Abstract
The hemagglutinin (HA) of fowl plague virus A/FPV/Rostock/34 (H7N1) carries two N-linked oligosaccharides attached to Asn123 and Asn149 in close vicinity to the receptor-binding pocket. In previous studies in which HA mutants lacking either one (mutants G1 and G2) or both (mutant G1,2) glycosylation sites had been expressed from a simian virus 40 vector, we showed that these glycans regulate receptor binding affinity (M. Ohuchi, R. Ohuchi, A. Feldmann, and H. D. Klenk, J. Virol. 71:8377–8384, 1997). We have now investigated the effect of these mutations on virus growth using recombinant viruses generated by an RNA polymerase I-based reverse genetics system. Two reassortants of influenza virus strain A/WSN/33 were used as helper viruses to obtain two series of HA mutant viruses differing only in the neuraminidase (NA). Studies using N1 NA viruses revealed that loss of the oligosaccharide from Asn149 (mutant G2) or loss of both oligosaccharides (mutant G1,2) has a pronounced effect on virus growth in MDCK cells. Growth of virus lacking both oligosaccharides from infected cells was retarded, and virus yields in the medium were decreased about 20-fold. Likewise, there was a reduction in plaque size that was distinct with G1,2 and less pronounced with G2. These effects could be attributed to a highly impaired release of mutant progeny viruses from host cells. In contrast, with recombinant viruses containing N2 NA, these restrictions were much less apparent. N1 recombinants showed lower neuraminidase activity than N2 recombinants, indicating that N2 NA is able to partly overrule the high-affinity binding of mutant HA to the receptor. These results demonstrate that N-glycans flanking the receptor-binding site of the HA molecule are potent regulators of influenza virus growth, with the glycan at Asn149 being dominant and that at Asn123 being less effective. In addition, we show here that HA and NA activities need to be highly balanced in order to allow productive influenza virus infection.
Influenza A and B viruses contain two spike glycoproteins, the hemagglutinin (HA) and the neuraminidase (NA). During the viral life cycle, both of these glycoproteins fulfill distinct functions in receptor binding and virus release which are crucial for establishing a productive infection (for a review, see reference 19). HA is the prototype of a class I transmembrane glycoprotein and is embedded in the viral membrane as a homotrimer (38). The HA monomer consists of a globular head region connected to a fibrous stalk domain (39). Both regions carry N-linked oligosaccharide side chains, with those attached to the stalk region being highly conserved and those at the tip of the molecule showing considerable variation in structure and number among different influenza A viruses. The tip of the globular region harbors the receptor-binding pocket, which mediates virus binding to sialic acid-containing receptors on the surface of host cells. N-glycans attached to the HA head domain in close proximity to this receptor-binding site have been suggested to modulate the receptor affinity (6, 10, 23). The HA of fowl plague virus (FPV; A/FPV/Rostock/34 [H7N1]) has two N-glycans flanking the receptor-binding pocket (17). They have been shown to significantly decrease the receptor-binding activity of transiently expressed FPV HA. HA mutants lacking either one or both of these glycans have an enhanced hemabsorbing activity as evidenced by an almost irreversible tight binding to erythrocytes (26).
NA is anchored in the viral envelope as a mushroom-shaped homotetramer with type II membrane topology (3). NA acts as a receptor-destroying enzyme by catalyzing the removal of sialic acids from viral and cellular components. NA activity has therefore been shown to promote the release of progeny viruses from host cells and to prevent virion aggregation (4, 11, 20, 28).
When different natural and laboratory-derived influenza viruses were analyzed for their HA and NA composition, it was striking to see that some combinations of antigenic subtypes occurred quite frequently while others were never detected (18, 32). In the latter case, the failure to produce stable high-yield reassortant strains with some HA and NA combinations has been attributed to an incompatibility between the opposing activities of these two glycoproteins leading to viral aggregation. The importance of a functional HA and NA match for productive infection was also suggested in a very recent study indicating that changes in HA receptor-binding activity occurring during adaptation to a new host are accompanied by concomitant changes in the NA sequence (22).
Since this concept of matching HA and NA combinations had so far only been deduced from analyses of naturally occurring viruses, our aim was to characterize the requirements for an effective interaction of HA and NA in more detail on the molecular level. To this end, we generated recombinant influenza viruses in which HA mutants lacking either one or both tip glycans (26) were combined with different NA subtypes and the growth properties of the resulting viruses were assayed in cell culture. For the production of these recombinant viruses, we used a recently described RNA polymerase (PolI)-based system (30, 40). We show that, as a consequence of the enhanced receptor affinity of the mutated HA, progeny virus release from host cells was significantly restricted, resulting in limited cell-to-cell spread. Therefore, the N-glycans at the tip of HA appear to be potent regulators of virus growth in cell culture. Interestingly, this effect was dependent on the nature of the accompanying viral NA. The high-activity N2-subtype NA was able to partly overcome increased binding of carbohydrate-deficient HA, while the low-activity N1-subtype NA was not. By employing specifically designed recombinant viruses, we provide direct experimental evidence that a functional balance of HA and NA is an important determinant of productive influenza virus infection.
MATERIALS AND METHODS
Cells and viruses.
Kidney cells from African green monkeys (CV1) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal calf serum (FCS) (Life Technologies GmbH, Karlsruhe, Germany), Madin-Darby bovine kidney (MDBK) cells were kept in DMEM with 4.5 g of glucose per liter supplemented with 10% FCS, and Madin Darby canine kidney cells (MDCK) were grown in minimal essential medium (MEM) containing 10% FCS. All cells were maintained at 37°C and 5% CO2.
Two reassortants of influenza viruses, A/WSN/33 (H1N1) and A/Hong Kong/8/68 (H3N2), were used. The reassortant WSN-HK (33) contains the N2-subtype NA gene of the Hong Kong virus and the residual genes of the WSN virus, and the reassortant HK-WSN (9) contains the H3-subtype HA gene of the Hong Kong virus and residual genes of the WSN virus. Both reassortants were amplified in 11-day-old embryonated chicken eggs.
Construction of plasmids.
The hemagglutinin gene of FPV (A/FPV/Rostock/34) (H7N1) was amplified from the pA11SVL3 vector (26) by PCR using the oligonucleotide GGCCGCTCTTCTATTAGTAGAAACAAGGGTG as a forward primer and the oligonucleotide GGCCGCTCTTCGGCCAGCAAAAGCAGGGGATACAAAATGAACACTCAAATCC as a reverse primer. Both oligonucleotides contained SapI restriction endonuclease recognition sites. PCR products were digested with SapI and ligated in the viral genome-sense orientation into the SapI sites of the PolI-SapI vector, resulting in the construct PolI-SapI/HA. The PolI-SapI vector (kindly provided by Adolfo Garcia-Sastre, New York, N.Y.) is a derivative of the pPolI-RT plasmid described earlier (30) and contains a truncated version of the human PolI promoter and a hepatitis virus delta ribozyme separated by SapI sites. Expression plasmids pHMG-PB1, pHMG-PB2, pHMG-PA, and pHMG-NP, encoding the proteins of the influenza virus polymerase complex under the control of a hydroxymethylglutaryl-coenzyme A reductase promoter were kindly provided by J. Pavlovic (University of Zürich, Zurich, Switzerland). The N-glycosylation sites in FPV HA at positions 123 and 149 were inactivated using the Quickchange mutagenesis kit (Stratagene, Amsterdam, The Netherlands) according to the manufacturer's protocol. Threonine-125 was exchanged for alanine using the oligonucleotide GGAATAAGGACCAACGGCGCCACTAGTGCATGTAGAAGATCAGG as a forward primer and the oligonucleotide CCTGATCTTCTACATGCACTAGTGGCGCCGTTGGTCCTTATTCC as a reverse primer to obtain the G1 mutant of FPV HA (construct PolI-SapI/G1). Primers contained a NarI restriction site to confer a genetic tag to the mutated HA sequence. Similarly, serine-151 was exchanged for glycine using the oligonucleotide CCTGTCAAATACAGACAATGCCGGCTTCCCACAAATGACAAAATCATA as a forward and the oligonucleotide GTATGATTTTGTCATTTGTGGGAAGCC-GGCATTGTCTGTATTTGAC AGG as a reverse primer, resulting in the G2 mutant of FPV HA (construct PolI-SapI/G2). These primers contained an NaeI genetic tag site. For generation of the double mutant G1,2 HA vector (construct PolI-SapI/G1,2), Quickchange mutagenesis was performed on the PolI-SapI/G1 plasmid with the latter primer pair. Thus, the G1,2 mutant HA sequence was modified to include both the NarI and NaeI genetic tag sites. To distinguish between HA from authentic FPV and plasmid-based wild-type FPV HA, the latter sequence was modified by the introduction of a PvuII site at position 1149 using the forward primer GGAGAAGGAACTGCAGCTGACTACAAAAGCACCCAATCGG and the reverse primer CCGATTGGGTGCTTTTGTAGTCAGCTGCAGTTCCTTCTCC in the Quickchange mutagenesis procedure.
Rescue of recombinant viruses.
CV1 cells were seeded in 6-cm dishes and grown to about 60 to 70% confluency. Cells were then transfected with plasmids pHMG-PB1 (1 μg), pHMG-PB2 (1 μg), pHMG-PA (1 μg), and pHMG-NP (2 μg) to express the influenza viral polymerase complex and with the PolI-SapI plasmid encoding the respective versions of the FPV HA sequence (5 μg). Transfection was performed using the Superfect transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer's instructions. At 36 h after transfection, the cells were infected with either the WSN-HK (H1N2) or the HK-WSN (H3N1) influenza virus reassortants at a multiplicity of infection (MOI) of 2. Progeny viruses were harvested at 18 h postinfection.
In infections with the HK-WSN helper virus, CV1 cells were treated with 10 mU of Vibrio cholerae neuraminidase (VCNA; Behring, Marburg, Germany) per ml of cell culture medium for 1 h at 37°C prior to virus harvest. Selection for recombinant viruses was then done using a specific neutralizing anti-H3-HA serum. To this end, progeny viruses from rescue experiments were adsorbed to MDBK cell monolayers for 1 h on ice, cells were washed two times with ice-cold PBS++ (135 mM NaCl, 2.5 mM KCl, 6.5 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM CaCl2, 0.5 mM MgCl2 [pH 7.2]) and grown in FCS-free DMEM supplemented with 0.2% bovine serum albumin (BSA; ICN, Aurora, Ill.) containing 0.05% serum directed against the X31 strain of influenza virus (kindly provided by Peter Palese, New York, N.Y.). When the reassortant WSN-HK was used as a helper virus, selection was achieved by passaging rescue supernatants on MDBK cell monolayers in the absence of trypsin. Infected MDBK cell monolayers were then monitored for the appearance of liquid plaques for the next few days. Recombinant viruses were purified by three plaque passages on MDBK cells under selection conditions. For the production of virus stock solutions, recombinant viruses were amplified in MDBK cell monolayers seeded in 10-cm dishes.
Genotypic characterization of recombinant viruses.
Plaque-purified recombinant viruses were used for the infection of MDCK cells seeded in 6-cm dishes. At 2 to 3 days postinfection, supernatants were collected and cleared of cellular debris by centrifugation at 2,000 × g. Viruses were subsequently pelleted from the supernatants by ultracentrifugation at 100,000 × g for 30 min. RNA was isolated from the virus pellet in a final volume of 50 μl of highly purified water by means of the High Pure RNA isolation kit (Roche Molecular Biochemicals, Mannheim, Germany) following the manufacturer's instructions. The isolated viral RNA (10 μl) was then subjected to reverse transcription (RT)-PCR employing the Titan One Tube RT-PCR system, supplied by Roche Molecular Biochemicals. The primers used in this procedure were GGCCAGTCCGGACGGATTGATTTTC (forward) and ATAGTGCACCGCATGTTTCCG (reverse) for wild-type (WT) plasmid-derived HA and GTATCAAATGGACCAAAGTAAAC (forward) and CGCAATTGGCATCAACCTGCACATCGC (reverse) for glycosylation-mutant forms of FPV HA. RT-PCR products were digested with PvuII (WT-HA), NarI (G1 mutant), NaeI (G2 mutant), or NarI and NaeI (G1,2 mutant), and cleavage products were examined by electrophoresis in a 1.4% agarose gel.
Phenotypic characterization of recombinant viruses.
MDCK cells (2 × 106) were infected with recombinant viruses at an MOI of 2. At 8 h after infection, the cells were washed with phosphate-buffered saline (PBS), and 20 μCi of Redivue Pro-mix l35S in vitro cell labeling mix (Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany) was added in 2 ml of MEM lacking methionine and cysteine. After 12 h, radioactively labeled viruses were pelleted from the supernatants. Viruses were lysed in 500 μl of radioimmunoprecipitation buffer (150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1% deoxycholate, 10 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 mM iodoacetamide, 5,000 U of aprotinin, 20 mM Tris-HCl [pH 8.8]). FPV HA was immunoprecipitated from the lysate by adding a monoclonal FPV HA-specific antibody (1:250) and 30 μg of protein A-Sepharose (Sigma, Deisenhofen, Germany). One half of the precipitated HA was digested for 6 h with 500 U of peptide:N-glycosidase (PNGase) F (New England Biolabs Inc., Schwalbach, Germany), while the other half remained untreated. Samples were run on SDS–10% polyacrylamide gel electrophoresis (PAGE), and HA bands were visualized by fluorography.
Analysis of virus growth.
For growth curves, MDCK cell monolayers were infected with recombinant viruses at an MOI of 0.001 in PBS containing 0.2% BSA for 1 h. Unbound viruses were washed away with PBS–0.2% BSA, and serum-free MEM–0.2% BSA was added. Cells were incubated at 37°C under 5% CO2. From then on, HA titers in the supernatant were periodically monitored with chicken red blood cells (1% in saline).
For plaque assays, confluent MDCK cell monolayers in 6-cm dishes were infected with 10-fold dilutions of recombinant viruses in a total volume of 1 ml of PBS–0.2% BSA for 1 h. Cells were washed with PBS–0.2% BSA and covered with an overlay of MEM containing 0.5% purified agar (Oxoid Ltd, Basingstoke, Hampshire, England), 0.02% BSA, and 0.001% DEAE-dextran. Cells were incubated at 37°C under 5% CO2, and plaques were stained 3 days postinfection with 0.1% crystal violet in a 10% formaldehyde solution.
Determination of viral NA activity.
The total protein content of viruses was determined using the BCA protein assay reagent (Pierce, Rockford, Ill.) following the supplier's instructions, with BSA serving as an internal standard. NA activity was measured with 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (MU-NANA) (Sigma) as a substrate (36). Defined amounts of viral proteins were diluted in 100 μl of 0.1 M sodium acetate buffer (pH 5.5). These mixtures were then incubated with 10 μl of 1 mM MU-NANA for 20 min at 37°C. The reactions were stopped by adding 1 ml of stop buffer (133 mM glycine, 60 mM NaCl, 40 mM Na2CO3 [pH 10.0]). The fluorescence of the released chromophore 4-methylumbelliferone was determined with a Perkin-Elmer luminescence spectrometer (λexc = 365 nm, λem = 450 nm) and was taken as a measure of the viral NA activity.
For the analysis of HA and NA incorporation, viruses were grown in MDCK cells in the presence of 50 μCi of d-[6-3H]glucosamine (Amersham Pharmacia) for 20 h. Viruses were pelleted from the culture medium by centrifugation at 100,000 × g and applied to SDS-PAGE on a 10% gel. Protein bands were visualized by fluorography and excised from the gel. Radioactivity incorporated in HA and NA bands was measured by liquid scintillation counting.
Virus elution from chicken erythrocytes.
Virus stocks were diluted serially in PBS, and 50-μl aliquots of these dilutions were incubated with 50 μl of chicken erythrocytes (1% in saline) on ice for 1 h in V-bottomed microtiter plates. Thereafter, the plates were transferred to 37°C, and the precipitation of agglutinated erythrocytes was monitored periodically for the next 24 h.
Release of progeny viruses from host cells.
Confluent MDCK cell monolayers were infected with recombinant viruses at an MOI of 5. At 12 h postinfection, virus titers in the culture medium were examined by plaque assay on MDCK cells as described above. In parallel, MDCK cells equally infected for 12 h were treated with 25 mU of VCNA per ml of medium for 1 h at 37°C in order to release all budded viruses from the cell surface. Again, virus titers in the culture medium were determined in a plaque assay on MDCK cells. Infections for plaque tests were done on ice to inhibit VCNA activity. Virus titers obtained in the presence of VCNA were regarded as the maximum virus yield and were therefore set at 100%.
RESULTS
Generation of recombinant viruses differing in HA glycosylation and neuraminidase subtypes.
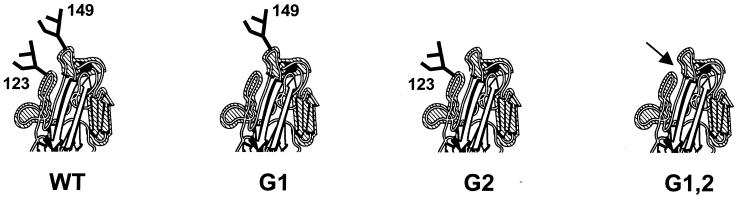
To investigate the possible effects of the oligosaccharides at Asn123 and Asn149 of FPV HA on the growth of intact viruses, glycan attachment sites were abolished from the HA sequence either individually or simultaneously by site-directed mutagenesis (Fig. 1). The HA wild-type and mutated cDNAs were placed in the genomic (antisense) orientation under the transcriptional control of a truncated version of the human PolI promoter so as to ensure the generation of a precise 5′ end of the transcript (25, 40). The correct 3′ end was brought about by a hepatitis virus delta ribozyme sequence included in the PolI-HA plasmid. For analytical reasons (see below), endonuclease restriction motifs were introduced as tag sites in the HA cDNAs. A PvuII site was added at position 1150 to the WT-HA cDNA. HA mutants G1 and G2 were modified by the introduction of a novel NarI site at position 445 and a novel NaeI site at position 523, respectively. Correspondingly, HA mutant G1,2 bears both artificial NarI and NaeI sites (Fig. 2A). In transfected CV1 cells, intranuclear PolI-based transcription produces a virus-like HA RNA gene that is encapsidated and amplified by the proteins of the influenza virus polymerase complex (PB1, PB2, PA, and NP) translated from expression plasmids which had been cotransfected along with the PolI-HA vector (30).
FIG. 1.
Head region of FPV HA. N-linked oligosaccharides adjacent to the receptor-binding pocket are indicated. Mutants G1 and G2 lack the glycosylation sites at Asn123 and Asn149, respectively. Both sites are absent in mutant G1,2. The arrow marks the entrance to the receptor-binding pocket.
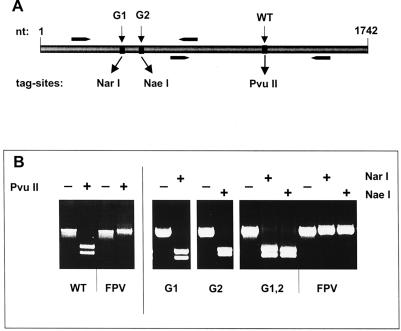
FIG. 2.
Restriction analysis of the HA cDNA derived from recombinant viruses. (A) Scheme of the HA cDNA used for the generation of recombinants. Positions of endonuclease recognition motifs introduced as genetic tag sites for individual mutants are indicated. The binding sites of specific oligonucleotide primers used in RT-PCR are indicated by arrows. nt, nucleotide. (B) RT-PCR analysis of RNA isolated from wild-type (WT), G1, G2, and G1,2 recombinant viruses of the N1 series. RT-PCR products were incubated with endonucleases as indicated and separated on an agarose gel. RNA isolated from FPV was used as a control.
To obtain recombinant viruses, CV1 cells were then infected with helper viruses. Two different reassortants of strains A/WSN/33 (H1N1) (WSN) and A/Hong Kong/8/68 (H3N2) (HK) were chosen for this purpose. The HK-WSN (H3N1) reassortant has all WSN genes except for the HA gene of subtype H3, which stems from the Hong Kong strain. Recombinants created with this reassortant as helper virus therefore carried the FPV HA gene within the context of the residual WSN genes. Selection for recombinants was achieved by passaging supernatants from rescue experiments on MDBK cells in the presence of a neutralizing anti-H3-HA serum, which specifically blocked the growth of the HK-WSN helper while leaving the recombinant FPV HA viruses unaffected.
The second helper virus used, WSN-HK (H1N2), contains all the WSN virus genes with the exception of the N2-subtype NA gene, which is derived from the Hong Kong virus. Tissue culture supernatants obtained with this reassortant as a helper virus were passaged in MDBK cells in the absence of trypsin. Because FPV HA is cleaved by the cellular protease furin (34), only the recombinant virus propagated under these conditions, whereas helper virus growth was inhibited in the absence of trypsin. The procedure described represents a novel system for the efficient selection of recombinant influenza viruses, taking advantage of specific functional properties of the FPV HA.
Following this approach, we rescued an N1 series and an N2 series of recombinant viruses, both carrying the glycosylation mutants of FPV HA within the WSN background: FPV/N1 recombinant viruses were derived from the HK-WSN helper, while FPV/N2 recombinants were derived from the WSN-HK helper. Rescued viruses were purified by three plaque passages on MDBK cells.
Molecular characterization of recombinant viruses.
The recombinant identity of the viruses obtained in rescue experiments was confirmed by RT-PCR analysis of viral RNA. To this end, isolated viral RNA was used as a template for RT-PCR with two sets of HA-specific primers encompassing the introduced genetic tag sites mentioned above (Fig. 2). RT-PCR products were subjected to restriction analysis with the respective endonucleases and analyzed by agarose gel electrophoresis. With RNA of the WT/N1 recombinant virus, RT-PCR yielded a product of about 780 nucleotides which was sensitive to PvuII treatment, whereas no cleavage occurred when FPV RNA was applied. The presence of the genetic tag sites was also confirmed in the recombinants containing HA mutants. RT-PCR products obtained from the G1/N1 virus were cleaved by NarI, those from the G2/N1 virus were cleaved by NaeI, and those from the G1,2/N1 mutant were sensitive to both NarI and NaeI. Again, no sensitivity to these enzymes was seen with RT-PCR products generated from FPV RNA. Identical results were obtained when viruses of the N2 series were assayed (data not shown). Thus, restriction analysis clearly revealed that the plasmid-based mutated FPV HA genes had been stably integrated into the rescued viruses.
Next, it was important to prove that N-glycans are in fact missing from the HA protein of the respective virus mutants. For this reason, HA was isolated by immunoprecipitation from radioactively labeled virus produced in MDCK cells. When examined by SDS-PAGE, the HA1 subunits from the G1 and G2 mutant viruses showed a reduced molecular mass compared to wild-type HA1. A larger reduction was observed with the G1,2 recombinant. PNGase F treatment confirmed that the differences in molecular weight resulted from loss of oligosaccharides (Fig. 3; data are shown for N2 recombinants only but were identical for viruses of the N1 series).
FIG. 3.
Analysis of the glycosylation pattern of HA from recombinant viruses. HA was immunoprecipitated from 35S-labeled viruses of the N2 group. One half of the material was treated with PNGase F (+), while the other half remained untreated (−). The protein profile was analyzed by SDS-PAGE, and bands were visualized by fluorography.
Growth of recombinant viruses in cell culture.
In view of the results obtained by solitary expression of HA glycosylation mutants in CV1 cells described above (26), it was of great interest to see if the lack of the tip glycans had an effect when the mutated HA constitutes an integral part of intact virions. MDCK cells were therefore inoculated at a low MOI with recombinant viruses, and the emergence of progeny viruses was monitored. Within the N2 series, wild-type as well as G1 and G2 viruses grew equally well on MDCK cells (Fig. 4). Only growth of the G1,2 mutant was moderately affected. However, with viruses of the N1 group, the loss of oligosaccharide side chains from the HA tip had striking effects on virus growth depending on the position and number of the deleted glycans. Titers obtained with the G2 mutant were reduced about fourfold compared to those reached by the WT/N1 and G1/N1 viruses. With a more than 20-fold reduction in virus titers and a delayed onset of virion release (see also Fig. 8), the G1,2/N1 virus exhibited a highly attenuated phenotype in MDCK cells.
FIG. 4.
Growth curves of recombinants in MDCK cells. Cell monolayers were infected at an MOI of 0.001 with recombinant viruses, and supernatants were monitored for HA titers at the time points indicated. (A) Viruses of the N2 series. (B) Viruses of the N1 series. ■, wild type; ▴, G1; ⧫, G2; ●, G1,2.
FIG. 8.
Release of recombinant viruses from MDCK cells. MDCK cells were infected at an MOI of 5 with recombinant viruses and incubated at 37°C overnight. One hour before virus harvest, VCNA was added to the culture medium of one half of the samples. Titers of progeny viruses released into the medium were determined by plaque assay. Levels of virus release in the absence of VCNA are presented as a percentage of the virus titers released after VCNA treatment.
The studies on the growth characteristics were extended by performing plaque assays on MDCK monolayers to track cell-to-cell spread of mutant viruses (Fig. 5). Results obtained by this approach closely corresponded to those described above for the growth curves. Within the N2 group, only the spread of the G1,2 mutant was inhibited. Yet again, there was a marked reduction in plaque size within the N1 group which was very distinct with the G1,2 mutant and less pronounced with the G2 mutant, demonstrating impaired cell-to-cell spread of these viruses.
FIG. 5.
Analysis of cell-to-cell spread of recombinant viruses by plaque assay. MDCK cell monolayers were infected with wild-type viruses (WT) or recombinant viruses of the N1 NA and N2 NA groups as indicated. Monolayers were covered with an agarose-containing overlay for 3 days and stained with crystal violet. Dilutions were chosen individually for each virus stock in order to obtain optimal plaque pictures.
These results indicate that N-glycans at the HA tip promote influenza virus replication in cell culture. Moreover, we could show that NA as well has a crucial impact on virus growth. This is evident from the finding that only the N2 subtype, but not N1 NA, was capable of abrogating the downregulating effects of missing N-glycans.
Release of recombinant viruses from receptors.
The data described so far suggested that the N1 series of recombinants differs from the N2 series in the efficiency of release from receptors. We therefore first compared the neuraminidase activities of recombinants WT/N1 and WT/N2 with MU-NANA as the substrate. The results shown in Fig. 6 indicate that neuraminidase activity is about six times higher in the N2 recombinant than in the N1 recombinant. In parallel, the HA and NA content of recombinant viruses was assayed by applying radioactively labeled virions to SDS-PAGE (Fig. 6). Liquid scintillation counting of excised protein bands revealed that the ratio of HA to NA in both virus types was about 6:1. The observed differences in NA activity are therefore not due to varying incorporation of NA molecules into virions but obviously reflect the weaker enzymatic activity of N1 NA.
FIG. 6.
Comparison of specific NA activities of WT/N1 and WT/N2 viruses. (A) Different amounts of purified virus were incubated with MU-NANA for 20 min at 37°C. The reaction was stopped, and NA activity was calculated by measuring the fluorescence of the liberated methylumbelliferone. The data are means of three experiments. They indicate that WT/N2 has a higher NA activity than WT/N1. (B) Analysis of equal amounts of purified WT/N1 (N1) and WT/N2 (N2) virions labeled with [3H]glucosamine by SDS-PAGE under nonreducing conditions. HA and NA bands were excised from the gel, and incorporated radioactivity was determined by liquid scintillation counting. The data show that both virus preparations contain equal amounts of HA and NA.
We then analyzed elution of viruses from erythrocytes. Viruses were adsorbed to chicken erythrocytes at 4°C, and release at 37°C was subsequently monitored (Fig. 7). Wild-type and G1 viruses of the N1 series eluted quickly from erythrocytes, with elution being complete after 2 h. However, the G2/N1 virus eluted to only about 50% even after 6 h of incubation at 37°C, and elution of the G1,2/N1 virus was totally blocked. This clearly demonstrated that the NA activity of N1 viruses was too weak to overcome the high receptor affinity of the G2 and G1,2 mutant HA. By contrast, NA activity in N2 viruses was able to elute wild-type, G1, and G2 viruses quantitatively from the erythrocytes within about an hour and only failed to fully release G1,2 viruses.
FIG. 7.
Virus elution from chicken erythrocytes. Twofold dilutions of recombinant viruses of the N2 NA group (A) and the N1 NA group (B) were incubated with equal volumes of chicken erythrocytes at 4°C for 1 h. Samples were then transferred to 37°C, and the reduction in HA titers was recorded periodically. Results are presented as percentages of the initial HA titer at 4°C. ■, wild type; ▴, G1; ⧫, G2; ●, G1,2.
Incomplete release of progeny viruses from infected cells might be overcome by VCNA treatment. Therefore, MDCK cells were infected with recombinant viruses at an MOI of 5. Before virus harvest, cells were treated with VCNA to quantitatively elute virus from the cell surface. Virus titers were determined by plaque assay on MDCK cells. Compared to virus release in the presence of VCNA, G2/N1 and G1,2/N1 were released in the absence of VCNA to only about 22 and 6%, respectively (Fig. 8). Release of G1/N1 was only moderately affected. Within the N2 NA group, elution of G1,2 closely resembled that of G2/N1, while the other members of this group were not significantly restricted in their release from host cells. By adding VCNA to the overlay of plaque assays with G2/N1 and G1,2/N1 viruses, it was possible to overcome the size restrictions described above (data not shown). This clearly indicated that VCNA was able to promote the spread and multicycle growth of these viruses.
DISCUSSION
HA-mediated attachment of influenza viruses to sialic acid-containing receptors on the host cell surface is the initial step in infection. Influenza virus HA contains at the tip a narrow crevice lined with highly conserved amino acids. By its ability to specifically bind sialic acids, this crevice has been identified as the receptor-binding site (7, 31, 37). The precise structure of this HA domain is known to be of crucial importance for the process of virus binding to its receptor. Accordingly, single-amino-acid substitutions in the binding pocket can result in altered receptor-binding specificity and altered host range of the viruses (1, 5, 35). Furthermore, in our previous study employing vector-expressed FPV HA, we could show that oligosaccharides flanking the binding site modulate receptor affinity (26). The aim of the present study was to evaluate the impact of each individual N-glycan at the FPV HA tip on the growth of intact viruses. To address this question, we generated recombinant influenza viruses containing the oligosaccharide-deleted HA mutants. Our studies demonstrate that the glycans flanking the receptor-binding pocket are potent regulators of virus growth in cell culture. The oligosaccharide attached to Asn149 (absent in mutant G2) plays a dominant role in controlling virus spread, while that attached to Asn123 (absent in mutant G1) is less effective. Growth of viruses lacking both N-glycans was found to be reduced in cell culture due to restricted release of progeny viruses from infected cells. These findings on the growth of recombinant viruses are an important extension of our previous work investigating the receptor interaction of transiently expressed HA. The results presented here provide experimental evidence for a distinct regulatory function of individual N-glycans located at the HA tip in the viral life cycle. By sequentially removing N-glycans from the vicinity of the HA receptor-binding site, we have delineated a novel approach to specifically generating influenza viruses with gradually greater degrees of attenuation in cell culture.
By removing terminal sialic acid residues from oligosaccharide side chains of glycoconjugates, the viral NA acts as a receptor-destroying enzyme in influenza viruses (3, 19). When NA activity was blocked by either antibodies (4), inhibitors (12, 27), or temperature-sensitive mutations (28), formation of large viral aggregates on the surface of infected cells was observed, as with virus lacking NA either partly (24) or completely (20). Accordingly, viral NA is regarded as an important factor for the release of progeny virus from host cells, promoting the efficient progression of an infection. In light of this, it was of special interest to examine how different NA subtypes affect the attenuated phenotype of recombinant viruses lacking N-glycans at the HA tip. Several N1 NAs have a deletion in the stalk region that is most extensive with FPV NA (14). NA enzymatic activity has been reported to vary according to the length of the stalk region of the molecule, with NA species containing a deletion in the stalk having lower activity (2, 8, 21, 22). By choosing appropriate helper viruses, we generated recombinants in which the HA mutants were combined with either the WSN virus NA (N1 subtype) containing a stalk deletion or the Hong Kong virus NA (N2 subtype) that has no deletion. When assayed for neuraminidase activity, recombinant viruses carrying N2 NA exceeded those with N1 NA at least sixfold. Thus, our set of recombinants was ideally suited to analyze in depth the impact of different NA activities on the growth of mutant influenza viruses specifically designed to show distinct receptor-binding activities. Using this system, we were able to demonstrate that the growth behavior of HA mutant viruses is governed by the nature of the accompanying viral NA.
Among the viruses with high-activity N2 NA, growth restriction was observed only when the G1,2 mutant was present, showing the highest receptor affinity, while recombinants containing G1 and G2 grew essentially like virus carrying wild-type HA. Yet the situation was different with viruses containing the low-activity N1 NA. Here, the growth of G1,2 mutant viruses was significantly impeded in cell culture due to restricted release from host cells. This effect was less pronounced with G2 mutant viruses but still evident. Obviously, unlike N2 NA, the lower-activity N1 NA is not able to overcome the high-affinity interaction of G1,2 and G2 HA with its receptor.
Hence, our data clearly point out that, for the establishment of productive infection, influenza viruses are strictly dependent on a highly balanced action of HA and NA. An increase in receptor-binding affinity apparently needs to be accompanied by a concomitant increase in the receptor-destroying activity of the viral NA. Otherwise, the enhanced receptor binding is a serious disadvantage in the late stage of infection because it prevents the release of progeny viruses from host cells. The need for such a match of HA and NA activities had so far only been deduced from studies analyzing natural virus isolates or laboratory-generated reassortants (15, 16, 22, 32). Taken together, our data represent the first concise study of the functional interrelationship of distinct HA and NA species and provide experimental evidence for the strict requirement of a fine tuning of HA receptor-binding and NA receptor-destroying activity in order to allow efficient influenza virus propagation (Fig. 9).
FIG. 9.
Regulation of virus binding and release by HA glycosylation and neuraminidase activity. Receptor affinity is controlled by the oligosaccharides adjacent to the receptor-binding site on HA. The efficiency of release depends on the activity of NA.
There is evidence that the N-glycans flanking the receptor-binding site not only modulate receptor affinity but also control receptor specificity. Thus, subtype H1 influenza virus strains with an oligosaccharide in such a position have been shown to bind preferentially to α2,3-linked neuraminic acid, whereas mutants lacking this oligosaccharide had a preference for the α2,6 linkage (10, 13). Furthermore, it has been shown recently that glycans carrying neuraminic acid in α2,3 or α2,6 linkages gain access to the receptor-binding pocket from opposite sides (7). Steric hindrance by a glycan adjacent to the receptor-binding site may therefore be a determinant of receptor specificity. Finally, the number and structure of N-glycans neighboring the receptor-binding pocket have been suggested to determine the host range and pathogenicity of influenza viruses (6, 10, 29). In view of these findings, it will now be interesting to employ our panel of recombinant viruses to elucidate the contributions of individual HA tip glycans to tissue tropism and host range.
ACKNOWLEDGMENTS
We are grateful to Peter Palese and Adolfo Garcia-Sastre for kindly providing the anti-H3-HA serum, the reassortant helper viruses, and the PolI-SapI vector.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 286) and from the Fonds der Chemischen Industrie. T.W. was a recipient of a fellowship of the Deutsches Krebsforschungszentrum (Infektionsforschung, AIDS-Stipendienprogramm).
REFERENCES
- 1.Aytay S, Schulze I T. Single amino acid substitutions in the hemagglutinin can alter the host range and receptor binding properties of H1 strains of influenza A virus. J Virol. 1991;65:3022–3028. doi: 10.1128/jvi.65.6.3022-3028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castrucci M R, Kawaoka Y. Biologic importance of neuraminidase stalk length in influenza A virus. J Virol. 1993;67:759–764. doi: 10.1128/jvi.67.2.759-764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colman P. Structure and function of the neuraminidase. In: Nicholson K G, Webster R G, Hay A J, editors. Textbook of influenza. London, England: Blackwell Science; 1998. pp. 65–73. [Google Scholar]
- 4.Compans R W, Dimmock N J, Meier-Ewert H. Effect of antibody to neuraminidase on the maturation and hemagglutinating activity of an influenza A2 virus. J Virol. 1969;4:528–534. doi: 10.1128/jvi.4.4.528-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor R J, Kawaoka Y, Webster R G, Paulson J C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 6.Deom C M, Caton A J, Schulze I T. Host cell-mediated selection of a mutant influenza A virus that has lost a complex oligosaccharide from the tip of the hemagglutinin. Proc Natl Acad Sci USA. 1986;83:3771–3775. doi: 10.1073/pnas.83.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisen M B, Sabesan S, Skehel J J, Wiley D C. Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallography. Virology. 1997;232:19–31. doi: 10.1006/viro.1997.8526. [DOI] [PubMed] [Google Scholar]
- 8.Els M C, Air G M, Murti K G, Webster R G, Laver W G. An 18-amino acid deletion in an influenza neuraminidase. Virology. 1985;142:241–247. doi: 10.1016/0042-6822(85)90332-0. [DOI] [PubMed] [Google Scholar]
- 9.Enami M, Palese P. High-efficiency formation of influenza virus transfectants. J Virol. 1991;65:2711–2713. doi: 10.1128/jvi.65.5.2711-2713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gambaryan A S, Marinina V P, Tuzikov A B, Bovin N V, Rudneva I A, Sinitsyn B V, Shilov A A, Matrosovich M N. Effects of host-dependent glycosylation of hemagglutinin on receptor-binding properties on H1N1 human influenza A virus grown in MDCK cells and in embryonated eggs. Virology. 1998;247:170–177. doi: 10.1006/viro.1998.9224. [DOI] [PubMed] [Google Scholar]
- 11.Griffin J A, Basak S, Compans R W. Effects of hexose starvation and the role of sialic acid in influenza virus release. Virology. 1983;125:324–334. doi: 10.1016/0042-6822(83)90205-2. [DOI] [PubMed] [Google Scholar]
- 12.Gubareva L V, Bethell R, Hart G J, Murti K G, Penn C R, Webster R G. Characterization of mutants of influenza A virus selected with the neuraminidase inhibitor 4-guanidino-Neu5Ac2en. J Virol. 1996;70:1818–1827. doi: 10.1128/jvi.70.3.1818-1827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Günther I, Glatthaar B, Doller G, Garten W. A H1 hemagglutinin of a human influenza A virus with a carbohydrate-modulated receptor binding site and an unusual cleavage site. Virus Res. 1993;27:147–160. doi: 10.1016/0168-1702(93)90078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausmann J, Kretzschmar E, Garten W, Klenk H D. Biosynthesis, intracellular transport and enzymatic activity of an avian influenza A virus neuraminidase: role of unpaired cysteines and individual oligosaccharides. J Gen Virol. 1997;78:3233–3245. doi: 10.1099/0022-1317-78-12-3233. [DOI] [PubMed] [Google Scholar]
- 15.Kaverin N V, Gambaryan A S, Bovin N V, Rudneva I A, Shilov A A, Khodova O M, Varich N L, Sinitsin B V, Makarova N V, Kropotkina E A. Postreassortment changes in influenza A virus hemagglutinin restoring HA-NA functional match. Virology. 1998;244:315–321. doi: 10.1006/viro.1998.9119. [DOI] [PubMed] [Google Scholar]
- 16.Kaverin N V, Klenk H D. Strain-specific differences in the effect of influenza A virus neuraminidase on vector-expressed hemagglutinin. Arch Virol. 1999;144:781–786. doi: 10.1007/s007050050543. [DOI] [PubMed] [Google Scholar]
- 17.Keil W, Geyer R, Dabrowski J, Dabrowski U, Niemann H, Stirm S, Klenk H D. Carbohydrates of influenza virus: structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H n.m.r. and methylation analysis. EMBO J. 1985;4:2711–2720. doi: 10.1002/j.1460-2075.1985.tb03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilbourne E D. Influenza. New York, N.Y: Plenum Press; 1987. [Google Scholar]
- 19.Lamb R A, Krug R M. Orthomyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1353–1395. [Google Scholar]
- 20.Liu C, Eichelberger M C, Compans R W, Air G M. Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding. J Virol. 1995;69:1099–1106. doi: 10.1128/jvi.69.2.1099-1106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo G, Chung J, Palese P. Alterations of the stalk of the influenza virus neuraminidase: deletions and insertions. Virus Res. 1993;29:141–153. doi: 10.1016/0168-1702(93)90055-r. [DOI] [PubMed] [Google Scholar]
- 22.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matrosovich M N, Gambaryan A S, Teneberg S, Piskarev V E, Yamnikova S S, Lvov D K, Robertson J S, Karlsson K A. Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology. 1997;233:224–234. doi: 10.1006/viro.1997.8580. [DOI] [PubMed] [Google Scholar]
- 24.Mitnaul L J, Castrucci M R, Murti K G, Kawaoka Y. The cytoplasmic tail of influenza A virus neuraminidase (NA) affects NA incorporation into virions, virion morphology, and virulence in mice but is not essential for virus replication. J Virol. 1996;70:873–879. doi: 10.1128/jvi.70.2.873-879.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann G, Zobel A, Hobom G. RNA polymerase I-mediated expression of influenza viral RNA molecules. Virology. 1994;202:477–479. doi: 10.1006/viro.1994.1365. [DOI] [PubMed] [Google Scholar]
- 26.Ohuchi M, Ohuchi R, Feldmann A, Klenk H D. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J Virol. 1997;71:8377–8384. doi: 10.1128/jvi.71.11.8377-8384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palese P, Compans R W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976;33:159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- 28.Palese P, Tobita K, Ueda M, Compans R W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61:397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 29.Perdue M L, Latimer J W, Crawford J M. A novel carbohydrate addition site on the hemagglutinin protein of a highly pathogenic H7 subtype avian influenza virus. Virology. 1995;213:276–281. doi: 10.1006/viro.1995.1571. [DOI] [PubMed] [Google Scholar]
- 30.Pleschka S, Jaskunas R, Engelhardt O G, Zurcher T, Palese P, Garcia-Sastre A. A plasmid-based reverse genetics system for influenza A virus. J Virol. 1996;70:4188–4192. doi: 10.1128/jvi.70.6.4188-4192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers G N, Paulson J C, Daniels R S, Skehel J J, Wilson I A, Wiley D C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 32.Rudneva I A, Sklyanskaya E I, Barulina O S, Yamnikova S S, Kovaleva V P, Tsvetkova I V, Kaverin N V. Phenotypic expression of HA-NA combinations in human-avian influenza A virus reassortants. Arch Virol. 1996;141:1091–1099. doi: 10.1007/BF01718612. [DOI] [PubMed] [Google Scholar]
- 33.Schulman J L, Palese P. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J Virol. 1977;24:170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stieneke-Gröber A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk H D, Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992;11:2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vines A, Wells K, Matrosovich M, Castrucci M R, Ito T, Kawaoka Y. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J Virol. 1998;72:7626–7631. doi: 10.1128/jvi.72.9.7626-7631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warner T G, O'Brien J S. Synthesis of 2′-(4-methylumbelliferyl)-alpha-d-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979;18:2783–2787. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 37.Weis W, Brown J H, Cusack S, Paulson J C, Skehel J J, Wiley D C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 38.Wiley D C, Skehel J J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- 39.Wilson I A, Skehel J J, Wiley D C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 40.Zobel A, Neumann G, Hobom G. RNA polymerase I catalysed transcription of insert viral cDNA. Nucleic Acids Res. 1993;21:3607–3614. doi: 10.1093/nar/21.16.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]