Summary
During COVID-19 in the US, social determinants of health (SDH) have driven health disparities. However, the use of SDH in COVID-19 vaccine modeling is unclear. This review aimed to summarize the current landscape of incorporating SDH into COVID-19 vaccine transmission modeling in the US. Medline and Embase were searched up to October 2022. We included studies that used transmission modeling to assess the effects of COVID-19 vaccine strategies in the US. Studies’ characteristics, factors incorporated into models, and approaches to incorporate these factors were extracted. Ninety-two studies were included. Of these, 11 studies incorporated SDH factors (alone or combined with demographic factors). Various sets of SDH factors were integrated, with occupation being the most common (8 studies), followed by geographical location (5 studies). The results show that few studies incorporate SDHs into their models, highlighting the need for research on SDH impact and approaches to incorporating SDH into modeling.
Funding
This research was funded by the Centers for Disease Control and Prevention (CDC).
Keywords: Social determinants of health, Health equity, Infectious disease modeling, COVID-19, Vaccine, Scoping review
Introduction
Since the onset of the COVID-19 pandemic, infectious disease modeling has played a pivotal role in supporting and informing policymakers, predicting the pandemic’s future, assessing the effects of interventions, and optimizing resource allocation (from screening and treatment to vaccination).1 To augment accuracy and precision in predicting outcomes and assessing strategy impacts, infectious disease models often incorporated demographic characteristics, such as age, because it is explicitly related to transmission rates and disease severity. However, the transmission of COVID-19 disease also encompasses a significant social dimension. Abundant evidence during the COVID-19 crisis demonstrated that social determinants of health (SDH) factors were significantly associated with higher case numbers, worse outcomes, and reduced vaccine accessibility.2,3
SDH are the non-medical factors in the environments where people are born, live, learn, work, play, and worship that affect various health outcomes and risk factors.4, 5, 6 SDH accounts for approximately 50% of health outcomes and are the major drivers of health inequities.7 In several instances, incorporating SDH has been shown to enhance the model’s ability to accurately capture COVID-19 disease dynamics among subpopulations.8,9 Better understanding of heterogeneous transmission outcomes within a population enables the evaluation of more effective and equitable response measures and policies, making it a crucial area for future research. Despite the apparent need and potential opportunities, progress toward incorporating these SDH factors in disease modeling has been limited. A few research works have been offered8,9 including a framework proposed by Quaife and colleagues in 2022 for incorporating equity factors into models.10 To better understand the current progress on integrating SDH factors into mathematical transmission modeling of the COVID-19 vaccine, this scoping review investigated to what extent these transmission models have incorporated these factors, factors types, and the methods for incorporating SDH variables.
Methods
We conducted our scoping review with guidance from the latest version of the JBI Manual for Evidence Synthesis.11 For transparency and reproducibility, we will adhere to the PRISMA reporting guidelines for scoping reviews and searches.12, 13, 14
Search strategy and selection criteria
The search strategies were developed by an information specialist (MMM) using a combination of keywords and database subject headings for the primary database, Medline, from sentinel studies and reviewers’ feedback, then translated the strategy to the other selected databases. Library colleagues peer-reviewed the strategies using the PRESS checklist.15 Then, we performed formal searching in two main databases, Medline (Ovid) 1946–2022 (from the database inception to Oct 6, 2022) and Embase (Elsevier) 1974–2022 (from database inception to Oct 29, 2022). We checked references of included studies for additional studies meeting our eligibility criteria. Pre-prints identified from the Embase (1974–2022) database were also included. No additional grey literature was included. Details of search strategies for each database are provided in Supplementary Methods S1 in the Supplementary Material.
The studies were included in this review if they satisfied the following criteria: 1) used mathematical transmission modeling (including compartmental, network, or agent-based models); 2) assessed the effects or impact of the COVID-19 vaccine, vaccine coverage, vaccine optimization strategies on COVID-19 transmission; and 3) studied in the US setting. We restricted our inclusion criteria in the US setting, vaccine-related interventions, and transmission models after searching and abstract screening because we found an intractably large number of modeling studies for the team to get a synthesis of evidence in a timely fashion. Additionally, given the surge in COVID-19 research and recent advancements in techniques, focusing exclusively on COVID-19 vaccines also allows us to better reflect the current landscape of SDH in transmission modeling. Conference abstracts and any non-English articles without an available translation were excluded. Each study was screened by two independent reviewers in our team reviewers for titles, abstracts, and full-texts. When there was discrepancy between two reviewers, a third reviewer was consulted if no consensus was reached.
We used Covidence, a web-based systematic review platform, to screen studies (Veritas Health Innovation, Melbourne, Victoria). Citation management and duplicate detection and removal were accomplished with EndNote (Clarivate Analytics.), Covidence was secondary duplicate removal. This manuscript reported a subset of results from a protocol written for a larger project, encompassing multiple aspects. The protocol was registered on the Open Science Framework (https://osf.io/dwqc4).
Data extraction and synthesis
The following detailed information was extracted from the included studies: 1) study characteristics: author, year of publication, study setting, study objectives, target population, interventions, and key findings; and 2) model characteristics: type of model, factors incorporated in the model, number of factors incorporated in the model, approach used to incorporate these factors.
Each article in the data extraction stage was reviewed independently by two reviewers from the review team. Discrepancies were resolved through consensus or the input of a third reviewer, as necessary. No quality assessment of included studies was conducted as our goal was to rapidly map the literature, which complied with the scoping review methodology.
A qualitative synthesis of extracted data was conducted to produce a narrative summary of the included literature. For study characteristics, the number of studies and frequency of each characteristic was quantified. For model characteristics, factors incorporated into the model were classified into demographic factors (e.g., age, gender, race, ethnicity, comorbidities) and SDH factors (e.g., occupation, geographical location, living condition).4 For approaches used to incorporate factors into the model, we classified each approach into three main approaches proposed by Quaife and colleagues, 2022: Approach 1) “Cases distributed through equity dimensions post-simulation” Approach 2) “Cases distributed through equity dimensions with parallel unlinked models”, and approach 3) “Cases distributed through equity dimensions integrated into the model”10 While these definitions make reference to cases, for our purposes, we also include studies that explicitly model other outcomes, such as infections, hospitalizations, deaths, disability-adjusted life years (DALYs), quality-adjusted life years (QALYs), or economic outcomes such as direct costs, indirect costs, or productivity losses. These broad approach categories differ in the way that SDH are represented in the model and, therefore, how SDH may impact model outcomes. Particularly, approach 1 models transmission without stratification based on SDH and then produces a post-simulation distribution of model outcomes across different groups defined by SDH. In approach 2, transmission is simulated independently for each SDH subgroup, each outbreak with its own parameter settings tailored to SDH of focus (i.e., a parallel unlinked modeling approach). Approach 3 links SDH attributes to specific agents that interact through a contact matrix or network or stratify the population via SDH-structured compartments within the model’s framework, with parameters designed to distinguish transmission–relevant interactions between individuals or groups based on SDH factors. For a thorough discussion of the implications of the modeling approach on the impact of SDH and modeled outcomes, please see.10
Data extraction was conducted in Microsoft Excel, and data analysis and visualization were conducted using R (version 4.1.1).
Role of the funding source
The funder had no role in the study design, study collection, analysis, interpretation of data, writing of the report, and the decision to submit the paper for publication.
Results
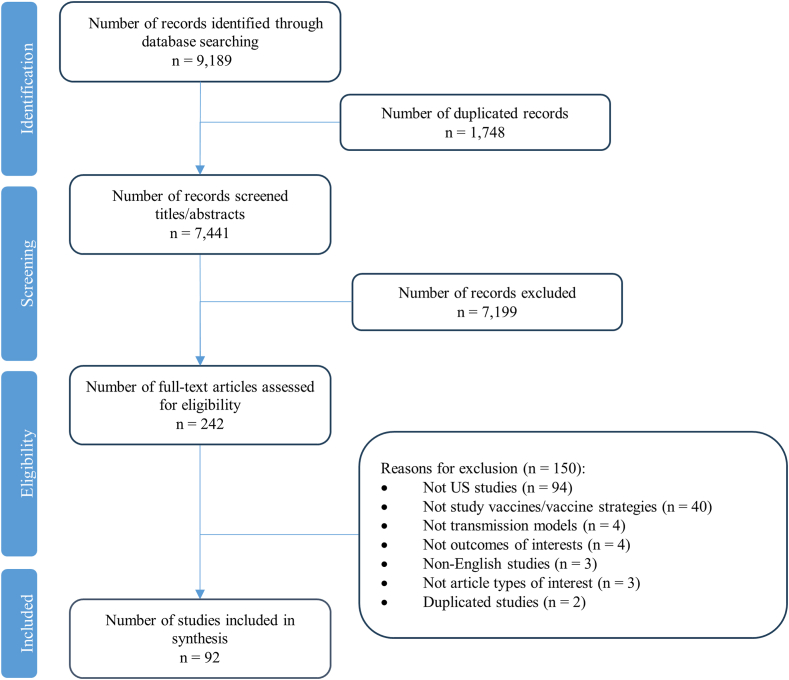
A total of 9189 citations were identified through data searching. Of these, 1748 duplicated records were excluded, 7199 were excluded in title/abstract screening, and 242 proceeded to full-text screening. During the full-text screening, 150 records were excluded. The most frequent reasons for exclusion were non-US settings (n = 94), followed by not studying vaccines/vaccine strategies (n = 40), non-transmission models (n = 4), not outcomes of interest (n = 4), non-English articles (n = 3), not article types of interest (n = 3), and duplicated studies (n = 2). Eventually, 92 studies that met the eligibility criteria proceeded to data extraction and synthesis. The PRISMA flow chart of study selection is presented in Fig. 1. A list of included studies in this review, and excluded studies with excluded reason is provided in Supplementary Methods S2 in Supplementary Material.
Fig. 1.
PRISMA-ScR flow chart.
Study characteristics
The majority of included studies were published between 2021 (n = 47) and 2022 (n = 43). The study setting was the US in 80 studies, and the US combined with other countries in 12 studies.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The target population was the general population in 68 studies and specific sub-populations in 24 studies, including university population (n = 11),28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 school population (n = 5),39, 40, 41, 42, 43 congregation setting population (n = 5),44, 45, 46, 47, 48 children (n = 2),18,49 and wildland firefighting workforce (n = 1).50 Vaccine intervention strategies were studied interventions in 69 studies, and vaccines combined with other strategies (including non-pharmaceutical interventions (NPIs) and screening) in 23 studies.23,27,29, 30, 31,33,35, 36, 37, 38, 39,41,44, 45, 46,50, 51, 52, 53, 54, 55, 56, 57 Seventy-three studies used compartmental models, and agent-based models were employed in 19 studies.23,24,30,32,33,37,39, 40, 41,43,45,46,50,57, 58, 59, 60, 61, 62, 63 Table 1 shows the summary of study characteristics included in this review, and a full description of included studies can be found in Supplementary Table S1 in Supplementary Material.
Table 1.
General characteristics of included studies.
| Characteristic | Number of studies (%) |
|---|---|
| Total studies included | 92 (100%) |
| Year | |
| 2020 | 2 (2%) |
| 2021 | 47 (51%) |
| 2022 | 43 (47%) |
| Study setting | |
| US only | 80 (87%) |
| US and other countries | 12 (13%) |
| Population | |
| General population | 68 (74%) |
| Specific population | |
| University populationa | 11 (12%) |
| School Populationb | 5 (7%) |
| Congregate setting populationc | 5 (5%) |
| Childrend | 2 (2%) |
| Wildland firefighting workforce | 1 (1%) |
| Intervention | |
| Vaccine strategies only | 69 (75%) |
| Vaccine strategies combined with other strategies | |
| NPIse | 19 (21%) |
| Screening | 4 (4%) |
| Model types | |
| Compartmental model | 73 (79%) |
| Agent-based model | 19 (21%) |
Including college/university.
Including elementary/secondary/high school.
Including nursing homes, jails.
Children aged less than 18 years in general (defined by the original study).
NPIs: Non-Pharmaceutical Interventions (for example, social distancing, masking).
Factors incorporated into models
Out of 92 studies, 27 studies incorporated demographic factors alone, 11 studies30,57,60,62, 63, 64, 65, 66, 67, 68, 69 incorporated SDH factors, 9 studies30,60,62,64, 65, 66, 67, 68, 69 incorporated both demographic and SDH factors, and 54 studies did not incorporate any demographic or SDH factors. Regarding demographic factors incorporated into the model, age was the most frequent demographic factor incorporated into models, (n = 36), followed by comorbidities (n = 5),47,59,66,68,70 race/ethnicity (n = 3),24,60,69 and gender (n = 1).24 Regarding SDH factors, 8 studies incorporated occupation, 5 included geographical location, and 2 included living conditions. Twenty-four studies incorporated 1 factor, 7, 4, 2, and 1 study incorporated 2, 3, 4, and 5 factors, respectively. Supplementary Table S2 in Supplementary Material shows the summary of characteristics of factors incorporated into the model.
Characteristics of models incorporating SDH factors
Among 11 studies that incorporated SDH factors (i.e., studies incorporating SDH factors alone or in combination with demographic factors), a combination of occupation-age (n = 3)30,64,67 was the most common one. The other combinations of factors were occupation-geographical location (n = 1),57 occupation-comorbidities-age (n = 1),68 occupation-geographical location-age (n = 1),62 occupation-race/ethnicity-age (n = 1)69; and occupation-comorbidities-living condition-age (n = 1).60 One study incorporated only geographical location.63 Regarding types of models, seven studies63, 64, 65, 66, 67, 68, 69 used compartmental models, and four used agent-based models.30,57,58,60 Regarding the approaches used to incorporate factors, the majority (n = 10) used cases distributed through equity dimension integrated into the model, and 1 study used cases distributed through paralleled unlinked models.68 More details are presented in Table 2, Table 3.
Table 2.
Characteristics of factors incorporated into the models.
| Characteristic | Number of studies (%) |
|---|---|
| Total studies included | 92 (100%) |
| Factors incorporated in the model | |
| Demographic factors | |
| Age | 36 (39%) |
| Comorbidities | 5 (5%) |
| Race/Ethnicity | 3 (3%) |
| Gender | 1 (1%) |
| SDH factors | |
| Occupation | 8 (9%) |
| Geographical location | 5 (5%) |
| Living condition | 2 (2%) |
| Models incorporated | |
| Demographic factors alone | 27 (29%) |
| SDH factors alone | 2 (2%) |
| SDH factors alone or in combination with demographic factors | 11 (12%) |
| Both demographic and SDH factors | 9 (10%) |
| Neither demographic nor SDH factors | 54 (59%) |
| Number of factors incorporated into the models | |
| 0 | 54 (59%) |
| 1 | 24 (26%) |
| 2 | 7 (8%) |
| 3 | 4 (4%) |
| 4 | 2 (2%) |
| 5 | 1 (1%) |
| Type of models in models incorporating SDH factors (11 studies) | |
| Compartmental model | 7 (64%) |
| Agent-based model | 4 (36%) |
| The approach used in models incorporating SDH factors (11 studies) | |
| Case distributed through equity dimensions integrated into the model | 10 (91%) |
| Case distributed through paralleled unlinked models | 1 (9%) |
SDH = Social determinants of health.
Table 3.
Characteristics of models incorporating social determinants of health factors.
| Author, Year | Study setting | Aim/Purpose | Model type | Target population | Intervention | Key findings | Factors incorporated into the model | Approach |
|---|---|---|---|---|---|---|---|---|
| Awad, 202163 | US (Ohio) | This study used an innovative spatiotemporal model to assess the impact of vaccination distribution strategies based on disease geospatial attributes and population-level risk assessment. | Compartmental model | General population | Vaccine strategies | In cases of limited vaccines, early phases of vaccine distribution should focus on areas with high infection intensity informed by geospatial attributes. Such an approach could be a cornerstone of effective vaccine microplanning. While traditional microplanning relies on census data to define target populations based on demographic attributes and geographical information systems (GIS), microplanning can also identify challenges and reach unreached people. | Geographical location | Case distributed through equity dimensions integrated into the model |
| Buckner, 202164 | US | To assess the optimal allocation of limited COVID-19 vaccine supply in the United States across sociodemographic groups differentiated by age and essential worker status | Compartmental model | General population | Vaccine strategies | The authors investigate three policy objectives: minimizing infections, years of life lost (YLL), or deaths. They find that the optimal policy is dynamic, with specific groups targeted each period, and these targets shift over time. The results highlight the importance of factors such as vaccine effectiveness and supply, rate of transmission, and the magnitude of initial infections in determining optimal prioritization strategies. |
Age, Occupation | Case distributed through equity dimensions integrated into the model |
| Islam, 202166 | US | To directly evaluate the CDC recommendation by comparing it to all potentially optimal allocation strategies that stagger the vaccine roll-out in up to four phases (17.5 million strategies) | Compartmental model | General population | Vaccine strategies | Under the developed model, the CDC allocation deviated from the optimal allocations by small amounts, with 0.19% more deaths, 4.0% more cases, 4.07% more infections, and 0.97% higher YLL than the respective optimal strategies. Prioritizing the vaccination of the working-age population generally led to fewer cases and infections at the expense of higher deaths and YLL, highlighting the anticipated trade-off in multi-objective decision-making. A higher prioritization of individuals with comorbidities in all age groups improved outcomes compared to the CDC allocation. | Age, Occupation, Comorbidities, Living condition | Case distributed through equity dimensions integrated into the model |
| Patel, 202160 | US (North Carolina) | To assess the association of simulated COVID-19 vaccine efficacy and coverage scenarios with and without NPIs with infections, hospitalizations, and deaths. | Agent-based model | General population | Vaccine strategies | Higher vaccination coverage with less efficacious vaccines can contribute to a larger reduction in the risk of SARS-CoV-2 infection compared to more efficacious vaccines at lower coverage. These findings highlight the importance of maintaining NPIs and achieving high vaccine coverage to reduce the spread of COVID-19 and prevent severe outcomes. |
Age, Race/Ethnicity, Living conditions, Geographical location | Case distributed through equity dimensions integrated into the model |
| Tatapudi, 202162 | US (Florida) | To assess the impact of vaccine prioritization strategies on mitigating COVID-19 | Agent-based model | General population | Vaccine strategies | A comparison of the prioritization strategies showed no significant difference in their impacts on pandemic mitigation. | Age, Occupation, Geographical location | Case distributed through equity dimensions integrated into the model |
| Tran, 202167 | US (Rhode Island and Massachusetts) | To evaluate age-based vaccine distributions during the pandemic | Compartmental model | General population | vaccine strategies | the study found that allocating a substantial proportion (>75%) of vaccine supply to individuals over the age of 70 is optimal in terms of reducing total cumulative deaths through mid-2021 | Age, Occupation | Case distributed through equity dimensions integrated into the model |
| Truszkowska, 202157 | US (New Rochelle, New York) | To evaluate the effects of testing and vaccination on the pandemic | Agent-based model | General population | vaccine strategies, NPIS | Until widespread vaccination efforts are underway, maintaining a balance between safety and normalcy during the current COVID-19 crisis requires the use of non-pharmaceutical prevention measures as well as efficient detection strategies | Occupation, Geographical location | Case distributed through equity dimensions integrated into the model |
| Chen, 202265 | US | To propose a framework for COVID-19 vaccine distribution that prioritizes disadvantaged communities based on community and societal risk indices. | Compartmental model | General population | vaccine strategies | The study found that social utility and equity can be simultaneously improved when vaccine access is prioritized for the most disadvantaged communities, even when such community’s manifest considerable vaccine reluctance. Nevertheless, equity among distinct demographic features may conflict; for example, low-income neighborhoods might have fewer older citizens. | Age, Geographical location | Case distributed through equity dimensions integrated into the model |
| Head, 202230 | US (California Bay Area) | To examine school reopening policies amidst ongoing transmission of the highly transmissible Delta variant | Agent-based model | University population | vaccine strategies, NPIS | Universal masking reduced infections by over 57% among students, and masking combined with 70% vaccination coverage resulted in fewer than 50 excess cases per 1000 students/teachers. | Age, Occupation | Case distributed through equity dimensions integrated into the model |
| Kadelka, 202269 | US | To investigate (i) how ethnic homophily and social interaction parameters affect the choice of optimal vaccine allocation strategy and (ii), notwithstanding possible ethical concerns, whether differentiating by ethnicity in these strategies can lead to better societal outcomes | Compartmental model | General population | vaccine strategies | This study highlights the importance of ethnic homophily on disease dynamics and, more specifically, on the design of optimal mass vaccine roll-out strategies. The most likely social context in the U.S. is very different from standard model assumptions, which do not account for ethnicity and ethnic homophily, and this difference significantly affects which vaccination strategy is optimal. It may thus be worth exploring options to better quantify social contact patterns between ethnic groups to better understand infectious disease spread. | Age, Race/Ethnicity, Occupation | Case distributed through equity dimensions integrated into the model |
| Walker, 202268 | US | To support the development of this guidance for ACIP (recommended phased allocation of SARS-CoV-2 vaccines) | Compartmental model | General population | vaccine strategies | Strategies of subsequently prioritizing adults aged ≥ 65 years, or a combination of essential workers and adults aged ≥ 75 years prevented the most deaths. Prioritizing adults with high-risk medical conditions immediately after HCP prevented the most infections. All three strategies prevented a similar fraction of hospitalizations. |
Age, Occupation, Comorbidities | Case distributed through paralleled unlinked models |
Characteristics of vaccine modeled in the studies incorporating SDH factors
Modeled vaccine characteristics were also summarized for the studies that incorporated SDH factors and presented in Table 4. Three studies57,60,65 conceptualized vaccine efficacy using a binary protection model, positing that a certain percentage of the vaccinated population achieves complete immunity (known as an “all-or-nothing” vaccine). Conversely, six studies30,63,66, 67, 68, 69 employed a partial immunity framework, hypothesizing that the entire vaccinated cohort obtains a uniform degree of protection, albeit not total immunity, from the vaccine (known as “leaky” vaccine). Two studies62,64 modeled both “all-or-nothing” and “leaky” vaccines. Two studies60,63 modeled the effectiveness of the vaccine on infection alone, two studies30,68 modeled the vaccine effect on infection and severity of disease, and one studies67 modeled the effectiveness of the vaccine on infection and transmission. Six studies57,62,64, 65, 66,69 modeled the effectiveness of vaccines on infection, severity of disease, and transmission. The modeled vaccine effectiveness on infection in these studies ranged from 50% to 90%, vaccine effectiveness on disease severity ranged from 60% to 95%, and vaccine effectiveness on disease transmission was 50%. Two studies assumed perfect protection (i.e., the vaccine’s effectiveness was 100% protection).57,65 Waning immunity to vaccination was modeled in one study.67
Table 4.
Characteristics of vaccine modeled in the studies incorporating social determinants of health factors.
| Author, year | Vaccine effectiveness modeled | Effectiveness of vaccine | Vaccine effectiveness on |
Waning immunity | ||
|---|---|---|---|---|---|---|
| Infection | Severity of disease | Transmission | ||||
| Awad, 202163 | Leaky vaccine | Reduce susceptibility to infection: 90% | Yes | No | No | No |
| Buckner, 202164 | Leaky vaccine, All-or-nothing vaccine | Reduce susceptibility to infection: 90% Reduce infectiousness: 90% Reduce fatality: 90% |
Yes | Yes | Yes | No |
| Islam, 202166 | Leaky vaccine | Reduce infection: 70%, 85% Reduce symptomatic infection: 75%, 95% Reduce contagiousness/transmissibility/infectiousness: 50% |
Yes | Yes | Yes | No |
| Patel, 202160 | All-or-nothing | Reduce susceptibility to infection: 90%, 50% | Yes | No | No | No |
| Tatapudi, 202162 | Leaky vaccine, All-or-no-thing vaccine | A linearly increasing partial immunity for susceptible after they received the first dose, attaining full immunity after 7 days after the second dose | Yes | Yes | Yes | No |
| Tran, 202167 | Leaky vaccine | Vaccine effectiveness is a function of the force of infection and reduction in susceptibility. | Yes | No | Yes | Yes |
| Truszkowska, 202157 | All-or-nothing | Perfect protection (move individuals from susceptible state to recovered/immune state) | Yes | Yes | Yes | No |
| Chen, 202265 | All or nothing | Perfect protection (move individuals from susceptible state to recovered/immune state) | Yes | Yes | Yes | No |
| Head, 202230 | Leaky vaccine | Reduce any infection: 77% Reduce symptomatic infection: 85% Reduce severe infection: 93% |
Yes | Yes | No | No |
| Kadelka, 202269 | Leaky vaccine | Reduce infection: 70% Reduce symptomatic infection: 66.7%% Reduce transmission: 50% |
Yes | Yes | Yes | No |
| Walker, 202268 | Leaky vaccine | Reduce infection (first, second dose): 70%, 85% Reduce severity of disease (first, second dose): 75%, 95% |
Yes | Yes | No | No |
Findings on studies incorporating SDH factors
None of the 11 studies explicitly evaluated the contribution of SDH factors to model performance as a primary objective. The objective in most of these studies was to find the optimal strategies given some defined goals, for example, minimizing infection cases, hospitalized cases, or death. We did find 3 studies that did incorporate SDH into the model and considered equity aspect. For instance, a study conducted by Award and colleagues, which incorporated geographical location, showed that focusing on areas with high infection intensity informed by geospatial attributes can be an effective vaccine microplan.63 In the model incorporating age, occupation, and comorbidities, Walter and colleagues compared different vaccine strategies. They found that prioritizing individuals with high-risk medical conditions after healthcare workers minimized the most infected cases while prioritizing individuals aged over 65 years old or 75 years old minimized the most deaths.68 Chen’s study in 2022 used a model that incorporated age and geographical location, which showed that vaccine access was prioritized for the most disadvantaged communities and can improve both health outcomes and equity.65
Discussion
A substantial body of research underscores the relevance of SDHs in COVID-19 vaccine uptake. Despite this, integrating SDHs into COVID-19 epidemiological modeling has not been prominent in scholarly literature. In the US, where population diversity is pronounced, SDHs have been identified as significant factors influencing and driving COVID-19 outcomes and health disparities. Effective interventions in prevention and treatment have reduced COVID-19 impact, but these reductions have not been uniformly distributed across different populations, subgroups, and geographical regions. This scoping review reveals that only 11 out of 92 modeling studies investigating COVID-19 vaccine in the US have incorporated SDHs into their analyses and even then, only a handful did so to consider equity. This study highlighted a need to incorporate SDH factors into COVID-19 transmission modeling.
While our review observed variability in SDH factors incorporated into COVID-19 modeling, occupation emerged as the most frequently integrated SDH factor. This focus on occupation is understandable, given the significant role of occupation-related risks in COVID-19 infection and its use as a criterion for vaccine prioritization during the first phase.71 Variations in the inclusion of occupation and other SDH factors can lead to dramatic differences in the assessment of risks and outcomes and resulting prioritization strategies. Not incorporating SDH, or incorporating different SDH factors, can dramatically change the prioritization of vaccine strategies. For example, Bubar et al.16 included only age into their model and concluded that prioritizing vaccination for adults aged over 60 would minimize mortality. In contrast, Walker et al.68 incorporated age, occupation, and comorbidities and concluded that prioritizing healthcare workers, followed by older adults at high risk, would prevent the most infections or deaths. Occupation (essential worker status) might be an important SDH to include when large differences in transmission rates by occupation status are expected. Meanwhile, Chen’s study65 which incorporated geographical data suggested that targeting the most disadvantaged groups based on community and societal risk indices may achieve optimal health outcomes and equity. Including geography might be important when access to quality healthcare is geographically segregated or there are large differences in health outcomes by geographical unit (e.g., zip code, census block). Ignoring such factors could lead to dramatic over or underprediction of public health outcomes and the effectiveness of public health strategies. This underscores the need to invest in the development of new models and approaches that explicitly consider SDH factors and how different sets of SDHs could impact the decision.
We found that the predominant methodological approach for integrating SDH into transmission models was to evaluate cases distributed through equity dimensions integrated into the model. This method, which allows subgroups with their linked equity-related factors to run through the same model, is generally preferred over unlinked parallel models where each subgroup runs through the model with its own set of parameters.10 The integrated approach facilitates a more realistic representation of intergroup and between-group correlations as opposed to treating subgroups as isolated entities. However, this integrated modeling requires extensive datasets and advanced computational techniques.10 The demand for high-quality, comprehensive data increases as more variables are introduced into the models, highlighting the critical need for improved data collection on SDH with associated diseases and health outcomes.
Incorporating SDHs into transmission models may encounter several challenges. The quantity and quality of data on SDH is the first one. SDH encompasses a broad spectrum of measures and metrics, necessitating extensive data collection efforts. Additionally, the complexity and multicausality of SDH present challenges in accurately capturing their association with related diseases or health outcomes. Next, computational capacity is required to incorporate multiple layers of SDH factors into modeling. Subsequently, the balance of the model - the trade-off between simplicity and complexity - is also a pivotal question for modelers. Furthermore, the difference in aims of building a model to understand disease ecology scientifically or to inform health policies will affect whether or not the incorporation of SDH is needed. There is an urgent need to establish guidelines or standard practices for when and how to incorporate SDH factors into transmission modeling.10,72,73
This scoping review was subject to certain limitations. Firstly, inherent in its nature as a scoping review, we did not assess the quality of the studies included. Secondly, we acknowledge the complexity and lack of consensus involved in classifying SDH versus demographic factors, including the non-exclusivity of these determinants. We categorized race and ethnicity as demographic factors, acknowledging their potential classification as SDH factor due to the significant correlations between these attributes and social-economic conditions.74 Additionally, while age is generally considered a demographic factor, the experience of ageism that accompanies age may also be considered a social determinant of health.75 Thirdly, our review focused on dynamic transmission infectious disease modeling, and we did not explore emerging technologies such as machine learning and artificial intelligence (AI). The rapid evolution of these techniques in the current era of generative AI shows promise for addressing challenges in dynamic transmission modeling, as an increasing number of studies investigate the application of AI and generative AI in infectious disease modeling and public health interventions.76,77 However, it is crucial to conduct additional research to assess their potential and limitations thoroughly. Additionally, groups of simulation models and theoretical models were not included in this review. We acknowledge that simulation techniques are popular in modeling. However, our focus is on dynamic transmission models that account for the effects of dynamic phenomena related to SDH and are common to infectious disease outbreaks in populations with variation in SDH. Also, while theoretical models are useful for understanding the hypothetical framework of incorporating SDH and assessing their impact on models, especially when data on SDHs are limited, however, this review that focused on studies in real-world settings provides answers to questions about the application of modeling. More specifically, it examines to what extent current models integrate SDHs in real-world settings. Fourthly, our study provides an overall picture of the integration of SDHs into transmission modeling without delving into the detailed examination of how these SDH-integrated models enhanced/impacted the model’s performance or outcomes. We recognize this as an important subsequent research area, warranting rigorous evaluation of model quality, performance, and its impact on predictions and public health outcomes. This initial review serves as a foundation for future inquiries and discussions in this domain. Fifthly, we did not specify the particular stage of COVID-19 vaccine modeling in our review, such as models intended for use in the initial stages or for booster vaccines. We acknowledge that at different stages of vaccine rollout, the necessity of considering the health equity aspect varies; thus, the incorporation of SDH may differ. Lastly, our review focuses on the US and COVID-19 so that the potential limitations in the generalizability of our findings are undeniable. Nonetheless, the COVID-19 pandemic presents a historical case study as the increased research, data collection, and attention towards modeling, coupled with a heightened focus on SDH and health equity, our findings can reflect the current state of SDH and COVID-19 transmission modeling. However, extending this research globally and across different diseases remains a critical area for future investigation.
Despite these limitations, this review provides a comprehensive overview of the integration of SDHs in COVID-19 modeling in the US. We hope to give modelers insights into the current landscape of transmission models that incorporate SDH considerations and foster awareness among the next generation of modelers to potential challenges they might face in future pandemics, thereby enhancing their preparedness. Additionally, this review aims to demonstrate that including SDHs in infectious disease modeling can aid policymakers in addressing equity issues when evaluating the impact of interventions. This consideration is increasingly pivotal in policy decisions regarding resource allocation to ensure both effectiveness and equity.78,79
In public health context, to better integrate SDH into infectious disease modeling, the following suggestions may be taken into consideration. Firstly, establishing standardized consensus guidelines for incorporating SDH into transmission models is necessary. These guidelines would provide a clear framework for researchers and modelers, ensuring consistency and comprehensiveness in how SDH factors are integrated into infectious disease dynamics. Secondly, data surveillance concerning SDHs and infectious diseases should be enhanced, so that models can more accurately reflect the complex interplay between SDH and disease transmission. Thirdly, monitoring SDHs and health equity metrics helps us properly assess the impact of health strategy on SDH and health equity. The World Health Organization recently released the operational framework for assessing SDH and health equity in early 2024, marking a significant step forward in monitoring SDH and health equity.7 Policymakers may consider building upon this foundation to develop more standardized approaches to integrating SDH into infectious disease modeling, thereby improving the effectiveness and equity of public health interventions. To implement these suggestions and ensure their utility in supporting health policy decisions, enhancing modeling capacity at the public health level is crucial. This can be achieved through close partnerships between academics, communities, and local and national public health departments, which may serve as a long-term strategy for immediate action and future preparedness.72
Conclusions
This review summarized the existing research on integrating SDH factors into mathematical modeling for the COVID-19 vaccine. The findings showed that there were few transmission modeling studies in the COVID-19 vaccine in the US incorporating SDH factors. We found that even when SDH factors were incorporated, equity evaluation was even rarer. We identified a gap in our understanding that future modelers could fill with investigations that can guide policymakers in leveraging modeling evidence for making decisions that are both efficient and fair.
Contributors
The study was conceived of by LV, DJAT, MJ, MS, and NC. KD, LV, SV, MMM, RN, BJ, AP, EC, KK, JL, GGVY, YZ, TW, ED, DJAT, MJ, MS, NC conceptualized the study. LV, MMM, MJ, MS, NC built search terms, search strategies and searched. KD, DN, LV, SV, EC, KK, JL, GGVY, YZ, ED, DJAT, MJ, MS screened articles. KD, DN, W Kategeaw, XL, and W Khaing extracted the data. KD wrote the first draft of the manuscript. All authors critically revised the manuscript for intellectual content. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The datasets generated during or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of interests
KD, DN, W Khaing, LV, SV, RN, BJ, AP, EC, KK, JL, GGVY, YZ, TW, ED, DJAT, MJ, MH, and NC received funding from Centers for Disease Control and Prevention (CDC) (SHEPheRD 2021 Domain 1-A015). MMM received funding from Systematic Review Core (SR Core), with funding in part from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UM1TR004409.
Acknowledgements
This work was supported by the Centers for Disease Control and Prevention (CDC) (SHEPheRD 2021 Domain 1-A015). This investigation was also supported by the Systematic Review Core (SR Core), with funding in part from the National Center for Advancing Translational Sciences, National Institutes of Health under Award Number UM1TR004409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC) or National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100806.
Appendix ASupplementary data
References
- 1.McBryde E.S., Meehan M.T., Adegboye O.A., et al. Role of modelling in COVID-19 policy development. Paediatr Respir Rev. 2020;35:57–60. doi: 10.1016/j.prrv.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams E.M., Szefler S.J. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020;8(7):659–661. doi: 10.1016/s2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seal K.H., Bertenthal D., Manuel J.K., Pyne J.M. Association of demographic, clinical, and social determinants of health with COVID-19 vaccination booster dose completion among US veterans. JAMA Netw Open. 2022;5(7) doi: 10.1001/jamanetworkopen.2022.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healthy people 2030. Social determinants of health. 2023. https://health.gov/healthypeople/priority-areas/social-determinants-health
- 5.Centers for Disease Control and Prevention Social determinants of health at CDC. 2022. https://www.cdc.gov/about/sdoh/index.html
- 6.World Health Organization . 2024. Social determinants of health.https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 [Google Scholar]
- 7.Operational framework for monitoring social determinants of health equity. World Health Organization; Geneva: 2024. https://www.who.int/publications/i/item/9789240088320 [Google Scholar]
- 8.Menkir T.F., Jbaily A., Verguet S. Incorporating equity in infectious disease modeling: case study of a distributional impact framework for measles transmission. Vaccine. 2021;39(21):2894–2900. doi: 10.1016/j.vaccine.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galanis G., Hanieh A. Incorporating social determinants of health into modelling of COVID-19 and other infectious diseases: a baseline socio-economic compartmental model. Soc Sci Med. 2021;274 doi: 10.1016/j.socscimed.2021.113794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaife M., Medley G.F., Jit M., et al. Considering equity in priority setting using transmission models: recommendations and data needs. Epidemics. 2022;41 doi: 10.1016/j.epidem.2022.100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters M.D.J., Marnie C., Tricco A.C., et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–2126. doi: 10.11124/jbies-20-00167. [DOI] [PubMed] [Google Scholar]
- 12.Rethlefsen M.L., Kirtley S., Waffenschmidt S., et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. J Med Libr Assoc. 2021;109(2):174–200. doi: 10.5195/jmla.2021.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tricco A.C., Lillie E., Zarin W., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 14.Page M.J., Moher D., Bossuyt P.M., et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Bubar K.M., Reinholt K., Kissler S.M., et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371(6352):916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi D., Chakravarty U. Predictive analysis of COVID-19 eradication with vaccination in India, Brazil, and USA. Infect Genet Evol. 2021;92 doi: 10.1016/j.meegid.2021.104834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavish N., Katriel G. The role of childrens’ vaccination for COVID-19—pareto-optimal allocations of vaccines. PLoS Comput Biol. 2022;18(2) doi: 10.1371/journal.pcbi.1009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabowski F., Kochańczyk M., Lipniacki T. The spread of SARS-CoV-2 variant Omicron with a doubling time of 2.0–3.3 days can be explained by immune evasion. Viruses. 2022;14(2):294. doi: 10.3390/v14020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He D., Ali S.T., Fan G., et al. Evaluation of effectiveness of global COVID-19 vaccination campaign. Emerg Infect Dis. 2022;28(9):1873. doi: 10.3201/eid2809.212226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jhun B., Choi H. Abrupt transition of the efficient vaccination strategy in a population with heterogeneous fatality rates. Chaos. 2022;32(9) doi: 10.1063/5.0087627. [DOI] [PubMed] [Google Scholar]
- 22.Ke R., Romero-Severson E., Sanche S., Hengartner N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J Theor Biol. 2021;517 doi: 10.1016/j.jtbi.2021.110621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar C.K., Balasubramanian R., Ongarello S., Carmona S., Laxminarayan R. SARS-CoV-2 testing strategies for outbreak mitigation in vaccinated populations. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q., Huang Y. Optimizing global COVID-19 vaccine allocation: an agent-based computational model of 148 countries. PLoS Comput Biol. 2022;18(9) doi: 10.1371/journal.pcbi.1010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X., Lv Z., Ding Y. Mathematical modeling and stability analysis of the time-delayed SAIM model for COVID-19 vaccination and media coverage. Math Biosci Eng. 2022;19(6):6296–6316. doi: 10.3934/mbe.2022294. [DOI] [PubMed] [Google Scholar]
- 26.Makhoul M., Chemaitelly H., Ayoub H.H., Seedat S., Abu-Raddad L.J. Epidemiological differences in the impact of COVID-19 vaccination in the United States and China. Vaccines. 2021;9(3):223. doi: 10.3390/vaccines9030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piccirillo V. COVID-19 pandemic control using restrictions and vaccination. Math Biosci Eng. 2022;19:1355–1372. doi: 10.3934/mbe.2022062. [DOI] [PubMed] [Google Scholar]
- 28.Brown R.A. A simple model for control of COVID-19 infections on an urban campus. Proc Natl Acad Sci USA. 2021;118(36) doi: 10.1073/pnas.2105292118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frazier P.I., Cashore J.M., Duan N., et al. Modeling for COVID-19 college reopening decisions: cornell, a case study. Proc Natl Acad Sci USA. 2022;119(2) doi: 10.1073/pnas.2112532119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Head J.R., Andrejko K.L., Remais J.V. Model-based assessment of SARS-CoV-2 Delta variant transmission dynamics within partially vaccinated K-12 school populations. Lancet Reg Health Am. 2022;5 doi: 10.1016/j.lana.2021.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hekmati A., Luhar M., Krishnamachari B., Matarić M. Simulating COVID-19 classroom transmission on a university campus. Proc Natl Acad Sci USA. 2022;119(22) doi: 10.1073/pnas.2116165119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junge M., Li S., Samaranayake S., Zalesak M. Safe reopening of university campuses is possible with COVID-19 vaccination. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motta F.C., McGoff K.A., Deckard A., et al. Assessment of simulated surveillance testing and quarantine in a SARS-CoV-2-vaccinated population of students on a university campus. JAMA Health Forum. 2021;2(10) doi: 10.1001/jamahealthforum.2021.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paltiel A.D., Schwartz J.L. Assessing COVID-19 prevention strategies to permit the safe opening of residential colleges in fall 2021. Ann Intern Med. 2021;174(11):1563–1571. doi: 10.7326/m21-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabil M.J., Tunc S., Bish D.R., Bish E.K. Benefits of integrated screening and vaccination for infection control. PLoS One. 2022;17(4) doi: 10.1371/journal.pone.0267388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabil M.J., Tunc S., Bish D.R., Bish E.K. Effective screening strategies for safe opening of universities under Omicron and Delta variants of COVID-19. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-25801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Q., Gruenbacher D.M., Scoglio C.M. Estimating data-driven coronavirus disease 2019 mitigation strategies for safe university reopening. J R Soc Interf. 2022;19(188) doi: 10.1098/rsif.2021.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao X., Tatapudi H., Corey G., Gopalappa C. Threshold analyses on combinations of testing, population size, and vaccine coverage for COVID-19 control in a university setting. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bilinski A., Ciaranello A., Fitzpatrick M.C., et al. Estimated transmission outcomes and costs of SARS-CoV-2 diagnostic testing, screening, and surveillance strategies among a simulated population of primary school students. JAMA Pediatr. 2022;176(7):679–689. doi: 10.1001/jamapediatrics.2022.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giardina J., Bilinski A., Fitzpatrick M.C., et al. Model-estimated relationship between elementary school-related SARS-CoV-2 transmission, mitigation interventions, and vaccination coverage across community incidence levels. Medrxiv. 2021 doi: 10.1101/2021.08.04.21261576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giardina J., Bilinski A., Fitzpatrick M.C., et al. Model-estimated association between simulated US elementary school–related SARS-CoV-2 transmission, mitigation interventions, and vaccine coverage across local incidence levels. JAMA Netw Open. 2022;5(2) doi: 10.1001/jamanetworkopen.2021.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGee R.S., Homburger J.R., Williams H.E., Bergstrom C.T., Zhou A.Y. Model-driven mitigation measures for reopening schools during the COVID-19 pandemic. Proc Natl Acad Sci USA. 2021;118(39) doi: 10.1073/pnas.2108909118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilinski A., Salomon J.A., Giardina J., Ciaranello A., Fitzpatrick M.C. Passing the test: a model-based analysis of safe school-reopening strategies. Ann Intern Med. 2021;174(8):1090–1100. doi: 10.7326/M21-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blumberg S., Lu P., Kwan A.T., et al. Modeling scenarios for mitigating outbreaks in congregate settings. PLoS Comput Biol. 2022;18(7) doi: 10.1371/journal.pcbi.1010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fosdick B.K., Bayham J., Dilliott J., Ebel G.D., Ehrhart N. Model-based evaluation of policy impacts and the continued COVID-19 risk at long term care facilities. Infect Dis Model. 2022;7(3):463–472. doi: 10.1016/j.idm.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gómez Vázquez J.P., García Y.E., Schmidt A.J., Martínez-López B., Nuño M. Testing and vaccination to reduce the impact of COVID-19 in nursing homes: an agent-based approach. BMC Infect Dis. 2022;22(1):477. doi: 10.1186/s12879-022-07385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryckman T., Chin E.T., Prince L., et al. Outbreaks of COVID-19 variants in US prisons: a mathematical modelling analysis of vaccination and reopening policies. Lancet Public Health. 2021;6(10):e760–e770. doi: 10.1016/S2468-2667(21)00162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh B.K., Walker J., Paul P., et al. De-escalation of asymptomatic testing and potential of future COVID-19 outbreaks in US nursing homes amidst rising community vaccination coverage: a modeling study. Vaccine. 2022;40(23):3165–3173. doi: 10.1016/j.vaccine.2022.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moghadas S.M., Fitzpatrick M.C., Shoukat A., Zhang K., Galvani A.P. Simulated identification of silent COVID-19 infections among children and estimated future infection rates with vaccination. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belval E.J., Bayham J., Thompson M.P., Dilliott J., Buchwald A.G. Modeling the systemic risks of COVID-19 on the wildland firefighting workforce. Sci Rep. 2022;12(1):8320. doi: 10.1038/s41598-022-12253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchin G., Dall’Anese E., Poveda J.I., Jacobson D., Carlton E.J., Buchwald A.G. Novel use of online optimization in a mathematical model of COVID-19 to guide the relaxation of pandemic mitigation measures. Sci Rep. 2022;12(1):4731. doi: 10.1038/s41598-022-08389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bracis C., Moore M., Swan D.A., et al. Improving vaccination coverage and offering vaccine to all school-age children allowed uninterrupted in-person schooling in King County, WA: modeling analysis. Math Biosci Eng. 2022;19(6):5699. doi: 10.3934/mbe.2022266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chowdhury M.M., Islam M.R., Hossain M.S., Tabassum N., Peace A. Incorporating the mutational landscape of SARS-COV-2 variants and case-dependent vaccination rates into epidemic models. Infect Dis Model. 2022;7(2):75. doi: 10.1016/j.idm.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hachtel G.D., Stack J.D., Hachtel J.A. Forecasting and modeling of the COVID-19 pandemic in the USA with a timed intervention model. Sci Rep. 2022;12(1):4339. doi: 10.1038/s41598-022-07487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ledder G. Incorporating mass vaccination into compartment models for infectious diseases. medRxiv. 2022;19:9457–9480. doi: 10.3934/mbe.2022440. [DOI] [PubMed] [Google Scholar]
- 56.Ngonghala C.N., Taboe H.B., Safdar S., Gumel A.B. Unraveling the dynamics of the Omicron and Delta variants of the 2019 coronavirus in the presence of vaccination, mask usage, and antiviral treatment. Appl Math Model. 2023;114:447–465. doi: 10.1016/j.apm.2022.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Truszkowska A., Behring B., Hasanyan J., et al. High-resolution agent-based modeling of COVID-19 spreading in a small town. Adv Theory Simul. 2021;4(3) doi: 10.1002/adts.202000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moghadas S.M., Vilches T.N., Zhang K., et al. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021;19(4) doi: 10.1371/journal.pbio.3001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moghadas S.M., Vilches T.N., Zhang K., et al. The impact of vaccination on coronavirus disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis. 2021;73(12):2257–2264. doi: 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel M.D., Rosenstrom E., Ivy J.S., et al. Association of simulated COVID-19 vaccination and nonpharmaceutical interventions with infections, hospitalizations, and mortality. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romero-Brufau S., Chopra A., Ryu A.J., et al. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ. 2021;373 doi: 10.1136/bmj.n1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tatapudi H., Das R., Das T.K. Impact of vaccine prioritization strategies on mitigating COVID-19: an agent-based simulation study using an urban region in the United States. BMC Med Res Methodol. 2021;21(1):1–14. doi: 10.1186/s12874-021-01458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awad S.F., Musuka G., Mukandavire Z., Froass D., MacKinnon N.J., Cuadros D.F. Implementation of a vaccination program based on epidemic geospatial attributes: covid-19 pandemic in Ohio as a case study and proof of concept. Vaccines. 2021;9(11):1242. doi: 10.3390/vaccines9111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buckner J.H., Chowell G., Springborn M.R. Dynamic prioritization of COVID-19 vaccines when social distancing is limited for essential workers. Proc Natl Acad Sci USA. 2021;118(16) doi: 10.1101/2020.09.22.20199174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Xu F., Han Z., et al. Strategic COVID-19 vaccine distribution can simultaneously elevate social utility and equity. Nat Hum Behav. 2022;6(11):1503–1514. doi: 10.1038/s41562-022-01429-0. [DOI] [PubMed] [Google Scholar]
- 66.Islam M.R., Oraby T., McCombs A., et al. Evaluation of the United States COVID-19 vaccine allocation strategy. PLoS One. 2021;16(11) doi: 10.1371/journal.pone.0259700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran T.N.-A., Wikle N.B., Albert E., et al. Optimal SARS-CoV-2 vaccine allocation using real-time attack-rate estimates in Rhode Island and Massachusetts. BMC Med. 2021;19:1–14. doi: 10.1186/s12916-021-02038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker J., Paul P., Dooling K., et al. Modeling strategies for the allocation of SARS-CoV-2 vaccines in the United States. Vaccine. 2022;40(14):2134–2139. doi: 10.1016/j.vaccine.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadelka C., Islam M.R., McCombs A., Alston J., Morton N. Ethnic homophily affects vaccine prioritization strategies. J Theor Biol. 2022;555 doi: 10.1016/j.jtbi.2022.111295. [DOI] [PubMed] [Google Scholar]
- 70.Rao I.J., Brandeau M.L. Optimal allocation of limited vaccine to control an infectious disease: simple analytical conditions. Math Biosci. 2021;337 doi: 10.1016/j.mbs.2021.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention . 2020. Vaccine recommendations and guidelines of the ACIP.https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19/evidence-table-phase-1b-1c.html [Google Scholar]
- 72.Buckee C., Noor A., Sattenspiel L. Thinking clearly about social aspects of infectious disease transmission. Nature. 2021;595(7866):205–213. doi: 10.1038/s41586-021-03694-x. [DOI] [PubMed] [Google Scholar]
- 73.Allen K S., Gilliam N.J., Kharrazi H., McPheeters M., Dixon B.E. In: Health information exchange. 2nd ed. Dixon B.E., editor. Academic Press; 2023. Chapter 19 - health information exchange: incorporating social and environmental determinants of health into health information exchange; pp. 423–433. [Google Scholar]
- 74.Centers for Disease Control and Prevention Social determinants of health. 2023. https://www.cdc.gov/places/measure-definitions/social-determinants-of-health/index.html
- 75.Mikton C., de la Fuente-Núñez V., Officer A., Krug E. Ageism: a social determinant of health that has come of age. Lancet. 2021;397(10282):1333–1334. doi: 10.1016/S0140-6736(21)00524-9. [DOI] [PubMed] [Google Scholar]
- 76.Cheng K., Li Z., He Y., et al. Potential use of artificial intelligence in infectious disease: take ChatGPT as an example. Ann Biomed Eng. 2023;51(6):1130–1135. doi: 10.1007/s10439-023-03203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malik Y.S., Sircar S., Bhat S., et al. How artificial intelligence may help the Covid-19 pandemic: pitfalls and lessons for the future. Rev Med Virol. 2021;31(5):1–11. doi: 10.1002/rmv.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chhibber A., Kharat A., Duong K., et al. Strategies to minimize inequity in COVID-19 vaccine access in the US: implications for future vaccine rollouts. Lancet Reg Health Am. 2022;7 doi: 10.1016/j.lana.2021.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt H., Weintraub R., Williams M.A., et al. Equitable allocation of COVID-19 vaccines in the United States. Nat Med. 2021;27(7):1298–1307. doi: 10.1038/s41591-021-01379-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.