Abstract
OBJECTIVES:
Intra-articular (IA) mineralization may contribute to OA structural progression. We studied the association of IA mineralization on knee CT with cartilage damage worsening on knee MRI, with a focus on location- and tissue-specific effects.
METHODS:
Participants from the Multicenter Osteoarthritis study with knee CTs and MRIs were included. Presence of IA mineralization on CT was defined as a Boston University Calcium Knee Score (BUCKS) >0 anywhere in the knee. Cartilage worsening on MRI was defined as any increase in MRI OA Knee Score (MOAKS) including incident damage. We evaluated the association of whole-knee, compartment-specific (i.e., medial or lateral), and subregion-specific (i.e., location-matched) IA mineralization at baseline with cartilage worsening at 2-year follow-up in the corresponding locations using binomial regression with generalized estimating equations, adjusting for age, sex, and body mass index (BMI).
RESULTS:
We included 1673 participants (mean age 60 years, 56% female, mean BMI 29 kg/m2). 9.0% had any IA mineralization in the knee and 47.4% had any cartilage worsening on follow-up. Mineralization of any tissue in the knee, regardless of location, was not associated with MRI cartilage worsening. However, cartilage mineralization was associated with 1.39 (95% CI 1.04, 1.88) times higher risk of cartilage worsening in the same compartment with similar results in subregion-specific analysis.
CONCLUSIONS:
CT-detected IA mineralization in the cartilage was associated with higher risk of MRI cartilage worsening in the same compartment and subregion over two years. These findings suggest potential localized, tissue-specific effects of IA mineralization on cartilage pathology in knee OA.
Keywords: mineralization, knee osteoarthritis, calcium crystal, cartilage, imaging, chondrocalcinsosis
INTRODUCTION
Intra-articular (IA) mineralization resulting from calcium crystal deposition is common in knee osteoarthritis (OA), with articular cartilage calcification universally present in advanced OA.1–3 However, its clinical significance remains unclear. Crystals that can deposit intra-articularly include calcium pyrophosphate (CPP), basic calcium phosphate (BCP), as well as monosodium urate. Each of these crystals may be associated with painful clinical conditions characterized by flares of joint pain and inflammation. IA mineralization due to crystal deposition may contribute to incident OA or disease worsening through inflammation, release of pro-catabolic factors, or altered cartilage biomechanical properties.4
Chondrocalcinosis on radiographs, reflecting calcium crystal deposition, has been variably associated with OA radiographic severity.3,5 BCP crystals are universally found in OA cartilage in studies of end-stage OA or cadaveric studies, raising questions of whether such mineralization is pathogenic, or merely a by-product of end-stage OA.3,6 Studies of the relationship between IA mineralization and OA structural progression longitudinally have shown mixed results.7–9 In the majority of prior studies, chondrocalcinosis has been visualized on radiography, which has a sensitivity of ~40%.10 This may have contributed to conflicting findings from earlier studies that attempted to evaluate chondrocalcinosis and its relation with structural changes.7–9 Further, prior studies focused on whole-knee effects of mineralization on structural changes (i.e., the impact of mineralization occurring anywhere in the joint with structural changes not tied to location of mineralization, rather than location-specific effects). Additionally, few, if any, studies have attempted to distinguish potential impact of mineralization of the meniscus versus hyaline articular cartilage on progression of cartilage damage. Thus, it remains unclear whether IA mineralization related to calcium crystal deposition is a cause or consequence of OA. If the former, whether this calcium deposition increases the risk of cartilage damage specifically as one mechanism by which it may contribute to OA pathogenesis is unclear.
We studied the relation of IA mineralization on knee CT, which has a markedly higher sensitivity for detection of mineralization than radiography11, to cartilage worsening over two years on knee MRI in a cohort of older adults with or at high risk of OA. In this study we paid particular attention to potential joint location- and tissue-specific effects of mineralization on cartilage damage.
METHODS
Data source and study sample.
The Multicenter Osteoarthritis (MOST) Study is a NIH-funded longitudinal study of community-dwelling adults. The original cohort was recruited in 2003–05 from Birmingham, Alabama and Iowa City, Iowa, and included adults between the ages of 50–79 years who had or were at risk of developing knee OA. A new cohort was recruited from the same communities during the 12th year study visit of the original cohort. The new cohort included adults between the ages of 45–69 years with Kellgren-Lawrence (KL) grade ≤2 in both knees, and either no knee pain or at most only mild intermittent knee pain. This 12th year visit was the baseline for this analysis as this was the first study visit at which CTs were obtained. The study was approved by the institutional review boards at the University of Iowa, University of Alabama at Birmingham, University of California at San Francisco, and Boston University Medical Center.
Imaging Assessments.
Radiography.
Weight-bearing, fixed-flexed posteroanterior and lateral views of the knees were obtained using an apparatus (Synaflexer) at baseline and each examination according to the MOST radiography protocol.12 Two experienced readers interpreted and graded all radiographs according to the Kellgren/Lawrence (K/L) scale using the PA view. In the case of disagreement between the 2 readers, readings were adjudicated by a panel of 3 readers (Piran Aliabadi, Burton Sack, DF).
Computed tomography.
CT examinations of both knees were performed at the 12th-year visit, using a dual energy CT system at both sites of the MOST study, i.e., at the University of Alabama at Birmingham and at the University of Iowa. A GE Discovery CT750HD scanner was used at the University of Alabama at Birmingham (sequential data acquisition mode at 80/140 kVp, 260 mAs, 0.9 mm pitch, 0.8 s exposure, rotation time 50 ms), while a dual-source Siemens SOMATOM Force scanner was used at the University of Iowa (80/150 kVp with tin filtration at 150 kVp, 250 mAs, 0.8 mm pitch, rotation time 15 ms). For this study, we utilized the 80 kVp images from both sites. The raw projection data were reconstructed using a slice thickness of 0.6 mm and a slice interval of 0.3 mm with a standard 512 × 512 matrix. Display field-of-view (DFOV) was standardized to approximately 14 cm for each respective knee data set, using the standard kernel (University of Alabama at Birmingham) and Qr40 kernel (University of Iowa). The DFOV provided an in-plane resolution of approximately 0.3 mm (x plane) × 0.3 mm (y plane) which corresponded to an isotropic voxel dimension of 0.3 mm × 0.3 mm × 0.3 mm when using a slice interval of 0.3 mm in the z-plane. The CT acquisition covered the distal 20% of femur and proximal 20% of tibia.13
A single musculoskeletal radiologist (MJ) scored CT images of both knees, using axial images along with multiplanar reformats (MPR) in the sagittal and coronal planes using the Boston University Calcium Knee Score (BUCKS). The BUCKS relies on a semi-quantitative scoring of extent of crystal mineralization 0–3 in WORMS cartilage and meniscus subregions, and binary scoring of the joint capsule, ligaments, and meniscal roots (present or absent).14 The intra-reader reliability, performed on a sample of 50 knees by a second musculoskeletal radiologist (AG), ranged from a kappa 0.92 for ligaments to 1.0 for joint capsule. The inter-reader reliability ranged from 0.94 for cartilage and ligaments to 1.0 for joint capsule.13 Details on scoring of IA mineralization have previously been described.13
Magnetic resonance imaging.
MRIs of the knee were acquired at each visit using a 1.5T magnet (OrthOne; ONI) and a circumferential extremity coil. All images were acquired without contrast. The original cohort had a single knee imaged at this visit, based upon which knee had previously been read longitudinally. The new cohort had both knees imaged, with a random knee MRI scored for budgetary reasons and because of the high rate of symmetry in knee MRIs.15
Cartilage damage was scored using the MRI OA Knee Score (MOAKS)16 in each MOAKS-defined subregion using ordinal scales (0–3) for each depth and extent of lesions by two readers with extensive experience in semiquantitative OA MRI assessment (FWR, MDC). Both the BUCKS and MOAKS cartilage and meniscus subregion definitions are based on MOAKS and correspond with one another.
Exposure at baseline.
At the 12th year study visit, we categorized presence of IA mineralization as a BUCKS score >0 as follows: 1) anywhere in the joint, including the hyaline cartilage, meniscus, capsule, and ligaments; 2) anywhere in the hyaline articular cartilage within the whole knee; 3) anywhere in the meniscus within the whole knee; 4) anywhere in the joint capsule; 5) anywhere in the ligaments. For cartilage and meniscus, we further classified mineralization as occurring in the medial and/or lateral tibiofemoral compartment for each of these two tissues, and additionally evaluated the specific subregion in which it was present (based upon MOAKS).
Additionally, the burden of mineralization anywhere in the joint (hyaline articular cartilage, meniscus, capsule, ligaments) was defined based upon the number of BUCKS subregions involved. The cut-points were determined by the distribution of number of regions affected (0 [referent], 1–5, and >5 subregions).
Outcome.
A MOAKS subregion was characterized as having cartilage worsening if the score for either the depth or extent of the lesions increased (including within grade increases and incident damage) between baseline and two-year follow-up. Subregions with baseline scores of 3 for depth and 3 for extent of lesions were excluded. For each compartment and the whole knee, we defined worsening as any worsening within the compartment or the knee, respectively.
Analyses.
We first examined the presence of any IA mineralization (i.e., anywhere in the joint in the tissues of interest (i.e., hyaline articular cartilage, meniscus, capsule, or ligaments)) and separately for any mineralization in these tissues, as well as the burden of mineralization (based upon the number of affected subregions involving any of these tissues) to the risk of MRI cartilage worsening at 2 years, regardless of location within the knee. We used binomial regression to estimate the risks of cartilage worsening due to mineralization. For the burden of mineralization, we performed tests for linear trend. Analyses were adjusted for age, sex, and body mass index (BMI).
In addition to the knee-level analyses above, we assessed compartment-specific associations of any cartilage or any meniscus mineralization with cartilage damage in the same tibiofemoral compartment. That is, we assessed whether the presence of mineralization in one compartment (i.e., medial or lateral tibiofemoral joint) was associated with cartilage worsening in the same compartment. We also performed subregion-specific analyses (based on MOAKS subregions) to evaluate the relation of cartilage mineralization to cartilage damage in their same MOAKS subregions. These analyses assessed whether mineralization in one subregion on CT was associated with cartilage worsening in the same subregion on MRI. We repeated subregion-specific analyses for meniscal mineralization in relation to cartilage damage in the subregions immediately overlying or underlying the particular meniscal subregion (Figure 1). We used generalized estimating equations for these analyses to account for correlations between locations within a knee.
Figure 1.
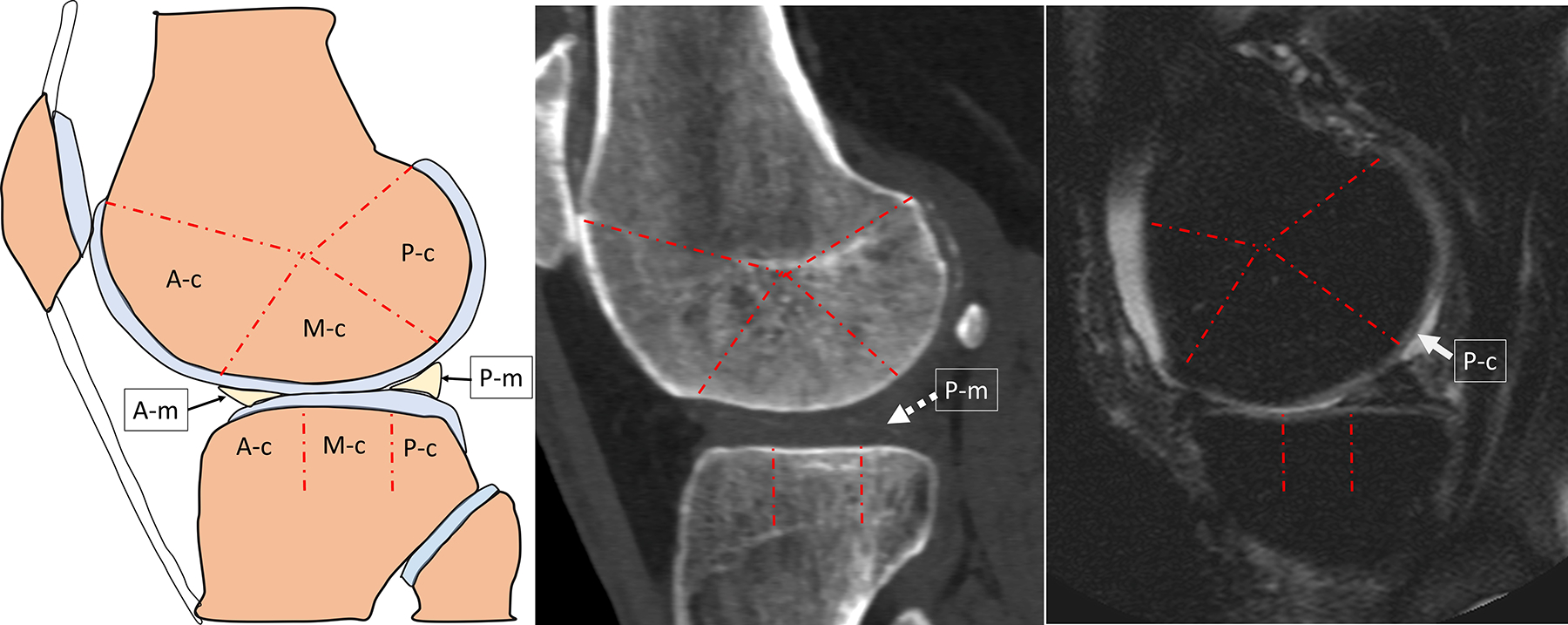
Schematic and CT and MR images illustrating the co-localization of CT-detected meniscal mineralization to MRI-detected cartilage damage used for subregion-specific analyses of CT-detected IA mineralization with MRI cartilage worsening.
A. Schematic of a sagittal section through the lateral compartment of the knee illustrating the subregional division of the lateral femoral condyle and tibial plateau cartilage in 3 subregions each (A-c: Anterior Cartilage, M-c: Mid Cartilage, P-c: Posterior Cartilage). The anterior and posterior horns of the lateral meniscus are designated as A-m and P-m, respectively. The meniscus body is better depicted on a coronal section, therefore not shown in this sagittal view. The co-localization of meniscal mineralization to cartilage damage refers to the co-localization of the anterior meniscus (A-m) to anterior femur/tibia (A-c), posterior meniscus (P-m) to posterior femur/tibia (P-c), and meniscus body (not shown) to mid femur/tibia (M-c). B. Sagittal reformat CT, and C. Sagittal proton density-weighted with fat suppression in the same person, same knee show a grade 1 mineralization of the articular cartilage (BUCKS grade 1) of the posterior horn of the lateral meniscus designated as P-m (dashed arrow), associated with partial thickness (MOAKS grade 2.0) cartilage loss of the overlying posterior cartilage subregion of the lateral femoral condyle designated as P-c (solid arrow).
To better address the question of whether IA mineralization is a cause or consequence of structural worsening in the knee, we performed the following sensitivity analyses: 1) Repeated analyses stratified by presence of baseline radiographic OA (defined as Kellgren-Lawrence ≥2) with our primary interest being associations noted in knees without OA at baseline; 2) Subregion-specific analyses restricted to subregions with MOAKS of 0 (no cartilage damage) at baseline. Given findings in a prior study of IA mineralization and knee OA progression suggesting effect modification by age,17 we stratified the main analyses (any IA mineralization, anywhere in the knee as the exposure) by age dichotomized at 60 years, in knee-level, compartment-specific, and subregion-specific analyses.
Analyses were performed using SAS 9.3 (Cary, NC).
Patient and public involvement.
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
RESULTS
We included 1673 participants (one knee/participant) (flow diagram in Supplemental Figure 1) who had a mean age 60.1±9.1 years, 56% were female, 81% were white, and mean BMI was 28.6±5.0 kg/m2 (Table 1). On conventional radiographs, 57% were Kellgren-Lawrence grade 0, 26% grade 1, 13% grade 2, and 4.6% grades 3 or 4. Overall, 9.0% had any IA mineralization in the knee; mineralization anywhere in the cartilage, meniscus, joint capsule, and ligaments was present in 6.3%, 7.1%, 4.0%, and 3.4%, respectively11. At the two-year follow-up, 47.4% had cartilage worsening anywhere in the knee.
Table 1.
Participant characteristics and imaging findings (n=1673, one knee per participant)
| Participant characteristics | |
|---|---|
| Age, years | 60.6 ± 9.1 |
| Women, n (%) | 937 (56%) |
| White race | 1360 (81%) |
| BMI, kg/m2 | 28.6 ± 5.0 |
| KL grade | |
| KL 0 | 956 (57%) |
| KL 1 | 433 (26%) |
| KL 2 | 209 (13%) |
| KL 3 | 68 (4.1%) |
| KL 4 | 7 (0.5%) |
| CT findings | |
| Any IA mineralization anywhere in the knee | 150 (9.0%) |
| Any cartilage mineralization | 105 (6.3%) |
| Any meniscus mineralization | 119 (7.1%) |
| Any capsular mineralization | 66 (4.0%) |
| Any ligament mineralization | 57 (3.4%) |
| MRI findings | |
| Any cartilage damage at baseline | 1504 (89.9%) |
| Any cartilage worsening* at 2-year follow-up | 778 (47.4%) |
Abbreviations: BMI, body mass index; KL, Kellgren-Lawrence; IA, intra-articular
Cartilage worsening defined as any within-grade increase in MOAKS from baseline to 2-year follow-up in any subregion
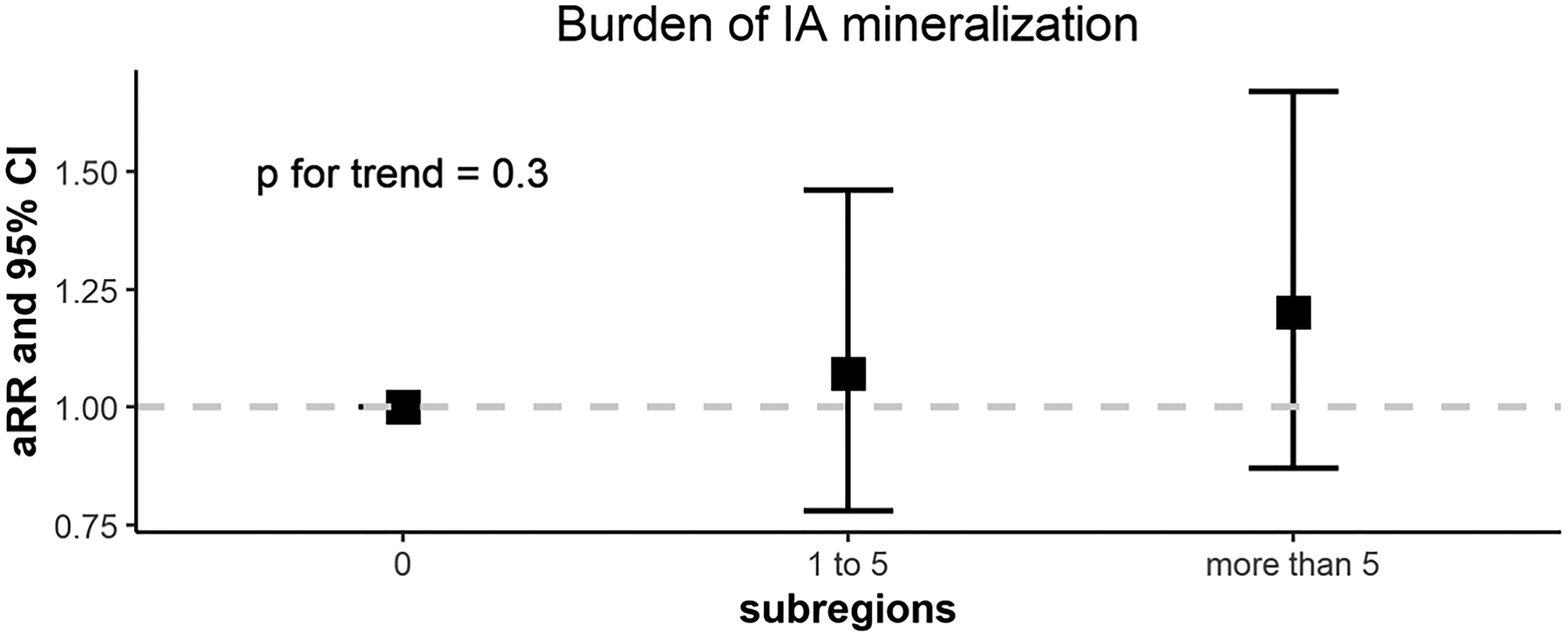
Mineralization of any tissue in the knee, regardless of location or tissue type was not associated with cartilage worsening at any location in the knee (RRs ranging from 1.09–1.27) (Figure 2A; Supplemental Table 1). There was also no association of burden of mineralization (of any tissue) with cartilage worsening (p for trend = 0.3; Supplemental Figure 2).
Figure 2.
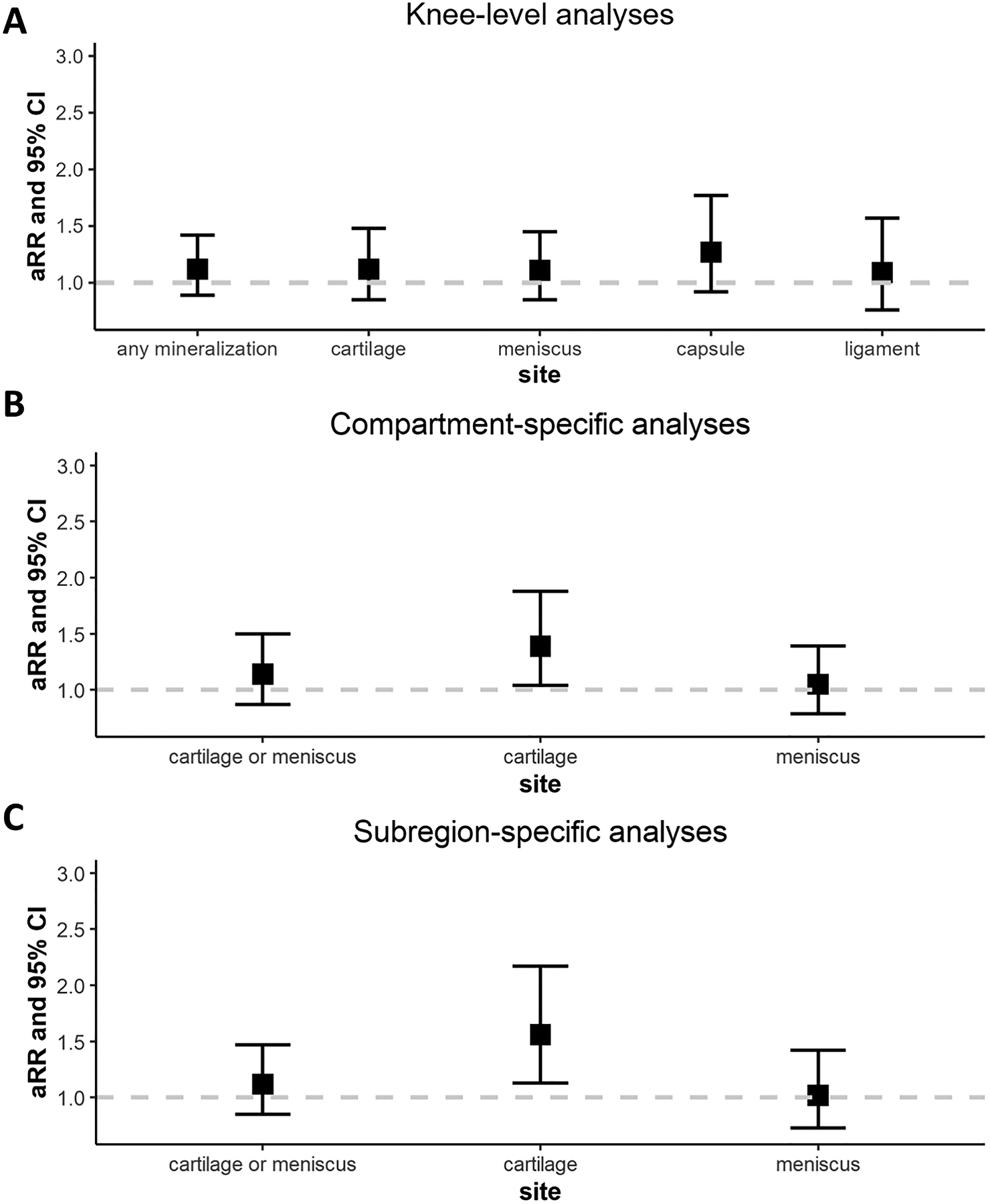
Longitudinal relation of intra-articular mineralization to MRI cartilage worsening in knee-level (A), compartment-specific (B), and subregion-specific analyses (C).
Analyses were adjusted for age, sex, and body mass index.
In compartment-specific analyses, the presence of cartilage mineralization was associated with a 1.39 times higher risk of cartilage damage in the same compartment (i.e., medial or lateral tibiofemoral) (95% CI 1.04–1.88). No association was noted for meniscal mineralization in relation to cartilage worsening at the compartment level (RR 1.05, 95% CI 0.79–1.39) (Figure 2B).
In subregion-specific analyses, the presence of cartilage mineralization was associated with a 1.56 times higher risk (95% CI 1.13–2.17) of cartilage worsening in the same subregion (Figure 2C). However, no association was noted for meniscal mineralization with cartilage worsening immediately overlying or underlying the meniscal subregion (RR 1.02, 95% CI 0.73–1.42).
In sensitivity analyses stratified by radiographic OA status at baseline, associations were not statistically significant for knee-level, compartment-specific, or subregion-specific analyses (Supplemental Table 2), although the effect estimates among knees without radiographic OA were similar in magnitude to the main results, particularly for the subregion-specific analysis of cartilage mineralization (RR 1.74, 95% CI 0.99–3.07). For subregion-specific analyses restricted to MRI subregions without any cartilage damage at baseline, cartilage mineralization was associated with a higher risk of incident cartilage worsening in the same subregion (RR 2.76, 95% CI 1.50–5.08), whereas there was no association for meniscal mineralization (RR 1.02, 95% CI 0.71–1.46) (Supplemental Table 3). In exploratory analyses stratified by age, the magnitude of association between the presence of any IA mineralization with cartilage worsening was stronger for the younger (age dichotomized at 60 years) group, which was particularly evident in the subregion-specific analyses (RR 1.95, 95% CI 1.11–3.41 for age <60 years versus RR 1.03, 95% CI 0.75–1.40 for age ≥60 years) (Supplemental Table 4).
DISCUSSION
CT-detected IA mineralization in the hyaline articular cartilage, but not in the meniscus, was associated with higher risk of cartilage worsening in the same compartment and in the same subregion over two years. These findings suggest tissue-specific and localized effects of IA mineralization on cartilage pathology in knee OA. Further, cartilage mineralization was associated with incident cartilage loss in the same subregion in analyses restricted to subregions without baseline cartilage damage, a finding that provides additional support to the hypothesis that IA mineralization may contribute to OA structural changes.
Chondrocalcinosis, the presence of cartilage calcification noted on imaging or histological examination,18 has been associated with OA severity2 and with higher levels of knee pain in some studies.19 Additionally, a prior study in the Osteoarthritis Initiative (OAI) found that chondrocalcinosis on baseline knee radiographs was significantly associated with change in MRI cartilage score over four years of follow-up.9 In contrast, in a French cohort with symptomatic knee and hip OA, chondrocalcinosis on knee radiographs at baseline was not significantly associated with the risk of joint replacement, radiographic progression, or changes in worsening pain or function over five years.8
Prior studies were limited by their use of conventional radiographs, which have a low sensitivity3,5,10 for identifying chondrocalcinosis (i.e., IA mineralization), leading to under-detection and misclassification. CT has been demonstrated in our previously published pilot study to be a feasible modality for the identification of IA mineralization in knees with OA with greater detectability than radiography,20 particularly given the two-dimensional nature of radiographs and their low overall resolution. Thus, use of CT enables improved ascertainment of IA mineralization, thereby overcoming limitations of relying on radiographs to study impact on OA outcomes11. Using CT-detected IA mineralization in the knee, our study did detect an increased risk of cartilage damage on follow-up, further supporting a pathogenic role for calcium crystal deposition within the joint. Further, some prior studies also relied on radiographs to identify OA progression, which also has low sensitivity. Our study using MRI for assessment of cartilage morphology provides greater sensitivity to detect OA progression as defined by cartilage worsening.
Whether IA mineralization may be a cause or consequence of OA has previously been disputed. Insights into whether IA crystals are “innocent bystanders” or “pathologic actors” would have important implications regarding efforts to target pathologic crystals and/or their effects, such as with cytokine-directed therapies. The finding that IA mineralization in the hyaline articular cartilage was associated with higher risk of cartilage worsening in subregion-specific analyses restricted to subregions without any baseline cartilage damage provides further evidence for IA mineralization as a contributor to OA structural damage. Furthermore, we have also demonstrated that IA mineralization on knee CT was associated with risk of having more frequent, persistent, and worsening knee pain in the same cohort,21 providing additional data supporting an active role of IA mineralization in the clinical presentation of OA.
If IA crystals do contribute to OA structure and/or symptoms, one mechanism is likely through innate immune pathways leading to inflammation. Calcium-containing crystals can act as damage-associated molecular patterns and activate the NLRP3 inflammasome, resulting in elaboration of IL-1β, a key pro-inflammatory cytokine implicated in OA.22–26 To date, however, small clinical trials targeting IL-1β and focusing on primary pain outcomes have been negative.27–29 In contrast, a post-hoc analysis of a large randomized trial of canakinumab, an IL-1β antagonist, among people with cardiovascular disease and elevated C-reactive protein demonstrated a lower risk of knee and hip replacements in the treatment arms compared with placebo.30 This observation has raised the intriguing possibility that targeting IL-1β or other cytokines may be an effective treatment strategy in OA if the appropriate phenotype is targeted, and that prior studies’ participants were too heterogeneous to detect the signal with their smaller sample sizes. Whether IA mineralization represents one such targetable phenotype with anti-IL-1 therapies merits further investigation.
Our findings also suggest location-specific effects of IA mineralization on cartilage damage or worsening. A main limitation of prior studies evaluating IA mineralization on OA structural outcomes was the focus on whole-knee level effects, i.e., whether mineralization anywhere in the knee was associated with radiographic progression (or, in one study, MRI cartilage damage) anywhere in the knee. A more granular focus on compartment- and subregion-specific associations with cartilage damage on MRI allows us to gain better insight into the potential pathogenesis of calcium crystal deposition in the knee, which may have a more localized effect.
This study is also the first to provide insights into whether IA mineralization in different tissues within the joint may have differential effects on cartilage damage. One prior study attempted to investigate meniscal calcification in an in vitro study using cultured meniscal cells from individuals with knee OA.31 They demonstrated that cultured meniscal cells produced more calcium deposition following induction with adenosine triphosphate than did non-OA controls or OA hyaline articular chondrocytes. The authors described three patterns of calcium crystal deposition in the OA menisci, including one pattern of crystal clusters at the edges of the meniscus, adjacent to areas of meniscal degeneration. However, this study did not report on negative effects of the calcium deposition on chondrocytes or other relevant tissues and was therefore unable to determine whether IA mineralization in the meniscus is pathogenic or part of a bystander effect related to OA structural damage.
Finally, in exploratory analyses stratified by age, the association of the presence of IA mineralization in the joint with cartilage damage was most apparent in the younger age group. These results are consistent with those of Ibad, et al using data from the Osteoarthritis Initiative.17 Due the high prevalence of IA mineralization and cartilage damage in the knees of older individuals,3,32–34 the findings in our study are more apparent in the younger age stratum. These results also complement the findings from our analysis that were restricted to knees without radiographic OA and to subregions without cartilage damage, in which we also noted a strong relation of IA mineralization to cartilage damage. Taken together, these results support a role for calcium crystal deposits on development and worsening of cartilage damage since these analyses evaluated the effects of the deposition at a stage without pre-existing cartilage damage or when such damage would be expected to be minimal.
Limitations of our study must be acknowledged. Although we included participants without OA as well as those with early OA, it is not known at what stages mineralization may be important. The majority (83%) of included knees in this study had Kellgren-Lawrence grade 0 or 1 indicating no or early radiographic OA; thus, our results may not generalize to a population with more severe structural disease. Nonetheless, our study sample with little or no radiographic OA provides a unique opportunity to investigate the effects of mineralization preceding more severe stages of OA. We acknowledge prior studies on the possible role of dual energy CT for the identification of different types of calcium crystals and differentiation between BCP and CPP; however, this was outside the scope of our study in which we relied on single energy images. Furthermore, more studies are needed to confirm the validity of dual energy CT for the purpose of calcium crystal classification. The limits of detection for smaller crystal deposits below the spatial resolution of CT must also be acknowledged.35
Strengths of our study include the use of the more sensitive modality of CT to assess IA mineralization, use of MRI to assess cartilage damage incidence and progression, inclusion of participants with earlier stages of OA (compared to studies focused on later stage OA), and longitudinal follow-up over two years. In addition to improved sensitivity, CT also allowed for the semiquantitative assessment of fibrocartilage mineralization as well as in the hyaline articular cartilage. We also focused on location-specific effects of IA mineralization on cartilage damage, rather than solely focusing on whole-knee effects.
In conclusion, these findings of tissue-specific and localized associations of IA mineralization with cartilage damage worsening in older adults with or at risk for knee OA suggest that IA mineralization may have a pathogenic effect on structural progression in knee OA. These findings complement the associations we have noted with regards to IA mineralization and knee pain.21 Our finding of no association between the presence of IA mineralization in the meniscus and in the joint capsule with cartilage damage further supports our hypotheses regarding site-/tissue-specific effects of calcium crystal deposition within the joint. Further studies are needed to confirm the validity of dual-energy CT data and multi-energy photon-counting CT for the identification of different calcium crystals, which in turn may provide novel insights regarding effects of specific crystal types.36 Nonetheless, our findings suggest that IA mineralization appears to have localized detrimental effects on hyaline articular cartilage.
Supplementary Material
Figure 3.
ACKNOWLEDGMENTS
The authors acknowledge the Boston Musculoskeletal Clinical Research Collaboratory Research ACCELERATOR group (P30 AR072571) for their insightful comments on this manuscript.
FUNDING
This work was supported by NIH P30 AR072571.
Jean Liew was supported by the Rheumatology Research Foundation Investigator Award.
Tuhina Neogi was supported by NIH K24 AR070892
The MOST Study was supported by U01-AG-18820, U01-AG-18832, U01-AG-18947, and U01-AG-19079.
Footnotes
DISCLOSURES / CONFLICTS OF INTEREST
Liew – Research grant from Pfizer, unrelated to this work
Jarraya – None
Guermazi – Shareholder of BICL, LLC; consultant to Pfizer, ICM, Coval, Medipost, TrialSpark, TissueGene and Novartis.
Roemer - Shareholder of BICL, LLC; consultant to, Grünenthal
Crema - None
Becce - Research agreement with Siemens Healthineers
Pascart - None
Wang – None
Rabasa – None
Nevitt - None
Torner - None
Lewis - None
Felson – None
Neogi –Novartis, Pfizer/Lilly, Regeneron
Contributor Information
Jean W. Liew, Section of Rheumatology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
Mohammed Jarraya, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA.
Ali Guermazi, Department of Radiology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
David Felson, Section of Rheumatology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
Michael Nevitt, University of California San Francisco, San Francisco, CA.
Cora E. Lewis, Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL.
James Torner, University of Iowa, Iowa City, IA.
Frank W. Roemer, Department of Radiology, Universitätsklinikum Erlangen & Friedrich-Alexander Universität Erlangen Nürnberg (FAU), Erlangen, Germany; Department of Radiology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
Michel D. Crema, Institut d’Imagerie du Sport, Institut National du Sport, de l’Expertise et de la Performance (INSEP), Paris, France
Na Wang, School of Public Health, Boston University, Boston, MA.
Gabriela Rabasa, Section of Rheumatology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
Fabio Becce, Department of Diagnostic and Interventional Radiology, Lausanne University Hospital, University of Lausanne, 1011 Lausanne, Switzerland.
Tristan Pascart, Department of Rheumatology, Lille Catholic Hospitals, University of Lille, Lomme, France.
Tuhina Neogi, Section of Rheumatology, Boston University Chobanian & Avedisian School of Medicine, Boston, MA.
REFERENCES
- 1.Schumacher HR. Crystals, inflammation, and osteoarthritis. American Journal of Medicine 1987;83:11–6. [DOI] [PubMed] [Google Scholar]
- 2.Ea HK, Nguyen C, Bazin D, et al. Articular cartilage calcification in osteoarthritis: Insights into crystal-induced stress. Arthritis Rheum 2011;63:10–8. [DOI] [PubMed] [Google Scholar]
- 3.Fuerst M, Bertrand J, Lammers L, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum 2009;60:2694–703. [DOI] [PubMed] [Google Scholar]
- 4.Denoble AE, Huffman KM, Stabler TV., et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A 2011;108:2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitsuyama H, Healey RM, Terkeltaub RA, et al. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage 2007;15:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen C, Bazin D, Daudon M, et al. Revisiting spatial distribution and biochemical composition of calcium-containing crystals in human osteoarthritic articular cartilage. Arthritis Res Ther 2013;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neogi T, Nevitt M, Niu J, et al. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: Results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum 2006;54:1822–8. [DOI] [PubMed] [Google Scholar]
- 8.Latourte A, Rat AC, Ngueyon Sime W, et al. Chondrocalcinosis of the knee and the risk of osteoarthritis progression: Data from the Knee and Hip Osteoarthritis Long-term Assessment cohort. Arthritis and Rheumatology 2020;72:726–32. [DOI] [PubMed] [Google Scholar]
- 9.Foreman SC, Gersing AS, von Schacky CE, et al. Chondrocalcinosis is associated with increased knee joint degeneration over 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2020;28:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirotti S, Becce F, Sconfienza LM, et al. Reliability and diagnostic accuracy of radiography for the diagnosis of calcium pyrophosphate deposition: performance of the novel definitions developed by an international multidisciplinary working group. Arthritis & Rheumatology 2022; [DOI] [PubMed] [Google Scholar]
- 11.Jarraya M, Guermazi A, Liew JW, et al. Prevalence of intra-articular mineralization on knee computed tomography: the multicenter osteoarthritis study. Osteoarthritis Cartilage [Internet] 2023; Available from: https://linkinghub.elsevier.com/retrieve/pii/S1063458423007586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehy L, Culham E, McLean L, et al. Validity and sensitivity to change of three scales for the radiographic assessment of knee osteoarthritis using images from the Multicenter Osteoarthritis Study (MOST). Osteoarthritis Cartilage 2015;23:1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guermazi A, Jarraya M, Lynch JA, et al. Reliability of a new scoring system for intraarticular mineralization of the knee: Boston University Calcium Knee Score (BUCKS). Osteoarthritis Cartilage 2020;28:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterfy CG, Guermazi A, Zaim S, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177–90. [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Niu J, Neogi T, et al. Synovitis and the risk of knee osteoarthritis: The MOST Study. Osteoarthritis Cartilage [Internet] 2016;24:458–64. Available from: 10.1016/j.joca.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Runhaar J, Schiphof D, van Meer B, et al. How to define subregional osteoarthritis progression using semi-quantitative MRI Osteoarthritis Knee Score (MOAKS). Osteoarthritis Cartilage 2014;22:1533–6. [DOI] [PubMed] [Google Scholar]
- 17.Ibad HA, Kwee RM, Ghotbi E, et al. Radiographically detectable intra-articular mineralization: Predictor of knee osteoarthritis outcomes or only an indicator of aging? A brief report from the osteoarthritis initiative. Osteoarthr Cartil Open 2023;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Doherty M, Bardin T, et al. European league against rheumatism recommendations for calcium pyrophosphate deposition. Part I: Terminology and diagnosis. Ann Rheum Dis 2011;70:563–70. [DOI] [PubMed] [Google Scholar]
- 19.Han BK, Kim W, Niu J, et al. Chondrocalcinosis in knee joints is associated with pain but not with synovitis: Data from the Osteoarthritis Initiative . Arthritis Care Res (Hoboken) 2017;69:1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra D, Guermazi A, Sieren JP, et al. CT imaging for evaluation of calcium crystal deposition in the knee: Initial experience from the Multicenter Osteoarthritis (MOST) study. Osteoarthritis Cartilage 2015;23:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liew J, Jarraya M, Guermazi A, et al. Relation of intra-articular mineralization to knee pain in knee osteoarthritis: A longitudinal analysis in the MOST Study . Arthritis and Rheumatology 2023; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–41. [DOI] [PubMed] [Google Scholar]
- 23.Page Thomas DP, King B, Stephens T, et al. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis 1991;50:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements KM, Price JS, Chambers MG, et al. Gene deletion of either interleukin-1β, interleukin-1β -converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of Knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partia. Arthritis Rheum 2003;48:3452–63. [DOI] [PubMed] [Google Scholar]
- 25.Caron JP, Fernandes JC, Martel-Pelletier J, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis: Suppression of collagenase-1 expression. Arthritis Rheum 1996;39:1535–44. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes J, Tardif G, Martel-Pelletier J, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints. Prevention of osteoarthritis progression. American Journal of Pathology 1999;154:1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chevalier X, Goupille P, Beaulieu AD, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res (Hoboken) 2009;61:344–52. [DOI] [PubMed] [Google Scholar]
- 28.Cohen SB, Proudman S, Kivitz AJ, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther 2011;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleischmann RM, Bliddal H, Blanco FJ, et al. A Phase II Trial of Lutikizumab, an Anti–Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis and Rheumatology 2019;71:1056–69. [DOI] [PubMed] [Google Scholar]
- 30.Schieker M, Conaghan PG, Mindeholm L, et al. Effects of interleukin-1β inhibition on incident hip and knee replacement: Exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2020;173:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Mauerhan DR, Honeycutt PR, et al. Calcium deposition in osteoarthritic meniscus and meniscal cell culture. Arthritis Res Ther 2010;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halverson PB, McCarty DJ. Patterns of radiographic abnormalities associated with basic calcium phosphate and calcium pyrophosphate dihydrate crystal deposition in the knee. Ann Rheum Dis 1986;45:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derfus BA, Kurian JB, Butler JJ, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. Journal of Rheumatology 2002;29:570–4. [PubMed] [Google Scholar]
- 34.Nalbant S, Martinez JAM, Kitumnuaypong T, et al. Synovial fluid features and their relations to osteoarthritis severity: New findings from sequential studies. Osteoarthritis Cartilage 2003;11:50–4. [DOI] [PubMed] [Google Scholar]
- 35.Budzik J, Marzin C, Legrand J, et al. Can Dual-Energy Computed Tomography Be Used to Identify Early Calcium Crystal Deposition in the Knees of Patients With Calcium Pyrophosphate Deposition? Arthritis & Rheumatology 2021;73:687–92. [DOI] [PubMed] [Google Scholar]
- 36.Bernabei I, Sayous Y, Raja A, et al. Multi-energy photon-counting computed tomography versus other clinical imaging techniques for the identification of articular calcium crystal deposition. Rheumatology 2021;60:2483–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


