Abstract
Objectives
Cytotoxic lesions of the corpus callosum (CLOCC) are a common magnetic resonance imaging (MRI) finding associated with various systemic diseases including COVID-19. Although an increasing number of such cases is reported in the literature, there is a lack of systematic evidence summarizing the etiology and neuroimaging findings of these lesions. Thus, the aim of this systematic review was to synthesize the applied nomenclature, neuroimaging and clinical features, and differential diagnoses as well as associated disease entities of CLOCC.
Materials and methods
A comprehensive literature search in three biomedical databases identified 441 references, out of which 324 were eligible for a narrative summary including a total of 1353 patients.
Results
Our PRISMA-conform systematic review identifies a broad panel of disease entities which are associated with CLOCC, among them toxic/drug-treatment-associated, infectious (viral, bacterial), vascular, metabolic, traumatic, and neoplastic entities in both adult and pediatric individuals. On MRI, CLOCC show typical high T2 signal, low T1 signal, restricted diffusion, and lack of contrast enhancement. The majority of the lesions were reversible within the follow-up period (median follow-up 3 weeks). Interestingly, even though CLOCC were mostly associated with symptoms of the underlying disease, in exceptional cases, CLOCC were associated with callosal neurological symptoms. Of note, employed nomenclature for CLOCC was highly inconsistent.
Conclusions
Our study provides high-level evidence for clinical and imaging features of CLOCC as well as associated disease entities.
Clinical relevance statement
Our study provides high-level evidence on MRI features of CLOCC as well as a comprehensive list of disease entities potentially associated with CLOCC. Together, this will facilitate rigorous diagnostic workup of suspected CLOCC cases.
Key Points
• Cytotoxic lesions of the corpus callosum (CLOCC) are a frequent MRI feature associated with various systemic diseases.
• Cytotoxic lesions of the corpus callosum show a highly homogenous MRI presentation and temporal dynamics.
• This comprehensive overview will benefit (neuro)radiologists during diagnostic workup.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00330-023-10524-3.
Keywords: Corpus callosum, Systematic review, Magnetic resonance imaging, Central nervous system diseases, COVID-19
Introduction
Transient magnetic resonance imaging (MRI) signal alterations of the corpus callosum are an increasingly discussed topic in neuroradiology [1]. They most frequently manifest as small round or oval lesions in or close to the midline of the splenium of the corpus callosum and are characterized by T2-weighted (w) hyperintensity without gadolinium enhancement [2]. They also commonly show decreased diffusivity [3], potentially because complex cell-cytokine interactions result in water influx into neurons resulting in cytotoxic edema [4].
These callosal lesions are associated with a broad variety of etiologies [5], such as viral or bacterial infections, drug-related, malignancies, or metabolic disorders [4]. Yet, despite the recent increase in interest in these lesions, the exact pathophysiology, their etiology, and their MRI features are not fully understood.
It is also noteworthy to acknowledge a high degree of inconsistency in employed nomenclature. These splenial lesions have been referred to as mild encephalitis/encephalopathy with reversible splenial lesion (MERS) or reversible splenial lesion syndrome (RESLES), or simply as transient splenial lesions (TSL). More recently, the term CLOCC (cytotoxic lesions of the corpus callosum) has been introduced by Starkey and colleagues to more objectively describe this lesions, as the lesions are not always strictly splenial, not always reversible, and not always associated with mild encephalopathy [3]. In our systematic review, we will refer to these lesions as CLOCC, with a discussion of nomenclature at the end of the paper.
Based on these shortcomings, the aim of this study is to perform a systematic review in patients with CLOCC to enhance the understanding of the imaging features and give a more detailed overview of this finding to facilitate diagnostic workup of suspected cases. In particular, we aim at answering the following questions: (1) What are the MRI features of CLOCC? (2) What is the currently used nomenclature for CLOCC including differences between terms and how commonly are these different terms used? (3) What is the imaging differential diagnosis of CLOCC? (4) What are underlying disease entities causing CLOCC? (5) What is the temporal evolution of CLOCC, that is do they change over time and how long does it persist? (6) What is the histopathological correlation of CLOCC? (7) Do patients with CLOCC present with neurological symptoms associated with corpus callosum lesions?
Materials and methods
We registered the study protocol in the International prospective register of systematic reviews (PROSPERO, CRD42022296487, https://www.crd.york.ac.uk/PROSPERO/) and used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Guidelines for reporting [6].
Search strategy
We searched for original observational studies published in full up to April 12, 2023, in Medline via PubMed, Web of Science, and Ovid EMBASE. See Supplementary Methods for the search string in each of these data bases.
Inclusion and exclusion criteria
We included all original publications including case reports that reported on cytotoxic lesions of the corpus callosum, as assessed by MRI, and their associations with any human disease. Exclusion criteria: animal studies, non-English articles, and papers which did not include quantitative data. Reviews were excluded but retained as source for additional references.
Study selection and data extraction
Titles and abstracts of studies were screened for their relevance in the web-based application Rayyan by two reviewers (S.M. and B.V.I.) [7] followed by full-text screening (S.M. and D.N.). Discrepancies were resolved by discussion.
Quality assessment
The quality of each study was assessed against pre-defined criteria by two reviewers using an adjusted version of the Newcastle–Ottawa scale [8].
Results
Eligible publications and general study characteristics
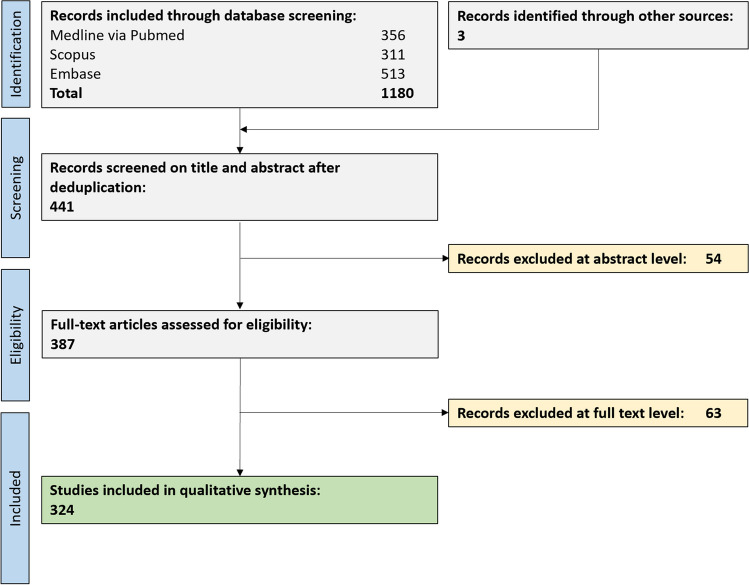
In total, 441 unique publications were retrieved from our comprehensive data base search. After full text screening, 324 publications were eligible for this systematic review (Fig. 1).
Fig. 1.
Flow chart depicting the study selection process
A total of 189 publications included adult subjects only, comprising a total of 416 patients (217 males [52%], 196 females [47%], three with unknown sex [1%]). Their median age was 37 years (interquartile range, IQR 24–46). One hundred twenty-five publications included children only, comprising a total of 937 children. Their median age was 5.1 years (IQR 4–12).
Study quality
Most publications (92%) showed a low risk of bias for the selection domain (that is, whether callosal lesions were defined according to acknowledged diagnostic criteria); see Supplementary Table 1. Many studies did not report on adjusting their statistical analyses for subject age, sex, or other potential confounders (comparability domain), thus potentially inducing biases.
Used nomenclature for callosal lesions
Out of the 324 publications, the most frequently used terms were MERS (mild encephalitis/encephalopathy with reversible splenial lesion) (134 publications, 41%), followed by the term reversible or transient splenial lesions (44, 14%), and RESLES (reversible splenial lesion syndrome) (41, 13%). CLOCC(s) (“Cytotoxic lesions of the corpus callosum”) was used in 26 publications (8%); this term was first proposed by Starkey and colleagues in 2017 [3].
Imaging/signal characteristics of callosal lesions
Splenial lesions had a highly homogenous MRI presentation (Table 1): they mostly presented with hyperintense signal on T2w/T2w-FLAIR (95%) and hypo- or isointense signal on T1w (70% hypointense, 27% isointense). In addition, the majority of lesions did show restricted diffusion (99%) but absent gadolinium enhancement (98%).
Table 1.
MRI Imaging characteristics of cytotoxic lesions of the corpus callosum (CLOCC)
| T1w |
70% hypointense (72) 27% isointense (28) 3% hyperintense (3) |
| T2w |
0% hypointense (0) 5% isointense (10) 95% hyperintense (185) |
| T2w-FLAIR |
0% hypointense (0) 4% isointense (7) 96% hyperintense (156) |
| DWI | > 99% restricted (274, one case unrestricted) |
| T1w post contrast (gadolinium) |
98% without enhancement (84) 2% with enhancement (2) |
The number of publications reporting this finding is listed in brackets
DWI diffusion-weighted imaging, FLAIR fluid-attenuated inversion recovery
Location of callosal lesions
CLOCC almost always involved the splenium of the corpus callosum (310/311 publications, > 99%) except in one case with callosal hypogenesis in which only the genu was affected [9]. In cases with more than one discrete location of involvement in the corpus callosum, the additional locations were most commonly the genu in 15 cases (5%), followed by the entire corpus callosum in six cases (2%) and the body in two cases (< 1%). There was no reported involvement of the rostrum of the corpus callosum. Extra-splenial callosal lesions predominantly affected children. Notably, lesions in the genu of the corpus callosum were found mainly in the pediatric population (13 of the 15 cases being children; median age, 8.5 years). Lesions spanning the entire corpus callosum were exclusively observed in children (n = 6 children). Only one child and one adult exhibited involvement in the body of the corpus callosum. There was no association of sex or disease entity with CLOCC location (Supplementary Table 4).
Temporal evolution of callosal lesions
In 283 of 324 (87%) publications, a follow-up MRI was performed. The follow-up time point ranged from a few days to several months with a median interval of 3 weeks (IQR 2–5 weeks).
Most publications (260/283 publications, 92%) reported a complete resolution of the lesion within the follow-up period. Of the 22 follow-up exams which were done after exactly 1 week, 19 (86%) showed a complete resolution. However, in some patients, CLOCC did not show complete resolution (n = 25; 9%); one case exhibited a persisting lesion as long as 10 months [10]. Of the persisting or residual cases, 22 were in adults and five in children. Rarely, CLOCC persisted without major changes (number of patients = 2; 1%) until their follow-up at 1 month [2] and 3 months [11], respectively.
Associated disease classes and entities
A variety of overarching pathology classes were associated with callosal lesions. Most common overarching classes in adults were drug/toxin-induced (including withdrawal of drugs) (26%, commonly by antiepileptic drugs), viral infections (18%, SARS-CoV-2, influenza, and others), (cerebro)vascular diseases (18%, mostly subarachnoid hemorrhage), bacterial infections (10%), and seizures (6%). In 20% of the adult patients, no associated disease could be identified. In children (< 18 years old), there was not a clear association with a disease entity in 42 subjects. Among the identified disease entities, callosal lesions were most frequently associated with viral infections (73%, mostly influenza and rotavirus). Bacterial infections (7%, mostly mycoplasma), seizures, and metabolic entities (3% each) were less common. A summary of associated disease classes and entities can be found in Table 2 for adults and Table 3 for children. For a complete list of associated disease entities, see Supplementary Table 2 for adults and Supplementary Table 3 for children.
Table 2.
Entities associated with cytotoxic lesions of the corpus callosum (CLOCC) in adults
| Drug or drug withdrawal or toxins (n = 88) (27%) |
Antiepileptic (n = 32) Neuroleptic (n = 12) Carbamazepine (n = 8) Chemotherapy (n = 8) Dietary supplement (n = 6) Metronidazole (n = 6) Immunomodulary drugs (n = 4) Toxins (n = 4) Other (n = 6) |
| Vascular (n = 60) (18%) |
Subarachnoid hemorrhage (n = 49) Stroke (n = 1) Other (n = 10) |
| Viral (n = 59) (18%) |
COVID-19 (n = 15) Influenza virus (n = 13) Dengue virus (n = 5) Epstein-Barr virus (n = 4) Other (n = 22) |
| Bacteria and plasmodia (n = 34) (10%) |
Plasmodium falciparum (n = 8) Mycoplasma pneumoniae (n = 7) Staphlyococcus aureus (n = 4) other (n = 15) |
| Seizure and epilepsy (n = 20) (6%) |
Status epilepticus (n = 1) Other (n = 19) |
| Metabolic (n = 10) (3%) | Hypo- and hyperglycemia (n = 10) |
| Pregnancy-associated (n = 8) (2%) |
Eclampsia (n = 1) other (n = 7) |
| Autoimmune (n = 6) (2%) |
GFAP antibodies (n = 2) Anti-NMDA encephalitis (n = 1) SLE (n = 1) Other (n = 2) |
| Traumatic (n = 4) (1%) | Head trauma (n = 4) |
| Neoplasia (n = 4) (1%) |
Insulinoma (n = 1) Leukemia (n = 1) Mantle cell lymphoma (n = 1) Melanocytoma (n = 1) |
| Various causes (n = 37) (11%) |
Alcoholism (n = 1) COVID-19 vaccine (n = 1) Deep brain stimulation (n = 1) High-altitude sickness (n = 1) Migraine (n = 1) Parkinsonism (n = 1) Other (n = 31) |
In 330 adult patients, an associated disease was identified; in 86 patients, no associated disease entity could be identified (number of subjects per associated disease is reported in brackets). Only diseases which occurred more than once or deemed of special interest are listed in this table, for a detailed list see supplementary data
Anti-NMDA encephalitis anti-N-methyl-d-aspartate-receptor encephalitis, GFAP antibodies glial fibrillary acidic protein antibodies, SLE systemic lupus erythematosus
Table 3.
Entities associated with cytotoxic lesions of the corpus callosum (CLOCC) in children
| Viral (n = 409) (75%) |
Influenza virus (A or B) (n = 187) Rotavirus (n = 140) HHV6 (n = 16) Adenovirus (n = 10) COVID 19 (n = 10) Mumps (n = 8) Respiratory-syncytial virus (n = 6) Epstein-Barr virus (n = 4) Viral gastroenteritis unspecified (n = 4) Other (n = 24) |
| Bacterial (n = 40) (7%) |
Mycoplasma pneumoniae (n = 15) Enterococcus faecalis (n = 5) Escheria coli (n = 4) Other (n = 16) |
| Seizure or epilepsy (n = 19) (4%) |
Benign infantile epilepsy (n = 2) Other (n = 17) |
| Electrolyte disbalance (n = 18) (3%) | Hyponatremia (n = 18) |
| Vascular (n = 13) (2%) | Kawasaki disease (n = 13) |
| Drugs, toxins, and vaccination (n = 12) (2%) |
Mumps vaccination (n = 6) other (n = 6) |
| Traumatic (n = 10) (2%) |
Diffuse axonal injury (n = 9) Unspecified (n = 1) |
| Autoimmune (n = 4) (1%) |
Anti-GFAP encephalitis (n = 2) Systematic lupus erythematosus (n = 2) |
| Various causes (n = 17) (3%) |
Acute encelopathy in congenital adrenal hyperplasia (n = 3) Thyroid crisis (n = 2) other (n = 12) |
In 542 pediatric patients, an associated disease was identified; in 395 patients, no associated disease entity could be identified (number of subjects per associated disease is reported in brackets). Only diseases which occurred more than once or are of special interest are listed in this table; for a detailed list, see the supplementary data
GFAP antibodies glial fibrillary acidic protein antibodies, HHV6 human herpes virus 6, SLE systemic lupus erythematosus
Callosal lesions and associated symptoms
In all but one publication, no neurological symptoms were described with tropism to the corpus callosum but rather symptoms of the associated disease entity. However, intriguingly, one single publication reported on one patient having a callosal lesion and clinically presenting with an alien hand syndrome [12].
MRI-histopathology correlation
Our systematic literature did not identify studies with MRI-histopathology correlation for callosal lesions.
Discussion
Main findings
Here, we systematically assessed MRI features of callosal lesions including their associated clinical disease entities and temporal evolution as well as their nomenclature. Callosal lesions show very homogenous MR signal characteristics: a typical lesion is T1w hypointense, T2w(-FLAIR) hyperintense, and exhibits restricted diffusion but not gadolinium enhancement. These lesions almost ubiquitously affect the splenium of the corpus callosum and are often transient in nature; i.e., they regress within one to few weeks. These lesions are associated with a broad class of disease entities including toxic/drug-treatment-associated, infectious (viral, bacterial), vascular, metabolic, traumatic, and neoplastic (Table 1). No studies were identified which assessed the tissue signature of these callosal lesions.
Findings in the context of existing evidence
Our systematic review identifies several used terms to denote callosal lesions. Early studies have most commonly used the terms MERS (“mild encephalopathy with reversible lesion in the splenium”) [13] or RESLES (“reversible splenial lesion syndrome”) [14]. In 2017, Starkey and colleagues proposed the term CLOCC (“cytotoxic lesions of the corpus callosum”) [3], mostly because these lesions are not strictly splenial, lesions are not always reversible, and the encephalopathy can also be more severe. Although some recent studies have adopted this nomenclature [15], some studies have retained prior terminology [16]. Some studies defined further subclassifications with small-type lesions isolated to the splenium and large-type lesions spread along the ventricles [17]. It is also noteworthy that the terms used to denote these lesions are sometimes not used interchangeably, with RESLES describing the radiological lesion and MERS the clinically mild course of infectious encephalitis with neurological symptoms like seizures and altered consciousness within the spectrum of RESLES [18, 19].
Our systematic review corroborates the typical and mostly homogenous MRI appearance of callosal lesions: hypointensity on T1w, hyperintensity on T2w, and restricted diffusion (Fig. 2). The few exceptions reported could be reflective of different stages of lesion evolution, underlying pathophysiological mechanisms, or individual patient factors. The lack of gadolinium enhancement is consistent with the cytotoxic, rather than vasogenic, nature of the edema typically associated with these lesions. Although MRI has a limited specificity of underlying tissue pathology, these characteristics are in line with a focal callosal edema. In fact, it has been speculated that a cytotoxic edema is the correlating tissue pathology of splenial lesions [20–22] (reviewed in [3]). The hypothesis suggests a cytokinopathy causing excitotoxicity resulting in an intracellular edema and hence restricted diffusion [23, 24]. This pathological cascade seems to preferentially affect the splenium of the corpus callosum, with its high density of glutamatergic and other excitatory amino acid receptors [25–27]. Even though this is a plausible hypothesis, our systematic review did not identify a single study with MRI-histopathology correlation for splenial lesions. Hence, histopathological validation of callosal lesions is warranted to investigate its actual tissue substrate. Alternatively, advanced MRI methods including diffusion tensor imaging, MR spectroscopy [28], or more specific myelin imaging approaches [29–31] could be harnessed to gain additional insight into pathophysiology of these lesions.
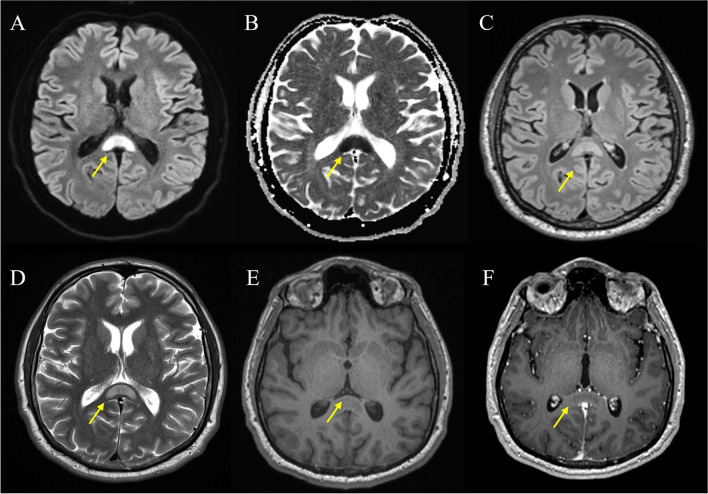
Fig. 2.
Typical structural MRI findings in cytotoxic lesions of the corpus callosum (CLOCC). Pictorial magnetic resonance imaging (MRI) examples of a 38-year-old patient who presents with a typical cytotoxic lesion of the corpus callosum (CLOCC) after head trauma. A DWI B0, oval hyperintensity throughout the splenium and into the adjacent hemispheres (“boomerang sign”). B ADC hypointensity due to restricted diffusion. C T2w-FLAIR, high signal. D T2 slightly hyperintense. E T1 native, pre-gadolinium uptake, shows a slight hypointensity in the region. F T1 post-gadolinium uptake, no enhancement. Abbreviations. DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery
Besides their homogenous MRI presentation, the vast majority of CLOCC (1) does affect the splenium of the corpus callosum and (2) is reversible within 1 to few weeks. In the majority of persisting cases, the follow-up period was notably brief, spanning few weeks; thus, it is possible that the lesion may have resolved in a subsequent period. In the rare instances where the lesion remains evident after several months, one must consider if a different etiology than CLOCC is at play. Biopsies of persistent CLOCC cases could provide further insights into the histopathology of CLOCC potentially distinguishing it from other etiologies.
It is also noteworthy that CLOCC were not described as being associated with specific corpus callosum symptoms, though in one case the CLOCC was associated with alien hand syndrome [12], which has been described before as being caused by lesions in the corpus callosum [32]. Additionally, none of the studies reported advanced neuropsychological testing which could pick up more subtle deficits. Advanced neuropsychological testing could be harnessed to gain additional insight into the neurological manifestations of these lesions.
Our systematic review identified several disease classes and entities which are associated with CLOCC, among them drug-induced (including withdrawal), viral infections, cerebrovascular diseases, and seizures. However, the exact etiology of splenial lesions is still under debate [14]. It has been hypothesized that trauma, infection, or inflammation may cause a macrophage-driven cytokinopathy; this in term would result in glutamatergic excitotoxicity leading to cytotoxic edema in the corpus callosum [3]. Although this pathogenic cascade seems plausible for neuroinflammatory or local traumatic insults, additional yet unknown pathomechanisms could be involved in this cascade for peripheral inflammatory diseases caused by, e.g., rotavirus or systemic bacterial infections. An as of yet to be defined unifying etiology is also possible, analogous to dentate nucleus T1w hyperintensity being attributable to various etiologies was eventually proven to be related to multiple administrations of gadolinium [33].
In diagnostic routine, CLOCC can be misdiagnosed (Table 4). Differential diagnostic entities include other diseases with common callosal/splenial involvement [5, 34, 35], among them (1) ischemic lesions (caused by rare distal occlusion of the anterior cerebral artery); (2) neuroinflammatory conditions such as acute disseminated encephalomyelitis (ADEM) or multiple sclerosis (MS) [36]; (3) neoplastic lesions such as lymphoma, glioblastoma, or metastases; and (4) Marchiafava–Bignami disease, induced by alcohol-related toxic effects on the brain [37]. Other, rare differential diagnoses include atypical presentations of posterior reversible encephalopathy syndrome (PRES), hypoxic-ischemic encephalopathy mainly in children, and changes in patients with hypoglycemic encephalopathy. Of note, CLOCC lesions do not show pathological signals in susceptibility-weighted imaging (SWI) sequences, unlike differential diagnoses such as post-traumatic or neoplastic changes of the corpus callosum [38–40]. Although differential diagnostic workup may be difficult, our systematic review emphasizes the mostly homogenous imaging presentation of CLOCC. In Table 4, we summarize the most common differential diagnosis including their detailed MRI characteristics. With this, a routine clinical MRI protocol is normally sufficient to discriminate splenial lesions from common imaging mimics. Advanced imaging techniques, such as quantitative susceptibility mapping (QSM), might be further helpful to differentiate among various entities, by measuring the concentration and distribution of substances that affect the magnetic susceptibility of tissues, such as iron, myelin, or calcium; however, no systematic analyses have been published to date.
Table 4.
Common differential diagnoses of cytotoxic lesions of the corpus callosum (CLOCC) including their magnetic resonance imaging (MRI) characteristics
| T1w | T2w-FLAIR | T1w Gd | SWI | DWI | Comments | |
|---|---|---|---|---|---|---|
| CLOCC | Hypointense | Hyperintense | No enhancement | No SWI artifacts | Diffusion restriction | Mostly reversible within a few weeks |
| Lymphoma, glioblastoma, metastases | Usually hypointense | Usually hyperintense | Gd enhancement in the majority of cases | Potential intratumoral susceptibility signals or hermorrhagic components | Diffusion restriction depends on the tumor cellularity | Often in line with a mass effect |
| Ischemic stroke | Hypointense | Demarcated ischemic tissue hyperintense |
Acute: No enhancement Subacute: Gd enhancement Chronic: No enhancement |
Usually no SWI artifacts, however hemorrhagic transformation possible | Diffusion restriction in acute and subacute cases | T2w-FLAIR hyperintensity not reversible |
| MS, ADEM | Hypointense | Hyperintense | Gd enhancement in active lesions | No SWI artifacts | Diffusion restriction might be visible in active lesions | Additional white matter lesions |
| Diffuse axonal injury (DAI) of the corpus callosum | Hypointense | Hyperintense | No enhancement | Blood products visible in SWI | Diffusion restriction might be visible in acute lesions | Associated with traumatic brain injury and indicates a poor prognosis |
| Marchiafava-Bignami disease | Hypointense | Usually hyperintense | No enhancement | No SWI artifacts | Diffusion restriction | Usually affects the central layers of the corpus callosum. Lesions resolve after successful therapy |
Abbreviations: ADEM acute demyelinating encephalomyelitis, DWI diffusion-weighted imaging, FLAIR fluid-attenuated inversion recovery, Gd gadolinium, MS multiple sclerosis, SWI susceptibility-weighted imaging
Limitations
First, there was considerable heterogeneity in the applied methodology for MRI of callosal lesions which could skew the interpretation of the narrative synthesis. Second, although our study does identify a comprehensive panel of disease entities associated with callosal lesions, no inference about causality can be made based on our analysis. However, of note, also primary studies were not able to discern direction of causality. Third, unsurprisingly, none of the included studies did histopathologically confirm the callosal lesions. Thus, callosal lesions might have been misinterpreted and erroneously included in our study. However, we did mitigate this by ascertaining study quality by acknowledged risk of bias assessment tools.
Conclusions
Our systematic review provides high level evidence for the homogenous MRI appearance of CLOCC as well as a comprehensive list of associated disease entities. Future research warrants histopathological validation of CLOCC as well as more and larger longitudinal cohorts to investigate causality of disease entities with CLOCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful for funding from the Swiss National Science Foundation and the UZH Alumni (to BVI).
Abbreviations
- ADEM
Acute disseminated encephalomyelitis
- CLOCC
Cytotoxic lesions of the corpus callosum
- FLAIR
Fluid-attenuated inversion recovery
- MERS
Mild encephalitis/encephalopathy with reversible splenial lesion
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- RESLES
Reversible splenial lesion syndrome
- TSL
Transient splenial lesions
Funding
Open access funding provided by University of Zurich. This work was supported by grants of the Swiss National Science Foundation (Grant Nr. P400PM_183884 to BVI) and the UZH Alumni Fellowship (To BVI). Role of the sponsor: The Swiss National Science Foundation or the UZH Alumni Foundation had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations
Guarantor
The scientific guarantor of this publication is Benjamin V. Ineichen.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a systematic review.
Ethical approval
Institutional Review Board approval was not required because the study is a systematic review.
Study subjects or cohorts overlap
No study subjects or cohorts overlap have been previously reported.
Methodology
• multicenter study
Footnotes
Sebastian Winklhofer and Benjamin V. Ineichen share senior authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blaauw J, Meiners LC. The splenium of the corpus callosum: embryology, anatomy, function and imaging with pathophysiological hypothesis. Neuroradiology. 2020;62:563–585. doi: 10.1007/s00234-019-02357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolshoven J, Fellows K, Ania R, Tabaac BJ (2021) Vertigo and cytotoxic lesions of the corpus callosum: report with review of the literature. Case Rep Neurol Med 18:5573822 [DOI] [PMC free article] [PubMed]
- 3.Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017;37:562–576. doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
- 4.Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav. 2019;9:e01440. doi: 10.1002/brb3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kontzialis M, Soares BP, Huisman T. Lesions in the splenium of the corpus callosum on MRI in children: a review. J Neuroimaging. 2017;27:549–561. doi: 10.1111/jon.12455. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells GA, Tugwell P, O’Connell D et al (2015) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses
- 9.Tsuji M, Chong PF, Yamashita F, Maeda K, Kira R. Cytotoxic lesion of the corpus callosum exclusively at the genu in a case of callosal hypogenesis. J Neuroradiol. 2019;46:222–223. doi: 10.1016/j.neurad.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Galnares-Olalde J, Vázquez-Mézquita A, Gómez-Garza G, et al. Cytotoxic lesions of the corpus callosum caused by thermogenic dietary supplements. AJNR Am J Neuroradiol. 2019;40:1304–1308. doi: 10.3174/ajnr.A6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda F, Yoshie Y, Aburano H, Hashimoto M, Matsui O, Gabata T. Splenial and white matter lesions showing transiently-reduced diffusion in mild encephalopathy monitored with MR spectroscopy and imaging. Magn Reson Med Sci. 2014;13:271–275. doi: 10.2463/mrms.2014-0011. [DOI] [PubMed] [Google Scholar]
- 12.Gellman SR, Ng Y-T. Transient corpus callosal lesion presenting with alien hand syndrome. Pediatr Neurol. 2018;89:66–67. doi: 10.1016/j.pediatrneurol.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Cho J-S, Ha S-W, Han Y-S, et al. Mild encephalopathy with reversible lesion in the splenium of the corpus callosum and bilateral frontal white matter. J Clin Neurol. 2007;3:53–56. doi: 10.3988/jcn.2007.3.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Monco JC, Cortina IE, Ferreira E, et al. Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging. 2011;21:e1–14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 15.Moreau A, Ego A, Vandergheynst F, et al. Cytotoxic lesions of the corpus callosum (CLOCCs) associated with SARS-CoV-2 infection. J Neurol. 2021;268:1592–1594. doi: 10.1007/s00415-020-10164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo K, Lai X, Liu Y, Zhou D, Hong Z. Anti-glial fibrillary acidic protein antibodies as a cause of reversible splenial lesion syndrome (RESLES): a case report. Neurol Sci. 2021;42:3903–3907. doi: 10.1007/s10072-021-05376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toi H, Yagi K, Matsubara S, Hara K, Uno M. Clinical features of cytotoxic lesions of the corpus callosum associated with aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2021;42:1046–1051. doi: 10.3174/ajnr.A7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W-X, Liu H-S, Yang S-D, et al. Reversible splenial lesion syndrome in children: retrospective study and summary of case series. Brain Develop. 2016;38:915–927. doi: 10.1016/j.braindev.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Azuma J, Nabatame S, Katsura T, et al. Marked elevation of urinary β2-microglobulin in patients with reversible splenial lesions: a small case series. J Neurol Sci. 2016;368:109–112. doi: 10.1016/j.jns.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 20.Kallenberg K, Bailey DM, Christ S, et al. Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. J Cereb Blood Flow Metab. 2007;27:1064–1071. doi: 10.1038/sj.jcbfm.9600404. [DOI] [PubMed] [Google Scholar]
- 21.Takayama H, Kobayashi M, Sugishita M, Mihara B. Diffusion-weighted imaging demonstrates transient cytotoxic edema involving the corpus callosum in a patient with diffuse brain injury. Clin Neurol Neurosurg. 2000;102:135–139. doi: 10.1016/S0303-8467(00)00079-2. [DOI] [PubMed] [Google Scholar]
- 22.Prilipko O, Delavelle J, Lazeyras F, Seeck M. Reversible cytotoxic edema in the splenium of the corpus callosum related to antiepileptic treatment: report of two cases and literature review. Epilepsia. 2005;46:1633–1636. doi: 10.1111/j.1528-1167.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi DW. Excitotoxicity: still hammering the ischemic brain in 2020. Front Neurosci. 2020;14:579953. doi: 10.3389/fnins.2020.579953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rho JM, Boison D. The metabolic basis of epilepsy. Nat Rev Neurol. 2022;18:333–347. doi: 10.1038/s41582-022-00651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen C, Niculescu I, Patel S, Krishnan A. COVID-19 and involvement of the corpus callosum: potential effect of the cytokine storm? AJNR Am J Neuroradiol. 2020;41:1625–1628. doi: 10.3174/ajnr.A6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moritani T, Smoker WR, Sato Y, Numaguchi Y, Westesson P-LA. Diffusion-weighted imaging of acute excitotoxic brain injury. AJNR Am J Neuroradiol. 2005;26:216–228. [PMC free article] [PubMed] [Google Scholar]
- 27.Ma D, Matute C. Expression of glutamate transporters in the adult bovine corpus callosum. Mol Brain Res. 1999;67:296–302. doi: 10.1016/S0169-328X(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 28.Henning A. Proton and multinuclear magnetic resonance spectroscopy in the human brain at ultra-high field strength: a review. Neuroimage. 2018;168:181–198. doi: 10.1016/j.neuroimage.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Weiger M, Pruessmann KP. Short-T(2) MRI: principles and recent advances. Prog Nucl Magn Reson Spectrosc. 2019;114–115:237–270. doi: 10.1016/j.pnmrs.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Weiger M, Froidevaux R, Baadsvik EL, Brunner DO, Rösler MB, Pruessmann KP. Advances in MRI of the myelin bilayer. Neuroimage. 2020;217:116888. doi: 10.1016/j.neuroimage.2020.116888. [DOI] [PubMed] [Google Scholar]
- 31.Baadsvik EL, Weiger M, Froidevaux R, Faigle W, Ineichen BV, Pruessmann KP (2022) Mapping the myelin bilayer with short‐T2 MRI: methods validation and reference data for healthy human brain. Magn Reson Med [DOI] [PMC free article] [PubMed]
- 32.Hassan A, Josephs KA. Alien hand syndrome. Curr Neurol Neurosci Rep. 2016;16:1–10. doi: 10.1007/s11910-016-0676-z. [DOI] [PubMed] [Google Scholar]
- 33.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–841. doi: 10.1148/radiol.13131669. [DOI] [PubMed] [Google Scholar]
- 34.Garg N, Reddel SW, Miller DH, et al. The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J Neurol Neurosurg Psychiatry. 2015;86:1374–1382. doi: 10.1136/jnnp-2014-309649. [DOI] [PubMed] [Google Scholar]
- 35.Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. Acquired lesions of the corpus callosum: MR imaging. Eur Radiol. 2006;16:905–914. doi: 10.1007/s00330-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 36.Ineichen BV, Beck ES, Piccirelli M, Reich DS. New prospects for ultra-high-field magnetic resonance imaging in multiple sclerosis. Invest Radiol. 2021 doi: 10.1097/rli.0000000000000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geibprasert S, Gallucci M, Krings T. Alcohol-induced changes in the brain as assessed by MRI and CT. Eur Radiol. 2010;20:1492–1501. doi: 10.1007/s00330-009-1668-z. [DOI] [PubMed] [Google Scholar]
- 38.Lu P-l, Hodes JF, Zheng X, Hu X-y. Reversible splenial lesion syndrome with some novel causes and clinical manifestations. Intern Med. 2020;59:2471–2480. doi: 10.2169/internalmedicine.4516-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao J-j, Zhang W-j, Wang D, et al. Susceptibility weighted imaging in the evaluation of hemorrhagic diffuse axonal injury. Neural Regen Res. 2015;10:1879. doi: 10.4103/1673-5374.170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabner G, Kiesel B, Wöhrer A, et al. Local image variance of 7 Tesla SWI is a new technique for preoperative characterization of diffusely infiltrating gliomas: correlation with tumour grade and IDH1 mutational status. Eur Radiol. 2017;27:1556–1567. doi: 10.1007/s00330-016-4451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.