Abstract
Entry of Epstein-Barr virus (EBV) into B cells is initiated by attachment of glycoprotein gp350 to the complement receptor type 2 (CR2). A complex of three glycoproteins, gH, gL, and gp42, is subsequently required for penetration. Gp42 binds to HLA class II, which functions as an entry mediator or coreceptor and, by analogy with other herpesviruses, gH is then thought to be involved virus-cell fusion. However, entry of virus into epithelial cells is thought to be different. It can be initiated by attachment by an unknown glycoprotein in the absence of CR2. There is no interaction between gp42 and HLA class II and instead a distinct complex of only the two glycoproteins gH and gL interacts with a novel entry mediator. Again, by analogy with other viruses gH is thought to be critical to fusion. To investigate further the different roles of gH in infection of the two cell types and to examine its influence on the assembly of the gH-gL-gp42 complex, we constructed two viruses, one in which the gH open reading frame was interrupted by a cassette expressing a neomycin resistance gene and the gene for green fluorescent protein and one as a control in which the neighboring nonessential thymidine kinase gene was interrupted with the same cassette. Virus lacking gH exited from cells normally, although loss of gH resulted in rapid turnover of gL and gp42 as well. The virus bound normally to B lymphocytes but could not infect them unless cells and bound virus were treated with polyethylene glycol to induce fusion. In contrast, virus that lacked the gH complex was impaired in attachment to epithelial cells and the effects of monoclonal antibodies to gH implied that this resulted from loss of gH rather than other members of the complex. These results suggest a role for gH in both attachment and penetration into epithelial cells.
The Epstein-Barr virus (EBV) gH-gL complex consists of three glycoproteins, gp85, the gH homolog which is the product of the BXLF2 open reading frame (ORF) (12, 26); gp25, the gL homolog which is the product of the BKRF2 ORF (38); and gp42, which is the product of the BZLF2 ORF (17). The complex behaves in many respects like its counterparts in other herpesviruses. Glycoprotein gH is dependent on gL for authentic processing and transport (38), and the complex as a whole has been implicated as important to the ability of virus to fuse with the cell membrane and penetrate into the cytoplasm (11, 22). The precise roles of the individual members of the complex are, however, still being elucidated and appear to depend in part on the cell type that the virus is infecting.
Infection of the B lymphocyte is dependent on an interaction between gp42 and HLA class II (32) which functions as an entry mediator for this cell type (16). A monoclonal antibody (MAb) to gp42 that blocks binding to HLA class II blocks virus-cell fusion (22), B cells that lack class II cannot be superinfected unless class II expression is restored (16), and a virus that lacks gp42 is unable to infect B cells unless a soluble form of gp42 that reassociates with the gH-gL complex is supplied in trans (36). These experiments have been interpreted to mean that following attachment of the virus glycoprotein gp350/220 (24, 34) to its primary B cell receptor, the complement receptor type 2 (CR2) (6, 25), gp42 binds to HLA class II (16). By analogy with other herpesviruses, the next step in virus entry is thought to involve a critical role for gH in the fusion process. However, no MAbs that react with gH and neutralize B-cell infection have yet been found, and thus there is currently no direct evidence for the involvement of gH in B-cell infection.
Infection of the epithelial cell is different in several important respects. First, although it may be initiated by attachment of virus to CR2 (5, 18), there is also evidence for CR2-independent attachment mediated by an as-yet-unknown EBV glycoprotein (15, 39). Second, the roles played by the members of the gH complex are different. Although supply of soluble gp42 in trans to wild-type virus or, in larger amounts, to virus deleted for expression of gp42 can inhibit infection as well or better than it can inhibit infection of B cells, the native glycoprotein is dispensable for infection of epithelial cells (37). In addition, MAbs that react with gH and which have no effect on B-cell transformation are capable of neutralizing infection of epithelial cells (17). These experiments were initially done with an epithelial cell line called SVKCR2 to which virus binds via an interaction between gp350 and CR2 and have been repeated with cell lines that lack CR2. They have been interpreted to mean that following attachment of virus to a primary receptor, gH interacts with a novel coreceptor or entry mediator via a domain that can be blocked by at least some neutralizing MAbs to gH or by the addition of gp42 to the gH-gL complex. Analysis of the gH-gL-gp42 complex in wild-type virus has indicated the presence of more gH and gL than gp42, further supporting a hypothesis in which virus maintains both a three-part complex that is required to infect B cells and a two-part complex, lacking gp42, that is required to infect epithelial cells (37). Analysis of virus lacking gp42 also reveals that gH and gL alone can form a detergent-stable complex (36). The effects of the loss of gH are, however, less certain. Velocity sedimentation analysis of the preformed complex has suggested that there may be an interaction between gp42 and gL (17), but it has not proven possible to demonstrate such an interaction in a transient-expression system.
Two types of recombinant virus were made in order to answer some of these questions that remain about the role of gH in assembly and function of the gH-gL-gp42 complex, one in which the BXLF2 ORF was disrupted and one in which the nonessential thymidine kinase encoded by the BXLF1 ORF was disrupted. In both viruses a cassette expressing neomycin resistance and a modified green fluorescence protein (GFP) was used for the disruption to facilitate identification of successfully penetrated cells. We report here that virus lacking gH also fails to express detectable amounts of gL and gp42. Not only does it fail to penetrate B cells but it is also impaired in attachment to CR2 negative epithelial cells.
MATERIALS AND METHODS
Cells.
Akata, a Burkitt lymphoma-derived cell line that carries EBV and can be induced to make virus (33) (a gift of John Sixbey, St. Jude Children's Research Hospital, Memphis, Tenn.) and EBV-negative Akata cells (a gift of Jeffrey Sample, St. Jude Children's Research Hospital) were grown in RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco-BRL Life Technologies, Grand Island, N.Y.). AGS cells (a gift of Shosuke Imai, Hokkaido University School of Medicine, Sapporo, Japan) were grown in Ham F-12 nutrient mixture (Gibco-BRL) supplemented with 10% heat inactivated fetal bovine serum. SVKCR2 cells (18) (a gift of A. B. Rickinson, University of Birmingham, Birmingham, England) were grown in Joklik modified Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) and 10 ng of cholera toxin (Sigma) per ml. Human leukocytes were obtained from heparinized adult peripheral blood by flotation on lymphocyte separation medium and depleted of T cells by a double cycle of rosetting with sheep erythrocytes as previously described (17).
Virus production.
EBV was obtained from the clarified culture medium of Akata cells that had been resuspended at a concentration of 4 × 106 per ml and induced with 50 μg of anti-human immunoglobulin G per ml for 5 days. Virus was either harvested under sterile conditions, passed through a 0.8-μm (pore-size) filter and used directly or else concentrated by high-speed centrifugation, resuspended in fresh medium, and sterilized by passing through a 0.45-μm (pore-size) filter.
Antibodies.
MAbs 72A1 reacting with gp350 (13), E2A5 reacting with gp78 (20), F-2-1 reacting with gp42 (17), and E1D1 (26), CL59, and CL40 reacting with gH were obtained from spent culture medium of hybridoma cells grown in RPMI 1640 supplemented with 20% heat-inactivated fetal bovine serum. MAb F-2-1 is of the immunoglobulin G2a subclass. All the other MAbs are of the immunoglobulin G1 subclass. MAbs E1D1, CL59, and CL40 all recognize different epitopes on gH. MAb CL59 reacts equally well in indirect immunofluorescence assays with recombinant gH expressed alone or gH expressed in combination with gL, whereas MAb CL40 reacts strongly with gH expressed in combination with gL and only weakly with gH expressed alone (data not shown). MAb E1D1 only reacts with gH that is coexpressed with gL in mammalian cells (17), but it does react with a truncated form of gH expressed in insect cells, confirming that the epitope it recognizes is present on gH. An antipeptide antibody (anti-gL) was made to a synthetic peptide corresponding to residues 125 to 137 of gL (38). All antibodies were purified by chromatography on protein A (Sigma) coupled to Affigel-15 (Bio-Rad, Richmond, Calif.).
Radiolabeling and immunoprecipitation.
EBV proteins were labeled biosynthetically with [3H]glucosamine (20 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) for 20 h at 6 h after induction with anti-human immunoglobulin G as previously described (38). Labeled cells were solubilized in radioimmunoprecipitation buffer (50 mM Tris-HCl, pH 7.2; 0.15 M NaCl; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate [SDS]; 0.1 mM phenylmethylsulfonyl fluoride; 100 U of aprotinin per ml) and immunoprecipitated with antibody and protein A-Sepharose CL4B (Sigma). Immunoprecipitated proteins were washed, dissociated by boiling in sample buffer containing 2-mercaptoethanol, and analyzed by SDS-polyacrylamide gel electrophoresis in 10% acrylamide cross-linked with 0.28% N,N′-diallyltartardiamide, followed by fluorography.
Derivation of viruses in which either the BXLF2 ORF or BXLF1 ORF is disrupted.
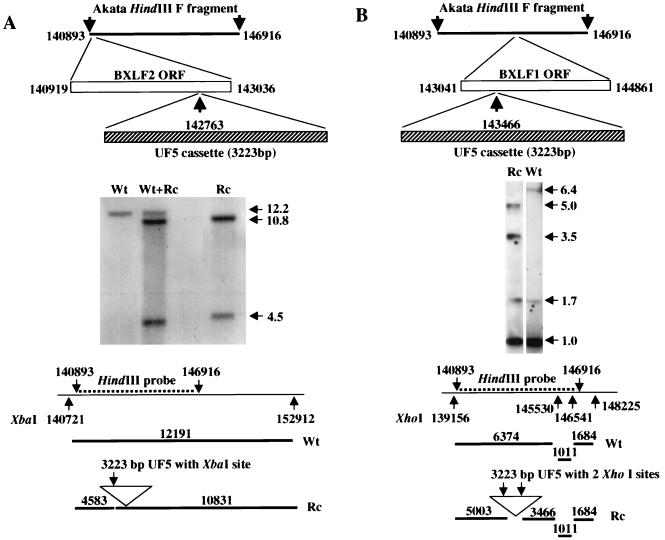
Dextran-purified virus harvested from the spent medium of 8 × 109 Akata cells was digested with proteinase K, and virion DNA was purified three times by centrifugation in cesium chloride. DNA that sedimented at a density of 1.718 g of cesium per ml was digested with HindIII. The 6-kb F fragment which corresponded to bp 140893 to 146916 of the B95-8 sequence (2) was cloned into pGEM (Promega) that had been modified to remove the HincII site in the multiple cloning site. A 3,223-bp SmaI fragment containing the neomycin resistance gene under control of a thymidine kinase promoter and a modified GFP gene under control of the cytomegalovirus promoter (UF5) was excised from plasmid pTR-UF5 (received from Nicholas Muzyczka, University of Florida, Gainesville, Fla.) which had been derived from pTRBS-UF3 (40). It was cloned either into a unique EcoRV site at bp 142763, 273 bp from the initiation codon of the BXLF2 ORF, or into a unique HincII site at bp 143466, 1,395 bp from the initiation codon of the BXLF1 ORF (Fig. 1). The HindIII F fragments, now 9.2 kb as a result of the insertions, were purified and used to transfect Akata cells using DEAE-dextran as described previously (36). Cells were then plated at 104 cells per well in 96-well tissue culture plates in medium containing 700 μg (500 μg active) per ml of G418 (Gibco-BRL/Life Technologies, Grand Island, N.Y.) and fed weekly with fresh drug-containing medium. Resistant clones began to emerge after approximately 3 weeks. Drug-resistant Akata cells were screened by Southern blotting for the presence of homologous or illegitimate recombination with cellular or viral DNA. To eliminate wild-type episomes in cells that screened positive for homologous recombination, virus was induced and rescued at a low multiplicity into EBV-negative Akata cells which were reselected with G418 (4).
FIG. 1.
Southern blot analysis of DNA from cells harboring wild-type virus (Wt), a mixture of wild type and recombinant virus (Wt+Rc), or pure recombinant virus (Rc). The diagrams above the blots show the strategy for insertion of the UF5 cassette; the diagrams below the blots show the fragments expected with the indicated probe. (A) DNA from virus with the UF5 cassette inserted into the BXLF2 ORF, digested with XbaI, and probed with the HindIII F fragment. (B) DNA from virus with the UF5 cassette inserted into the BXLF1 ORF, digested with XhoI, and probed with the HindIII F fragment. The numbering of the base pairs corresponds to the B95-8 sequence except in panel A. B95-8 virus has a 11,801-bp deletion that begins at bp 152012 (2). The XbaI site at position 152912 was therefore predicted from the sequence of this deletion in the Raji strain of virus (28). Sizes in kilobases are indicated by horizontal arrows.
Southern blotting.
Cells were digested overnight at 56°C with proteinase K (1 mg/ml in 100 mM NaCl–10 mM Tris-HCl (pH 8.0)–25 mM EDTA–0.5% SDS), and DNA was purified by phenol-chloroform extraction and ethanol precipitation. Purified DNA was digested overnight with XbaI to identify insertion into the BXLF2 ORF and with XhoI to identify insertion into the BXLF1 ORF. DNA was separated by agarose gel electrophoresis in 0.7% agarose, transferred to a nylon membrane (Magnacharge), and cross-linked and hybridized with the 6-kb HindIII F fragment which had been labeled with 32P.
Slot blot assays.
The amount of EBV DNA in cells or virion particles was measured by hybridization with the BamHI W fragment of EBV DNA labeled with 32P as previously described (36) and quantified by scanning with a Molecular Dynamics Storm PhosphorImager.
Flow cytometric analysis of virus binding and entry.
To examine virus binding, cells were fixed in ice-cold 0.1% paraformaldehyde and incubated with virus for 1 h on ice. Binding was visualized with MAb 72A1 to gp350 and sheep anti-mouse immunoglobulin coupled to fluorescein isothiocyanate (ICN Biomedical, Inc., Costa Mesa, Calif.). Cells incubated with both antibodies but no virus were used as controls. Infection of cells with virus expressing GFP was measured directly by flow-cytometric analysis of unfixed cells. Infection of EBV-negative Akata cells was analyzed 72 h after infection, and infection of AGS cells was analyzed at 5 days postinfection.
Gardella gel analysis of virus binding.
Two million Akata cells or 106 AGS cells were incubated with virus for 1 to 2 h on ice, after which cells were washed repeatedly with medium. Cells were pelleted at 325 × g for 4 min and resuspended in 40 μl of buffer containing 90 mM Tris-borate, 2 mM EDTA, 20% Ficoll 400, and 0.01% bromophenol blue. Each sample was then transferred to the well of a Gardella gel (8) for analysis of the amount of virion DNA bound to cells. Gels were run as described earlier (14), depurinated, alkalinized, and neutralized before transfer to a Nytran membrane (Schleicher and Schuell, Inc., Keene, N.H.). EBV DNA was visualized by probing with a BamHI W fragment of EBV DNA labeled with 32P as described above. In experiments with MAbs, virus and MAbs were preincubated for 1 h at 37°C.
Assays for infection.
Five million T-cell-depleted peripheral blood leukocytes were incubated at 37°C with 300 μl of dilutions of filtered culture supernatant from induced Akata cells. After 2 h, the volume was brought to 5 ml with medium containing serum and the cells were reincubated. A total of 5 × 105 EBV-negative Akata cells were incubated with 150 μl of virus for 2 h at 37°C, after which the volume was brought to 3 ml and the cells were reincubated. AGS cells and SVKCR2 cells were plated in six-well tissue culture dishes grown to 80 to 90% confluency. Then, 600 μl of virus was added to the cells for 4 h, followed by approximately 2.5 ml of medium. Five days later both leukocytes and Akata cells were harvested and analyzed for expression of EBNA-1 by Western blotting. In addition, 6 × 105 T-cell-depleted peripheral blood leukocytes were incubated for 1 h at 37°C with 240 μl of virus, plated in quintuplicate at 105 cells per well in 96-well tissue culture plates and reincubated for 4 weeks, at which time wells were examined for the presence of transforming foci.
Polyethylene glycol-mediated infection.
Samples of 5 × 106 T-cell-depleted peripheral blood leukocytes were incubated for 2 h on ice with wild-type or recombinant virus or growth medium. Cells were washed once and gently resuspended in 1 ml of 35% polyethylene glycol 1500 (Boehringer Mannheim) or serum-free medium for 5 min. Then, 10 ml of medium was added, cells were centrifuged at 400 × g, washed, resuspended in fresh growth medium, and incubated for 14 days before harvesting and Western blot analysis.
Western blotting.
Proteins were electrophoresed in polyacrylamide and then electrically transferred onto nitrocellulose membranes (0.45-μm pore size; Schleicher and Schuell) at 125 mA for 6 h. The transferred sheets were reacted overnight with blocking buffer (10 mM Tris-HCl, pH 7.2; 0.15 M NaCl; 5% skim milk; 0.05% sodium azide) containing a 1/500 dilution of EBNA-1-positive human serum. They were then washed five times with wash buffer (10 mM Tris-HCl, pH 7.2; 0.15% NaCl; 0.3% Tween 20) for 10 min each time. The washed sheets were reacted with alkaline phosphatase-conjugated goat anti-human antibodies (HyClone) for 2.5 h, and the bound anti-human antibodies were detected by reacting with substrate 5-bromo-4-chloro-3-indolylphosphate and Nitro Blue Tetrazolium (Sigma).
RESULTS
Generation of recombinant viruses with the BXLF1 or BXLF2 ORFs disrupted by UF5.
An Akata HindIII F fragment into which the UF5 cassette had been inserted either into the BXLF1 or the BXLF2 ORF was transfected into Akata cells carrying EBV episomes, and cells in which recombination had occurred were obtained by selection in the presence of G418. Clones in which homologous as opposed to illegitimate recombination had occurred were identified by Southern blotting (Fig. 1). To derive cells that contained only recombinant episomes in the absence of wild-type episomes, cells from each were induced with anti-human immunoglobulin, and virus harvested from the spent culture medium was used to infect EBV-negative Akata cells. Drug-resistant clones that grew out after infection with virus from each parental clone were tested for the ability to be induced to make virus and for the presence of recombinant but not wild-type episomes (e.g., Fig. 1). One inducible clone from each of two independently isolated parents was selected for further study. The phenotype of recombinant virus with UF5 inserted into the BXLF1 ORF (Rc-TK) was, as expected (31), indistinguishable from that of wild-type virus (data not shown). Both recombinants with UF5 inserted into the BXLF2 ORF (Rc-gH) had the same phenotype, which was defective in several respects relative to wild-type virus. These defects are described in detail below. GFP was expressed in all cells harboring virus carrying the UF5 cassette.
Disruption of the BXLF2 ORF affects expression of the entire gH-gL-gp42 complex.
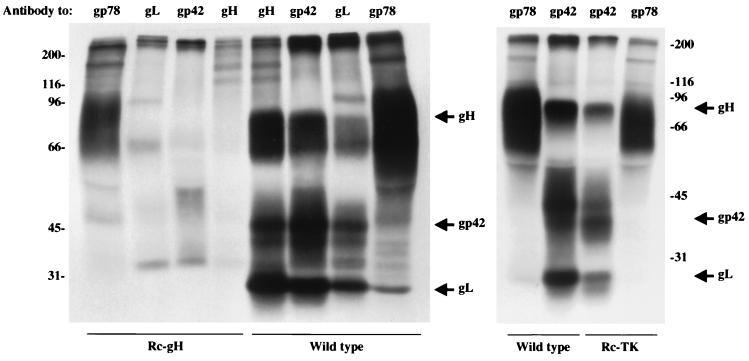
To examine the effect of disrupting the BXLF2 ORF on the expression of the gH-gL-gp42 complex, cells carrying Rc-gH, Rc-TK, or wild-type virus were induced with anti-human immunoglobulin, labeled with [3H]glucosamine, and immunoprecipitated with MAb E2A5 against gp78, MAb F-2-1 against gp42, MAb CL59 against gH, or anti-gL, an antibody made to a peptide derived from the predicted gL sequence. No detectable gH could be immunoprecipitated from Rc-gH virus (Fig. 2). In addition, antibodies to gL and gp42 were unable to specifically immunoprecipitate their respective targets. The amounts of radioactivity immunoprecipitated from cells harboring wild-type and recombinant viruses by antibody to gp78, a glycoprotein encoded by the BILF2 ORF (20), were 166,720 and 39,460 cpm, respectively, indicating that induction and labeling of wild-type virus proteins was better than for recombinant proteins. However, repeated experiments and longer exposures of autoradiographs confirmed that the Rc-gH virus was deficient in all three components of the gH-gL-gp42 complex. No defects in expression of the gH complex were seen in the Rc-TK virus.
FIG. 2.
Electrophoretic analysis of proteins immunoprecipitated by MAb E2A5 to gp78, MAb F-2-1 to gp42, MAb CL59 to gH, or rabbit anti-peptide antibodies to gL from Akata cells harboring wild-type episomes or episomes in which the UF5 cassette was inserted into the BXLF2 (Rc-gH) or the BXLF1 (Rc-TK) ORFs. The cells were induced with anti-human immunoglobulin and labeled with [3H]glucosamine. Numbers at the left indicate the sizes in kilodaltons.
Virus lacking gH exit cells normally.
To examine whether the loss of the gH complex in the Rc-gH virus influenced its ability to egress from cells, a cell blot assay was used to assess the total amount of virion DNA associated with induced cells and the DNase-resistant, encapsidated DNA that could be pelleted from spent culture medium after it had been filtered through a 1.2-μm-pore-size filter to remove cells. A comparison of the ratios of the two for two Rc-gH clones and for wild-type virus showed that, although they varied from induction to induction, there was no evidence that cells harboring recombinant virus consistently released more or less encapsidated virion DNA than did those harboring wild-type virus (Table 1).
TABLE 1.
Comparison of the efficiency of egress of wild-type virus and two independently isolated recombinant viruses that lack gH
| Expt. no. | Extracellular virion DNA (% intracellular virus DNA at 72 h postinfection)
|
||
|---|---|---|---|
| Wild-type virus | Rc-BXLF2 1 | Rc-BXLF2 2 | |
| 1 | 25 | 18 | 12 |
| 2 | 13 | NDa | 12 |
| 3 | 10 | ND | 21 |
| 4 | 10 | 20 | 15 |
| Avg | 14.5 | 19 | 15 |
ND, not determined.
Virus lacking gH binds normally to B cells but is impaired in binding to epithelial cells.
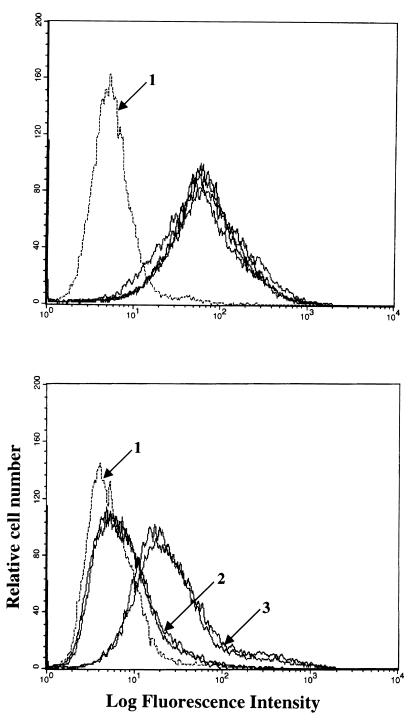
Binding of virus to B cells (EBV-negative Akata cells) and AGS cells was examined by flow cytometry using viruses that had been equilibrated by slot blot analysis for virus DNA content. No differences in the binding of wild-type virus, Rc-TK, and two independently isolated Rc-gH viruses could be detected (Fig. 3, top panel). However, both Rc-gH viruses were significantly impaired in their ability to bind to AGS cells (Fig. 3, bottom panel). To determine whether the small amount of residual binding represented low-level expression of CR2 which has been reported for some epithelial cell lines (5), virus was preincubated with MAb 72A1, which blocks virus binding to CR2 (13, 22) before addition to AGS cells or EBV-negative Akata cells which express CR2. Binding to AGS cells was very slightly increased by MAb 72A1 although, despite the fact that large amounts of virus were used to provide a strong signal in the flow cytometer, it significantly reduced virus binding to the EBV-negative Akata cells (Fig. 4).
FIG. 3.
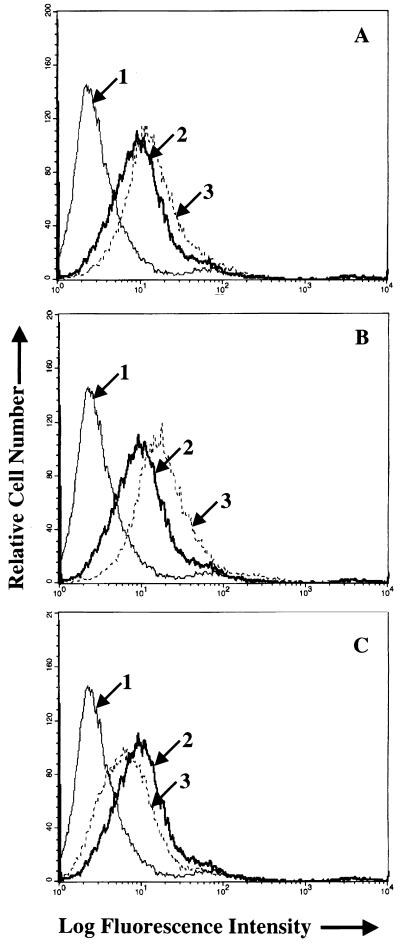
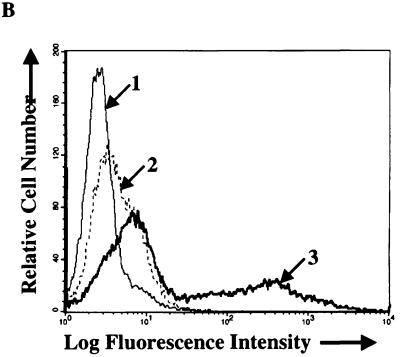
Flow cytometric analysis of binding of equal amounts of wild-type, Rc-TK, or Rc-gH virus to EBV-negative Akata cells (top panel) or AGS cells (bottom panel). In the top panel, arrow 1 indicates the histogram of cells incubated with MAb 72A1 to gp350 and fluorescein-conjugated sheep anti-mouse immunoglobulin alone; all remaining histograms represent cells incubated with wild-type, Rc-TK, or two independent isolates of Rc-gH virus. In the bottom panel, arrow 1 indicates the histogram of cells incubated with MAb 72A1 to gp350 and fluorescein-conjugated sheep anti-mouse immunoglobulin alone; arrow 2 indicates two histograms representing cells incubated with two independent isolates of Rc-gH; arrow 3 indicates two histograms representing cells incubated with wild-type virus and Rc-TK.
FIG. 4.
Flow-cytometric analysis of the effects of MAb 72A1 to gp350 on virus binding to AGS cells (A and B) or EBV-negative Akata cells (C). The virus used in panels A and C is Rc-TK; the virus used in panel B is Rc-gH. In each panel arrow 1 indicates the histogram of cells incubated with MAb 72A1 to gp350 and fluorescein-conjugated sheep anti-mouse immunoglobulin alone; arrow 2 indicates the virus bound after preincubation with phosphate-buffered saline and visualized with MAb 72A1 and fluorescein-conjugated sheep anti-mouse immunoglobulin; arrow 3 indicates the virus bound after preincubation with MAb 72A1 and visualized with MAb 72A1 and fluorescein-conjugated sheep anti-mouse immunoglobulin.
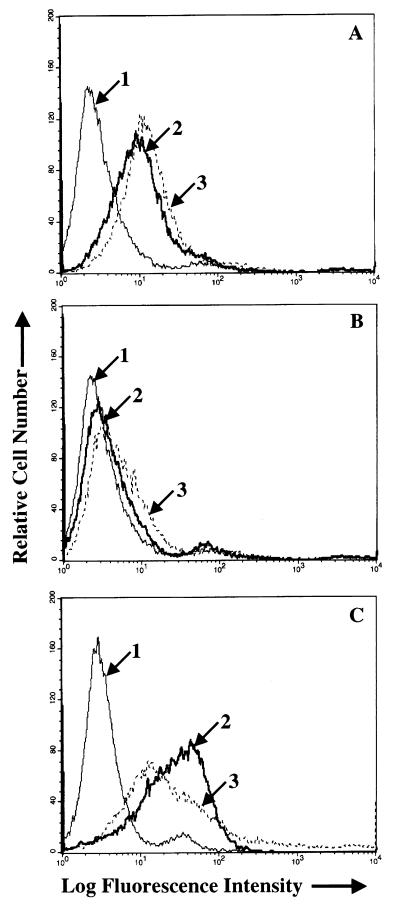
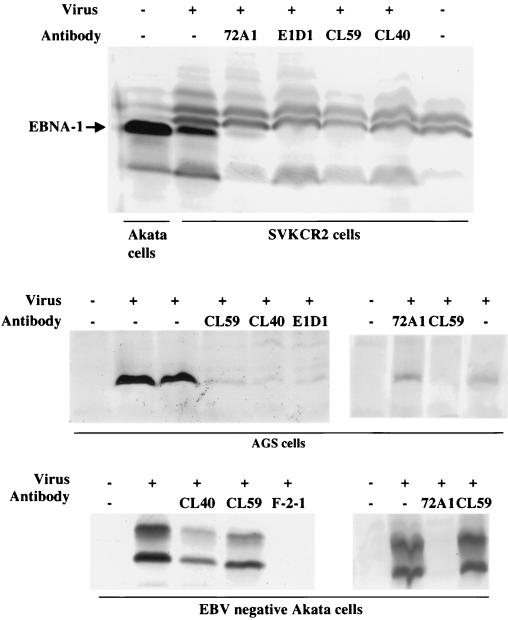
Since gH appeared to play a major role in attachment of virus to AGS cells, the effects of the three MAbs to gH that neutralized infection of AGS cells were also studied further. We have previously reported on the biological effects of one of these antibodies to gH, MAb E1D1. This antibody has no effect on B-cell infection, but it inhibits infection of SVKCR2 cells. Virus binds to SVKCR2 cells via an interaction between gp350 and CR2, and thus neutralization is not mediated by inhibition of binding to a primary receptor. Rather, it has been hypothesized that MAb E1D1 blocks the interaction of gH with a novel epithelial cell coreceptor or entry mediator (37). Two newer anti-gH antibodies, MAbs CL59 and CL40 behaved in the same way as MAb E1D1. Both antibodies neutralized virus infection of AGS cells and SVKCR2 cells but did not inhibit infection of EBV-negative Akata cells, although a control MAb F-2-1 to gp42 did so quite efficiently (Fig. 5). As previously shown, MAb 72A1 to gp350 could neutralize infection of both SVKCR2 cells and EBV-negative Akata cells. However, as expected from the flow cytometry experiments shown in Fig. 4, it was not able to neutralize infection of AGS cells. The effects of MAbs E1D1, CL40, and CL59 on virus binding to AGS cells were then also examined by flow cytometry. Neither MAb CL40 nor MAb CL59 inhibited virus binding, as judged by this assay, instead both MAbs, particularly MAb CL59, appeared to increase virus binding by a small amount (Fig. 6). However, in contrast, virus binding to AGS cells was reduced by MAb E1D1.
FIG. 5.
Western blot analysis of induction of EBNA-1 in SVKCR2 cells, AGS cells, and EBV-negative Akata cells by virus preincubated with growth medium or with MAbs 72A1 to gp350, E1D1, CL59 and CL40 to gH, or F-2-1 to gp42, as indicated. Blots were reacted with human serum containing antibodies to EBNA-1 and with goat anti-human immunoglobulin conjugated to alkaline phosphatase. Uninduced Akata cells were included on the far left of the top blot to demonstrate the electrophoretic mobility of EBNA-1 in this strain of virus. The virus used in the leftmost panel of EBV-negative Akata cells was virus released into the supernatant of virus-producing cells. The virus used in the other panels was concentrated 30-fold from this supernatant by centrifugation.
FIG. 6.
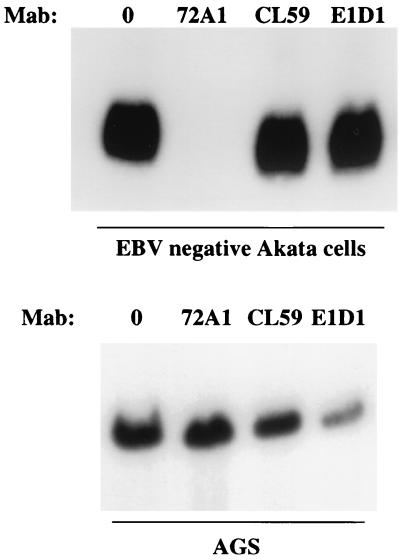
Flow-cytometric analysis of the effects of MAbs CL40 (A), CL59 (B), and E1D1 (C) on binding of wild-type Akata virus to AGS cells. In each panel arrow 1 indicates the histogram of cells incubated with MAb to 72A1 to gp350 and fluorescein-conjugated sheep anti-mouse immunoglobulin alone; arrow 2 indicates the virus bound after preincubation with phosphate-buffered saline and visualized with MAb 72A1 and fluorescein-conjugated sheep anti-mouse immunoglobulin; arrow 3 indicates the virus bound after preincubation with MAb C140, CL59, or E1D1 and visualized with MAb to 72A1 and fluorescein-conjugated sheep anti-mouse immunoglobulin.
The slight increase in virus binding to AGS cells measured by flow cytometry after preincubation with either MAb 72A1 (Fig. 4A and B) or CL59 and CL40 (Fig. 6A and B) might have reflected an increase in MAb bound to virus that was available for detection by the fluorescein-conjugated sheep anti-mouse immunoglobulin used for detection. Both 72A1 and CL59 provide very strong fluorescence signals when reacted with virus alone. Virus binding assays were therefore repeated using Gardella gels to analyze the amount of virus DNA that associated with each cell type. Virus was preincubated with medium or antibody before incubation with EBV-negative Akata cells or AGS cells that had been briefly fixed in ice-cold paraformaldehyde. Excess virus was removed by washing, and the cells were lysed and digested in the wells of a Gardella gel. Linear virion DNA was run into the gel, Southern blotted, and probed. MAb 72A1 to gp350 blocked virus binding to EBV-negative Akata cells but not to AGS cells. MAb CL59 had little effect on binding to either cell type (Fig. 7). In contrast, although MAb E1D1 had no effect on virus binding to EBV-negative Akata cells, it did reduce virus binding to AGS cells, a finding consistent with the effects measured by flow cytometry. If the Southern blots of the AGS binding assay were scanned at low exposure with a Molecular Dynamics Storm PhosphorImager, the percent virus binding in the presence of the antibodies relative to binding in the presence of medium alone was 118% for MAb 72A1, 77% for MAb CL59, and 35% for MAb E1D1.
FIG. 7.
Southern blot of Gardella gel analyses of the amounts of Akata virus bound to EBV-negative Akata (top panel) or AGS cells (bottom panel) in the presence of growth medium, MAb 72A1 to gp350, or MAbs CL59 and E1D1 to gH.
Virus lacking gH cannot penetrate cells but can be rescued by the exogenous fusogen polyethylene glycol.
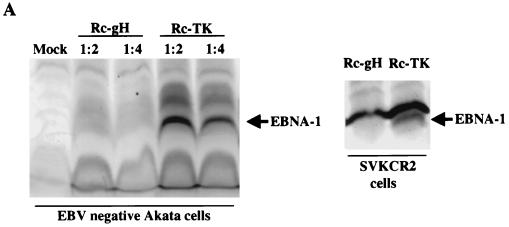
Since Rc-gH viruses were apparently unimpaired in their ability to bind to B cells, they were tested for their ability to penetrate and infect. The failure of virus to bind to AGS cells and the concomitant failure of virus to infect AGS cells, as judged by the most sensitive measure of expression of GFP (data not shown), prohibited examination of whether virus was also impaired in the penetration of epithelial cells with this cell type. However, the stable expression of CR2 by the SVKCR2 cell line provided a surrogate experimental model. When equal amounts of Rc-gH and Rc-TK viruses were added to EBV-negative Akata cells or SVKCR2 cells, only the Rc-TK virus induced expression of EBNA-1 (Fig. 8). In addition, only Rc-TK induced the expression of GFP in Akata cells (Fig. 8B). Wild-type virus exhibited transforming activity over the entire range of dilutions tested from 1/5 to 1/2,000, whereas over the same range of dilutions the virus that lacked gH had no detectable transforming activity. To determine whether this defect was restricted to virus penetration, freshly isolated B cells were incubated with Rc-gH virus or a virus that lacks gp42 and has been previously shown to be defective in penetration (36) and treated with the exogenous fusogen polyethylene glycol. Both viruses induced EBNA-1 in these cells in the presence, but not the absence, of polyethylene glycol (Fig. 9).
FIG. 8.
(A) Western blot analysis of the ability of equal amounts of Rc-TK and Rc-gH viruses to induce EBNA-1 in EBV-negative Akata cells (left panel) or SVKCR2 cells (right panel). Blots were reacted with human serum containing antibodies to EBNA-1 and with goat anti-human immunoglobulin conjugated to alkaline phosphatase. (B) Flow-cytometric analysis of the ability of equal amounts of Rc-TK and Rc-gH viruses to infect and express GFP in EBV-negative Akata cells. Cells were analyzed 72 h after infection. Arrow 1 indicates the histogram of uninfected cells; arrow 2 indicates the histogram of cells infected with Rc-gH virus; arrow 3 indicates the histogram of cells infected with Rc-TK. The increase in cells in the right tail of the uninfected cell profile after infection with either Rc-gH or Rc-TK represents an increase in dead cells which can be seen by fluorescent microscopy to become slightly yellow.
FIG. 9.
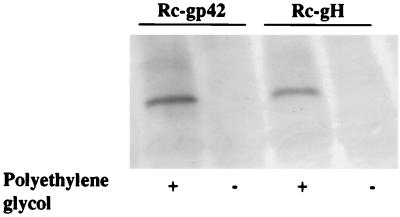
Western blot analysis of T-cell-depleted leukocytes infected with Rc-gH or a recombinant virus lacking gp42, the product of the BZLF2 ORF (36) (Rc-gp42) in the presence or absence of polyethylene glycol. Blots were reacted with human serum containing antibodies to EBNA-1 and with goat anti-human immunoglobulin conjugated to alkaline phosphatase.
DISCUSSION
Essentially all of the gH homologs that have been studied to date in many different herpesviruses have been implicated as playing a role in virus penetration, and null mutants have been made to confirm this role in herpes simplex virus (7), pseudorabies virus (1, 29), and bovine herpesvirus 1 (21, 30). The evidence for involvement of the EBV gH in penetration has been indirect. Removal of the entire gH-gL-gp42 complex from virosomes made from EBV proteins resulted in vesicles that retained the ability to bind to receptor-positive B cells but not to fuse (11), but no antibodies to gH have been identified that block entry into B cells. The current study was in part an attempt to address the question directly by construction of an EBV recombinant that lacks gH. The results reported here are consistent with a role for gH in penetration into B cells but retain some ambiguity, since the loss of gH apparently resulted in a rapid turnover of gL and gp42 as well. Thus, while it seems extremely unlikely that either gL or gp42 are central to virus-cell fusion, since both are type 2 membrane proteins with sequences that are not predicted to be particularly hydrophobic, the possibility cannot be completely ruled out, and the complex is still probably best considered the functional unit.
More enlightening and surprising were the observations made with the epithelial cell line AGS. Binding of B cells by virus lacking gH was unimpaired. This was expected since the glycoprotein gp350 has a very high affinity for its B-cell receptor CR2 (23). However, it is clear that gH plays a major role in attachment of virus to epithelial cells such as the AGS line which can be infected in a CR2-independent manner (15, 39). Our own unpublished observations confirm that expression of CR2 cannot be detected on these cells by indirect immunofluorescence with appropriate MAbs or by reverse transcription and PCR. In addition, as shown here, virus binding is unaffected by MAb 72A1, which binds to gp350 and efficiently inhibits attachment to CR2. The recombinant virus that lacks gH was, however, almost completely unable to bind to AGS cells and was also unable to infect them.
Two issues are immediately raised by this finding. The first issue is whether or not it is gH or another member of the complex that mediates binding to epithelial cells. Since a recombinant virus that lacks gp42 (36) can infect AGS cells in a CR2-independent manner (data not shown), the choices are between gH and gL. The most straightforward explanation of the ability of MAb E1D1 and perhaps to a lesser extent MAb CL59 to inhibit virus binding would suggest that it is gH itself that functions in attachment. The second issue relates to the identity of the molecule(s) with which gH is interacting on AGS cells. Previous work with the SVKCR2 cell line had already suggested that gH interacted with a novel epithelial coreceptor that was not present on B cells (37). The primary data in support of this probability were that MAb to gH inhibited SVKCR2 infection without influencing the infection of B cells and that conversion of all two-part gH-gL to three-part gH-gL-gp42 complexes in virus had the same effect. Addition of MAb and gp42 were both interpreted as effecting a physical block of a critical site in gH. The block was in this case obviously not affecting virus binding, since SVKCR2 cells by virtue of the transfected CR2 receptor can bind virus via gp350. In addition, since SVKCR2 cells lack the B-cell coreceptor major histocompatibility complex (MHC) class II (10, 16) and can be infected with a virus that lacks gp42 (37), the MHC class II ligand (32), the existence of a novel epithelial-cell coreceptor was considered a reasonable possibility. A key question now is whether the receptor that binds a gH-expressing, but not a virus-lacking gH to epithelial cells in the absence of CR2 is the same entity as this postulated coreceptor or is yet another unidentified molecule. Again, the most straightforward interpretation of the partial blocking of binding by MAbs E1D1 and CL59, both of which also neutralize infection of epithelial cells that express CR2, is that the two receptors are the same.
However, unfortunately, neither the issue of identity of the attachment protein nor the issue of potential overlap of receptor and coreceptor can be definitively addressed by antibody studies, with the second of the two issues perhaps being the most in doubt. Each of the MAbs, which recognize different epitopes on gH, might affect the conformation of gH and thus disrupt a distal interactive site just as well as it might simply block direct access to a binding site by a receptor. By the same token, an MAb might do both at the same time, that is, block an interaction with a primary receptor at the epitope to which it binds and alter conformation elsewhere in the molecule where there is an interaction with a coreceptor or vice versa. Indeed, if the primary receptor and the coreceptor are in fact the same molecule, then either this molecule is expressed at much higher levels on AGS cells than on cells such as the CR2-negative parent of SVKCR2 cells, SVK, to which virus binding cannot be visualized with antibody (18), or the molecule is expressed in an altered form that creates a much higher affinity for virus. Infection of SVK cells in the absence of transfected CR2 is not detectable. Infection of the AGS cell line is considerably less efficient than infection of B cells in that a much higher concentration of virus is required. However, it is not much less efficient than infection of SVKCR2 cells, to which virus binds with high efficiency. Clearly, cloning and sequencing of the receptor and/or coreceptor are the critical, although by no means trivial, next steps.
The role that epithelial cells play in the normal biology of infection with EBV has been called into question (35), but there are several diseases, including nasopharyngeal carcinoma (19), oral hairy leukoplakia (9), gastric carcinoma (27), and breast cancer (3), where virus is found in this cell type. The studies described here suggest that just as EBV maintains expression of both a three-part gH-gL-gp42 complex that is essential for infection of B cells and a two-part gH-gL complex that is essential for infection of epithelial cells, so it has one attachment protein, gp350, that binds virus efficiently to B cells via CR2 and a second attachment protein, probably gH, which can mediate infection of at least some epithelial cells that lack CR2. Access to the lymphoid system at mucosal surfaces would certainly be facilitated by at least a transient infection of mucosal epithelium, and it would appear that the virus in its evolution as a lymphotropic gammaherpesvirus has at the least retained or evolved the means with which to effect such an event.
ACKNOWLEDGMENT
This work was supported by grant AI20662 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A, Mettenleiter T C. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 2.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet M, Guinebretiere J-M, Kremmer E, Grunewald V, Benhamou E, Contesso G, Joab I. Detection of Epstein-Barr virus in invasive breast cancers. J Natl Cancer Inst. 1999;91:1376–1381. doi: 10.1093/jnci/91.16.1376. [DOI] [PubMed] [Google Scholar]
- 4.Borza C, Hutt-Fletcher L M. Epstein-Barr virus recombinant lacking expression of glycoprotein gp150 infects B cells normally but is enhanced for infection of the epithelial line SVKCR2. J Virol. 1998;72:7577–7582. doi: 10.1128/jvi.72.9.7577-7582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fingeroth J D, Diamond M E, Sage D R, Hayman J, Yates J L. CD-21 dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J Virol. 1999;73:2115–2125. doi: 10.1128/jvi.73.3.2115-2125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingeroth J D, Weis J J, Tedder T F, Strominger J L, Biro P A, Fearon D T. Epstein-Barr virus receptor of human B lymphocytes is the C3d complement CR2. Proc Natl Acad Sci USA. 1984;81:4510–4516. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardella T, Medveczky P, Sairenji T, Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984;50:248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenspan J S, Greenspan D, Lennette E T, Abrams D I, Conant M A, Petersen V, Freese U K. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. New Engl J Med. 1985;313:1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 10.Haan K M, Kwok W W, Longnecker R, Speck P. Epstein-Barr virus entry utilizing HLA-DP or HLA-DQ as a coreceptor. J Virol. 2000;74:2451–2454. doi: 10.1128/jvi.74.5.2451-2454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad R S, Hutt-Fletcher L M. Depletion of glycoprotein gp85 from virosomes made with Epstein-Barr virus proteins abolishes their ability to fuse with virus receptor-bearing cells. J Virol. 1989;63:4998–5005. doi: 10.1128/jvi.63.12.4998-5005.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heineman T, Gong M, Sample J, Kieff E. Identification of the Epstein-Barr virus gp85 gene. J Virol. 1988;62:1101–1107. doi: 10.1128/jvi.62.4.1101-1107.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman G J, Lazarowitz S G, Hayward S D. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc Natl Acad Sci USA. 1980;77:2979–2983. doi: 10.1073/pnas.77.5.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurley E A, Thorley-Lawson D A. B cell activation and the establishment of Epstein-Barr virus latency. J Exp Med. 1988;168:2059–2075. doi: 10.1084/jem.168.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai S, Nishikawa J, Takada K. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol. 1998;72:4371–4378. doi: 10.1128/jvi.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q X, Spriggs M K, Kovats S, Turk S M, Comeau M R, Nepom B, Hutt-Fletcher L M. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q X, Turk S M, Hutt-Fletcher L M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q X, Young L S, Niedobitek G, Dawson C W, Birkenbach M, Wang F, Rickinson A B. Epstein-Barr virus infection and replication in a human epithelial system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 19.Liebowitz D. Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin Oncol. 1994;21:376–381. [PubMed] [Google Scholar]
- 20.Mackett M, Conway M J, Arrand J R, Haddad R S, Hutt-Fletcher L M. Characterization and expression of a glycoprotein encoded by the Epstein-Barr virus BamHI 1 fragment. J Virol. 1990;64:2545–2552. doi: 10.1128/jvi.64.6.2545-2552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer G, Hanon E, Georlette D, Pastoret P P, Thiry E. Bovine herpesvirus type 1 glycoprotein H is essential for penetration and propagation in cell culture. J Gen Virol. 1998;79:1983–1987. doi: 10.1099/0022-1317-79-8-1983. [DOI] [PubMed] [Google Scholar]
- 22.Miller N, Hutt-Fletcher L M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988;62:2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore M D, DiScipio R G, Cooper N R, Nemerow G R. Hydrodynamic, electron microscopic and ligand binding analysis of the Epstein-Barr virus/C3dg receptor (CR2) J Biol Chem. 1989;34:20576–20582. [PubMed] [Google Scholar]
- 24.Nemerow G R, Mold C, Keivens Schwend V, Tollefson V, Cooper N R. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J Virol. 1987;61:1416–1420. doi: 10.1128/jvi.61.5.1416-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemerow G R, Wolfert R, McNaughton M, Cooper N R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2) J Virol. 1985;55:347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oba D E, Hutt-Fletcher L M. Induction of antibodies to the Epstein-Barr virus glycoprotein gp85 with a synthetic peptide corresponding to a sequence in the BXLF2 open reading frame. J Virol. 1988;62:1108–1114. doi: 10.1128/jvi.62.4.1108-1114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osato T, Imai S. Epstein-Barr virus and gastric carcinoma. Semin Cancer Biol. 1996;7:175–182. doi: 10.1006/scbi.1996.0024. [DOI] [PubMed] [Google Scholar]
- 28.Parker B D, Bankier A T, Satchwell S C, Barrell B G, Farrell P J. Sequence and transcription of Raji Epstein-Barr virus DNA spanning the B95-8 deletion region. Virology. 1990;179:339–346. doi: 10.1016/0042-6822(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 29.Peeters B, Dewind N, Broer R, Gielkins A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroder C, Keil G M. Bovine herpesvirus 1 requires glycoprotein H for infectivity and direct spreading and glycoproteins gH (W450) and gB for glycoprotein D-independent cell-to-cell spread. J Gen Virol. 1999;80:57–61. doi: 10.1099/0022-1317-80-1-57. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu N, Yoshiyama H, Takada K. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J Virol. 1996;70:7260–7263. doi: 10.1128/jvi.70.10.7260-7263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spriggs M K, Armitage R J, Comeau M R, Strockbine L, Farrah T, MacDuff B, Ulrich D, Alderson M R, Mullberg J, Cohen J I. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR beta chain and inhibits antigen presentation. J Virol. 1996;70:5557–5563. doi: 10.1128/jvi.70.8.5557-5563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada K. Cross-linking of cell surface immunoglobulin induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 34.Tanner J, Weis J, Fearon D, Whang Y, Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping and endocytosis. Cell. 1987;50:203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- 35.Thorley-Lawson D A, Miyashita E M, Khan G. Epstein-Barr virus and the B cell: that's all it takes. Trends Microbiol. 1996;4:204–208. doi: 10.1016/s0966-842x(96)90020-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Hutt-Fletcher L M. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J Virol. 1998;72:158–163. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Kenyon W J, Li Q X, Mullberg J, Hutt-Fletcher L M. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaswen L R, Stephens E B, Davenport L C, Hutt-Fletcher L M. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology. 1993;195:387–396. doi: 10.1006/viro.1993.1388. [DOI] [PubMed] [Google Scholar]
- 39.Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997;71:5688–5691. doi: 10.1128/jvi.71.7.5688-5691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]