Abstract
Canine distemper virus (CDV) infection of ferrets is clinically and immunologically similar to measles, making this a useful model for the human disease. The model was used to determine if parenteral or mucosal immunization of infant ferrets at 3 and 6 weeks of age with attenuated vaccinia virus (NYVAC) or canarypox virus (ALVAC) vaccine strains expressing the CDV hemagglutinin (H) and fusion (F) protein genes (NYVAC-HF and ALVAC-HF) would induce serum neutralizing antibody and protect against challenge infection at 12 weeks of age. Ferrets without maternal antibody that were vaccinated parenterally with NYVAC-HF (n = 5) or ALVAC-HF (n = 4) developed significant neutralizing titers (log10 inverse mean titer ± standard deviation of 2.30 ± 0.12 and 2.20 ± 0.34, respectively) by the day of challenge, and all survived with no clinical or virologic evidence of infection. Ferrets without maternal antibody that were vaccinated intranasally (i.n.) developed lower neutralizing titers, with NYVAC-HF producing higher titers at challenge (1.11 ± 0.57 versus 0.40 ± 0.37, P = 0.02) and a better survival rate (6/7 versus 0/5, P = 0.008) than ALVAC-HF. Ferrets with maternal antibody that were vaccinated parenterally with NYVAC-HF (n = 7) and ALVAC-HF (n = 7) developed significantly higher antibody titers (1.64 ± 0.54 and 1.28 ± 0.40, respectively) than did ferrets immunized with an attenuated CDV vaccine (0.46 ± 0.59; n = 7) or the recombinant vectors expressing rabies glycoprotein (RG) (0.19 ± 0.32; n = 8, P = 7 × 10−6). The NYVAC vaccine also protected against weight loss, and both the NYVAC and attenuated CDV vaccines protected against the development of some clinical signs of infection, although survival in each of the three vaccine groups was low (one of seven) and not significantly different from the RG controls (none of eight). Combined i.n.-parenteral immunization of ferrets with maternal antibody using NYVAC-HF (n = 9) produced higher titers (1.63 ± 0.25) than did i.n. immunization with NYVAC-HF (0.88 ± 0.36; n = 9) and ALVAC-HF (0.61 ± 0.43; n = 9, P = 3 × 10−7), and survival was also significantly better in the i.n.-parenteral group (3 of 9) than in the other HF-vaccinated animals (none of 18) or in controls immunized with RG (none of 5) (P = 0.0374). Multiple routes were not tested with the ALVAC vaccine. The results suggest that infant ferrets are less responsive to i.n. vaccination than are older ferrets and raises questions about the appropriateness of this route of immunization in infant ferrets or infants of other species.
Despite the availability of a safe and efficacious vaccine, measles causes approximately 1 million deaths per year worldwide. Most deaths occur in infants and children due to the disease itself or its immunosuppressive sequelae (30). Very young children (<4 months of age) are usually protected from disease by the presence of transplacentally derived maternal antibody. However, this maternal antibody interferes with the effective vaccination and protection of infants with the currently available attenuated live-virus vaccines (14). Vaccination with higher-titer vaccines can overcome some of the maternal antibody interference, but this has led to increased non-measles-related mortality in some settings, possibly due to vaccine-induced immunosuppression (1, 15, 19, 40).
Measles virus (MV) and canine distemper virus (CDV) are closely related members of the Morbillivirus family. Both viruses cause diseases with similar clinical and immunological presentations: fever, rash, leukocytopenia, conjunctivitis, and possible progression to pneumonia and encephalitis. European ferrets (Mustela putorius furo) are exquisitely sensitive to canine distemper infection, inevitably dying of CDV-induced pneumonia or encephalitis after exposure unless adequately protected by vaccination. CDV infections of ferrets is thus a useful model for testing MV vaccine strategies (41, 49).
Much like MV vaccines, attenuated CDV vaccines are available for use in ferrets; however, they are insufficient to induce protection when administered in the presence of maternal antibody that is acquired via colostrum in the first few days of life. Such passive antibody titers wane with age and are undetectable by 12 weeks but can interfere with effective vaccination through 10 weeks of age (2, 41). Use of recombinant poxvirus vaccines, rather than attenuated CDV vaccines, has been suggested as a possible method of overcoming the interference of maternally acquired antibody (20, 45). Vaccinia virus-MV protein recombinants have been shown to be immunogenic following intranasal (i.n.) or intrajejunal vaccination in mice (12) and intramuscular (i.m.) injection in macaques (47). Other poxvirus recombinants have been shown to be immunogenic in various species, including ferrets, by both parenteral and mucosal routes (21, 27, 28, 43). We have previously shown that recombinant poxvirus vectors expressing the CDV hemagglutinin (H) and fusion (F) genes can effectively protect ferrets from virulent CDV challenge after parenteral (41) and i.n. (49) immunization.
Because the administration of recombinant vaccines by either the parenteral or mucosal route might overcome maternal antibody inhibition of vaccination of infant ferrets, we tested the efficacy of the two recombinant poxvirus vaccines which we have previously used in juvenile ferrets (41, 49), NYVAC-HF (the highly attenuated NYVAC strain of vaccinia virus expressing the CDV H and F proteins) and ALVAC-HF (the canarypox recombinant expressing the same CDV proteins) in infant ferrets with maternally derived antibody to CDV. The ALVAC-CDV vaccine has recently been licensed for use in dogs as a combo vaccine (32). Vaccines were given by parenteral and mucosal routes or by both routes simultaneously. Age-matched controls born to jills not vaccinated against CDV were also included to determine whether infant ferrets without passive antibody protection respond adequately to vaccination.
MATERIALS AND METHODS
Ferrets.
Pregnant, CDV-vaccinated European ferrets (M. putorius furo) were purchased from Marshall Farms (North Rose, N.Y.), and kitted in our colony. Dams of kits with no maternal antibody to CDV were purchased at age 6 weeks, raised in our colony until breeding age, and bred to an unrelated male ferret. After weaning and during challenge studies, ferrets were housed in small groups.
Generation of NYVAC- and ALVAC-based recombinant CDV vaccines.
The Onderstepoort strain of CDV was obtained from M. Appel (James A. Baker Institute for Animal Health, Cornell University, Ithaca, N.Y.). CDV H and F genes from this strain were derived as described previously (41) and were inserted into NYVAC and ALVAC vectors by standard methods previously described (42, 45). In each vector, both H and F genes are inserted in a 5′-to-5′ orientation with both genes under the transcriptional control of the early/late vaccinia virus H6 promoter, which has been described previously (35). Appropriate expression of the CDV F and H genes was confirmed by immunoprecipitation analysis performed essentially as described by Tartaglia et al. (42) with a polyclonal CDV-immune serum derived from a dog. Generation of the NYVAC-RG and ALVAC-RG recombinant vaccines has been described by Tartaglia et al. (42) and Taylor et al. (44), respectively.
Vaccinations.
Ferrets were vaccinated at 3 and 6 weeks of age with 108 PFU of the NYVAC-HF and ALVAC-HF constructs with 0.2 ml per dose. One group of ferrets received an attenuated CDV vaccine (Distem-RTC; Schering Corp., Union, N.J.) prepared in chicken tissue culture for use in mink. This vaccine has been extensively tested in ferrets (2). Ferrets were vaccinated subcutaneously (s.c.) at age 3 weeks, due to lack of adequate muscle mass for i.m. injection. At age 6 weeks, ferrets were injected into the posterior thigh musculature. Ferrets were vaccinated i.n. by slowly dripping the vaccine into the nares using a micropipette. A group of ferrets was vaccinated by both i.n. and parenteral routes, with the total dose of the vaccine being divided between the two sites.
Challenge infection.
Ferrets vaccinated parenterally were challenged i.n. at 12 weeks of age with 1,000 50% tissue culture infective dose (TCID50) units of the Snyder Hill strain of CDV in 0.2 ml while under anesthesia to prevent sneezing (5 to 10 mg each of tiletamine and zolazepam per kg of body weight). Ferrets vaccinated i.n. were challenged with 100 TCID50 units of CDV. The virus was dripped into each nostril using a micropipette while the ferret's nose was pointed upward to allow the inoculum to drain into the nasal passages and trachea. Preliminary work indicated that 0.5 TCID50 unit did not cause symptomatic infection within 4 weeks of challenge but that 5 TCID50 units produced typical distemper within 18 days.
Monitoring clinical course of distemper.
After challenge, animals were monitored at least daily. Rectal temperature, body weight, and activity level (normal or diminished) were recorded, as was the presence or absence of any of the following five clinical signs of distemper: conjunctivitis, a chin rash typical of distemper, generalized erythema, decreased activity, and central nervous system (CNS) signs (seizures and circling behavior). Aural temperature was measured during the mucosal immunization experiments. Since ferrets do not recover from CNS involvement (which eventually causes protracted seizures), animals with CNS signs were immediately euthanized. Animals which became moribund without showing CNS signs (e.g., due to pneumonia) were also euthanized. Leukocytopenia was detected by enumerating total peripheral blood leukocytes using a Coulter Counter. Subjective evaluation of clinical signs was blinded by identifying animals by number only, without reference to vaccine group.
Blood.
Blood from 3-week-old kits was collected by snipping approximately 1 cm from the tail and collecting into heparinized capillary tubes. At age 6 weeks and thereafter, blood was collected from anesthetized (5 to 10 mg each tiletamine and zolazepam per kg) ferrets by venipuncture of the cephalic or saphenous vein or terminally by cardiac puncture.
TCID50 assays for CDV.
The Onderstepoort strain of CDV was grown and titered in Vero cells essentially as described previously (4). We used the fifth virus passage after plaque purification. The Snyder Hill strain of CDV was purchased from the American Type Culture Collection, Manassas, Va. (VR-526) and was grown and titered in canine peripheral blood lymphocytes as described previously (3).
Virus-neutralizing antibody.
CDV-neutralizing titers were determined in Vero cells using a TCID50 format assay based on the method of Appel and Robson (4). In a 96-well plate, duplicate twofold dilutions (from 1:2 through 1:4,096) of heat-inactivated sera were added to a standard inoculum (20 TCID50 units) of the Onderstepoort strain of CDV diluted in media (Dulbecco's modified Eagle's medium containing 5% heat-inactivated, newborn calf serum, and 25 mM HEPES buffer). After incubation at room temperature for 2 h, 1.2 × 104 Vero cells were added to each well. The plates were then incubated at 37°C in 5% CO2 for 5 to 6 days. Endpoints were determined by examining plates for syncytia with phase-contrast optics and an inverted microscope.
PBMC preparation.
Ferret peripheral blood mononuclear cells (PBMCs) were collected on days 5, 10, 15, 20, and 28 postinfection in the parenteral vaccination experiment and on days 7, 10, 14, 21, and 28 in the mucosal vaccination experiment. PBMCs were separated from 1.5 ml of heparinized whole blood by layering over 1.0 ml of Histopaque-1077 (Sigma Diagnostics, St. Louis, Mo.) and centrifuging at 184 × g for 40 min. The PBMCs were then washed twice with cold phosphate-buffered saline, resuspended in 400 μl of the same, and frozen at −85°C.
RT-PCR assay.
CDV nucleocapsid-specific primers from the Onderstepoort strain were chosen from the nucleocapsid gene (36). The upstream primer, CDV-15 (5′-GGTCGGAGAATTTAGAATGAAC-3′), and the downstream primer, CDV-23 (5′-CCAAGAGCCGGATACATNG-3′), yielded a 240-bp product spanning nucleotides 588 through 827 in the nucleocapsid gene (GenBank accession number X02000 M10242). RNA was extracted from 50 μl of a suspension of ferret PBMCs using the guanidinium thiocyanate method developed by Boom et al. (8) as modified by Park et al. (33), as previously described (41). Reverse transcription (RT) was performed on 8 μl of eluted RNA in a 20-μl reaction volume using 50 U of Moloney murine leukemia virus reverse transcriptase (Superscript II; Life Technologies, Gaithersburg, Md.), 1 mM nucleoside triphosphates, 1.25 μM upstream primer (CDV-23), and 2 μl of 0.1 M dithiothreitol in 1× reaction buffer provided by the manufacturer. Five microliters of cDNA from the RT reaction was used in the 50-μl PCR, with 1.25 U of Taq DNA polymerase (Promega, Madison, Wis.), 2.5 mM MgCl, 1 mM nucleoside triphosphates, and 0.25 μM each CDV-15 and CDV-23 in 1× PCR buffer provided by the manufacturer. The reaction was carried out for 40 cycles using a Perkin-Elmer Cetus DNA thermal cycler (Perkin-Elmer Corp., Norwalk, Conn.) with the following temperature profile: 94°C for 1 min, 51°C for 1 min, and 72°C for 1 min. Then 15-μl aliquots of the product were run on a 3% NuSieve 3:1 agarose gel (FMC, Rockland, Maine) in 1× TAE buffer at 135 V for 54 min and examined by UV transillumination following staining with ethidium bromide. The limit of detection of this assay was 0.02 TCID50 units of Snyder Hill strain CDV in dog PBMCs. In the mucosal immunization experiments, we performed RT-PCR on PBMCs collected only on day 10 postchallenge. Previous work indicated that a viremia detected on day 10 correlated most closely with the eventual survival of the animal.
Statistical analysis.
Analysis was done with the program SigmaStat for Windows, version 1.00 (Jandel Scientific, San Raphael, Calif.). Binomially distributed variables were analyzed by the Fisher exact test. Normally distributed two-group comparisons were made using the paired or unpaired Student's t test. Nonnormal two-group comparisons were made using the Mann-Whitney rank sum test. Multiple comparisons of continuous variables (neutralizing titers, leukocyte counts, body temperature, and body weight) were made using analysis of variance (ANOVA) followed by pairwise comparisons with the Student-Newman-Keuls test or, when normality or variance tests failed, ANOVA on ranks followed by pairwise comparisons with the Dunnett's test (only P values of <0.05 are reported as significantly different). Due to the large number of comparisons in some instances (e.g., daily body weights), only the final result (significantly different or not by the Student-Newman-Keuls test) was often reported. Neutralizing titers were transformed to log10 values in order to normalize their distribution for statistical analysis. In many instances, the statistical power of the tests to detect the differences actually seen between groups was <0.80. This does not affect positive results (i.e., where a significant difference is found between groups at P < 0.05) but indicates that negative results should be interpreted cautiously. In order to correct for the heavier body weight of males (e.g., mean ± standard deviation [SD] body weights [in grams] for the 13 males and 26 females in all groups in the parenteral vaccine experiments on the day of challenge were 780 ± 130 and 561 ± 81, respectively), body weights were normalized to the weight of each individual on the day of challenge. All animals were the same age within a 1-day range. Control animals receiving the NYVAC-RG and ALVAC-RG were grouped together for most analyses, as there were no significant differences in their responses to vaccination or challenge.
RESULTS
Neutralizing antibody titers. (i) Parenteral immunization.
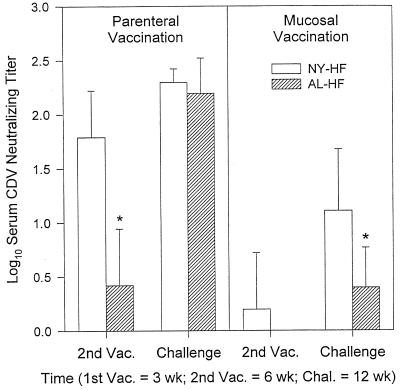
The two recombinant vaccines expressing CDV H and F proteins, NYVAC-HF and ALVAC-HF, were both immunogenic when administered to infant ferrets without maternal antibody protection (Fig. 1). Ferret kits born to unvaccinated jills were vaccinated parenterally (s.c. at 3 weeks and i.m. at 6 weeks of age) with NYVAC-HF (n = 5) and ALVAC-HF (n = 4). There was no detectable CDV-neutralizing antibody at 3 weeks of age in any kit born to an unvaccinated jill. At age 6 weeks, animals vaccinated with NYVAC-HF had developed significant neutralizing antibody (log10 titer ± SD = 1.79 ± 0.43) compared to their titer at age 3 weeks (P = 0.0007). Some ALVAC-HF ferrets had measurable neutralizing titers by 6 weeks of age, but the mean was low and there was no statistically significant increase in neutralizing titers between ages 3 and 6 weeks (0.0 ± 0.0 versus 0.42 ± 0.52). At age 6 weeks, animals vaccinated with NYVAC-HF had a significantly higher log10 titer (1.79 ± 0.43) than animals vaccinated with ALVAC-HF (0.42 ± 0.52) (P = 0.004). However, the second ALVAC-HF vaccination had a significant booster effect, and at age 12 weeks there were no differences in mean log10 titers between the NYVAC-HF (2.30 ± 0.12) and ALVAC-HF groups (2.20 ± 0.34).
FIG. 1.
Serum CDV-neutralizing antibody titers in ferrets without maternal antibody that were vaccinated parenterally at ages 3 (s.c.) and 6 (i.m.) weeks or mucosally (i.n.) at the same time points. Neutralizing titers were undetectable at 3 weeks of age in all animals. Vaccines included NYVAC (NY-HF; n = 5, parenteral; n = 7, mucosal) and ALVAC (AL-HF; n = 4, parenteral; n = 5, mucosal) vectors expressing CDV HF proteins. Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Values shown are mean ± SD inverse log10 titers from samples collected just prior to vaccination (Vac.) or challenge (Chal.), as indicated. The asterisk indicates that the group mean titer was significantly lower than the mean of the other vaccine at the same time point.
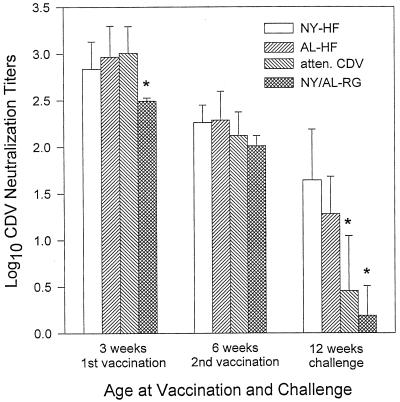
The NYVAC-HF and ALVAC-HF vaccines were also immunogenic when administered in the presence of maternal antibody and produced significantly greater neutralizing antibody titers than did an attenuated, live-virus CDV vaccine (Fig. 2). Ferrets with maternal antibody protection were vaccinated parenterally (s.c. at 3 weeks and i.m. at 6 weeks of age) with one of three CDV vaccines—NYVAC-HF, ALVAC-HF, or attenuated CDV—or with control vaccines expressing rabies glycoprotein (RG) (NYVAC- or ALVAC-RG). Passively acquired antibody titers were high at 3 weeks of age, before the first vaccination, in all four groups (Fig. 2). By chance, the RG control animals had significantly lower log10 titers (2.49 ± 0.03, n = 7) than the other groups (ANOVA; P = 0.002), but this difference had disappeared by 6 weeks of age, when there were no significant differences among the groups. At 12 weeks of age, animals vaccinated with NYVAC-HF or ALVAC-HF had significantly higher mean log10 titers (1.64 ± 0.54 [n = 7] and 1.28 ± 0.40 [n = 7], respectively) than did animals vaccinated with attenuated CDV (0.46 ± 0.59, n = 7) or with the RG vaccines (0.19 ± 0.32, n = 8) (ANOVA; P = 7 × 10−6). The mean titer for the attenuated CDV group did not differ significantly from that for the RG control group, indicating that the attenuated CDV vaccine did not induce a statistically significant increase in neutralizing antibody in the presence of maternal antibody.
FIG. 2.
Serum CDV-neutralizing antibody titers in ferrets vaccinated parenterally at ages 3 (s.c.) and 6 (i.m.) weeks in the presence of maternal antibody. Vaccines included NYVAC (NY-HF; n = 7) and ALVAC (AL-HF; n = 7) vectors expressing CDV H and F proteins, an attenuated, live-virus CDV vaccine (atten. CDV; n = 7), and the NYVAC and ALVAC vectors expressing RG (AL-RG [n = 4] and NY-RG [n = 4]) (NY/AL-RG). Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Values shown are mean ± SD inverse log10 titers from samples collected just prior to vaccination or challenge, as indicated. Asterisks indicate means that are significantly lower than the other means at the same time.
Maternal antibody inhibited the efficacy of the parenterally administered recombinant vaccines in eliciting a neutralizing antibody response. At the time of challenge, animals vaccinated with NYVAC-HF in the absence of maternal antibody had a greater response (2.30 ± 0.12) than did those vaccinated in the presence of maternal antibody (1.64 ± 0.54, P = 0.026). The same was also true of the ALVAC-HF vaccine (2.20 ± 0.34 versus 1.28 ± 0.40, P = 0.004) (see Fig. 1 and 2).
(ii) Mucosal immunization.
Vaccination of infant ferrets without maternal antibody by the i.n. route was less effective in eliciting neutralizing antibody titers than was vaccination via the parenteral route (Fig. 1). Significant titer increases were not seen after one dose of either vaccine. However, titers in the i.n. NYVAC-HF group (n = 7) rose significantly after two doses (paired t test; P = 0.002); titers in the i.n. ALVAC-HF group (n = 5) also rose, but the increase was not statistically significant (paired t test; P = 0.07). By the day of challenge, i.n. vaccination with NYVAC-HF yielded a log10 mean titer (1.11 ± 0.57) that was significantly greater than the mean titer in the i.n. ALVAC-HF group (0.4 ± 0.37) or the unvaccinated control group (0 ± 0, n = 2) (ANOVA; P = 0.02). Parenteral vaccination with two doses of NYVAC-HF produced higher titers by 12 weeks of age than did i.n. vaccination (2.30 ± 0.12 versus 1.11 ± 0.57, P = 0.0011), and the same was true of the ALVAC-HF vaccine (2.20 ± 0.33 versus 0.40 ± 0.37, P = 0.0001), as seen in Fig. 1.
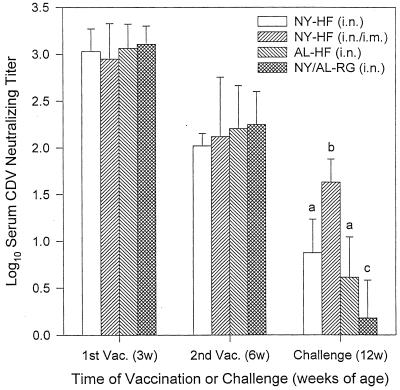
When administered mucosally (i.n.) in the presence of maternal antibody, both the NYVAC-HF and ALVAC-HF vaccines were immunogenic, although administering the same dose of NYVAC-HF via both parenteral and mucosal routes (s.c./i.n. at 3 weeks and i.m./i.n. at 6 weeks) produced the highest neutralizing titers by 12 weeks of age (Fig. 3). Maternally derived antibody titers were high at 3 weeks of age, before vaccination, in all vaccine groups, and mean titers did not differ among the groups—NYVAC-HF i.n. (n = 9), NYVAC-HF i.n./i.m. (n = 9), ALVAC-HF i.n. (n = 9), and NYVAC-RG (n = 3) plus ALVAC-RG (n = 2; the RG vaccinates were pooled for analysis) i.n. Titers decreased by 6 weeks of age, the time of the second vaccination but again did not differ among the groups. At 12 weeks of age, 6 weeks after the second vaccination, animals vaccinated in the presence of maternal antibody with NYVAC-HF i.n./i.m. developed a mean log10 neutralizing titer (1.63 ± 0.25) that was significantly higher than the log10 titers elicited by i.n. NYVAC-HF (0.88 ± 0.36) or ALVAC-HF i.n. (0.61 ± 0.43); all three CDV-vaccinated groups had significantly higher neutralizing titers than did the RG control groups (0.181 ± 0.404) (ANOVA; P = 3 × 10−7).
FIG. 3.
Serum CDV-neutralizing antibody titers in ferrets vaccinated mucosally (i.n.) at ages 3 and 6 weeks (3w and 6w) in the presence of maternal antibody. Vaccines included i.n. NYVAC (NY-HF; n = 9) and i.n. ALVAC (AL-HF; n = 9) vectors expressing CDV H and F proteins, NYVAC-HF given via the parenteral and mucosal route at each vaccination [NY-HF (i.n./i.m.); n = 9], and the NYVAC and ALVAC vectors expressing RG (AL-RG [n = 2] and NY-RG [n = 3]) (NY/AL-RG). Ferrets were challenged i.n. with virulent CDV at 12 weeks (12w) of age. Values shown are mean ± SD inverse log10 titers from samples collected just prior to vaccination (Vac.) or challenge, as indicated. Different letter superscripts at the time of challenge indicate means that are significantly different from one another (P < 0.05).
Maternal antibody did not significantly decrease the neutralizing antibody response to mucosal vaccination. On the day of challenge, there was no statistically significant difference in the log10 mean titers of animals vaccinated with NYVAC-HF i.n. in the presence or absence of maternal antibody (0.88 ± 0.36 versus 1.11 ± 0.57, respectively). Likewise, vaccination with ALVAC-HF i.n. in the presence or absence of maternal antibody did not elicit significantly different titers (0.61 ± 0.43 versus 0.40 ± 0.37, respectively).
With respect to efficacy in eliciting neutralizing antibody in animals with maternal antibody protection, the parenteral route and the combined parenteral/mucosal route are both superior to the i.n. route. Administration of ALVAC-HF parenterally produced significantly higher neutralizing titers on the day of challenge infection (1.28 ± 0.40) than did administering the same dose of vaccine mucosally (0.61 ± 0.43, P = 0.007). When the three routes of administration of the NYVAC-HF vaccine were compared by ANOVA, the means for the parenteral and combined parenteral/mucosal routes did not differ (1.64 ± 0.54 and 1.63 ± 0.25, respectively) but were both significantly greater than the mean of the mucosal group (0.88 ± 0.36, P = 0.0005).
Survival.
All animals vaccinated parenterally with NYVAC-HF and ALVAC-HF in the absence of maternal antibody survived challenge infection at 12 weeks of age (Table 1), while control animals vaccinated with either NYVAC-RG or ALVAC-RG died by day 16 after challenge. However, these vaccines were not effective in protecting animals when given parenterally in the presence of maternal antibody; only one animal (14%) survived in each of the three CDV vaccine groups.
TABLE 1.
Survival of infant ferrets vaccinated parenterally with the indicated vaccines following i.n. challenge infection with CDV
| Vaccine | Maternal antibody | % Surviving (surviving/total) | P valuea (Fisher exact test) |
|---|---|---|---|
| NYVAC-HF | No | 100 (5/5) | 0.0008 |
| ALVAC-HF | No | 100 (4/4) | 0.0020 |
| NYVAC-HF | Yes | 14.3 (1/7) | 0.47 |
| ALVAC-HF | Yes | 14.3 (1/7) | 0.47 |
| Attenuated CDV | Yes | 14.3 (1/7) | 0.47 |
| NY/AL-RG | Yes | 0 (0/8) | NA |
Comparing each vaccine group to NYVAC-RG and ALVAC-RG (NY/AL-RG) controls. NA, not applicable.
When animals were vaccinated mucosally in the absence of maternal antibody, six of seven vaccinated NYVAC-HF i.n. survived; however, there were no survivors in the ALVAC-HF i.n. group (Table 2). Of the animals vaccinated in the presence of maternal antibody, there were no survivors in the NYVAC-HF i.n. or ALVAC i.n. group, but 33% of the ferrets vaccinated by the combined parenteral/mucosal route with NYVAC-HF survived challenge, a significant difference from the 0% survival in the RG control group.
TABLE 2.
Survival of infant ferrets immunized mucosally with the indicated vaccines following i.n. challenge infection with CDV
| Vaccine | Maternal antibody | % Surviving (surviving/total) | P value (χ2 test) |
|---|---|---|---|
| NYVAC-HF i.n. | Yes | 0 (0/9) | 0.0374a |
| NYVAC-HF i.n./i.m. | Yes | 33 (3/9) | |
| ALVAC-HF i.n. | Yes | 0 (0/9) | |
| NY/AL-RG controls | Yes | 0 (0/5) | |
| NYVAC-HF i.n. | No | 86 (6/7) | 0.005b |
| ALVAC-HF i.n. | No | 0 (0/5) | |
| Unvaccinated controls | No | 0 (0/2) |
Comparing survival among the four groups with maternal antibody.
Comparing survival among the three groups without maternal antibody.
Fever.
No statistically significant differences in body temperature were seen among the different vaccine groups using any route of vaccination—parenteral, i.n., or combined—either in the presence or in the absence of maternal antibody (data not shown). Most animals that did not survive challenge infection were febrile at some point during the observation period, but there were no synchronized spikes of fever in the negative control animals (i.e., RG vaccine or no vaccine), as we have previously seen in older ferrets (22 weeks of age), following challenge infection with CDV (41, 49).
Leukocytopenia. (i) Parenteral immunization.
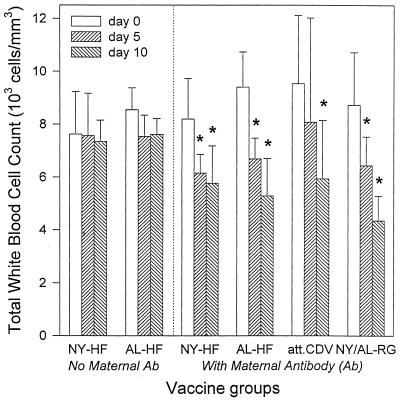
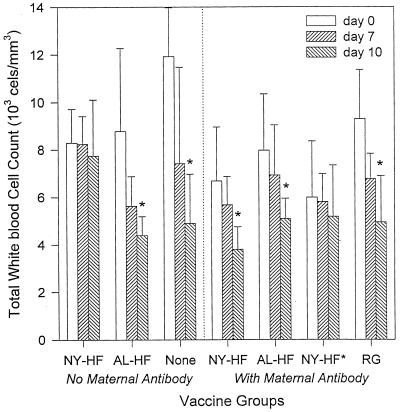
Leukocytopenia, a typical sign of CDV infection in ferrets (40), was not seen in ferrets vaccinated parenterally in the absence of maternal antibody on any day after infection but was seen in all groups vaccinated parenterally in the presence of maternal antibody, as shown in Fig. 4.
FIG. 4.
Mean ± SD total white blood cell counts in ferrets vaccinated parenterally in the presence and absence of maternal antibody with the indicated vaccines. Vaccines given in the absence of maternal antibody (Ab) included NYVAC (NY-HF; n = 5) and ALVAC (AL-HF; n = 4) vectors expressing CDV H and F proteins. Vaccines given in the presence of maternal antibody included NY-HF (n = 7), AL-HF (n = 7), an attenuated, live-virus CDV vaccine (att. CDV; n = 7), and the NYVAC (n = 4) and ALVAC (n = 4) vectors expressing RG (NY/AL-RG). Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Asterisks indicate days on which the mean is significantly lower (P < 0.05) than the corresponding day 0 mean by paired t test. Specific P values are as follows: for NYVAC-HF, P = 0.02 on day 5 and P = 0.03 on day 10; for ALVAC-HF, P = 0.003 on day 5 and P = 0.0002 on day 10; for attenuated CDV, P = 0.0007 on day 10; for control animals receiving the RG vaccines, P = 0.004 on day 5 and P = 0.0001 on day 10.
(ii) Mucosal immunization.
In the absence of maternal antibody, mucosal vaccination with NYVAC-HF protected against the development of leukocytopenia (Fig. 5), while white blood cell counts were significantly decreased in both the ALVAC-HF (P = 0.03) and unvaccinated control (P = 0.001) groups by day 10 after challenge. In animals vaccinated in the presence of maternal antibody, only the combined mucosal/parenteral NYVAC-HF vaccine protected against the development of significant leukocytopenia through 10 days after challenge infection (Fig. 5). However, the parenteral/mucosal NYVAC-HF did become leukocytopenic on day 14, after one animal had died, when the mean count for survivors was 4,274 ± 1,164, significantly lower than the day 0 value for these 8 animals (6,353 ± 2,237, P = 0.013). The three surviving animals still had low counts on day 21 (P = 0.048), but the mean count increased by day 28 and was no longer significantly different than the day 0 value.
FIG. 5.
Mean ± SD total white blood cell counts in ferrets vaccinated mucosally (i.n.) in the presence and absence of maternal antibody with the indicated vaccines. Vaccines given in the absence of maternal antibody included NYVAC (NY-HF; n = 7) and ALVAC (AL-HF; n = 5) vectors expressing CDV H and F proteins. Two animals remained unvaccinated (None) in the absence of maternal antibody. Vaccines given in the presence of maternal antibody included i.n. NY-HF (n = 9), i.n. AL-HF (n = 9), NY-HF given via a combined parenteral/mucosal route (NY-HF*; s.c./i.n. at 3 weeks and i.m./i.n. at 6 weeks) and the NYVAC (n = 3) and ALVAC (n = 2) vectors expressing RG given i.n. (NY/AL-RG). Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Asterisks indicate days on which the mean is significantly lower (P < 0.05) than the corresponding day 0 mean by paired t test. Specific P values are as follows: for the NYVAC-HF i.n. group, P = 0.01 on day 10; for the ALVAC-HF i.n. group, P = 0.005 on day 10; for the RG group, P = 0.032 on day 10.
Body weight. (i) Parenteral immunization.
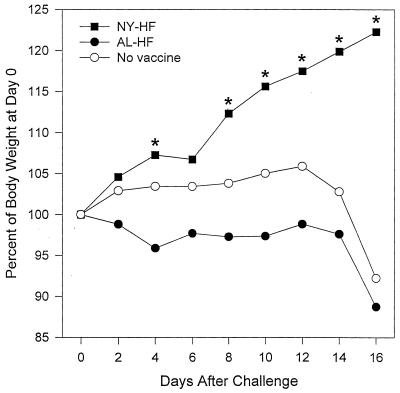
Animals vaccinated with NYVAC-HF and ALVAC-HF in the absence of maternal antibody continued to gain weight at a normal rate after challenge infection (data not shown). Animals vaccinated with NYVAC-HF in the presence of maternal antibody gained weight more rapidly after challenge than did other vaccine groups and thus were significantly heavier than the RG control, ALVAC-HF, and attenuated CDV animals on most days, as shown in Fig. 6.
FIG. 6.
Mean body weights of ferrets vaccinated parenterally with the indicated CDV vaccines in the presence of maternal antibody. Vaccines included NYVAC (NY-HF; n = 7) and ALVAC (AL-HF; n = 7) vectors expressing CDV H and F proteins, an attenuated, live-virus CDV vaccine (atten. CDV; n = 7), and the NYVAC and ALVAC vectors expressing rabies RG (AL-RG [n = 4] and NY-RG [n = 4]) (NY/AL-RG). Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Asterisks indicate days on which the mean weights were significantly greater than mean weights of the other groups by ANOVA and the Student-Newman-Keuls test for pairwise comparisons.
(ii) Mucosal immunization.
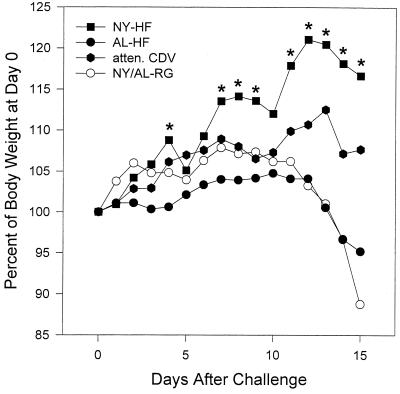
Ferrets vaccinated i.n. with NYVAC-HF in the absence of maternal antibody had significantly higher body weights (ANOVA; P < 0.01) than did the ALVAC-HF and unvaccinated control groups on most days after challenge infection (Fig. 7). In the groups vaccinated in the presence of maternal antibody, there were no significant differences in body weight after challenge, although the NYVAC-HF i.n./i.m. vaccinates had slightly higher body weights from day 8 through the end of the observation period (data not shown).
FIG. 7.
Mean body weights of ferrets vaccinated mucosally with the indicated CDV vaccines in the absence of maternal antibody. Vaccines included NYVAC (NY-HF; n = 7) and ALVAC (AL-HF; n = 5) vectors expressing CDV H and F proteins; two animals remained unvaccinated (None). Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Asterisks indicate days on which the mean weights were significantly greater than mean weights of the other groups by ANOVA and the Student-Newman-Keuls test for pairwise comparisons.
Clinical signs. (i) Parenteral immunization.
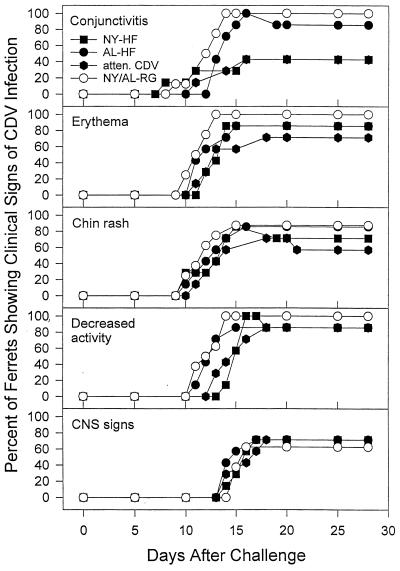
Animals vaccinated with either NYVAC-HF or ALVAC-HF in the absence of maternal antibody did not develop clinical signs of distemper (data not shown). In addition, when these vaccines, or attenuated CDV, were administered in the presence of maternal antibody, all three vaccines significantly decreased or delayed the occurrence of at least some clinical signs of distemper (Fig. 8). Control animals vaccinated with NYVAC- or ALVAC-RG in the presence of maternal antibody developed clinical signs as early as day 9 after challenge. Animals vaccinated with NYVAC-HF in the presence of maternal antibody were significantly less likely to develop conjunctivitis than controls (Fisher exact test; three of seven vaccinates versus eight of eight controls, P = 0.03), as were animals vaccinated with attenuated CDV (Fisher exact test; three of seven vaccinates versus eight of eight controls, P = 0.03). The mean and median times to first occurrence of conjunctivitis were significantly longer in the groups vaccinated with ALVAC-HF and attenuated CDV (respectively) in the presence of maternal antibody (t test for ALVAC-HF, 14.0 days versus 12.4 for controls, t = 2.23, df = 13, P = 0.04; Mann-Whitney rank sum test for attenuated CDV, 18.0 days versus 12.4 days for controls, T = 76.0, P = 0.02). The median time to first occurrence of erythema was significantly longer in animals vaccinated with NYVAC-HF (14.0 days versus 11.5 for controls, T = 77.0, P = 0.01) and in animals vaccinated with attenuated CDV (13.0 days versus 11.5 for controls, T = 74.0, P = 0.04), as determined by Mann-Whitney rank sum test. The mean time to first occurrence of decreased activity was significantly longer in animals vaccinated with NYVAC-HF (t test; 15.3 days versus 12.5 for controls, t = −4.65, df = 13, P = 0.0005). The median time to first occurrence of decreased activity was significantly longer in animals vaccinated with attenuated CDV (Mann-Whitney rank sum test; 16.0 days versus 12.5 for controls, T = 75.5, P = 0.02). All three animals that survived challenge after being vaccinated in the presence of maternal antibody developed at least one clinical sign of distemper. The NYVAC-HF vaccinate developed a chin rash and decreased activity of 4 days duration; the ALVAC-HF vaccinate developed a conjunctivitis that lasted 3 days; and the attenuated CDV vaccinate developed a chin rash of 3 days duration.
FIG. 8.
Prevalence of indicated clinical signs of distemper among ferrets vaccinated parenterally with the indicated vaccines. Vaccines included NYVAC (NY-HF; n = 7) and ALVAC (AL-HF; n = 7) vectors expressing CDV HF proteins, an attenuated, live-virus CDV vaccine (atten. CDV; n = 7), and the NYVAC and ALVAC vectors expressing RG (AL-RG [n = 4] and NY-RG [n = 4]) (NY/AL-RG). Ferrets were challenged i.n. with virulent CDV at 12 weeks of age. Animals that died remained in both the numerator and denominator when prevalence rates were calculated.
(ii) Mucosal immunization.
In the absence of maternal antibody, all animals vaccinated i.n. with ALVAC-HF developed all clinical signs of distemper, while those vaccinated i.n. with NYVAC-HF had lower prevalence rates of some clinical signs (Table 3), although only the lower incidence of CNS signs and decreased activity were statistically significant (P = 0.0013 and 0.0076, respectively) compared to the ALVAC-HF group. Of the six surviving ferrets vaccinated i.n. with NYVAC-HF, one showed no clinical evidence of infection, four had conjunctivitis, five had erythema, four had a chin rash, and none lost weight or had a decrease in activity level. All clinical signs of infection resolved by day 21 in these survivors.
TABLE 3.
Percent of infant ferrets vaccinated i.n. with the indicated vaccines that developed clinical signs of distemper at any point after i.n. CDV challenge infection
| Sign | % with sign
|
|||||
|---|---|---|---|---|---|---|
| With maternal antibody
|
Without maternal antibody
|
|||||
| NY-HF (n = 9) | AL-HF (n = 9) | NY-HF i.n./i.m. (n = 9) | NY/AL- RG (n = 7) | NY-HF (n = 7) | AL-HF (n = 5) | |
| Conjunctivitis | 100 | 100 | 78 | 100 | 57 | 100 |
| Chin rash | 100 | 100 | 78 | 100 | 57 | 100 |
| Erythema | 100 | 100 | 56a | 100 | 85 | 100 |
| Decreased activity | 78 | 67 | 56 | 100 | 14a | 100 |
| CNS involvement | 56 | 45 | 56 | 71 | 0a | 100 |
P < 0.05, compared to the other vaccine groups in the same maternal antibody category.
In the presence of maternal antibody, most animals developed multiple clinical signs of distemper (Table 3). The differences in the percentages of animals exhibiting a specific clinical sign are not statistically significant, except for the lower incidence of erythema seen in the parenteral/mucosal NYVAC-HF group (P = 0.0056). No significant differences in time to onset of signs were seen. Of the three surviving mucosal/parenteral NYVAC-HF vaccinates, all had conjunctivitis, two had a chin rash, one had erythema, and two had decreased activity. All of these signs were transient and resolved by day 20 postchallenge.
Detection of CDV RNA in PBMCs by RT-PCR. (i) Parenteral immunization.
None of the ferrets vaccinated with NYVAC-HF and ALVAC-HF in the absence of maternal antibody had viremia detectable by RT-PCR at any time after challenge (Table 4). RT-PCR evidence of viral RNA was found on days 5, 10, and 15 in all ferrets vaccinated in the presence of maternal antibody that eventually succumbed to the challenge infection. The surviving ferret vaccinated with NYVAC-HF in the presence of maternal antibody had evidence of viremia only on day 5 after challenge. The surviving attenuated CDV vaccinate had CDV-specific RT-PCR product on days 5, 10, and 15 but no detectable viremia on days 20 or 28. The surviving ferret vaccinated with ALVAC-HF had no detectable viremia at any point after challenge.
TABLE 4.
Detection of CDV RNA in PBMCs by RT-PCR in infant parenteral vaccinates after i.n. CDV challenge infection
| Vaccine | Maternal antibody | No. of ferrets positive/total no. after challenge (days)a
|
||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 28 | ||
| NYVAC-HF | No | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| ALVAC-HF | No | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| NYVAC-HF | Yes | 7/7 | 6/7 | 6/7 | 0/1 | 0/1 |
| ALVAC-HF | Yes | 6/7 | 6/7 | 6/7 | 0/1 | 0/1 |
| Attenuated CDV | Yes | 7/7 | 7/7 | 7/7 | 0/1 | 0/1 |
| NY/AL-RG | Yes | 8/8 | 8/8 | 8/8 | ||
All control ferrets died between days 15 and 20 postchallenge.
(ii) Mucosal immunization.
In these animals RT-PCR was carried out only on PBMCs collected on day 10 after challenge because previous work (41, 49) indicates that viremia detected on day 10 correlated most closely with survival. Of the animals vaccinated in the absence of maternal antibody, viremia was detected in one of seven animals in the NYVAC-HF i.n. group, all five of the ALVAC-HF i.n. ferrets, and both of the unvaccinated controls. The six surviving NYVAC-HF animals had no detectable viremia. Among animals vaccinated in the presence of maternal antibody, viremia was detected in all nine ferrets given NYVAC-HF i.n., all nine given ALVAC-HF i.n., six of nine given NYVAC-HF by the mucosal/parenteral route, and all five of the NYVAC-RG and ALVAC-RG controls. The three surviving animals in the mucosal/parenteral group had no detectable viremia.
DISCUSSION
Morbillivirus subunit vaccines that include the H and F proteins have been shown to protect against challenge infection in various species (11, 21, 28, 31). We have recently shown that NYVAC-HF and ALVAC-HF are both immunogenic and protect against disease and death from CDV infection in juvenile ferrets vaccinated parenterally (41) or i.n. (49) after the waning of maternal antibody.
Vaccinia virus vectors are capable of replication in the presence of serum antibody directed against recombinant antigens (13). Although passive antibody directed against such antigens can diminish the magnitude of the serum antibody response to that antigen (20), the decrease is dose dependent. This suggests that the response to parenteral immunization with vaccinia virus recombinants will be inhibited by very high titers of maternal antibody but that such a vaccine could still be efficacious, particularly when administered after titers diminish somewhat. In addition, since most interference in infants is thought to be from circulating antibodies acquired transplacentally or transcolostrally, it is possible that vaccinating via a mucosal route may protect against infection and disease. For these reasons, we elected to evaluate the efficacy of NYVAC-HF and ALVAC-HF given by both parenteral and mucosal routes in infant ferrets protected by maternal antibody at two time points: 3 weeks of age, when maternal antibody titers were quite high (i.e., log10 mean values of approximately 3.0), and at 6 weeks, after titers had diminished somewhat (i.e., to approximately 2.0).
When vaccinated parenterally with NYVAC-HF or ALVAC-HF in the absence of maternal antibody, ferret kits developed CDV-neutralizing antibody titers comparable to those seen in juveniles (41). NYVAC-HF elicited high titers after one vaccination at age 3 weeks, and titers did not increase significantly after a second vaccination. Kits vaccinated with ALVAC-HF had little rise in titer after the first vaccination, but the second vaccination increased their titer to the level of the NYVAC-HF vaccinates. When these vaccines were given to juveniles (41), the ALVAC-induced neutralizing antibody response was also lower than the NYVAC-induced response after one vaccination, although the difference was not statistically significant. Similarly, the responses were equivalent after the second vaccination. The reason for the higher initial titer seen in response to the NYVAC vaccine is not clear. Replication of NYVAC is restricted in a range of cell lines from a number of species, including humans (42). There are no data available on NYVAC replication in ferret cells. Other factors which may be involved in the difference include the relative level of expression of the foreign gene products from ferret cells, or differential stimulation of the immune system by the parent vectors, perhaps by production of gamma interferon or interleukin-12, which could result in higher antibody titers when the NYVAC-HF construct is used.
Infant ferrets vaccinated mucosally with NYVAC-HF and ALVAC-HF in the absence of maternal antibody responded with significantly lower neutralizing antibody titers, even after two vaccinations, than did the ferrets vaccinated parenterally with the same vaccines. In contrast, i.n. vaccination in juvenile ferrets (49) produced neutralizing titers after two vaccine doses that were equivalent to or higher than those seen with parenteral vaccination of juveniles (41). This suggests that infants are less responsive to i.n. vaccination than are older ferrets, which raises questions about the appropriateness of this route of immunization in infant ferrets or infants of other species. One possible explanation for the decreased efficacy of i.n. immunization with either vector is that nonspecific, antiviral factors found in breast milk (ferret kits were breast-feeding through 6 weeks of age) may have been present in the nasopharynx and thus could have interfered with NYVAC or ALVAC viability or uptake by cells (2). Such interference could have diminished the response to both vaccines in nursing ferrets.
The infant ferret immune system is capable of responding to the recombinant antigens expressed by the poxvirus vectors in either the absence or presence of interfering maternal antibody, although the magnitude of the response is diminished by the presence of maternal antibody. In addition, the neutralizing antibody response to the recombinant vaccines was better than that seen in response to the attenuated CDV vaccine. These vaccines—NYVAC-HF, ALVAC-HF, and attenuated CDV—had undistinguishable neutralizing antibody responses when administered parenterally to juvenile ferrets (41). Since CDV-neutralizing antibody may directly neutralize the infectivity of the attenuated CDV vaccine, it is not surprising that the response to this vaccine was blunted to a greater degree by neutralizing antibody than were the responses to NYVAC-HF and ALVAC-HF. These results indicate that the recombinant CDV vaccines were superior to the attenuated CDV in inducing a neutralizing antibody response in the presence of maternal neutralizing antibody, although survival rates did not differ among the groups.
Intranasal vaccination with two doses of NYVAC-HF or ALVAC-HF in the presence of maternal antibody elicited equivalent titers of neutralizing antibody; these titers were significantly higher than seen with the control RG vaccine preparations. In addition, titers in response to i.n. immunization in the presence of maternal antibody were not significantly lower than those induced in the absence of maternal antibody, indicating that CDV-specific maternal antibody did not interfere with i.n. immunization. However, the lack of interference of maternal antibody with this route of immunization is not particularly encouraging with regard to finding a useful route of immunization, since mean titers both in the presence and in the absence of maternal antibody were very low (i.e., log10 titers were below 1.5). In addition, none of the animals vaccinated i.n. with ALVAC-RG or NYVAC-RG survived challenge infection with CDV or had a significant decrease or delay in clinical signs of disease, indicating that the low titers of neutralizing antibody did not reflect an induction of protective immunity. Although corresponding data on the response to i.n. vaccination with recombinant vaccines are not available from humans, live attenuated measles vaccines have been administered by aerosol, although seroconversion rates were lower in infants under 6 months of age (23, 36, 38), probably as the result of the presence of higher levels of maternal antibody. Thus, while i.n. immunization is efficacious in older animals, administration of these vectors via the i.n. route was less effective in these studies than was parenteral administration.
One modification of i.n. vaccine administration which did show some promise in these studies was combined parenteral/mucosal immunization with NYVAC-HF. In the presence of maternal antibody, this vaccine regimen yielded significantly higher neutralizing antibody titers than did i.n. immunization alone, and survival after challenge infection was correspondingly higher (33%) than when the same vaccine was given by the mucosal route alone (0%) or parenteral route alone (14%) (although the difference between the former and latter survival rates was not statistically significant). Surviving animals in the parenteral/mucosal NYVAC-HF group did not have significantly higher titers than did nonsurvivors, indicating that the presence of neutralizing antibody was not a significant correlate of protection. Elicitation of cytotoxic T lymphocytes (CTLs) could account for the increased survival; although efforts to measure CTL responses in these animals were not successful (data not shown) and their role remains uncertain, CTLs clearly contribute to recovery from other Morbillivirus infections (17, 24, 25, 28, 48). It would also be useful to evaluate the combined parental/mucosal method of vaccination with the ALVAC-HF vaccine.
All infant ferrets vaccinated parenterally with NYVAC-HF or ALVAC-HF in the absence of maternal antibody survived challenge, with no clinical signs of distemper or RT-PCR evidence of CDV viremia. Essentially identical results were seen when these vaccines were administered parenterally to juvenile ferrets (41). Thus, two doses of either vaccine are equally efficacious in infant or juvenile ferrets, when administered in the absence of passively acquired, neutralizing antibody to CDV.
Although parenteral vaccination with NYVAC-HF, ALVAC-HF, and attenuated CDV in the presence of maternal antibody did not protect against death from distemper, all three vaccines showed some beneficial effects in slowing disease progression. Compared to RG controls, animals vaccinated with NYVAC-HF in the presence of maternal antibody had a significantly lower incidence of conjunctivitis and a delayed onset of erythema and decreased activity, and they continued to grow at normal rate until just before death. ALVAC-HF vaccinates had a delayed onset of conjunctivitis. Animals vaccinated with attenuated CDV, despite very little production of neutralizing antibody, had a decreased incidence and delayed onset of conjunctivitis, as well as a delayed onset of erythema and decreased activity. This suggests that the amelioration of the clinical signs of disease was not completely due to the presence of neutralizing antibody. Nonneutralizing antibody may be present and could confer protection by enhancing virus phagocytosis, through aggregation of virus particles or antibody-dependent cell-mediated cytotoxicity. The vaccines may also have induced a CTL response that slowed progression of the disease but was not sufficient to prevent infection of the CNS, which inevitably leads to seizures and death in ferrets (10). CDV infection in ferrets is highly neurotropic, much more so than measles; so, relatively more protective antibody may be needed to prevent fatal CDV disease in ferrets than would be needed for protection against symptomatic measles infection in humans. In addition, there is considerable evidence for the importance of cell-mediated immunity in the clearance of Morbillivirus infections (17, 24, 25, 39, 48). While there is a known T-cell epitope in the fusion protein of MV that is immunodominant in mice and humans (34), there has not been one reported in CDV. However, there is a similar amino acid sequence in the CDV fusion protein (6) that may function as a T-cell epitope in ferrets, to prime the cell-mediated response to CDV. The addition of other CDV proteins with known T-cell epitopes (such as the nucleocapsid protein) (7) to the recombinant poxvirus vectors may improve cell-mediated response, even in animals with existing passive immunity. The ability of these CDV vaccines to delay the clinical course of disease in ferrets suggests that such vaccines may be even more efficacious when used to prevent clinical disease in other Morbillivirus infections, such as measles, where CNS involvement is not such a prominent feature of disease pathogenesis.
CDV infection of ferrets is a useful animal model for testing Morbillivirus vaccine strategies, both because it employs a natural host-virus system and because the clinical course of disease is such that many clinical endpoints may be monitored to evaluate vaccine efficacy. Other animal models are also available. While MV infects only humans and nonhuman primates by the natural, i.n. route, intracerebral inoculation of selected MV strains into rodents or ferrets does produce a disease similar to subacute sclerosing panencephalitis (5, 9, 46, 50). Cotton rats can be infected with MV by the i.n. route, resulting in limited MV replication but no clinical disease (51), although some MV-induced immunosuppression is observed (29). Transgenic mice expressing the cellular MV receptor CD46 have been developed but are not susceptible to MV infection in vivo, although their cells can be infected in vitro (18). MV pathogenesis and immune response in rhesus macaques has recently been well described (26, 47, 52). The disease progression and immune response to MV infection in rhesus macaques and humans are very similar, making macaques an important model for the study of measles pathogenesis but, perhaps, impractical for many studies. Although ferrets cannot be infected with MV, they can be infected via the natural route with the closely related CDV and develop a clinical course comparable to measles, with rash, conjunctivitis, fever, malaise, leukopenia, and immunosuppression (22). The immune response after vaccination is also similar. Virus-neutralizing antibody against the hemagglutinin proteins of both MV and CDV correlates strongly with protection against infection, and antifusion antibody can also provide some protection against challenge with MV in mice (16). If not protected by vaccination, ferrets inevitably succumb to CDV encephalitis or pneumonia, which provides a clear-cut measure of vaccine efficacy. The CDV-ferret model offers clear advantages over other available small-animal models for studies of protection against development of symptomatic Morbillivirus infection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R01 AI35142 from the National Institute for Allergy and Infectious Disease and grant T32 RR07003 from the Division of Research Resources.
We thank Sharon Blount for excellent technical assistance.
REFERENCES
- 1.Aaby P, Samb B, Simondon F, Knudsen K, Seck A M C, Bennett J, Whittle H. Divergent mortality for male and female recipients of low-titer and high-titer measles vaccines in rural Senegal. Am J Epidemiol. 1993;138:746–755. doi: 10.1093/oxfordjournals.aje.a116912. [DOI] [PubMed] [Google Scholar]
- 2.Appel M J, Harris W V. Antibody titers in domestic ferret jills and their kits to canine distemper virus vaccine. J Am Vet Med Assoc. 1988;193:332–333. [PubMed] [Google Scholar]
- 3.Appel M J G, Pearce-Kelling S, Summers B A. Dog lymphocyte cultures facilitate the isolation and growth of virulent canine distemper virus. J Vet Diagn Investig. 1992;4:258–263. doi: 10.1177/104063879200400306. [DOI] [PubMed] [Google Scholar]
- 4.Appel M, Robson D S. A microneutralization test for canine distemper virus. Am J Vet Res. 1973;34:1459–1463. [PubMed] [Google Scholar]
- 5.Bankamp B, Brinckmann U G, Reich A, Niewiesk S, ter Meulen V, Liebert U G. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol. 1991;65:1695–1700. doi: 10.1128/jvi.65.4.1695-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett T, Clarke D K, Evans S A, Rima B K. The nucleotide sequence of the gene encoding the F protein of canine distemper: a comparison of the deduced amino acid sequence with other paramyxoviruses. Virus Res. 1987;8:373–386. doi: 10.1016/0168-1702(87)90009-8. [DOI] [PubMed] [Google Scholar]
- 7.Beauverger P, Buckland R, Wild T F. Measles virus antigens induce both type-specific and canine distemper virus cross-reactive cytotoxic T lymphocytes in mice: localization of a common Ld-restricted nucleoprotein epitope. J Gen Virol. 1993;74:2357–2363. doi: 10.1099/0022-1317-74-11-2357. [DOI] [PubMed] [Google Scholar]
- 8.Boom R, Sol C J A, Salimans M M M, Jansen C L, Dillen P M E W, Noordaa J V. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinckmann U G, Bankamp B, Reich A, ter Meulen V, Liebert U G. Efficacy of individual measles virus structural proteins in the protection of rats from measles encephalitis. J Gen Virol. 1991;72:2491–2500. doi: 10.1099/0022-1317-72-10-2491. [DOI] [PubMed] [Google Scholar]
- 10.Crook E, Gorham J R, McNutt S H. Experimental distemper in mink and ferrets. I. Pathogenesis. Am J Vet Res. 1958;19:955–957. [PubMed] [Google Scholar]
- 11.de Vries P, VanBinnendijk R S, VanDerMarcel P, Wezel A L, Voorma H O, Sundquist B, Uytdehaag F G, Osterhaus A D. Measles virus fusion protein presented in an immune-stimulating complex (iscom) induces haemolysis-inhibiting antibodies, virus-specific T-cells and protection in mice. J Gen Virol. 1988;69:549–559. doi: 10.1099/0022-1317-69-3-549. [DOI] [PubMed] [Google Scholar]
- 12.Etchart N, Wild F, Kaiserlian D. Mucosal and systemic immune responses to measles virus haemagglutinin in mice immunized with a recombinant vaccinia virus. J Gen Virol. 1996;77:2471–2478. doi: 10.1099/0022-1317-77-10-2471. [DOI] [PubMed] [Google Scholar]
- 13.Flexner C, Murphy B R, Rooney J F, Wohlenberg C, Yuferov V, Notkins A L, Moss B. Successful vaccination with a polyvalent live vector despite existing immunity to an expressed antigen. Nature. 1988;335:259–262. doi: 10.1038/335259a0. [DOI] [PubMed] [Google Scholar]
- 14.Garenne M, Aaby P. Pattern of exposure and measles mortality in Senegal. J Infect Dis. 1990;161:1088–1094. doi: 10.1093/infdis/161.6.1088. [DOI] [PubMed] [Google Scholar]
- 15.Garenne M, Leroy O, Beau J P, Sene I. Child mortality after high-titre measles vaccines: prospective study in Senegal. Lancet. 1991;338:903–907. doi: 10.1016/0140-6736(91)91771-l. [DOI] [PubMed] [Google Scholar]
- 16.Giraudon P, Wild T F. Correlation between epitopes on hemagglutinin of measles and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985;144:46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Good R A, Zak S J. Disturbances in gamma globulin synthesis as “experiments of nature.”. Pediatrics. 1956;18:109–122. [PubMed] [Google Scholar]
- 18.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infection. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussey G D, Goddard E A, Hughes J, Ryon J J, Kerran M, Carelse E, Strebel P M, Markowitz L E, Moodie J, Barron P, Latief Z, Sayed R, Beatty D, Griffin D E. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune responses in infants. J Infect Dis. 1996;173:1320–1326. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 20.Johnson M P, Meitin C A, Bender B S, Small P A. Recombinant vaccinia immunization in the presence of passively administered antibody. Vaccine. 1993;11:665–669. doi: 10.1016/0264-410x(93)90314-n. [DOI] [PubMed] [Google Scholar]
- 21.Jones L, Tenorio E, Gorham J, Yilma T. Protective vaccination of ferrets against canine distemper with recombinant pox virus vaccines expressing the HA or F genes of rinderpest virus. Am J Vet Res. 1997;58:590–593. [PubMed] [Google Scholar]
- 22.Kauffman C A, Bergman A G, O'Connor R P. Distemper virus infection in ferrets: an animal model of measles-induced immunosuppression. Clin Exp Immunol. 1982;47:617–625. [PMC free article] [PubMed] [Google Scholar]
- 23.Khanum S, Uddin N, Garelick H, Mann G, Tomkins A. Comparison of Edmonston-Zagreb and Schwarz strains of measles vaccine given by aerosol or subcutaneous injection. Lancet. 1987;i:150–153. doi: 10.1016/s0140-6736(87)91978-7. [DOI] [PubMed] [Google Scholar]
- 24.Kreth H W, ter Meulen V, Eckert G. Demonstration of HLA restricted killer cells in patients with acute measles. Med Microbiol Immunol. 1979;165:203–214. doi: 10.1007/BF02152920. [DOI] [PubMed] [Google Scholar]
- 25.Lucas C J, Biddison W E, Nelson D L, Shaw S. Killing of measles virus-infected cells by human cytotoxic T cells. Infect Immun. 1982;38:226–232. doi: 10.1128/iai.38.1.226-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McChesney M B, Miller C J, Rota P A, Zhu Y, Antipa L, Lerche N W, Ahmed R, Bellini W J. Experimental measles. I. Pathogenesis in the normal and immunized host. Virology. 1997;233:74–84. doi: 10.1006/viro.1997.8576. [DOI] [PubMed] [Google Scholar]
- 27.Murphy B R, Collins P L, Lawrence L, Zubak J, Chanock R M, Prince G A. Immunosuppression of the antibody response to respiratory syncytial virus (RSV) by pre-existing serum antibodies: partial prevention by topical infection of the respiratory tract with vaccinia virus-RSV recombinants. J Gen Virol. 1989;70:2185–2190. doi: 10.1099/0022-1317-70-8-2185. [DOI] [PubMed] [Google Scholar]
- 28.Ngichabe C, Wamway H, Barrett T, Ndungu E, Black D, Bostock C. Trial of a capripox-rinderpest recombinant vaccine in African cattle. Epidemiol Infect. 1997;118:63–70. doi: 10.1017/s0950268896007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niewiesk S, Eisenhuth I, ter Muelen V. Protective immunity, but suppressed immune responses to third party antigens are generated in cotton rats during measles virus infection. Biochem Soc Trans. 1997;25:355S. doi: 10.1042/bst025355s. [DOI] [PubMed] [Google Scholar]
- 30.Norrby E, Oxman M N. Measles virus. In: Fields B N, Knipe D M, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Fields virology. 2nd ed. Vol. 1. New York, N.Y: Raven Press; 1990. pp. 1013–1044. [Google Scholar]
- 31.Norrby E, Utter G, Orvell C, Appel M J. Protection against canine distemper virus in dogs after immunization with isolated fusion protein. J Virol. 1986;58:536–541. doi: 10.1128/jvi.58.2.536-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pardo M C, Bauman J E, Mackowiak M. Protection of dogs against canine distemper by vaccination with a canarypox virus recombinant expressing canine distemper virus fusion and hemagglutinin glycoproteins. Am J Vet Res. 1997;58:833–836. [PubMed] [Google Scholar]
- 33.Park J, Peters C J, Rollin P E, Ksiazek T G, Gray B, Waites K B, Stephensen C B. Development of an RT-PCR assay for diagnosis of lymphocytic choriomeningitis virus (LCMV) infection and its use in a prospective surveillance study. J Med Virol. 1997;51:107–114. [PubMed] [Google Scholar]
- 34.Partidos C D, Steward M W. Prediction and identification of a T-cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990;71:2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- 35.Perkus M E, Limbach K, Paoletti E. Cloning and expression of foreign genes in vaccinia virus using a host-range selection system. J Virol. 1989;36:3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozenblatt S, Eizenberg O, Ben-Levy R, Lavie V, Bellini W J. Sequence homology within the morbilliviruses. J Virol. 1985;53:684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabin A B, Albrecht P, Takeda A K, Ribeiro E M, Veronesi R. High effectiveness of aerosolized chick embryo fibroblast measles vaccine in seven-month-old and older infants. J Infect Dis. 1985;152:1231–1237. doi: 10.1093/infdis/152.6.1231. [DOI] [PubMed] [Google Scholar]
- 38.Sabin A B, Flores Arechiga A, Fernandez de Castro J, Sever J L, Madden D L, Shekarchi L, Albrecht P. Successful immunization of children with and without maternal antibody by aerosolized measles vaccine. I. Different results with undiluted human diploid cell and chick embryo fibroblast vaccines. JAMA. 1983;249:2651–2662. [PubMed] [Google Scholar]
- 39.Sethi K K, Stroehmann I, Brandis H. Generation of cytolytic T-cell cultures displaying measles virus specificity and human histocompatibility leukocyte antigen restriction. Infect Immun. 1982;36:657–661. doi: 10.1128/iai.36.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smedman L, Joki A, da Silva A P J, Troye-Blomberg M, Aronsson B, Perlmann P. Immunosuppression after measles vaccination. Acta Paediatr. 1994;83:164–168. doi: 10.1111/j.1651-2227.1994.tb13043.x. [DOI] [PubMed] [Google Scholar]
- 41.Stephensen C B, Welter J, Thaker S, Taylor J, Tartaglia J, Paoletti E. Canine distemper virus (CDV) infection of ferrets as a model for testing Morbillivirus vaccine strategies: NYVAC- and ALVAC-based CDV recombinants protect against symptomatic infection. J Virol. 1997;71:1506–1513. doi: 10.1128/jvi.71.2.1506-1513.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tartaglia J, Perkus M E, Taylor J, Norton E K, Audonnet J C, Cox W I, Davis S W, van der Hoeven J, Meignier B, Riviere M, Languet B, Paoletti E. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992;188:217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- 43.Taylor J, Pincus S, Tartaglia J, Richardson C, Alkhatib G, Briedis D, Appel M, Norton E, Paoletti E. Vaccinia virus recombinants expressing either the measles virus fusion or hemagglutinin glycoprotein protect dogs against canine distemper virus challenge. J Virol. 1991;65:4263–4274. doi: 10.1128/jvi.65.8.4263-4274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor J, Meignie B, Tartaglia J, Languet B, VanderHoeven J, Franchini G, Trimarchi C, Paoletti E. Biological and immunogenic properties of a canarypox-rabies recombinant ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995;13:539–549. doi: 10.1016/0264-410x(94)00028-l. [DOI] [PubMed] [Google Scholar]
- 45.Taylor J, Tartaglia J, Rivière M, Duret C, Languet B, Chappuis G, Paoletti E. Applications of canarypox (ALVAC) vectors in human and veterinary vaccination. Dev Biol Stand. 1994;82:131–135. [PubMed] [Google Scholar]
- 46.Thormar H, Mehta P D, Barshatzky M R, Brown H R. Measles virus encephalitis in ferrets as a model for subacute sclerosing panencephalitis. Lab Anim Sci. 1985;35:229–232. [PubMed] [Google Scholar]
- 47.vanBinnendijk R S, Poelen M C M, van Amerongen G, de Vries P, Osterhaus A D M E. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J Infect Dis. 1997;175:524–532. doi: 10.1093/infdis/175.3.524. [DOI] [PubMed] [Google Scholar]
- 48.vanBinnendijk R S, Poelen M C M, Kuijpers K C, Osterhaus A D M E, Uytdehaag F G C M. The predominance of CD8+ T-cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. J Immunol. 1990;144:2394–2399. [PubMed] [Google Scholar]
- 49.Welter J, Taylor J, Tartaglia J, Paoletti E, Stephensen C B. Mucosal vaccination with recombinant poxvirus vaccines protects ferrets against symptomatic CDV infection. Vaccine. 1999;17:308–313. doi: 10.1016/s0264-410x(98)00211-4. [DOI] [PubMed] [Google Scholar]
- 50.Wild T F, Bernard A, Spehner D, Villeval D, Drillien R. Vaccination of mice against canine distemper virus-induced encephalitis with vaccinia virus recombinants encoding measles or canine distemper virus antigens. Vaccine. 1993;11:438–444. doi: 10.1016/0264-410x(93)90285-6. [DOI] [PubMed] [Google Scholar]
- 51.Wyde P R, Ambrose M W, Voss T G, Meyer H L, Gilbert B E. Measles virus replication in lungs of hispid cotton rats after intranasal inoculation. Proc Soc Exp Biol Med. 1992;201:80–87. doi: 10.3181/00379727-201-43483. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y, Heath J, Collins J, Greene T, Antipa L, Rota P, Bellini W, McChesney M. Experimental measles. II. Infection and immunity in the rhesus macaque. Virology. 1997;233:85–92. doi: 10.1006/viro.1997.8575. [DOI] [PubMed] [Google Scholar]