Abstract
Mice harbor a family of endogenous retroviruses, the mouse mammary tumor viruses (MMTV), which encode superantigens. These superantigens are responsible for the deletion of T cells expressing certain Vβ chains of the T-cell receptor in the thymus. Human T cells are able to recognize MMTV-encoded superantigens presented by human major histocompatibility complex class II-positive cells. Owing to this and to the similarity of the human and murine immune systems, it was speculated that human endogenous retroviruses might also code for superantigens. Recently, it was reported that a proviral clone (IDDMK1,222) of the human endogenous retrovirus family HTDV/HERV-K encodes a superantigen. The putative superantigen gene was located within the env region of the virus. Stimulated by these findings, we amplified by PCR and cloned into eucaryotic expression vectors open reading frames (ORFs) which were identical or very similar to IDDMK1,222. When we transfected these vectors into A20 cells, a murine B-cell lymphoma, we were able to demonstrate mRNA expression and protein production. However, we did not find any evidence that the ORF stimulated human or murine T cells in a Vβ-specific fashion, the most prominent feature of superantigens.
Superantigens (Sags) are characterized as stimulating a large fraction of peripheral T cells expressing a certain Vβ chain of the T-cell receptor (TCR) (21). If they are expressed within the thymus, they induce a Vβ-specific deletion of thymocytes (3). To activate T cells or delete thymocytes, Sags have to be presented by major histocompatibility complex class II (MHC-II)-positive cells (11, 12). They are produced by gram-positive bacteria, in particular Staphylococcus aureus and Streptococcus pyogenes (21), as well as by a family of murine endogenous retroviruses, the mouse mammary tumor viruses (MMTV) (1, 2). Until now no other retrovirus was shown convincingly to encode a Sag. Since human T cells are stimulated in a Vβ-specific pattern by MMTV-encoded Sags presented by human MHC-II-positive cells (17), and since the human and murine immune systems are very similar, it was speculated that human endogenous Sags might also exist. By analogy to the murine situation, it was envisaged that these endogenous human Sags are encoded by endogenous retroviral elements which reside in the human genome. It was suggested repeatedly that the postulated endogenous human Sags might be responsible for the initiation of human autoimmune diseases like systemic lupus erythematosus, multiple sclerosis, or type I diabetes mellitus. Indeed, in the murine model of experimental autoimmune encephalitis, relapsing paralysis can be triggered by bacterial Sags (7), and, in humans, expression of an endogenous retrovirus family (MSRV) was shown to be associated with the occurrence of multiple sclerosis (23). Furthermore, Conrad et al. showed that expression of a proviral sequence designated IDDMK1,222 of the human endogenous retrovirus family HTDV/HERV-K was associated with the onset of type I diabetes mellitus (10). This association, however, could not be confirmed in other studies (18, 20, 22). Conrad et al. also demonstrated that IDDMK1,222 encodes a Sag function and suggested that the Sag might be responsible for the initiation of type I diabetes mellitus, since the Sag activated Vβ7+ T cells in a test system (10). Vβ7 T-cell expansion was also detected in patients with type I diabetes mellitus (9).
Intrigued by the hypothesis that a human endogenous Sag might exist, we cloned the retroviral open reading frame (ORF) described by Conrad et al. and tested the activity of its product as a Sag. Here we report experiments to test whether a Vβ-specific subset of human T cells was activated by the ORF product presented by murine A20 cells. To circumvent the problem of allogenic stimulation, which is unavoidable in a human-based test system, we had previously developed an assay system consisting of syngeneic murine cells (30), including the B-cell lymphoma line A20 as an antigen-presenting cell (APC) and T cells. In this study we also took advantage of this test system to elucidate whether the endogenous retroviral ORF product represents a Sag, i.e., stimulates T cells in a Vβ-specific fashion.
MATERIALS AND METHODS
Reagents and antibodies.
Anti-Flag monoclonal antibody M2 (MAb) (catalog no. F3165) came from Sigma (Deisenhofen, Germany). The fluorescein isothiocyanate (FITC)-labeled anti-human Vβ7 MAb (clone ZOE) was purchased from Coulter (Hialeah, Fla.). The biotinylated anti-human CD3 MAb (clone UCHT1), the biotinylated anti-murine Vβ8.1 and -8.2 MAb (clone MR5-2), the phycoerythrin (PE)-labeled anti-murine Vβ10 MAb (clone B21.5), the biotinylated anti-murine Vβ14 MAb (clone 14-2), and the FITC-labeled anti-murine CD3ɛ MAb (clone 145-2C11) were bought from Pharmingen (San Diego, Calif.). The hybridoma KT4, which produces an anti-murine Vβ4 MAb, was kindly donated by H. Hengartner (Zürich, Switzerland). The antibody was purified and biotinylated according to standard protocols. PE-conjugated streptavidin was purchased from Jackson ImmunoResearch (West Grove, Pa.), and staphylococcal enterotoxin B (SEB) (BT202) was from Toxin Technology (Sarasota, Fla.).
Cell culture.
The murine B-cell lymphoma A20 (16) cell line was cultured in Clicks RPMI medium (Seromed Biochrom, Berlin, Germany) with 10% fetal calf serum (FCS; BioWhittaker, Verviers, Belgium), 2 mM glutamine (Seromed Biochrom), 100 U of penicillin and 100 μg of streptomycin (Seromed Biochrom) per ml, 50 μM 2-mercaptoethanol (GIBCO BRL, Paisley, Scotland), and 1 μg of indomethacine (Sigma) per ml.
Human T cells were isolated from a healthy blood donor's buffy coat by Ficoll (density, 1.077; Seromed Biochrom) separation followed by negative selection via a magnetic cell sorting column with the Pan T-cell isolation kit (Miltenyi Biotec, Auburn, Calif.). The purity of the T-cell fraction was over 90%, as assessed by CD3-CD14 double staining and flow cytometry.
Murine lymphocytes were freshly prepared from the mesenteric lymph nodes of BALB/c mice (Harlan, Borchen, Germany) by teasing through a mesh metal sieve. Over 85% of the viable cells were CD3+ T cells.
Plasmids and primers.
pEGFP-N1 was kindly donated by H. Häcker (Munich, Germany), pMMTV2Sag was a gift from W. Günzburg (Vienna, Austria), pCIneo-DMSag was generously provided by B. Conrad (Geneva, Switzerland), and pRK5 came from Pharmingen.
The putative Sag gene in the plasmid pHKputSag1 was amplified from the genome of a patient with type I diabetes mellitus using the primers HERV-1 (ACCCATCAGAGATGCAAAGAAAAGC) and R300 (CTTTACAAAGCAGTATTGCTGC), resulting in a PCR product of approximately 2.2 kb. To obtain the coding region of the putative Sag gene, a second PCR was performed using the primers 5′ Sag (ATCCGCGGATCCCACCATGGTAACACCAGTCACATG) and 3′Sag (CTCGAGCTAATCTATAATAGTTCCGAA). The PCR product was cloned into the mammalian expression vector pRK5. The resulting plasmid, pHKputSag1, contained only the endogenous retrovirus Sag ORF with a Kozak sequence in the vector pRK5. The plasmid pHKputSag1-FLAG contains the Flag sequence but is otherwise identical to pHKputSag1.
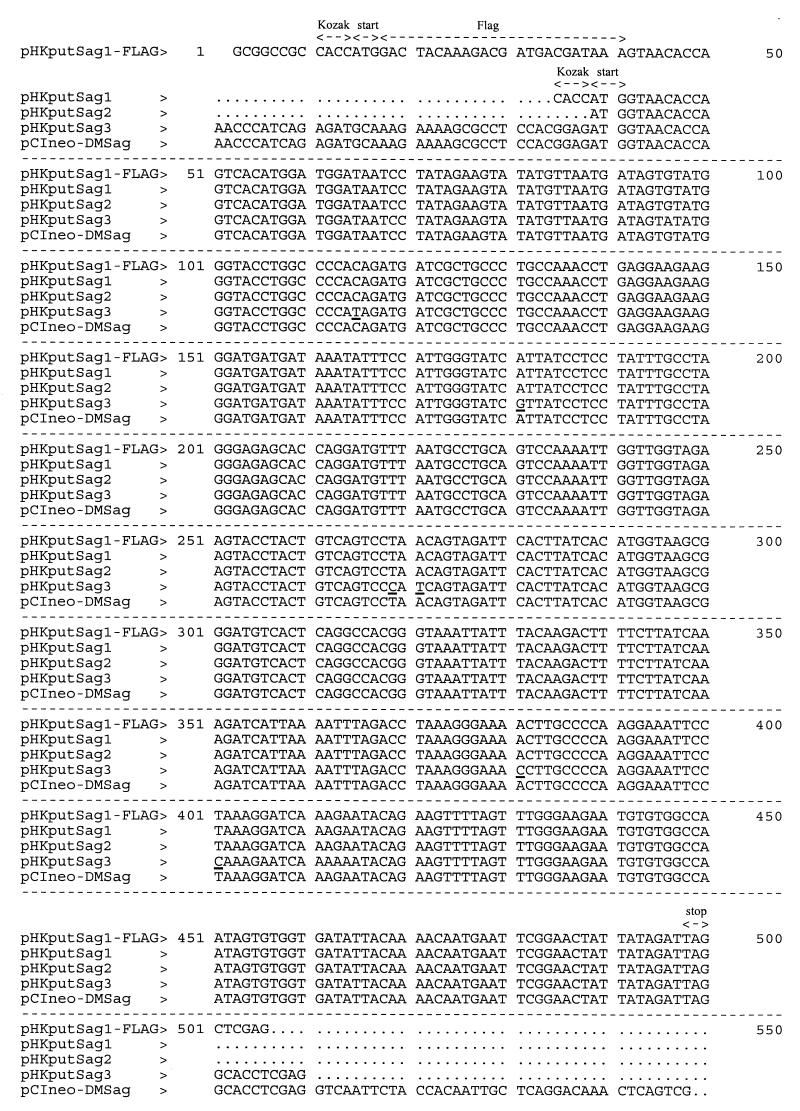
Plasmid pHKputSag2 contained the same sequence of the endogenous retrovirus Sag ORF as pHKputSag1 but was cloned into the HindIII and XbaI sites of the vector pRC/CMV (Invitrogen, Carlsbad, Calif.) after amplification with primers IDMM3 (TCCAAGCTTATGGTAACACCAGTCACATGG) and IDDM4 (ACGTCTAGACTAATCTATAATAGTTCCGAATTC). pHKputSag3 also contained the same endogenous Sag ORF but had a slightly different sequence (see Fig. 1). This plasmid was constructed as detailed in reference 10, relying on the same primers (IDDM1, GACTAAGCTTAAGAACCCATCAGAGATGC, and IDDM2, AGACTGGATCCGTTAAGTCGCTATCGACAGC) and subcloning into pCIneo (Promega, Madison, Wis.). Primers were purchased from TIB Molbiol (Berlin, Germany) and ARK Scientific Biosystems (Darmstadt, Germany).
FIG. 1.
Sequence comparison of the IDDMK1,222-borne ORF and ORFs cloned from different type I diabetes mellitus patients. Underlined residues indicate point mutations in pHKputSag3.
Transfection.
Twenty-four hours after passaging, 107 A20 cells in 400 μl of RPMI medium–10% FCS with 20 μg of plasmid DNA were electroporated in a 0.4-cm cuvette at 960 μF and 280 V using a gene pulser (Bio-Rad, Hercules, Calif.). Transfection efficiency was controlled with the pEGFP-N1 vector by flow cytometry 24 h posttransfection.
Detection of mRNA by reverse transcription and PCR.
Transfected cells (5 × 106 A20 cells, 20 μg of plasmid DNA) were cultured overnight (37°C). After centrifugation (300 × g for 5 min) the pellet was lysed with 600 μl of RLT (RNA lysis/thiocyanate) buffer (Qiagen, Hilden, Germany). RNA was extracted using the RNeasy kit (no. 74103) from Qiagen. The amount of RNA was quantified photometrically (260 nm). Afterward, any contaminating DNA was digested with DNase (20 U/2 μg of RNA, 10 min, 37°C; Roche, Mannheim Diagnostics GmbH, Mannheim, Germany), and cDNA was synthesized using 1 μl of oligo(dT) (10 μM; Gibco Life Technology Inc., Rockville, Md.), 1 μl of hexamer random primer (10 μM; Gibco), 4 μl of 5× transcription buffer (Gibc), 2 μl of dithiothreitol (0.1 M; Gibco), 2 μl deoxynucleoside triphosphate (200 μM; Roche), 0.5 μl of Rnasin (Promega), and 1 μl of Superscript II Plus (Gibco). The reaction mixture was incubated for 60 min at 37°C and subsequently heated to 95°C for 5 min.
Two microliters of the generated cDNA was used for PCR amplification to detect the transcribed retroviral ORF using the 5′ primer 5′-Sag and the 3′ primer 3′-Sag. The PCR mixture consisted of 10 μl of deoxynucleoside triphosphate (1 mM; Roche), 1.5 μl of each of the primers 5′-Sag and 3′-Sag (50 μM; TIB Molbiol), 29 μl of H2O, 1 μl of Pfu Turbo (Invitrogen), and 5 μl of 10× buffer (Invitrogen). The cycle conditions were 94°C and 30 s for denaturation, 55°C and 60 s for annealing, and 72°C and 120 s for elongation. The PCR was run for 30 cycles. The PCR products were visualized with an ethidium bromide-stained 1% agarose gel.
Western blotting.
Transfected cells (5 × 106 A20 cells, 20 μg of plasmid DNA) were cultured overnight (37°C) and subsequently lysed in NP-40 (1%, vol/vol). Cell lysates were separated by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis using Laemmli sample buffer (15.1 g of Tris per liter, 9.4 g of glycine per liter, 0.5% sodium dodecyl sulfate). After being transferred onto nitrocellulose by semidry electroblotting for 2 h (250 mA), the filters were blocked (5% milk powder in TBST [2.4 g of Tris per liter, 8 g of NaCl per liter, 0.1% Tween, pH 7.6], 1 h at 37°C and 1 h at room temperature) and incubated with the anti-Flag MAb M2 (diluted 1:1,000, overnight, 4°C). After three washings with 1× TBST, goat anti-mouse serum coupled with peroxidase was added (diluted 1:5,000, 1 h, room temperature; Dianova, Hamburg, Germany). The blot was washed again three times with 1× TBST and visualized by using the enhanced chemiluminescence reagent (NEN Life Science Products, Boston, Mass.) as described by the manufacturer.
Mixed-lymphocyte culture.
Twenty-four hours after transfection, the A20 cells were arrested in growth by a 1-h incubation with 80-ng/ml mitomycin C. After three washings they were subsequently cocultured at a stimulator-responder ratio of 2:1 or 1:1 with human T cells in RPMI medium containing 5% autologous human serum for a total culture period of 8 days with 10 U of interleukin-2 (IL-2) per ml added after 3 days of culture. The BALB/c lymphocytes were cocultured with the mitomycin-treated A20 cells at a stimulator-responder ratio of 2:1 in RPMI medium–10% FCS with 10 U of IL-2 per ml for 4 to 5 days.
FACS analysis.
After the mixed-lymphocyte culture, viable T lymphocytes were retrieved from the coculture by gradient centrifugation with Ficoll at a density of 1.077 for human and 1.090 for murine lymphocytes. The cells were then stained with the anti-Vβ-antibodies (diluted 1:100) mentioned above and counterstained with anti-CD3 (diluted 1:100). In the case of biotinylated antibodies, a second staining step with streptavidin-PE (diluted 1:100) was added. The fluorescence-activated cell sorter (FACS) analysis was performed with a Facscalibur cytometer (Becton Dickinson, Franklin Lakes, N.J.).
Proliferation assay.
The proliferative response of murine T cells was measured on day 4 or 5 by pulsing microcultures with 37 kBq of [3H]thymidine (NEN, Zaventem, Belgium) for 8 h. Thereafter, cultures were harvested on fiberglass filters, and the amount of [3H]thymidine incorporation was determined by direct beta counting (Matrix 96; Packard Instrument Co., Meriden, Conn.).
RESULTS
Cloning and sequencing of the ORF sequence.
The putative HTDV/HERV-K Sag was amplified from the genome of peripheral blood lymphocytes from a patient with type I diabetes mellitus using the primers listed in Materials and Methods. PCR products of the appropriate size were obtained from patients as well as healthy blood donors (data not shown). The PCR products were cloned into the mammalian expression vector pRK5, resulting in the plasmid pHKputSag1; into the vector pRC/CMV, resulting in the plasmid pHKputSag2; and into the vector pCIneo, resulting in the plasmid pHKputSag3. As demonstrated in Fig. 1, the sequences of the ORFs encoding the putative Sag are identical or very similar to the one published by Conrad et al. (10), reflecting the multicopy nature of the endogenous retrovirus family HTDV/HERV-K. The plasmids and a plasmid containing the established Sag gene from MMTV2 were used to transiently transfect A20 cells for the functional characterization of the ORF. The different vector backbones were used to exclude potential unspecific activation of transfected cells due to nonphysiological high expression of a foreign protein by potent eucaryotic promoters. Besides the MMTV2-encoded Sag, the bacterial Sag SEB, was included as a positive control.
Transcription and protein expression of the ORF in A20 cells.
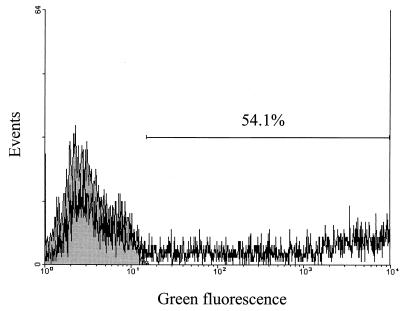
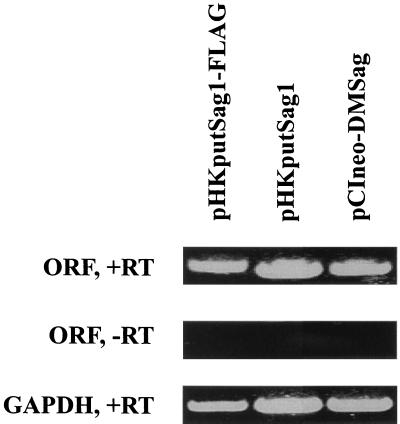
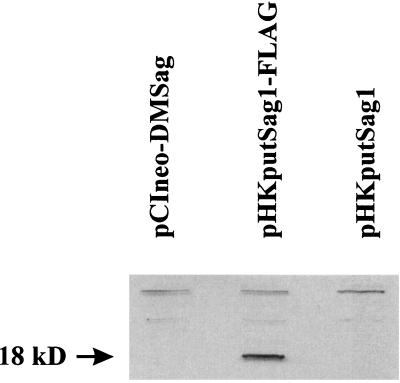
At first we verified that the plasmids were transcribed in the transfected cells. A plasmid encoding the enhanced green fluorescent protein (EGFP) was used to measure the transfection efficiency of A20 cells. As shown in Fig. 2, around 50% of all living cells were transfected. Using reverse transcription and PCR, we showed (Fig. 3) that A20 cells electroporated with the different plasmids expressed mRNA encoding the respective ORF products. Since there is no antibody available which would recognize the product of the ORFs, we inserted a Flag sequence at the 5′ end of the coding sequence, giving rise to the plasmid pHKputSag1-FLAG (Fig. 1). A protein with the predicted size of 18 kDa was detected by Western blotting using an anti-Flag MAb only in A20 cells transfected with pHKputSag1-FLAG (Fig. 4).
FIG. 2.
Transfection efficiency of A20 cells. A20 cells were transfected with the EGFP-encoding plasmid pEGFP-N1. After 24 h the cells were analyzed by FACS for the expression of EGFP. Shadowed area, cells transfected with an empty vector; solid line, EGFP-transfected cells.
FIG. 3.
Transcription of pHKputSag1, pHKputSag1-FLAG, and pCIneo-DMSag in A20 cells. A20 cells were transfected with pHKputSag1, pHKputSag1-FLAG, and pCIneo-DMSag (20 μg of DNA/20 × 106 cells). After 24 h RNA was prepared. After reverse transcription (+RT) and treatment with DNase, ORF cDNA was amplified by PCR. All three plasmids are transcribed (top panel). The omission of reverse transcription (−RT) prevents detection by PCR (middle panel), indicating that the PCR products were not derived from the transfected plasmid. The glyceraldehyde-3-phosphate-dehydrogenase gene (GAPDH) was also amplified to show that approximately equal amounts of RNA were used for reverse transcription and PCR amplification (bottom panel). The experiment was repeated twice with identical results.
FIG. 4.
Translation of the Flag ORF in A20 cells. A20 cells were transfected with the indicated plasmids. After 24 h, cells were lysed with NP-40 and the cell lysate was analyzed by Western blotting using MAb M2, which is specific for the Flag epitope. A predicted 18-kDa band is visible in the case of the flagged ORF (pHKputSag1-FLAG). pCIneo-DMSag and pHKputSag1 served as negative controls. The experiment was repeated with identical results.
Test for Sag function using human T cells.
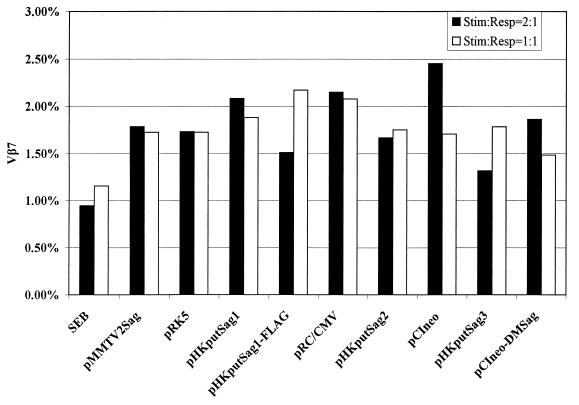
Since the postulated Sag function was described in humans, we initially stimulated human T cells with murine A20 cells transfected with the vectors described above, in a manner similar to a protocol described by B. Conrad (personal communication). Twenty-four hours after transfection, A20 cells were arrested in their growth and used to stimulate purified human T cells (see Materials and Methods). Because Conrad et al. had reported that human Vβ7+ T cells were induced to proliferate (10), this human T-cell subpopulation was analyzed by FACS 8 days after stimulation of the culture. However, in comparison to the vector controls (pRK5, pRC/CMV, and pCIneo), we were unable to observe any increase of the Vβ7+ T-cell population, although, as depicted in Fig. 5, we tested a variety of different ORF-containing plasmids including the construct pCIneo-DMSag, which was kindly provided by Conrad and which he had used in his studies.
FIG. 5.
Human peripheral blood Vβ7+ T cells do not expand upon stimulation with different ORF constructs. Purified human peripheral blood T cells (1.25 × 106 or 2.5 × 106 cells/well) were stimulated with mitomycin C (80 μg/ml)-treated A20 cells (2.5 × 106 cells/well) transfected with the indicated ORF-bearing constructs. T cells were cultured for 8 days. IL-2 (10 U/ml) was added for the last 5 days. After double staining with an FITC-labeled anti-Vβ7 MAb and PE-labeled anti-CD3 MAb, the percentage of Vβ7+ T cells was measured by FACS. pRK5 is the vector backbone for pHKputSag1 and pHKputSag1-FLAG; pRC/CMV is the backbone for pHKputSag2; and pCIneo is the backbone for pHKputSag3 and pCIneo-DMSag. Stim:Resp, stimulator-responder ratio.
Test of Sag function using the murine test system.
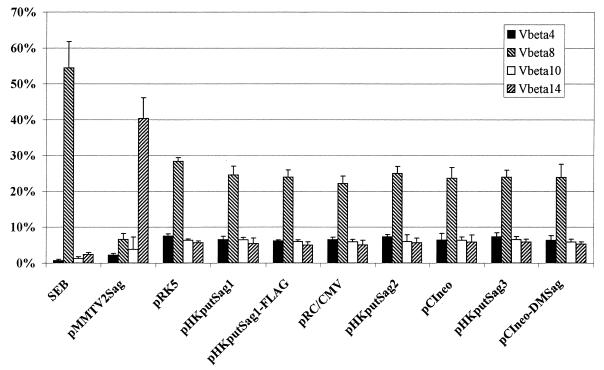
All established Sags of bacterial origin have been found to stimulate human as well as murine T cells. As mentioned earlier, the Sags encoded by MMTV stimulate human and murine T cells (17). The Vβ chains of the TCRs involved in the recognition of the different Sags are conserved between humans and mice (15, 17). At present there is no evidence that a species-specific Sag exists, i.e., a Sag which is active only in one species. We therefore used our well-defined murine test system, which circumvents the problem of alloreactivity encountered in human-based test systems or of xenoreactivity observed in human- and murine-based test systems. In order to analyze whether the ORF product of IDDMK1,222 belongs to the Sag family, syngeneic murine peripheral T cells were stimulated with transfected A20 cells for 5 days and subsequently analyzed by FACS for a Vβ-specific outgrowth of T cells. As already mentioned, the system was controlled by two established Sags: first, the exogenous bacterial Sag, SEB, which stimulates all murine Vβ8+ T cells (21), and second and more important, the Sag ORF product of MMTV2, which stimulates all murine Vβ14+ T cells (3, 14). The positive control plasmid bearing the ORF of MMTV2 was used in exactly the same way as all the plasmids bearing ORFs of human endogenous retroviruses. A typical result of the T-cell stimulation assay is demonstrated in Fig. 6. As was expected, SEB expanded all Vβ8+ T cells, which resulted in a relative reduction of the other three tested T-cell subsets, consisting of Vβ4-, -10-, and -14-positive T cells. The MMTV2-encoded Sag stimulated all Vβ14+ cells; again, the relative numbers of the other T-cell subsets were reduced. In contrast, none of the constructs encoding the putative endogenous human Sag changed the Vβ repertoire of peripheral T cells (Fig. 4), as determined by comparison with peripheral T cells which were stimulated by A20 cells transfected with the noncoding parental plasmids pRK5, pRC/CMV, and pCIneo. Notably, the Sag-encoding construct kindly provided by B. Conrad also did not stimulate T cells in a Vβ-specific manner.
FIG. 6.
Murine peripheral T cells are not stimulated Vβ-specifically by different ORF constructs. A20 cells were transfected with the indicated plasmids (20 μg of DNA/107 cells). After 24 h the cells were treated with mitomycin C (80 μg/ml) and used as APCs at a density of 5 × 106 cells/well. Murine peripheral lymph node cells were added (2.5 × 106 cells/well) and after 5 days of culture in the presence of 10 U of IL-2 per ml, the cells were harvested. After double staining with an MAb specific for the Vβ4, Vβ8, Vβ10, or Vβ14 chains of the TCR and with an MAb specific for CD3ɛ protein, the individual T-cell subpopulations were quantified by FACS (■, Vβ4+; ▧, Vβ8+; □, Vβ10+; ▨, Vβ14+ T cells). Each bar represents the mean of at least five independent experiments; error bars indicate standard errors. pRK5 is the vector backbone for pHKputSag1 and pHKputSag1-FLAG; pRC/CMV is the backbone for pHKputSag2; and pCIneo is the backbone for pHKputSag3 and pCIneo-DMSag.
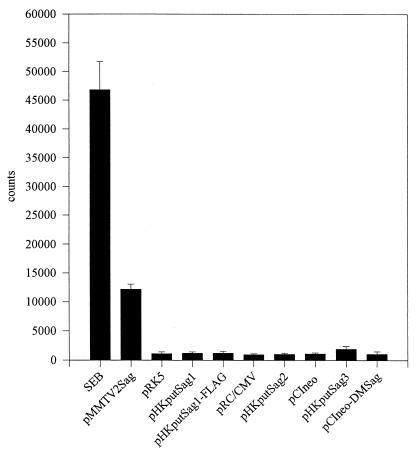
Since in this approach, we analyzed the expansion of a substantial portion of but not the entire Vβ repertoire, we might have missed the responding subset of T cells. Therefore, we also measured the induced proliferation of the peripheral T cells as a parameter for Sag response. As depicted in Fig. 7, only the control Sags, SEB and the ORF product encoded by MMTV2, stimulated T cells to proliferate above background levels (levels reached with the expression vectors lacking a coding sequence, pRK5, pRC/CMV, and pCIneo). None of the plasmids encoding the putative endogenous retrovirus Sag, including the plasmid supplied by Conrad, induced proliferation of the T cells. In addition, a more extensive analysis of the Vβ repertoire, including the Vβ4, -6, -7, -8, -9, -10, -13, and -14 T-cell subsets, also failed to show a Vβ-specific expansion of T cells (data not shown).
FIG. 7.
Proliferation of murine T cells stimulated by different ORF-bearing plasmids. The experiment was performed as described for Fig. 6. After a culture period of 4 days, the cells were pulsed for 8 h with [3H]thymidine (37 kBq) and the amount of incorporation of [3H]thymidine was determined. Each bar represents the mean of quadruplicates; error bars indicate standard errors. The experiment was repeated twice with comparable results.
MHC-II expression is not changed by the ORF.
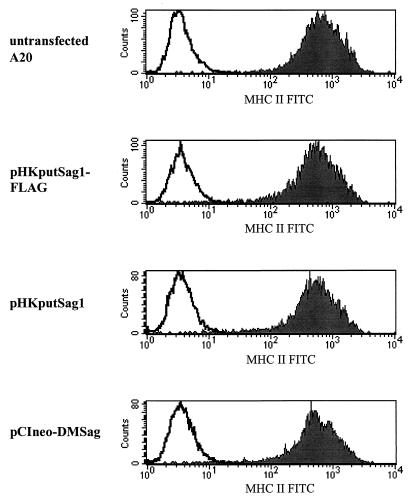
In order to activate T cells, bacterial as well as retroviral Sags need to be presented by MHC-II-expressing APCs (1, 21). To exclude the possibility that, during transfection or due to the expression of the ORF itself, MHC-II molecules are modulated from the surface of A20 cells, the expression of MHC-II molecules 24 h after transfection was analyzed. Figure 8 and Table 1 show that the expression level of MHC-II molecules is not influenced by the ORF.
FIG. 8.
Expression of MHC-II molecules on A20 cells is not influenced by the ORF. Transfected A20 cells were analyzed for the expression of MHC-II molecules 24 h after electroporation. Cells were stained with an FITC-labeled anti-MHC-II antibody and the expression level of MHC-II was recorded with the cytometer. Solid lines, isotype controls; shadowed areas, expression of MHC-II. Four representative histograms are shown; the other transfected cells expressed MHC-II at the same level (Table 1).
TABLE 1.
MHC-II expression is not influenced by different ORF constructs
| Constructa | MHC-II FITC fluorescence (mean) | Coefficient of variation (SD/mean) (%) |
|---|---|---|
| None | 790.27 | 65.67 |
| pMMTV2Sag | 659.29 | 68.24 |
| pRK5 | 640.52 | 69.67 |
| pHKputSag1 | 624.55 | 68.55 |
| pHKputSag1-FLAG | 649.85 | 68.73 |
| pRC/CMV | 645.16 | 67.61 |
| pHKputSag2 | 624.18 | 68.08 |
| pCIneo | 624.68 | 68.25 |
| pHKputSag3 | 628.62 | 68.74 |
| pCIneo-DMSag | 659.11 | 70.61 |
Plasmid used to transfect A20 cells.
DISCUSSION
It is well known that many retroviral sequences are inherent to the human genome, including the B/D-type retrovirus-related family HTDV/HERV-K, which consists of approximately 50 proviruses with full-length genomes (28). Although all proviruses sequenced so far are defective and noninfectious, retroviral proteins like reverse transcriptase and proteins of the env and gag regions are expressed in germ cell tumors (5, 13, 24, 26, 27, 29). Furthermore, the expression of HTDV/HERV-K proviruses in germ cell tumor cell lines leads to the formation of retrovirus particles (6, 19). The existence of these retroviral products, in particular the possibility that such proteins might possess a Sag function, has stimulated the idea that they might be involved in triggering autoimmunity in humans.
A recent paper stated that a novel sequence of the human endogenous retrovirus family HTDV/HERV-K was associated with the onset of type I diabetes mellitus and that this sequence encoded a Sag which activated human Vβ7+ T cells (10). Stimulated by this hypothesis, we tried to repeat these interesting findings. Although we and others could not confirm that the development of type 1 diabetes depends on the specific expression of HTDV/HERV-K proviruses (18, 20, 22), in that study we could not exclude the possibility that the expressed sequences harbor a Sag function (20). In the present study we have cloned into eucaryotic expression vectors sequences identical or very similar to the sequence published by Conrad et al. Together with an IDDMK1,222 expression clone kindly provided by Conrad, we tested these plasmids in several systems that would reveal a Sag function. None of the test systems indicated the existence of a Sag function in the HERV-K sequences, although the MMTV2-derived sequence exerted a Sag function similar to that of the bacterial Sag.
Several issues should be considered to ensure that the negative results obtained were not caused by the experimental design. First, did we use the wrong species for the evaluation of the ORF as a Sag? Until now, all established Sags have stimulated T cells of different species, i.e., Sags are not species specific (17, 21). In order to avoid the stimulation of responder T cells by alloantigen (since the frequency of V segments of the TCR in peripheral blood lymphocytes seems to be influenced by HLA genes) (4), we chose to use a murine test system instead of the human test system used by Conrad et al. The system used here responded rapidly to the MMTV2-encoded Sag as well as to the bacterial Sag SEB (Fig. 6). Although one could expect to observe an expansion of murine T-cell subsets expressing the Vβ4 or Vβ10 chain of the TCR because these chains are closely related to the human Vβ7 chain (8), we were unable to detect such an expansion with the IDDMK1,222 expression plasmid or with any related plasmid. To minimize the chance that the IDDMK1,222-borne ORF is the first species-specific Sag, we used a xenogeneic system consisting of human T cells and murine A20 cells as APCs. As shown by Subramanyam et al., Sags can be presented by xenogeneic APCs (25).
In addition, when Conrad sent us the pCIneo-DMSag expression plasmid for testing, he recommended A20 cells as the most convenient APCs and human T cells as the responder cell system, as described in this article. However, we were unable to detect any Sag function of the IDDMK1,222-borne ORF with any of the systems tested.
Second, the APC used here was the transiently transfected B-cell line A20, whereas Conrad et al. utilized the transiently transfected monocytic cell line THP1 (10). They also used the stably transfected B-lymphoblastoid cell line Raji as the APC. Comparing both APCs revealed that the Vβ7-specific expansion of human peripheral T cells induced by the Raji cell line was weaker than that induced by THP1 cells (10). Unfortunately, we did not receive the stable transfectants. In addition, we were unable to achieve a satisfactory transfection efficiency with the THP1 cell line, although we tested several reagents recommended by manufacturers (data not shown). The use of the B-cell line A20 as the APC might yield false-negative results if the expression of the transgene failed and if the cell line was not able to present a Sag. However, importantly, A20 cells can present the MMTV2-encoded Sag with the induction of a Vβ14-specific expansion of T cells (Fig. 6). Furthermore, we were able to confirm that the putative Sags were indeed expressed in A20 cells, since mRNAs of the ORFs were transcribed (Fig. 3) and protein was produced (Fig. 4).
Third, the amount or the type of MHC-II molecules may be inadequate to present the putative Sag. THP1 cells had to be stimulated with gamma interferon to allow the upregulation of MHC-II molecules and presentation of the putative Sag (10). In contrast, the A20 cell line used in this study as APC did express high levels of MHC-II molecules and this did not change upon the transfection of different ORF constructs (Fig. 8; Table 1). The presentation of Sags is not MHC restricted. However, a hierarchy exists in that human HLA-DR class II isotypes present Sags much better than HLA-DQ and HLA-DP isotypes (21). The same is true for their murine counterparts, since H2-IE is superior to H2-IA (2, 21). Since the A20 cell line is derived from BALB/c mice, the MHC-II isotypes H2-IE and H2-IA are expressed. Therefore, this cell line should be able to present Sags successfully to T cells, and this is shown here for SEB and the MMTV2-encoded Sag.
Fourth, could we have missed the murine T-cell subset, which was activated by the ORF, since we did not analyze the complete Vβ repertoire? In that case, one should expect a relative reduction in the T-cell subsets tested. As shown in Fig. 6, we observed a relative reduction of the T-cell subsets Vβ4, Vβ10, and Vβ14 in response to the expansion of Vβ8 by SEB and a relative reduction of Vβ4, Vβ8, and Vβ10 in response to the expansion of Vβ14 by the MMTV2-encoded Sag. In addition, we were unable to induce a substantial proliferation of murine T cells upon stimulation by the putative endogenous retroviral Sag (Fig. 7), which also indicated that no undefined T-cell subset had expanded. In contrast, SEB- and pMMTV2Sag-transfected cells induced a significant proliferation of the murine responder T cells. To rule out that the endogenous retrovirus Sag might be the first species-specific Sag, we stimulated human T cells and again obtained no evidence that these ORF products activated a Vβ-specific T-cell repertoire (Fig. 5). Surprisingly, the plasmid pCIneo-DMSag also showed no evidence of Sag activity.
Fifth, did the Flag in the pHKputSag1-FLAG construct impede Sag activity of the IDDMK1,222-borne ORF? We used a flagged ORF to demonstrate translation of the ORF in A20 cells (Fig. 4). Since we were worried that the Flag influences the function of the protein, we added the Flag to either the 5′ or 3′ end of the ORF. Both constructs as well as the unflagged ORF were negative in the T-cell stimulation assay (Fig. 5 and 6; also data not shown). However, in A20 cells transfected with the construct containing the Flag sequence at the 3′ end of the ORF, we were unable to detect the formation of mRNA in a nonnested PCR approach, indicating that this construct was less efficiently transcribed than that with the 5′ Flag (data not shown). One could argue that the 5′-flagged protein lost its Sag activity due to the Flag and that the normal ORF gave rise to an unstable message. However, we demonstrated that ORF mRNA levels were similar in A20 cells transfected with pHKputSag1-FLAG, pHKputSag1, or pCIneo-DMSag (Fig. 3). Since we tested the flagged ORF together with four different unflagged ORF constructs in three different vectors and failed to detect Sag activity, we are convinced that the Flag is not responsible for our failure.
In summary we can only conclude from our data that the ORF located in the env region of IDDMK1,222 does not belong to the family of Sags.
ACKNOWLEDGMENTS
This work was supported by the Bundesministerium für Bildung und Forschung grant 01 GB9403.
We thank Walter Günzburg and Hans Häcker for donating MMTV2 Sag- and EGFP-expressing plasmids, respectively. We also thank Bernard Conrad for providing the plasmid pCIneo-DMSag.
REFERENCES
- 1.Acha-Orbea H. Retroviral superantigens. Chem Immunol. 1992;55:65–86. [PubMed] [Google Scholar]
- 2.Acha-Orbea H, Palmer E. Mls—a retrovirus exploits the immune system. Immunol Today. 1991;12:356–361. doi: 10.1016/0167-5699(91)90066-3. [DOI] [PubMed] [Google Scholar]
- 3.Acha-Orbea H, Shakov A N, Scarpellino L, Kolb E, Müller V, Vessaz-Shaw A, Fuchs R, Blöchlinger K, Rollini P, Billotte J, Sarafidou M, MacDonald H R, Diggelmann H. Clonal deletion of Vβ14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991;350:207–211. doi: 10.1038/350207a0. [DOI] [PubMed] [Google Scholar]
- 4.Akolkar P N, Gulwani-Akolkar B, Pergolizzi R, Bigler R D, Silver J. Influence of HLA genes on T cell receptor V segment frequencies and expression levels in peripheral blood lymphocytes. J Immunol. 1993;150:2761–2773. [PubMed] [Google Scholar]
- 5.Boller K, Janssen O, Schuldes H, Tönjes R R, Kurth R. Characterization of the antibody response specific for the human endogenous retrovirus HTDV/HERV-K. J Virol. 1997;71:4581–4588. doi: 10.1128/jvi.71.6.4581-4588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boller K, Konig H, Sauter M, Mueller Lantzsch N, Lower R, Lower J, Kurth R. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 1993;196:349–353. doi: 10.1006/viro.1993.1487. [DOI] [PubMed] [Google Scholar]
- 7.Brocke S, Gaur A, Piercy C, Gautam A, Gijbels K, Fathman C G, Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993;365:642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- 8.Clark S P, Arden B, Kabelitz D, Mak T W. Comparison of human and mouse T-cell receptor variable gene segment subfamilies. Immunogenetics. 1995;42:531–540. doi: 10.1007/BF00172178. [DOI] [PubMed] [Google Scholar]
- 9.Conrad B, Weidmann E, Trucco G, Rudert W A, Behboo R, Ricordi C, Rodriquez-Rilo H, Finegold D, Trucco M. Evidence for superantigen involvement in insulin-dependent diabetes mellitus aetiology. Nature. 1994;371:351–355. doi: 10.1038/371351a0. [DOI] [PubMed] [Google Scholar]
- 10.Conrad B, Weissmahr R N, Boni J, Arcari R, Schupbach J, Mach B. A human endogenous retroviral superantigen as candidate autoimmune gene in type I diabetes. Cell. 1997;90:303–313. doi: 10.1016/s0092-8674(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 11.Dellabona P, Peccoud J, Kappler J, Marrack P, Benoist C, Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990;62:1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- 12.Fraser J D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 13.Gotzinger N, Sauter M, Roemer K, Mueller-Lantzsch N. Regulation of human endogenous retrovirus-K Gag expression in teratocarcinoma cell lines and human tumours. J Gen Virol. 1996;77:2983–2990. doi: 10.1099/0022-1317-77-12-2983. [DOI] [PubMed] [Google Scholar]
- 14.Gunzburg W H, Heinemann F, Wintersperger S, Miethke T, Wagner H, Erfle V, Salmons B. Endogenous superantigen expression controlled by a novel promoter in the MMTV long terminal repeat. Nature. 1993;364:154–158. doi: 10.1038/364154a0. [DOI] [PubMed] [Google Scholar]
- 15.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 16.Kim K J, Kanellopoulos-Langevin C, Merwin R M, Sachs D H, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979;122:549–554. [PubMed] [Google Scholar]
- 17.Labrecque N, McGrath H, Subramanyam M, Huber B T, Sekaly R P. Human T cells respond to mouse mammary tumor virus-encoded superantigen: V beta restriction and conserved evolutionary features. J Exp Med. 1993;177:1735–1743. doi: 10.1084/jem.177.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan M S, Mason A, Coutant R, Chen Q Y, Vargas A, Rao J, Gomez R, Chalew S, Garry R, Maclaren N K. HERV-K10s and immune-mediated (type 1) diabetes. Cell. 1998;95:14–16. doi: 10.1016/s0092-8674(00)81777-8. [DOI] [PubMed] [Google Scholar]
- 19.Lower R, Boller K, Hasenmaier B, Korbmacher C, Muller-Lantzsch N, Lower J, Kurth R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc Natl Acad Sci USA. 1993;90:4480–4484. doi: 10.1073/pnas.90.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lower R, Tonjes R R, Boller K, Denner J, Kaiser B, Phelps R C, Lower J, Kurth R, Badenhoop K, Donner H, Usadel K H, Miethke T, Lapatschek M, Wagner H. Development of insulin-dependent diabetes mellitus does not depend on specific expression of the human endogenous retrovirus HERV-K. Cell. 1998;95:11–14. doi: 10.1016/s0092-8674(00)81776-6. [DOI] [PubMed] [Google Scholar]
- 21.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 22.Murphy V J, Harrison L C, Rudert W A, Luppi P, Trucco M, Fierabracci A, Biro P A, Bottazzo G F. Retroviral superantigens and type 1 diabetes mellitus. Cell. 1998;95:9–11. doi: 10.1016/s0092-8674(00)81775-4. [DOI] [PubMed] [Google Scholar]
- 23.Perron H, Garson J A, Bedin F, Beseme F, Paranhos-Baccala G, Komurian-Pradel F, Mallet F, Tuke P W, Voisset C, Blond J L, Lalande B, Seigneurin J M, Mandrand B The Collaborative Research Group on Multiple Sclerosis. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. Proc Natl Acad Sci USA. 1997;94:7583–7588. doi: 10.1073/pnas.94.14.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schommer S, Sauter M, Krausslich H G, Best B, Mueller-Lantzsch N. Characterization of the human endogenous retrovirus K proteinase. J Gen Virol. 1996;77:375–379. doi: 10.1099/0022-1317-77-2-375. [DOI] [PubMed] [Google Scholar]
- 25.Subramanyam M, McLellan B, Labrecque N, Sekaly R P, Huber B T. Presentation of the Mls-1 superantigen by human HLA class II molecules to murine T cells. J Immunol. 1993;151:2538–2545. [PubMed] [Google Scholar]
- 26.Tonjes R R, Boller K, Limbach C, Lugert R, Kurth R. Characterization of human endogenous retrovirus type K virus-like particles generated from recombinant baculoviruses. Virology. 1997;233:280–291. doi: 10.1006/viro.1997.8614. [DOI] [PubMed] [Google Scholar]
- 27.Tönjes R R, Limbach C, Löwer R, Kurth R. Expression of human endogenous retrovirus type K envelope glycoprotein in insect and mammalian cells. J Virol. 1997;71:2747–2756. doi: 10.1128/jvi.71.4.2747-2756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonjes R R, Lower R, Boller K, Denner J, Hasenmaier B, Kirsch H, Konig H, Korbmacher C, Limbach C, Lugert R, Phelps R C, Scherer J, Thelen K, Lower J, Kurth R. HERV-K: the biologically most active human endogenous retrovirus family. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl. 1):S261–S267. doi: 10.1097/00042560-199600001-00039. [DOI] [PubMed] [Google Scholar]
- 29.Vogetseder W, Dumfahrt A, Mayersbach P, Schonitzer D, Dierich M P. Antibodies in human sera recognizing a recombinant outer membrane protein encoded by the envelope gene of the human endogenous retrovirus K. AIDS Res Hum Retrov. 1993;9:687–694. doi: 10.1089/aid.1993.9.687. [DOI] [PubMed] [Google Scholar]
- 30.Wintersperger S, Indraccolo S, Miethke T, Gunzburg W H, Salmons B. A transient assay for gene expression studies in B lymphocytes and its use for superantigen assays. BioTechniques. 1994;16:882–886. [PubMed] [Google Scholar]