Abstract
An extensive literature shows that race information can impact cognitive performance. Two key findings include an attentional bias to Black racial cues in U.S. samples and diminished recognition of other-race faces compared to same-race faces in predominantly White adult samples. Yet face stimuli are increasingly used in psychological research often unrelated to race (Conley et al., 2018) or without consideration for how race information may influence cognitive performance, especially among developmental participants from different racial groups. In the current study we used open-access data from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study® 4.0.1 release to test for developmentally similar other- and same-race effects of Black and White face stimuli on attention, working memory, and recognition memory in 9- and 10-year-old Black and White children (n=5,659) living in the U.S. Black and White children showed better performance when attending to Black versus White faces. We also show an advantage in recognition memory of same-race compared to other-race faces in White children that did not generalize to Black children. Together the findings highlight how race information, even when irrelevant to an experiment, may indirectly lead to misinterpretation of group differences in cognitive performance in children of different racial backgrounds.
Keywords: Attention, Children, Cognition, Memory, Racial bias
1. Introduction
Race is a social construct that can influence how we perceive and respond to others (e.g., Goff et al., 2008; Hetey and Eberhardt, 2014; Hughes et al., 2019; Kubota and Ito, 2017). Social categorization based on racial group membership is thought to be the byproduct of evolutionary adaptations originally intended to identify those who may be allies or adversaries (Cosmides et al., 2003, Tajfel, 1982), and has been shown to be present early in development (Baron and Banaji, 2006, Newheiser and Olson, 2012). Social categorization can be based on any number of attributes, including age, gender, nationality, and religion (Tajfel, 1978, Tajfel and Turner, 1979). However, in the United States, race has historically been and remains a salient and culturally relevant basis for social categorization, often judged based on readily observable physical features (e.g., skin tone, hair texture) (Maddox and Chase, 2004, Maddox and Gray, 2002; but also see Richeson and Sommers, 2016). Indeed, people in the U.S. often automatically categorize others into racial groups and use the associated, relevant category information to prepare to respond and/or interact with them (Allport, 1954, Dovidio et al., 1986, Fiske and Neuberg, 1990, Macrae and Bodenhausen, 2000). In the current study, we test for: 1) developmentally similar effects of Black and White race information on cognitive performance in White youth as previously observed in White adults; and 2) whether these effects generalize to Black youth in the large open-access ABCD study that is following brain and behavioral development in children in the U.S.
Perhaps not surprisingly, there is a large psychological literature showing that race-related information can significantly impact cognition and behavior (see review Amodio, 2014). Faces, which portray racial and other social categories as well as state information (e.g., gender, age, emotions), are frequently used as stimuli in psychological research, with over 15,000 studies reported in 2018 alone (Conley et al., 2018) and in rapidly emerging, large open access datasets [e.g., ABCD Study® (Casey et al., 2018), Human Connectome Project (HCP) (Barch et al., 2013), and United Kingdom (UK) Biobank (Miller et al., 2016)]. However, most studies that use these face stimuli rarely examine the influence of either the stimulus race or whether it may vary based on the race of the participant.
1.1. Limited racial diversity in face stimulus sets
Although the racial and ethnic diversity in the U.S is increasing, with White individuals making up only 61% of the population in 2020 compared to 72% in 2010 (Jones et al., 2021), many face stimulus sets used in psychological and neuroimaging research remain racially homogeneous with the majority consisting of 90–100% White faces (e.g., Biehl et al., 1997; Calvo and Lundqvist, 2008; Ekman and Friesen, 1975; Russell and Bullock, 1985). Table 1 summarizes a list of static, non-computer-generated face stimulus sets and their percentages in diversity of the models. Systematic surveys of face stimulus sets used in psychological research often fail to address this lack of diversity in available stimulus sets (Cook and Over, 2021). However, recently, we and others have created validated stimulus sets of racially and ethnically diverse faces (Conley et al., 2018, Strohminger et al., 2016, DeBruine and Jones, 2017a) to in part help address this problem.
Table 1.
Face stimulus sets by race of models. Adapted from https://rystoli.github.io/FSTC.html
| Face stimuli dataset | Citation | % Diversity in Models |
|---|---|---|
| RADIATE | Conley et al., (2018) | 74% |
| MR2 Database | Strohminger et al., (2016) | 70% |
| Face Research Lab London Set | DeBruine and Jones, 2017a | 60% |
| Chicago Face Database (3.0) | Ma et al., (2015) | 54% |
| NimStim | Tottenham et al., (2009) | 42% |
| Center for Vital Longevity | Minear and Park, (2004) | 24% |
| 10k US Adult Faces Database | Bainbridge et al., (2013) | 16% |
| Developmental Emotional Faces Stimulus Set | Meuwissen et al., (2017) | 10% |
| NIMH-ChEFS adolescent face stimulus set | Coffman et al., (2015) | 8% |
| Pictures of Facial Affect | Ekman and Friesen, (1976) | 0% |
| KDEF | Lundqvist et al., (1998) | 0% |
| Max Planck FACES Database | Ebner et al., (2010) | 0% |
| Radboud Faces Database | Langner et al., (2010) | 0% |
| Young Adult White Faces with Manipulated Versions | DeBruine and Jones, (2017b) | 0% |
| Social perception of bodies [neutral faces] | Morrison et al., (2017) | 0% |
| High quality white fe/male stimuli | Jones et al., (2018) | 0% |
| Basel Face Database | Walker et al., (2018) | 0% |
| Oslo Face Database | Chelnokova et al., (2014) | not reported |
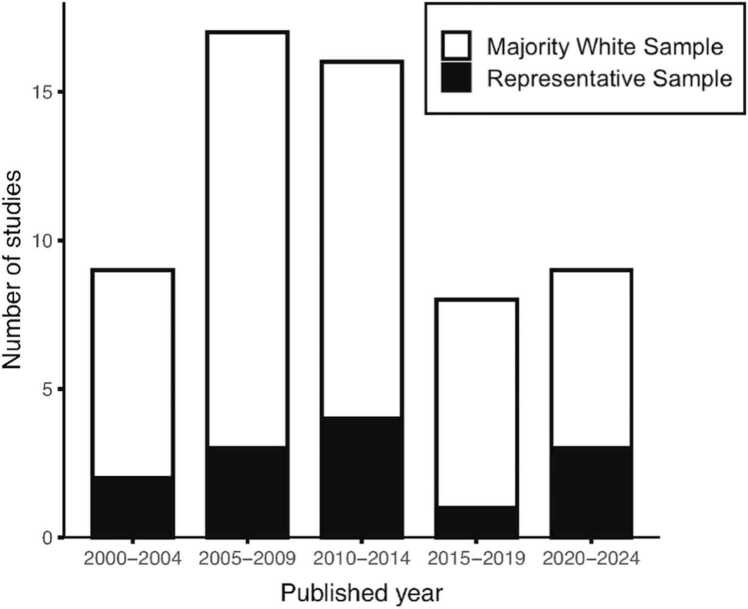
Not only are face stimulus sets largely homogenous and predominantly pictures of White people, but research participant samples also lack racial diversity (Ricard et al., 2023, Roberts et al., 2020) diminishing the generalizability of findings. Homogenous samples are especially an issue for studies that specifically examine the effects of race information on behavior (Ricard et al., 2023, Roberts et al., 2020). In the context of the ABCD Study that examines both behavioral and brain responses, we reviewed the sample demographics of neuroimaging studies that specifically tested for Black and White race information effects on brain and behavior from 2000 to 2024 (n=59 studies, See Supplemental Text and Table S1). Fig. 1 shows that on average less than 25% of studies included a representative sample - a pattern that has not changed in nearly 25 years. Studies examining responses to race information in nonrepresentative samples cannot make general claims about in-group or out-group effects in the general population, given that effects are only tested in participants of one race.
Fig. 1.
Number of neuroimaging studies published between 2000 and 2024 (N=59) examining the effects of race information on brain and behavior with and without representative samples. Representative samples are defined as those having an equal representation of Black and White participants.
Another important concern is how a mismatch between stimulus and sample racial demographics might inadvertently lead to performance differences between racial groups when race information is not the focus of the study. These group differences may be misinterpreted as related to the race of the participant rather than to interactive effects of stimulus race and participant race. It is this concern that the ABCD study design potentially raises (i.e., a mismatch in stimuli and participant race that inadvertently leads to the perception of group differences in performance).
1.2. Biased attention to racial cues
Limited racial diversity in stimuli and samples is surprising given the large adult literature demonstrating that race information can significantly and differentially influence psychological and cognitive processes as a function of participant race. One such set of studies has focused mainly on Black racial cues (e.g., Black faces or individuals) and have been conducted largely in the U.S. where race is particularly salient given its historical context and present systemic racial biases. The findings show biased attention toward Black, compared to White, faces are associated with faster, more accurate responses and/or greater distractibility by these cues in both Black and White adults (Bean et al., 2012, Correll et al., 2002, Correll et al., 2006, Donders et al., 2008, Richeson et al., 2003, Richeson and Shelton, 2003, Rubien-Thomas et al., 2021, Trawalter et al., 2008). This bias of attention toward Black facial cues is associated with early stages of rapid visual and face processing (Brosch et al., 2013, Contreras et al., 2013, Golby et al., 2001, Hughes et al., 2019, Natu et al., 2011, Ratner et al., 2013, Reggev et al., 2020, Rubien-Thomas et al., 2021) and thought to be related to negative stereotypes of Black individuals as threatening (Brosch et al., 2013, Stolier and Freeman, 2016, Rubien-Thomas et al., 2023), consistent with cues of physiological threat being processed early and rapidly (Öhman et al., 2001). These basic attentional effects also may be related to heightened arousal due to the social threat of appearing prejudiced in response to racial cues (Richeson and Trawalter, 2008).
Racial biases have been observed early in development. Infants process race-relevant cues and even young children categorize people into racial in-groups and out-groups (Dunham et al., 2018, Katz and Kofkin, 1997, Nesdale, 2001). Furthermore, assigning negative attributes to out-group individuals, even those based on arbitrary distinctions, is evident by early childhood (Dunham et al., 2011, Patterson and Bigler, 2006, Rutland et al., 2005) and White children as young as six demonstrate negative associations with Black faces compared with White faces in the U.S. (Baron and Banaji, 2006, Williams and Steele, 2019). Although the number of studies including Black youth is limited, there is some evidence of negative associations and rejection of Black faces and dolls relative to White ones among these youth (Meltzoff and Gilliam, 2024, Kleider-Offutt et al., 2018). Together, these studies suggest that racial biases emerge early in development for both Black and White Americans, but the impact of racial information (e.g. Black and White faces) on visual attention and information processing has not been directly assessed to date.
1.3. Other race-effect in recognition memory
Race information also affects differentiation and recognition, in a well-documented phenomenon known as the other-race effect. This effect reflects worse ability of individuals, in overwhelmingly White samples, at recognizing and differentiating faces from a racial or ethnic out-group compared to their own racial in-group (White faces; Meissner and Brigham, 2001; Stelter and Degner, 2018). The other-race effect appears to be less robust in Black adults living in the U.S., a finding attributed to their having significant contact with, as well as a greater motivation to individuate, White people, given the group’s status as both the numerical majority and dominant racial group in the U.S. population (Hugenberg et al., 2013). Still, the other-race effect is observed early in development, emerging by the first year of life (Katz and Kofkin, 1997, Kelly et al., 2007), presumably due to less familiarity with out-group faces and, thus, depends in part on the racial environments to which children are exposed (Bar-Haim et al., 2006).
Other-race effects have been reported in other forms of memory beyond recognition memory. For example, working memory performance for same-race faces has been shown to be enhanced compared to other-race faces in adults (Gonzalez and Schnyer, 2019, Stelter and Degner, 2018), but the literature is somewhat mixed (c.f. Sessa and Dalmaso, 2016). Together these findings suggest that race information can influence memory performance, typically with better memory for same-race faces.
1.4. The current study
The current study attempts to fill a gap in the literature associated with limited racial diversity in stimuli and samples by testing for differential effects of race information as a function of participant race on different cognitive processes in children. Specifically, we examine whether stimulus race (i.e., Black and White faces) influences measures of attention, working memory, and recognition memory performance in Black and White children from the ABCD Study in similar ways as previously observed in adults. First, we test if in- and out-group effects on cognition previously observed in predominantly White adult samples is present in White youth. Second, and importantly, we test if these in- and out-group effects generalize to Black youth. We chose the ABCD open access dataset for the following reasons: First, the dataset includes racially diverse face stimuli in the experimental design of two cognitive tasks: the fMRI n-back task with 2 memory load conditions (0- and 2-back); and a subsequent behavioral recognition memory task for items from the n-back task. Together these tasks tap aspects of three cognitive domains: attention, working memory, and recognition memory (see Methods for operational definitions). Second, the ABCD Study sample of nearly 12,000 youth was designed to estimate the diversity of the U.S. population including on race (Garavan et al., 2018), providing relatively large samples of youth from different racial groups. As such, this open access dataset provides an opportunity to examine the less researched question of how race information impacts cognitive performance in children from different racial backgrounds.
Based on the existent literature, we had two primary hypotheses: 1) Given the majority of prior evidence of attentional bias to Black faces relative to White faces in both Black and White participants (Bean et al., 2012, Donders et al., 2008, Trawalter et al., 2008), we predicted better attentional performance to Black relative to White faces regardless of participant race; and 2) given prior findings of other-race effects on recognition memory (Bar-Haim et al., 2006, Meissner and Brigham, 2001, Stelter and Degner, 2018), we predicted worse recognition memory for other-race compared to same-race faces for both Black and White participants, but especially for White participants. Given the mixed literature on stimulus race effects for working memory, we had no directional predictions, but included this cognitive domain to test for specific versus general effects of race information on cognitive performance.
2. Methods
2.1. Sample
Participants were 9- and 10-year-old Black and White youth with data in the ABCD Study data release 4.0.1 (https://ABCDStudy.org, https://nda.nih.gov/abcd; DOI: 10.15154/1523041). Primary exclusion criteria for this study included those who did not complete both the n-back and subsequent recognition memory tasks (n=99) or had a diagnosis of autism spectrum disorder, cerebral palsy, schizophrenia, intellectual disability, or history of epilepsy, seizures, or brain trauma (n=476). Subjects who performed below chance (60% accuracy at p <.05) on the n-back task (n=633) were also excluded. Finally, we subset the data to only include participants whose parents identified them as either “Black” (n=1,022) or “White” (n=4,637) for a total sample of n=5,659 youth in the primary analysis. Demographics are reported in Table S2 (left panel). The primary results reported in the paper are based on this sample.
Because Black and White participant groups varied on a variety of demographics (Table S2, left panel), we performed a secondary analysis on group-matched Black and White participants across age, sex, parent education, parent income, and fluid intelligence (Matrix Reasoning; Wechsler Intelligence Scale for Children (5th Edition), 2014) using propensity scores (Ho et al., 2011). This secondary analysis resulted in a subsample of 998 participants (50% Black, 51.6% female), reported in Table S2 (right panel).
2.2. Behavioral paradigms
We assessed attention and working memory with the ABCD n-back task that includes a low load (0-back) and a high load (2-back) memory condition (Casey et al., 2018, see specific task details below). For the purposes of this study, we operationally defined attention as performance on the 0-back condition of the n-back task, which requires participants to detect a rare face stimulus (target) among sequentially presented non-target face stimuli in a manner similar to a continuous performance task, a traditional measure of attention (Kardan et al., 2022, Lin et al., 1999). We defined working memory as performance on the 2-back condition of the n-back task, which requires participants to maintain and update stimulus information to detect whether a current stimulus is the same as the one presented 2 trials back. We used performance on the n-back task to test our first hypothesis of better attentional performance to Black relative to White faces regardless of participant race [i.e., we predicted a stimulus race X task condition where the 0-back condition (attention), but not the 2-back condition (working memory), was predicted to show better performance to Black compared White faces.]
Recognition memory was assessed by the ability to subsequently recognize old stimuli from the n-back task relative to new stimuli (see specific task details below). We used performance on this task to test our second hypothesis of worse recognition memory for other-race compared to same-race faces for both Black and White participants (i.e., participant race X stimulus race interaction). For both the n-back task and the recognition memory task, race of the face stimulus was incidental and not part of the task instructions. Together, the n-back task and the recognition memory task allowed us to examine the effects of stimulus race as a function of participant race for three cognitive domains: attention (0-back), working memory (2-back), and recognition memory.
2.3. N-back task
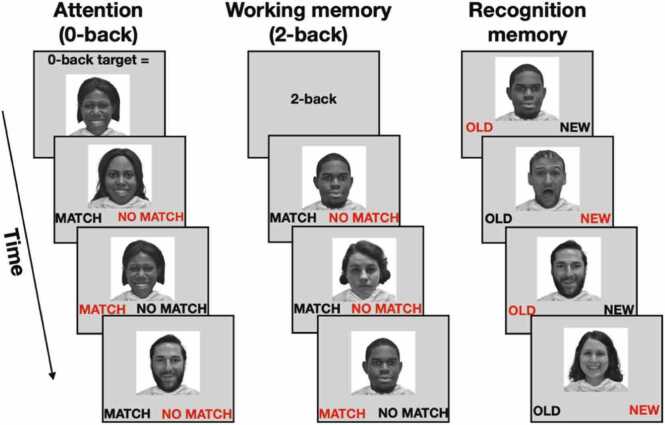
The n-back task (Casey et al., 2018; Fig. 2) included two runs of eight blocks each. On each trial, participants were asked to respond as to whether the picture was a “Match” or “No Match.” Participants were told to make a response on every trial. In each run, there were four blocks of the high memory load (2-back) condition for which participants were instructed to respond “match” when the current stimulus was the same as the one shown two trials back; and four blocks were the low load memory load (0-back) condition for which participants were instructed to respond “match” when the current stimulus was the same as the target presented at the beginning of the block. A 2.5 s cue at the beginning of each block indicates the task condition to be performed as shown in Fig. 2.
Fig. 2.
Cognitive Task Design. Illustration of the n-back and recognition memory paradigms. The n-back task included two memory load conditions: the 0-back (left panel) and the 2-back (middle panel). The 0-back condition required sustained attention in order to detect a rare target. The 2-back condition required maintenance and updating of any stimulus that repeated itself 2 trials later (working memory). The recognition memory task (right panel) tested for memory of items from the n-back task. Words in red represent correct responses.
Blocks of trials consisted of happy, fearful, and neutral facial expressions as well as places. Individual faces were unique to only one facial expression and were not repeated for the other expressions. Each block consisted of 10 trials and each stimulus was presented for 2 s during which time the participant had to respond, followed immediately by a 500 ms fixation cross (i.e., 10 trials of 2.5 s each). Of the 10 trials in each block, 2 were targets and the rest were nontargets. There were 160 trials total with 96 unique stimuli of 4 different stimulus types (24 unique stimuli per type) presented in separate blocks in each run. In total there were 80 trials that assessed sustained attention (0-back condition) and 80 trials that assessed working memory (2-back condition), and 20 trials for each stimulus type in each of the two memory load conditions. Four versions of the task that pseudo-randomized the order of conditions and stimulus types within each block were randomly assigned to participants. Participants completed the n-back task either in the scanner (n=5649) or on a laptop/testing computer (n=10). Test environment information was included as a covariate (see Results and Table S6 and S10). Prior to completing the task, participants practiced both the 0-back and 2-back conditions based on unique face and place stimuli not included in the actual task.
2.4. Recognition memory task
The recognition memory task (Casey et al., 2018; Fig. 2) was completed within an hour of completing the n-back task. The task examined recognition memory for the stimuli from the n-back task (described above). The recognition memory task included 48 old stimuli presented during the emotional n-back task and 48 new stimuli, with equal numbers of each stimulus type in the old and new stimulus sets (12 each of happy, fearful, and neutral facial expressions as well as places in each set). Only face stimuli were analyzed in the present study. A total of 96 trials were presented during the recognition memory test. Participants were asked to rate each stimulus as either “Old” or “New”. Each stimulus was presented for 2 s during which time the participant was required to respond and followed immediately by a 1 s presentation of a fixation cross. All participants completed the recognition memory task on laptops or testing computers. Instructions and a 2-trial practice (one “Old” from the task practice and one “New” stimulus) preceded the recognition memory task.
For both the n-back task and recognition memory task, places were excluded from the analysis. The remaining data were collapsed across face stimulus type (fearful, happy, neutral faces) blocks due to an insufficient number of trials with Black and White faces to analyze stimulus race by emotional categories (neutral, positive, and negative facial expressions) separately. The mean proportion of Black stimuli (among Black and White stimuli) was .49 (SD=.030) in the n-back task and .53 (SD=.017) in the recognition memory task. This Black to White stimulus ratio was treated as a covariate in secondary analysis of performance on each task (See Results).
2.5. Analytical approach
Analyses were conducted using R version 4.3.1 (R Core Team, 2023). We used d-prime (d’) as our dependent measure for both the n-back (0- and 2-back) task and recognition memory task. D-prime is an index of signal detection sensitivity between signal and noise (Stanislaw and Todorov, 1999) and was calculated using the “psycho” package as d’ = z(H) – z(F), where z(H) and z(F) are the z transforms of hit rate and false alarm rate, respectively. In the n-back task, d-prime was calculated separately for the 0-back and 2-back conditions and for Black and White faces based on the number of hits and false alarms in identification of targets and nontargets. For the recognition memory task, d-prime was calculated separately for Black and White faces based on the number of correct and incorrect detections of old (i.e. items from the n-back task) and new stimuli.
The effects of participant race, stimulus race, task condition (0-back, 2-back), and their interactions on n-back and recognition memory performance were tested with mixed-effect models using the “lme4” package (Bates et al., 2015). In the n-back model, since the two conditions (0-back and 2-back) were interleaved blocks during data collection, we included the two conditions in a single model to control for their effects in performance (i.e., control for variance in performance on the 2-back in the 0-back measure and control for variance in performance on the 0-back in the 2-back measure). Random effects for subjects and family nested within-site were included in each model as random intercepts to account for ABCD Study sampling design (Saragosa-Harris et al., 2022).
Given our hypothesis of enhanced attention (0-back) performance to Black faces relative to White faces, regardless of the participant race, and no predicted differences in working memory (2-back performance), we expected to see an interaction of stimulus race X task condition (0- or 2-back) in the mixed-effect model for the n-back task. In the recognition memory model, the condition factor represents the condition (0- or 2-back) in which stimuli were originally presented in the n-back task. Given our hypothesis of the other-race effect in recognition memory, we predicted worse performance of the other-race stimuli in the recognition memory task for both Black and White participants (i.e., an interaction between stimulus race and participant race in the mixed-effect model). To interpret significant interactions, post-hoc analyses were performed using non-parametric Monte Carlo t-tests with 10,000 permutations with Bonferroni correction for multiple comparisons.
3. Results
3.1. N-back task
Main effects. The mixed-effect model testing participant race (Black and White), stimulus race (Black and White), and task condition (0- and 2-back) on behavioral performance (d-prime) during the n-back task showed main effects of task condition (F(1)= 673.53, p <.001), stimulus race (F(1)= 360.07, p <.001) and participant race (F(1)= 191.44, p <.001) on d-prime (Table 2). D-prime was higher for the 0-back (1.68±0.95) than the 2-back condition (1.32±0.89; p <.001); for Black (1.61±0.96) than White stimuli (1.39±0.90; p <.001); and for White (1.56±0.93) than Black participants (1.23±0.94; p <.001).
Table 2.
Mixed-effect model examining task condition, stimulus (Stim) race, and participant (PA) race for the n-back memory task (n=5659).
|
N-back Memory Task Dependent variable: d-prime |
|||
|---|---|---|---|
| Predictors | Estimates | 95% CI | p |
| Condition (0-back) |
0.17 | 0.15 – 0.18 | <0.001 |
| Stim race (White) |
-0.12 | -0.14 – -0.11 | <0.001 |
| PA race (White) |
0.16 | 0.14 – 0.18 | <0.001 |
| Condition × Stim race (0-back × White) |
-0.09 | -0.10 – -0.08 | <0.001 |
| Condition × PA race (0-back × White) |
0.01 | -0.00 – 0.03 | 0.053 |
| Stim race × PA race (White × White) | 0.02 | 0.00 – 0.03 | 0.015 |
| Condition × Stim race × PA race (0-back×White×White) |
-0.00 | -0.01 – 0.01 | 0.743 |
| Observations | 22636 | ||
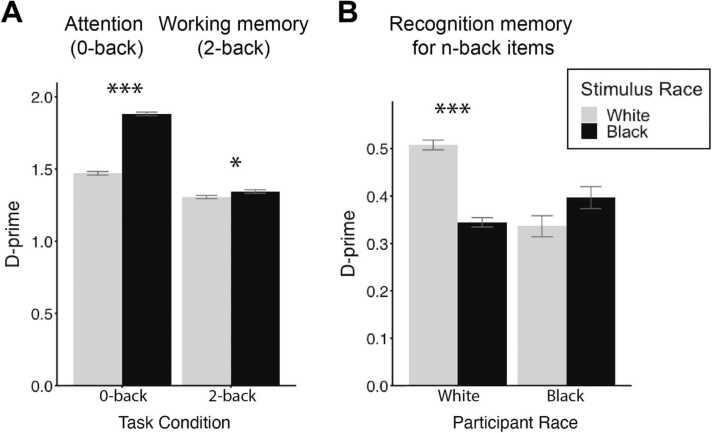
Interactions. Consistent with our first hypothesis of better attention (0-back) performance to Black relative to White faces, regardless of the participant race; and no predicted differences in working memory (2-back) performance (d-prime), we observed a stimulus race X task condition (0- or 2-back) interaction (F(1)= 202.22, p <.001) (See Fig. 3A and Table 3). There was also a stimulus race X participant race interaction (F(1)= 5.93, p <.05; see Table S3).
Fig. 3.
Performance on the n-back and recognition memory tasks. A) D-prime for the 0-back (attention) and 2-back (working memory) conditions by memory load task condition. Participants show better performance to Black, compared to White, faces in the 0-back condition and to a lesser extent in the 2-back condition. B) Recognition memory as measured by d-prime by participant and stimulus race. Only White participants show a significant same-race face advantage in recognition memory. Error bars represent standard errors. *p <.05, ***p <.001.
Table 3.
Post-hoc analysis of stimulus race X memory load task condition for d-prime on the n-back task (n=5659).
| Condition | Black Stimuli | White Stimuli | t-statistic | Observations | Effect size (Cohen’s d) |
|---|---|---|---|---|---|
| 0-back | 1.88 ± 0.93 | 1.47 ± 0.92 | < 2.2 ×10-16 | 5659 | 0.44 |
| 2-back | 1.34 ± 0.90 | 1.31 ± 0.88 | = 0.0192 | 5659 | 0.04 |
For the stimulus race X task condition interaction, we performed post-hoc analysis separately for Black and White stimuli in each of the two task conditions (0- or 2-back). The results are depicted in Fig. 3A and Table 3. They show that as predicted, all participants, regardless of race, were more sensitive (higher d-prime) to Black compared to White faces in the 0-back (attention) condition. For the 2-back (working memory) condition, all participants showed only a slight improvement in d-prime for Black compared to White stimuli (effect size .04). Thus, the higher sensitivity to Black than White faces for both Black and White participants was driven by the 0-back (attention) condition consistent with our first hypothesis of better performance when attending to Black faces regardless of the participant’s race.
Given group differences in participant demographics that could potentially impact performance, we performed two subsidiary analyses to control for these group differences. First, we group-matched Black and White participants for age, sex, parent education, parent income, and fluid intelligence to minimize the differences, resulting in equal but fewer numbers of Black (n=499) and White (n=499) participants (n=998, See the right panel of Table S2 for demographics of this sample by participant race). Even with a smaller subset of the data, the regression model showed similar results and importantly the predicted stimulus race X task condition interaction (p <.001) (see Table S4, S5). Second, we tested our mixed-effect model on the original sample (n=5659) but included the covariates of age, sex, parent education, parent income, test environment (in-scanner and on laptop/testing computer), Black to White stimulus ratio and fluid intelligence. This analysis also revealed similar results including the hypothesized stimulus race X task condition interaction (p <.001) (see Table S6).
3.2. Recognition memory task
Main Effects. Our mixed-effect model testing the effects of stimulus race (Black, White), task condition (0-back, 2-back), and participant race (Black, White), and their interactions on d-prime showed main effects of task condition (F(1)=26.16, p <.001), stimulus race (F(1)=44.35, p <.001), and participant race (F(1)=5.27, p <.05) (Table 4). D-prime was higher for items from the 0-back (0.45±0.77) than the 2-back condition (0.38±0.79; p <.001); for White (0.49±0.78) than Black stimuli (0.34±0.77; p <.001); and for White (0.42±0.77) than Black participants (0.37±0.81; p <.001).
Table 4.
Mixed-effect models examining task condition, stimulus (Stim) race, and participant (PA) race for recognition memory task (n=5659).
|
Recognition Memory Task Dependent variable: d-prime |
|||
|---|---|---|---|
| Predictors | Estimates | 95% CI | p |
| Condition (0-back) |
0.03 | 0.02 – 0.04 | <0.001 |
| Stim race (White) |
0.04 | 0.03 – 0.05 | <0.001 |
| PA race (White) |
0.02 | 0.00 – 0.04 | 0.022 |
| Condition × Stim race (0-back × White) |
-0.00 | -0.02 – 0.01 | 0.400 |
| Condition × PA race (0-back × White) |
0.02 | 0.00 – 0.03 | 0.005 |
| Stim race × PA race (White × White) | 0.06 | 0.05 – 0.07 | <0.001 |
| Condition × Stim race × PA race (0-back×White×White) |
0.00 | -0.01 – 0.01 | 0.580 |
| Observations | 22636 | ||
Interactions. Consistent with our second hypothesis of an other-race effect on memory recognition, there was an interaction of stimulus race X participant race (F(1)=106.54, p <.001) on recognition memory performance as measured by d-prime (Fig. 3B and Table 5). There was also an interaction of participant race X task condition (0-/2-back) (F(1)= 7.81, p <.01.) (Table S7).
Table 5.
Post-hoc analysis of stimulus race X participant race for d-prime on the recognition memory task (n=5659).
| Participant race | Black Stimuli | White Stimuli | t-statistics | Observations | Effect size (Cohen’s d) |
|---|---|---|---|---|---|
| Black | 0.39±0.82 | 0.35±0.79 | 0.05291 | 2044 | 0.05 |
| White | 0.33±0.76 | 0.52±0.78 | <.001 | 9274 | 0.24 |
The post-hoc analysis of the interaction of stimulus race X participant race is reported in Table 5. These results reveal greater recognition memory in White participants for same-race (White) faces (0.52±0.78) than for other-race (Black) faces (0.33±0.76; p <.001; Fig. 3B). Black participants showed a similar but nonsignificant pattern of greater recognition memory for Black faces (0.39±0.82) over White faces (0.35±0.79, p >.05; Fig. 3B). Effect sizes of .24 and .05 for White and Black participants, respectively, indicate the interaction was driven by a same-race stimulus advantage in White participants (Table 5).
To control for differences in Black and White participant demographics on performance, we again performed two additional analyses using the group-matched samples (Table S8) and mixed-effect model with covariates (Table S10). We observed similar results, including the predicted participant race X stimulus race interaction (p <.001) for both models. We showed an advantage for same-race faces on recognition memory performance for White participants (p <.001) and not Black participants (p =.32) in the group-matched sample (Table S9). Together the results show an added benefit for White participants only in recognition of same-race (White) faces.
4. Discussion
Here, we used the large, open access ABCD Study dataset to examine interactive effects of stimulus and participant race on cognitive performance in children. We found that the effects of race information on cognitive performance varied by cognitive domain and participant race. Specifically, both Black and White children showed enhanced attention to Black faces compared to White faces. We also showed an other-race effect for recognition memory, but only for White youth (i.e., better performance to same-race White faces than to other-race Black faces). Finally, both Black and White participants showed slightly better working memory performance to Black compared to White faces. Together, these findings highlight that race information can differentially influence aspects of cognitive performance in relation to the race of the participant and potentially benefit performance for some groups of youth over others. Importantly, the results underscore the need for considering stimulus and sample demographics in study design, analysis, and interpretation of findings.
4.1. Attentional bias to race information
As predicted, both Black and White children showed a higher sensitivity to Black compared to White faces when detecting a rare target face among sequentially presented non-target faces during the 0-back condition of the n-back task. The sequential search for a rare target is characteristic of continuous performance tasks that have traditionally served as measures of attention (Kardan et al., 2022, Lin et al., 1999). The findings of enhanced attention to Black faces, regardless of participant race, in the current study with this measure, is consistent with the adult literature (Bean et al., 2012, Richeson and Trawalter, 2008, Trawalter et al., 2008) and with studies showing advantages in early or rapid processing of Black faces over White faces in U.S. samples (Brosch et al., 2013, Contreras et al., 2013, Golby et al., 2001, Hughes et al., 2019, Natu et al., 2011, Ratner et al., 2013, Reggev et al., 2020, Rubien-Thomas et al., 2021). Advantages in processing of Black faces or individuals has been interpreted as reflecting negative stereotypes of Black individuals as threatening (Brosch et al., 2013, Stolier and Freeman, 2016, Rubien-Thomas et al., 2023) just as cues of physiological threat are processed early and rapidly (Öhman et al., 2001). These basic attentional effects also may reflect heightened arousal due to the social threat of appearing prejudiced in response to racial cues (Richeson and Trawalter, 2008). Importantly, we see these effects even though stimulus race was incidental to the task and not part of the task instructions.
Our results extend adult findings to a developmental population. Prior studies have provided evidence that young children assign negative attributes to out-group individuals (Dunham et al., 2011, Patterson and Bigler, 2006, Rutland et al., 2005) and that White children in the U.S. demonstrate negative associations with Black faces compared with White faces (Baron and Banaji, 2006, Williams and Steele, 2019). While few studies have included Black youth, those studies suggest similar negative and threatening associations with Black racial stimuli relative to White ones in U.S. samples (Meltzoff and Gilliam, 2024, Kleider-Offutt et al., 2018). However, in the current study, we uniquely show a specific racial bias in attention to Black faces that is not only seen in White children, but in Black children too – a pattern that has only been shown in adults to date.
4.2. Other-race effect in recognition memory
Our findings on the impact of race information on recognition memory are in part consistent with the well-documented other-race effect. The literature has shown that participants, in predominantly White samples, are worse at recognizing and differentiating racial out-group members compared to their own racial in-group (White faces; Meissner and Brigham, 2001; Stelter and Degner, 2018) - an effect observed early in development (Katz and Kofkin, 1997, Kelly et al., 2007). In the current study, we found that White children showed worse recognition of other-race (Black) faces (i.e., better recognition of White faces by White participants). Black children showed a somewhat similar, but nonsignificant pattern (i.e., worse recognition of White compared to Black faces) of only a nominal effect size (Cohen’s d =.05).
The lack of an other-race effect for Black children may be related to the racial environments to which children are exposed (Bar-Haim et al., 2006). In the U.S., White people make up 61% of the population, whereas Black people represent only 14% of the U.S. population (Jones et al., 2021). As such there is likely over-exposure of Black youth to White people. Historically and presently, White people hold more positions of authority which could also necessitate differentiation and recognition of White individuals by Black youth (Hugenberg et al., 2013). Thus, Black children in the current U.S.-based sample may have developed expertise in recognizing other-race (White) individuals that they encounter.
4.3. Influence of race information on working memory
We had no hypothesis for whether race information would influence working memory performance. The literature to date is mixed on how same- or other-race information influences working memory (Gonzalez and Schnyer, 2019, Sessa and Dalmaso, 2016, Stelter and Degner, 2018). However, both Black and White participants showed a small advantage in performance to Black faces relative to White faces in the 2-back condition of the working memory task. This observed advantage in working memory performance for both Black and White children may simply reflect upstream attentional effects on working memory as measured by the n-back task. This interpretation is consistent with evidence of enhanced attention to Black versus White faces on the 0-back (attention) condition of the n-back task by both Black and White children.
4.4. Limitations
The current study provides novel evidence that stimulus race can differentially impact cognitive performance as a function of participant race in children, but there are potential limitations of this study to consider. First, while attempts were made to match the Black and White participants on family education and income and other variables, the groups still differed on a number of factors (Table S1). The limited ability to match groups, in part, reflects sampling issues of the ABCD Study (Compton et al., 2019), but also the inequality of structural racial discrimination in the U.S. from which the sample was obtained (Krieger, 2012, Richeson and Sommers, 2016). Second, the ABCD Study was not designed to test for stimulus race effects. However, the large number of Black and White face stimuli and similarity in Black-to-White stimulus ratio for the n-back and recognition tasks allowed us to test for potential stimulus race effects. Further, Black to White stimulus ratio was treated as a covariate in our models. So the observed results are unlikely due to differences in the number of Black versus White face stimuli in either task. Third, we focused our analyses only on the effects of Black and White stimuli for Black and White participants due to fewer face stimuli of other races and ethnicities in the n-back and recognition memory tasks. As such, the specificity of our findings and their generalizability to other racial and ethnic groups in the U.S. cannot be determined. We also collapsed across face stimulus type (fearful, happy, neutral faces) because of an insufficient number of trials with Black and White faces to analyze stimulus race by emotional categories (neutral, positive, and negative facial expressions) separately which in part hinders our interpretation on whether attentional bias to Black faces reflects negative stereotypes of Black individuals as threatening. However, in studies where only neutral Black and White faces are used as stimuli (e.g. on a go/nogo task), an attentional bias to Black versus White faces in both Black and White participants is observed too (Rubien-Thomas et al., 2021). Moreover, in studies that include Black and White participants and face stimuli in emotional conditions of threat, reward and nonarousal, greater attentional bias to Black than White faces in both Black and White participants has been shown in the threat relative to the reward and nonarousal conditions (Rubien-Thomas et al., 2023). These findings, although from adults, are consistent with an interpretation of racial attentional bias as related to negative stereotypes of Black individuals as threatening. Fourth, although our findings are, in part, consistent with the literature for White participants, with benefits in performance to both same- and other-race stimuli across different cognitive domains (Meissner and Brigham, 2001, Stelter and Degner, 2018, Bean et al., 2012, Richeson and Trawalter, 2008, Trawalter et al., 2008), the interpretation of our findings for Black participants is more limited. Measures currently available in the ABCD Study dataset do not allow us to test for potential factors that may contribute to the observed effects (e.g., intergroup contact, race of the experimenter, implicit racial bias, stereotype threat, etc.). Finally, while the current study examined stimulus race effects of Black and White faces, other forms of demographic bias could have been present or interacted with race (e.g., gender bias, Johnson et al., 2012). Future intersectional frameworks and methodology will be important and necessary for understanding how the interaction of different aspects of stimuli and participant identities influences performance on cognitive tasks.
5. Conclusion
Racial biases that indirectly impact behavior emerge early in development although most research in this area has focused on adults and predominantly White participants and none to date have examined the influence that racial stimuli have on different domains of cognitive performance. Here, we use a large, open access dataset to illustrate interactive effects of stimulus and participant race on cognitive performance in both Black and White children. Specifically, both Black and White children showed enhanced attention to, and modest improvements in working memory for, Black faces compared to White faces (i.e., better accuracy detecting a rare Black face target). This finding is consistent with the literature on attentional bias to Black faces that has been associated with negative racial stereotypes associated with threat in U.S. adult samples (Rubien-Thomas et al., 2023). White children also showed an advantage in recognition memory performance for same-race faces compared to other-race faces. This finding is consistent with the documented other-race effect in recognition of out-group faces (i.e., worse differentiation and recognition of other-race faces) observed in predominantly White samples (Meissner and Brigham, 2001, Stelter and Degner, 2018). Importantly, Black children did not show this advantage in recognition performance. Such interactive effects of stimulus race with participant race may lead to misinterpretation of performance differences between racial groups.
Together the findings highlight: 1) the importance of racially diverse samples when assessing stimulus race effects; and 2) that race information in face stimuli can differentially influence aspects of cognitive performance in children from different racial backgrounds, even when the study is not theoretically motivated or experimentally designed to specifically examine the effects of same- and other-race stimuli. Moreover, our findings underscore the need to consider stimulus and participant race when analyzing data from studies like the ABCD study that include diversity in both the sample and stimuli. In sum, just as descriptions of sample demographics in empirical studies are imperative, and now a requirement for publication in many scientific journals, so too is the need for descriptions of stimuli demographics. Moreover, analyses and/or consideration of how a mismatch in stimuli and participant demographics may inadvertently contribute to group differences in performance may help constrain interpretations of empirical findings across development.
CRediT authorship contribution statement
BJ Casey: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Arielle Baskin-Sommers: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition. Yen-Chu Lin: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis. Arya Adake: Writing – review & editing. Hailey Kopp: Writing – review & editing. Dylan G. Gee: Writing – review & editing, Writing – original draft, Funding acquisition. Jennifer A. Richeson: Writing – review & editing, Writing – original draft, Conceptualization. Ivan Chan: Writing – review & editing, Writing – original draft, Formal analysis. Estée Rubien-Thomas: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lena Skalaban: Writing – review & editing. May I. Conley: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported in part by NIH U01DA041174 (BJC, ABS, DGG). The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2024.101393.
Contributor Information
Yen-Chu Lin, Email: ylin@barnard.edu.
BJ Casey, Email: bcasey@barnard.edu.
Appendix A. Supplementary material
Supplementary material.
Data availability
The authors do not have permission to share data.
References
- Allport G.W. Addison-Wesley; 1954. The nature of prejudice. [Google Scholar]
- Amodio D.M. The neuroscience of prejudice and stereotyping. Nat. Rev. Neurosci. 2014;15(10):670–682. doi: 10.1038/nrn3800. [DOI] [PubMed] [Google Scholar]
- Bainbridge W.A., Isola P., Oliva A. The intrinsic memorability of face photographs. J. Exp. Psychol.: Gen. 2013;142(4):1323–1334. doi: 10.1037/a0033872. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Burgess G.C., Harms M.P., Petersen S.E., Schlaggar B.L., Corbetta M., Glasser M.F., Curtiss S., Dixit S., Feldt C., Nolan D., Bryant E., Hartley T., Footer O., Bjork J.M., Poldrack R., Smith S., Johansen-Berg H., Snyder A.Z., Van Essen D.C. Function in the human connectome: Task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Ziv T., Lamy D., Hodes R.M. Nature and nurture in own-race face processing. Psychol. Sci. 2006;17(2):159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Baron A.S., Banaji M.R. The development of implicit attitudes: evidence of race evaluations from ages 6 and 10 and adulthood. Psychol. Sci. 2006;17(1):53–58. doi: 10.1111/j.1467-9280.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1) doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bean M.G., Slaten D.G., Horton W.S., Murphy M.C., Todd A.R., Richeson J.A. Prejudice concerns and race-based attentional bias: new evidence from eyetracking. Soc. Psychol. Personal. Sci. 2012;3(6):722–729. doi: 10.1177/1948550612436983. [DOI] [Google Scholar]
- Biehl M., Matsumoto D., Ekman P., Hearn V., Heider K., Kudoh T., Ton V. Matsumoto and Ekman’s Japanese and Caucasian facial expressions of emotion (JACFEE): reliability data and cross-national differences. J. Nonverbal Behav. 1997;21(1):3–21. doi: 10.1023/A:1024902500935. [DOI] [Google Scholar]
- Brosch T., Bar-David E., Phelps E.A. Implicit race bias decreases the similarity of neural representations of black and white faces. Psychol. Sci. 2013;24(2):160–166. doi: 10.1177/0956797612451465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M.G., Lundqvist D. Facial expressions of emotion (KDEF): Identification under different display-duration conditions. Behav. Res. Methods. 2008;40(1):109–115. doi: 10.3758/BRM.40.1.109. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M., Dale A.M. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O., Laeng B., Eikemo M., Riegels J., Løseth G., Maurud H., Willoch F., Leknes S. Rewards of beauty: the opioid system mediates social motivation in humans. Mol. Psychiatry. 2014;19(7):746–747. doi: 10.1038/mp.2014.1. [DOI] [PubMed] [Google Scholar]
- Coffman M.C., Trubanova A., Richey J.A., White S.W., Kim-Spoon J., Ollendick T.H., Pine D.S. Validation of the NIMH-ChEFS adolescent face stimulus set in an adolescent, parent, and health professional sample. Int. J. Methods Psychiatr. Res. 2015;24(4):275–286. doi: 10.1002/mpr.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton W.M., Dowling G.J., Garavan H. Ensuring the best use of data: the adolescent brain cognitive development study. JAMA Pediatr. 2019;173(9):809–810. doi: 10.1001/jamapediatrics.2019.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley M.I., Dellarco D.V., Rubien-Thomas E., Cohen A.O., Cervera A., Tottenham N., Casey B. The racially diverse affective expression (RADIATE) face stimulus set. Psychiatry Res. 2018;270:1059–1067. doi: 10.1016/j.psychres.2018.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras J.M., Banaji M.R., Mitchell J.P. Multivoxel patterns in fusiform face area differentiate faces by sex and race. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R., Over H. Why is the literature on first impressions so focused on White faces? R. Soc. Open Sci. 2021;8(9) doi: 10.1098/rsos.211146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll J., Park B., Judd C.M., Wittenbrink B. The police officer’s dilemma: Using ethnicity to disambiguate potentially threatening individuals. J. Personal. Soc. Psychol. 2002;83(6):1314–1329. doi: 10.1037/0022-3514.83.6.1314. [DOI] [PubMed] [Google Scholar]
- Correll J., Urland G.R., Ito T.A. Event-related potentials and the decision to shoot: the role of threat perception and cognitive control. J. Exp. Soc. Psychol. 2006;42(1):120–128. doi: 10.1016/j.jesp.2005.02.006. [DOI] [Google Scholar]
- Cosmides L., Tooby J., Kurzban R. Perceptions of race. Trends Cogn. Sci. 2003;7(4):173–179. doi: 10.1016/S1364-6613(03)00057-3. [DOI] [PubMed] [Google Scholar]
- DeBruine, L., & Jones, B. (2017a). Face Research Lab London Set (p. 88208545 Bytes) [dataset]. figshare. https://doi.org/10.6084/M9.FIGSHARE.5047666.V2.
- DeBruine, L., & Jones, B. (2017b). Young Adult White Faces with Manipulated Versions.
- Donders N.C., Correll J., Wittenbrink B. Danger stereotypes predict racially biased attentional allocation. J. Exp. Soc. Psychol. 2008;44(5):1328–1333. doi: 10.1016/j.jesp.2008.04.002. [DOI] [Google Scholar]
- Dovidio J.F., Evans N., Tyler R.B. Racial stereotypes: the contents of their cognitive representations. J. Exp. Soc. Psychol. 1986;22(1):22–37. doi: 10.1016/0022-1031(86)90039-9. [DOI] [Google Scholar]
- Dunham Y., Baron A.S., Casey S. Consequences of “Minimal” Group Affiliations in Children: Minimal Group Affiliations in Children. Child Development. 2011;82(3):793–811. doi: 10.1111/j.1467-8624.2021.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham Y., Durkin A., Tyler T.R. The development of a preference for procedural justice for self and others. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-36072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner N.C., Riediger M., Lindenberger U. FACES—A database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav. Res. Methods. 2010;42(1):351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Ekman, P., & Friesen, W.V. (1975). Unmasking the face: A guide to recognizing emotions from facial clues. Prentice-Hall.
- Ekman P., Friesen W.V. Consulting psychologists Press; 1976. Pictures of Facial Affect. [Google Scholar]
- Fiske S.T., Neuberg S.L. Vol. 23. Elsevier; 1990. A Continuum of Impression Formation, from Category-Based to Individuating Processes: Influences of Information and Motivation on Attention and Interpretation; pp. 1–74. (In Advances in Experimental Social Psychology). [DOI] [Google Scholar]
- Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Jernigan T., Potter A., Thompson W., Zahs D. Recruiting the ABCD sample: design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff P.A., Steele C.M., Davies P.G. The space between us: stereotype threat and distance in interracial contexts. J. Personal. Soc. Psychol. 2008;94(1):91–107. doi: 10.1037/0022-3514.94.1.91. [DOI] [PubMed] [Google Scholar]
- Golby A.J., Gabrieli J.D.E., Chiao J.Y., Eberhardt J.L. Differential responses in the fusiform region to same-race and other-race faces. Nat. Neurosci. 2001;4(8):845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Gonzalez G.D.S., Schnyer D.M. Attention and working memory biases to black and asian faces during intergroup contexts. Front. Psychol. 2019;9:2743. doi: 10.3389/fpsyg.2018.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetey R.C., Eberhardt J.L. Racial disparities in incarceration increase acceptance of punitive policies. Psychol. Sci. 2014;25(10):1949–1954. doi: 10.1177/0956797614540307. [DOI] [PubMed] [Google Scholar]
- Ho D.E., Imai K., King G., Stuart E.A. MatchIt: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011;42(8) doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- Hugenberg K., Wilson J.P., See P.E., Young S.G. Towards a synthetic model of own group biases in face memory. Vis. Cogn. 2013;21(9–10):1392–1417. doi: 10.1080/13506285.2013.821429. [DOI] [Google Scholar]
- Hughes B.L., Camp N.P., Gomez J., Natu V.S., Grill-Spector K., Eberhardt J.L. Neural adaptation to faces reveals racial outgroup homogeneity effects in early perception. Proc. Natl. Acad. Sci. 2019;116(29):14532–14537. doi: 10.1073/pnas.1822084116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.L., Freeman J.B., Pauker K. Race is gendered: How covarying phenotypes and stereotypes bias sex categorization. J. Personal. Soc. Psychol. 2012;102(1):116–131. doi: 10.1037/a0025335. [DOI] [PubMed] [Google Scholar]
- Jones B.C., Hahn A.C., Fisher C.I., Wang H., Kandrik M., Han C., Fasolt V., Morrison D., Lee A.J., Holzleitner I.J., O’Shea K.J., Roberts S.C., Little A.C., DeBruine L.M. No compelling evidence that preferences for facial masculinity track changes in women’s hormonal status. Psychol. Sci. 2018;29(6):996–1005. doi: 10.1177/0956797618760197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, N., Marks, R., Ramirez, R., & Ríos-Vargas, M. (2021). 2020 Census Illuminates Racial and Ethnic Composition of the Country. https://www.census.gov.
- Kardan O., Stier A.J., Cardenas-Iniguez C., Schertz K.E., Pruin J.C., Deng Y., Chamberlain T., Meredith W.J., Zhang X., Bowman J.E., Lakhtakia T., Tindel L., Avery E.W., Lin Q., Yoo K., Chun M.M., Berman M.G., Rosenberg M.D. Differences in the functional brain architecture of sustained attention and working memory in youth and adults. PLOS Biol. 2022;20(12) doi: 10.1371/journal.pbio.3001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P.A., Kofkin J.A. In: Developmental psychopathology: Perspectives on adjustment, risk, and disorder. Luthar S.S., Burack J.A., Cicchetti D., Weisz J.R., editors. Cambridge University Press; 1997. Race, gender, and young children. [Google Scholar]
- Kelly D.J., Quinn P.C., Slater A.M., Lee K., Ge L., Pascalis O. The other-race effect develops during infancy: evidence of perceptual narrowing. Psychol. Sci. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleider-Offutt H.M., Bond A.D., Williams S.E., Bohil C.J. When a face type is perceived as threatening: using general recognition theory to understand biased categorization of Afrocentric faces. Mem. Cogn. 2018;46(5):716–728. doi: 10.3758/s13421-018-0801-0. [DOI] [PubMed] [Google Scholar]
- Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am. J. Public Health. 2012;102(5):936–944. doi: 10.2105/AJPH.2011.300544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota J.T., Ito T. Rapid race perception despite individuation and accuracy goals. Soc. Neurosci. 2017;12(4):468–478. doi: 10.1080/17470919.2016.1182585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H.J., Hawk S.T., Van Knippenberg A. Presentation and validation of the Radboud faces database. Cogn. Emot. 2010;24(8):1377–1388. doi: 10.1080/02699930903485076. [DOI] [Google Scholar]
- Lin C.C.H., Hsiao C.K., Chen W.J. Development of sustained attention assessed using the continuous performance test among children 6-15 years of age. J. Abnorm. Child Psychol. 1999;27(5):403–412. doi: 10.1023/A:1021932119311. [DOI] [PubMed] [Google Scholar]
- Lundqvist, D., Flykt, A., & Öhman, A. (1998). The Karolinska Directed Emotional Faces—KDEF [CD ROM]. [dataset].
- Ma D.S., Correll J., Wittenbrink B. The Chicago face database: a free stimulus set of faces and norming data. Behav. Res. Methods. 2015;47(4):1122–1135. doi: 10.3758/s13428-014-0532-5. [DOI] [PubMed] [Google Scholar]
- Macrae C.N., Bodenhausen G.V. Social cognition: thinking categorically about others. Annu. Rev. Psychol. 2000;51(1):93–120. doi: 10.1146/annurev.psych.51.1.93. [DOI] [PubMed] [Google Scholar]
- Maddox K.B., Chase S.G. Manipulating subcategory salience: exploring the link between skin tone and social perception of Blacks. Eur. J. Soc. Psychol. 2004;34(5):533–546. doi: 10.1002/ejsp.214. [DOI] [Google Scholar]
- Maddox K.B., Gray S.A. Cognitive representations of black americans: reexploring the role of skin tone. Personal. Soc. Psychol. Bull. 2002;28(2):250–259. doi: 10.1177/0146167202282010. [DOI] [Google Scholar]
- Meissner C.A., Brigham J.C. Thirty years of investigating the own-race bias in memory for faces: a meta-analytic review. Psychol., Public Policy, Law. 2001;7(1):3–35. doi: 10.1037/1076-8971.7.1.3. [DOI] [Google Scholar]
- Meltzoff A.N., Gilliam W.S. Young children & implicit racial biases. Daedalus. 2024;153(1):65–83. doi: 10.1162/daed_a_02049. [DOI] [Google Scholar]
- Meuwissen A.S., Anderson J.E., Zelazo P.D. The creation and validation of the developmental emotional faces stimulus set. Behav. Res. Methods. 2017;49(3):960–966. doi: 10.3758/s13428-016-0756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.L., Alfaro-Almagro F., Bangerter N.K., Thomas D.L., Yacoub E., Xu J., Bartsch A.J., Jbabdi S., Sotiropoulos S.N., Andersson J.L.R., Griffanti L., Douaud G., Okell T.W., Weale P., Dragonu I., Garratt S., Hudson S., Collins R., Jenkinson M., Smith S.M. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016;19(11):1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear M., Park D.C. A lifespan database of adult facial stimuli. Behav. Res. Methods, Instrum., Comput. 2004;36(4):630–633. doi: 10.3758/BF03206543. [DOI] [PubMed] [Google Scholar]
- Morrison D., Wang H., Hahn A.C., Jones B.C., DeBruine L.M. Predicting the reward value of faces and bodies from social perception. PLOS ONE. 2017;12(9) doi: 10.1371/journal.pone.0185093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu V., Raboy D., O’Toole A.J. Neural correlates of own- and other-race face perception: spatial and temporal response differences. NeuroImage. 2011;54(3):2547–2555. doi: 10.1016/j.neuroimage.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Nesdale D. Language and the development of children’s Ethnic Prejudice. J. Lang. Soc. Psychol. 2001;20(1–2):90–110. doi: 10.1177/0261927X01020001005. [DOI] [Google Scholar]
- Newheiser A.-K., Olson K.R. White and black American children’s implicit intergroup bias. J. Exp. Soc. Psychol. 2012;48(1):264–270. doi: 10.1016/j.jesp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A., Flykt A., Esteves F. Emotion drives attention: Detecting the snake in the grass. J. Exp. Psychol.: Gen. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Patterson M.M., Bigler R.S. Preschool Children’s Attention to Environmental Messages About Groups: Social Categorization and the Origins of Intergroup Bias. Child Development. 2006;77(4):847–860. doi: 10.1111/j.1467-8624.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- Ratner K.G., Kaul C., Van Bavel J.J. Is race erased? Decoding race from patterns of neural activity when skin color is not diagnostic of group boundaries. Soc. Cogn. Affect. Neurosci. 2013;8(7):750–755. doi: 10.1093/scan/nss063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggev N., Brodie K., Cikara M., Mitchell J.P. Human face-selective cortex does not distinguish between members of a racial outgroup. Eneuro. 2020;7(3) doi: 10.1523/ENEURO.0431-19.2020. ENEURO.0431-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard J.A., Parker T.C., Dhamala E., Kwasa J., Allsop A., Holmes A.J. Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nat. Neurosci. 2023;26(1):4–11. doi: 10.1038/s41593-022-01218-y. [DOI] [PubMed] [Google Scholar]
- Richeson J.A., Baird A.A., Gordon H.L., Heatherton T.F., Wyland C.L., Trawalter S., Shelton J.N. An fMRI investigation of the impact of interracial contact on executive function. Nat. Neurosci. 2003;6(12):1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Richeson J.A., Shelton J.N. When prejudice does not pay: effects of interracial contact on executive function. Psychol. Sci. 2003;14(3):287–290. doi: 10.1111/1467-9280.03437. [DOI] [PubMed] [Google Scholar]
- Richeson J.A., Sommers S.R. Toward a social psychology of race and race relations for the twenty-first century. Annu. Rev. Psychol. 2016;67(1):439–463. doi: 10.1146/annurev-psych-010213-115115. [DOI] [PubMed] [Google Scholar]
- Richeson J.A., Trawalter S. The threat of appearing prejudiced and race-based attentional biases. Psychol. Sci. 2008;19(2):98–102. doi: 10.1111/j.1467-9280.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- Roberts S.O., Bareket-Shavit C., Dollins F.A., Goldie P.D., Mortenson E. Racial inequality in psychological research: trends of the past and recommendations for the future. Perspect. Psychol. Sci. 2020;15(6):1295–1309. doi: 10.1177/1745691620927709. [DOI] [PubMed] [Google Scholar]
- Rubien-Thomas E., Berrian N., Cervera A., Nardos B., Cohen A.O., Lowrey A., Daumeyer N.M., Camp N.P., Hughes B.L., Eberhardt J.L., Taylor-Thompson K.A., Fair D.A., Richeson J.A., Casey B.J. Processing of task-irrelevant race information is associated with diminished cognitive control in black and white individuals. Cogn., Affect., Behav. Neurosci. 2021;21(3):625–638. doi: 10.3758/s13415-021-00896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubien-Thomas E., Berrian N., M. Rapuano K., J. Skalaban L., Cervera A., Nardos B., Cohen A.O., Lowrey A., M. Daumeyer N., Watts R., Camp N.P., Hughes B.L., Eberhardt J.L., Taylor-Thompson K.A., Fair D.A., Richeson J.A., Casey B.J. Uncertain threat is associated with greater impulsive actions and neural dissimilarity to Black versus White faces. Cogn., Affect., Behav. Neurosci. 2023;23(3):944–956. doi: 10.3758/s13415-022-01056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.A., Bullock M. Multidimensional scaling of emotional facial expressions: similarity from preschoolers to adults. J. Personal. Soc. Psychol. 1985;48(5):1290–1298. doi: 10.1037/0022-3514.48.5.1290. [DOI] [Google Scholar]
- Rutland A., Cameron L., Bennett L., Ferrell J. Interracial contact and racial constancy: A multi-site study of racial intergroup bias in 3–5 year old Anglo-British children. Journal of Applied Developmental Psychology. 2005;26(6):699–713. doi: 10.1016/j.appdev.2005.08.005. [DOI] [Google Scholar]
- Saragosa-Harris N.M., Chaku N., MacSweeney N., Guazzelli Williamson V., Scheuplein M., Feola B., Cardenas-Iniguez C., Demir-Lira E., McNeilly E.A., Huffman L.G., Whitmore L., Michalska K.J., Damme K.S., Rakesh D., Mills K.L. A practical guide for researchers and reviewers using the ABCD Study and other large longitudinal datasets. Dev. Cogn. Neurosci. 2022;55 doi: 10.1016/j.dcn.2022.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa P., Dalmaso M. Race perception and gaze direction differently impair visual working memory for faces: an event-related potential study. Soc. Neurosci. 2016;11(1):97–107. doi: 10.1080/17470919.2015.1040556. [DOI] [PubMed] [Google Scholar]
- Stanislaw H., Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods, Instrum., Comput. 1999;31(1):137–149. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- Stelter M., Degner J. Investigating the other-race effect in working memory. Br. J. Psychol. 2018;109(4):777–798. doi: 10.1111/bjop.12304. [DOI] [PubMed] [Google Scholar]
- Stolier R.M., Freeman J.B. Neural pattern similarity reveals the inherent intersection of social categories. Nat. Neurosci. 2016;19(6):795–797. doi: 10.1038/nn.4296. [DOI] [PubMed] [Google Scholar]
- Strohminger N., Gray K., Chituc V., Heffner J., Schein C., Heagins T.B. The MR2: A multi-racial, mega-resolution database of facial stimuli. Behav. Res. Methods. 2016;48(3):1197–1204. doi: 10.3758/s13428-015-0641-9. [DOI] [PubMed] [Google Scholar]
- Tajfel H. Differentiation between social groups. Academic Press; London: 1978. The achievement of inter-group differentiation; pp. 77–100. [Google Scholar]
- Tajfel H. Social Psychology of Intergroup Relations. Annu. Rev. Psychol. 1982;33(1):1–39. doi: 10.1146/annurev.ps.33.020182.000245. [DOI] [Google Scholar]
- Tajfel H., Turner J.C. Intergroup relations: Essential readings. Brooks/Cole; Monterey, CA: 1979. An integrative theory of inter-group conflict; pp. 94–109. [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Marcus D.J., Westerlund A., Casey B., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawalter S., Todd A.R., Baird A.A., Richeson J.A. Attending to threat: race-based patterns of selective attention. J. Exp. Soc. Psychol. 2008;44(5):1322–1327. doi: 10.1016/j.jesp.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M., Schönborn S., Greifeneder R., Vetter T. The Basel Face Database: a validated set of photographs reflecting systematic differences in Big Two and Big Five personality dimensions. PLOS ONE. 2018;13(3) doi: 10.1371/journal.pone.0193190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler Intelligence Scale for Children (5th Edition). (2014). [Computer software]. https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Gifted-%26-Talented/Wechsler-Intelligence-Scale-for-Children-%7C-Fifth-Edition-/p/100000771.html.
- Williams A., Steele J.R. Examining children’s implicit racial attitudes using exemplar and category-based measures. Child Dev. 2019;90(3) doi: 10.1111/cdev.12991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
The authors do not have permission to share data.