Abstract
Treatment of human osteosarcoma cells, expressing CD4 and various chemokine receptors, with the glucosylceramide synthase inhibitor 1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (PPMP), blocked target membrane glycosphingolipid (GSL) biosynthesis and reduced the susceptibility of cells to infection and fusion mediated by envelope glycoproteins from a variety of human immunodeficiency virus type 1 (HIV-1) isolates that utilize CXCR4 and/or CCR5. PPMP treatment of the cell lines did not significantly change the cell surface expression of CD4, CXCR4, and/or CCR5, nor did it alter the chemokine receptor association with CD4. PPMP-treated cells exhibited no changes in chemokine-induced Ca2+ mobilization and chemotaxis. However, massive envelope glycoprotein conformational changes triggered by CD4 and the appropriate chemokine receptor on the target membrane were inhibited when the target cells were treated with PPMP. Addition of various purified GSLs to PPMP-treated target cells showed that for all isolates tested, globotriaosylceramide (Gb3) was the most potent GSL in restoring the fusion susceptibility of target cells with cells expressing HIV-1 envelope glycoproteins; addition of the monosialoganglioside GM3 yielded a slight enhancement of fusion susceptibility. Our data are consistent with the notion that a limited number of specific GSL species serve as crucial elements in organizing gp120-gp41, CD4, and an appropriate chemokine receptor into a membrane fusion complex.
Human immunodeficiency virus type 1 (HIV-1) infects susceptible cells by fusion of the viral membrane with the cell plasma membrane. This process is mediated by the interactions of the HIV-1 envelope glycoprotein gp120-gp41 (11, 45, 46) with CD4 on the host cell surface (26) and requires additional coreceptors such as CXCR4 (X4) and CCR5 (R5) (4, 5, 14, 28), which determine the tropism of different HIV-1 isolates. Several viral envelope glycoprotein oligomers then assemble into a viral fusion machine (18, 23), forming a molecular scaffold that brings the viral and target cell membranes into close apposition and allow the actual fusion event (29). Previously reported work suggests that glycosphingolipids (GSLs) may be involved in the assembly and functioning of the HIV-1 fusion machine. This is based on the demonstration that inhibition of target cell GSL biosynthesis affects HIV-1 envelope glycoprotein-mediated cell-cell fusion (37) and that fusion activity can be recovered following addition of human erythrocyte membrane components (15, 38) or purified GSLs to the impaired cells (35). Moreover, studies using reconstituted monolayers of purified GSLs at the air-water interface provide evidence for CD4-induced interactions between HIV-1 gp120 and the GSLs globotriaosylceramide (Gb3) and the monosialoganglioside GM3 (21). These observations have led to the hypothesis that plasma membrane GSL microdomains are preferential sites for assembly of the HIV-1 fusion machine (21, 36).
In this study, we have examined the effect of treating a variety of cell lines with an inhibitor of GSL biosynthesis, 1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol (PPMP), on HIV-1 entry and HIV-1 envelope glycoprotein-mediated fusion. We show here that the presence of GSL in the target membrane is required for HIV-1 infection, that the viral requirement for target membrane GSL is independent of viral isolate tropism, and that addition of Gb3 to the target membrane preferentially restores the susceptibility of cells to fusion by envelopes of different tropisms, including primary isolates. These data show that the HIV-1 fusion machine may utilize target membrane GSLs.
MATERIALS AND METHODS
Materials.
Fluorescent probes were obtained from Molecular Probes (Eugene, Oreg.), and tissue culture media were obtained from Gibco-BRL (Gaithersburg, Md.). Phospholipids were purchased from Avanti Polar Lipids (Alabaster, Ala.), and PPMP, GSLs, and the monosialoganglioside mixture were obtained from Matreya (Pleasant Gap, Pa.). pNL4-3Luc r−e−, pBsTAT, pCMVsREV, pNL1.5E, pHCMV-G, and pJRCSF were the gift of George Pavlakis and Margherita Rosati (National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, Md.). pJRFL, pADA, pBAL, p89.6, pSV-A-MLV-env, and pHXB2 were the gift of Daniel Littman and Vineet KewalRamani (New York University). The monoclonal antibodies (MAbs) 5C7 and 4G10 were gifts from L. Wu (Leukocyte, Inc., Cambridge, Mass.) and Chris Broder (U.S. Uniformed Health Services University, Bethesda, Md.). Other reagents were from Sigma (St. Louis, Mo.).
Cell culture.
Human osteosarcoma (HOS) cells that stably express CD4 as well as CXCR4 or CCR5 were obtained from the National Institutes of Health (NIH) AIDS Reagent Program. HOS cells which had also been transduced with a construct containing a humanized S65T mutant of the green fluorescent protein (GFP) under the inducible control of the HIV-2ROD long terminal repeat enhancer-promoter (9) (GHOST-X4, GHOST-R5, and GHOST-345), NIH 3T3-CD4/X4 and NIH 3T3-CD4/R5 cells, and 293T cells were the gift of Dan Littman and Vineet KewalRamani. HeLa cells were from John Silver (National Institute of Allergy and Infectious Diseases, Bethesda, Md.), and TF228 cells were from Zdenka L. Jonak (SmithKline Beecham, King of Prussia, Pa.). HeLa cells were grown in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (FBS) (D10). NIH 3T3-CD4/X4 and NIH 3T3-CD4/R5 were grown in D10 plus 3 μg of puromycin per ml. GHOST-X4, R5, and 345 cells were grown in D10 plus 500 μg of G418 per ml, 100 μg of hygromycin per ml, and 1 μg of puromycin per ml. All cells were grown in the presence of penicillin and streptomycin. HIV-1 envelope proteins were transiently expressed on the surface of HeLa cells using the recombinant vaccinia virus constructs vPE16 (IIIB; X4 utilizing) (16), vCB43 (Ba-L; R5 utilizing) (7), and vDC-1 (89.6; X4/R5 utilizing) (10), as described previously (23). Cells were grown for at least 7 days in medium containing PPMP before being used in a fusion assay. GHOST-X4, GHOST-R5, and GHOST-345 cells were grown in medium containing 10 μM PPMP; NIH 3T3-CD4/X4 and NIH 3T3-CD4/R5 cells were grown in medium containing 7.5 μM PPMP.
Infection assays.
Single-round infection assays using viral particles containing genomes with a defective env gene were conducted by the method of Cecilia et al. (9). Viral stocks were prepared by transfecting a plasmid containing the NL4-3 genome (pNL4-3Luc r−e−) (13), pBsTAT, and pCMVsREV along with a plasmid to supply the envelope in trans into 293T cells by using calcium phosphate. 293T is a highly transfectable subclone of the 293 cell line, which is itself an adenovirus type 5 DNA-transformed human kidney cell line. The plasmids that supplied the envelope in trans are as follows: the envelope glycoprotein of amphotropic murine leukemia virus (A-MLV) comes from pSV-A-MLV-env; the envelope glycoprotein of vesicular stomatitis virus (VSV-G) comes from pHCMV-G; HXB2 comes from pHXB2; 89.6 comes from p89.6; BAL comes from pBAL; ADA comes from pADA; JRFL comes from pJRFL; JRCSF comes from pJRCSF. The sources of the various plasmids are given above. After 48 h, the supernatant, containing HIV-1 particles with genomes derived from pNL4-3Luc r−e− and gp120-gp41 from the envelope plasmid, was harvested, sterile filtered, and added to GHOST cells plated on 12-well plates (1 × 104 to 2 × 104/well). In these cells, the very low basal expression of GFP is induced manyfold upon infection with HIV-1 or HIV-2. Three to four days after infection, the cells were examined using an IX70 inverted microscope (Olympus, New Hyde Park, N.Y.) with a 20× objective and a special GFP filter cube (exciter, HQ510/10X; dichroic mirror, Q5201p; emitter, HQ535/20M) (Chroma Technology, Brattleboro, Vt.). Infectivity was calculated as follows: % infectivity = 100 × (number of clusters of GFP-positive cells × average number of cells per GFP-positive cluster)/total number of cells observed. All infection assays used the following internal controls: mock infection, viral particles without env, viral particles with nonfusogenic env containing the V2E mutation (18), and, as a positive control, viral particles containing envelope glycoproteins from VSV and A-MLV.
HIV-1 envelope glycoprotein-mediated cell-cell fusion.
Target cells were labeled with the cytoplasmic dye 5- and 6-([(4-chloromethyl)benzoyl]-amino)tetramethylrhodamine (CMTMR) at 10 μM for 1 h at 37°C. When GSLs were added to the cells, labeling was performed before addition of GSLs to the cell surface. Envelope-expressing cells were labeled with 5 μM calcein AM for 1 h at 37°C. Calcein-labeled effector cells were cocultured with CMTMR-labeled target cells for 2 h at 37°C, and dye redistribution was monitored microscopically as described previously (35). The extent of fusion was calculated as: % fusion = 100 × number of bound cells positive for both dyes/number of bound cells positive for CMTMR. When fusion assays were performed on PPMP-treated cells, all media contained PPMP.
Extraction and analysis of cellular GSLs.
Total GSLs were extracted from cultured cells as described by Bligh and Dyer (6). Briefly, 107 GHOST-345 cells, suspended with trypsin-EDTA in phosphate-buffered saline (PBS) from Gibco-BRL (Gaithersburg, Md.), were pelleted at 450 × g for 5 min. The cell pellet was resuspended in 0.5 ml of H2O, which was added to 2 ml of CHCl3-CH3OH (2:1, vol/vol). After vortexing, 0.5 ml CHCl3 and 0.5 ml H2O were added, and the suspension was vortexed and centrifuged at 100 × g for 5 min to separate the two phases. The extract in the lower phase was then removed for storage, and the CHCl3-H2O step was repeated twice with the aqueous phase. Extracted GSLs were pooled, dried under N2, resuspended in 100 μl of CHCl3-CH3OH (2:1, vol/vol), and stored at −20°C until use. The total GSL composition of cells before and after treatment with PPMP was analyzed by thin-layer chromatography (TLC) developed in CHCl3–CH3OH–10% KCl(aq) (50:40:10, vol/vol/vol). At the end of the run, the plate was air dried, sprayed with resorcinol (24), heated at 100°C for 20 min to develop the spots, and scanned with a Fluor-S MultiImager (Bio-Rad, Hercules, Calif.).
Flow cytometry.
GHOST-345 cells, harvested with trypsin-EDTA in PBS, were centrifuged at 450 × g and resuspended at 106 cells/ml in PBS–5% FBS–5% normal mouse serum. After incubation for 15 min at room temperature, the cells were washed twice in PBS–0.1% bovine serum albumin and resuspended at 107 cells/ml (in 100 μl) in PBS–5% FBS–5% normal mouse serum. Phycoerythrin (PE)-conjugated mouse immunoglobulin G (IgG) anti-CD4 (RPA-T4), PE-conjugated mouse IgG anti-CXCR4 (12G5), or PE-conjugated mouse IgG anti-CCR5 (2D7) from Pharmingen (San Diego, Calif.) was then added to each sample at a 1:5 dilution. Cells were incubated at 4°C for 1 hour and washed twice in PBS–0.1% BSA. Samples were fixed in PBS–1% paraformaldehyde and resuspended in 1 ml of PBS to be read by a FACScalibur instrument (Becton Dickinson, San Jose, Calif.) at 10,000 events/sample with respect to unlabeled cells.
CD4-chemokine receptor association.
Immunoprecipitation was done by a previously reported procedure (47) with some modifications. Briefly, untreated and PPMP-treated NIH 3T3-CD4/X4 or NIH 3T3-CD4/R5 cells were collected and washed with PBS once. The cells were suspended in ice-cold PBS at a final density of 5 × 106/ml. A 1-ml volume of the cell suspension was used for one immunoprecipitation sample. The CD4-CCR5 complexes were immunoprecipitated by the anti-CCR5 MaB, 5C7, and CD4-CXCR4 complexes were immunoprecipitated by the anti-CXCR4 MaB, 4G10. Antibodies were added to the cell suspension at a final concentration of 3 μg/ml and incubated with gentle mixing for 4 h at 4°C. Cells were then collected by centrifugation and lysed using a lysis buffer (47). After 40 min of incubation with gentle mixing, the supernatant was obtained by centrifugation at top speed for 25 min in a refrigerated Eppendorf centrifuge. A 10-μl sample of protein G-Sepharose beads (Sigma) prewashed with PBS was added to each sample, and the samples were incubated at 4°C for 14 h. The protein G-Sepharose beads were then washed four times, each with 1 ml of ice-cold lysis buffer. Samples were then eluted by adding 4× sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and boiled for 5 min. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide), SDS-PAGE and Western blotting was performed using the Supersignal chemiluminescent substrate from Pierce (Rockford, Ill.).
Chemotaxis assay.
The migration of HOS-CD4/X4 and HOS-CD4/R5 cells was assessed by a 48-well microchamber technique (3). Different concentrations of SDF-1α or MIP-1β (Peprotech) were placed in the lower wells of the chamber. The HOS cells (50 μl, 106/ml) were loaded in the upper wells. The lower and upper wells were separated by a polycarbonate filter (PVPF; pore size, 10 μm; Poretics) precoated with 50 μg of collagen type 1 per ml for 2 h at 37°C. The chamber was incubated at 37°C for 5 h in humidified air with 5% CO2. At the end of the incubation, after removal of nonmigrating cells, the filter was fixed and stained with Diff-Quik (Biochemical Sciences). Using three high-power fields under light microscopy, the cells migrating across the filter were counted in triplicate with all samples coded. The chemotaxis index was calculated as follows: chemotaxis index = number of cells migrating to chemokines/number of cells migrating to medium. The significance of the difference between test and control groups was analyzed by a paired Student t test.
Measurement of CD4- and CXCR4-induced conformational changes.
Fluorescence changes of the hydrophobicity-sensitive dye 4,4-dianilino-1,1-binaphthyl-5,5-disulfonic acid (bis-ANS), resulting from conformational changes in gp120-gp41 were monitored by a procedure described previously (23) with some modifications. Untreated and PPMP-treated HeLaCD4 cells were plated on 35-mm dishes with coverslip cutouts in the center. TF228 cells, which constitutively express the X4-utilizing (IIIB) gp120-gp41, were labeled with calcein-AM as described above and then added to the culture dish. bis-ANS was added at 2 μg/ml to culture medium without serum. Once the TF228 cells were touching the target cells as determined by bright-field and calcein fluorescence, images were recorded using the quantitative light microscopy setup described previously (23). The fluorescence intensity averaged from regions of interest drawn around individual gp120-gp41-expressing cells was monitored at 37°C as a function of time following contact of effector and target cells.
Addition of GSLs to CD4+ cells.
The addition of GSLs to the plasma membrane of cells was performed as described previously (35). Briefly, liposomes containing Egg phosphatidylcholine:Egg phosphatidylethanolamine: GSL (3:1.5:1, wt/wt) were prepared in PBS (Ca and Mg free) by extrusion through a 0.2-μm-pore-size filter using an extruder from Lipex Biomembranes (Vancouver, Canada) to a final lipid concentration of 0.9 mg/ml. Target cells plated on 35-mm dishes with coverslip cutouts in the center (at 5 × 104/dish) were infected with the recombinant influenza virus strain X-31 (H3N2) (34) overnight at 37°C. Target cells were treated with 5 μg of trypsin per ml for 5 min at room temperature to activate the hemagglutinin (HA) on the cell surface. Liposomes were allowed to bind to the HA-expressing target cells for 30 min at room temperature. Liposome-cell fusion was induced by a 60-s exposure of the cells to pH 5.2, followed by incubation in D10 (pH 7.4) for 30 min at room temperature. The modified cells were then used as targets in the cell-cell fusion assays described above.
RESULTS
Inhibition of GSL biosynthesis blocks target cell susceptibility to HIV-1 infectivity by a broad variety of HIV-1 isolates.
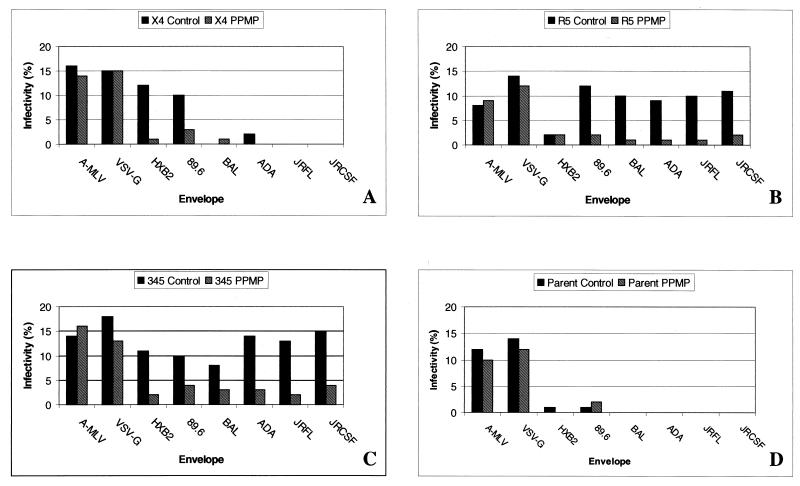
The synthesis of most GSLs begins with glucosylation of ceramide to form glucosylceramide (GlcCer), the precursor for hundreds of different GSLs (22). This cerebroside is synthesized from UDP-glucose and ceramide by the glucosyltransferase GlcCer synthase. One way to better understand the functions of GSLs is to selectively inhibit cellular GlcCer formation. Abe et al. have synthesized a variety of specific inhibitors of GlcCer synthase (1). We have used one of these, PPMP, in our examination of the role of GSLs in HIV-1 entry. Previously, we had shown that inhibition of GSL biosynthesis in HeLaCD4 and SupT1 cells by treatment with PPMP reduced their susceptibility to CXCR4-dependent HIV-1 fusion (35, 37). To examine susceptibility to HIV-1 infection, we used GHOST(3) cell lines, which constitute an HIV-1 or HIV-2 indicator cell panel whose individual lines were engineered to express a Tat-dependent GFP reporter cassette in conjunction with CD4 and a specific chemokine receptor (9). To examine the role of GSL in HIV-1 infection, we used env-complemented HIV-1 pseudotypes and CD4+ target cells that stably express CXCR4 (GHOST-X4), CCR5 (GHOST-R5), or CCR3, CXCR4, and CCR5 (GHOST-345). HIV-1 infection resulted in GFP expression, which was monitored by counting stained cells in the fluorescence microscope. Figure 1 shows the results for the pseudotypes containing env expression vectors for HIV-1 HXB2, 89.6, ADA, BaL, JRFL, and JRCSF. In all cases, treatment of GHOST cells with PPMP inhibited HIV-1 infection. Treatment of cells with PPMP did not affect the entry of viruses pseudotyped with the envelope glycoprotein of VSV or A-MLV.
FIG. 1.
Effect of GSL depletion on HIV-1 infection. HIV-1 particles containing the NL4-3r−e− genome and having gp120-gp41 derived from different isolates were prepared as described in Materials and Methods. GHOST-X4 (A), GHOST-R5 (B), GHOST-345 (C), and GHOST(3) parent cells expressing CD4 only (D) were grown for at least 7 days prior to the assay in medium containing 10 μM PPMP. Cells were plated in 12-well plates (1.5 × 104 cells/well), and medium containing viral particles was added. Four days after infection, the cells were examined microscopically for GFP expression as described in Materials and Methods. The percent infectivity = 100 × (number of clusters of GFP-positive cells × average number of cells per GFP-positive cluster)/total number of cells observed. Three separate experiments yielded the same results.
Effect of PPMP treatment on target cell lipid biosynthesis and cell surface receptor expression.
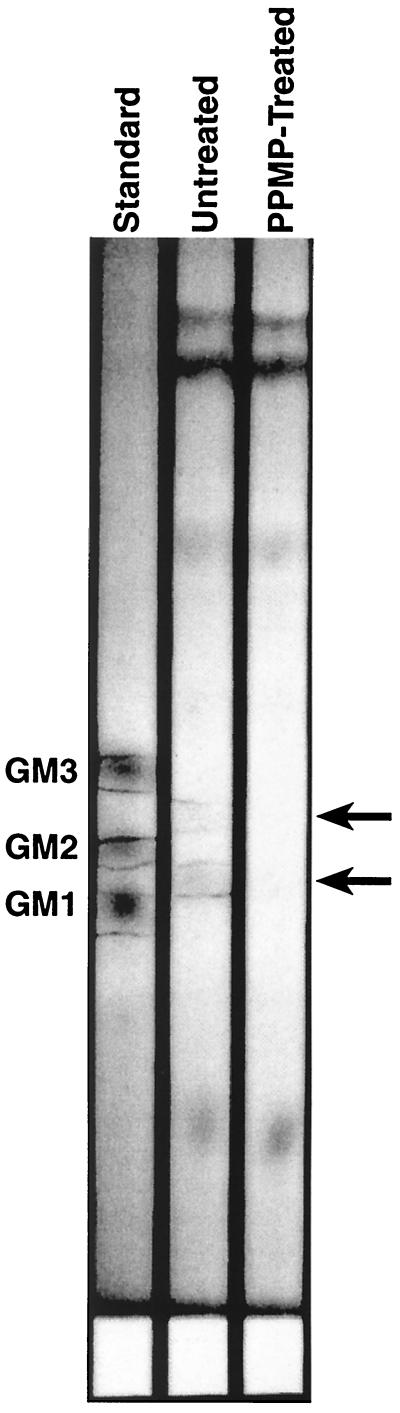
Figure 2 shows thin-layer chromatograms of GSLs isolated from GHOST-345 cells that were either untreated or treated for at least 7 days in culture medium containing 10 μM PPMP as well as monosialoganglioside standard. The bands, indicative of GSLs interacting with resorcinol, are outlined in the standard and untreated lanes. The shift in alignment of cell-derived GSLs with the standards is most probably because the standards and the cell-derived GSLs are purified from different species. GSLs with equivalent head groups have very different acyl chain distributions, which will cause them to run differently in a chromatogram. However, it is clear from the TLC results that the PPMP treatment resulted in a marked reduction of GSLs in these cells. Phospholipid and cholesterol compositions were unchanged following PPMP treatment (data not shown).
FIG. 2.
TLC of GSL levels in control and PPMP-treated cells. GSLs were isolated as described in Materials and Methods from GHOST-345 cells that were either untreated or treated for at least 7 days in culture medium containing 10 μM PPMP. Monosialoganglioside standard (10 μg), containing GM3, GM2, and GM1, and lipid extracts from 5 × 106 cells were spotted onto a 20- by 20-cm silica gel TLC plate (Fisher, Malvern, Pa.), which was developed in CHCl3–CH3OH–10% KCl(aq) (50:40:10, vol/vol/vol). After being run, the plate was air dried, sprayed with resorcinol, and heated to develop the spots. Images were taken on a Fluor-S MultiImager using white light epi-illumination. Purple bands, indicative of GSLs interacting with resorcinol, are outlined in the standard and untreated lanes. We have quantified the extent of GSL depletion by determining the integrated grey levels from regions of interest of the Fluor-S MultiImager scans corresponding to the arrows. For the top arrow, the integrated grey levels are 77,806 and 32,295 in lanes 2 and 3, respectively, and for the bottom arrow, they are 120,063 and 38,531 in lanes 2 and 3, respectively.
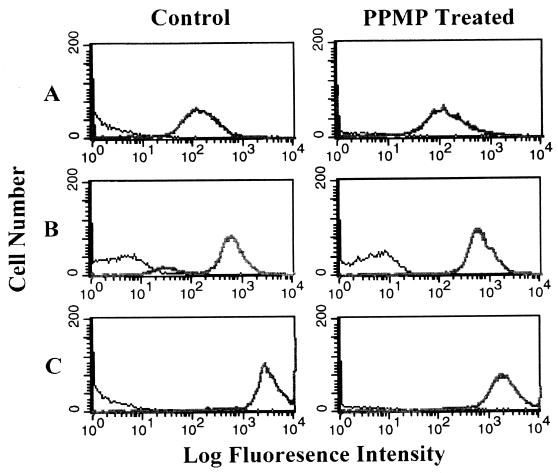
Figure 3 shows the cell surface expression levels of CD4, CXCR4, and CCR5 on control and PPMP-treated cells. Although there was no significant change in cell surface expression of CD4 or CXCR4, the level of CCR5 expression was reduced to about 60% of control following treatment of these cells with PPMP.
FIG. 3.
Flow cytometry of CD4 and chemokine receptor expression in control and PPMP-treated cells. Expression levels of CD4, CXCR4, and CCR5 in GHOST-345 cells were examined by using control (left panel) and PPMP-treated (right panel) cells. Cells were incubated for 1 h at 4°C with PE-conjugated mouse IgG anti-CD4 (RPA-T4) (A), PE-conjugated mouse IgG anti-CXCR4 (12G5) (B), or PE-conjugated mouse IgG anti-CCR5 (2D7) (C). Fluorescence was examined with a Becton Dickinson FACScalibur at 10,000 events/sample. Control and PPMP-treated unlabeled cells were run as background. The surface concentrations of CD4, CXCR4, and CCR5 estimated from the observed median fluorescence intensities relative to median intensities for cells with known amounts of those receptors bound to their specific antibodies are about 5.2 × 104, 2.3 × 105, and 1.1 × 106 molecules/cell, respectively.
Effect of PPMP treatment on the physiology of target cells.
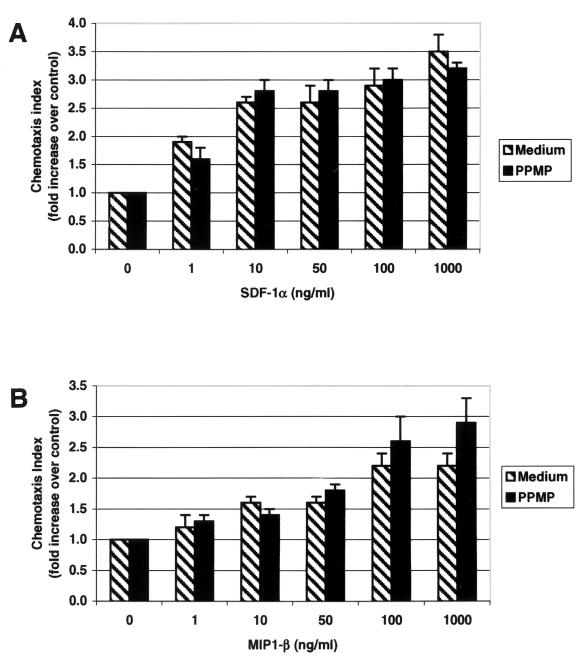
Chemokines and their seven-transmembrane-domain G-protein-coupled receptors constitute a large and highly differentiated signaling system involved in many biological processes, including development, hematopoiesis, angiogenesis, and regulation of specific leukocyte trafficking (2). The activity of the chemokine receptors has been examined by monitoring chemotaxis in response to specific ligands. Figure 4 shows chemotaxis of HOS-CD4/X4 cells in response to SDF1-α, the ligand for CXCR4, and of HOS-CD4/R5 in response to MIP1-β, a ligand for CCR5. Treatment of these cells with PPMP, which inhibited HIV-1 entry, did not affect their ability to respond to SDF1-α or MIP1-β. The ability of SDF1-α to trigger Ca2+ mobilization in the HOS-CD4/X4 cells and of MIP1-β to trigger Ca2+ mobilization in the HOS-CD4/R5 cells was also unaffected by pretreatment with PPMP (data not shown).
FIG. 4.
Migration of HOS-CD4 cells expressing X4 or R5 in response to chemokines. Different concentrations of SDF-1α (A) or MIP1-β (B) were placed in the lower wells of the chemotaxis chamber, and HOS-CD4/X4 (A) or HOS-CD4/R5 (B) cells were placed in the upper wells; the upper and lower wells were separated by a polycarbonate filter. The results are expressed as a chemotaxis index (CI), representing the fold increase of migrating cells in response to SDF-1α over the response to control medium. Significant cell migration (P < 0.05) was detected with ≥10 ng of chemoattractant per ml.
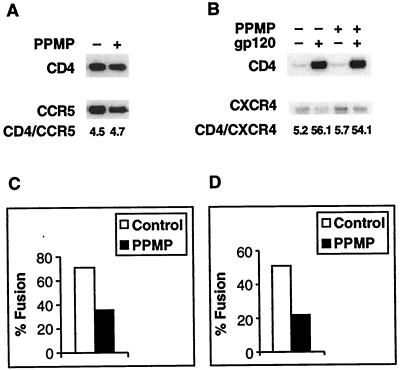
Effect of GSLs on the association between CD4 and CXCR4 or CCR5.
It has been demonstrated that CXCR4 may directly associate with the complex between CD4 and the HIV-1 envelope glycoprotein, suggesting that the complex between these three molecules plays a critical role in the initial stages of the entry process (25). More recently, it has been shown that cell surface CD4 associates with CCR5 in the absence of gp120 or other chemokine receptor- or CD4-specific ligands and that there is a functional correlation between this association and HIV-1 envelope glycoprotein-mediated fusion (47). Since these molecules may be associated in membrane domains, we examined whether GSLs are involved in this association. We used NIH 3T3-CD4/X4 and NIH 3T3-CD4/R5 for these experiments because coimmunoprecipitation of CD4 with CXCR4 or CCR5 in these cells yielded a better signal in Western blots. Figure 5 shows that treatment of NIH 3T3-CD4/X4 and NIH 3T3-CD4/R5 cells with PPMP for 7 days reduced fusion yields with cells expressing HIV-1 gp120-gp41 of the appropriate specificity. The coimmunoprecipitation data show that treatment of these cells with PPMP had no effect on the association of CD4 with CXCR4 or CCR5. Moreover, treatment with PPMP did not affect the amount of gp120-induced coimmunoprecipitation of CD4 and CXCR4. This indicates that the formation of the trimolecular gp120-CD4-CXCR4 complex, which presumably occurs at an early stage in the fusion cascade (25), is not dependent on the presence of GSLs in the target membrane.
FIG. 5.
Effect of GSL depletion on the CD4-chemokine receptor association. (A) CD4-CCR5 association. 3T3-CD4-CCR5 cells treated (+) and not treated (−) with PPMP were solubilized, and CD4 was isolated by coimmunoprecipitation by the anti-CCR5 antibody 5C7. The gels shown are Western blots obtained with rabbit anti-CD4 and goat anti-CCR5 antibody. The numbers below the gels represent intensity ratios determined using a Molecular Imager (Bio-Rad). (B) CD4-CXCR4-gp120 association. 3T3-CD4-CXCR4 cells treated (+) and not treated (−) with PPMP were solubilized, and CD4 was isolated by coimmunoprecipitation by anti-CXCR4 antibody 4G10 in the presence (+) or absence (−) of rgp120IIIB. The gels shown are Western blots obtained with rabbit anti-CD4 and mouse anti-CXCR4 antibody. The numbers below the gels represent intensity ratios determined using a Molecular Imager. (C) Inhibition of fusion with cells expressing the R5-utilizing envelope glycoprotein (Ba-L). (D) Inhibition of fusion with cells expressing the X4-utilizing envelope glycoprotein (IIIB).
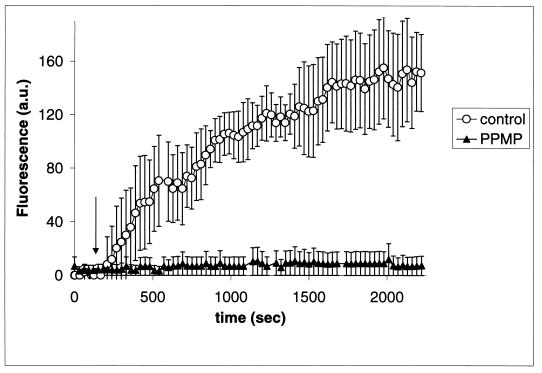
Effect of PPMP treatment on CD4 and chemokine receptor-induced conformational changes in Env.
In a previous study, we continuously monitored conformational changes of cell surface-expressed HIV-1 gp120-gp41 in situ using bis-ANS, a fluorescent probe that binds to hydrophobic groups (23). These conformational changes, which lead to membrane fusion, are highly cooperative, requiring both CD4 and an appropriate chemokine receptor. We have used the bis-ANS technique to monitor the interactions between gp120-gp41-expressing cells and CD4+ CXCR4+ target cells, which are GSL depleted and fusion incompetent. Figure 6 shows that the GSL-depleted cells failed to produce a response in the bis-ANS assay. These observations, taken together with the results of the coimmunoprecipitation experiments (Fig. 5), demonstrate that while the GSLs have no effect on the intrinsic associations between individual molecules of CD4 and CXCR4 or CCR5, they are necessary to trigger the supramolecular associations and massive conformational changes in the envelope glycoprotein required for membrane fusion.
FIG. 6.
Kinetics of exposure of hydrophobic binding sites upon addition of HeLaCD4 cells to gp120-gp41IIIB-expressing TF228 cells. The average changes in the bis-ANS fluorescence intensity of 7 to 13 individual cells are shown against time following addition of the TF228 cells. The arrow indicates the time at which the effector cells made contact with the target cells.
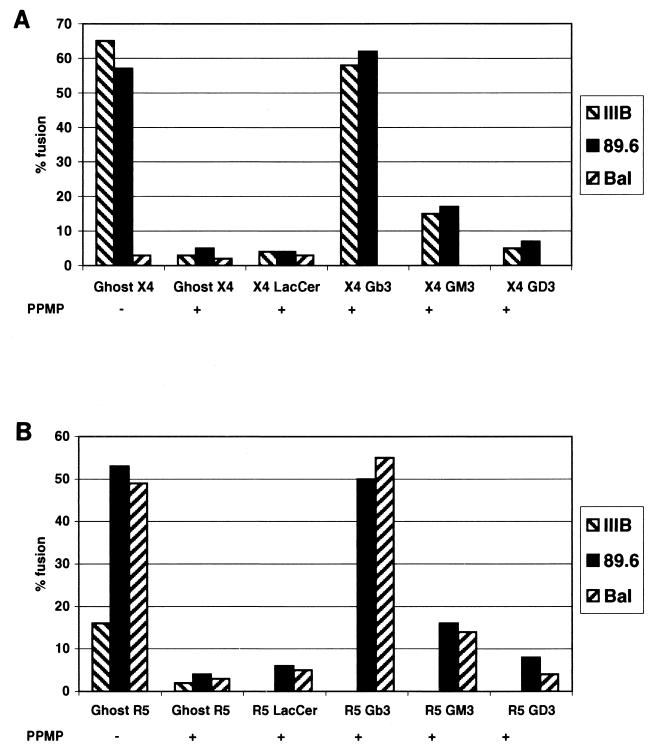
Recovery of fusion activity of GSL-depleted cells following reconstitution with purified GSLs.
Purified GSLs were reconstituted into PPMP-treated cells by influenza virus HA-mediated fusion of liposomes containing a specific GSL with the target cells. It has previously been shown that addition of exogenous GM1 (31) or GM3 (19) to target cells leads to down-regulation of CD4 and, for GM1, to inhibition of HIV-1 infectivity (12). However, control experiments with untreated cells showed no change in cell surface CD4 expression or susceptibility to HIV-1 envelope glycoprotein-mediated fusion following incorporation of GSLs using our method (data not shown). We had previously reported that Gb3 was the most potent GSL in its ability to restore fusion activity with cells expressing the CXCR4-utilizing envelope glycoprotein (35). To examine possible strain specificity of Gb3, we tested various isolates for their ability to fuse with GSL-depleted GHOST-X4 or R5 cells. Figure 7 shows the specificity of these cells for the envelope glycoproteins of their respective HIV-1 isolates and their inhibition by pretreatment of the target cells with PPMP. Although Gb3 appeared to be the most potent in restoring the fusion activity of CXCR4 and/or CCR5-utilizing envelope glycoproteins, some enhanced activity over background was also seen upon reconstitution with GM3.
FIG. 7.
Recovery of HIV-1 fusion activity by reconstitution of PPMP-treated cells with GSLs. Lipids were incorporated into liposomes and transferred to control and PPMP-treated GHOST-X4 and GHOST-R5 cells as described in Materials and Methods. Fusion activity was monitored using vaccinia virus vectors which express gp120-gp41 from an X4-utilizing isolate (IIIB, vpE16), an R5-utilizing isolate (Ba-L, vcB43), and an X4R5-utilizing isolate (89.6, vDC-1) in HeLa cells. (A) GHOST-X4 cells as targets; (B) GHOST-R5 cells as targets. PPMP (−) GHOST-X4 and GHOST-R5 are the untreated cells; PPMP (+) GHOST-X4 and GHOST-R5 are the PPMP-treated cells without addition of GSL; PPMP (+) GHOST-X4 and GHOST-R5 + GSL are the PPMP-treated cells with addition of the indicated GSL.
DISCUSSION
In this study we demonstrated that GSLs are involved in the entry of a broad range of HIV-1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. We discovered this result by inhibiting the synthesis of GlcCer, the precursor for a plethora of different GSLs (22). GlcCer-based sphingolipids have been identified as important mediators of a variety of cellular functions, including proliferation, differentiation, development, and cell-cell recognition (20). We show that cell lines cultured in the presence of PPMP, a competitive inhibitor of GlcCer synthase (1), exhibit a reduction in overall GSL content (Fig. 2). The inhibition of GSL biosynthesis did not affect cell surface expression of CD4 or CXCR4 but caused a slight decrease in the level of CCR5 expression. Since it has been shown that the susceptibility of cells with high surface concentrations of CD4 and CCR5 to infection by HIV-1 R5 isolates is independent of CCR5 levels (33), the observed inhibition of infectivity of R5 isolates (Fig. 1) by PPMP treatment is not caused by the observed reduction of CCR5 levels. Because PPMP is very specific for GlcCer synthase (1), it is unlikely that it affects the cell surface complement of glycosoaminoglycans or other adhesion factors which are known to play important roles in virus-cell attachment prior to initiation of the fusion reaction (44). This specificity also implies that there are no changes in tyrosine sulfation of the N-terminal domains of CCR5, which recently has been shown to facilitate HIV-1 entry (17). Our observation that the susceptibility of PPMP-treated cells to fuse with cells expressing R5 envelope glycoprotein can be completely recovered following reconstitution with purified GSLs (see Fig. 7) indicates that neither the reduced level of CCR5, nor a possible modification of tyrosine sulfation of CCR5, nor a change in the cell surface complement of glycosoaminoglycans or other adhesion factors is a probable cause of the fusion inhibition. Moreover, signaling of these cells via chemokine receptors was not altered by inhibition of GSL biosynthesis (Fig. 4).
Although formation of the trimolecular gp120-CD4-chemokine receptor complex was not affected by PPMP treatment (Fig. 5), lack of GSLs on the target membrane did block the massive conformational changes in gp120-gp41 which result from specific interactions between gp120-gp41, CD4, and chemokine receptor (Fig. 6). Inhibition of GSL biosynthesis in the target membrane reduced fusion activity with env-expressing cells (Fig. 7), as well as the infection by HIV-1 from a variety of isolates (Fig. 1). PPMP treatment of envelope glycoprotein-expressing cells did not affect their subsequent fusion with appropriate (untreated) target cells (data not shown), indicating that the GSL effect is unidirectional. Inhibition of GSL biosynthesis did not affect the entry of virus pseudotyped with the envelope glycoproteins from VSV or A-MLV (Fig. 1). Moreover, HIV-2 envelope glycoprotein-mediated fusion is not affected by treatment of target cells with PPMP (37). Although alphaviruses depend on target cell sphingolipids (and cholesterol) for their entry into the cells (30, 41) while paramyxoviruses and orthomyxoviruses (39, 43) depend on specific gangliosides, the combination of GSLs with receptors and coreceptors to form a fusion complex appears to be quite unique to HIV-1 entry.
We found that Gb3 ≫ GM3 > GD3 in their ability to restore the fusion activity of all HIV-1 isolates tested. In contrast, Hammache et al. (21) reconstituted monolayers of purified GSLs at the air-water interface and observed that Gb3 was stronger than GM3 in its interaction with the X4 gp120 whereas GM3 interacted preferentially with the X4R5 gp120. In T lymphocytes, the monosialoganglioside GM3 represents the main ganglioside constituent of the plasma membrane (72% of total gangliosides); Gb3 is not detectable (data not shown). Nevertheless, HIV-1 envelope glycoprotein-mediated cell fusion is inhibited following PPMP treatment of SupT1 cells (a T-cell line) and other cell lines normally devoid of Gb3. Although addition of Gb3 to GSL-depleted cells results in fusion recovery, even in backgrounds where Gb3 is normally absent, other glycosphingolipids, presumably GM3, may fulfill the necessary role in mediating HIV-1 fusion.
How could GSLs play a role in HIV-1 entry? Recent studies suggest that sphingolipid-rich and cholesterol-rich domains may exist as phase-separated “rafts” in the membrane, which serve as sites enriched in signal transduction assemblies (8, 40). According to a model proposed by Hammache et al. (21), the GSLs recognized by both CD4 and gp120 induce the formation of a trimolecular complex of CD4, GSL, and gp120 in such rafts. The observation that CD4 is found in GM3-enriched domains on the lymphocyte plasma membrane (27, 42) is consistent with this hypothesis. Typically GSLs exhibit long saturated acyl chains, which are thought to drive self-assembly with cholesterol to form liquid ordered domains capable of organizing membrane proteins such as CD4, CXCR4, and CCR5. Although PPMP inhibits the assembly of the oligosaccharide head group in GSLs, the cells are not depleted of ceramide, which may continue to function in domain formation (32). Consequently, domain formation is not sufficient for GSL function in HIV-1 entry. Rather, a Gb3-like oligosaccharide head group acts specifically with CD4 and CCR5 or CXCR4 and gp120-gp41 to facilitate membrane fusion. These complexes are presumably enriched in rafts, leading to a higher local concentration within a cell surface microdomain. In the absence of GSLs, the trimolecular gp120-CD4-chemokine receptor complexes are still formed (Fig. 5) but the massive conformational changes required for fusion do not occur (Fig. 6). We hypothesize that secondary interactions between the V3 loop of gp120 and the polar heads of GSL molecules lead to the conformational changes in gp120-gp41 that allow the dissociation of gp120 from gp41. This step enables gp41 to form the viral hairpin (11, 45) and promotes assembly of the gp41 molecules into the fusion complex. Further experiments are needed to test the validity of this hypothesis.
ACKNOWLEDGMENTS
We are grateful to G. Pavlakis, M. Rosati, L. Wu, C. Broder, Z. Jonak, V. KewalRamani, D. Littman, J. Silver, and the NIH AIDS Reference and Reagent Program for supplying cell lines and reagents. We also thank Alicia Mazouat and Wang-Hua Gong for technical assistance.
REFERENCES
- 1.Abe A, Inokuchi J, Jimbo M, Shimeno H, Nagamatsu A, Shayman J A, Shukla G S, Radin N S. Improved inhibitors of glucosylceramide synthase. J Biochem. 1992;111:191–196. doi: 10.1093/oxfordjournals.jbchem.a123736. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Baruch A, Xu L, Young P R, Bengali K, Oppenheim J J, Wang J M. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J Biol Chem. 1995;270:22123–22128. doi: 10.1074/jbc.270.38.22123. [DOI] [PubMed] [Google Scholar]
- 4.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 5.Berson J F, Doms R W. Structure-function studies of the HIV-1 coreceptors. Semin Immunol. 1998;10:237–248. doi: 10.1006/smim.1998.0130. [DOI] [PubMed] [Google Scholar]
- 6.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Canadian J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Broder C C, Berger E A. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc Natl Acad Sci USA. 1995;92:9004–9008. doi: 10.1073/pnas.92.19.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown D A, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Cecilia D, KewalRamani V N, O'Leary J, Volsky B, Nyambi P, Burda S, Xu S, Littman D R, Zolla-Pazner S. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J Virol. 1998;72:6988–6996. doi: 10.1128/jvi.72.9.6988-6996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabot D J, Zhang P F, Quinnan G V, Broder C C. Mutagenesis of CXCR4 identifies important domains for human immunodeficiency virus type 1 X4 isolate envelope-mediated membrane fusion and virus entry and reveals cryptic coreceptor activity for R5 isolates. J Virol. 1999;73:6598–6609. doi: 10.1128/jvi.73.8.6598-6609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 12.Chieco-Bianchi L, Calabro M L, Panozzo M, De Rossi A, Amadori A, Callegaro L, Siccardi A. CD4 modulation and inhibition of HIV-1 infectivity induced by monosialoganglioside GM1 in vitro. AIDS. 1989;3:501–507. doi: 10.1097/00002030-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov D S. How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity. Cell. 1997;91:721–730. doi: 10.1016/s0092-8674(00)80460-2. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Picard L, Alizon M. Proteinase-resistant factors in human erythrocyte membranes mediate CD4-dependent fusion with cells expressing human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1995;69:1013–1018. doi: 10.1128/jvi.69.2.1013-1018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earl P L, Koenig S, Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991;65:31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 18.Freed E O, Delwart E L, Buchschacher G L, Jr, Panganiban A T. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci USA. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garofalo T, Sorice M, Misasi R, Cinque B, Giammatteo M, Pontieri G M, Cifone M G, Pavan A. A novel mechanism of CD4 down-modulation induced by monosialoganglioside GM3. Involvement of serine phosphorylation and protein kinase c delta translocation. J Biol Chem. 1998;273:35153–35160. doi: 10.1074/jbc.273.52.35153. [DOI] [PubMed] [Google Scholar]
- 20.Hakomori S. New insights in glycosphingolipid function: “glycosignaling domain,” a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology. 1998;8:xi–xix. doi: 10.1093/oxfordjournals.glycob.a018822. [DOI] [PubMed] [Google Scholar]
- 21.Hammache D, Yahi N, Maresca M, Pieroni G, Fantini J. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3) J Virol. 1999;73:5244–5248. doi: 10.1128/jvi.73.6.5244-5248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikawa S, Hirabayashi Y. Glucosylceramide synthase and glycosphingolipid synthesis. Trends Cell Biol. 1998;8:198–202. doi: 10.1016/s0962-8924(98)01249-5. [DOI] [PubMed] [Google Scholar]
- 23.Jones P L, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. doi: 10.1074/jbc.273.1.404. [DOI] [PubMed] [Google Scholar]
- 24.Kundu S K. Thin-layer chromatography of neutral glycosphingolipids and gangliosides. Methods Enzymol. 1981;72:185–204. doi: 10.1016/s0076-6879(81)72012-3. [DOI] [PubMed] [Google Scholar]
- 25.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 26.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 27.Millan J, Cerny J, Horejsi V, Alonso M A. CD4 segregates into specific detergent-resistant T-cell membrane microdomains. Tissue Antigens. 1999;53:33–40. doi: 10.1034/j.1399-0039.1999.530104.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieva J L, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Offner H, Thieme T, Vandenbark A A. Gangliosides induce selective modulation of CD4 from helper T lymphocytes. J Immunol. 1987;139:3295–3305. [PubMed] [Google Scholar]
- 32.Ostermeyer A G, Beckrich B T, Ivarson K A, Grove K E, Brown D A. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. Methyl-beta-cyclodextrin does not affect cell surface transport of a GPI-anchored protein. J Biol Chem. 1999;274:34459–34466. doi: 10.1074/jbc.274.48.34459. [DOI] [PubMed] [Google Scholar]
- 33.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophage tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri A, Booy F, Doms R W, White J M, Blumenthal R. Conformational changes and fusion activity of influenza hemagglutinin of the H2 and H3 subtypes: effects of acid pretreatment. J Virol. 1990;64:3824–3832. doi: 10.1128/jvi.64.8.3824-3832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puri A, Hug P, Jernigan K, Barchi J, Kim H Y, Hamilton J, Wiels J, Murray G J, Brady R O, Blumenthal R. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1998;95:14435–14440. doi: 10.1073/pnas.95.24.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puri A, Hug P, Jernigan K, Rose P, Blumenthal R. Role of glycosphingolipids in HIV-1 entry: requirement of globotriaosylceramide (Gb3) in CD4/CXCR4-dependent fusion. Biosci Rep. 1999;19:317–325. doi: 10.1023/a:1020554509642. [DOI] [PubMed] [Google Scholar]
- 37.Puri A, Hug P, Munoz-Barroso I, Blumenthal R. Human erythrocyte glycolipids promote HIV-1 envelope glycoprotein-mediated fusion of CD4+ cells. Biochem Biophys Res Commun. 1998;242:219–225. doi: 10.1006/bbrc.1997.7941. [DOI] [PubMed] [Google Scholar]
- 38.Puri A, Morris S J, Jones P, Ryan M, Blumenthal R. Heat-resistant factors in human erythrocyte membranes mediate CD4-dependent fusion with cells expressing HIV-1 envelope glycoproteins. Virology. 1996;219:262–267. doi: 10.1006/viro.1996.0244. [DOI] [PubMed] [Google Scholar]
- 39.Rogers G N, Paulson J C, Daniels R S, Skehel J J, Wilson I A, Wiley D C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 40.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 41.Smit J M, Bittman R, Wilschut J. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J Virol. 1999;73:8476–8484. doi: 10.1128/jvi.73.10.8476-8484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorice M, Parolini I, Sansolini T, Garofalo T, Dolo V, Sargiacomo M, Tai T, Peschle C, Torrisi M R, Pavan A. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J Lipid Res. 1997;38:969–980. [PubMed] [Google Scholar]
- 43.Suzuki Y, Suzuki T, Matsunaga M, Matsumoto M. Gangliosides as paramyxovirus receptor. Structural requirement of sialo-oligosaccharides in receptors for hemagglutinating virus of Japan (Sendai virus) and Newcastle disease virus. J Biochem. 1985;97:1189–1199. doi: 10.1093/oxfordjournals.jbchem.a135164. [DOI] [PubMed] [Google Scholar]
- 44.Ugolini S, Mondor I, Sattentau Q J. HIV-1 attachment: another look. Trends Microbiol. 1999;7:144–149. doi: 10.1016/s0966-842x(99)01474-2. [DOI] [PubMed] [Google Scholar]
- 45.Weissenhorn W, Dessen A, Calder L J, Harrison S C, Skehel J J, Wiley D C. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 46.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 47.Xiao X, Wu L, Stantchev T S, Feng Y R, Ugolini S, Chen H, Shen Z, Riley J L, Broder C C, Sattentau Q J, Dimitrov D S. Constitutive cell surface association between CD4 and CCR5. Proc Natl Acad Sci USA. 1999;96:7496–7501. doi: 10.1073/pnas.96.13.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]