ABSTRACT
Background and Objectives
Pancreatic cancer (PC) is the third cause of cancer-related deaths. Early detection and interception of premalignant pancreatic lesions represent a promising strategy to improve outcomes. We evaluated risk factors of focal pancreatic lesions (FPLs) in asymptomatic individuals at hereditary high risk for PC.
Methods
This is an observational single-institution cohort study conducted over a period of 5 years. Surveillance was performed through imaging studies (EUS or magnetic resonance imaging/magnetic resonance cholangiopancreatography) and serum biomarkers. We collected demographic characteristics and used univariate and multivariate logistic regression models to evaluate associations between potential risk factors and odd ratios (ORs) for FPL development.
Results
A total of 205 patients completed baseline screening. Patients were followed up to 53 months. We detected FPL in 37 patients (18%) at baseline; 2 patients had lesions progression during follow-up period, 1 of them to PC. Furthermore, 13 patients developed new FPLs during the follow-up period. Univariate and multivariate analyses revealed that new-onset diabetes (NOD) is strongly associated with the presence of FPL (OR, 10.94 [95% confidence interval, 3.01–51.79; P < 0.001]; OR, 9.98 [95% confidence interval, 2.15–46.33; P = 0.003]). Follow-up data analysis revealed that NOD is also predictive of lesions progression or development of new lesions during screening (26.7% vs. 2.6%; P = 0.005).
Conclusions
In a PC high-risk cohort, NOD is significantly associated with presence of FPL at baseline and predictive of lesions progression or new lesions during surveillance.
Key words: Early detection, New-onset diabetes, Focal pancreatic lesions, Germline mutations, EUS, MRI/MRCP
INTRODUCTION
Pancreatic cancer (PC) is one of the most lethal solid malignancies. According to the National Cancer Institute, the estimated number of new PC cases in 2022 is 62,210, with 49,830 predicted deaths.[1] Most PC patients present with local invasion or metastatic disease at diagnosis. Only 11% of the cases are diagnosed in the localized stage, for which the 5-year survival rate is 39%. When all stages are considered, only 10% of patients with PC survive for 5 years or longer from the date of diagnosis.[2,3]
Previous studies suggest a window of opportunity for early detection of PC. A period greater than 10 years is estimated from tumor initiation to acquisition of metastatic capacity.[4] Current screening strategies have many challenges in effectively detecting PC at early stages because of limitations of imaging and serum biomarkers.[5–7] Recent studies have reported an overall survival benefit of routine surveillance in patients diagnosed with PC during active surveillance versus those individuals who were lost to surveillance (85% vs. 25% survival at 3 years; P < 0.0001).[8]
This article reports the observations of the first 5 years of screening from a high-risk cohort followed at MD Anderson Cancer Center. We aimed to identify factors associated with focal pancreatic lesion (FPL) at baseline and predict the development of new lesions during follow-up with the goal of finding biomarkers to guide future screening strategies.
MATERIALS AND METHODS
Patient selection and enrollment
Asymptomatic adults (>18 years old) have been enrolled into a pancreatic cancer high risk cohort since January 2015. This study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. The following 2 categories of patients were considered to have hereditary high risk and were therefore eligible for screening: (1) patients with 2 first-degree relatives with PC or 1 first-degree relative plus 1 second-degree relative with PC on the same side of the family, and (2) patients with germline mutations in genes associated with higher susceptibility for PC (BRCA1, BRCA2, PALB2, EPCAM, MLH1, MSH2, MSH6, ATM, TP53, CDKN2A, APC, STK11, and PRSS1), many of whom also had relatives affected with PC.
Screening procedures
A multidisciplinary team agreed on the imaging methodology and follow-up timing based on each patient’s initial imaging and laboratory testing results. The multidisciplinary team consisted of a gastrointestinal medical oncologist, a gastroenterologist, a gastrointestinal surgeon, and a magnetic resonance imaging (MRI) expert radiologist. The screening methods used were the following: (A) Laboratory testing: serum CA19-9, plasma fasting or random blood glucose levels, hemoglobin A1c (HbA1c), serum lipase, and amylase levels. (B) MRI/magnetic resonance cholangiopancreatography (MRCP): performed using a pancreas-dedicated protocol, including multiple-pulse sequences and T1 weighting combined with T2-weighted sequences. Intravenous gadolinium was used as a contrast agent, and MRCP reconstruction was performed. (C) EUS: performed using upper endoscopes (Olympus America, Center Valley, PA), linear array echoendoscopes (Olympus America), and ultrasound processors (Hitachi Aloka Medical, Wallingford, CT). At each screening, patients had either MRI/MRCP or EUS plus laboratory testing. Depending on testing results, patients were followed annually or every 6 months.
Data collection
Data variables collected included sex and race/ethnicity of the patients, history of smoking and alcohol use, history of chronic diabetes, new-onset diabetes (NOD within the first 3 years of their diabetes diagnosis.), history of pancreatitis, family history of cancer, personal history of cancer, presence of germline mutations, laboratory values, and imaging results at the first clinical visit (baseline) and during a 5-year follow-up period.
Description of pancreatic lesions detected during screening
Lesions were categorized as FPL or diffuse changes for improved characterization of the results. Two types of FPLs were included: solid and cystic lesions. Solid lesions were defined in the MRI/MRCP radiology report as areas of hypointensity relative to the regular pancreatic parenchyma on fat-suppressed T1-weighted pregadolinium and dynamic postcontrast T1-weighted imaging.[9,10] Cystic lesions were T1 hypointense and T2 bright and well defined on the radiology report. Diffuse changes in the pancreas are nonspecific changes such as lobularity, septations, dilated side branches, and heterogeneity of the parenchyma.
Statistical methods
We reported frequencies and percentages for categorical variables. Summary statistics, such as means, medians, SDs, and minimum and maximum values, were calculated for continuous data. χ2 and Fisher exact tests were used to evaluate associations between the groups. Wilcoxon rank sum test was used to compare the distributions of continuous variables between 2 different groups. Univariate and multivariate logistic regression models were performed to identify any risk factors associated with FPL. The clinically and statistically important risk factors (age, alcohol consumption, NOD, PC family history, and presence of any germline mutation) were included in the models. Odds ratios (ORs) and 95% confidence intervals (CIs) from logistic regression models were calculated. Thirteen patients with unknown genetics data were excluded from the multivariate logistic analysis. P values less than 0.05 were considered significant. All statistical analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC).
RESULTS
Patient demographics and characteristics
Two hundred five patients completed baseline screening with laboratory testing and imaging studies between January 2015 and January 2020. The median age of the participants was 52 years (27–81 years), and 161 patients (78.5%) were female. Most patients (78%) were White, 13.7% were Hispanic, 5.9% were African American, and 2.4% were Asian. The cohort included 73.7% never smokers, 21.9% former smokers, and 4.4% current smokers. Sixty-six percent of patients reported occasional alcohol intake, whereas 34% had no alcohol intake history. Fifty-six percent of the patients had a personal cancer history, 60.5% had a family history of PC, and 79% were carriers of a germline mutation. The most common germline mutation detected was BRCA2 (42.9%), followed by BRCA1 (8.8%) and TP53 (8.8%) mutations. Eighty-two patients (40%) had a family history of PC in addition to carrying a germline mutation [Table 1]. Ninety-three patients completed at least 1 follow-up screening during the 5-year period (2015–2020). Demographic characteristics of the subgroup who had follow-up screening can also be found in Table 1.
Table 1.
Demographic, medical and family history factors in PCHRC
| Characteristics | Patients With Baseline Screening (n = 205) |
Patients With Follow-Up Screening (n = 93) |
|---|---|---|
| Age, y | ||
| Mean (SD) | 52.88 (±12.4) | 55.28 (±13.3) |
| Median (min–max) | 52 (27–81) | 56 (28–81) |
| Sex | ||
| Female | 161 (78.5%) | 69 (74.2%) |
| Male | 44 (21.5%) | 24 (25.8%) |
| Race and ethnicity | ||
| White | 160 (78%) | 75 (80.6%) |
| Hispanic | 28 (13.7%) | 11 (11.8%) |
| African American | 12 (5.9%) | 4 (4.3%) |
| Asian | 5 (2.4%) | 3 (3.3%) |
| Smoking | ||
| Never | 151 (73.7%) | 75 (80.6%) |
| Past | 45 (21.9%) | 18 (19.4%) |
| Current | 9 (4.4%) | — |
| Alcohol | ||
| Nondrinker | 70 (34%) | 32 (34.4%) |
| Drinker | 135 (66%) | 61 (65.6%) |
| Personal history of cancer | ||
| Yes | 115 (56%) | 55 (59.1%) |
| No | 90 (44%) | 38 (40.9%) |
| Family history of pancreatic cancer | ||
| Yes | 124 (60.5%) | 60 (64.5%) |
| No | 81 (39.5%) | 33 (35.5%) |
| Presence of germline mutation | ||
| Yes | 162 (79%) | 77 (82.8%) |
| No | 30 (14.6%) | 12 (12.9%) |
| Unknown | 13 (6.4%) | 4 (4.3%) |
| BRCA2 | 88 (42.9%) | 41 (44.5%) |
| BRCA1 | 18 (8.8%) | 11 (11.9%) |
| P53 | 18 (8.8%) | 10 (10.8%) |
| PALB2 | 16 (7.8%) | 7 (7.5%) |
| CDKN2A | 10 (4.9%) | 5 (5.4%) |
| ATM | 9 (4.4%) | 4 (4.4%) |
| STK11 | 3 (1.5%) | 3 (3.3%) |
| MLH1 | 2 (1%) | 1 (1.1%) |
| APC | 1 (0.5%) | — |
| CFTR | 2 (1%) | — |
| PRSS1 | 1 (0.5%) | — |
| Presence of germline mutation and family history | 82 (40%) | 45 (49%) |
PCHRC: pancreatic cancer high risk cohort.
Screening outcomes
From 205 patients eligible for analysis with baseline screening completed, 51 had findings on imaging studies: 37 patients (18%) with FPL and 14 patients (6.8%) with diffuse findings. Specifically, from the 37 patients with FPL, 33 had cystic lesions, and 4 had solid lesions (2%): 2 of them were diagnosed with a pancreatic neuroendocrine tumor, and 2 patients had fine-needle aspiration reporting no malignant cells content. The patients with cystic lesions included 27 (13.2%) with sub-cm cystic lesions with no malignant suspicious features, 5 patients (2.4%) with cystic lesions larger than 1 cm, and 1 patient (0.5%) with a serous cystadenoma [Figure 1].
Figure 1.
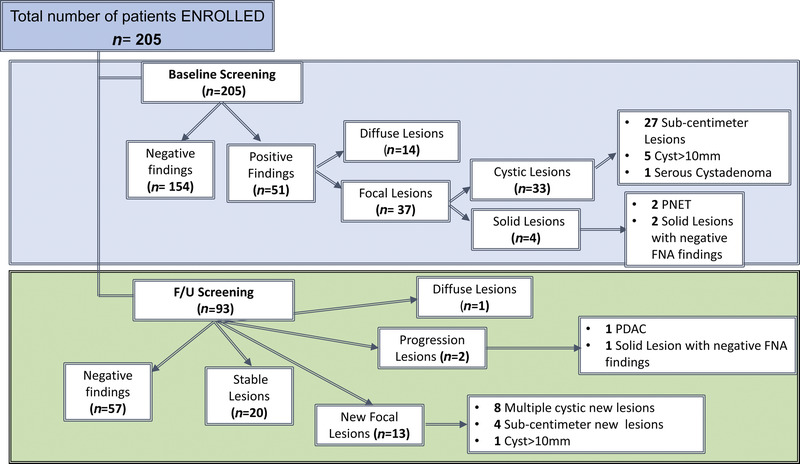
Screening results based on imaging findings. FNA: fine-needle aspiration; PC: pancreatic cancer; PNET: pancreatic neuroendocrine tumor.
A total of 93 patients completed at least 1 follow-up cycle during the 5 years of the study observation, with the following outcomes: 13 patients developed new pancreatic focal lesions (14%), 4 of whom already had lesions at baseline, and 2 patients (2.1%) had progression of lesions detected at baseline: 1 with progression to PDAC and 1 with a solid lesion developed over an existing cyst without atypia on fine-needle aspiration biopsy. Also, 1 patient had diffuse pancreatic changes during follow-up. Twenty patients (21.5%) had stable lesions from baseline, whereas 57 (61.3%) continued to have negative screening [Figure 1].
Risk factors associated with FPLs at baseline
When we assessed for factors associated with the presence of FPL (including cystic and solid lesions), we found that patients with FPL were much more likely to have NOD compared with the patients with negative findings (18.9% vs. 1.9%; P = 0.0005; Table 2). As previously reported, patients with FPL were older than those with negative findings (median age, 61 vs. 50.5 years; P > 0.00001; Table 2). Family history of PC and the presence of germline mutations were not associated with FPL detection. When we separately assessed risk factors for the development of diffuse pancreatic lesions, we found that smoking history (P = 0.01) and male sex (P = 0.0009) were significantly enriched in patients with diffuse lesions compared with patients with negative findings (Supplementary Table 1, http://links.lww.com/ENUS/A358).
Table 2.
Factors associated with FPL at baseline
| Variables | Negative Results (n = 154) | Focal Pancreatic Lesions (n = 37) | P* |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 51 (±12.3) | 60.95 (±10.1) | <0.0001 |
| Median (min–max) | 50.5 (27–79) | 61 (38–81) | |
| Sex | |||
| Female | 125 (81.2%) | 30 (81.1%) | >0.99 |
| Male | 29 (18.8%) | 7 (18.9%) | |
| Race/ethnicity | |||
| White | 114 (74%) | 33 (89.2%) | |
| Hispanic | 23 (15%) | 4 (10.8%) | 0.14 |
| African American | 12 (7.8%) | — | |
| Asian | 5 (3.2%) | — | |
| Smoking | |||
| Never | 118 (76.6%) | 26 (70.3%) | 0.21 |
| Past | 30 (19.5%) | 11 (29.7%) | |
| Current | 6 (3.9%) | — | |
| Alcohol consumption | |||
| Nondrinker | 59 (38.3%) | 9 (24.3%) | 0.12 |
| Drinker | 95 (61.7%) | 28 (75.7%) | |
| Personal medical history | |||
| Personal history of cancer | 86 (55.8%) | 23 (62.2%) | 0.58 |
| New-onset diabetes | 2 (1.9%) | 8 (18.9%) | 0.0005 |
| New-onset diabetes and prediabetes | 6 (3.9%) | 9 (24.3%) | 0.0003 |
| Chronic diabetes | 8 (5.2%) | 1 (2.7%) | >0.99 |
| Episodes of pancreatitis | 5 (3.2%) | 3 (8.1%) | 0.18 |
| Family history of PC | 90 (58.4%) | 23 (62.2%) | 0.71 |
| Presence of germline mutation | |||
| Yes | 129 (83.8%) | 26 (70.3%) | 0.14 |
| No | 16 (10.4%) | 8 (21.6%) | |
| Unknown | 9 (5.8%) | 3 (8.1) | |
| Germline mutations | |||
| BRCA2 | 67 (45.9%) | 17 (43.5%) | 0.70 |
| BRCA1 | 16 (10.4%)† | 2 (5.4%) | 0.53 |
| P53 | 15 (9.7%) | 2 (5.4%) | 0.53 |
| PALB2 | 15 (9.7%) | 1 (2.7%) | 0.31 |
| CDKN2A | 9 (5.8%) | — | 0.21 |
| ATM | 5 (3.2%)‡ | 3 (8.1%) | 0.17 |
| STK11 | 3 (1.9%) | — | >0.99 |
| MLH1 | 2 (1.3%) | — | >0.99 |
| APC | 1 (0.6%) | — | >0.99 |
| CFTR | 2 (1.3%)§ | — | >0.99 |
| PRSS1 | — | 1 (2.7%) | >0.99 |
*P value compares the focal pancreatic lesions to negative results.
†Two patients also have CDKN2A mutation, and 1 patient also has a BRCA2 mutation.
‡One patient also has P53 mutation, and 1 patient also has PALB2 mutation.
§One patient also has APC mutation.
Risk factors predictive of new or progressing pancreatic lesions during follow-up
As expected, we found that patients with new or progressing lesions were older than those with stable or negative findings (median age, 66 vs. 55 years; P = 0.01). Similar to baseline, patients with NOD also had a higher risk for developing new or progressing lesions than those without NOD (26.7% vs. 2.6%; P = 0.005) during follow-up. From 15 patients who developed new or progressing lesions during follow-up, 4 had NOD (26.7%), whereas 2 patients (2.6%) with stable or negative findings had NOD [Table 3].
Table 3.
Factors predictive of new or progressing pancreatic lesions during follow-up
| Variables | Negative Results or Stable Lesions (n = 77) |
New Lesions or Progression (n = 15) | P* |
|---|---|---|---|
| Age, y | |||
| Mean (SD) | 53.8 (±13.5) | 63.1 (±9.3) | 0.01 |
| Median (min–max) | 55 (28–81) | 66 (48–73) | |
| Sex | |||
| Female | 59 (76.6%) | 13 (86.7%) | 0.5 |
| Male | 18 (23.4%) | 2 (13.3%) | |
| Race/ethnicity | |||
| White | 62 (80.5%) | 12 (80%) | |
| Hispanic | 10 (13%) | 1 (6.7%) | 0.73 |
| African American | 3 (3.9%) | 1 (6.6%) | |
| Asian | 2 (2.6%) | 1 (6.7%) | |
| Smoking | |||
| Never | 61 (79.2%) | 13 (86.7%) | 0.72 |
| Past | 16 (20.8%) | 2 (13.3%) | |
| Current | — | — | |
| Alcohol consumption | |||
| Nondrinker | 28 (36.3%) | 3 (20%) | 0.37 |
| Drinker | 49 (63.7%) | 12 (80%) | |
| Personal medical history | |||
| Personal history of cancer | 45 (58.4%) | 9 (60%) | >0.99 |
| New-onset diabetes | 2 (2.6%) | 4 (26.7%) | 0.005 |
| New-onset diabetes and prediabetes | 2 (2.6%) | 5 (33.3%) | 0.001 |
| Chronic diabetes | 4 (5.2%) | 1 (6.7%) | >0.99 |
| Episodes of pancreatitis | 1 (1.3%) | 1 (6.7%) | 0.3 |
| Family history of PC | 46 (59.7%) | 13 (86.7%) | 0.07 |
| Presence of germline mutation | |||
| Yes | 66 (85.7%) | 11 (73.3%) | 0.46 |
| No | 9 (11.7%) | 3 (20%) | |
| Unknown | 2 (2.6%) | 1 (6.7%) | |
| Germline mutations | |||
| BRCA2 | 35 (45.4%) | 6 (40%) | >0.99 |
| BRCA1 | 9 (11.7%)† | 2 (13.3%) | 0.68 |
| P53 | 10 (12.9%) | — | 0.35 |
| PALB2 | 6 (7.8%) | 1 (6.7%) | >0.99 |
| CKN2A | 4 (5.2%) | 1 (6.7%) | 0.58 |
| ATM | 3 (3.9%)‡ | 1 (6.7%) | 0.5 |
| STK11 | 2 (2.6%) | — | >0.99 |
| MLH1 | 1 (1.3%) | — | >0.99 |
| APC | — | — | |
| CFTR | — | — | |
| PRSS1 | — | — |
*P value compares the new lesions or progression lesions to negative results or stable lesions.
†Two patients also have CDKN2A mutation.
‡One patient also has PALB2 mutation, and 1 patient also has P53 mutation.
Bold values represent the statistically significant results.
NOD is the main risk factor associated with FPL at baseline and during follow-up
Univariate and multivariate logistic regression analyses were performed to explore the predictive value of clinically and statistically important risk factors (NOD, alcohol consumption, PC family history, and presence of any germline mutation) in patients with FPL. In the univariate analysis, the odds of having FPL were significantly higher in patients with NOD (OR, 10.94; 95% CI, 3.01–0.79; P < 0.001), and this finding was still significant in the multivariate analysis (OR, 9.98; 95% CI, 2.15–46.33; P = 0.003) when accounting for other clinically relevant factors like age, alcohol, PC family history, and presence of germline mutations [Table 4].
Table 4.
Logistic regression analysis for predictive risk factors of focal pancreatic lesions
| Variable (Level) | Univariate Analysis, OR (95% CI) | P | Multivariate Analysis, OR (95% CI) | P |
|---|---|---|---|---|
| Age | 1.08 (1.04–1.11) | <0.001* | 1.08 (1.04–1.12) | <0.001* |
| Alcohol | 1.87 (0.91–4.13) | 0.089 | 2.28 (0.92–5.6) | 0.072 |
| New-onset diabetes | 10.94 (3.01–51.79) | <0.001* | 9.98 (2.15–46.33) | 0.003* |
| Pancreatic cancer family history | 1.46 (0.74–2.99) | 0.27 | 1.18 (0.49–2.88) | 0.708 |
| Presence of any germline mutation | 0.51 (0.22–1.23) | 0.13 | 0.79 (0.29–2.15) | 0.65 |
* marks statistically significance.
DISCUSSION
National Comprehensive Cancer Network guidelines recommend PC screening for high-risk individuals at experienced high-volume centers with appropriate expertise, using a multidisciplinary approach.[11] Individuals get risk assessment, and screening is discussed after being identified as high risk.[12] We presented baseline results and 5-year follow-up of our PC high-risk cohort. Overall, our study found that the prevalence of pancreatic abnormalities in high-risk patients was 22%, consistent with the other screening programs.[13–15] Studies have shown survival improvement with detection of PC at early stages.[16] Furthermore, reports from long-standing high-risk programs have shown survival benefit in patients enrolled in surveillance.[17]
Recent studies have demonstrated that age older than 60 years, multiple cysts, and dilated main ducts at baseline were robust predictors of the radiologic and neoplastic progression of pancreatic lesions.[8] Age of onset is an important predictor of PC risk in familial kindreds. Similarly, we found that the incidence of FPL was higher in older individuals. Only 6 patients (2.7%) with FPL in our study were younger than 50 years. Furthermore, all patients detected with solid lesions in our study were older than 64 years.
Increasing evidence suggests that NOD precedes PC diagnosis,[18–20] and 85% of patients with PC can have hyperglycemia, which manifests as early as 3 years before the cancer diagnosis.[21–23] Shah et al. [24] noticed a similar trend in a cohort of high-risk individuals undergoing PC screening, where 20% of high-risk individuals had abnormal fasting blood sugars, of which only 1 patient was diagnosed with NOD; however, it did not statistically correlate with the presence of focal lesions. Similarly, another retrospective study of high-risk individuals found an HbA1c >5.7% associated with the presence of pancreatic cysts but not with diabetes (HbA1c ≥6.5%) because of a small number of patients (n = 4).[25] In our high-risk cohort, the most robust clinical risk factor for detecting pancreatic lesions was NOD diagnosis, which maintained statistical significance after multivariate analyses. Moreover, our study is the first one to show that NOD is predictive of the development of new pancreatic lesions and the progression of lesions during follow-up.
Our study had several limitations: relatively small size, young cohort, single institution, and a short follow-up time. This was likely the reason for only 1 patient in our cohort developing PC, compared with previous reports.[8,26] Although some FPL may progress to malignancy, some could regress or stay stable. Whether these focal lesions predict a more substantial field defect with a greater chance of malignancy in another region of the pancreas compared with the ones who did not have an FPL can only be determined with longer-term follow-up. More extended surveillance will give us more data to make more definitive conclusions. Our population had more female individuals and was enriched with Whites, so this disparity may also influence the study’s findings.
In conclusion, NOD is a significant risk factor for the presence of FPL and is also predictive development of new or progressing pancreatic lesions in a high-risk cohort during 5-year follow-up. More extensive studies with a more diverse population are needed to validate these findings further and understand their clinical implications.
Conflicts of Interest
A.M. receives royalties from Cosmos Wisdom Biotechnology and Thrive Earlier Detection, an Exact Sciences Company, and is a consultant for Freenome and Tezcat. F.M. is an SAB Member at Neological Biosciences. Dr Bhutani is Walter H Wriston Distinguished Professor for Pancreatic Cancer Research.
Manoop S. Bhutani is a senior associate editor of the journal. This article was subject to the journal's standard procedures, with peer review handled independently of the editor and his research group.
Financial Support and Sponsorship
Dr F. McAllister received support from the National Cancer Institute (1R37CA237384), Cancer Prevention Research Institute of Texas (RP200173), Shelby Levine Pancreatic Scholar Program, V Foundation (Translational Award), Sabin Family Foundation, Robert L. Fine Cancer Research Foundation, and MD Anderson Moonshot Philanthropic Funds.
Author Contributions
S.B., C.H., and F.M. designed, extracted data, performed analysis, and wrote the manuscript. F.M. and M.S.B. supervised the study. W.D. and L.F performed statistical analysis. S.B. and M.H had worked on data tabulation and extraction. M.H., M.M, P.Q., I.C., A.A.L.C., M.M, Y.N.Y., B.A., E.V., P.B, M.H.G.K, S.T.C., A.M., E.P.T., M.P.K., M.S.B., and F.M. contributed with patients’ data, study revision, analysis, and writing revision. S.B., C.H., F.M. and M.S.B contributed by the critical analysis of manuscript and contribution in resubmission write up.
Footnotes
Received: 1 June 2023; Accepted: 13 December 2023.
Published online: 10 April 2024
S.B. and C.M. contributed equally to the article.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Web site (www.eusjournal.com).
Contributor Information
Seyda Baydogan, Email: sbaydogan@mdanderson.org.
Chirayu Mohindroo, Email: cmohindroo@mdanderson.org.
Merve Hasanov, Email: mhasanov@mdanderson.org.
Maria F. Montiel, Email: mfmontiel@mdanderson.org.
Pompeyo Quesada, Email: pquesada@mdanderson.org.
Irina M. Cazacu, Email: irina.cazacu89@gmail.com.
Adrianna A. Luzuriaga Chavez, Email: aaluzuriaga@mdanderson.org.
Maureen E. Mork, Email: memork@mdanderson.org.
Wenli Dong, Email: wdong@mdanderson.org.
Lei Feng, Email: leifeng@mdanderson.org.
Y. Nancy You, Email: ynyou@mdanderson.org.
Banu Arun, Email: barun@mdanderson.org.
Eduardo Vilar, Email: evilar@mdanderson.org.
Powel Brown, Email: pbrown22@mdanderson.org.
Matthew H. G. Katz, Email: mhgkatz@mdanderson.org.
Suresh T. Chari, Email: stchari@mdanderson.org.
Anirban Maitra, Email: amaitra@mdanderson.org.
Eric P. Tamm, Email: etamm@mdanderson.org.
Michael P. Kim, Email: mkim@mdanderson.org.
Manoop S. Bhutani, Email: manoop.bhutani@mdanderson.org.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute. Cancer 2014;120(Suppl 23):3755–3757. [DOI] [PubMed] [Google Scholar]
- 4.Yachida S Jones S Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467(7319):1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko AH, Hwang J, Venook AP, Abbruzzese JL, Bergsland EK, Tempero MA. Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 2005;93(2):195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol 1990;85(4):350–355. [PubMed] [Google Scholar]
- 7.Young MR Wagner PD Ghosh S, et al. Validation of biomarkers for early detection of pancreatic cancer: summary of the Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop. Pancreas 2018;47(2):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canto MI Almario JA Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 2018;155(3):740–51.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics 2011;31(4):993–1015. [DOI] [PubMed] [Google Scholar]
- 10.Tummala Md P, Rao Md S, Agarwal Md B. Differential diagnosis of focal non-cystic pancreatic lesions with and without proximal dilation of pancreatic duct noted on CT scan. Clin Transl Gastroenterol 2013;4(11):e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network NCC . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Version 1.2022 2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed August 11, 2021. [DOI] [PubMed]
- 12.Lucas AL, Kastrinos F. Screening for pancreatic cancer. JAMA 2019;322(5):407–408. [DOI] [PubMed] [Google Scholar]
- 13.Verna EC Hwang C Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010;16(20):5028–5037. [DOI] [PubMed] [Google Scholar]
- 14.Vasen HF Wasser M van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 2011;140(3):850–856. [DOI] [PubMed] [Google Scholar]
- 15.Del Chiaro M Verbeke CS Kartalis N, et al. Short-term results of a magnetic resonance imaging–based Swedish screening program for individuals at risk for pancreatic cancer. JAMA Surg 2015;150(6):512–518. [DOI] [PubMed] [Google Scholar]
- 16.Torisu Y, Takakura K, Kinoshita Y, Tomita Y, Nakano M, Saruta M. Pancreatic cancer screening in patients with presumed branch-duct intraductal papillary mucinous neoplasms. World J Clin Oncol 2019;10(2):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dbouk M Katona BW Brand RE, et al. The multicenter cancer of pancreas screening study: impact on stage and survival. J Clin Oncol 2022;40(28):3257–3266. 10.1200/JCO.22.00298, PMID 35704792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toki M Yamaguchi Y Kurata I, et al. Diabetes mellitus as a risk factor for pancreatic cancer-for realization of efficient screening of pancreatic cancer in patients with diabetes mellitus. Gastroenterology 2013;144(5):S659–S. [Google Scholar]
- 20.Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995;273(20):1605–1609. [PubMed] [Google Scholar]
- 21.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129(2):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munigala S, Singh A, Gelrud A, Agarwal B. Predictors for pancreatic cancer diagnosis following new-onset diabetes mellitus. Clin Transl Gastroenterol 2015;6:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10(7):423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah I Wadhwa V Bilal M Germansky KA Sawhney MS, Pancreas Cancer Screening Study Group . Prospective assessment for prediabetes and new-onset diabetes in high-risk individuals undergoing pancreatic cancer screening. Gastroenterology 2021;161(5):1689–1691.e1. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Mashiah A, Aronson A, Naparst M, DiMaio CJ, Lucas AL. Elevated hemoglobin A1c is associated with the presence of pancreatic cysts in a high-risk pancreatic surveillance program. BMC Gastroenterol 2020;20(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overbeek KA Levink IJM Koopmann BDM, et al. Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 2022;71(6):1152–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]


