Key Clinical Message
VEXAS syndrome (vacuoles, E1 enzyme, X‐linked, autoinflammatory, somatic) is a novel autoinflammatory syndrome. We describe a case of VEXAS syndrome with upper airway and oral cavity involvement which are not well described in the literature.
Keywords: autoinflammation, Ludwigs angina, periorbital cellulitis, UBA1 mutation, venous thromboembolism, VEXAS syndrome
1. INTRODUCTION
First described in 2020, VEXAS syndrome (vacuoles, E1 enzyme, X‐linked, autoinflammatory, and somatic), is a disease of adulthood caused by somatic mutations in the ubiquitin‐like modifier activating enzyme 1 (UBA1) gene located on the X chromosome. 1 VEXAS syndrome is a progressive inflammatory syndrome, typically affecting males aged over 60 years. 2 Typical clinical features include fever, chondritis, pulmonary infiltrates, venous thromboembolism (VTE), and hematological abnormalities, however, clinical heterogeneity is common. 1 , 3 We describe an unusual case of VEXAS syndrome with predominantly head and neck manifestations.
2. CASE HISTORY
A Caucasian 78‐year‐old male presented (day 0) with left sided pre‐septal orbital cellulitis. His past medical history included hypertension and dyslipidaemia. He was treated with empiric antimicrobial therapy with good clinical response. Two further episodes were subsequently observed within 1 month (days 14–30). Magnetic resonance imaging (MRI) demonstrated extensive orbital cellulitis involving the medial rectus muscle. Orbital muscle biopsy showed evidence of organizing inflammation but no evidence of malignancy, vasculitis, or granulomas. Repeat antimicrobial treatment was commenced with apparent good clinical response.
Approximately 1 month later (day 75), the patient presented with new, rapid‐onset floor of mouth swelling and submandibular gland swelling. Flexible laryngoscopy demonstrated no critical airway edema. A diagnosis of Ludwig's angina was established but no source of infection was identified. The patient was treated with antimicrobial therapy and corticosteroids resulting in gradual symptom resolution.
3. METHODS (DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT)
The patient presented again (day 90) with a further episode of periorbital cellulitis. On this occasion, the patient reported mild odynophagia. Flexible laryngoscopy highlighted prominent supraglottic edema affecting the arytenoid cartilages bilaterally without other airway edema. The patient was again treated with antimicrobial therapy and corticosteroids. Laboratory investigations showed anemia with hemoglobin of 7.6 g/dL, mean corpuscular volume of 91.7 fL, and C‐reactive protein of 111 mg/L. Haematinics, serum protein electrophoresis, erythropoietin level, and reticulocyte count were within normal range. A bone marrow aspirate and trephine biopsy were performed (day 97) and morphologically were consistent with reactive change. Flow cytometry was negative for blasts, and karyotype was noted as normal.
The patient developed pyrexia (day 98) and was recommenced on antimicrobial therapy. Computed tomography (CT) of his neck (Figure 1A) was performed due to mild persistent neck stiffness and showed an asymmetric soft tissue prominence of the right parapharyngeal space. MRI demonstrated soft tissue thickening of the right palatine tonsil. Flexible laryngoscopy and clinical ENT examination were again performed which demonstrated no focal lesion. Interval CT imaging showed an increase in asymmetric soft tissue thickening of the right parapharyngeal space (Figure 1B). Panendoscopy was performed (day 133) but demonstrated diffuse edema in the parapharyngeal space only. Intraoperative parapharyngeal biopsy returned benign squamous epithelial cells. A significant improvement in parapharyngeal edema was observed with antimicrobial therapy and steroids, in keeping with earlier episodes.
FIGURE 1.
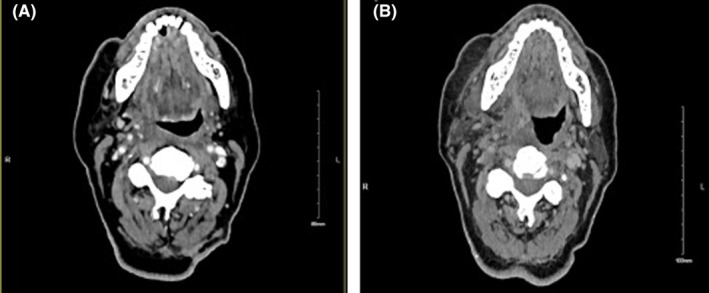
(A) Computed tomography (CT) showing asymmetrical soft tissue prominence extending from the right‐sided pharyngeal recess. (B) Interval computed tomography (CT) showing an increase in the asymmetric soft tissue thickening in the right pharyngeal mucosal space.
The patient suffered multiple thrombotic events during his hospital admissions and a diagnosis of thrombophlebitis migrans was made. He underwent extensive investigations in the pursuit of a unifying diagnosis, including CT thorax, abdomen and pelvis and MRI brain, which yielded no abnormalities. Positron emission tomography (PET) CT revealed no occult malignancy as a cause of a paraneoplastic phenomenon. A comprehensive infection and autoimmune screen was performed with all results negative.
4. CONCLUSION AND RESULTS (OUTCOME AND FOLLOW‐UP)
On day 134 after initial presentation, the results of a targeted next generation sequencing assay performed on his bone marrow became available, identifying a UBA1 mutation (p.Met41Val) at a variant allele frequency of 56% enabling a unifying diagnosis of VEXAS syndrome. No additional somatic myelodysplastic syndrome (MDS)‐defining mutations were identified. Retrospective review of the marrow morphology showed subtle vacuolation.
The patient was commenced on prednisolone and had a significant clinical and biochemical improvement. Ruxolitinib was commenced as a steroid sparing agent. At follow up review, the patient was doing well with a striking improvement in his anemia and inflammatory markers.
5. DISCUSSION
We describe an unusual case of VEXAS (vacuoles, E1 enzyme, X‐linked, autoinflammatory, and somatic) syndrome presenting with multifocal, relapsing head and neck inflammation. VEXAS syndrome was first described in 2020 by Beck et al. using a genotype‐driven approach. 1 It is a novel inflammatory syndrome caused by a somatic mutation in the UBA1 gene on the X chromosome.
VEXAS‐associated orbital and periorbital inflammation have been described in the literature, as well as pinna and nasal chondritis. 3 , 4 , 5 The ENT manifestations in this case (inflammation of the parapharyngeal space, arytenoids, floor of mouth and submandibular glands) are less commonly described. Oral cavity involvement has not been described in the literature and only limited cases refer to airway involvement. (Table 1).
TABLE 1.
Reported cases of VEXAS syndrome with airway involvement.
VEXAS syndrome is also associated with a broad spectrum of hematological findings such as cytoplasmic vacuoles in hematopoietic precursor cells in the bone marrow, high rates of VTE, macrocytic anemia, and an increased risk of hematological malignancy. 2 Our patient had relatively atypical hematological findings with normocytic anemia and only subtle vacuolation on bone marrow biopsy. However, he did have multiple episodes of thrombosis which is well described. 1 , 3
There are no standardized treatment guidelines for VEXAS syndrome at present but the initial approach of therapy is to control inflammatory symptoms with systemic corticosteroids. 2 They are highly effective but sustained courses have an unacceptable safety profile and the addition of steroid‐sparing agents is crucial. Our patient was commenced on ruxolitinib, a janus kinase inhibitor, which has demonstrated treatment efficacy in a series by Heiblig et al. 11 Hematopoietic stem cell transplantation is the only current treatment strategy with curative intent but it was not considered in our patient's case due to his advanced chronological age, lack of MDS and favorable response to initial therapy.
This case demonstrates the clinical heterogeneity of VEXAS syndrome and aims to raise awareness of this potential diagnosis when confronted with atypical head and neck inflammation.
AUTHOR CONTRIBUTIONS
Aoife Heeney: Writing – original draft; writing – review and editing. Rachael Wu: Writing – review and editing. Conall Fitzgerald: Writing – review and editing. Nina Orfali: Writing – review and editing. Nadim Akasheh: Writing – review and editing. Conor Magee: Supervision; writing – review and editing.
FUNDING INFORMATION
The authors have no sources of funding to declare.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
This study has been approved by the St James' Hospital Research and Innovation Office.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
I would like to thank all members of staff involved in the care of this patient.
Heeney A, Wu R, Fitzgerald C, Orfali N, Akasheh N, Magee C. VEXAS syndrome as a cause for multifocal, relapsing head and neck inflammation. Clin Case Rep. 2024;12:e9126. doi: 10.1002/ccr3.9126
DATA AVAILABILITY STATEMENT
Data are available on request.
REFERENCES
- 1. Beck DB, Ferrada MA, Sikora KA, et al. Somatic mutations in UBA1 and severe adult‐onset autoinflammatory disease. N Engl J Med. 2020;383(27):2628‐2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grayson PC, Patel BA, Young NS. VEXAS syndrome. Blood. 2021;137(26):3591‐3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Georgin‐Lavialle S, Terrier B, Guedon AF, et al. Further characterization of clinical and laboratory features in VEXAS syndrome: large‐scale analysis of a multicentre case series of 116 French patients. Br J Dermatol. 2022;186(3):564‐574. [DOI] [PubMed] [Google Scholar]
- 4. Beecher MB, Tong JY, Halliday LA, Hissaria P, Selva D. Recurrent orbital inflammation associated with VEXAS syndrome. Orbit. 2022;1‐4:350‐353. [DOI] [PubMed] [Google Scholar]
- 5. Martin‐Nares E, Vargas‐Serafin C, Delgado‐de la Mora J, et al. Orbital and periorbital inflammation in VEXAS syndrome. Scand J Rheumatol. 2022;51(4):338‐341. [DOI] [PubMed] [Google Scholar]
- 6. Beaumesnil S, Boucher S, Lavigne C, Urbanski G, Lacombe V. Ear, nose, throat, and bronchial involvements in VEXAS syndrome: specifying the Spectrum of clinical features. JAMA Otolaryngol Head Neck Surg. 2022;148(3):284‐286. [DOI] [PubMed] [Google Scholar]
- 7. Guerrero‐Bermudez CA, Cardona‐Cardona AF, Ariza‐Parra EJ, et al. Vacuoles, E1 enzyme, X‐linked, autoinflammatory, somatic syndrome (VEXAS syndrome) with prominent supraglottic larynx involvement: a case‐based review. Clin Rheumatol. 2022;41(11):3565‐3572. [DOI] [PubMed] [Google Scholar]
- 8. Islam S, Cullen T, Sumpton D, et al. VEXAS syndrome: lessons learnt from an early Australian case series. Intern Med J. 2022;52(4):658‐662. [DOI] [PubMed] [Google Scholar]
- 9. Tsuchida N, Kunishita Y, Uchiyama Y, et al. Pathogenic UBA1 variants associated with VEXAS syndrome in Japanese patients with relapsing polychondritis. Ann Rheum Dis. 2021;80(8):1057‐1061. [DOI] [PubMed] [Google Scholar]
- 10. Khitri MY, Guedon AF, Georgin‐Lavialle S, et al. Comparison between idiopathic and VEXAS‐relapsing polychondritis: analysis of a French case series of 95 patients. RMD Open. 2022;8(2):e002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heiblig M, Ferrada MA, Koster MJ, et al. Ruxolitinib is more effective than other JAK inhibitors to treat VEXAS syndrome: a retrospective multicenter study. Blood. 2022;140(8):927‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.


