Helicobacter pylori is a small, curved, highly motile, Gram negative bacillus that colonises only the mucus layer of the human stomach. Since its discovery in 1984, it has been recognised as the principal cause of peptic ulcer disease and as the main risk factor for the development of gastric cancer. However, most infected people (>70%) are asymptomatic. We therefore need to discover how infection is acquired, why ulcers or cancer occur in so few of those infected, and how this subgroup can be identified and treated.
Epidemiology of H pylori infection
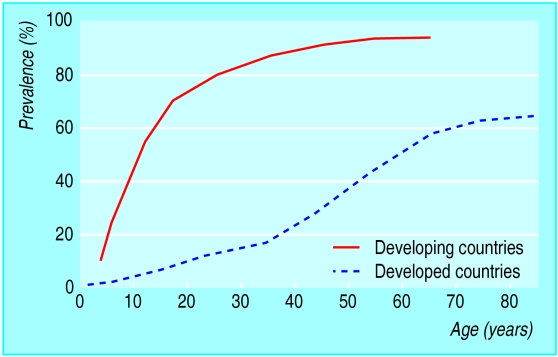
H pylori is one of the commonest bacterial pathogens in humans. The prevalence of infection varies but is falling in most developed countries. Seropositivity increases with age and low socioeconomic status. Retrospective seroepidemiological studies have shown a cohort effect consistent with the hypothesis that infection is mainly acquired in early childhood. Until recently, however, it has been difficult to assess accurately the incidence (or route) of infection because of the inaccuracy and cost of detecting (non-invasively) H pylori in young children. Primary acquisition in adults, or reinfection after successful eradication, does occur but is less common, with an annual incidence of 0.3-0.7% in developed countries and 6-14% in developing countries.
How H pylori is usually acquired and its route of transmission are unknown. Since humans are the only known reservoir of infection, it is likely that in developed countries H pylori is picked up from siblings, other children, or parents, predominantly via the gastro-oral route. In developing countries faecal-oral transmission may also occur. Various risk factors are associated with H pylori infection, but the extent to which these are simply markers of childhood socioeconomic deprivation is unclear. H pylori infection is an occupational hazard for gastroenterologists and is associated with performing endoscopy.
Why is H pylori a chronic infection?
Although H pylori initially induces an acute inflammatory gastritis, this immunological response by the host is generally not sufficient to clear infection, which persists for life. In addition, infection with one strain of H pylori does not protect against subsequent co-infection with a different strain. Infection with multiple strains is quite common and occurs more frequently in developing countries. Polyclonal infection allows DNA to be exchanged between different strains, which could promote the spread of genes encoding important virulence factors or resistance to antibiotics.
H pylori is not a new bacteria species and, by virtue of its urease enzyme and other products, has become extremely well adapted to its unique niche within the gastric mucus. It also has considerable genetic heterogeneity (no two strains are identical), and studies have suggested that this diversity may allow each strain to become uniquely adapted to each host to an extent that, for some subjects, it may be considered as a commensal bacteria.
Peptic ulcer and gastric cancer
About 15% of infected individuals will develop peptic ulcer (duodenal or gastric) or gastric cancer as a long term consequence of infection. The outcome of infection depends mainly on the severity and topography of histological gastritis, which may be determined by the age at which infection is acquired. Infection in infancy is thought to lead to pangastritis, whereas acquisition in later childhood may lead to a predominantly antral gastritis only.
With antral gastritis there is loss of regulatory feedback (but with an intact and undamaged acid secreting gastric corpus), and the high acid load reaching the duodenum leads to the development of duodenal gastric metaplasia. The islands of gastric metaplasia are subsequently colonised by H pylori, leading to duodenitis and a high risk of duodenal ulcer.
In contrast, pangastritis, with an inflamed corpus, is associated with the loss of acid secreting cells, which leads to an increased risk of gastric ulcer and gastric cancer—similar to that seen with autoimmune gastritis in pernicious anaemia.
Diagnosis
Invasive tests
H pylori can be detected at endoscopy by histology, culture, or urease tests, each with inherent advantages and disadvantages. All these biopsy based methods for detecting H pylori are liable to sampling error because infection is patchy. Up to 14% of infected patients do not have antral infection but have H pylori elsewhere in the stomach, especially if they have gastric atrophy, intestinal metaplasia, or bile reflux. In addition, after partially effective eradication treatment, low levels of infection can easily be missed by endoscopic biopsy, leading to overestimates of the efficacy of eradication treatment and reinfection rates. Proton pump inhibitors affect the pattern of H pylori colonisation of the stomach and compromise the accuracy of antral biopsy. Consensus guidelines therefore recommend that multiple biopsies are taken from the antrum and corpus for histology and for one other method (either culture or urease testing).
Histology—Although H pylori may be recognised on sections stained with haematoxylin and eosin alone, supplementary stains (such as Giemsa, Genta, Gimenez, Warthin-Starry silver, Creosyl violet) are needed to detect low levels of infection and to show the characteristic morphology of H pylori. An important advantage of histology is that, in addition to the historical record provided, sections from biopsies (or even additional sections) can be examined at any time, and that gastritis, atrophy, or intestinal metaplasia can also be assessed. Biopsy specimens from other parts of the stomach can be retained in formalin to be processed only if antral histology is inconclusive.
Culture—Microbiological isolation is the theoretical gold standard for identifying any bacterial infection, but culture of H pylori can be unreliable. Risks of overgrowth or contamination make it the least sensitive method of detection, and it is the least readily available test for use with endoscopy. Although only a few centres routinely offer microbiological isolation of H pylori, the prevalence of multiresistant strains makes it increasing likely that culture and antibiotic sensitivity testing may become a prerequisite for patients with persistent infection after initial or repeated treatment failure.
Urease tests are quick and simple tests for detecting H pylori infection but indicate only the presence or absence of infection. The CLO test and cheaper “home made” urease tests are of similar sensitivity and specificity. However, the sensitivity of urease tests is often higher than that of other biopsy based methods because the entire biopsy specimen is placed in the media, thereby avoiding the additional sampling or processing error associated with histology or culture. The sensitivity of biopsy urease tests seems to be much lower (∼60%) in patients with upper gastrointestinal bleeding, but this can be improved by placing multiple biopsy samples into the same test vial.
Comparative accuracy, availability, and costs of tests for H pylori infection
| Test | Sensitivity | Specificity | Availability | Cost |
| Invasive | ||||
| Histology | 88-95% | 90-95% | ++++ | ££££ |
| Culture | 80-90% | 95-100% | ++ | £££ |
| Urease test | 90-95% | 90-95% | ++++ | £-££ |
| Non-invasive | ||||
| 13C-UBT | 90-95% | 90-95% | ++++ | £££ |
| 14C-UBT | 86-95% | 86-95% | +++ | ££ |
| Serology: | ||||
| ELISA | 80-95% | 80-95% | +++ | £ |
| NPT | 60-90% | 70-85% | ++++ | ££ |
| Stool antigen | 90-95% | 90-95% | ++ | ££ |
UBT=urea breath test. NPT=near patient test.
Non-invasive tests
Serology
H pylori infection elicits a local mucosal and a systemic antibody response. Circulating IgG antibodies to H pylori can be detected by enzyme linked immunosorbent assay (ELISA) antibody or latex agglutination tests. These tests are generally simple, reproducible, inexpensive, and can be done on stored samples. They have been used widely in epidemiological studies, including retrospective studies to determine the prevalence or incidence of infection.
Individuals vary considerably in their antibody responses to H pylori antigens, and no single antigen is recognised by sera from all subjects. The accuracy of serological tests therefore depends on the antigens used in the test, making it essential that H pylori ELISA is locally validated. In elderly people with lifelong infection, underlying atrophic gastritis has been associated with false negative results. Consumption of non-steroidal anti-inflammatory drugs has also been reported to affect the accuracy of ELISAs.
Antibody titres fall only slowly after successful eradication, so serology cannot be used to determine H pylori eradication or to measure reinfection rates. Although titres of IgM antibodies to H pylori fall with age, there are no reliable IgM assays to indicate recent acquisition, which, since this is usually asymptomatic, makes it difficult to identify cases of primary infection and thus establish possible routes of transmission.
An important advantage of serological methods over other tests for H pylori infection has been the development of simple finger prick tests that use a fixed, solid phase assay to detect the presence of H pylori immunoglobulins. These “near patient tests” (NPT) can be performed in primary care and are much simpler than the 13C-urea breath test, which is the only other test for H pylori that is currently used as a NPT. However, the accuracy of the serological NPT is lower than that reported for standard ELISA tests using the same antigen preparations. These tests are often used to reassure patients, but to date no studies have compared the accuracy, cost effectiveness, and reassurance value of the 13C-urea breath test with the serological NPT in primary care.
Further reading
Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer disease and gastric cancer: a model. Gastroenterology 1997;113:1983-91
Misiewicz JJ, Harris A. Clinician's manual on Helicobacter pylori. London: Science Press, 1995
Urea breath test
Non-invasive detection of H pylori by the 13C-urea breath test is based on the principle that a solution of urea labelled with carbon-13 will be rapidly hydrolysed by the urease enzyme of H pylori. The resulting CO2 is absorbed across the gastric mucosa and hence, via the systemic circulation, excreted as 13CO2 in the expired breath. The 13C-urea breath test detects current infection and is not radioactive. It can be used as a screening test for H pylori, to assess eradication and to detect infection in children. The similar but radioactive 14C-urea breath test cannot be performed in primary care.
Faecal antigen test
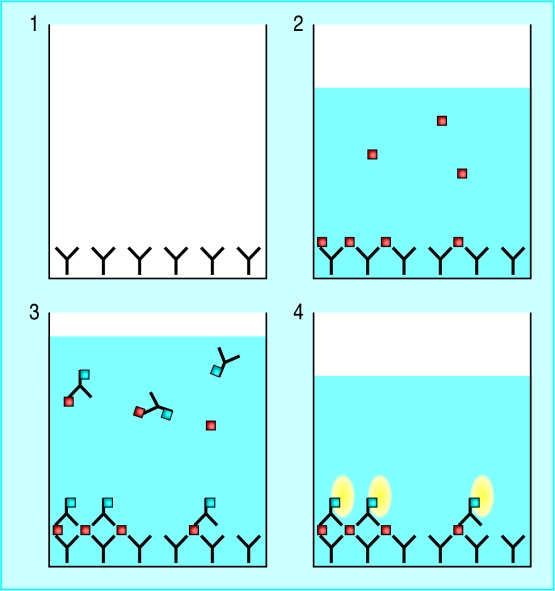
In the stool antigen test a simple sandwich ELISA is used to detect the presence of H pylori antigens shed in the faeces. Studies have reported sensitivities and specificities similar to those of the 13C-urea breath test (>90%), and the technique has the potential to be developed as a near patient test. The main advantage of the test, however, is in large scale epidemiological studies of acquisition of H pylori in children.
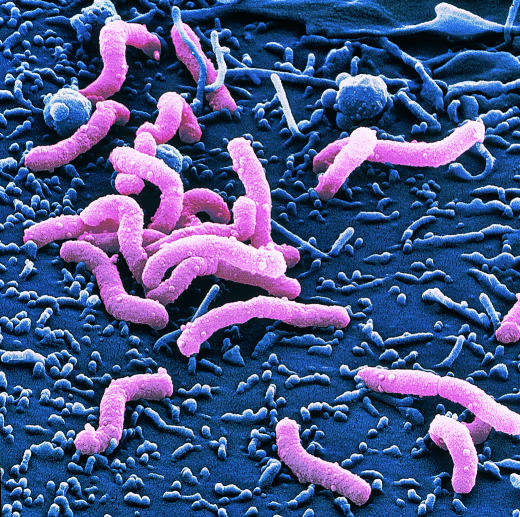
Figure.
Coloured scanning electron micrograph of H pylori on surface of gastric cell
Figure.
Prevalence of H pylori infection by age in developing and developed countries
Figure.
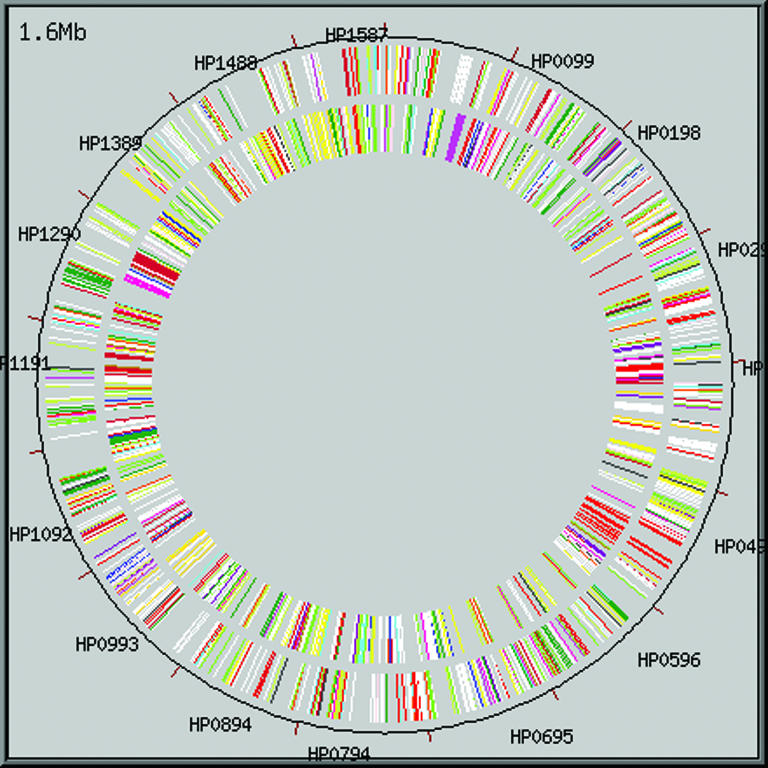
Circular display of the H pylori genome. The recent sequencing of the whole genome of two separate H pylori strains will help in developing new treatments and emphasises the importance of H pylori as a human pathogen
Figure.
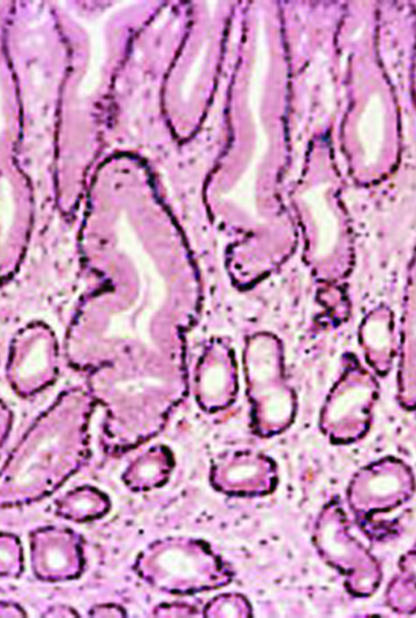
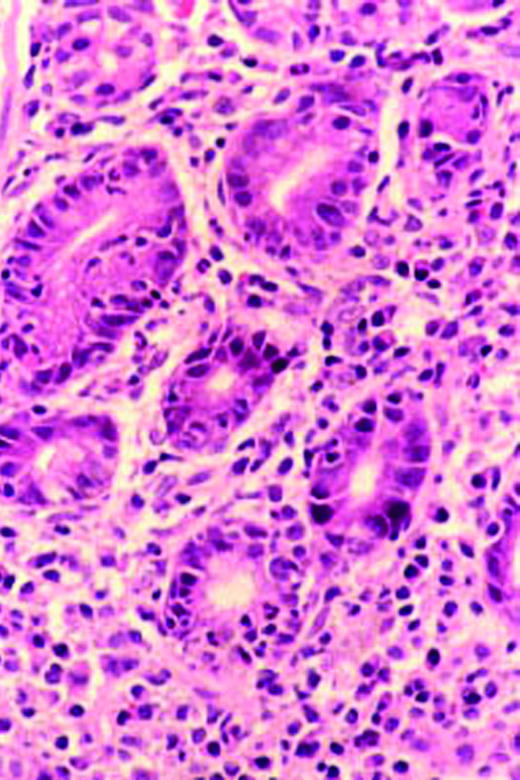
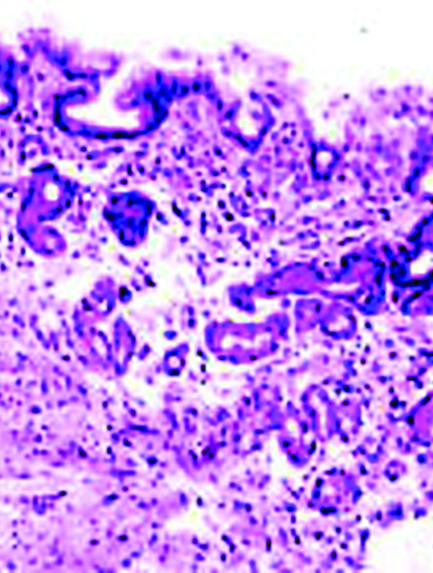
Histology of gastric mucosa. Top left: normal antral mucosa, with sparse infiltrate of lymphocytes in lamina propria. Top right: active gastritis with neutrophils infiltrating epithelium and marked infiltrate of lymphocytes in lamina propria. Bottom: atrophy of antral mucosa with loss of specialised glands near muscularis mucosa
Figure.

Culture of H pylori tested for antibiotic sensitivity with an E strip that is impregnated with a scale of increasing concentrations of metronidazole
Figure.
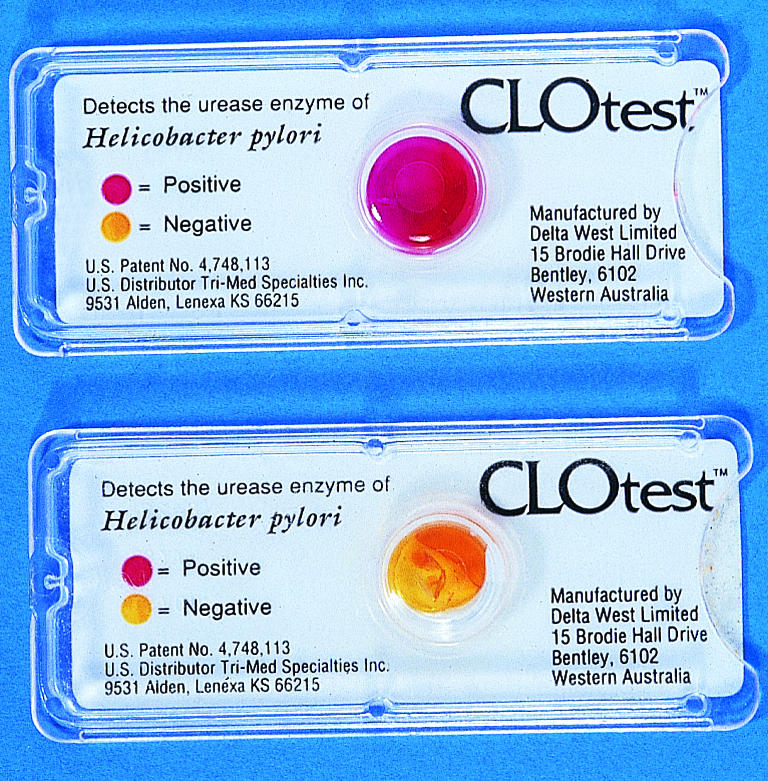
Positive and negative results of CLO test for H pylori. The urease of H pylori hydrolyses urea to release ammonia, which is detected colorimetrically
Figure.
Principle of the faecal antigen test. Polyclonal antibody to H pylori is adsorbed to microwells (1). Diluted patient samples are added to the wells, and any H pylori in the faecal sample is bound to the adsorbed antibody (2). A second H pylori antibody conjugated to peroxidase is added and binds to H pylori (3). After unbound material is washed off, a substrate is added that reacts with bound peroxidase enzyme to produce a yellow colour (4), the intensity of which can be measured to estimate H pylori levels
Acknowledgments
The electron micrograph of H pylori is reproduced with permission of Juergen Berger (Max-Planck Institute) and Science Photo Library.
Footnotes
Robert P H Logan is senior lecturer in the division of gastroenterology, University Hospital, Nottingham. Marjorie M Walker is senior lecturer in the department of histopathology, Faculty of Medicine, Imperial College, St Mary's Campus, London.
The ABC of upper gastrointestinal tract is edited by Robert Logan, Adam Harris, consultant physician and gastroenterologist, Kent and Sussex Hospital, Tunbridge Wells, J J Misiewicz, honorary consultant physician and joint director of the department of gastroenterology and nutrition, Central Middlesex Hospital, London, and J H Baron, honorary professorial lecturer at Mount Sinai School of Medicine, New York, USA, and former consultant gastroenterologist, St Mary's Hospital, London.