Abstract
Background
Bullous pemphigoid (BP) is a rare, autoimmune, blistering skin disease associated with high disease burden, profoundly decreased quality of life and increased morbidity. Emerging evidence supports an important role for type 2 inflammation in disease pathogenesis. Current management relies on topical and/or systemic corticosteroids, non-selective immunosuppressants and antibiotics with anti-inflammatory properties, which are all limited by side effects and toxicities. Therefore, targeted, efficacious and safe therapies are needed. Dupilumab blocks the shared receptor component for interleukin (IL)-4 and IL-13, key and central drivers of type 2 inflammation. Several reports of patients successfully treated with dupilumab have been published; however, dupilumab has not been formally assessed in a double-blind, placebo-controlled trial.
Objectives
We report the design of LIBERTY-BP ADEPT, a multicenter, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of dupilumab in adults with BP.
Methods
LIBERTY-BP ADEPT comprises a 35-day screening, 52-week treatment and 12-week follow-up period. Approximately 98 adults aged 18–90 years with moderate-to-severe BP are being enrolled at 51 sites on 4 continents and randomized 1:1 to subcutaneous dupilumab or placebo every 2 weeks. All participants will receive concomitant oral corticosteroids (OCS).
Planned Outcomes
The primary endpoint is the proportion of patients achieving complete remission off steroid therapy at week 36. Key secondary endpoints include total cumulative OCS dose to week 36, percent change and proportion of patients with ≥ 4-point reduction in the weekly average of daily Peak Pruritus Numerical Rating Scale from baseline to week 36 and percent change in Bullous Pemphigoid Area Index score from baseline to week 36.
Conclusion
The trial results will provide evidence on whether the efficacy and safety of dupilumab support its use as a potential novel treatment approach for BP and will provide new insights into the role of type 2 inflammation in BP pathogenesis.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT04206553.
Keywords: Autoimmune blistering disorder, Bullous pemphigoid, Dupilumab, Oral corticosteroids
Key Summary Points
| Dupilumab is a fully human monoclonal antibody that inhibits the activity of both interleukin (IL)-4 and IL-13, which play a key role in type 2 inflammation in multiple diseases, including bullous pemphigoid (BP) |
| In recent case report studies, dupilumab successfully treated patients with recalcitrant moderate-to-severe BP |
| LIBERTY-BP ADEPT (NCT04206553) is an ongoing, randomized, phase 2/3 clinical trial designed to assess the efficacy and safety of dupilumab in patients with moderate-to-severe BP |
| LIBERTY-BP ADEPT will enroll approximately 98 patients from 51 global sites in 4 continents (North America, Europe, Australia and Asia) |
Introduction
Bullous pemphigoid (BP) is the most common autoimmune skin-blistering disease. BP is associated with autoantibodies directed against the hemidesmosome transmembrane BP180 and/or intracellular BP230 proteins, which are responsible for adhesion and integrity of the dermal-epidermal junction [1]. The disease incidence varies globally, with a recent meta-analysis estimating a global incidence of 8.2–13.3 per million people [2]. The incidence has reportedly increased in the past 2 decades, likely because of an aging population and the availability of more sensitive diagnostic methods [3]. A growing amount of evidence also suggests that a number of therapies can increase the risk of developing BP and a greater use of these therapies in recent years may contribute to the rising incidence of BP [3]. A recent systematic review identified 89 drugs linked to drug-associated BP, the most common of which included gliptins, programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors, loop diuretics and penicillin [4]. Other external factors that have been linked to the development or exacerbation of BP include radiotherapy, ultraviolet irradiation, burns (including thermal, chemical, or electrical burns), surgery, trauma, transplants and infections [5, 6].
BP predominantly affects the elderly population, with typical onset at > 70 years of age, and it often has a long disease course of months or years [7–9]. It characteristically clinically manifests with tense blisters over urticarial plaques, usually located on the trunk and limbs, accompanied by severe pruritus [1, 10, 11]. Patients with BP experience severely reduced quality of life due to intense itching and skin pain, sleep deprivation and lesions, which have a negative impact on their self-confidence, self-image and social activities [11–13]. BP can be life-threatening for elderly patients with associated comorbidities [1, 14–16], and its current management (which includes the use of non-selective immunosuppressants) poses safety concerns, including increased risk of infections and side effects and/or complications from polypharmacy. Concomitant diabetes and heart disease, which are common in the elderly, are specifically linked to increased mortality in patients with BP [16].
The mechanisms underpinning BP development are not completely understood. However, mounting evidence indicates that a type 2 inflammatory response may play a key role in disease development. Studies demonstrate elevated levels of the type 2 cytokines interleukin [IL]-4, IL-5 and IL-13, elevated levels of the chemokine eotaxin-1 and greater numbers of eosinophils in BP lesions and peripheral blood as well as increased serum immunoglobulin E (IgE) in patients with BP [17]. In addition, serum levels of anti-BP180 IgE were shown to correlate with the extent of affected body area [18]. Cells producing IL-4 and IL-13 are observed at a greater frequency in blister fluid than in the peripheral blood of patients with BP [19]. A potential role for type 2 inflammation in the disease pathogenesis of BP is also supported by the clinical characteristics of pruritus in BP, which resembles in intensity and frequency that seen in highly pruritic Th2-response diseases such as prurigo nodularis (PN), atopic dermatitis (AD) and chronic spontaneous urticaria (CSU) [20], where dupilumab has shown benefit. Therefore, inhibiting the type 2 inflammatory pathway is a potential therapeutic target for BP.
Currently, there are no approved drugs for BP in the US; oral corticosteroids (OCS) are approved in some countries in the European Union, and human immunoglobulin is approved in Japan for BP inadequately controlled with corticosteroids. Topical corticosteroids (TCS) are commonly indicated as first-line treatment [10, 21, 22], with variable results depending on the extent and severity of the lesions. A Cochrane Review of the evidence-based literature found that potent TCS are effective in treating BP with minimal size effects, but there are significant practical barriers associated with this treatment, including the difficulty of applying TCS to large areas of skin and a potentially greater financial cost of the treatment itself (relative to OCS) as well as the potential cost of nursing care that may be required to assist in TCS application [23]. Additionally, TCS application for extensive lesions, especially in elderly patients, is difficult to manage and carries the risk of adverse effects because of systemic absorption. Moreover, chronic use of TCS is associated with skin atrophy and increased risk of infections [23]. OCS are considered the mainstay treatment for patients with BP and are first line in certain regions; however, their use is time-limited and associated with relapses and increased mortality with long-term use [22].
Off-label non-steroid immunosuppressants (methotrexate, azathioprine, mycophenolate mofetil) and antibiotics with anti-inflammatory properties (tetracycline, sulfone) are used as adjuvant steroid-sparing therapy or independently when OCS are contraindicated; all are associated with various toxicities, including renal, mucocutaneous, pulmonary, hematologic and neurologic [10, 24–27]. Among targeted BP therapies, anti-B cell rituximab and anti-IgE omalizumab monoclonal antibodies, used off-label, were reported to achieve variable degrees of disease control in several case series [28–30]; however, their efficacy and safety in BP have not been assessed in randomized trials.
Dupilumab, a fully human VelocImmune®-derived monoclonal antibody, blocks the shared receptor component for IL-4 and IL-13, key and central drivers of type 2 inflammation in multiple diseases [31–33]. Dupilumab has been shown to decrease the type 2 inflammatory biomarkers IgE, thymus and activation-regulated chemokine, eotaxin-3 and periostin in specific diseases, including AD, PN, asthma, chronic rhinosinusitis with nasal polyposis and eosinophilic esophagitis (EoE) [34]. In multiple studies, dupilumab has demonstrated rapid, significant and clinically meaningful improvement of type 2 inflammation, pruritus and skin lesions in AD and PN [35–37]. In patients with AD, it was shown that dupilumab also reduced total and allergen-specific IgE [38, 39]. In patients with CSU, dupilumab significantly and clinically improved CSU symptoms, including severe itch [40]. In two independent randomized trials in adults and adolescents with EoE, dupilumab reduced tissue eosinophils and improved symptoms associated with EoE [41]. Furthermore, in a population with severe corticosteroid-dependent asthma, dupilumab treatment significantly reduced the use of steroids while significantly improving lung capacity and reducing exacerbations [42].
Dupilumab safety has been extensively studied and is favorable across its approved indications, including in patients > 60 years [43, 44]. Given the findings from studies of dupilumab in other diseases (i.e., a reduction in both pruritus and eosinophils), dupilumab may be effective in BP. Consistent with this idea, recent publications report successful treatment of BP, including recalcitrant and moderate-to-severe BP, with dupilumab [17, 45–49]. Here, we report the study design and rationale of the LIBERTY-BP ADEPT (NCT04206553) randomized, placebo-controlled clinical trial, designed to investigate the efficacy and safety of dupilumab in patients with BP.
Methods
Study Design
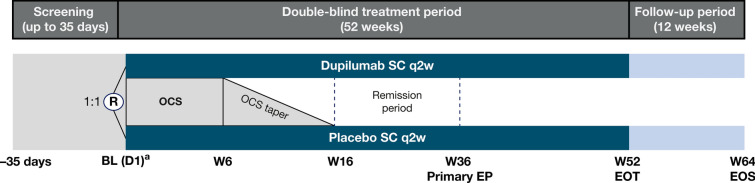
LIBERTY-BP ADEPT (NCT04206553) is a global, randomized, double-blind, placebo-controlled, parallel group, phase 2/3 study designed to assess the efficacy, safety, tolerability, pharmacokinetics and immunogenicity of dupilumab in patients with moderate-to-severe BP. The study comprises a 35-day pre-treatment screening period, a 52-week double-blind treatment period and a 12-week post-treatment follow-up period (Fig. 1). Participants included in the study will be off OCS or medium/higher potency TCS for at least 1 week before randomization.
Fig. 1.
Design of the LIBERTY-BP ADEPT study. A diagram illustrating the LIBERTY-BP ADEPT study design compromised of a screening period (up to 35 days), a treatment period (52 weeks) and a follow-up period (12 weeks). aDupilumab loading dose (or equivalent placebo dose) given at day 1. BL baseline, D1 day 1, EOS end of study, EOT end of treatment, EP endpoint, OCS oral corticosteroids, q2w every 2 weeks, R randomization, SC subcutaneous, W week
At baseline, patients will be randomized 1:1 to subcutaneous dupilumab or placebo every 2 weeks. In parallel, all patients will receive a concomitant standard-of-care regimen of OCS (prednisone or prednisolone), which will be tapered between week 6 and week 16.
For patients who achieve complete remission off steroid therapy for at least 2 months, the interval between week 16 and week 36 will be considered the remission period, during which patients will be assessed for relapses. A relapse is defined as the appearance of at least one new eczematous lesion or urticarial plaque with diameter > 10 cm that does not heal within a week, or as the appearance of at least three smaller new lesions in the last month (blisters, eczematous lesions or urticarial plaques) [50].
Patient Population Selection
Approximately 98 patients will be recruited at 51 global sites. At baseline, eligible patients will have confirmed histopathologic, immunopathologic and serologic diagnosis of BP; clinical BP signs (urticarial or eczematous/erythematous plaques, bullae); Bullous Pemphigoid Disease Area Index (BPDAI) activity score ≥ 24; Peak Pruritus Numerical Rating Scale (NRS) for maximum itch intensity ≥ 4; Karnofsky Performance Status Scale score ≥ 50%. Patients with other pemphigoid diseases, systemic or parasitic infections, BP secondary to medications or severe concomitant illnesses will be excluded at screening. Detailed inclusion and exclusion criteria are presented in Table 1.
Table 1.
Key inclusion and exclusion criteria of the LIBERTY-BP ADEPT study
| Key inclusion criteria | Key exclusion criteria |
|---|---|
|
•A Karnofsky Performance Status Scale score ≥ 50% at screening •Baseline Peak Pruritus NRS score for maximum itch intensity ≥ 4 (based on the average of daily NRS scores for maximum itch intensity during the 7 days before randomization) •A BPDAI activity score ≥ 24 at baseline and screening visits •Clinical features of BP with a confirmed diagnosis based on histopathology, immunopathology and serology •Aged between 18 and 90 years (aged 20–90 years for study sites in Japan) |
•Forms of pemphigoid other than classic BP •Previous treatment with IL-4 or IL-13 antagonists •Within 7 days of baseline visit, treatment with: –Systemic or medium-to-high potency topical corticosteroids –BP-directed antibiotics such as doxycycline or dapsone –Nicotinamide (although use of multivitamins containing nicotinamide is allowed) •Treatment with certain BP-directed biologics after a specified washout period prior to baseline •History of immunosuppression or HIV •Severe concomitant illnesses that would adversely affect the patient’s participation in the study •Certain laboratory abnormalities |
BP bullous pemphigoid, BPDAI bullous pemphigoid disease area index, HIV human immunodeficiency virus, IL interleukin, NRS numerical rating scale
Planned Outcomes
The primary endpoint is the proportion of patients achieving sustained remission at week 36. Sustained remission is defined by concomitant achievement of complete tapering off OCS and complete remission (no new lesions and epithelialization of old lesions) by week 16, no relapse by week 36 and no rescue treatments by week 36.
Key secondary endpoints include total cumulative dose of OCS from baseline to week 36; percent change in weekly average of daily Peak Pruritus NRS from baseline to week 36; proportion of patients with improvement (reduction) in weekly average of daily Peak Pruritus NRS ≥ 4 from baseline to week 36; percent change in BPDAI score from baseline to week 36.
Other secondary endpoints at week 36 and week 52 address additional disease activity signs and symptoms, patient-reported outcomes and changes in BP180/BP230 IgG titers from baseline. Clinical pharmacology and immunogenicity endpoints include serum concentration of dupilumab over time and incidence of treatment-emergent antidrug antibodies and titers from baseline up to week 64 (end of study).
Assessment Scales
Peak Pruritus NRS is a patient-reported outcome of the maximum itch intensity on a scale from 0 (“no itch”) to 10 (“worst itch imaginable”). Patients will be asked to record a daily maximum score; the weekly average of daily scores will be used to assess efficacy. Peak Pruritus NRS is widely used in clinical trials to assess itch intensity in chronic pruritus [51] and is validated in other pruritic dermatoses, such as AD, with a threshold of clinically meaningful reduction of ≥ 2–4 points [52].
BPDAI is a validated instrument in BP [53, 54], with a total score, which includes skin activity and mucosal activity, ranging from 0 to 360. Total BPDAI is calculated as the sum of scores for cutaneous blisters/erosions, cutaneous urticaria/erythema and mucosal blisters/erosions (each ranging from 0 to 120). Higher scores indicate higher disease activity. The minimal clinically important difference for BPDAI activity score is a 4-point reduction for assessing improvement and a 3-point increase for assessing deterioration [53].
The Autoimmune Bullous Disease Quality of Life (ABQOL) questionnaire is a 17-item validated quality of life instrument, which has demonstrated responsiveness in BP in multiple cultures and languages [55–62].
Data Collection
All data generated from this study will be recorded and reported in accordance with the protocol, Good Clinical Practice (GCP) and any applicable regulatory requirement in accordance with International Council for Harmonisation (ICH) E6. All case report form data will be collected with an electronic data capture system.
Data Analysis
A hierarchical testing procedure will be used for multiplicity control, with each endpoint sequentially tested at a two-sided significance level until the hierarchy is broken.
Ethics
This clinical trial will be conducted in accordance with the ethical principles originating in the Declaration of Helsinki and consistent with the ICH guidelines for GCP, the Japanese GCP and applicable regulatory requirements. All participants must sign an Informed Consent Form prior to their participation in the study, reviewed and approved by the institutional review board (IRB) and the ethics committee (EC). Any suspected unexpected serious adverse reaction during the study will be reported to the health authorities, EC, IRB and participating investigators. Final study results will be published on a public clinical trial website according to applicable local guidelines and regulations.
The safety profile of dupilumab has been well established in several clinical trials across multiple indications; however, the LIBERTY-BP ADEPT study is the first primary clinical trial to be conducted in elderly patients with a severe, potentially life-threatening disease. Therefore, an additional independent data monitoring committee, composed of members who are independent from the sponsor and the study investigators, will monitor the safety of dupilumab in patients by performing formal reviews of accumulated safety data that will be blinded by treatment group.
Strengths and Limitations
Strengths of the study include the fact that it is the first multicenter, randomized, placebo-controlled trial to assess efficacy and safety of dupilumab as steroid-sparing treatment for moderate-to-severe BP. Additionally, the 1-year treatment duration, with dupilumab-/placebo-only exposure for 52 weeks, considers the typically prolonged disease course and allows for observation of treatment effects beyond the timing of the primary endpoint at week 36.
Limitations include the concomitant use of OCS, which limits the assessment of dupilumab in achieving disease control as monotherapy; however, the inclusion of OCS is necessary, as patients in the placebo group require some degree of treatment.
Discussion and Dissemination
BP is a severe disease of an elderly population, and therefore LIBERTY-BP-ADEPT was designed to assess the ability of dupilumab to achieve sustained remission in patients with BP, while being able to fully taper OCS. Dupilumab has shown a favorable safety profile in several clinical trials, highlighting its tolerability and positive risk-benefit profile [35–37, 41, 42, 63].
In clinical practice, the average time to cessation of corticosteroid use is an important aspect of patient management, given that prolonged OCS use is associated with toxicity and increased mortality, particularly in elderly patients [10, 22, 24]. Although the optimal duration of OCS use is unclear, experts recommend a total treatment duration of 4–12 months [21]. Even low doses of OCS may result in OCS-related adverse events such as osteoporosis, heart failure, diabetes and infections [64], highlighting the importance of minimizing OCS treatment duration. In clinical practice, the duration of continuous treatment with OCS is often much longer; a recent prospective cohort study of 2,312 patients with BP in the UK found that a substantial proportion of patients (39.7%) were continuously treated with OCS for over a year, with 14.7% of patients treated with OCS for > 3 years, 5.0% for > 5 years and 1.7% for > 10 years [65].
The aim of the LIBERTY BP-ADEPT study (i.e., to assess whether and to what extent treatment with dupilumab can sustain disease remission once OCSs are tapered off by week 16 or earlier) is reflected in the choice of the primary and key secondary endpoints: the proportion of patients with sustained remission at week 36 (at least 20 weeks after OCS are tapered off at week 16 or sooner) and the cumulative dose of OCS from baseline to week 36. The primary endpoint in this study is quite stringent, particularly given the short timeline to achieve this endpoint relative to treatment durations commonly used in clinical practice, which are often substantially longer than 16 weeks [65].
Pruritus is an important symptom and a major factor affecting quality of life in patients with BP [1, 10, 13]. In a multicenter study, 85% of patients with BP experienced daily itch, with a mean intensity of 5.2/10 [13]. In the LIBERTY BP-ADEPT study, the key secondary endpoints of percent change in Peak Pruritus NRS from baseline at week 36 and proportion of patients with ≥ 4-point improvement in Peak Pruritus NRS at week 36 will investigate the effect of dupilumab on significant and clinically meaningful itch improvement in patients with BP.
In previous studies of BP treated with standard-of-care OCS, BPDAI score reached a minimum point at week 16 (4 months) after treatment started [66]. In the LIBERTY BP-ADEPT study design, the key secondary endpoint of percent change in BPDAI score from baseline to week 36 was chosen to assess the effect of dupilumab after early OCS discontinuation on blister, urticarial and mucosal activity.
In summary, LIBERTY-BP ADEPT is a landmark trial designed to assess the efficacy and safety of dupilumab as a steroid-sparing therapy in BP and to provide insights into the role of type 2 inflammation in the disease’s pathophysiology. The results of this study will be disseminated through peer-reviewed journals and dermatology congresses.
Acknowledgments
Medical Writing, Editorial and Other Assistance
Medical writing/editorial assistance was provided by Moataz Badawi, PhD, and Iulia Oprea, MD, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals Inc., according to the Good Publication Practice guidelines.
Author Contributions
All authors contributed to this manuscript and read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research and the journal’s Rapid Service and Open Access Fees were sponsored by Sanofi and Regeneron Pharmaceuticals, Inc.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Dédée F. Murrell is an investigator, advisory board member for Argenx, Principia Biopharma, Regeneron Pharmaceuticals Inc., Roche; investigator for AstraZeneca; co-creator of BPDAI; creator of ABQOL. Pascal Joly is a consultant for Amgen, Argenx, AstraZeneca, Innovaderm, Principia Biopharma, Roche, Sanofi-Regeneron Pharmaceuticals Inc., Thermo Fisher Scientific. Victoria P. Werth is a consultant for AbbVie, Argenx, AstraZeneca, Janssen, Kyowa Hakko Kirin, Eli Lilly, Principia Biopharma, Regeneron Pharmaceuticals Inc., Roche-Genentech; reports grants from Argenx, Roche-Genentech, Sanofi-Regeneron Pharmaceuticals Inc., Syntimmune; co-creator of BPDAI. Hideyuki Ujiie is a consultant for Argenx, Ishin Pharma. Margitta Worm reports consultancy for AbbVie, Aimmune Therapeutics, ALK-Abelló, Amgen, AstraZeneca, Boehringer Ingelheim, DBV Technologies, Kymab, LEO Pharma, Lilly, GSK, Mylan, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., Sanofi-Aventis. Aaron R. Mangold reports consultancy for Argenx, Boehringer Ingelheim, Bristol Myers Squibb, Clarivate, Eli Lilly, Incyte, Janssen, Kyowa Kirin, Momenta, Pfizer, PHELEC, Regeneron Pharmaceuticals Inc., Soligenix; grants from AbbVie, Akari Therapeutics, Argenx, Corbus, Eli Lilly, Elorac, Incyte, Janssen, Kyowa Kirin, Merck, Miragen, Novartis, Palvella, Pfizer, Priovant Therapeutics, Regeneron Pharmaceuticals Inc., Soligenix, Sun Pharma, UCB; patents for Methods and Materials for Assessing and Treating Cutaneous Squamous Cell Carcinoma (provisional 63-423254), Use of oral JAKi in Lichen Planus (provisional 63/453,065), Topical Ruxolitinib in Lichen Planus (WO2022072814A1). Elena Avetisova, Jennifer Maloney, and Arsalan Shabbir are employees and shareholders of Regeneron Pharmaceuticals Inc. Elizabeth Laws and Ariane Dubost-Brama are Sanofi employees and may hold stock and/or stock options in the company. Eric Mortensen was an employee and shareholder of Regeneron Pharmaceuticals Inc. during the development of this manuscript, and currently is an employee at AstraZeneca.
Ethical Approval
This clinical trial will be conducted in accordance with the ethical principles originating in the Declaration of Helsinki, and consistent with the International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP), the Japanese GCP, and applicable regulatory requirements. All participants must sign an informed consent form prior to their participation in the study, reviewed and approved by the institutional review board (IRB) and the ethics committee (EC). Any suspected unexpected serious adverse reaction during the study will be reported to the health authorities, EC, IRB, and participating investigators. Final study results will be published on a public clinical trial website according to applicable local guidelines and regulations. The safety profile of dupilumab has been well established in several clinical trials across multiple indications; however, the LIBERTY-BP ADEPT study is the first primary clinical trial to be conducted in elderly patients with a severe, potentially life-threatening disease. Therefore, an additional independent data monitoring committee, composed of members who are independent from the sponsor and the study investigators, will monitor the safety of dupilumab in patients by performing formal reviews of accumulated safety data that will be blinded by treatment group.
Footnotes
The original online version of this article was revised due to update in text.
Change history
5/24/2024
A Correction to this paper has been published: 10.1007/s12325-024-02886-x
References
- 1.Schmidt E, Seitz CS, Benoit S, Bröcker EB, Goebeler M. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol. 2007;156(2):352–356. doi: 10.1111/j.1365-2133.2006.07646.x. [DOI] [PubMed] [Google Scholar]
- 2.Persson MSM, Begum N, Grainge MJ, Harman KE, Grindlay D, Gran S. The global incidence of bullous pemphigoid: a systematic review and meta-analysis. Br J Dermatol. 2022;186(3):414–425. doi: 10.1111/bjd.20743. [DOI] [PubMed] [Google Scholar]
- 3.Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne) 2018;5:220. doi: 10.3389/fmed.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verheyden MJ, Bilgic A, Murrell DF. A systematic review of drug-induced pemphigoid. Acta Derm Venereol. 2020;100(15):adv00224. [DOI] [PMC free article] [PubMed]
- 5.Moro F, Fania L, Sinagra JLM, Salemme A, Di Zenzo G. Bullous pemphigoid: trigger and predisposing factors. Biomolecules. 2020;10(10):1432. doi: 10.3390/biom10101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mul VE, van Geest AJ, Pijls-Johannesma MC, et al. Radiation-induced bullous pemphigoid: a systematic review of an unusual radiation side effect. Radiotherapy Oncol. 2007;82(1):5–9. doi: 10.1016/j.radonc.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Joly P, Baricault S, Sparsa A, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132(8):1998–2004. doi: 10.1038/jid.2012.35. [DOI] [PubMed] [Google Scholar]
- 8.Jung M, Kippes W, Messer G, Zillikens D, Rzany B. Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J Am Acad Dermatol. 1999;41(2 Pt 1):266–268. doi: 10.1016/S0190-9622(99)70061-7. [DOI] [PubMed] [Google Scholar]
- 9.Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJP, West J. Bullous pemphigoid and pemphigus vulgaris—incidence and mortality in the UK: population-based cohort study. BMJ. 2008;337(7662):a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol. 2017;18(4):513–528. doi: 10.1007/s40257-017-0264-2. [DOI] [PubMed] [Google Scholar]
- 11.Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid. A case control study. An Bras Dermatol. 2016;91(5):601–3. [DOI] [PMC free article] [PubMed]
- 12.Wang EQ, Castrillón Velásquez MA, Murrell DF. The effects of autoimmune blistering diseases on work productivity: a review. Int J Womens Dermatol. 2018;4(3):131–138. doi: 10.1016/j.ijwd.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briand C, Gourier G, Poizeau F, et al. Characteristics of pruritus in bullous pemphigoid and impact on quality of life: a prospective cohort study. Acta Derm Venereol. 2020;100(18):adv00320. [DOI] [PMC free article] [PubMed]
- 14.Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133–146. doi: 10.1590/abd1806-4841.20199007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.della Torre R, Combescure C, Cortés B, et al. Clinical presentation and diagnostic delay in bullous pemphigoid: a prospective nationwide cohort. Br J Dermatol. 2012;167(5):1111–7. [DOI] [PubMed]
- 16.Chen X, Zhang Y, Luo Z, et al. Prognostic factors for mortality in bullous pemphigoid: a systematic review and meta-analysis. PLoS ONE. 2022;17(4):e0264705. doi: 10.1371/journal.pone.0264705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidman JS, Eichenfield DZ, Orme CM. Targeting type 2 inflammation for treatment of bullous pemphigoid. J Dermatol Skin Sci. 2020;2(1):29–33. [Google Scholar]
- 18.Kalowska M, Ciepiella O, Kowalewski C, Demkow U, Schwartz RA, Wozniak K. Enzyme-linked immunoassay index for anti-NC16a IgG and IgE auto-antibodies correlates with severity and activity of bullous pemphigoid. Acta Dem Venereol. 2016;96(2):191–196. doi: 10.2340/00015555-2101. [DOI] [PubMed] [Google Scholar]
- 19.Russo R, Cozzani E, Gasparini G, Parodi A. Targeting interleukin 4 receptor α: a new approach to the treatment of cutaneous autoimmune bullous diseases? Dermatol Ther. 2020;33(1):e13190. doi: 10.1111/dth.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcovitch S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines (Basel) 2021;9(3):303. doi: 10.3390/vaccines9030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feliciani C, Joly P, Jonkman MF, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172(4):867–877. doi: 10.1111/bjd.13717. [DOI] [PubMed] [Google Scholar]
- 22.Joly P, Roujeau J-C, Benichou J, et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346(5):321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 23.Kirtschig G, Middleton P, Bennet C, Murell DF, Wojnarowska F, Khumalo NP. Interventions for bullous pemphigoid. Cochrane Database Syst Rev. 2010;(10):CD002292. [DOI] [PMC free article] [PubMed]
- 24.Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G. British Association of Dermatologists' guidelines for the management of bullous pemphigoid 2012. Br J Dermatol. 2012;167(6):1200–1214. doi: 10.1111/bjd.12072. [DOI] [PubMed] [Google Scholar]
- 25.Khalid SN, Khan ZA, Ali MH, Almas T, Khedro T, Nagarajan VR. A blistering new era for bullous pemphigoid: a scoping review of current therapies, ongoing clinical trials, and future directions. Ann Med Surg (Lond) 2021;70:102799. doi: 10.1016/j.amsu.2021.102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fichel F, Barbe C, Joly P, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014;150(1):25–33. doi: 10.1001/jamadermatol.2013.5757. [DOI] [PubMed] [Google Scholar]
- 27.Gaies E, Jebabli N, Trabelsi S, et al. Methotrexate side effects: review article. J Drug Metabol Toxicol. 2012;3(4):123–125. doi: 10.4172/2157-7609.1000125. [DOI] [Google Scholar]
- 28.Hall RP, 3rd, Streilein RD, Hannah DL, et al. Association of serum B-cell activating factor level and proportion of memory and transitional B cells with clinical response after rituximab treatment of bullous pemphigoid patients. J Invest Dermatol. 2013;133(12):2786–2788. doi: 10.1038/jid.2013.236. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 30.Fairley JA, Baum CL, Brandt DS, Messingham KAN. Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol. 2009;123(3):704–705. doi: 10.1016/j.jaci.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy. 2020;75(5):1188–1204. doi: 10.1111/all.14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111:5147–5152. doi: 10.1073/pnas.1323896111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111:5153–5158. doi: 10.1073/pnas.1324022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton JD, Harel S, Swanson BN, et al. Dupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases. Clin Exp Allergy. 2021;51(7):915–931. doi: 10.1111/cea.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koskeridis F, Evangelos E, Ntzani EE, Kostikas K, Tsabouri S. Treatment with dupilumab in patients with atopic dermatitis: systematic review and meta-analysis. J Cutan Med Surg. 2022;26(6):613–621. doi: 10.1177/12034754221130969. [DOI] [PubMed] [Google Scholar]
- 36.Silverberg JI, Yosipovitch G, Simpson EL, et al. Dupilumab treatment results in early and sustained improvements in itch in adolescents and adults with moderate to severe atopic dermatitis: analysis of the randomized phase 3 studies SOLO1 and SOLO2, AD ADOL and CHRONOS. J Am Acad Dermatol. 2020;82(6):1328–1336. doi: 10.1016/j.jaad.2020.02.060. [DOI] [PubMed] [Google Scholar]
- 37.Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29(5):1180–1190. doi: 10.1038/s41591-023-02320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geba GP, Li D, Xu M, et al. Attenuating the atopic march: meta-analysis of the dupilumab atopic dermatitis database for incident allergic events. J Allergy Clin Immunol. 2023;151(3):756–766. doi: 10.1016/j.jaci.2022.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Siegfried EC, Cork MJ, Boguniewicz M, et al. Dupilumab treatment reduces total IgE levels in patients 6 months and older with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2023;151(2 Suppl):AB149, Poster No. 456.
- 40.Maurer M, Casale T, Saini S, et al. Dupilumab significantly reduces itch and hives in patients with chronic spontaneous urticaria: results from a phase 3 trial (LIBERTY-CSU CUPID Study A). J Allergy Clin Immunol. 2022;149(2 Suppl):AB312.
- 41.Dellon ES, Rothenberg ME, Collins MH, et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N Engl J Med. 2022;387(25):2317–2330. doi: 10.1056/NEJMoa2205982. [DOI] [PubMed] [Google Scholar]
- 42.Domingo C, Maspero JF, Castro M, et al. Dupilumab efficacy in steroid-dependent severe asthma by baseline oral corticosteroid dose. J Allergy Clin Immunol Pract. 2022;10(7):1835–1843. doi: 10.1016/j.jaip.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Silverberg JI, Lynde CW, Abuabara K, et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023;24(3):469–483. doi: 10.1007/s40257-022-00754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patruno C, Fabbrocini G, Longo G, et al. Effectiveness and safety of long-term dupilumab treatment in elderly patients with atopic dermatitis: a multicenter real-life observational study. Am J Clin Dermatol. 2021;22(4):581–586. doi: 10.1007/s40257-021-00597-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154(10):1225–1226. doi: 10.1001/jamadermatol.2018.2526. [DOI] [PubMed] [Google Scholar]
- 46.Seyed Jafari SM, Feldmeyer L, Bossart S, Simon D, Schlapbach C, Borradori L. Case report: combination of omalizumab and dupilumab for recalcitrant bullous pemphigoid. Front Immunol. 2021;11:611549. doi: 10.3389/fimmu.2020.611549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleh M, Reedy M, Torok H, Weaver J. Successful treatment of bullous pemphigoid with dupilumab: a case and brief review of the literature. Dermatol Online J. 2021;27(4):13030/qt0dv3f9h6. [PubMed]
- 48.Abdat R, Waldman RA, de Bedout V, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52. doi: 10.1016/j.jaad.2020.01.089. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Front Immunol. 2021;12:738907. doi: 10.3389/fimmu.2021.738907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murrell DF, Daniel BS, Joly P, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66(3):479–485. doi: 10.1016/j.jaad.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topp J, Apfelbacher C, Ständer S, Augustin M, Blome C. Measurement properties of patient-reported outcome measures for pruritus: an updated systematic review. J Invest Dermatol. 2022;142(2):343–354. doi: 10.1016/j.jid.2021.06.032. [DOI] [PubMed] [Google Scholar]
- 52.Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181(4):761–769. doi: 10.1111/bjd.17744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wijayanti A, Zhao CY, Boettiger D, et al. The reliability, validity and responsiveness of two disease scores (BPDAI and ABSIS) for bullous pemphigoid: which one to use? Acta Derm Venereol. 2017;97(1):24–31. doi: 10.2340/00015555-2473. [DOI] [PubMed] [Google Scholar]
- 54.Patsatsi A, Kyriakou A, Pavlitou-Tsiontsi A, Giannakou A, Sotiriadis D. Association of autoantibodies to BP180 with disease activity in Greek patients with bullous pemphigoid. Clin Dev Immunol. 2012;2012:854795. doi: 10.1155/2012/854795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sebaratnam DF, Hanna AM, Chee SN, et al. Development of a quality-of-life instrument for autoimmune bullous disease: the Autoimmune Bullous Disease Quality of Life questionnaire. JAMA Dermatol. 2013;149(10):1186–1191. doi: 10.1001/jamadermatol.2013.4972. [DOI] [PubMed] [Google Scholar]
- 56.Tjokrowidjaja A, Daniel BS, Frew JW, et al. The development and validation of the treatment of autoimmune bullous disease quality of life questionnaire, a tool to measure the quality of life impacts of treatments used in patients with autoimmune blistering disease Br J Dermatol. 2013;169(5):1000–6. Erratum in Br J Dermatol. 2014;170(2):481–3. [DOI] [PubMed]
- 57.Ferries L, Gillibert A, Duvert-Lehembre S, et al. Sensitivity to change and correlation between the autoimmune bullous disease quality-of-life questionnaires ABQOL and TABQOL, and objective severity scores. Br J Dermatol. 2020;183(5):944–945. doi: 10.1111/bjd.19173. [DOI] [PubMed] [Google Scholar]
- 58.Yang B, Chen G, Yang Q, et al. Reliability and validity of the Chinese version of the autoimmune bullous disease quality of life (ABQOL) questionnaire. Health Qual Life Outcomes. 2017;15(1):31. doi: 10.1186/s12955-017-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saleh MA, Zaraa I, Doss N, Saleh NA, Murrell DF. Assessment of the quality of life of Egyptian and Tunisian autoimmune bullous diseases' patients using an Arabic version of the autoimmune bullous disease quality of life and the treatment of autoimmune bullous disease quality of life questionnaires. An Bras Dermatol. 2019;94(4):399–404. doi: 10.1590/abd1806-4841.20197198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teimourpour A, Hedayat K, Salarvand F, et al. Autoimmune Bullous Disease Quality of Life (ABQoL) questionnaire: validation of the translated Persian version in pemphigus vulgaris. Int J Womens Dermatol. 2020;6(4):306–310. doi: 10.1016/j.ijwd.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patsatsi A, Kokolios M, Kyriakou A, et al. Quality of life in Greek patients with autoimmune bullous diseases assessed with ABQOL and TABQOL indexes. Acta Derm Venereol. 2017;97(9):1145–1147. doi: 10.2340/00015555-2737. [DOI] [PubMed] [Google Scholar]
- 62.Kalinska-Bienias A, Jakubowska B, Kowalewski C, Murrell DF, Wozniak K. Measuring of quality of life in autoimmune blistering disorders in Poland. Validation of disease-specific Autoimmune Bullous Disease Quality of Life (ABQOL) and the Treatment Autoimmune Bullous Disease Quality of Life (TABQOL) questionnaires. Adv Med Sci. 2017; 62(1):92–6. [DOI] [PubMed]
- 63.Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(2):131–143. doi: 10.1001/jamadermatol.2019.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silverberg JI, Yang F, Alemao E, et al. The risk of adverse events in US bullous pemphigoid patients treated with oral corticosteroids. Oral presentation at the 25th World Congress of dermatology; Singapore; July 3–8, 2023.
- 65.Persson MSM, Harman KE, Thomas KS, et al. Long-term oral prednisolone exposure in primary care for bullous pemphigoid: population-based study. Br J Gen Pract. 2021;71(713):e904–e911. doi: 10.3399/BJGP.2020.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cole EF, DeGrazia T, Sun Y, Liu Y, Feldman RJ. Assessing disease outcome measures in bullous pemphigoid on standard-of-care therapies. JID Innov. 2021;1(4):100050. doi: 10.1016/j.xjidi.2021.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.