Introduction
Post-transplant lymphoproliferative disorders (PTLDs) are a heterogenous set of unregulated lymphoid cell proliferations after organ or tissue transplant (1, 2). While many cancers occur at higher frequency in the setting of reduced immune surveillance because of the non-specific immune suppression by the immunosuppressant medications used to prevent acute rejections (3), PTLDs are among the most common (4, 5) and most feared. Some of these unregulated lymphoid proliferations can evolve into full-fledged cancers such as frank lymphomas, but even other subtypes carry a significant risk of morbidity and mortality.
Epidemiology:
PTLDs are relatively rare, more so after kidney transplantation. Their frequency is best expressed as an incidence density, with time under immunosuppression as a denominator. Renal allografts that were lost early and had their immunosuppression stopped early would otherwise be inappropriately be counted in the denominator if raw proportions are used. From the Organ Procurement and Transplant Network database in the USA, the incidence density for PTLD after kidney transplant was reported as 1.58 per 1000 person-years (6), while from the United Kingdom, the incidence density of the PTLD sub-type non-Hodgkin lymphoma was 2.6 per 1000 person-years (7). The ANZDATA registry reported a 25-year cumulative PTLD incidence of 3.3% in adults and 3.6% in children, adjusted for competing risks such as death (8). In children after kidney transplant, PTLD incidence density jumped in the 1990s-2000s (9), but more recent eras are associated with a reduction in incidence across both children and adults (8, 10). In a very recent prospective multicentre study, where 944 children were enrolled and 872 received some type of solid organ transplant (including higher risk intestinal transplant), only 34 PTLDs had occurred in a group followed till at least 1 year (11).
The risk factors for PTLDs that are consistently reported can be divided into 2 groups: non-modifiable and modifiable. Among the non-modifiable risk factors are: a) the extremes of recipient age – the youngest and oldest are at higher risk. Since cancers are much less common in children, their standardized incidence ratios are magnified 20–40 fold (8, 12); b) recipient Epstein-Barr virus (EBV) seronegativity (13, 14) (see Pathogenesis section for a detailed discussion of EBV relationship to PTLD); c) recipient race/ethnicity – white recipients are at higher risk than Black, Hispanic or Asian recipients, even after multivariable adjustments for recipient EBV serostatus (13); d) type of organ transplant – intestinal, lung and heart transplant recipients are at comparatively higher risk than liver, kidney or pancreas transplant recipients (15).
Among the modifiable risk factors are: a) the overall intensity of immunosuppression – a greater number of agents or higher doses/levels increase the risk (16–18). Though many studies report on individual immunosuppressive agents, the only agents that consistently showed a higher risk were the monoclonal antibody OKT3 (19) or the fusion protein belatacept (20). For the latter, PTLD rates were 10-fold higher in EBV-seronegative recipients (5% rate vs 0.5% in EBV-seropositive recipients), and CNS localization of PTLDs were seen in 9/15 (60%) of patients who received belatacept, a very high proportion compared to 10–12% in other series (9, 18, 21, 22). Most cases occurred within the first 18 months. Other agents such as anti-thymocyte globulin induction, de novo sirolimus use or tacrolimus-mycophenolate combinations are inconsistently reported, with conflicting data on risk, suggesting that totality of immunosuppression plays the major role. Other inconsistently reported risk factors are recipient HLA alleles or the use of anti-viral prophylaxis (23, 24).
Pathogenesis:
Majority of PTLD cases are associated with EBV (25, 26). EBV infection is almost ubiquitous, with more than 90% of adults showing EBV seropositivity (27). EBV has several differential gene expression patterns, defining the distinct latency programs. During type III latency, which occurs shortly after infection and is also known as proliferation program, full range of EBV latent genes is expressed including six nuclear antigens (EBNA-1, −2, −3A, −3B, −3C, and -LP) and three latent membrane proteins (LMP-1, −2A, and −2B) as well as several untranslated RNAs (28, 29). In immunocompetent individual, the proliferation of EBV-infected B-cells is controlled by vigorous cytotoxic T-lymphocyte response which rapidly clears the vast majority of the infected B cells (30, 31). A small subpopulation of EBV infected B-cells persists for lifetime of the host by downregulating viral antigen expression and escaping immune surveillance (28).
In the post-transplant setting, these latent EBV-positive B cells may give rise to EBV-positive PTLD, as a consequence of immunosuppression-induced deficiencies in EBV-specific T cells. Type III latency is found in majority of EBV-positive PTLDs (29, 32, 33), with full expression of EBV latent genes providing critical growth and survival signals for infected B-cells. Several of EBV-encoded proteins, including LMP1, have been shown to be essential for B-cell transformation (32). LMP1 is the major EBV oncogene, that mimics CD40 receptor signaling pathway (34) and prevents apoptosis by upregulating anti-apoptotic protein Bcl-2 by activating NF-kB pathway (35, 36). Interactions between LMP1 and adaptor molecules also lead to signal transduction through the MAPK p38, ERK, and JNK, and PI3K signaling pathways (37–40). LMP-1 can increased expression of regulatory cytokine IL-10 (41) leading to the attenuation of anti-viral adoptive immune and proliferation of EBV-positive B-cells (42). Expression of LMP1 in B cells in genetically engineered mice was sufficient to induce immune surveillance of the LMP1-positive cells by cells of the adaptive immune system, and weakening of this surveillance mechanism by depleting T cells resulted in the rapid development of B cell lymphomas in LMP1-positive mice model (43).
Recent studies have utilized advanced sequencing techniques to focus on EBV genome diversity and gain additional insights into pathogenesis of EBV-positive PTLD. Sequencing of EBV genomes from FFPE PTLD samples revealed that EBNA-1, EBNA-LP and EBNA3C genes had increased number of variations compared to other EBV genes (44). Peripheral blood sequencing from solid organ transplant recipients revealed that EBV genomes from PTLD patients had significantly more variations than EBV genome from transplant recipients without PTLD, including variations within EBNA3C and LMP2A epitopes targeted by T-cell response. Computational modeling suggested that the PTLD-associated variations could impact antigen presentation and subsequent T-cell response, altering host immunity to EBV and promoting escape from the immune response (45). Multicenter prospective study demonstrated that presence of G212S and S366T mutations in LMP1 is associated with a significantly increased risk of EBV-positive PTLD in pediatric solid organ transplant recipients, while absence of both mutations is associated with low probability of developing PTLD (11).
EBV-negative PTLD is less common than EBV-positive PTLD but accounts for up to 40% of PTLD cases, with incidence of EBV-negative PTLD increasing over the years. In contrast to EBV-positive PTLD, EBV-negative PTLD cases occur more commonly in adults and later after transplantation, with higher proportion of monomorphic PTLD cases (46, 47). Despite these differences, proportion of EBV-negative cases may respond to decreased immunosuppression, and are considered a subset of PTLD category (48).
Pathogenesis of EBV-negative PTLD is uncertain, with several proposed etiologies including another viral infection, undetectable EBV infection due to loss of EBV or technical reasons, prolonged immunosuppression, and transplant-related chronic antigenic stimulation (49). With the exception of rare human herpesvirus 8 (HHV8)-positive PTLDs (50, 51), association of PTLD development with viral infection other than EBV remains unproven. In a metagenomic shot gun sequencing analysis of 69 PTLD tissue samples, no viruses were detected in higher proportion of EBV-negative PTLD cases as compared to EBV-positive PTLDs (44). Further evidence that viral oncogenesis is not a key pathological pathway in EBV‐negative PTLD comes from the studies comparing gene expression profiles and genomic aberrations of EBV-positive and EBV-negative diffuse large B-cell lymphoma (DLBCL) PTLD to that of DLBCL arising in immunocompetent hosts. These studies have shown that EBV-negative DLBCL PTLD cases have molecular signatures that cluster with that of DLBCL in non-immunosuppressed patients and are distinct from EBV-positive DLBCL PTLD (32, 52–54).
PTLD Classifications:
Since first histological description of PTLD more than 50 years ago (55), PTLD designation has come to encompass a spectrum of lymphoid or plasmacytic proliferations, ranging from usually EBV-positive polyclonal proliferations to EBV-positive or EBV-negative malignancies that have the same histological appearance as non-Hodgkin or Hodgkin lymphoma in immunocompetent patients. Due to this broad range of histological and immunophenotypic findings and varying degree of tissue destructiveness and clinical behavior, PTLD lesions require further classification. Several editions of the World Health Organization (WHO) classification of Tumours of Haematopoietic and Lymphoid Tissues have incorporated diagnostic criteria for different types of PTLD. Revised 4th edition of WHO classification recognized four major categories of PTLD: non-destructive PTLD, polymorphic PTLD, monomorphic PTLD, and Classic Hodgkin Lymphoma PTLD (48).
As the name suggests, non-destructive PTLDs preserve architecture of the involved tissue, commonly tonsils and lymph nodes. This group of PTLDs was previously designated as “early lesions” but this term was retired due to confusion with PTLD cases that occur shortly after transplantation. These lesions are polyclonal mixed lymphocytic and plasmacytic infiltrates showing a spectrum of B-cell maturation. They lack of histological features diagnostic for a malignant lymphoma. Non-destructive PTLD category is further divided into three histologic subtypes: infectious mononucleosis, plasmacytic hyperplasia, and florid follicular hyperplasia. Infectious mononucleosis PTLD has histological appearance associated with primary EBV infection in immunocompetent host, with interfollicular expansion composed of small lymphocytes, plasma cells and usually frequent larger transformed lymphoid cells known as immunoblasts. Plasmacytic hyperplasia is composed of polytypic plasma cells, small lymphocytes and occasional immunoblasts. Florid follicular hyperplasia is composed of reactive secondary follicles with prominent germinal centers (Figure 1). Most non-destructive PTLD cases are EBV-positive and form mass lesions, and these characteristics help to distinguish plasmacytic hyperplasia and florid follicular hyperplasia, which otherwise have non-specific histological findings, from reactive lymphoid or plasmacytic proliferations (48, 49).
Figure 1.

Non-destructive PTLD, florid follicular hyperplasia (revised 4th WHO classification/ICC) / Florid follicular hyperplasia, EBV-positive, post-transplant (5th edition of WHO classification). Low-power view of tonsil with preserved architecture and florid follicular hyperplasia with multiple reactive secondary follicles ((A) H&E stain, original magnification 2x). Germinal center ((B) H&E stain, original magnification 20x) with increased EBV-positive cells ((C) In situ hybridization for EBV (EBER), original magnification 20x).
Polymorphic PTLDs are also heterogeneous mixtures of lymphocytic and plasmacytic components that show a full range of B-cell maturation, including small- to intermediate-sized lymphocytes, plasma cells and immunoblasts (Figure 2). However, unlike non-destructive PTLD, polymorphic PTLD cases are associated with effacement of underlying tissue architecture and usually show clonal IgH rearrangements, reflecting emergence of clonal B-cell population. The histological findings in polymorphic PTLD do not fulfill the criteria of any of the recognized types of lymphoma described in immunocompetent hosts but they may have necrosis and/or increased large lymphoid cells or Reed-Sternberg-type cells, making distinction from monomorphic PTLD difficult in some cases (48, 49).
Figure 2.
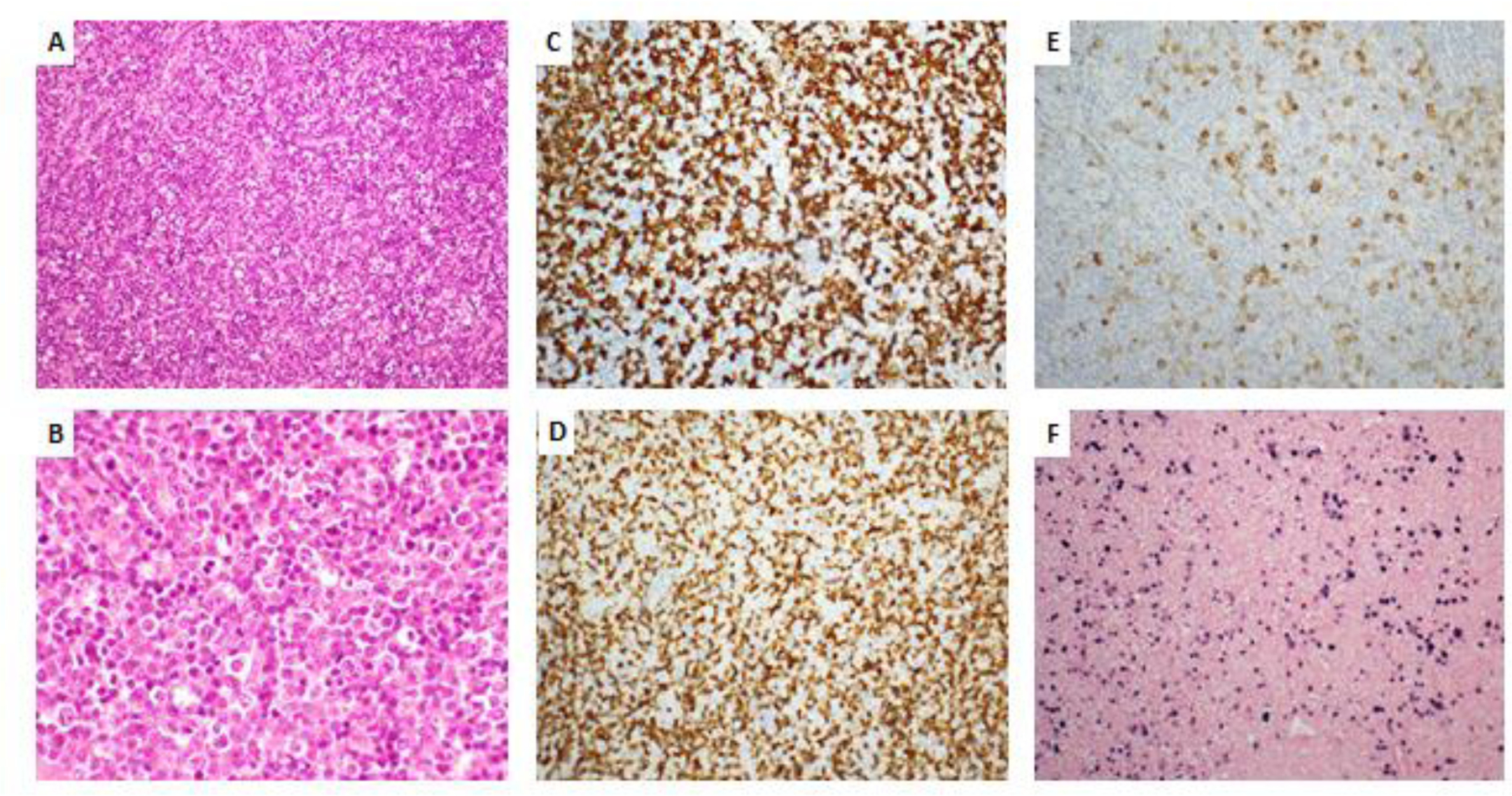
Polymorphic PTLD (revised 4th WHO classification/ICC) / Polymorphic lymphoproliferative disorder, EBV-positive, post-transplant (5th edition of WHO classification). Effacement of architecture ((A)H&E stain, original magnification 20x) by heterogeneous lymphoid proliferation ((B) H&E stain, original magnification 50x) composed of mixture by CD20-positive B cells ((C), original magnification 20x) and CD3-positive T cells ((D), original magnification 20x) with scattered admixed CD30-positive immunoblasts ((E), original magnification 20x). In situ hybridization for EBV ((F), original magnification 20x) highlights frequent positive cells.
Monomorphic PTLDs, which account for most cases of PTLD, are clonal lymphoid or plasmacytic proliferations that fulfill the diagnostic criteria for one of the non-Hodgkin lymphomas or plasma cell neoplasms recognized in non-immunosuppressed patients. The monomorphic PTLD cases may be of B-cell or T/NK-cell origin and are further categorized according to the lymphoma they resemble in immunocompetent hosts. The majority of cases of monomorphic PTLD are B-cell neoplasms, most commonly diffuse large B-cell lymphoma. Histologically, these are overtly malignant proliferations composed of large atypical lymphoid cells expressing B-cell markers (Figure 3) and usually exhibiting increased proliferation rate, apoptosis and necrosis. Post-transplant DLBCLs may be EBV-positive and EBV-negative. Gene-expression profile and immunohistochemical staining have been used to classify DLBCL cases in immunocompetent subjects into germinal center cell or non–germinal center cell origin (56, 57). In PTLD setting, EBV-positive DLBCL cases predominantly have non-germinal center cell immunophenotype, while significant subset of EBV-negative DLBCL cases is of germinal center cell origin (32, 58). It is important to note that indolent B-cell neoplasms arising in the post-transplant setting, with the exception of uncommon EBV-positive MALT lymphomas occurring preferentially in skin or subcutaneous tissue, are not considered PTLD.
Figure 3.

Monomorphic B-cell PTLD, diffuse large B-cell lymphoma (revised 4th WHO classification/ICC) / Diffuse large B-cell lymphoma, EBV-positive, post-transplant (5th edition of WHO classification). Diffuse proliferation ((A) H&E stain, original magnification 20x) of large atypical cells with irregular nuclear contours and prominent nucleoli ((B) H&E stain, original magnification 50x) positive for B-cell marker CD20 ((C) original magnification 20x) and EBV ((D) in situ hybridization for EBV (EBER), original magnification 20x).
Monomorphic T-cell PTLDs are less frequent and are more commonly EBV-negative. Most of subtypes of T/NK-cell lymphomas have been reported in the post-transplant setting, with peripheral T-cell lymphoma, not otherwise specified, representing the most common subtype, followed by hepatosplenic T-cell lymphoma (48).
Classic Hodgkin lymphoma PTLD is the least common category of PTLD. These PTLDs meet the diagnostic criteria for classic Hodgkin Lymphoma, with relatively low numbers of malignant Reed-Sternberg cells in a non-neoplastic inflammatory background (Figure 4) and are overwhelmingly EBV-positive. Because Reed-Sternberg-like cells may be seen in other subtypes of PTLD, diagnosis of classic Hodgkin lymphoma PTLD requires presence of both morphological and immunophenotypic findings characteristic of classic Hodgkin lymphoma outside of post-transplant setting (48, 49).
Figure 4.

Classic Hodgkin Lymphoma PTLD (revised 4th WHO classification/ICC) / Classic Hodgkin Lymphoma, EBV-positive, post-transplant (5th edition of WHO classification). Non-neoplastic inflammatory background composed of small lymphocytes and histiocytes ((A), H&E stain, original magnification 20x) with scattered admixed Reed-Strernberg cells ((B) H&E stain, original magnification 50x) positive for CD30 ((C), original magnification 20x) and EBV ((D) in situ hybridization for EBV (EBER), original magnification 20x).
Two new/updated classifying proposals of hematolymphoid neoplasms have been published in 2022: 5th edition of the WHO classification and International Consensus Classification (ICC) (59, 60). Updated WHO classification has integrated PTLD categories into overarching framework for immunodeficiency-associated lymphoproliferative disorders arising in the different settings of immune dysfunction (60) (Table 1). It has been previously observed that PTLD-like lesions arise in patients are immunosuppressed due to other iatrogenic or genetic causes. In prior classifications including revised 4th edition of WHO classification (48), these lymphoproliferative disorders were organized according to the disease background in which they arose, with separate topics pertaining to post-transplantation, HIV infection, other iatrogenic immunodeficiencies and primary immunodeficiencies. Citing the evidence that morphological features and possibly the pathogenesis of many of these entities overlap, 5th edition of WHO classification introduced the term immunodeficiency and dysregulation associated lymphoproliferative disorders (IDD- LPSDs) (60). Integrated classification of IDD-LPSDs was first proposed the Workshop on Immunodeficiency and Dysregulation in 2015 (61, 62). The new standardized multi-part nomenclature for reporting the diagnosis organizes information around common histologic and pathogenetic features IDD-LPSDs and includes: (1) histological diagnosis (hyperplasia, polymorphic LPD, lymphoma as for immunocompetent patients); (2) presence or not of virus such as EBV; (3) clinical setting/immunodeficiency background (post-transplant, HIV, iatrogenic/autoimmune) (60).
Table 1.
Different PTLD classification systems
| WHO classification, revised 4th edition | International Consensus Classification | WHO classification, 5th edition |
|---|---|---|
| Non-destructive PTLD Subtypes: ● Plasmacytic hyperplasia ● Infectious mononucleosis ● Florid follicular hyperplasia |
Non-destructive PTLD Subtypes: ● Plasmacytic hyperplasia ● Infectious mononucleosis ● Florid follicular hyperplasia |
Hyperplasia arising in immune deficiency/dysregulation Subtypes: ● Plasmacytic hyperplasia ● Infectious mononucleosis Florid follicular hyperplasia |
| Polymorphic PTLD | Polymorphic PTLD | Polymorphic lymphoproliferative disorder arising in immune deficiency/dysregulation |
| Monomorphic B- and T-cell PTLD further categorized based on classification of B-cell and T-cell lymphomas in immunocompetent hosts | Monomorphic B- and T-cell PTLD further categorized based on classification of B-cell and T-cell lymphomas in immunocompetent hosts | Lymphomas arising in immune deficiency/dysregulation further categorized based on classification of B-cell and T-cell lymphomas in immunocompetent hosts |
| Classic Hodgkin Lymphoma, PTLD | Classic Hodgkin Lymphoma, PTLD | Lymphomas arising in immune deficiency/dysregulation further categorized as classic Hodgkin lymphoma |
In contrast to the 5th edition of WHO classification changes, consensus ICC opinion acknowledged that EBV-positive B-cell lymphoproliferative disorders arising in different immunodeficiency backgrounds share some histological findings but concluded that further studies are needed. Thus, in ICC system PTLD category remains separate and unaltered from revised 4th edition of WHO classification (Table) due to significant differences in clinical management (63).
Clinical features and diagnosis:
The clinical features for PTLD can be both diverse and non-specific, necessitating a high index of suspicion. Routine physical examination should include looking for any visible or palpable lymph node masses in the neck, axilla, groin or abdomen. Non-specific clinical features can include fever, night chills, weight loss or unexplained anemia. Some of the clinical features relate to the location of the lymphoid mass, such that gastrointestinal symptoms (diarrhea, vomiting) are seen if the PTLD is within the intestine. Similarly, central nervous system tumors may show symptoms and signs related to the location of the mass and the areas of the nervous system that are compressed.
With the increasing use of regular EBV nucleic acid testing in peripheral circulation, the diagnosis of PTLD can often be suspected even when asymptomatic. While in general, higher EBV DNA loads associate to higher PTLD risk (64), the specificity of individual prediction is low (65). The reasons for this relate to the varying sample sources (lymphocytes, whole blood or plasma), variations in the EBV PCR assay methodologies across laboratories (66) and the differing values and units (67), now attenuated but still quite variable after the implementation of an international EBV standard and increasing use of international units/ml (68). Both EBV peak load and rate of rise in EBV load exhibit areas-under-the-curve value in the 0.6–0.7 range, not high enough to be diagnostic in a given individual (69). For EBV-negative PTLDs, the EBV peripheral load is not useful.
Imaging, especially with radiation, should be reserved for those situations where suspicion of PTLD is high. Imaging can identify which lesions are amenable to biopsy. A tissue diagnosis is still the gold standard, though in some inaccessible locations such as the brain, a combination of clinical, laboratory and imaging features are used to make a presumptive PTLD diagnosis.
Prevention and pre-emptive interventions for PTLD
Given the morbidity and mortality of PTLDs, prevention is highly desirable. Unfortunately, no EBV vaccine has reached the clinical realm. One vaccine to the EBV glycoprotein gp350 was tested in children with chronic kidney disease who were to receive a kidney transplant, but did not prevent either EBV replication or PTLD (70). Vaccines with other mechanisms are under development. The use of intravenous immunoglobulin or CMV-Ig, both of which would carry some EBV-directed antibodies, has conflicting results. Large retrospective data suggested benefit (71), whereas small prospective studies did not (72). Chemoprophylaxis with antiviral agents such as acyclovir, ganciclovir or their analogues is similarly controversial. A meta-analysis by AlDabbagh et al showed no benefit in PTLD prevention (23), but European registry data do show a benefit in reducing primary infection EBV DNAemia (73) and another group showed a benefit in preventing late PTLDs, those occurring after the first year post-transplant (24).
Rituximab use has become established in hematopoietic stem cell transplants as an effective pre-emptive measure at the time of EBV DNAemia. But unlike in kidney transplants, 90% of PTLDs are EBV positive in these populations, and the EBV DNAemia is more monophasic, occurring predominantly within the first year post-transplant. Two retrospective studies, one small (74) and one larger (75), have suggested that rituximab can prevent the progression to PTLD in adult kidney transplants recipients too. The use of EBV-directed cytotoxic T lymphocytes as a pre-emptive measure at the time of EBV DNAemia is attractive conceptually, but remains to be studied.
Treatment of PTLD
Treatment of PTLD typically involves a stepwise approach, balancing control of the PTLD with allograft preservation and side effects of therapy. Collaboration between specialists is essential for evaluating timing of PTLD diagnosis in relation to transplant, degree of necessary immunosuppression, allograft function, histological subtype, and overall health of the patient when considering available treatment options. The first line treatment is reduction of immunosuppression (RIS) to enhance alloreactive T-cell immunity. Response rates to RIS alone range widely from 43 to 63% in retrospective studies and are higher in patients with non-destructive and EBV positive PTLD (76, 77). Close monitoring is necessary due to the increased risk of organ rejection and graft loss with RIS. In rare circumstances when PTLD is localized, surgery may also play a role in treatment.
For patients who either do not respond to RIS, or are at high risk of developing rejection and are not candidates for RIS, rituximab monotherapy is often the next line of treatment for CD20 positive PTLD. Rituximab is an anti-CD20 monoclonal antibody which targets CD20, a transmembrane protein present on almost all B cells, and decreases B cell proliferation. Prospective trials of rituximab monotherapy evaluating 3 or 4 weekly doses reported overall response rates ORR of 44–79% with complete response of 25%–53% (78–81). Long-term survival for patients who achieve a CR to rituximab alone is excellent with disease-specific survival at 10 years of 88% (82).
The international prospective phase II PTLD-1 trial of adults with solid organ transplant PTLD introduced a risk stratified sequential treatment approach (83). Patients with CR after four doses of rituximab induction then received for additional doses every 21 days with estimated three-year progression-free survival (PFS) of 89% and overall survival (OS) of 91%. Patients with a partial response (PR) or progressive disease then received chemotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone). For this group of patients requiring chemotherapy, ORR was 88%, with 70% CR, and treatment-related mortality of 8%, highlighting the risk of chemotherapy toxicity in this patient population. Since PTLD arises from an underlying immunodeficient environment, treatment regimens developed for acquired immunodeficiency syndrome‐associated lymphomas, such as DA-EPOCH-R (dose adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) have also been trialed in advanced PTLD with comparable efficacy and tolerability to R-CHOP (84, 85). Overall, the use of risk stratified sequential treatment in patients with PTLD, especially polymorphic or diffuse large B-cell (DLBCL) sub-type, has led to good outcomes and decreased treatment-related mortality.
In pediatric patients with EBV positive PTLD, two phase-two trials have evaluated the use of low-dose chemotherapy including cyclophosphamide and prednisone for six cycles with or without rituximab (86, 87). Patients treated with cyclophosphamide, prednisone and rituximab had a 2-year EFS of 71%, including functioning original allograft, and OS of 83% with median follow-up of 4.8 years (87). Response-adapted sequential treatment strategy with rituximab +/− chemotherapy in pediatric patients with CD20+ PTLD has also been investigated (88). Patients received three weekly infusions of rituximab and if at least a PR was obtained they then received three additional rituximab doses. 64% of patients attained a CR with rituximab alone and 81% of responders remain alive and in continuous complete remission with a median follow up of 4.9 years. Patients with stable or progressive disease then received a moderate chemotherapy regimen mCOMP (vincristine, prednisone, cyclophosphamide and methotrexate). 66% of patients who received chemotherapy achieved a CR with two-year EFS 67% and OS 86%. Notably patients with liver or renal transplant had superior EFS to patients after thoracic organ transplantation 79% vs 44%.
Rare subtypes of PTLD including Hodgkin lymphoma, T-cell lymphoma, and central nervous system (CNS) lymphoma typically do not respond to RIS or rituximab monotherapy and should be treated with the standard of care chemoimmunotherapy for the specific histology. Patients with Hodgkin lymphoma PTLD treated with standard Hodgkin chemotherapy protocols have favorable outcomes, though not as good as for non-immunocompromised patients treated on similar protocols (89, 90). Chemotherapy dose modifications are common due to patient comorbidities. T-cell lymphoma PTLD, including peripheral T-cell lymphoma, hepatosplenic T-cell lymphoma, and anaplastic large cell lymphoma are often not associated with EBV, occur later than B-cell PTLD, and typically have a poor prognosis (91, 92). PTLD involving the CNS also has a poor prognosis. Treatment approaches include chemotherapy with high-dose methotrexate, higher dose intravenous and intrathecal rituximab, radiotherapy, and EBV cytotoxic T-lymphocytes (EBV-CTLs) (93, 94).
Adoptive T-cell therapy using EBV-CTLs is a newer treatment approach designed to address the underlying immunological defect. For EBV positive PTLD and other immune deficiency–related lymphoproliferations, the goal of treatment is to eradicate EBV infected B-cells and recover the EBV-specific T-cell immune response. EBV-CTLs for patients following solid organ transplant can be autologous, generated from the patient‟s own cells, or third party CTLs, generated from healthy HLA matched, EBV seropositive donors. The benefit of third party CTLs is that they are rapidly available for patients, while autologous cells take several weeks to generate. Products are chosen based on best HLA match and HLA restriction of viral activity. Both types of EBV-CTLs are generally well tolerated and generate good responses without causing organ rejection (95–97). Maintaining adequate immunosuppression throughout treatment is critical for preventing organ rejection. Concurrent use of steroids has not demonstrated a significant difference in response to EBV-CTLs, though most studies have limited the dose to <0.5 mg/kg prednisone or its equivalent in order to maintain CTL functionality (98). EBV-CTLs have been used as consolidation following chemotherapy and used prophylactically to treat EBV viremia (99). Several Phase II/III trials of EBV-CTLs in PTLD patients are currently underway to further evaluate the efficacy of this approach.
The use of other immunotherapies in PTLD treatment have been tried with caution due to the potential risks of T-cell activation and allograft rejection. Chimeric antigen receptor T-cell (CAR-T) therapy targeting CD19 is being increasingly used for treatment of lymphomas, but has not been studied systematically in PTLD. Questions remain about use of immunosuppressive therapy during CAR-T, its impact of efficacy of treatment, and risk of rejection (100). Most patients with PTLD receiving CAR-T in a real world case series had their immunosuppression discontinued prior to CAR-T infusion and a few remained on prednisone 5mg daily, resuming immunosuppression after a median of 2.2 months (101). Of the 20 patients included in the series, seven remain in remission after two years and three experienced kidney allograft rejection with two requiring hemodialysis and one patient dying due to PTLD progression. Similarly, checkpoint inhibitors are being incorporated into lymphoma treatment, however, the PD-1 and CTLA-4 pathways are important for allograft tolerance, raising concerns about risk of rejection with use of these therapies. In a single center retrospective review of patients post-SOT who received checkpoint inhibitors as part of their treatment for a range of cancers, primarily metastatic melanoma, 41% of patients (16/39) experienced organ rejection and 81% of those experienced graft loss (102). For patients with PTLD, at this point in time the risks of checkpoint inhibitors likely outweigh the benefits.
Prognosis
Imaging plays a critical role in diagnosis, staging, and response assessment of PTLD. 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) is the most sensitive and specific imaging modality for Hodgkin and non-Hodgkin lymphoma and is increasingly being used for patients with PTLD (103). PET scans may be helpful in cases of suspected PTLD. A retrospective study of 125 PET scans performed for differentiating PTLD from other diseases found a sensitivity of 90%, specificity of 89%, positive predictive value of 85% and negative predictive value of 93% (104). For staging, PET is more appropriate than a conventional computed tomography (CT) scan because PTLD often has extra-nodal involvement that is not always adequately detailed on CT (105). A meta-analysis of three studies evaluating PET response in PTLD showed that PET detected more lesions than CT in 15% of the scans and showed no metabolic activity in 32% of the lesions detected on CT, ultimately changing treatment decisions in 29% of patients (106). End of therapy (EOT) PET may also be useful for identifying patients at low risk of relapse. In a retrospective study of patients with CD20+ PTLD treated with rituximab or rituximab and chemotherapy, the positive predictive value of EOT PET for PTLD relapse was 38%, and the negative predictive value was 92%. Further studies are needed to better understand the prognostic utility of EOT PET.
A number of prognostic indices have been investigated in adults with PTLD. The International Prognostic Index (IPI), developed as a predictive model for aggressive non-Hodgkin Lymphoma, is based on the baseline parameters age, stage of disease, Eastern Cooperative Oncology Group (ECOG) performance status, involvement of extranodal sites, and LDH (107). However, several retrospective studies of patients with PTLD demonstrated that the IPI and clinical prognostic factors used for NHL in immunocompetent patients may not be as accurate for immunosuppressed patients with PTLD (108–111). Alternate prognostic indices for patients with PTLD have been developed, utilizing elements of the IPI and other PTLD specific factors such as monomorphic disease and graft involvement (Table 2). Notably, the ‘Renal-PTLD’ Index was specifically developed for renal transplant and includes elevated LDH and B-symptoms as negative prognostic markers (111). The applicability of these indices has changed over time with the evolution of PTLD treatment, especially the introduction of rituximab to upfront therapy. The PTLD-1 study which used a risk-stratified sequential treatment approach evaluated the use of these scoring systems for predicting outcome and found that the IPI, PTLD Prognostic Index, and Ghobrial Score were predictive for overall survival (112). Age and ECOG performance status had the highest prognostic impact in the different scoring systems. Other variables not included in the scores that were found to impact overall survival and disease progression were the type of transplant and the response to rituximab at interim staging. Further refinement and validation of these scores through a prospective trial may help guide treatment stratification.
Table 2:
Comparison of PTLD prognostic indices
| Variable | IPI | PTLD Prognostic Index | Leblond Score | Ghobrial Score | Renal-PTLD Index |
|---|---|---|---|---|---|
| Age >60 | X | X | |||
| Ann Arbor Stage III-IV | X | ||||
| ECOG >=2 | X | X | X | X | |
| LDH > 1x norrmal | X | X | X | ||
| > 1 Extranodal site* | X | ||||
| > 1 Disease site | X | ||||
| Monomorphic disease | X | ||||
| Graph involvement | X | ||||
| B-symptoms | X | ||||
| Score | 0–5 | 0–3 | 0–2 | 0–3 | 0–2 |
Extranodal = Bone marrow, GI tract, liver, lung, CNS, skin, testes, Waldeyer’s ring
Prognostic risk factors in pediatric PTLD are poorly characterized with no published prognostic scores due to its rarity. Studies of pediatric patients with PTLD have identified multiple prognostic factors including age at transplantation, EBV status, deceased donor graft, HLA mismatch, intensity of immunosuppression, organ rejection, bone marrow or CNS involvement, and PTLD time of onset (113–117). Lack of response to initial PTLD therapy has also been shown to be a poor prognostic factor (117). All of these factors are important to take into account when considering the best treatment approach for pediatric patients with PTLD.
Concluding remarks:
While we have learned much about PTLD etiopathogenesis in the last 2 decades, many knowledge gaps still remain. While some pre-emptive therapies and treatment strategies are available have reasonable success are available, they do not eliminate the high morbidity and significant mortality after PTLD.
Acknowledgements:
VRD, MR and LM acknowledge support from the PTLD-MSMS study grant from the NIH (1R01 AI142135).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
No relevant recent disclosures.
References:
- 1.Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers 2016;2:15088. [DOI] [PubMed] [Google Scholar]
- 2.Dierickx D, Habermann TM. Post-Transplantation Lymphoproliferative Disorders in Adults. N Engl J Med 2018;378(6):549–62. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan A, Wong G, Teixeira-Pinto A, Lim WH. Incidence and Outcomes of Early Cancers After Kidney Transplantation. Transplant international : official journal of the European Society for Organ Transplantation 2022;35:10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharnidharka VR. Comprehensive review of post-organ transplant hematologic cancers. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18(3):537–49. [DOI] [PubMed] [Google Scholar]
- 5.Allen UD, Preiksaitis JK, Practice ASTIDCo. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33(9):e13652. [DOI] [PubMed] [Google Scholar]
- 6.Sampaio MS, Cho YW, Qazi Y, Bunnapradist S, Hutchinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients: an analysis of the U.S. National Transplant Database. Transplantation 2012;94(10):990–8. [DOI] [PubMed] [Google Scholar]
- 7.Morton M, Coupes B, Roberts SA, Klapper PE, Byers RJ, Vallely PJ, et al. Epidemiology of posttransplantation lymphoproliferative disorder in adult renal transplant recipients. Transplantation 2013;95(3):470–8. [DOI] [PubMed] [Google Scholar]
- 8.Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, Wong G. Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: a registry study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2018;33(5):881–9. [DOI] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Araya CE. Post-transplant lymphoproliferative disease. Pediatr Nephrol 2009;24(4):731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med 2014;371(6):549–58. [DOI] [PubMed] [Google Scholar]
- 11.Martinez OM, Krams SM, Robien MA, Lapasaran MG, Arvedson MP, Reitsma A, et al. Mutations in latent membrane protein 1 of Epstein-Barr virus are associated with increased risk of posttransplant lymphoproliferative disorder in children. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2023;23(5):611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA : the journal of the American Medical Association 2011;306(17):1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, et al. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2007;7(11):2619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharnidharka VR, Lamb KE, Gregg JA, Meier-Kriesche HU. Associations between EBV serostatus and organ transplant type in PTLD risk: an analysis of the SRTR National Registry Data in the United States. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2012;12(4):976–83. [DOI] [PubMed] [Google Scholar]
- 15.Dharnidharka VR, Tejani AH, Ho PL, Harmon WE. Post-transplant lymphoproliferative disorder in the United States: young Caucasian males are at highest risk. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2002;2(10):993–8. [DOI] [PubMed] [Google Scholar]
- 16.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004;18(4):446–9. [DOI] [PubMed] [Google Scholar]
- 17.Herzig KA, Juffs HG, Norris D, Brown AM, Gill D, Hawley CM, et al. A single-centre experience of post-renal transplant lymphoproliferative disorder. Transplant international : official journal of the European Society for Organ Transplantation 2003;16(7):529–36. [DOI] [PubMed] [Google Scholar]
- 18.Opelz G, Dohler B. Lymphomas After Solid Organ Transplantation: A Collaborative Transplant Study Report. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2004;4(2):222–30. [DOI] [PubMed] [Google Scholar]
- 19.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med 1990;323(25):1723–8. [DOI] [PubMed] [Google Scholar]
- 20.Grinyo J, Charpentier B, Pestana JM, Vanrenterghem Y, Vincenti F, Reyes-Acevedo R, et al. An integrated safety profile analysis of belatacept in kidney transplant recipients. Transplantation 2010;90(12):1521–7. [DOI] [PubMed] [Google Scholar]
- 21.Trofe J, Buell JF, Beebe TM, Hanaway MJ, First MR, Alloway RR, et al. Analysis of factors that influence survival with post-transplant lymphoproliferative disorder in renal transplant recipients: the Israel Penn International Transplant Tumor Registry experience. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2005;5(4 Pt 1):775–80. [DOI] [PubMed] [Google Scholar]
- 22.Caillard S, Lelong C, Pessione F, Moulin B. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2006;6(11):2735–42. [DOI] [PubMed] [Google Scholar]
- 23.AlDabbagh MA, Gitman MR, Kumar D, Humar A, Rotstein C, Husain S. The Role of Antiviral Prophylaxis for the Prevention of Epstein-Barr Virus-Associated Posttransplant Lymphoproliferative Disease in Solid Organ Transplant Recipients: A Systematic Review. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2017;17(3):770–81. [DOI] [PubMed] [Google Scholar]
- 24.Ville S, Imbert-Marcille BM, Coste-Burel M, Garandeau C, Meurette A, Cantarovitch D, et al. Impact of antiviral prophylaxis in adults Epstein-Barr Virus-seronegative kidney recipients on early and late post-transplantation lymphoproliferative disorder onset: a retrospective cohort study. Transplant international : official journal of the European Society for Organ Transplantation 2018;31(5):484–94. [DOI] [PubMed] [Google Scholar]
- 25.Tanner JE, Alfieri C. The Epstein-Barr virus and post-transplant lymphoproliferative disease: interplay of immunosuppression, EBV, and the immune system in disease pathogenesis. Transpl Infect Dis 2001;3(2):60–9. [DOI] [PubMed] [Google Scholar]
- 26.Nourse JP, Jones K, Gandhi MK. Epstein-Barr Virus-related post-transplant lymphoproliferative disorders: pathogenetic insights for targeted therapy. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2011;11(5):888–95. [DOI] [PubMed] [Google Scholar]
- 27.Cohen JI. Epstein-Barr virus infection. N Engl J Med 2000;343(7):481–92. [DOI] [PubMed] [Google Scholar]
- 28.Thorley-Lawson DA. EBV Persistence--Introducing the Virus. Curr Top Microbiol Immunol 2015;390(Pt 1):151–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang MS, Kieff E. Epstein-Barr virus latent genes. Experimental & molecular medicine 2015;47(1):e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hislop AD, Kuo M, Drake-Lee AB, Akbar AN, Bergler W, Hammerschmitt N, et al. Tonsillar homing of Epstein-Barr virus-specific CD8+ T cells and the virus-host balance. The Journal of clinical investigation 2005;115(9):2546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O’Callaghan CA, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. The Journal of experimental medicine 1998;187(9):1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morscio J, Dierickx D, Ferreiro JF, Herreman A, Van Loo P, Bittoun E, et al. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013;13(5):1305–16. [DOI] [PubMed] [Google Scholar]
- 33.Saha A, Robertson ES. Mechanisms of B-Cell Oncogenesis Induced by Epstein-Barr Virus. J Virol 2019;93(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam N, Sugden B. CD40 and its viral mimic, LMP1: similar means to different ends. Cell Signal 2003;15(1):9–16. [DOI] [PubMed] [Google Scholar]
- 35.Thornburg NJ, Kulwichit W, Edwards RH, Shair KH, Bendt KM, Raab-Traub N. LMP1 signaling and activation of NF-kappaB in LMP1 transgenic mice. Oncogene 2006;25(2):288–97. [DOI] [PubMed] [Google Scholar]
- 36.Zhao B, Barrera LA, Ersing I, Willox B, Schmidt SC, Greenfeld H, et al. The NF-kappaB genomic landscape in lymphoblastoid B cells. Cell reports 2014;8(5):1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliopoulos AG, Blake SM, Floettmann JE, Rowe M, Young LS. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol 1999;73(2):1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gires O, Kohlhuber F, Kilger E, Baumann M, Kieser A, Kaiser C, et al. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. The EMBO journal 1999;18(11):3064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 1995;80(3):389–99. [DOI] [PubMed] [Google Scholar]
- 40.Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. The Journal of biological chemistry 2003;278(6):3694–704. [DOI] [PubMed] [Google Scholar]
- 41.Lambert SL, Martinez OM. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. Journal of immunology 2007;179(12):8225–34. [DOI] [PubMed] [Google Scholar]
- 42.Martinez OM, Krams SM. The Immune Response to Epstein Barr Virus and Implications for Posttransplant Lymphoproliferative Disorder. Transplantation 2017;101(9):2009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang B, Kracker S, Yasuda T, Casola S, Vanneman M, Homig-Holzel C, et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell 2012;148(4):739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dharnidharka VR, Ruzinova MB, Chen CC, Parameswaran P, O’Gorman H, Goss CW, et al. Metagenomic analysis of DNA viruses from posttransplant lymphoproliferative disorders. Cancer Med 2019;8(3):1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maloney EM, Busque VA, Hui ST, Toh J, Fernandez-Vina M, Krams SM, et al. Genomic variations in EBNA3C of EBV associate with posttransplant lymphoproliferative disorder. JCI Insight 2020;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, et al. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015. [DOI] [PMC free article] [PubMed]
- 47.Leblond V, Davi F, Charlotte F, Dorent R, Bitker MO, Sutton L, et al. Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1998;16(6):2052–9. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow SH, Webber SA, Chadburn A, Ferry JA. Immunodeficiency-associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. , editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 2. 4th ed. Lyon: International Agency for Research on Cancer; 2017. p. 453–62. [Google Scholar]
- 49.Swerdlow SH, Craig FE. Iatrogenic Immunodeficiency-Associated Lymphoproliferative Disorders. In: Jaffe ES, Harris NL, Vardiman JW, editors. Hematopathology: Elsevier Saunders; 2016. p. 1013–25. [Google Scholar]
- 50.Dotti G, Fiocchi R, Motta T, Facchinetti B, Chiodini B, Borleri GM, et al. Primary effusion lymphoma after heart transplantation: a new entity associated with human herpesvirus-8. Leukemia 1999;13(5):664–70. [DOI] [PubMed] [Google Scholar]
- 51.Matsushima AY, Strauchen JA, Lee G, Scigliano E, Hale EE, Weisse MT, et al. Posttransplantation plasmacytic proliferations related to Kaposi’s sarcoma-associated herpesvirus. The American journal of surgical pathology 1999;23(11):1393–400. [DOI] [PubMed] [Google Scholar]
- 52.Ferreiro JF, Morscio J, Dierickx D, Vandenberghe P, Gheysens O, Verhoef G, et al. EBV-Positive and EBV-Negative Posttransplant Diffuse Large B Cell Lymphomas Have Distinct Genomic and Transcriptomic Features. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2016;16(2):414–25. [DOI] [PubMed] [Google Scholar]
- 53.Craig FE, Johnson LR, Harvey SA, Nalesnik MA, Luo JH, Bhattacharya SD, et al. Gene expression profiling of Epstein-Barr virus-positive and -negative monomorphic B-cell posttransplant lymphoproliferative disorders. Diagn Mol Pathol 2007;16(3):158–68. [DOI] [PubMed] [Google Scholar]
- 54.Guney E, Lucas CG, Singh K, Pekmezci M, Fernandez-Pol S, Mirchia K, et al. Molecular profiling identifies at least 3 distinct types of posttransplant lymphoproliferative disorder involving the CNS. Blood Adv 2023;7(13):3307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplantation proceedings 1969;1(1):106–12. [PMC free article] [PubMed] [Google Scholar]
- 56.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103(1):275–82. [DOI] [PubMed] [Google Scholar]
- 57.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 2008;359(22):2313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Courville EL, Yohe S, Chou D, Nardi V, Lazaryan A, Thakral B, et al. EBV-negative monomorphic B-cell post-transplant lymphoproliferative disorders are pathologically distinct from EBV-positive cases and frequently contain TP53 mutations. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2016;29(10):1200–11. [DOI] [PubMed] [Google Scholar]
- 59.Campo E, Jaffe ES, Cook JR, Quintanilla-Martinez L, Swerdlow SH, Anderson KC, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood 2022;140(11):1229–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022;36(7):1720–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Natkunam Y, Goodlad JR, Chadburn A, de Jong D, Gratzinger D, Chan JK, et al. EBV-Positive B-Cell Proliferations of Varied Malignant Potential: 2015 SH/EAHP Workshop Report-Part 1. American journal of clinical pathology 2017;147(2):129–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Natkunam Y, Gratzinger D, Chadburn A, Goodlad JR, Chan JKC, Said J, et al. Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood 2018;132(18):1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Falini B, Martino G, Lazzi S. A comparison of the International Consensus and 5th World Health Organization classifications of mature B-cell lymphomas. Leukemia 2023;37(1):18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holman CJ, Karger AB, Mullan BD, Brundage RC, Balfour HH Jr. Quantitative Epstein-Barr virus shedding and its correlation with the risk of post-transplant lymphoproliferative disorder. Clin Transplant 2012;26(5):741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dharnidharka VR. Peripheral Blood Epstein-Barr Viral Nucleic Acid Surveillance as a Marker for Posttransplant Cancer Risk. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2017;17(3):611–6. [DOI] [PubMed] [Google Scholar]
- 66.Preiksaitis JK, Pang XL, Fox JD, Fenton JM, Caliendo AM, Miller GG. Interlaboratory comparison of epstein-barr virus viral load assays. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2009;9(2):269–79. [DOI] [PubMed] [Google Scholar]
- 67.Gartner B, Preiksaitis JK. EBV viral load detection in clinical virology. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2010;48(2):82–90. [DOI] [PubMed] [Google Scholar]
- 68.Rychert J, Danziger-Isakov L, Yen-Lieberman B, Storch G, Buller R, Sweet SC, et al. Multicenter comparison of laboratory performance in cytomegalovirus and Epstein-Barr virus viral load testing using international standards. Clin Transplant 2014;28(12):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho YU, Chi HS, Jang S, Park SH, Park CJ. Pattern analysis of Epstein-Barr virus viremia and its significance in the evaluation of organ transplant patients suspected of having posttransplant lymphoproliferative disorders. American journal of clinical pathology 2014;141(2):268–74. [DOI] [PubMed] [Google Scholar]
- 70.Rees L, Tizard EJ, Morgan AJ, Cubitt WD, Finerty S, Oyewole-Eletu TA, et al. A phase I trial of epstein-barr virus gp350 vaccine for children with chronic kidney disease awaiting transplantation. Transplantation 2009;88(8):1025–9. [DOI] [PubMed] [Google Scholar]
- 71.Opelz G, Daniel V, Naujokat C, Fickenscher H, Dohler B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol 2007;8(3):212–8. [DOI] [PubMed] [Google Scholar]
- 72.Green M, Michaels MG, Katz BZ, Burroughs M, Gerber D, Shneider BL, et al. CMV-IVIG for prevention of Epstein Barr virus disease and posttransplant lymphoproliferative disease in pediatric liver transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2006;6(8):1906–12. [DOI] [PubMed] [Google Scholar]
- 73.Hocker B, Bohm S, Fickenscher H, Kusters U, Schnitzler P, Pohl M, et al. (Val-)Ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation. Transplant international : official journal of the European Society for Organ Transplantation 2012;25(7):723–31. [DOI] [PubMed] [Google Scholar]
- 74.Martin SI, Dodson B, Wheeler C, Davis J, Pesavento T, Bumgardner GL. Monitoring infection with Epstein-Barr virus among seromismatch adult renal transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2011;11(5):1058–63. [DOI] [PubMed] [Google Scholar]
- 75.Walti LN, Mugglin C, Sidler D, Mombelli M, Manuel O, Hirsch HH, et al. Association of antiviral prophylaxis and rituximab use with posttransplant lymphoproliferative disorders (PTLDs): A nationwide cohort study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2021;21(7):2532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder( bigstar). Am J Transplant 2011;11(2):336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan K, Franke AJ, Skelton WPt, Bishnoi R, Shah C, Dang NH, et al. Reduction of immunosuppression for post-transplant lymphoproliferative disorder (PTLD): a single-center experience of allograft survival outcomes. Leuk Lymphoma 2021;62(5):1123–8. [DOI] [PubMed] [Google Scholar]
- 78.Oertel SH, Verschuuren E, Reinke P, Zeidler K, Papp-Vary M, Babel N, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2005;5(12):2901–6. [DOI] [PubMed] [Google Scholar]
- 79.Blaes AH, Peterson BA, Bartlett N, Dunn DL, Morrison VA. Rituximab therapy is effective for posttransplant lymphoproliferative disorders after solid organ transplantation: results of a phase II trial. Cancer 2005;104(8):1661–7. [DOI] [PubMed] [Google Scholar]
- 80.Choquet S, Leblond V, Herbrecht R, Socie G, Stoppa AM, Vandenberghe P, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood 2006;107(8):3053–7. [DOI] [PubMed] [Google Scholar]
- 81.Gonzalez-Barca E, Domingo-Domenech E, Capote FJ, Gomez-Codina J, Salar A, Bailen A, et al. Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica 2007;92(11):1489–94. [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez-Barca E, Capote FJ, Gomez-Codina J, Panizo C, Salar A, Sancho JM, et al. Long-term follow-up of a prospective phase 2 clinical trial of extended treatment with rituximab in patients with B cell post-transplant lymphoproliferative disease and validation in real world patients. Ann Hematol 2021;100(4):1023–9. [DOI] [PubMed] [Google Scholar]
- 83.Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, et al. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol 2017;35(5):536–43. [DOI] [PubMed] [Google Scholar]
- 84.DeStefano CB, Malkovska V, Rafei H, Shenoy A, Fitzpatrick K, Aggarwal A, et al. DA-EPOCH-R for post-transplant lymphoproliferative disorders. Eur J Haematol 2017;99(3):283–5. [DOI] [PubMed] [Google Scholar]
- 85.Rubinstein JD, Shah R, Breese EH, Burns KC, Mangino JL, Norris RE, et al. Treatment of posttransplant lymphoproliferative disorder with poor prognostic features in children and young adults: Short-course EPOCH regimens are safe and effective. Pediatr Blood Cancer 2021;68(8):e29126. [DOI] [PubMed] [Google Scholar]
- 86.Gross TG, Bucuvalas JC, Park JR, Greiner TC, Hinrich SH, Kaufman SS, et al. Low-dose chemotherapy for Epstein-Barr virus-positive post-transplantation lymphoproliferative disease in children after solid organ transplantation. J Clin Oncol 2005;23(27):6481–8. [DOI] [PubMed] [Google Scholar]
- 87.Gross TG, Orjuela MA, Perkins SL, Park JR, Lynch JC, Cairo MS, et al. Low-dose chemotherapy and rituximab for posttransplant lymphoproliferative disease (PTLD): a Children’s Oncology Group Report. Am J Transplant 2012;12(11):3069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maecker-Kolhoff B, Beier R, Zimmermann M, Schlegelberger B, Baumann U, Mueller C, et al. Response-Adapted Sequential Immuno-Chemotherapy of Post-Transplant Lymphoproliferative Disorders in Pediatric Solid Organ Transplant Recipients: Results from the Prospective Ped-PTLD 2005 Trial. Blood 2014;124:4468-. [Google Scholar]
- 89.Rosenberg AS, Klein AK, Ruthazer R, Evens AM. Hodgkin lymphoma post-transplant lymphoproliferative disorder: A comparative analysis of clinical characteristics, prognosis, and survival. American journal of hematology 2016;91(6):560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Twist CJ, Hiniker SM, Gratzinger D, Gutkin PM, Merriott DJ, Iagaru A, et al. Treatment and outcomes in classic Hodgkin lymphoma post-transplant lymphoproliferative disorder in children. Pediatr Blood Cancer 2019;66(8):e27803. [DOI] [PubMed] [Google Scholar]
- 91.Yang F, Li Y, Braylan R, Hunger SP, Yang LJ. Pediatric T-cell post-transplant lymphoproliferative disorder after solid organ transplantation. Pediatr Blood Cancer 2008;50(2):415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swerdlow SH. T-cell and NK-cell posttransplantation lymphoproliferative disorders. American journal of clinical pathology 2007;127(6):887–95. [DOI] [PubMed] [Google Scholar]
- 93.Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, et al. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2013;13(6):1512–22. [DOI] [PubMed] [Google Scholar]
- 94.Taj MM, Maecker-Kolhoff B, Ling R, Bomken S, Burkhardt B, Chiang AKS, et al. Primary post-transplant lymphoproliferative disorder of the central nervous system: characteristics, management and outcome in 25 paediatric patients. Br J Haematol 2021;193(6):1178–84. [DOI] [PubMed] [Google Scholar]
- 95.Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood 2006;108(9):2942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 2007;110(4):1123–31. [DOI] [PubMed] [Google Scholar]
- 97.Toner K, Bollard CM. EBV+ lymphoproliferative diseases: opportunities for leveraging EBV as a therapeutic target. Blood 2022;139(7):983–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prockop S, Doubrovina E, Suser S, Heller G, Barker J, Dahi P, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest 2020;130(2):733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Comoli P, Maccario R, Locatelli F, Valente U, Basso S, Garaventa A, et al. Treatment of EBV-related post-renal transplant lymphoproliferative disease with a tailored regimen including EBV-specific T cells. Am J Transplant 2005;5(6):1415–22. [DOI] [PubMed] [Google Scholar]
- 100.Mamlouk O, Nair R, Iyer SP, Edwards A, Neelapu SS, Steiner RE, et al. Safety of CAR T-cell therapy in kidney transplant recipients. Blood 2021;137(18):2558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J, Narendranath E, Ghobadi A, Lazaryan A, Ibrahim U, Jacobson C, et al. Real World Evidence (RWE) of Safety, Efficacy, and Outcomes of CD19 CAR-T Therapy in 20 Patients with Solid Organ Transplant (SOT)-Related Post-Transplant Lymphoproliferative Disorder (PTLD). Blood 2021;138(Supplement 1):3853-. [Google Scholar]
- 102.Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer 2019;7(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dierickx D, Tousseyn T, Requile A, Verscuren R, Sagaert X, Morscio J, et al. The accuracy of positron emission tomography in the detection of posttransplant lymphoproliferative disorder. Haematologica 2013;98(5):771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bakker NA, Pruim J, de Graaf W, van Son WJ, van der Jagt EJ, van Imhoff GW. PTLD visualization by FDG-PET: improved detection of extranodal localizations. Am J Transplant 2006;6(8):1984–5. [DOI] [PubMed] [Google Scholar]
- 106.Montes de Jesus FM, Kwee TC, Nijland M, Kahle XU, Huls G, Dierckx R, et al. Performance of advanced imaging modalities at diagnosis and treatment response evaluation of patients with post-transplant lymphoproliferative disorder: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;132:27–38. [DOI] [PubMed] [Google Scholar]
- 107.International Non-Hodgkin’s Lymphoma Prognostic Factors P. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993;329(14):987–94. [DOI] [PubMed] [Google Scholar]
- 108.Choquet S, Oertel S, LeBlond V, Riess H, Varoqueaux N, Dorken B, et al. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol 2007;86(8):599–607. [DOI] [PubMed] [Google Scholar]
- 109.Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol 2001;19(3):772–8. [DOI] [PubMed] [Google Scholar]
- 110.Ghobrial IM, Habermann TM, Maurer MJ, Geyer SM, Ristow KM, Larson TS, et al. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005;23(30):7574–82. [DOI] [PubMed] [Google Scholar]
- 111.Hourigan MJ, Doecke J, Mollee PN, Gill DS, Norris D, Johnson DW, et al. A new prognosticator for post-transplant lymphoproliferative disorders after renal transplantation. British journal of haematology 2008;141(6):904–7. [DOI] [PubMed] [Google Scholar]
- 112.Trappe RU, Choquet S, Dierickx D, Mollee P, Zaucha JM, Dreyling MH, et al. International prognostic index, type of transplant and response to rituximab are key parameters to tailor treatment in adults with CD20-positive B cell PTLD: clues from the PTLD-1 trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2015;15(4):1091–100. [DOI] [PubMed] [Google Scholar]
- 113.Maecker B, Jack T, Zimmermann M, Abdul-Khaliq H, Burdelski M, Fuchs A, et al. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol 2007;25(31):4902–8. [DOI] [PubMed] [Google Scholar]
- 114.Dharnidharka VR, Martz KL, Stablein DM, Benfield MR. Improved survival with recent Post-Transplant Lymphoproliferative Disorder (PTLD) in children with kidney transplants. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2011;11(4):751–8. [DOI] [PubMed] [Google Scholar]
- 115.Hocker B, Fickenscher H, Delecluse HJ, Bohm S, Kusters U, Schnitzler P, et al. Epidemiology and morbidity of Epstein-Barr virus infection in pediatric renal transplant recipients: a multicenter, prospective study. Clin Infect Dis 2013;56(1):84–92. [DOI] [PubMed] [Google Scholar]
- 116.Laurent A, Klich A, Roy P, Lina B, Kassai B, Bacchetta J, et al. Pediatric renal transplantation: A retrospective single-center study on epidemiology and morbidity due to EBV. Pediatr Transplant 2018;22(3):e13151. [DOI] [PubMed] [Google Scholar]
- 117.Bosse RC, Franke AJ, Paul Skelton Wt, Woody LE, Bishnoi R, Wang Y, et al. Post Transplant Lymphoproliferative Disorder risk factors in children: Analysis of a 23-year single-institutional experience. Pediatr Transplant 2020;24(5):e13747. [DOI] [PubMed] [Google Scholar]


