Abstract
The COVID-19 pandemic generated significant life stress and increases in internalizing disorders. Moreover, COVID-related stressors disproportionately impacted women, consistent with outcomes showing a gender gap in stress-related disorders. Gender-related stress vulnerability emerges in adolescence alongside gender-specific changes in neuroendocrine signaling. Most research on the neuroendocrinology of stress-related disorders has focused on differences in the hypothalamic-pituitary-adrenal (HPA) axis effector hormone cortisol. More recent studies, however, emphasize dehydroepiandrosterone (DHEA), a neuroprotective and neuroactive hormone released concurrently with cortisol that balances its biobehavioral actions during stress. Notably, women show lower cortisol responses and higher DHEA responses to stress. However, lower cortisol and higher DHEA are associated with internalizing disorders in women, while those associations are opposite in men. Thus, gender-specific factors perhaps result in a neuroendocrine profile that places women at greater risk for stress-related disorders. The current study prospectively examined socially evaluated cold-pressor task (SECPT) induced neuroendocrine responses at age 15 and internalizing symptoms during the COVID-19 pandemic at age 21 in a cohort of 175 primarily Black low-socioeconomic status participants, while controlling for internalizing symptoms at age 15. The association between COVID-related stress and internalizing symptoms was not stronger in women. Lower DHEA-cortisol ratios were associated with a weaker relationship between COVID-related stress and internalizing symptoms in women, while higher ratios were associated with a weaker relationship in men. These findings suggest gender differences in the relationship between DHEA and cortisol and internalizing outcomes during a stressful period, and support differential neuroendocrine protective and risk pathways for young men and women.
Keywords: HPA axis, stress, gender differences, internalizing symptoms, COVID-19 pandemic
1. Introduction
The COVID-19 pandemic has been a global catastrophic event that contributed to millions of deaths and hospitalizations, as well as a significant disruption in employment, education, home environment, and social relationships (Grasso et al., 2021). COVID-related stressors have been linked to internalizing symptoms (Kujawa et al., 2020). Consequently, rates of internalizing symptoms increased dramatically worldwide after the onset of the pandemic. For example, epidemiological and longitudinal studies have shown a two-fold increase in depression and anxiety during the first year of the pandemic (Crandall et al., 2022).
Moreover, the pandemic disproportionately impacted women in their professional and personal lives. Women were more likely to experience unemployment, work postponement due to caretaking, discontinuation of education, and gender-based violence during the pandemic (Flor et al., 2022). Women also experienced more restrictions in everyday and recreational activities and social relationships (Jellen & Ohlbrecht, 2020). Not surprisingly, there has been a greater increase in internalizing symptoms among women since the beginning of the pandemic (Hawes et al., 2021).
These findings are consistent with a broader literature on gender differences in the rates of stress-related disorders. For example, women report greater life stress, are at greater risk for depression, and may also have greater stress sensitivity (see review by Cohen et al., 2019). Notably, these differences emerge in adolescence and continue throughout adulthood. A leading model of this phenomenon suggests that women are exposed to greater levels of adversity and that there may be sex-specific affective, biological, and cognitive vulnerabilities that all converge in adolescence to increase the risk for stress-related disorders (Hyde et al., 2008). Specifically, within the biological domain, there is evidence that sex differences in neuroendocrine signaling that emerge in adolescence may contribute to gender-related stress vulnerability (Roberts & Lopez-Duran, 2019).
Individual differences in the functioning of the primary neuroendocrine stress system, the hypothalamic-pituitary-adrenal (HPA) axis, are an important driver of stress-related vulnerability. Most research on the neuroendocrinology of stress-related disorders has focused on the effector hormone cortisol because it is a reliable index of HPA-axis activation and is critical in modulating the biobehavioral stress response and regulating the axis. Cortisol activates mineralocorticoid receptors (MR) during basal HPA-axis functioning, and it increases and binds glucocorticoid receptors (GR) during activation of the stress response, which promotes physiological and cognitive adaptation and regulates cortisol production through negative feedback. An increase in cortisol from tonic to phasic levels is critical for adaptive biobehavioral stress response and homeostasis (de Kloet & Joëls, 2023). However, prolonged cortisol exposure can exhaust biological (Karen et al, 2020) and cognitive actions required to meet the demands of stress (McEwen et al. 2016). For example, hypocortisolemia and hypercortisolemia are both associated with risk for stress-related disorders (Gunnar, 2021). More recent studies, however, have highlighted the importance of the neuroprotective and neuroactive hormone dehydroepiandrosterone (DHEA) released in concert with cortisol, which balances its biobehavioral actions during stress (Kamin & Kertes, 2017). For example, increased DHEA is associated with greater emotion regulation (Sripada et al., 2013) and decision-making (Shields et al., 2016).
Given the coordinated release of cortisol and DHEA and their partially opposite effects, DHEA-cortisol ratios may more accurately capture the dynamics of the HPA-axis stress response. Higher ratios may reflect greater availability of DHEA to offset cortisol and balance the stress response, while lower ratios may reflect heightened cortisol and dysregulation (Lambert et al., 2020). Not surprisingly, lower DHEA-cortisol ratios are associated with depression (Asadikaram et al., 2019) and anxiety (Clemens et al., 2020). Thus, research must account for the relationship between cortisol and DHEA to examine gender-related stress vulnerability more precisely.
Gender differences in the dynamics of cortisol and DHEA may contribute to gender differences in the development of stress-related disorders. For example, there is evidence of a greater cortisol response to psychosocial stress in men compared to women (Liu et al., 2017), but a greater DHEA response in women compared to men (Dutheil et al., 2021). Furthermore, meta-analyses show a positive relationship between cortisol response and internalizing symptoms in men, but a negative relationship in women (Zorn et al., 2017), as well as a negative relationship between DHEA response and internalizing symptoms in men, but a positive relationship (Ullsperger & Nikolas, 2017) or no relationship in women (Kurita et al., 2013). These gender differences may reflect gender-specific factors that impact the development of the HPA axis, resulting in a neuroendocrine profile that places women at greater risk for stress-related disorders (Bangasser & Valentino, 2014). For example, chronic stress can down-regulate the HPA axis, and this down-regulation is stronger in women (Moisan, 2021). Combined with greater DHEA response in women, perhaps HPA-axis activation is blunted below the “threshold” necessary for adaptive biobehavioral action in response to stress. Thus, studies are needed that not only examine gender differences in response to stress and gender differences in DHEA-cortisol ratios, but also whether DHEA-cortisol ratios might interact with gender to modulate the links between stress and mental health outcomes.
In the current study, we first examined whether gender moderated the association between COVID-related stress and internalizing symptoms during COVID at age 21. We then examined whether socially evaluated cold-pressor task (SECPT; Schwabe et al., 2008) induced peak DHEA-cortisol ratios at age 15 moderated gender differences in the association between COVID-related stress and internalizing symptoms. This study expands previous research by examining the association between neuroendocrine profiles in adolescence when gender-related stress vulnerability arises, and the relationship between life stress and internalizing symptoms in young adulthood while controlling for earlier internalizing symptoms. Given the gender gap in stress-related disorders, we expected that the association between COVID-related stress and internalizing symptoms would be stronger in young women versus men. Furthermore, considering the negative and null associations between DHEA and internalizing disorders in women, we expected that higher peak DHEA-cortisol ratios would be associated with a weaker relationship between COVID-related stress and internalizing symptoms in young men versus women. We tested these hypotheses in a sample of 175 primarily Black young adults from low-income backgrounds, who were likely to experience significant hardship during the COVID-19 pandemic.
2. Methods
2.1. Participants
Participants were young adults from the Study of Adolescent to Adult Neural Development (SAND) who participated both in in-person study visits at the University of Michigan at age 15 and online/phone surveys during the COVID-19 pandemic at age 21. Two-hundred and thirty-seven (237) adolescents (52% female) were recruited for the SAND from the Detroit, Toledo, and Chicago cohorts of the Future of Families and Child Wellbeing Study (FFCWS; formerly the Fragile Families and Child Wellbeing Study). The FFCWS is a multi-stage, stratified, probability sample of births from large cities in the United States with populations greater than 200,000. Original recruitment occurred between 1998 and 2000 and oversampled for non-marital births (3:1).
At age 15 participants were invited to the University of Michigan to participate in a full-day study visit where, after assent and parental consent, the adolescents completed questionnaires, interviews, and biological samples including saliva samples. All measures collected were approved by the University of Michigan Institutional Review Board. Transportation or travel expenses were provided for all participants. Participants who did not provide saliva samples (n=10) or who had elevated cortisol concentrations (greater than 3 SD from the mean) resulting from drink/food consumption or improper saliva collection (n=5) were excluded. The final sample from the SAND-15 time point was 222 adolescents.
Six-hundred and eighty-eight (688) youth were recruited from the FFCWS (including all SAND participants) for online or phone surveys during the COVID-19 pandemic. Surveys were collected between April 2020 and September 2021 and participants were on average 21 years old. Participants were recruited using phone calls, text messages, emails, and mailings. 1,007 participants were contacted with a response rate of 68.3%. Surveys were administered via a Qualtrics link or phone interviews.
The current study included 175 primarily Black participants (n = 75 young women) in their early 20s from low-socioeconomic status backgrounds with useable neuroendocrine data from the SAND-15 in-person visit and useable COVID-related stress and internalizing symptoms data from the COVID online surveys (see Table 1). Gender was self-reported male or female status at age 15.
Table 1.
Usable age 15 and age 21 COVID wave data
| n | Mean (SD) | Min | Max | Skew | Kurtosis | |
|---|---|---|---|---|---|---|
| Gender | 237 | 1.48 (0.50) | 1 | 2 | 0.09 | −2.00 |
| COVID-related stress | 178 | 8.40 (5.99) | 0 | 24 | 0.18 | 0.17 |
| DHEA-Cortisol | 173 | 20.45 (4.25) | 7.60 | 31.97 | 0.17 | 0.32 |
| Depression | 175 | 11.10 (9.03) | 0.00 | 35.00 | 0.65 | −0.38 |
| Anxiety | 176 | 10.05 (10.94) | 0.00 | 42.00 | 1.28 | 0.88 |
3.1. Measures
3.2. COVID-related stress.
The Epidemic-Pandemic Impacts Inventory (EPII) consists of 92 checklist items measuring the tangible impacts of the epidemic and pandemic on economic, social, and personal domains over the course of the pandemic (Grasso et al., 2021). The EPII has demonstrated utility in capturing objective COVID-related life stressors during the pandemic. For example, in a person-centered latent class analysis (LCA), EPII profiles (e.g., high vs low cumulative scores) were able to differentiate sociodemographic characteristics that have been associated with greater life stress during the pandemic, such as young adults, unemployed workers, and caregivers (Grasso et al., 2021). Moreover, historically marginalized groups, such as women and low-income individuals have been shown to score higher on the EPII, which is consistent with broader disparities in life stress (Thomas et al., 2022). Thus, these findings suggest that the EPII score is correlated with overall exposure to adversity and has a nomologic network that supports the validity of the measure. The domains are Work/Employment (11 items), Education (2 items), Home Life (13 items), Social Activities (10 items), Economic (5 items), Emotional Health (8 items), Physical Health (8 items), Physical Distancing/Quarantine (8 items), Infection History (8 items; not administered in this study), and Positive Change (19 items). Participants responded to each item by indicating whether they had experienced it or not (Yes = 1, No = 0). Sixty-five items assessed negative changes in life domains during the COVID-19 pandemic, and 19 assessed positive changes. This study created COVID-related stress by summing all endorsed negative change items except the Emotional Health domain items because of the overlap with internalizing symptoms. Thus, our COVID-related stress score has a potential range of 0–57 negative change items endorsed. COVID-related stress was winsorized at 2% to account for outliers (n = 3 out of 178 observations).
3.3. Neuroendocrine Data.
3.3.1. Socially evaluated cold-pressor task (SECPT).
The SECPT is a modification of the traditional cold-water hand immersion task that adds social evaluation to more reliably activate the HPA axis (Schwabe et al., 2008; Schwabe & Schächinger, 2018). During the SECPT, participants immerse their non-dominant hand in ice water (1–3 °Celsius) while being watched by a researcher and videotaped (Schwabe et al., 2008). Peckins and colleagues (2020) previously described the SECPT and its efficacy in the current sample. Consistent with the current recommendations for administering the SECPT (Schwabe & Schächinger, 2018), all participants spent a minimum of 45 minutes at the lab prior to completing the SECPT. To avoid time of day effects, all participants completed the SECPT in the afternoon (M = 14:16 h, SD =38.96 min, Range = 13:11–16:07). Following the task, participants watched a calming video alone for 60 minutes (n = 116) or completed questionnaires on neutral topics (n = 42). Saliva samples were collected 30 minutes before the task (sample 0), immediately before starting the task (0 minutes, sample 1), at the end of the task (3 minutes, sample 2), at 10, 15, 25, 30, 35, 40, 45, and at 60 minutes post-task (samples 3–10). These times were chosen because they allow for the assessment of peak salivary cortisol levels which generally occur between 25 and 45 minutes post stress-induction (Dickerson & Kemeny, 2004). DHEA was only assayed from saliva at times 0 min, 25 min, and 35 min.
Saliva samples were stored within 1 hour of collection in a −20c freezer for up to a year, without freeze-thaw cycles, until the time of assay. Salivary cortisol samples have been shown to be stable for at least 1 year at −20c, though, to our knowledge, there have been no specific stability studies on salivary DHEA (Giacomello et al., 2020). However, best practices recommend avoiding freezing-thawing cycles when examining DHEA (d’Amone et al., 2021). Saliva samples were assayed for cortisol by the University of Michigan Core Assay Facility with a high-sensitivity enzyme immunoassay kit (Salimetrics, State College, PA). The assay sensitivity for cortisol was < 0.007 μg/dL and the assay range was 0.012–3.000 μg/dL. The inter-assay and intra-assay coefficients of variance for cortisol were 9.72% and 6.25%, respectively. Across all participants and samples, only four cortisol samples were missing due to insufficient saliva volume. The assay sensitivity for DHEA was < 5 pg/mL and the assay range was 10.2 pg/mL - 1000 pg/mL. The inter-assay and intra-assay coefficients of variance for DHEA were 10.31% and 7.02%, respectively. All cortisol and DHEA assays were within the expected ranges.
3.3.2. Salivary cortisol and DHEA.
Peckins and colleagues (2020) previously found that seventy-six participants (47.8%) in the current sample showed a 15.5% increase or greater in cortisol levels from baseline to peak and were classified as responders. As we aimed to investigate neuroendocrine functioning in gender-related stress vulnerability, including blunted cortisol responsivity, both responders and non-responders were included in all analyses. Peak cortisol and DHEA levels were winsorized at 2% (n = 4 out of 222 and n = 4 out of 177, respectively). We used the 25-minute time points (the mode peak time of “responders”) as the expected cortisol peak values for non-responders.
3.4. Internalizing symptoms.
During the COVID wave, participants were administered the Beck Depression Inventory (Beck et al., 1961) and Beck Anxiety Inventory (Beck et al., 1988). The BDI consists of 21 items assessing depressive symptoms that scale from 0 to 3. As the surveys were administered virtually, one item assessing suicidality was removed. Two items assessing sleep and appetite changes are scaled from 0 to 7. BDI was winsorized at 2% to account for outliers (n = 3 out of 175 observations). The BAI consists of 21 items assessing symptoms of anxiety that scale from 0 to 3. Missing items were excluded from the BDI and BAI composite scores. BAI was winsorized at 2% to account for outliers (n = 3 out of 176 observations).
3.5. Covariates
All models controlled for several covariates associated with cortisol reactivity in a lab paradigm (Kudielka et al., 2009): participants’ age, race/ethnicity, medication use, pubertal development, income-to-needs ratio, and depression and anxiety at age 15. Self-reported race/ethnicity was coded as one dummy code for the largest group in the SAND sample (Non-Hispanic African American [75.5%] = 1). Current self-reported medication use of asthma medication or birth control was also dummy coded (Medication Use: 1 = Yes [16.06%]). The Pubertal Development Scale (Petersen et al., 1988) was used to measure self-reported pubertal development. Total pubertal development score was calculated as a mean of the five items for females (M = 3.58, SD = 0.46) and males (M = 2.86, SD = 0.50). The income-to-needs ratio was calculated using the relationship of parent-reported family income to the census poverty line for families of distinct sizes, considering family income and family size. Values above and below 1 represent the percentage each family is above or below the poverty line. Adolescent depression was measured using the Child Depression Inventory (Kovacs, 1978) consisting of 27 depression symptoms items that scale from 0 to 3. Adolescent anxiety was measured using the Screen for Child Anxiety Related Disorders (Hale et al., 2005) consisting of 41 anxiety symptoms items that scale from 0 to 2.
3.6. Statistical Analysis
Mplus® software (Version 8.5) was used to run full information maximum likelihood (FIML) to account for missing data in all models. This method can transform missing not completely at random (MNCAR) data into missing at random (MAR) and reduce missing data bias by including all variables of interest that are correlated with missingness in models (Newman, 2014). Raw peak DHEA values were divided by raw peak cortisol values to create peak DHEA-cortisol ratios. Peak DHEA-cortisol ratios were normalized using a Box-Cox power transformation to account for the positive skew (Miller & Plessow, 2013). Predictor variables were mean centered to reduce multicollinearity and assist in the interpretation of interactions (Aiken & West, 1991). Predictor variables were analyzed for multicollinearity, and all variance inflation factors (VIF) were within the acceptable range. There were no influential cases (all Cook’s distances < 1). The outcome variables, depression and anxiety symptoms, showed overdispersion due to excess zeros, and approximately twice as few zeros at one level of one of the predictor variables, gender. Thus, hurdle negative binomial (HNB) regressions were used to account for zero inflation in the outcome count data, and zero deflation in the outcome data among the subject group of women (Feng, 2021). First, we ran separate HNB regression models with 2-way interaction terms to examine gender differences in the association between COVID-related stress and depression and anxiety symptoms. Next, we ran separate HNB regression models with 3-way interaction terms to examine gender differences in the association between COVID-related stress and depression and anxiety symptoms as a function of peak DHEA-cortisol ratios. All predictors and 2-way and 3-way interaction terms were included in the HNB 3-way interaction models for depression and anxiety. A Bonferroni adjustment was used to correct for multiple comparisons (i.e., the depression and anxiety models). Lastly, simple slope analyses were conducted using the model constraint command in MPlus to include new/additional parameters for the HNB 3-way interaction models at 1 standard deviation below the mean, at the mean, and 1 standard deviation above the mean for men and women. Models were also run with standard regression with multiple imputation, and this approach yielded highly similar results (see Supplemental).
4. Results
4.1. Gender differences
4.1.1. COVID-related stress.
There was a significant association between gender and COVID-related stress (β = 1.201, z = 2.443, p = 0.015), with young women (M = 9.571) reporting greater COVID-related stress than young men (M = 6.712). These findings were not significant when controlling for covariates (β = −0.011, z = −0.018, p = 0.985).
4.1.2. Neuroendocrine functioning.
There were no gender differences in peak DHEA-cortisol ratios at age 15 between young men (M = 20.627) and women (M = 20.257), (β = −0.369, t = −0.574, p = 0.566). There was also no association between gender and peak DHEA-cortisol ratios at age 15 when controlling for covariates (β = −0.102, t = −0.136, p = 0.892).
4.1.3. Internalizing symptoms.
There was no significant association between gender and depression symptoms at age 21 (β = 0.920, z = 1.894, Bonferroni-adjusted p = 0.116). There was a marginally significant association between gender and depression symptoms at age 21 when controlling for covariates, which included concurrent depression and anxiety symptoms at age 15 (β = 1.370, z = 2.083, Bonferroni-adjusted p = 0.074), with young women (M = 11.886) reporting greater depression symptoms than young men (M = 9.914) during the COVID-19 pandemic. There was a significant association between gender and anxiety symptoms at age 21 (β = 0.915, z = 2.450, Bonferroni-adjusted p = 0.028), with young women (M = 11.514) reporting greater anxiety symptoms than young men (M = 7.873) during the COVID-19 pandemic. This finding was not significant when controlling for covariates, which included concurrent anxiety and depression symptoms at age 15 (β = 0.756, z = 1.461, Bonferroni-adjusted p = 0.288).
4.2. Are there gender differences in the association between COVID-related stress and internalizing symptoms?
Gender did not moderate the association between COVID-related stress and depression (β = 0.127, z = 1.144, p = 0.265) or anxiety symptoms (β = 0.109, z = 1.233, p = 0.218) at age 21.
4.3. Do neuroendocrine profiles moderate the association between COVID-related stress and internalizing symptoms?
Peak DHEA-cortisol ratios at age 15 did not moderate the association between COVID-related stress and depression (β = 0.060, z = 0.870, p = 0.384) or anxiety symptoms (β = 0.004, z = 0.399, p = 0.690) at age 21.
4.4. Do neuroendocrine profiles moderate the association between COVID-related stress and depression symptoms differently in men versus women?
Peak DHEA-cortisol ratios at age 15 moderated the association between COVID-related stress and depression symptoms at age 21 differently in young men versus women (β = 0.091, z = 2.740, Bonferroni-adjusted p = 0.012). Simple slopes analyses were run to examine the association between COVID-related stress and depression at different levels of peak DHEA-cortisol ratios in young men and women (see Figure 1). In young women, the association between COVID-related stress and depression was negative and statistically significant at 1 standard deviation below mean levels of peak DHEA-cortisol ratios (β = −0.489, z = −3.061, p = 0.002) and at mean levels (β = −0.212, z = −2.589, p = 0.010), but was positive and not statistically significant at 1 standard deviation above mean levels (β = 0.065, z = 0.536, p = 0.592). That is, for women, greater COVID-related stress predicted less steep increases in depression symptoms for women who had relatively low or average (but not high) DHEA-cortisol ratios. In young men, the association between COVID-related stress and depression was positive but not statistically significant at 1 standard deviation below mean levels of peak DHEA-cortisol ratios (β = 0.018, z = 0.227, p = 0.821), and was negative and not statistically significant at mean levels (β = −0.089, z = −0.970, p = 0.332) and 1 standard deviation above mean levels (β = −0.196, z =− 1.439, p = 0.150). That is, for men, there did not appear to be a statistically significant relationship between COVID-related stress and changes in depression symptoms at any level of DHEA-cortisol ratios within the central distribution of the data (i.e., from +1 SD to −1SD around the mean).
Figure 1.
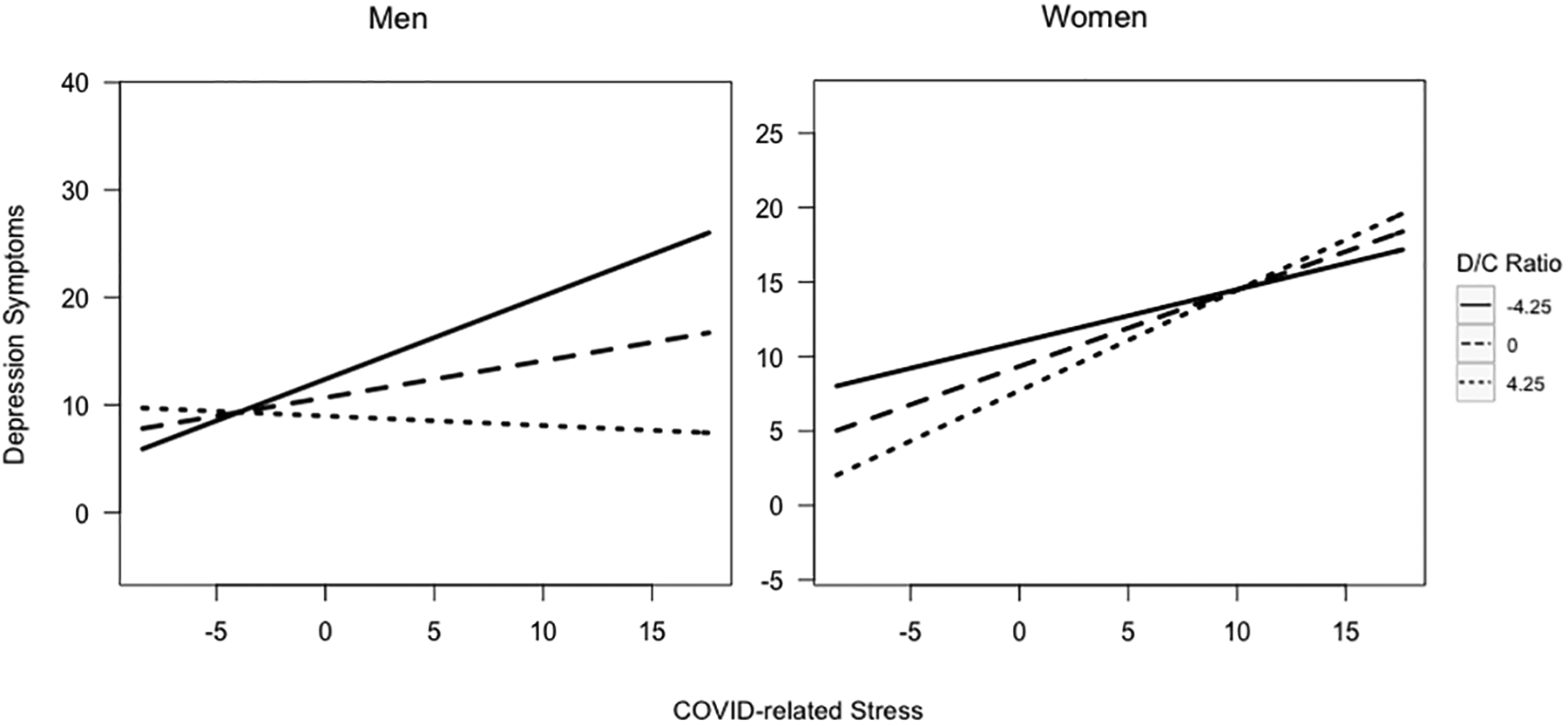
Peak DHEA-cortisol ratios at age 15 moderating the association between COVID-related stress and depression symptoms by gender at age 21
Fig. 1: Significant 3-way interaction between COVID-related stress, gender, and peak DHEA-cortisol ratios predicting depression symptoms. Model controlled for medication use, age, race/ethnicity, pubertal development, income-to-needs ratio, and depression and anxiety symptoms at age 15.
4.5. Do neuroendocrine profiles moderate the association between COVID-related stress and anxiety symptoms differently in men versus women?
Peak DHEA-cortisol ratios at age 15 moderated the association between COVID-related stress and anxiety symptoms differently in young men versus women (β = 0.081, z = 3.435, Bonferroni-adjusted p = 0.002). Simple slopes analyses were run to examine the association between COVID-related stress and anxiety at different levels of peak DHEA-cortisol ratios in young men and women (see Figure 2). In young women, the association between COVID-related stress and anxiety was negative and statistically significant at 1 standard deviation below mean levels of peak DHEA-cortisol ratios (β = −0.371, z = −2.876, p = 0.004) and at mean levels (β = −0.173, z = −2.368, p = 0.018), but was positive and not significant at 1 standard deviation above mean levels (β = 0.025, z = 0.318, p = 0.751). Thus, similar to depression, for women with relatively low or average (but not high) levels of DHEA-cortisol ratios, greater COVID-related stress predicted a less steep increase in anxiety symptoms. In young men, the association between COVID-related stress and anxiety was positive but was not statistically significant at 1 standard deviation below mean levels of peak DHEA-cortisol ratios (β = 0.078, z = 1.117, p = 0.264), negative and not statistically significant at mean levels (β = −0.068, z = −1.159, p = 0.246), and negative and statistically significant at 1 standard deviation above mean levels (β = −0.214, z =− 2.129, p = 0.033). That is, for men, greater COVID-related stress predicted less steep increases in anxiety symptoms for those who had relatively high (but not low or average) DHEA-cortisol ratios.
Figure 2.
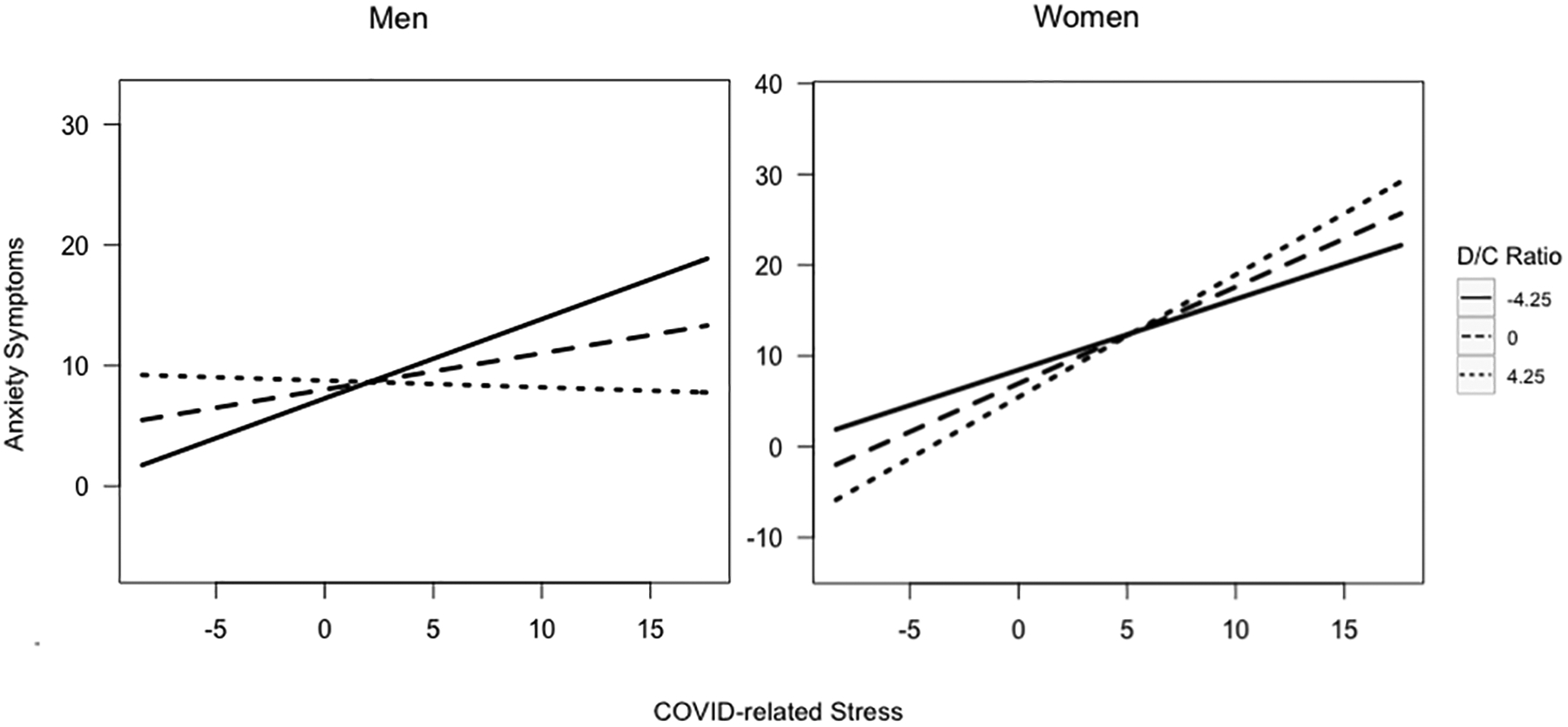
Peak DHEA-cortisol ratios at age 15 moderating the association between COVID-related stress and anxiety symptoms by gender at age 21
Fig. 2: Significant 3-way interaction between COVID-related stress, gender, and peak DHEA-cortisol ratios predicting anxiety symptoms. Model controlled for medication use, age, race/ethnicity, pubertal development, income-to-needs ratio, and depression and anxiety symptoms at age 15.
5. Discussion
In this study, we examined whether the association between COVID-related stress and internalizing symptoms was stronger in young women than in young men during the COVID-19 pandemic at age 21. Furthermore, we examined whether differences in DHEA-cortisol ratios in response to the SECPT at age 15 were associated with gender differences in the relationship between COVID-related stress and internalizing symptoms. In a sample of primarily Black young adults from low-income backgrounds, we found that there were no gender differences in the association between COVID-related stress and internalizing symptoms. Moreover, we found that the relationship between stress-induced peak DHEA-cortisol ratios measured in adolescence and the association between COVID-related stress and internalizing symptoms in early adulthood varied by gender. COVID-related stress predicted a less steep increase in depression and anxiety symptoms for women with low and average peak DHEA-cortisol ratios, but not high ratios. In men, COVID-related stress predicted a less steep increase in anxiety symptoms for those with high peak DHEA-cortisol ratios, but not average and low ratios. COVID-related stress did not appear to predict increased depression among men, regardless of their DHEA-cortisol ratios. These findings suggest that a more tightly coupled DHEA and cortisol response to stress in adolescence may predict less stress sensitivity in young adulthood for women, but that a greater DHEA response relative to cortisol response may predict less stress sensitivity in young adulthood for men.
Contrary to our first hypothesis, we did not find a stronger relationship between COVID-related stress and internalizing symptoms in young women compared to young men. Although young women reported greater COVID-related stress and internalizing symptoms, they did not show a stronger relationship between life stress and depression and anxiety as demonstrated in previous literature (e.g., Hanken et al., 2007; Ruscio et al., 2015). However, studies on gender-related stress vulnerability have largely focused on adolescents. Compared to the association between child and adolescent life stress and adult stress-related disorders, the association between life stress and stress-related disorders in adulthood may be weaker (Dunn et al., 2017). Furthermore, research suggests that gender differences in the development of behavioral responses to stress (e.g., support seeking, emotion expression) from adolescence to adulthood, which is the least developed in adolescence, may potentially mitigate some of the negative effects of stress in adult women (Zimmermann & Iwanski, 2014). Thus, the lack of gender differences in stress sensitivity to the disruptions of the COVID-19 pandemic in our study may reflect a more nuanced developmentally influenced response to stress (i.e., biopsychosocial interplay) in young adulthood. Moreover, though men and women may be equally responsive to stress, because women are more likely to be exposed to greater stress, this difference in levels (versus difference in etiology), can help explain the gender gap in internalizing diagnoses and symptoms. Alternatively, the greater reporting of negative events by individuals with internalizing disorders (women endorsed greater symptoms) and/or the less accurate reporting of past negative (i.e., stressful) events (suggested to be stronger in men [Zoladz et al., 2014]), may have biased the measure of COVID-related stress and obscured potential gender differences in stress sensitivity, although the measure assessed tangible stressors during the pandemic (e.g., job loss, housing transitions, education discontinuation) that were answered by either yes or no (as opposed to ratings of stressors).
In support of our second hypothesis, we found that higher SECPT-induced peak DHEA-cortisol ratios during adolescence were associated with a weaker relationship between COVID-related stress and depression and anxiety symptoms in young adult men compared to young adult women. Specifically, we found that COVID-related stress predicted a less steep increase in internalizing symptoms for women with low and average DHEA-cortisol ratios, but not high ratios. In men, we found that COVID-related stress predicted a less steep increase in anxiety symptoms for those with high DHEA-cortisol ratios, but not average or low ratios. We did not find COVID-related stress to predict increased depression symptoms among men, regardless of their DHEA-cortisol ratios. These findings support past research identifying neuroendocrine profiles as robust prospective predictors of stress-related disorders (e.g., Mocking et al., 2015; Bendezú et al., 2021). Moreover, these findings are generally consistent with previous studies that found a positive association between DHEA-cortisol ratios and symptoms of stress-related disorders in women (Gill et al., 2008), and a negative association in men (Kimonis et al., 2019). These findings suggest that a more tightly coupled DHEA and cortisol response to stress in adolescence may be associated with a weaker relationship between later life stress and internalizing symptoms in young women, but that a greater DHEA response relative to cortisol response in adolescence may predict less stress sensitivity for men later in life. The lack of gender differences in peak DHEA-cortisol ratios and the non-significant association between stress sensitivity and neuroendocrine profile ignoring gender suggest that stress vulnerability in women may be more influenced by gender differences in neuroendocrine functioning (e.g., levels and metabolism of individual hormones), rather than the overall ratio of DHEA to cortisol in response to stress. For example, there is evidence that DHEA biologically inactivates cortisol at a greater rate in women (Kroboth et al., 2003), and findings from animal models show that DHEA increases glucose uptake in the brain more in males (Vieira-Marques et al., 2017), which supports the stronger positive effect of DHEA on cognitive and socioemotional functioning found in men compared to women (Chen et al., 2018). Therefore, lower DHEA-cortisol ratios during stress may reflect less suppression of cortisol biobehavioral action and better adaptability to stress in young women, while higher DHEA-cortisol ratios may reflect a greater offset of cortisol and better stress adaptability in men, given gender-specific balances in neuroendocrine signaling and metabolism of neuroendocrine hormones. In the context of chronic stress (e.g., the COVID-19 pandemic), perhaps the greater inactivation of cortisol by DHEA in women suppresses the gender-specific, lower cortisol response below the threshold for HPA-axis activation and the biological and cognitive action required to meet the demands of the environment and maintain homeostasis. In men, a greater DHEA response may be needed to balance the gender-specific, higher cortisol response and reduce hyperactivation of the HPA axis and exhaustion of biological and cognitive resources.
This study has multiple strengths, which include the use of stress-induced neuroendocrine data, a measure of a ubiquitous and novel stressor, prospective, longitudinal statistical control of earlier internalizing symptoms, and a focus on participants with identities often excluded or under-represented in biomedical research (i.e., most participants were from lower-income families and were Black/African American). However, these findings must be interpreted while considering three limitations. First, it is possible that our predictor variable, COVID-related stress, may have been confounded with our outcome variables, depression and anxiety symptoms, because of the negative memory bias associated with internalizing disorders and the significant gender differences in internalizing symptoms in our sample. Furthermore, potential gender differences in the effects of stress on memory may have biased reports of past stressors during the COVID-19 pandemic, which the literature suggests may be stronger in men. Therefore, further investigation is required to determine potential gender differences in the dynamics of DHEA and cortisol and the development of stress-related disorders. Second, research has suggested that the HPA axis can undergo recalibration during adolescence in the presence of a supportive and enriched environment, potentially reversing the effects of early life stress (Gunnar et al., 2019). Thus, our sample’s risk profiles may differ from those in populations with changing physical and social environments. Third, approximately half (47.8%) of our sample showed a 15.5% increase or greater in cortisol levels from baseline to peak and were labeled responders, compared to a 75% responder rate with a 45% increase (e.g., Schwabe & Schächinger, 2018). Therefore, our sample has a greater proportion of participants showing blunted responses and may be less generalizable to other populations, particularly those with more access to resources and those who have not been exposed as extensively to poverty-related stressors.
In sum, using a prospective longitudinal design, we found that young women were not more sensitive than young men to the stressors of the COVID-19 pandemic. Furthermore, we found that the association between neuroendocrine profiles in adolescence and the relationship between COVID-related stress and internalizing symptoms in early adulthood were different for young men versus women. Notably, we found that young women with low and average DHEA-cortisol ratio stress responses in adolescence had a weaker relationship between stress and internalizing symptoms in young adulthood. In men, adolescents with high DHEA-cortisol ratio stress responses had a weaker relationship between stress and anxiety (but not depression) symptoms in young adulthood. This study highlights potential gender-specific neuroendocrine risk profiles that may help explain gender differences in stress-related vulnerabilities and previous discrepancies in the literature on the role of cortisol and DHEA in the development of stress-related psychopathology. Given the transaction between neuroendocrine functioning, internalizing disorders, and life stress, one important contribution our study makes is examining the association between neuroendocrine functioning and later gender-related stress vulnerability while controlling for earlier internalizing symptoms. Moreover, as the study leverages individual differences in exposure to stressors related to the COVID-19 pandemic, it can help inform our understanding of gendered individual differences in response to major stressors and identify who is at the most risk for negative mental health outcomes during these exposures.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Mental Health, 5R01MH103761-05 (Monk) and 1R01MH121079-01 (Monk, Hyde, & Mitchell). We are thankful for the prior research of the Future of Families and Child Wellbeing Study, the parents and adolescents for participating and sharing their lived experiences, and the study staff for supporting this research.
Footnotes
Declaration of Interest: None
References
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. sage. [Google Scholar]
- Asadikaram G, Khaleghi E, Sayadi A, Foulady S, Ghasemi MS, Abolhassani M, … & Nematollahi MH (2019). Assessment of hormonal alterations in major depressive disorder: a clinical study. PsyCh journal, 8(4), 423–430. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, & Valentino RJ (2014). Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Frontiers in neuroendocrinology, 35(3), 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, & Steer RA (1988). An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology, 56(6), 893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward C, Mendelson M, Mock J, & Erbaugh JJAGP (1961). Beck depression inventory (BDI). Arch Gen Psychiatry, 4(6), 561–571. [DOI] [PubMed] [Google Scholar]
- Bendezú JJ, Howland M, Thai M, Marceau K, Shirtcliff EA, Hastings PD, … & Klimes-Dougan B (2021). Adolescent cortisol and DHEA responses to stress as prospective predictors of emotional and behavioral difficulties: A person-centered approach. Psychoneuroendocrinology, 132, 105365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wu CC, Huang YC, Hung CF, & Wang LJ (2018). Gender differences in the relationships among neurosteroid serum levels, cognitive function, and quality of life. Neuropsychiatric Disease and Treatment, 14, 2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens V, Bürgin D, Eckert A, Kind N, Doelitzsch C, Fegert JM, & Schmid M (2020). Hypothalamic-pituitary-adrenal axis activation in a high-risk sample of children, adolescents and young adults in residential youth care–associations with adverse childhood experiences and mental health problems. Psychiatry research, 284, 112778. [DOI] [PubMed] [Google Scholar]
- Cohen S, Murphy ML, & Prather AA (2019). Ten surprising facts about stressful life events and disease risk. Annual review of psychology, 70, 577–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall A, Daines C, Hanson CL, & Barnes MD (2022). The effects of COVID‐19 stressors and family life on anxiety and depression one‐year into the COVID‐19 pandemic. Family Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Amone L, Matzeu G, & Omenetto FG (2021). Stabilization of salivary biomarkers. ACS Biomaterials Science & Engineering, 7(12), 5451–5473. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, & Joëls M (2023). The cortisol switch between vulnerability and resilience. Molecular Psychiatry, 1–15. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dunn EC, Nishimi K, Powers A, & Bradley B (2017). Is developmental timing of trauma exposure associated with depressive and post-traumatic stress disorder symptoms in adulthood?. Journal of psychiatric research, 84, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil F, de Saint Vincent S, Pereira B, Schmidt J, Moustafa F, Charkhabi M, … & Clinchamps M (2021). DHEA as a biomarker of stress: A systematic review and meta-analysis. Frontiers in Psychiatry, 12, 688367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CX (2021). A comparison of zero-inflated and hurdle models for modeling zero-inflated count data. Journal of statistical distributions and applications, 8(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor LS, Friedman J, Spencer CN, Cagney J, Arrieta A, Herbert ME, … & Gakidou E (2022). Quantifying the effects of the COVID-19 pandemic on gender equality on health, social, and economic indicators: a comprehensive review of data from March, 2020, to September, 2021. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello G, Scholten A, & Parr MK (2020). Current methods for stress marker detection in saliva. Journal of pharmaceutical and biomedical analysis, 191, 113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Vythilingam M, & Page GG (2008). Low cortisol, high DHEA, and high levels of stimulated TNF‐α, and IL‐6 in women with PTSD. Journal of Traumatic Stress: Official Publication of The International Society for Traumatic Stress Studies, 21(6), 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, Briggs‐Gowan MJ, Carter AS, Goldstein BL, & Ford JD (2021). Profiling COVID‐related experiences in the United States with the epidemic‐pandemic impacts inventory: Linkages to psychosocial functioning. Brain and Behavior, 11(8), e02197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR (2021). Forty years of research on stress and development: What have we learned and future directions. American Psychologist, 76(9), 1372. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, DePasquale CE, Reid BM, Donzella B, & Miller BS (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proceedings of the National Academy of Sciences, 116(48), 23984–23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale WW III, Raaijmakers Q, Muris P, & Meeus WIM (2005). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in the general adolescent population. Journal of the American Academy of Child & Adolescent Psychiatry, 44(3), 283–290. [DOI] [PubMed] [Google Scholar]
- Hawes MT, Szenczy AK, Klein DN, Hajcak G, & Nelson BD (2021). Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychological Medicine, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, & Abramson LY (2008). The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological review, 115(2), 291. [DOI] [PubMed] [Google Scholar]
- Jellen J, & Ohlbrecht H (2020). Parenthood in a Crisis: Stress Potentials and Gender Differences of Parents during the Corona Pandemic. International Dialogues on Education: Past and Present, 7, 44–51. [Google Scholar]
- Kamin HS, & Kertes DA (2017). Cortisol and DHEA in development and psychopathology. Hormones and behavior, 89, 69–85. [DOI] [PubMed] [Google Scholar]
- Karin O, Raz M, Tendler A, Bar A, Korem Kohanim Y, Milo T, & Alon U (2020). A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Molecular systems biology, 16(7), e9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis ER, Fleming GE, Wilbur RR, Groer MW, & Granger DA (2019). Dehydroepiandrosterone (DHEA) and its ratio to cortisol moderate associations between maltreatment and psychopathology in male juvenile offenders. Psychoneuroendocrinology, 101, 263–271. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1978). The Children’s Depression Inventory: A self-rated scale for school children. Unpublished manuscript, University of Pittsburgh. [Google Scholar]
- Kroboth PD, Amico JA, Stone RA, Folan M, Frye RF, Kroboth FJ, … & Hakala C (2003). Influence of DHEA administration on 24-hour cortisol concentrations. Journal of clinical psychopharmacology, 23(1), 96–99. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Green H, Compas BE, Dickey L, & Pegg S (2020). Exposure to COVID‐19 pandemic stress: Associations with depression and anxiety in emerging adults in the United States. Depression and anxiety, 37(12), 1280–1288. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, & Wüst S (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34(1), 2–18. [DOI] [PubMed] [Google Scholar]
- Kurita H, Maeshima H, Kida S, Matsuzaka H, Shimano T, Nakano Y, … & Arai H (2013). Serum dehydroepiandrosterone (DHEA) and DHEA-sulfate (S) levels in medicated patients with major depressive disorder compared with controls. Journal of affective disorders, 146(2), 205–212. [DOI] [PubMed] [Google Scholar]
- Lambert K, Hunter RG, Bartlett AA, Lapp HE, & Kent M (2020). In search of optimal resilience ratios: differential influences of neurobehavioral factors contributing to stress-resilience spectra. Frontiers in neuroendocrinology, 56, 100802. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology, 41(1), 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking RJT, Pellikaan CM, Lok A, Assies J, Ruhé HG, Koeter MW, … & Schene AH (2015). DHEAS and cortisol/DHEAS-ratio in recurrent depression: state, or trait predicting 10-year recurrence?. Psychoneuroendocrinology, 59, 91–101. [DOI] [PubMed] [Google Scholar]
- Moisan MP (2021). Sexual dimorphism in glucocorticoid stress response. International Journal of Molecular Sciences, 22(6), 3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DA (2014). Missing data: Five practical guidelines. Organizational Research Methods, 17(4), 372–411. [Google Scholar]
- Peckins MK, Roberts AG, Hein TC, Hyde LW, Mitchell C, Brooks-Gunn J, … & Lopez-Duran NL (2020). Violence exposure and social deprivation is associated with cortisol reactivity in urban adolescents. Psychoneuroendocrinology, 111, 104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of youth and adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Roberts AG, & Lopez-Duran NL (2019). Developmental influences on stress response systems: Implications for psychopathology vulnerability in adolescence. Comprehensive psychiatry, 88, 9–21. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Gentes EL, Jones JD, Hallion LS, Coleman ES, & Swendsen J (2015). Rumination predicts heightened responding to stressful life events in major depressive disorder and generalized anxiety disorder. Journal of abnormal psychology, 124(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, & Schachinger H (2008). HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology, 33(6), 890–895. [DOI] [PubMed] [Google Scholar]
- Schwabe L, & Schächinger H (2018). Ten years of research with the Socially Evaluated Cold Pressor Test: Data from the past and guidelines for the future. Psychoneuroendocrinology, 92, 155–161. [DOI] [PubMed] [Google Scholar]
- Shields GS, Lam JC, Trainor BC, & Yonelinas AP (2016). Exposure to acute stress enhances decision-making competence: Evidence for the role of DHEA. Psychoneuroendocrinology, 67, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rajaram N, Garfinkel SN, Abelson JL, & Liberzon I (2013). DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology, 38(9), 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AS, Osbourne M, Appelhans BM, Roisman GI, Booth‐LaForce C, & Bleil ME (2022). Disparities in covid‐19–related stressful life events in the united states: Understanding who is most impacted. Health & social care in the community, 30(3), 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Marques C, Arbo BD, Cozer AG, Hoefel AL, Cecconello AL, Zanini P, … & Ribeiro MFM (2017). Sex-specific effects of dehydroepiandrosterone (DHEA) on glucose metabolism in the CNS. The Journal of steroid biochemistry and molecular biology, 171, 1–10. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, & Iwanski A (2014). Emotion regulation from early adolescence to emerging adulthood and middle adulthood: Age differences, gender differences, and emotion-specific developmental variations. International journal of behavioral development, 38(2), 182–194. [Google Scholar]
- Zoladz PR, Peters DM, Kalchik AE, Hoffman MM, Aufdenkampe RL, Woelke SA, … & Talbot JN (2014). Brief, pre-learning stress reduces false memory production and enhances true memory selectively in females. Physiology & behavior, 128, 270–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


