Abstract
Objectives
In patients with an unruptured intracranial aneurysm, gadolinium enhancement of the aneurysm wall is associated with growth and rupture. However, most previous studies did not have a longitudinal design and did not adjust for aneurysm size, which is the main predictor of aneurysm instability and the most important determinant of wall enhancement. We investigated whether aneurysm wall enhancement predicts aneurysm growth and rupture during follow-up and whether the predictive value was independent of aneurysm size.
Materials and methods
In this multicentre longitudinal cohort study, individual patient data were obtained from twelve international cohorts. Inclusion criteria were as follows: 18 years or older with ≥ 1 untreated unruptured intracranial aneurysm < 15 mm; gadolinium-enhanced aneurysm wall imaging and MRA at baseline; and MRA or rupture during follow-up. Patients were included between November 2012 and November 2019. We calculated crude hazard ratios with 95%CI of aneurysm wall enhancement for growth (≥ 1 mm increase) or rupture and adjusted for aneurysm size.
Results
In 455 patients (mean age (SD), 60 (13) years; 323 (71%) women) with 559 aneurysms, growth or rupture occurred in 13/194 (6.7%) aneurysms with wall enhancement and in 9/365 (2.5%) aneurysms without enhancement (crude hazard ratio 3.1 [95%CI: 1.3–7.4], adjusted hazard ratio 1.4 [95%CI: 0.5–3.7]) with a median follow-up duration of 1.2 years.
Conclusions
Gadolinium enhancement of the aneurysm wall predicts aneurysm growth or rupture during short-term follow-up, but not independent of aneurysm size.
Clinical relevance statement
Gadolinium-enhanced aneurysm wall imaging is not recommended for short-term prediction of growth and rupture, since it appears to have no additional value to conventional predictors.
Graphical abstract
Key Points
• Although aneurysm wall enhancement is associated with aneurysm instability in cross-sectional studies, it remains unknown whether it predicts risk of aneurysm growth or rupture in longitudinal studies.
• Gadolinium enhancement of the aneurysm wall predicts aneurysm growth or rupture during short-term follow-up, but not when adjusting for aneurysm size.
• While gadolinium-enhanced aneurysm wall imaging is not recommended for short-term prediction of growth and rupture, it may hold potential for aneurysms smaller than 7 mm.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-023-10388-7.
Keywords: Brain, Intracranial aneurysm, Magnetic resonance imaging, Risk factors
Introduction
In a patient with a newly diagnosed unruptured intracranial aneurysm (UIA), the risk of aneurysm rupture needs to be weighed against the risk of treatment complications from preventive endovascular or neurosurgical aneurysm treatment [1, 2]. Important predictors for aneurysm rupture are size, site, and shape of the aneurysm, family history, smoking, and hypertension [1, 3–6]. Size is a consistent predictor and is included in scores predicting future rupture risk, with smaller intracranial aneurysms having a lower risk of rupture [1, 3]. Paradoxically, most episodes of aneurysmal subarachnoid haemorrhage (SAH) result from rupture of small aneurysms. This is partially explained by the higher prevalence of small aneurysms [7]. To better advise patients with a small aneurysm, it is important to identify the subset of those patients who have an increased risk of growth or rupture.
Gadolinium-enhanced MRI aneurysm wall imaging is a promising technique to improve risk prediction in UIAs. After administration of gadolinium, enhancement of the aneurysm wall may occur, which is thought to represent wall inflammation, slow flow, or wall permeability [8–15]. Because aneurysm wall enhancement occurs in almost all recently ruptured aneurysms compared to approximately 30% of small unruptured aneurysms, it may be a novel biomarker to identify unstable aneurysms [16]. Since most previous studies had a cross-sectional design, comparing ruptured with unruptured aneurysms or comparing unstable with stable aneurysms, the higher proportion of enhancement in ruptured or growing aneurysms could be the result of either the growth or rupture, or a risk factor for growth or rupture [17, 18]. Therefore, it remains unclear whether aneurysm wall enhancement predicts future growth or rupture. In addition, previous studies did not adjust for aneurysm size. Larger aneurysms, with an inherent increased risk of growth or rupture, more often show aneurysm wall enhancement than smaller aneurysms [16, 19–24]. Thus, to assess whether wall enhancement is an independent predictor of growth or rupture, it is necessary to adjust for aneurysm size in the analysis.
Our primary aim was to investigate if aneurysm wall enhancement predicts aneurysm growth or rupture during follow-up in a large international cohort of patients with a UIA < 15 mm. Our secondary aim was to investigate if wall enhancement predicts aneurysm growth or rupture independent of aneurysm size.
Methods
Ethics
The protocol of the study was approved by the Institutional Review Board. This analysis of existing data from cohorts of patients did not require formal approval from an ethics committee, according to the accredited Medical Research Ethics Committee. The individual institutions received approval for retrospective review of the imaging and clinical data, with waiver of consent because data were de-identified.
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants
Individual patient and aneurysm data were retrospectively obtained of patients with at least one saccular UIA who had baseline aneurysm wall imaging and MRA between November 2012 and November 2019 from twelve cohorts of the following countries: the USA (two centres), UK (one centre), The Netherlands (one centre), France (one centre), China (five centres), and Japan (two centres). Inclusion criteria were as follows: (1) 18 years or older with one or more untreated unruptured intracranial aneurysms; (2) gadolinium-enhanced MRI aneurysm wall imaging and MRA at baseline; and (3) MRA or rupture during follow-up. Exclusion criteria were as follows: (1) extradural aneurysm; (2) fusiform aneurysm; and (3) arteriovenous malformation-related aneurysm. We also excluded aneurysms ≥ 15 mm since these aneurysms have a high risk of rupture and therefore usually undergo preventive endovascular or neurosurgical treatment. As a result, the research question of the current study has limited value in that subgroup of aneurysms. We used individual patient data from unpublished and published cohorts (eTable 1) [15, 25, 26]. The data of the published cohorts were updated for the purpose of this study and patients who did not fulfil the inclusion criteria were excluded.
Measurements
The local investigators collected the following data from medical records: patients’ age, sex, population (Japanese, or other), history of hypertension (yes/no), earlier SAH (yes/no), current smoking (yes/no), family history of SAH (yes/no), date of first aneurysm wall imaging, field strength of aneurysm wall imaging (1.5, 3, or 7 T), date of last follow-up MRA, aneurysm growth during follow-up (yes/no), date of MRA that observed growth, date of last MRA before growth, aneurysm rupture during follow-up (yes/no), date of aneurysm rupture, and follow-up duration in patients without growth or rupture (until last MRA).
Imaging
All patients had gadolinium-enhanced MRI aneurysm wall imaging and MRA at baseline. The technique parameters are described in eTable 2. The local neuroradiologist compared the pre- and post-contrast imaging, to assess the presence of wall enhancement (yes/no), irrespective of pattern of enhancement. The follow-up MRA was performed according to the local protocol for the timing of follow-up imaging. The local neuroradiologist recorded the aneurysm size (maximum diameter on a 0.1-mm scale), aneurysm site, and aneurysm irregularity (yes/no) at baseline and follow-up imaging. Irregular aneurysm shape was defined as the presence of blebs, aneurysm wall protrusions, daughter sacs, or multiple lobes [27]. For patients with two or more intracranial aneurysms, each aneurysm was evaluated separately.
Outcome assessment
The primary outcome was aneurysm growth or rupture during follow-up. Aneurysm growth was defined as a ≥ 1 mm increase in at least one direction [28].
Power calculation
A Cox regression of the log hazard ratio (HR) on a covariate with a standard deviation of 0.48 based on a fixed number of 559 aneurysms was used to detect a regression coefficient equal to 1.0986, which corresponds with a HR of 3.0. The calculation was adjusted since a multiple regression of the variable of interest, aneurysm wall enhancement, on the other covariate, aneurysm size, in the Cox regression is expected to have an R-squared of 0.20 [8, 19–22]. Assuming a risk of aneurysm growth or rupture of 10%, 94% power will be achieved at a 0.05 significance level.
Statistical analyses
A descriptive analysis of the studied population was performed. All continuous variables were checked for normality. The analyses were aneurysm-based since our determinant, aneurysm wall enhancement (AWE), and our outcomes, growth and rupture, were all aneurysm-specific. In our primary analysis, we investigated the risk of aneurysm growth or rupture during follow-up of aneurysms with and without gadolinium enhancement of the aneurysm wall at baseline. The risk difference between the two groups was determined with a 95%CI with the Clopper-Pearson test. A Cox proportional hazard model was used to determine the HR of aneurysm growth or rupture during follow-up in aneurysms with and without wall enhancement. The assumptions of proportional hazard were tested. A stratified analysis was performed to investigate potential sex differences in the risk of growth or rupture. In our secondary analysis, the HR of wall enhancement on aneurysm growth or rupture was calculated when adjusting for aneurysm size. In this bivariable model, aneurysm size was analysed as a continuous variable. To examine the absolute risk of growth or rupture for aneurysms with and without aneurysm wall enhancement, the Kaplan-Meier estimate was used, and the hazard function was visualised with a Kaplan-Meier curve.
Furthermore, we performed a subgroup analysis for small aneurysms, defined as aneurysms with a diameter < 7 mm. These aneurysms have a low risk of rupture and therefore usually do not undergo preventive endovascular or neurosurgical treatment. Instead, they are often monitored radiologically. Nevertheless, because < 7 mm aneurysms are much more prevalent than larger aneurysms, most instances of subarachnoid haemorrhage result from rupture of an aneurysm < 7 mm. In this subgroup analysis, we calculated both unadjusted and adjusted hazard ratios. The log-rank test pooled over strata was used to test the equality of the survival distributions of the different levels. A p value less than .05 indicated statistical significance.
Finally, we performed a subgroup analysis for patients who had 3T aneurysm wall imaging, excluding those who were scanned on another field strength (1.5T or 7T). In this subgroup analysis, we calculated unadjusted and adjusted hazard ratios.
Four variables (history of hypertension, earlier SAH, current smoking, and family history of SAH) showed missing values. As we did not include these variables in the analysis to answer the primary and secondary research questions, the final analyses were based on complete data. Statistical analyses were performed in R version 4.0.2 (The R Foundation) using the packages car (Fox J, Weisberg S (2002)), dplyr (Wickham H (2014)), ggplot2 (Wickham H (2007)), survival (Therneau TM, Grambsch PM (1997)), and survminer (Kassambara A (2017)).
Results
We included 455 patients with 559 aneurysms. Patient and aneurysm characteristics are shown in Table 1. The mean age of the included patients was 60 years, and 323 patients (71%) were female. The total follow-up duration was 994 aneurysm-years (median 1.2 years [IQR 0.6–2.4]). Aneurysm wall enhancement was observed in 194 (35%) aneurysms. The median size of aneurysms with wall enhancement at baseline was 5.6 mm compared to 3.5 mm for aneurysms without wall enhancement. Of the 81 patients with multiple aneurysms (2 aneurysms: n= 66; 3 aneurysms: n= 11; 4 aneurysms: n= 4), 44 patients had at least 1 aneurysm with wall enhancement.
Table 1.
Descriptive data for baseline stratified to wall enhancement
| Wall enhancement at baseline | ||||
| Patient characteristics | All (N = 455) | N, missing | Present (N = 168) | Absent (N = 287) |
| Female sex | 323 (71%) | 0 | 127 (76%) | 196 (68%) |
| Mean age, years (SD) | 60 (13) | 0 | 63 (12) | 59 (13) |
| Japanese | 99 (22%) | 0 | 29 (17%) | 70 (24%) |
| Previous SAH from another aneurysm | 48 (11%) | 8 | 11 (7%) | 37 (13%) |
| Hypertension | 201 (44%) | 4 | 78 (46%) | 123 (43%) |
| Current smoker | 110 (24%) | 6 | 36 (21%) | 74 (26%) |
| Family history of SAH | 26 (6%) | 8 | 9 (5%) | 17 (6%) |
| Aneurysm characteristics | All (N = 559) | N, missing | Present (N = 194) | Absent (N = 365) |
| Site | ||||
| ICA | 174 (31%) | 0 | 46 (24%) | 128 (35%) |
| MCA | 209 (37%) | 0 | 81 (42%) | 128 (35%) |
| Acom | 76 (14%) | 0 | 28 (14%) | 48 (13%) |
| Pcom | 33 (6%) | 0 | 11 (6%) | 22 (6%) |
| Vertebrobasilar arteries (excluding basilar tip) | 28 (5%) | 0 | 11 (6%) | 17 (5%) |
| ACA | 24 (4%) | 0 | 11 (6%) | 13 (4%) |
| Basilar tip | 15 (3%) | 0 | 6 (3%) | 9 (3%) |
| Median size, mm (IQR) | 4 (3–6) | 0 | 6 (4–7) | 4 (3–4) |
| Irregular shape | 145 (26%) | 0 | 78 (40%) | 67 (18%) |
| Field strength | ||||
| 1.5T | 115 (21%) | 0 | 32 (16%) | 83 (23%) |
| 3T | 443 (79%) | 0 | 161 (83%) | 282 (77%) |
| 7T | 1 (0%) | 0 | 1 (1%) | 0 (0%) |
| Median follow-up duration, years (IQR) | 1.2 (0.6–2.4) | 0 | 1.1 (0.5–2.2) | 1.3 (0.7–2.5) |
Abbreviations: SD standard deviation, SAH subarachnoid haemorrhage, ICA internal carotid artery, MCA middle cerebral artery, Pcom posterior communicating artery, Acom anterior communicating artery, IQR interquartile range
Risk of growth and rupture in aneurysms with and without wall enhancement
During follow-up, 21 patients had seventeen growing aneurysms without rupture (10/17 had wall enhancement) and five ruptured aneurysms (3/5 had wall enhancement) (Table 2). Two of the 21 patients with aneurysm growth or rupture had multiple aneurysms. One patient had four aneurysms; the two largest aneurysms (12 mm middle cerebral artery (MCA) and 9 mm anterior cerebral artery aneurysm) had AWE at baseline and grew during follow-up, while the two smaller aneurysms (4 mm anterior communicating artery and 2 mm MCA aneurysm) had no AWE at baseline and did not grow during follow-up. The other patient had three aneurysms (4 mm, 3 mm, and 2 mm internal carotid artery aneurysms), of which only the smallest showed AWE at baseline and grew during follow-up.
Table 2.
Descriptive data for stable and unstable aneurysms during follow-up
| Baseline characteristics | Primary outcome | |
|---|---|---|
| Stable during FU | Growth or rupture during FU | |
| Patient characteristics | N = 435 | N = 20 |
| Female sex | 308 (71%) | 15 (75%) |
| Mean age (SD) | 60 (13) | 65 (13) |
| Japanese | 94 (23%) | 5 (25%) |
| Previous SAH from another aneurysm † | 47 (11%) | 1 (5%) |
| Hypertension ‡ | 189 (43%) | 12 (60%) |
| Current smoker § | 104 (24%) | 6 (30%) |
| Family history of SAH ∥ | 26 (6%) | 0 (0%) |
| Aneurysm characteristics | N = 537 | N = 22 |
| Site | ||
| ICA | 168 (31%) | 6 (27%) |
| MCA | 203 (38%) | 6 (27%) |
| Pcom | 32 (6%) | 1 (5%) |
| Acom | 73 (14%) | 3 (14%) |
| ACA | 21 (4%) | 3 (14%) |
| Basilar tip | 13 (2%) | 2 (9%) |
| Vertebrobasilar arteries | 27 (5%) | 1 (5%) |
| Median size, mm (IQR) | 4 (3–6) | 5.5 (4–10.5) |
| Irregular shape | 137 (26%) | 8 (36%) |
| Aneurysm wall enhancement | 181 (34%) | 13 (59%) |
| Mean PHASES score (SD) | 4 (3) | 6 (5) |
| Median follow-up duration, years (IQR) | 1.2 (0.6–2.3) | 1.1 (0.7–1.6) |
† 8 missing values, ‡ 4 missing values, § 6 missing values, ∥ 8 missing values. Abbreviations: FU follow-up, SD standard deviation, SAH subarachnoid haemorrhage, ICA internal carotid artery, MCA middle cerebral artery, Pcom posterior communicating artery, Acom anterior communicating artery, IQR interquartile range, PHASES Population, Hypertension, Age, Size, Earlier subarachnoid haemorrhage, and Site
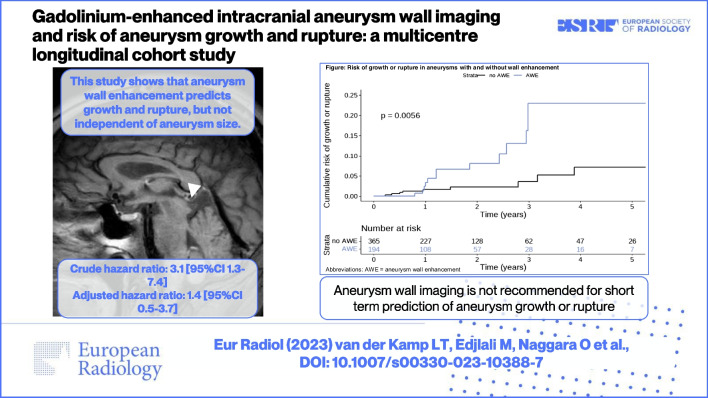
Aneurysm growth or rupture occurred after a median follow-up of 1.1 years (IQR 0.5–2.1) in 13/194 (6.7%) aneurysms with wall enhancement and after a median follow-up of 1.1 years (IQR 0.7–2.5) in 9/365 (2.5%) aneurysms without wall enhancement (absolute overall risk difference 4.2% [95%CI 0.3–8.1%]). The assumptions of proportional hazard were met. The crude HR of wall enhancement for aneurysm growth or rupture was 3.1 [95%CI 1.3–7.4]. The Kaplan-Meier curve is shown in Figure 1 (log-rank test: p = 0.0053). When adjusting for aneurysm size, the HR was 1.4 [95%CI 0.5–3.7] (Table 3). A stratified analysis in male and female patients did not show sex differences in the risk of growth or rupture.
Fig. 1.
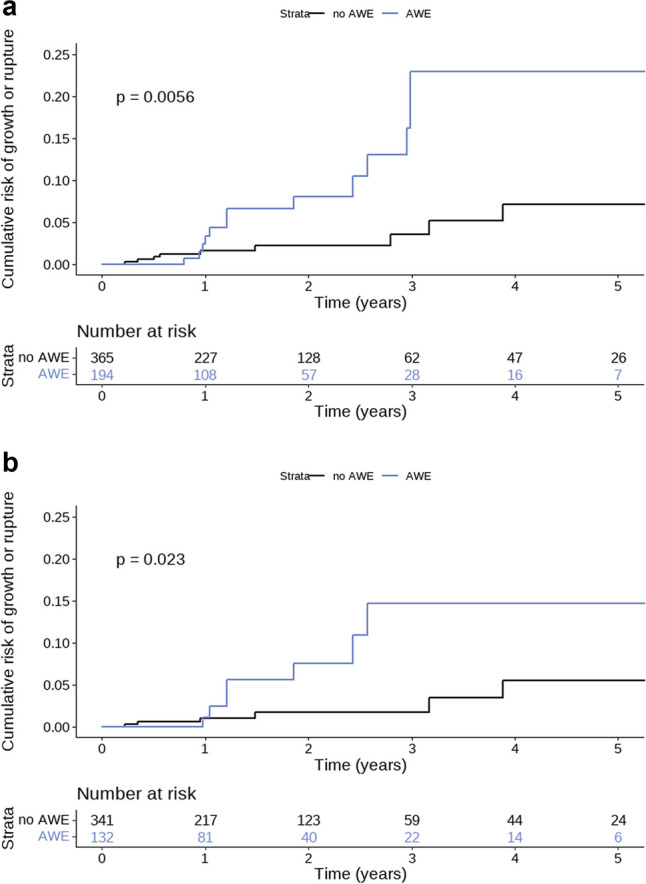
Risk of growth or rupture in aneurysms with and without wall enhancement. a Hazard function on aneurysm growth or rupture. b Hazard function on aneurysm growth or rupture in a subgroup of aneurysms < 7 mm. Legends: p value represents the outcome of the log-rank test. Abbreviations: AWE aneurysm wall enhancement
Table 3.
Cox regression analysis for aneurysm growth or rupture
| Analysis | Predictor | Univariable [95%CI] | Bivariable [95%CI] |
|---|---|---|---|
| Main analysis: n = 559 aneurysms | Aneurysm wall enhancement | 3.1 [1.3–7.4] | 1.4 [0.5–3.7] |
| Aneurysm size | 1.4 [1.2–1.5] | 1.3 [1.2–1.6] | |
| Subgroup analysis: small (< 7 mm) aneurysms; n = 473 aneurysms | Aneurysm wall enhancement | 3.3 [1.1–9.7] | 2.7 [0.8–8.9] |
| Aneurysm size | 2.5 [0.9–2.0] | 1.2 [0.8–1.8] | |
| Subgroup analysis: 3T field strength; n = 443 aneurysm | Aneurysm wall enhancement | 2.5 [0.9–6.8] | 1.2 [0.4–4.1] |
| Aneurysm size | 1.3 [1.1–1.5] | 1.3 [1.1–1.5] |
Bold indicates statistical significance at an alpha level of 0.05
Subgroup analysis for aneurysms < 7 mm
In this subgroup analysis, 473 aneurysms with a diameter < 7 mm were included. The median follow-up duration was 1.3 years (IQR 0.7–2.6). Aneurysm growth occurred in 11 (2.3%) aneurysms and rupture occurred in 2 (0.4%) aneurysms during follow-up. Aneurysm growth or rupture occurred in 7/132 (5.3%) aneurysms with wall enhancement and in 6/341 (1.8%) aneurysms without wall enhancement (absolute overall risk difference 3.5% [95%CI−0.5–7.6%]). The HR of aneurysm wall enhancement in this subgroup was 3.3 (95% CI 1.1–9.9). The adjusted HR of aneurysm wall enhancement for risk of aneurysm growth or rupture was 2.7 (95%CI 0.8–8.5). The Kaplan-Meier curve is shown in Figure 1 (log-rank test: p = 0.025).
Subgroup analysis for patients scanned on 3T field strength
In this subgroup analysis, 443 aneurysms were included. The median follow-up duration was 1.2 years (IQR 0.7–2.5). Aneurysm growth occurred in 13 (2.9%) aneurysms and rupture occurred in 2 (0.5%) aneurysms during follow-up. Aneurysm growth or rupture occurred in 8/161 (5.0%) aneurysms with wall enhancement and in 7/282 (2.4%) aneurysms without wall enhancement (absolute overall risk difference 3.1% [95%CI −0.9–7.0%]). The crude HR was 2.5 (95%CI 0.9–6.8) and the adjusted HR of aneurysm wall enhancement for risk of aneurysm growth or rupture was 1.2 (95%CI 0.4–4.1).
Discussion
This study shows that in patients with an unruptured intracranial aneurysm, gadolinium enhancement is a predictor for growth or rupture during short-term follow-up, but not independent of aneurysm size. Larger aneurysms more often have enhancement of the aneurysm wall and have a higher risk of future aneurysm growth or rupture than smaller aneurysms.
Although many previous studies investigated gadolinium-enhanced aneurysm wall imaging, only three studies had a longitudinal design [15, 25, 26]. In the first study, 57 patients with 65 aneurysms were included [26]. During a median follow-up of 27 months, aneurysm growth or rupture was observed in 4/19 (21%) aneurysms with wall enhancement and 0/46 without wall enhancement. The risk difference of growth and rupture between aneurysms with and without enhancement was 21% (95%CI 3–39%), but no adjustment for aneurysm size was made. In the second study, nine aneurysms were longitudinally followed, of which two (22%) ruptured within 1 year of follow-up [15]. One of the two ruptured aneurysms had wall enhancement at baseline. It was found that the contrast extravasation rate, indicating a higher permeability of the aneurysm wall, was associated with a higher risk of aneurysm rupture. Since no data were given on aneurysm wall enhancement of the other seven longitudinally followed aneurysms, it was not possible to assess whether enhancement was a predictor for growth or rupture. In the most recent study, 129 patients with 145 UIAs were included [25]. During a median follow-up of 24 months, aneurysm growth or morphological change, but not rupture, occurred in 10/65 (15%) aneurysms with wall enhancement at baseline, and in 2/80 (2.5%) aneurysms without enhancement. Presented relative risks were not adjusted for aneurysm size.
Our data suggest that gadolinium enhancement of the aneurysm wall may serve as an additional predictor in the subgroup of aneurysms < 7 mm, because in this subgroup the HR was statistically significant in univariate analysis and the point estimate of the HR only slightly decreased when adjusting for aneurysm size, with a 95% confidence interval slightly crossing 1. It may be that with a larger sample size or a longer follow-up duration, the confidence interval becomes smaller resulting in a statistically significant difference. This subgroup of small aneurysms is the most important to find additional predictors for, because this is the largest group of patients with unruptured aneurysms, and larger aneurysms are usually preventively treated. Especially in this group, it is clinically relevant to find predictors that add value to conventional predictors, which can then be used to improve existing risk prediction models for rupture and growth of unruptured intracranial aneurysms.
A strength of this study is that it is the largest longitudinal aneurysm wall imaging study to date. Previous longitudinal studies did not adjust for aneurysm size. Due to the inclusion of individual patient data of twelve published and unpublished cohorts from three different continents, we had sufficient patient numbers with aneurysm growth or rupture to adjust for the confounder aneurysm size. In addition, several cross-sectional studies used the PHASES score as a surrogate to assess the risk of rupture, since follow-up data were lacking. The current study overcomes these limitations of previous studies, since all aneurysms were followed over time. Furthermore, the radiologists assessed the aneurysm wall, size, and shape prospectively and were therefore blinded for future aneurysm growth or rupture, our primary outcome. Moreover, we used pre-defined inclusion criteria and uniform definitions from the NIH Common Data Elements project on Unruptured Intracranial Aneurysms and Subarachnoid Hemorrhage [28]. This approach added to the generalizability of the study results.
We also need to address a few limitations. First, selection bias inevitably occurred, since only patients who had no preventive aneurysm treatment and had radiological follow-up could be included. This selection of conservatively treated UIA patients might differ between cohorts, since the decision on whether follow-up imaging is advised could differ between centres and countries. However, this selection did not affect our implications, because patients for whom it is decided that follow-up imaging is advised are the targeted group to consider for aneurysm wall imaging. The inclusion of 12 cohorts of patients from different centres adds to the generalizability of the results. Second, our sample size was powered to adjust for one variable and not for other determinants of aneurysm wall enhancement, such as aneurysm shape. However, aneurysm size has been shown to be the strongest determinant of aneurysm wall enhancement [16], thus most important to adjust for. In addition, we had a lower proportion of aneurysms with growth or rupture during follow-up than expected beforehand, which could be explained by a short follow-up period and selection of low-risk aneurysms. Due to the limited number of patients with long-term follow-up and the overall median follow-up period of 1.2 years, our findings cannot be extrapolated to assess the predictive value of aneurysm wall enhancement in the long term. The selection of low-risk aneurysms is reflected by relatively small numbers of patients with an aneurysm at the anterior cerebral artery, posterior communicating artery, or vertebrobasilar arteries. Third, we might not have captured all outcomes since rupture can result in sudden out-of-hospital death, possibly misclassified as sudden cardiac arrest. This could underestimate (if those who died outside the study had aneurysm wall enhancement), overestimate (if those who died outside the study had no wall enhancement), or not affect (in case of an equal distribution of out-of-hospital death from aneurysm rupture in patients with and without aneurysm wall enhancement) the HR in the univariate analysis. The HR when adjusting for size would likely not change, as aneurysm size is the strongest predictor of rupture, and the association between wall enhancement and size holds. Thus, our main outcome is most likely not affected by missing patients with out-of-hospital death who were misclassified as sudden cardiac arrest. Fourth, due to a lack of international consensus on the imaging protocol for aneurysm wall imaging, the imaging protocols differed between sites. This variation in imaging parameters may have affected the visualisation of wall enhancement and the presence of flow artefacts mimicking wall enhancement. We could not include the site as a random effect in our analysis since the number of patients was too small in most of the 12 sites. In addition, the presence of wall enhancement for each aneurysm was not centrally assessed but determined based on a local MR protocol by a local neuroradiologist. This may have resulted in interobserver variability, yet previous studies showed that the interobserver variability for qualitative assessment of wall enhancement is negligible [21, 29].
Conclusions
Although gadolinium enhancement of the aneurysm wall is a predictor for aneurysm growth and rupture during short-term follow-up, this association was not independent of aneurysm size. As such, aneurysm wall enhancement appears to have no additional value to conventional predictors. Future studies with a longer follow-up period are needed, especially in the subgroup of aneurysms < 7 mm, in order to obtain more precise risk estimates.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- Acom
Anterior communicating artery
- AWE
Aneurysm wall enhancement
- HR
Hazard ratio
- ICA
Internal carotid artery
- IQR
Interquartile range
- MCA
Middle cerebral artery
- Pcom
Posterior communicating artery
- SAH
Subarachnoid haemorrhage
- UIA
Unruptured intracranial aneurysm
Funding
This study was supported by a Clinical Established Investigator grant by the Dutch Heart Foundation (2018T076) to Dr M. D. I. Vergouwen. We acknowledge the support from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON2015-08 ERASE. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Declarations
Guarantor
The scientific guarantor of this publication is Mervyn D. I. Vergouwen.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Mervyn D. I. Vergouwen received grant support from Bayer (investigator-initiated research). This company produces GADOVIST®, a contrast agent to perform vessel wall imaging. Bayer had no role in the design and conduct of the study. All other authors had no relevant conflicts of interest.
Statistics and biometry
Two of the authors, Laura T. van der Kamp and Irene C. van der Schaaf, have significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The protocol of the study was approved by the Institutional Review Board of the UMC Utrecht. This analysis of existing data from cohorts of patients did not require formal approval from an ethics committee, according to the accredited Medical Research Ethics Committee NedMec (REC number: 21- 145). The individual institutions received approval for retrospective review of the imaging and clinical data, with waiver of consent because data were de-identified.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in Stroke 2020 (129 patients), AJNR 2018 (57 patients), and AJNR 2019 (29 patients). We included data of 455 patients from 12 centres. In total, data of 176 patients from 3 centres were previously published. This is outlined in eTable 1, which also includes references to the papers of the 3 monocentre studies. The current study differs from the 3 previous studies: first, this was a multicentre study instead of a monocentre study. Second, we used uniform definitions across all participating centres. Third, we updated the available data which increased the follow-up duration of the previously published patients.
Methodology
• retrospective
• observational
• multicentre study
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greving JP, Wermer MJH, Brown RD, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66. doi: 10.1016/S1474-4422(13)70263-1. [DOI] [PubMed] [Google Scholar]
- 2.Algra AM, Lindgren A, Vergouwen MDI, et al. Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis. JAMA Neurol. 2019;76:282–293. doi: 10.1001/jamaneurol.2018.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tominari S, Morita A, Ishibashi T, et al. Prediction model for 3-year rupture risk of unruptured cerebral aneurysms in Japanese patients. Ann Neurol. 2015;77:1050–1059. doi: 10.1002/ana.24400. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi T, Murayama Y, Urashima M, et al. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke. 2009;40:313–316. doi: 10.1161/STROKEAHA.108.521674. [DOI] [PubMed] [Google Scholar]
- 5.Vlak MHM, Rinkel GJE, Greebe P, Algra A. Risk of rupture of an intracranial aneurysm based on patient characteristics: a case-control study. Stroke. 2013;44:1256–1259. doi: 10.1161/STROKEAHA.111.000679. [DOI] [PubMed] [Google Scholar]
- 6.Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001;32:485–491. doi: 10.1161/01.STR.32.2.485. [DOI] [PubMed] [Google Scholar]
- 7.Vlak MHM, Algra A, Brandenburg R, Rinkel GJE. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 8.Hu P, Yang Q, Wang DD, et al. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiol. 2016;58:979–985. doi: 10.1007/s00234-016-1729-3. [DOI] [PubMed] [Google Scholar]
- 9.Larsen N, Von Der Brelie C, Trick D, et al. Vessel wall enhancement in unruptured intracranial aneurysms: an indicator for higher risk of rupture? High-resolution MR imaging and correlated histologic findings. AJNR Am J Neuroradiol. 2018;39:1617–1621. doi: 10.3174/ajnr.A5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong W, Su W, Li T, et al. Aneurysm wall enhancement in unruptured intracranial aneurysms: a histopathological evaluation. J Am Heart Assoc: 2021 doi: 10.1161/jaha.120.018633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelissen BMW, Leemans EL, Slump CH, et al. Vessel wall enhancement of intracranial aneurysms: fact or artifact? Neurosurg Focus. 2019 doi: 10.3171/2019.4.FOCUS19236. [DOI] [PubMed] [Google Scholar]
- 12.Kalsoum E, Chabernaud Negrier A, Tuilier T, et al. Blood flow mimicking aneurysmal wall enhancement: a diagnostic pitfall of vessel wall MRI using the postcontrast 3D turbo spin-echo MR imaging sequence. AJNR Am J Neuroradiol. 2018;39:1065–1067. doi: 10.3174/ajnr.A5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pravdivtseva MS, Gaidzik F, Berg P, et al. Pseudo-enhancement in intracranial aneurysms on black-blood MRI: effects of flow rate, spatial resolution, and additional flow suppression. J Magn Reson Imaging. 2021;54:888–901. doi: 10.1002/jmri.27587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American society of neuroradiology. AJNR Am J Neuroradiol. 2017;38:218–229. doi: 10.3174/ajnr.A4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi H, Liu X, Liu P, et al. Complementary roles of dynamic contrast-enhanced MR imaging and postcontrast vessel wall imaging in detecting high-risk intracranial aneurysms. AJNR Am J Neuroradiol. 2019;40:490–496. doi: 10.3174/ajnr.A5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backes D, Hendrikse J, van der Schaaf I, et al. Determinants of gadolinium-enhancement of the aneurysm wall in unruptured intracranial aneurysms. Neurosurg. 2018;83:719–725. doi: 10.1093/neuros/nyx487. [DOI] [PubMed] [Google Scholar]
- 17.Edjlali M, Guédon A, Ben HW, et al. Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability. Radiology. 2018;289:181–187. doi: 10.1148/radiol.2018172879. [DOI] [PubMed] [Google Scholar]
- 18.Omodaka S, Endo H, Niizuma K, et al. Circumferential wall enhancement in evolving intracranial aneurysms on magnetic resonance vessel wall imaging. J Neurosurg. 2018;131:1262–1268. doi: 10.3171/2018.5.JNS18322. [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Qi H, Liu A, et al. Relationship between aneurysm wall enhancement and conventional risk factors in patients with unruptured intracranial aneurysms: a black-blood MRI study. Interv Neuroradiol. 2016;22:501–505. doi: 10.1177/1591019916653252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv N, Karmonik C, Chen S, et al. Relationship between aneurysm wall enhancement in vessel wall magnetic resonance imaging and rupture risk of unruptured intracranial aneurysms. Clin Neurosurg. 2019;84:E385–E391. doi: 10.1093/neuros/nyy310. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C, Wang X, Degnan AJ, et al. Wall enhancement of intracranial unruptured aneurysm is associated with increased rupture risk and traditional risk factors. Eur Radiol. 2018;28:5019–5026. doi: 10.1007/s00330-018-5522-z. [DOI] [PubMed] [Google Scholar]
- 22.Petridis AK, Filis A, Chasoglou E, et al. Aneurysm wall enhancement in black blood MRI correlates with aneurysm size. Black blood MRI could serve as an objective criterion of aneurysm stability in near future. Clin Pract. 2018;8:73–79. doi: 10.4081/cp.2018.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zwarzany Ł, Tyburski E, Poncyljusz W. High-resolution vessel wall magnetic resonance imaging of small unruptured intracranial aneurysms. J Clin Med. 2021 doi: 10.3390/jcm10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roa JA, Zanaty M, Ishii D, et al. Decreased contrast enhancement on high-resolution vessel wall imaging of unruptured intracranial aneurysms in patients taking aspirin. J Neurosurg. 2020;6:1–7. doi: 10.3171/2019.12.JNS193023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gariel F, Ben Hassen W, Boulouis G, et al. Increased wall enhancement during follow-up as a predictor of subsequent aneurysmal growth. Stroke. 2020;51:1868–1872. doi: 10.1161/STROKEAHA.119.028431. [DOI] [PubMed] [Google Scholar]
- 26.Vergouwen MDI, Backes D, Van Der Schaaf IC, et al. Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: a follow-up study. AJNR Am J Neuroradiol. 2019;40:1112–1116. doi: 10.3174/ajnr.A6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backes D, Vergouwen MDI, Tiel Groenestege AT, et al. PHASES score for prediction of intracranial aneurysm growth. Stroke. 2015;46:1221–1226. doi: 10.1161/STROKEAHA.114.008198. [DOI] [PubMed] [Google Scholar]
- 28.Hackenberg KAM, Algra A, Salman RAS, et al. Definition and prioritization of data elements for cohort studies and clinical trials on patients with unruptured intracranial aneurysms: proposal of a multidisciplinary research group. Neurocrit Care. 2019;30:87–101. doi: 10.1007/s12028-019-00729-0. [DOI] [PubMed] [Google Scholar]
- 29.Fu Q, Wang Y, Zhang Y, et al. Qualitative and quantitative wall enhancement on magnetic resonance imaging is associated with symptoms of unruptured intracranial aneurysms. Stroke. 2021;52:213–222. doi: 10.1161/STROKEAHA.120.029685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.