muscle in response to exercise training. J Physiol. 2023;601(3):419–434. doi: 10.1113/JP279499. [DOI] [PubMed] [Google Scholar]
- 97.Ryan AS, et al. Sex differences in insulin regulation of skeletal muscle glycogen synthase and changes during weight loss and exercise in adults. Obesity (Silver Spring) 2024;32(4):667–677. doi: 10.1002/oby.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chehab O, et al. Endogenous sex hormone levels and myocardial fibrosis in men and postmenopausal women. JACC Adv. 2023;2(3):e100320. doi: 10.1016/j.jacadv.2023.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ancona R, Pinto SC. Epidemiology of aortic valve stenosis (AS) and of aortic valve incompetence (AI): is the prevalence of AS/AI similar in different parts of the world? e-Journal Cardiol Pract. 2020;18:10 [Google Scholar]
- 100.Nkomo VT et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 101.Iribarren AC, et al. Sex differences in aortic stenosis: Identification of knowledge gaps for sex-specific personalized medicine. Am Heart J Plus. 2022;21:100197. doi: 10.1016/j.ahjo.2022.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cramariuc D, et al. Sex differences in cardiovascular outcome during progression of aortic valve stenosis. Heart. 2015;101(3):209–214. doi: 10.1136/heartjnl-2014-306078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ito S, et al. Sex differences in LV remodeling and hemodynamics in aortic stenosis: sex-specific criteria for severe stenosis? JACC Cardiovasc Imaging. 2022;15(7):1175–1189. doi: 10.1016/j.jcmg.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 104.Carroll JD, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86(4):1099–1107. doi: 10.1161/01.CIR.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 105.Cramariuc D, et al. Factors influencing left ventricular structure and stress-corrected systolic function in men and women with asymptomatic aortic valve stenosis (a SEAS Substudy) Am J Cardiol. 2008;101(4):510–515. doi: 10.1016/j.amjcard.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 106.Singh A, et al. Symptom onset in aortic stenosis: relation to sex differences in left ventricular remodeling. JACC Cardiovasc Imaging. 2019;12(1):96–105. doi: 10.1016/j.jcmg.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 107.Douglas PS, et al. Gender differences in left ventricle geometry and function in patients undergoing balloon dilatation of the aortic valve for isolated aortic stenosis. NHLBI Balloon Valvuloplasty Registry. Br Heart J. 1995;73(6):548–554. doi: 10.1136/hrt.73.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luchner A, et al. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc Res. 2002;53(3):720–727. doi: 10.1016/S0008-6363(01)00510-7. [DOI] [PubMed] [Google Scholar]
- 109.Aurigemma GP, et al. Impact of chamber geometry and gender on left ventricular systolic function in patients > 60 years of age with aortic stenosis. Am J Cardiol. 1994;74(8):794–798. doi: 10.1016/0002-9149(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 110.Toyofuku M, et al. Sex differences in severe aortic stenosis- clinical presentation and mortality. Circ J. 2017;81(8):1213–1221. doi: 10.1253/circj.CJ-16-1244. [DOI] [PubMed] [Google Scholar]
- 111.Kararigas G, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail. 2014;16(11):1160–1167. doi: 10.1002/ejhf.171. [DOI] [PubMed] [Google Scholar]
- 112.deAlmeida AC, et al. Transverse aortic constriction in mice. J Vis Exp. 2010;(38):1729. doi: 10.3791/1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Furihata T, et al. The experimental model of transition from compensated cardiac hypertrophy to failure created by transverse aortic constriction in mice. Int J Cardiol Heart Vasc. 2016;11:24–28. doi: 10.1016/j.ijcha.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Douglas PS, et al. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32(4):1118–1125. doi: 10.1016/S0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 115.Witt H, et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med (Berl) 2008;86(9):1013–1024. doi: 10.1007/s00109-008-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weinberg EO, et al. Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol. 1999;34(1):264–273. doi: 10.1016/S0735-1097(99)00165-5. [DOI] [PubMed] [Google Scholar]
- 117.Kararigas G, et al. Comparative proteomic analysis reveals sex and estrogen receptor β effects in the pressure overloaded heart. J Proteome Res. 2014;13(12):5829–5836. doi: 10.1021/pr500749j. [DOI] [PubMed] [Google Scholar]
- 118.Fliegner D et al. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1597–R1606. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- 119.Montalvo C, et al. Androgens contribute to sex differences in myocardial remodeling under pressure overload by a mechanism involving TGF-β. PLoS One. 2012;7(4):e35635. doi: 10.1371/journal.pone.0035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kararigas G, et al. Role of the estrogen/estrogen-receptor-beta axis in the genomic response to pressure overload-induced hypertrophy. Physiol Genomics. 2011;43(8):438–446. doi: 10.1152/physiolgenomics.00199.2010. [DOI] [PubMed] [Google Scholar]
- 121.Skavdahl M, et al. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288(2):H469–H476. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- 122.Malahfji M, et al. Sex differences in myocardial remodeling and extracellular volume in aortic regurgitation. Sci Rep. 2023;13(1):11334. doi: 10.1038/s41598-023-37444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tower-Rader A, et al. Sex-based differences in left ventricular remodeling in patients with chronic aortic regurgitation: a multi-modality study. J Cardiovasc Magn Reson. 2022;24(1):12. doi: 10.1186/s12968-022-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kammerlander AA, et al. Sex differences in left ventricular remodeling and outcomes in chronic aortic regurgitation. J Clin Med. 2020;9(12):1. doi: 10.3390/jcm9124100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang B, et al. Association of bioprosthetic aortic valve leaflet calcification on hemodynamic and clinical outcomes. J Am Coll Cardiol. 2020;76(15):1737–1748. doi: 10.1016/j.jacc.2020.08.034. [DOI] [PubMed] [Google Scholar]
- 126.Akintoye E, et al. Impact of age and sex on left ventricular remodeling in patients with aortic regurgitation. J Am Coll Cardiol. 2023;81(15):1474–1487. doi: 10.1016/j.jacc.2023.02.037. [DOI] [PubMed] [Google Scholar]
- 127.Bening C, et al. Sex differences in volume overload in skinned fibers. BMC Cardiovasc Disord. 2016;16(1):197. doi: 10.1186/s12872-016-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gardner JD, et al. Gender differences in cardiac remodeling secondary to chronic volume overload. J Card Fail. 2002;8(2):101–107. doi: 10.1054/jcaf.2002.32195. [DOI] [PubMed] [Google Scholar]
- 129.You J, et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am J Physiol Heart Circ Physiol. 2018;314(3):H552–H562. doi: 10.1152/ajpheart.00212.2017. [DOI] [PubMed] [Google Scholar]
- 130.Nowbar AN, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12(6):e005375. doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aimo A, et al. Sex-related differences in ventricular remodeling after myocardial infarction. Int J Cardiol. 2021;339:62–69. doi: 10.1016/j.ijcard.2021.07.036. [DOI] [PubMed] [Google Scholar]
- 132.Anand SS, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29(7):932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 133.Cenko E, et al. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern Med. 2018;178(5):632–639. doi: 10.1001/jamainternmed.2018.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lam CSP, et al. Sex differences in clinical characteristics and outcomes after myocardial infarction: insights from the Valsartan in Acute Myocardial Infarction Trial (VALIANT) Eur J Heart Fail. 2015;17(3):301–312. doi: 10.1002/ejhf.238. [DOI] [PubMed] [Google Scholar]
- 135.Kosmidou I, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur Heart J. 2017;38(21):1656–1663. doi: 10.1093/eurheartj/ehx159. [DOI] [PubMed] [Google Scholar]
- 136.Vaccarino V, et al. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 137.van der Bijl P, et al. Left ventricular remodelling after ST-segment elevation myocardial infarction: sex differences and prognosis. ESC Heart Fail. 2020;7(2):474–481. doi: 10.1002/ehf2.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crabbe DL, et al. Gender differences in post-infarction hypertrophy in end-stage failing hearts. J Am Coll Cardiol. 2003;41(2):300–306. doi: 10.1016/S0735-1097(02)02710-9. [DOI] [PubMed] [Google Scholar]
- 139.Garber L, et al. Predictors of left ventricular remodeling after myocardial infarction in patients with a patent infarct related coronary artery after percutaneous coronary intervention (from the post-myocardial infarction remodeling prevention therapy [PRomPT] Trial) Am J Cardiol. 2018;121(11):1293–1298. doi: 10.1016/j.amjcard.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 140.Stone G, et al. Sex differences in gene expression in response to ischemia in the human left ventricular myocardium. Hum Mol Genet. 2019;28(10):1682–1693. doi: 10.1093/hmg/ddz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jain M, et al. Influence of gender on the response to hemodynamic overload after myocardial infarction. Am J Physiol Heart Circ Physiol. 2002;283(6):H2544–H2550. doi: 10.1152/ajpheart.00338.2002. [DOI] [PubMed] [Google Scholar]
- 142.Cavasin MA, et al. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75(18):2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 143.Gabel SA, et al. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38(2):289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 144.Wang M, et al. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol. 2006;40(2):205–212. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 145.Pullen AB, et al. Molecular and cellular differences in cardiac repair of male and female mice. J Am Heart Assoc. 2020;9(8):e015672. doi: 10.1161/JAHA.119.015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fang L, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 2007;43(5):535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 147.Patten RD, et al. 17 Beta-estradiol differentially affects left ventricular and cardiomyocyte hypertrophy following myocardial infarction and pressure overload. J Card Fail. 2008;14(3):245–253. doi: 10.1016/j.cardfail.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith PJW, et al. Effects of estrogen replacement on infarct size, cardiac remodeling, and the endothelin system after myocardial infarction in ovariectomized rats. Circulation. 2000;102(24):2983–2989. doi: 10.1161/01.CIR.102.24.2983. [DOI] [PubMed] [Google Scholar]
- 149.Nahrendorf M, et al. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc Res. 2003;57(2):370–378. doi: 10.1016/S0008-6363(02)00701-0. [DOI] [PubMed] [Google Scholar]
- 150.Cahill TJ, et al. Genetic cardiomyopathies causing heart failure. Circ Res. 2013;113(6):660–675. doi: 10.1161/CIRCRESAHA.113.300282. [DOI] [PubMed] [Google Scholar]
- 151.Argirò A, et al. Sex-related differences in genetic cardiomyopathies. J Am Heart Assoc. 2022;11(9):e024947. doi: 10.1161/JAHA.121.024947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marian AJ. Molecular Genetic Basis of Hypertrophic Cardiomyopathy. Circ Res. 2021;128(10):1533–1553. doi: 10.1161/CIRCRESAHA.121.318346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ommen SR, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;22(25):e558–e631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 154.Lakdawala NK, et al. Associations between female sex, sarcomere variants, and clinical outcomes in hypertrophic cardiomyopathy. Circ Genom Precis Med. 2021;14(1):e003062. doi: 10.1161/CIRCGEN.120.003062. [DOI] [PubMed] [Google Scholar]
- 155.Geske JB, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017;38(46):3434–3440. doi: 10.1093/eurheartj/ehx527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao H, et al. Is There a sex difference in the prognosis of hypertrophic cardiomyopathy? A systematic review and meta-analysis. J Am Heart Assoc. 2023;12(11):e026270. doi: 10.1161/JAHA.122.026270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim M, et al. Sex differences in the prognosis of patients with hypertrophic cardiomyopathy. Sci Rep. 2021;11(1):4854. doi: 10.1038/s41598-021-84335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Page SP, et al. Cardiac myosin binding protein-C mutations in families with hypertrophic cardiomyopathy: disease expression in relation to age, gender, and long term outcome. Circ Cardiovasc Genet. 2012;5(2):156–166. doi: 10.1161/CIRCGENETICS.111.960831. [DOI] [PubMed] [Google Scholar]
- 159.Lorenzini M, et al. Penetrance of hypertrophic cardiomyopathy in sarcomere protein mutation carriers. J Am Coll Cardiol. 2020;76(5):550–559. doi: 10.1016/j.jacc.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Butters A, et al. Sex Differences in hypertrophic cardiomyopathy: interaction with genetics and environment. Curr Heart Fail Rep. 2021;18(5):264–273. doi: 10.1007/s11897-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nijenkamp LLAM, et al. Sex differences at the time of myectomy in hypertrophic cardiomyopathy. Circ Heart Fail. 2018;11(6):e004133. doi: 10.1161/CIRCHEARTFAILURE.117.004133. [DOI] [PubMed] [Google Scholar]
- 162.Batzner A, et al. Sex-related differences in symptomatic patients with hypertrophic obstructive cardiomyopathy – Time for a new definition? Int J Cardiol. 2021;328:117. doi: 10.1016/j.ijcard.2020.12.039. [DOI] [PubMed] [Google Scholar]
- 163.Vikstrom KL, et al. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med. 1996;2(5):556–567. doi: 10.1007/BF03401640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Olsson MC, et al. Gender and aging in a transgenic mouse model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2001;280(3):H1136–H1144. doi: 10.1152/ajpheart.2001.280.3.H1136. [DOI] [PubMed] [Google Scholar]
- 165.Freeman K, et al. Progression from hypertrophic to dilated cardiomyopathy in mice that express a mutant myosin transgene. Am J Physiol Heart Circ Physiol. 2001;280(1):H151–H159. doi: 10.1152/ajpheart.2001.280.1.H151. [DOI] [PubMed] [Google Scholar]
- 166.McNally EM, Mestroni L. Dilated cardiomyopathy: genetic determinants and mechanisms. Circ Res. 2017;121(7):731–748. doi: 10.1161/CIRCRESAHA.116.309396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duan D, et al. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Owen R, et al. Sex differences in the clinical presentation and natural history of dilated cardiomyopathy. JACC Heart Fail. 2023;12(2):352–363. doi: 10.1016/j.jchf.2023.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nishiuchi S, et al. Gene-based risk stratification for cardiac disorders in LMNA mutation carriers. Circ Cardiovasc Genet. 2017;10(6):e001603. doi: 10.1161/CIRCGENETICS.116.001603. [DOI] [PubMed] [Google Scholar]
- 170.Akhtar MM, et al. Clinical phenotypes and prognosis of dilated cardiomyopathy caused by truncating variants in the TTN Gene. Circ Heart Fail. 2020;13(10):e006832. doi: 10.1161/CIRCHEARTFAILURE.119.006832. [DOI] [PubMed] [Google Scholar]
- 171.Wahbi K, et al. Development and validation of a new risk prediction score for life-threatening ventricular tachyarrhythmias in laminopathies. Circulation. 2019;140(4):293–302. doi: 10.1161/CIRCULATIONAHA.118.039410. [DOI] [PubMed] [Google Scholar]
- 172.Herman DS, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366(7):619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mazzarotto F, et al. Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation. 2020;141(5):387–398. doi: 10.1161/CIRCULATIONAHA.119.037661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vissing CR, et al. Dilated cardiomyopathy caused by truncating titin variants: long-term outcomes, arrhythmias, response to treatment and sex differences. J Med Genet. 2021;58(12):832–841. doi: 10.1136/jmedgenet-2020-107178. [DOI] [PubMed] [Google Scholar]
- 175.van Rijsingen IAW et al. Gender-specific differences in major cardiac events and mortality in lamin A/C mutation carriers. Eur J Heart Fail. 2013;15(4):376–384. doi: 10.1093/eurjhf/hfs191. [DOI] [PubMed] [Google Scholar]
- 176.Arimura T, et al. Nuclear accumulation of androgen receptor in gender difference of dilated cardiomyopathy due to lamin A/C mutations. Cardiovasc Res. 2013;99(3):382–394. doi: 10.1093/cvr/cvt106. [DOI] [PubMed] [Google Scholar]
- 177.Yang F, et al. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20(5):614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feyen DAM, et al. Metabolic maturation media improve physiological function of human iPSC-derived cardiomyocytes. Cell Rep. 2020;32(3):107925. doi: 10.1016/j.celrep.2020.107925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.DeFilippis EM, Van Spall HGC. Is it time for sex-specific guidelines for cardiovascular disease? J Am Coll Cardiol. 2021;78(2):189–192. doi: 10.1016/j.jacc.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 180.Ober C, et al. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]