Abstract
Background
The high levels of healthcare worker shortage is recognised as a severe impediment to increasing patients' access to antiretroviral therapy. This is particularly of concern where the burden of disease is greatest and the access to trained doctors is limited.This review aims to better inform HIV care programmes that are currently underway, and those planned, by assessing if task‐shifting care from doctors to non‐doctors provides both high quality and safe care for all patients requiring antiretroviral treatment.
Objectives
To evaluate the quality of initiation and maintenance of HIV/AIDS care in models that task shift care from doctors to non‐doctors.
Search methods
We conducted a comprehensive search to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress) from 1 January 1996 to 28 March 2014, with major HIV/AIDS conferences searched 23 May 2014. We had also contacted relevant organizations and researchers. Key words included MeSH terms and free‐text terms relevant to 'task shifting', 'skill mix', 'integration of tasks', 'service delivery' and 'health services accessibility'.
Selection criteria
We included controlled trials (randomised or non‐randomised), controlled‐before and after studies, and cohort studies (prospective or retrospective) comparing doctor‐led antiretroviral therapy delivery to delivery that included another cadre of health worker other than a doctor, for initiating treatment, continuing treatment, or both, in HIV infected patients.
Data collection and analysis
Two authors independently screened titles, abstracts and descriptor terms of the results of the electronic search and applied our eligibility criteria using a standardized eligibility form to full texts of potentially eligible or uncertain abstracts. Two reviewers independently extracted data on standardized data extraction forms. Where possible, data were pooled using random effects meta‐analysis. We assessed evidence quality with GRADE methodology.
Main results
Ten studies met our inclusion criteria, all of which were conducted in Africa. Of these four were randomised controlled trials while the remaining six were cohort studies.
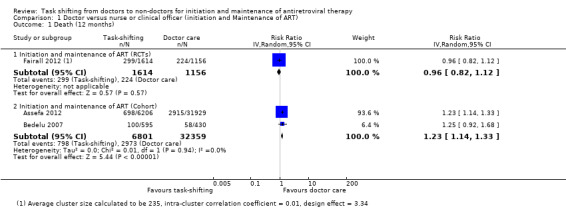
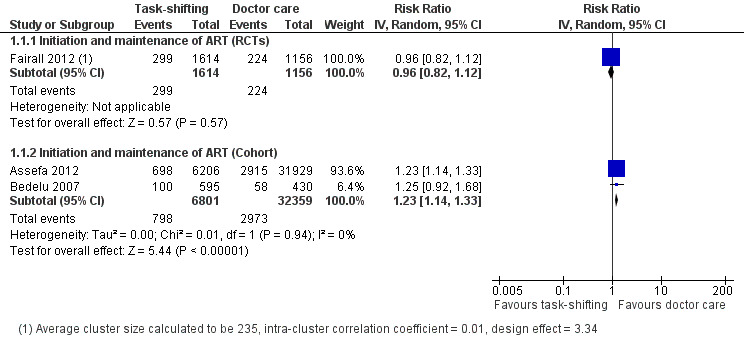
From the trial data, when nurses initiated and provided follow‐up HIV therapy, there was high quality evidence of no difference in death at one year, unadjusted risk ratio was 0.96 (95% CI 0.82 to 1.12), one trial, cluster adjusted n = 2770. There was moderate quality evidence of lower rates of losses to follow‐up at one year, relative risk of 0.73 (95% CI 0.55 to 0.97). From the cohort data, there was low quality evidence that there may be an increased risk of death in the task shifting group, relative risk 1.23 (95% CI 1.14 to 1.33, two cohorts, n = 39 160) and very low quality data reporting no difference in patients lost to follow‐up between groups, relative risk 0.30 (95% CI 0.05 to 1.94).
From the trial data, when doctors initiated therapy and nurses provided follow‐up, there was moderate quality evidence that there is probably no difference in death compared with doctor‐led care at one year, relative risk of 0.89 (95% CI 0.59 to 1.32), two trials, cluster adjusted n = 4332. There was moderate quality evidence that there is probably no difference in the numbers of patients lost to follow‐up at one year, relative risk 1.27 (95% CI 0.92 to 1.77), P = 0.15. From the cohort data, there is very low quality data that death at one year may be lower in the task shifting group, relative risk 0.19 (95% CI 0.05 to 0.78), one cohort, n = 2772, and very low quality evidence that loss to follow‐up was reduced, relative risk 0.34 (95% CI 0.18 to 0.66).
From the trial data, for maintenance therapy delivered in the community there was moderate quality evidence that there is probably no difference in mortality when doctors deliver care in the hospital or specially trained field workers provide home‐based maintenance care and antiretroviral therapy at one year, relative risk 1.0 (95% CI 0.62 to 1.62), 1 trial, cluster adjusted n = 559. There is moderate quality evidence from this trial that losses to follow‐up are probably no different at one year, relative risk 0.52 (0.12 to 2.3), P = 0.39. The cohort studies did not report on one year follow‐up for these outcomes.
Across the studies that reported on virological and immunological outcomes, there was no clear evidence of difference whether a doctor or nurse or clinical officer delivered therapy. Three studies report on costs to patients, indicating a reduction in travel costs to treatment facilities where task shifting was occurring closer to patients homes. There is conflicting evidence regarding the relative cost to the health system, as implementation of the strategy may increase costs. The two studies reporting the patient and staff perceptions of the quality of care, report good acceptability of the service by patients, and general acceptance by doctors of the shifting of roles. One trial reported on the time to initiation of antiretroviral therapy, finding no clear evidence of a difference between groups. The same trial reports on new diagnosis of tuberculosis which favours nurse initiation of HIV care for increasing the numbers of diagnoses of tuberculosis made.
Authors' conclusions
Our review found moderate quality evidence that shifting responsibility from doctors to adequately trained and supported nurses or community health workers for managing HIV patients probably does not decrease the quality of care and, in the case of nurse initiated care, may decrease the numbers of patients lost to follow‐up.
Keywords: Humans; Africa; Anti‐HIV Agents; Anti‐HIV Agents/therapeutic use; Cohort Studies; Delegation, Professional; Delegation, Professional/standards; General Practice; General Practice/standards; HIV Infections; HIV Infections/drug therapy; HIV Infections/mortality; Health Services Accessibility; Health Services Accessibility/economics; Induction Chemotherapy; Induction Chemotherapy/standards; Lost to Follow‐Up; Maintenance Chemotherapy; Maintenance Chemotherapy/standards; Practice Patterns, Nurses'; Practice Patterns, Nurses'/standards; Practice Patterns, Physicians'; Practice Patterns, Physicians'/standards; Randomized Controlled Trials as Topic
Plain language summary
Shifting HIV care from doctors to non‐doctors to improve access to therapy for people living with HIV
Background
High levels of healthcare worker shortage has limited HIV infected patients access to antiretroviral therapy in lower and middle‐income countries. This occurs most where the burden of HIV disease is greatest and where access to trained doctors is limited. We wanted to assess if task shifting of care from doctors to non‐doctors provides both high quality and safe care for all patients requiring antiretroviral treatment.
Study characteristics
We searched for studies up to March 2014. We found 10 studies, including four randomised controlled trials and 6 cohort studies collecting data from HIV care programmes. All the studies were conducted in Africa in adults who were followed up for up to one year. We describe three types of care:
‐ Doctor versus nurse or clinical officer care for initiation and maintenance of antiretrovirals
‐ Doctor versus nurse or clinical officer care for maintenance of antiretroviral therapy
‐ Doctor versus community health workers for maintenance of antiretroviral therapy.
Key Results.
We found high quality evidence from trial data that when nurses initiated and provided follow‐up HIV therapy, there was no difference in death and lower rates of losses to follow up at one year, (n = 2770). However, lower quality data from two cohort studies suggests that there may be an increased risk of death in the task shifting group, (n = 39 160) but no difference in patients lost to follow‐up between groups,
We found moderate quality evidence from two trials that when doctors initiated therapy and nurses provided follow‐up, that there was probably no difference in death or number of patients lost to follow up at one year (n = 4332). Lower quality evidence from the cohort study showed that death as well as the number of patients lost to follow‐up at one year may be lower in the group treated by nurses.
Compared to doctor led care, we found moderate quality evidence from a single trial that when antiretroviral therapy was provided in the community, by trained field workers, there was probably no difference in death or losses to follow‐up (n= 559).
Summary of findings
Summary of findings for the main comparison. Doctor versus nurse or clinical officer for initiation and maintenance of antiretroviral therapy for HIV‐infected patients.
| Doctor versus nurse or clinical officer for initiation and maintenance of antiretroviral therapy for HIV‐infected patients | ||||||
| Patient or population: HIV‐infected patients Settings: Lower and middle income countries Intervention: Doctor versus nurse or clinical officer for initiation and maintenance of antiretroviral therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Doctor versus nurse or clinical officer for initiation and maintenance of antiretroviral therapy | |||||
| Death (RCTs) Follow‐up: 12 months | 194 per 1000 | 186 per 1000 (159 to 217) | RR 0.96 (0.82 to 1.12) | 2770 (1 study) | ⊕⊕⊕⊕ high1 | |
| Death (Cohorts) Follow‐up: 12 months | 92 per 1000 | 113 per 1000 (105 to 122) | RR 1.23 (1.14 to 1.33) | 39160 (2 studies) | ⊕⊕⊝⊝ low2 | |
| Lost to follow‐up (RCTs) Follow‐up: 12 months | 77 per 1000 | 56 per 1000 (42 to 75) | RR 0.73 (0.55 to 0.97) | 2770 (1 study) | ⊕⊕⊕⊝ moderate3 | |
|
Lost to follow‐up (cohorts) Follow‐up: 12 months |
297 per 1000 | 89 per 1000 (15 to 577) | RR 0.3 (0.05 to 1.94) | 39156 (2 studies) | ⊕⊝⊝⊝ very low4 | |
|
Death or loss to follow‐up (RCTs) Follow‐up: 12 months |
271 per 1000 |
241 per 1000 (214 to 273) |
RR 0.89 (0.79 to 1.01) |
2770 (1 study) |
⊕⊕⊕⊕ high |
|
|
Death or loss to follow‐up (Cohorts) Follow‐up: 12 months |
389 per 1000 |
280 per 1000 (187 to 416) |
RR 0.72 (0.48 to 1.07) |
39160 (2 studies) |
⊕⊝⊝⊝ very low5,6 |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The confidence interval is narrow and does not include appreciable harm or benefit. 2 Not downgraded for risk of bias. Two retrospective cohorts provided data. Bedelu 2007 included patients with higher CD4 counts at the health centre. As this is likely to favour the intervention, we did not downgrade for risk of bias. 3 Downgraded by 1 for imprecision. There was a low number of events after adjusting for clustering (<300 events). 4 Downgraded by 1 for imprecision. The confidence interval includes both appreciable harm and appreciable benefit.
5 Downgraded by 1 for imprecision. 95% CI includes appreciable benefit and null value
6Not downgraded for inconsistency. Despite quantitative heterogeneity, both studies showed that attrition was decreased with task shifting of ART initiation and maintenance to nurses or clinical officers.
Summary of findings 2. Doctor versus nurse or clinical officer for maintenance of antiretroviral therapy for HIV infected patients on antiretroviral therapy.
| Doctor versus nurse or clinical officer for maintenance of antiretroviral therapy for HIV infected patients on antiretroviral therapy | ||||||
| Patient or population: HIV infected patients on antiretroviral therapy Settings: Lower and middle‐income countries Intervention: Doctor versus nurse or clinical officer for maintenance of antiretroviral therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Doctor versus nurse or clinical officer for maintenance of antiretroviral therapy | |||||
| Death (RCTs) Follow‐up: 12 months | 23 per 1000 | 20 per 1000 (14 to 30) | RR 0.89 (0.59 to 1.32) | 4332 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Death (Cohorts) Follow‐up: 12 months | 15 per 1000 | 3 per 1000 (1 to 12) | RR 0.19 (0.05 to 0.78) | 2772 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Lost to follow‐up (RCTs) Follow‐up: 12 months | 28 per 1000 | 36 per 1000 (26 to 49) | RR 1.27 (0.92 to 1.77) | 4332 (2 studies) | ⊕⊕⊕⊝ moderate3 | |
| Lost to follow‐up (Cohorts) Follow‐up: 12 months | 42 per 1000 | 14 per 1000 (8 to 28) | RR 0.34 (0.18 to 0.66) | 2772 (1 study) | ⊕⊝⊝⊝ very low2 | |
| Death or loss to follow‐up (RCTs) Follow‐up: 12 months | 51 per 1000 |
56 per 1000 (44 to 72) |
RR 1.1 (0.86 to 1.41) |
4332 (2 studies) |
⊕⊕⊕⊝ moderate3 |
|
| Death or loss to follow‐up (Cohorts) Follow‐up: 12 months | 57 per 1000 |
17 per 1000 (10 to 31) |
RR 0.3 (0.17 to 0.54) |
2772 (1 study) |
⊕⊝⊝⊝ very low2 |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for imprecision. There was a low number of events (<300) and the 95% confidence interval includes appreciable harm and benefit. 2 Downgraded by 1 for imprecision, due to low event numbers (<300). 3 Downgraded by 1 for imprecision. There was a low number of events (<300) after adjusting for clustering in the Fairall 2012 study.
Summary of findings 3. Doctor versus community health worker for maintenance of antiretroviral therapy for HIV infected patients on antiretroviral therapy.
| Doctor versus community health worker for maintenance of antiretroviral therapy for HIV infected patients on antiretroviral therapy. | ||||||
| Patient or population: HIV infected patients on antiretroviral therapy. Settings: Lower and middle‐income countries Intervention: Doctor versus community health worker for maintenance of antiretroviral therapy | ||||||
| Outcomes2 | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Doctor versus community health worker for maintenance of antiretroviral therapy | |||||
| Death (RCTs) Follow‐up: 12 months | 109 per 1000 | 109 per 1000 (68 to 177) | RR 1 (0.62 to 1.62) | 559 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Lost to follow‐up (RCTs) Follow‐up: 12 months | 17 per 1000 | 9 per 1000 (2 to 40) | RR 0.52 (0.12 to 2.3) | 559 (1 study) | ⊕⊕⊕⊝ moderate1 | |
|
Death or lost to follow‐up (RCTs) Follow‐up: 12 months |
127 per 1000 |
118 per 1000 (76 to 185) |
RR 0.93 (0.6 to 1.46) |
559(1 study) | ⊕⊕⊕⊝ moderate1 |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for imprecision.This cluster randomised trial was adjusted for design effect having assumed an intra‐cluster correlation coefficient (0.05) as none was provided in the report. The resultant 95% confidence interval included appreciable benefit and harm.
2 No cohorts were reported for this comparison
Background
Description of the condition
Currently, there are more than 34 million people worldwide infected with HIV, of these, eight million are accessing antiretroviral therapy (ART) leaving approximately 14 million of those eligible for ART still in need of treatment ( UNAIDS 2011).Combination antiretroviral therapy has long been recognised as effective for reducing morbidity and mortality of people infected with HIV and for maximising their quality of life and longevity (Palella 1998). The World Health Organization (WHO) supports the roll‐out of antiretroviral therapy to ensure these positive health outcomes and increasingly treatment is recommended earlier in the course of the disease, both for the health of those affected and to reduce the risks of transmission especially in discordant couples, pregnant women and key populations (WHO 2013; Anglemyer 2013; Cohen 2011Siegfried 2010). Despite the requirement for increasing access to HIV therapy, settings with the highest burden of the disease, tend to have the least access to care (Ford 2011). Shortages of human resources for health have hampered the scale‐up of antiretroviral therapy in regions most affected by the pandemic.This leaves a substantial shortfall of people living with HIV/AIDS who currently or shortly will require antiretroviral treatment. Task shifting of care from doctors to other cadres of health care workers is proposed to address the inequitable access to antiretroviral therapy initiation and maintenance (WHO 2008).
Conventionally, most countries applied the doctor‐led model in the management of HIV due to the perceived complexity of the ARVs and as a policy requirement from donor organisations despite the huge disparity in the doctor : patient ratio in many high burden settings (WHO 2006). Several studies conducted in high income settings supported the role of experienced doctors in caring for patients with HIV (Kitahata 1996; Landon 2005). Although this may apply in resource rich settings, it does not adequately address the issues facing poor countries with resource limitations and the highest burdens of HIV disease. In the settings most affected by HIV, access to HIV treatment needs to be addressed as a priority, which requires innovative methods to address health worker shortages (UNAIDS 2011).
The human resource shortage evident in most resource‐poor settings is due to a multitude of factors: notable amongst which are the low rate of recruitment and training of health workers, the inequitable distribution of health workers (in terms of both the urban‐rural as well as the public‐private care disparity) and the emigration of health professionals from resource‐poor to resource‐rich settings (Schneider 2006). A discrete‐time model used to estimate the human resource requirement necessary to achieve universal coverage of antiretroviral therapy by 2017, illustrated that this could only be achieved if the current population of health workers in sub‐Saharan Africa was doubled each year for the next ten years, with factors such as the emigration of health workers being kept to a minimum (Barnighausen 2007). While it is imperative to increase the rate of recruitment and training of health workers as well as improve working conditions to reduce attrition and emigration, the HIV pandemic requires more urgent measures to address the critical skills shortage. The restructuring of the health service model from the traditional doctor‐led model to one that allows the shifting of tasks to non‐doctors and introduction of other cadres of health workers has been identified as a way to solve the skills shortage. By reducing the workload on doctors and aiming to reduce the cost of health care. Other cadres of health workers (e.g. nurses, clinical officers or health assistants) may be engaged in the process of delivering antiretroviral care in a more sustained way.
How the intervention might work
Task shifting is the process whereby specific tasks are transferred to different cadres of health workers with shorter training and fewer qualifications. The definition of the various cadres of non‐physician health workers differs from country to country (Mullan 2007). Task shifting makes efficient use of existing health care workers in order to ease delays in service delivery (WHO 2008). Task shifting may also include the delegation of clearly outlined duties to various levels of health workers who receive specific, skills‐based training. Task shifting aims to provide an equivalent standard of care to that delivered by higher cadres of health workers.
A Cochrane systematic review (16 studies) appraising nurse‐led versus doctor‐led models of general primary health care reported that 25‐75% of activities performed by doctors at the primary healthcare level could be reallocated to nurses without significantly negatively influencing clinical outcomes and quality of care (Laurant 2004). Similar results regarding quality of care were reported in a South African treatment programme, with a trial that reported that nurses can provide care that is at least as good as doctors (Sanne 2010). Substituting nurses for doctors in primary care did not necessarily result in a reduction in health care costs when one considers consultation time, requests for further laboratory investigations and prescription practices in addition to the unit cost of labour (Hollinghurst 2006; Laurant 2004).
In addition to task shifting of antiretroviral initiation and maintenance, the model of care may be accompanied by a process of decentralisation of care, where care is delivered at primary health care centres or in the community by various cadres of health workers instead of centralised hospital setting at district or higher level hospitals . In this review we summarise and outline the tiers of healthcare facilities and the possible health workers found at each of these levels (Table 4). A Cochrane review on decentralisation of care evaluated the issue of retention and quality of care in models to improve access to HIV services, finding that there were lower losses to follow‐up when care was delivered at a health centre, rather than at a hospital. They also report and that there was probably no difference both in the attrition and the quality of care when antiretrovirals were delivered in the community by trained community health workers, rather than at the health centre (Kredo 2013).
1. Framework: health service nomenclature in lower and middle‐income countries.
| Tier | Highest cadre | Terms often used | Facility and staff | Equipment facilities |
| Community |
Individual with maximum of few months training, paid or unpaid | Family led care | Family member | HIV tests, counselling, replenish drugs |
| Community volunteer | Trained volunteer; health assistants | |||
| Primary care clinic | Nurse aide or community health workers | |||
| Health centre | clinical officer or nurse (2+ years training) | Health centres; district hospitals | Purpose built with at least one paramedic or nurse with some health assistants | HIV tests; antiretrovirals; opportunistic infections medicines; point of care laboratories |
| Health centre (enhanced) | Clinical officer or nurse (2 + years training) | Health centres, primary health care clinics, district hospitals | Purpose built with at least one paramedic or nurse with some health assistants, with input from a doctor (may be via mobile support service) | HIV tests; antiretrovirals; opportunistic infections medicines; point of care laboratories |
| Hospital | Doctor | Health centres; district hospitals | Purpose built with at least one medical doctor with nurses / paramedics and assistants | CD4 count Medicines Not viral load |
| Hospital (advanced) | Specialist doctor | District hospital; referral hospital | Purpose built with at least 2 specialist doctors with nurses / paramedics and assistants | Viral load and full investigations |
Why it is important to do this review
A previous narrative review of the literature provides a framework of the policy, practice and relevant issues being addressed by task shifting of care, including: efficiency (i.e. time saved where higher level health workers direct attention to patients with more severe illness ); access to care (i.e. scaling up those patients able to access care); quality of care and health outcomes (i.e. mortality, virological and immunological responses) and team dynamics (i.e. social dynamics between cadres of carers and definitions of roles, access to training) (Callaghan 2010). However, the review did not provide definitive evidence to inform policy and practice, but rather a description of current programmes. In addition, since it's publication several new studies have been published (Assefa 2012; Brennan 2011; Jaffar 2009; Kipp 2010) which report on programmatic findings of task shifting in lower‐ and middle‐income countries. A systematic review, Emdin 2013, provided results about the the quality of care and retention, suggesting that these outcomes were not worse in task shifted models of care. However, although they did evaluate risk of bias for the various study designs, they chose to pool the data from various study designs which may introduce bias in the reporting of the results.
In some countries with the greatest burden of HIV, task shifting is already underway ‐ driven by the urgent need to increase numbers of patients receiving antiretroviral therapy and the dire doctor : patient ratios in many resource constrained countries (Bedelu 2007; Jaffar 2009). This review aims to better inform the HIV care programmes that are currently underway, and those planned, on whether task shifting of care from doctors to non‐doctors provides both high quality and safe care as well as good retention for all patients requiring antiretroviral treatment.
Objectives
The objective of this review was to evaluate the quality of care of initiation and maintenance of HIV/AIDS therapy in HIV care models that task shift care from doctors to non‐doctors.
The most clinically important outcome in delivery of ART care is death, therefore quality of care in the context of this review refers to death after being considered eligible for treatment, or during treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, non‐randomised and controlled before and after studies. Prospective and retrospective cohort studies with a comparison between standard delivery of HIV treatment by doctors and one where components of HIV treatment and care was delegated to a lower cadre of health carers. Comparators needed to be health care delivery that was contemporary with the new model (delivered at the same time), in the same country, and geographically adjacent (such as adjacent districts within a province).
Types of participants
HIV‐infected patients at the point of initiating treatment.
HIV‐infected patients on treatment requiring maintenance and follow‐up.
Types of interventions
Intervention
A model of care that involves the initiation or maintenance of ART by another cadre of health worker other than a doctor [Table 4: Health Service Nomenclature in lower‐ and middle‐income countries].
Control
Care delivered by a doctor
Types of outcome measures
Primary outcomes
Quality of care: Death after being considered eligible for treatment, or during treatment
Secondary outcomes
Clinical
Loss to follow‐up: Any measure of comparative retention between study populations at set time points after the intervention as defined by the study authors
Attrition: composite of death and loss to follow‐up
Time to initiation of ART
Diagnosis of tuberculosis after entry into HIV care
Occurrence of a new AIDS defining illness (A newly diagnosed WHO clinical stage 4 illness)
Laboratory
Virologic response to ART (The proportion of participants that reach or maintain a pre‐defined level of viral load suppression, as defined by the study authors)
Immunologic response to ART (The mean change in the concentration of CD4+ lymphocytes from baseline, as expressed in cells/μL)
Cost
Cost of care to the provider
Cost of care to the patient and family
Programme important outcomes
Patient satisfaction with care (Defined by the study authors include qualitative analysis if available)
Any negative impact on other programme and health care delivery reported by the authors
Search methods for identification of studies
See: Collaboration HIV Review Group search strategy
Electronic searches
In collaboration with the Trial Search coordinator of the Cochrane HIV/AIDS Review Group, we developed a comprehensive search strategy to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress). We searched the following electronic databases from 1 January 1996 (the advent of triple ART) to 16 April 2013:
MEDLINE (Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 2)
EMBASE (Appendix 3)
LILACS
CINAHL
Web of Science
Conference on Retroviruses and Opportunistic Infections Conference
International AIDS Society Conference
Key words included MeSH terms and free‐text terms relevant to task shifting, skill mix, integration of tasks, service delivery, health services accessibility and others.
The search was repeated in the key databases including MEDLINE, CENTRAL, EMBASE and in the registries of ongoing trials on 28 March 2014. We searched the Conference on Retroviruses and Opportunistic Infections 2014 and International AIDS Society Conference 2013 on 23 May 2014.
Searching other resources
Researchers and relevant organisations. We contacted individual researchers working in the field and policymakers based in inter‐governmental organizations including the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) to identify additional studies either completed or ongoing. Reference lists. We checked the reference lists of all studies identified by the above methods and examined the bibliographies of any systematic reviews, meta‐analyses, or current guidelines which we identified during the search process. Ongoing studies. We searched www.clinicaltrials.gov (Appendix 4) and the WHO International Clinical Trials Registry Platform search portal (Appendix 5) for information on unpublished and on‐going trials.
Data collection and analysis
The methodology for data collection and analysis was based on the guidance of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Abstracts of all trials identified by electronic or bibliographic scanning were examined by two authors working independently. Where necessary, the full text was obtained to determine the eligibility of studies for inclusion.
Selection of studies
We removed duplicate references using reference management software. Following this a Cochrane research specialist conducted a broad review of results, excluding those that were clearly irrelevant. Two authors (FBA and TK) independently selected potentially relevant studies by scanning the titles, abstracts, and descriptor terms of the remaining references and applied the inclusion criteria. Irrelevant reports were discarded, and the full article or abstract obtained for all potentially relevant or uncertain reports. The two authors independently applied the inclusion criteria. Studies were reviewed for relevance, based on study design, types of participants, exposures and outcomes measures. A neutral third party adjudicated any disagreements that could not be resolved by discussion.
Data extraction and management
After initial search and article screening, two reviewers independently extracted data and entered information from each selected study onto standardised data extraction forms.
Extracted information included:
Study details: citation, start and end dates, location, study design and details.
Participant details: study population eligibility (inclusion and exclusion) criteria, ages, population size, attrition rate, details of HIV care and disease progression and any clinical, immunologic or virologic staging, tuberculosis or laboratory information.
Interventions details: any form of health care delivery by cadres of health workers other than doctors
Outcome details: mortality, loss to follow‐up, time to initiation of care, diagnosis of tuberculosis, virological outcomes, immunological outcomes, occurrence of new AIDS defining illness, patient satisfaction with care, cost of care to patient and to service provider,other negative impact on service delivery.
The interventions were carefully and systematically described, so that all of the interventions and co‐interventions reported were captured.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias within the included studies against key criteria described below. This was adapted from the methods recommended by the Cochrane Effective Practice and Organisation of Care Group (EPOC), Newcastle Ottawa Scale (Newcastle‐Ottawa Scale) and The Cochrane Collaboration (Higgins 2008). The following judgments were used: low risk of bias, high risk of bias or unclear risk of bias (either due to lack of information or uncertainty over the potential for bias). We resolved disagreements by consensus.
Risk of bias for studies with a separate control group
Randomised controlled trials (RCTs); non‐randomised controlled trials (NRCTs) and controlled before‐after (CBA) studies:
Nine standard criteria are suggested for all RCTs, NRCTs and CBA studies from EPOC. Further information can be obtained from the Cochrane Handbook section on risk of bias (Higgins 2008A). We adapted these criteria to best address the included studies and potential risk of bias presented by them as follows:
Adequate generation of the allocation sequence [Trials]
Adequate allocation concealment [Trials]
Baseline CD4 count measurements were similar [All studies]
Other baseline characteristics were similar [All studies]
The study was adequately protected against contamination [Trials]
Data collection methods (i.e. retrospective or prospective) [Cohorts]
The study was free from other risks of bias [we have specified co‐interventions as possibly introducing bias] [All studies]
Patient selection bias [Cohorts]
Assessment of Quality of Evidence Across Studies We assessed the quality of evidence across a body of evidence (i.e., multiple studies with similar interventions and outcomes) with the GRADE approach (Guyatt 2011), defining the quality of evidence for each outcome as “the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest” (Higgins 2008). The quality rating across studies has four levels: high, moderate, low or very low. Randomised controlled trials are categorised as high quality but can be downgraded; similarly, other types of controlled trials and observational studies are categorised as low quality but can be upgraded. Factors that decrease the quality of evidence include limitations in design, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results or high probability of publication bias. Factors that can increase the quality level of a body of evidence include a large magnitude of effect, if all plausible confounding would lead to an underestimation of effect and if there is a dose‐response gradient.
Measures of treatment effect
We used ReviewManager software (RevMan) provided by the Cochrane Collaboration for statistical analysis and GRADEpro software (GRADEpro 2008) to produce GRADE Summary of Findings tables and GRADE evidence profiles. We summarised dichotomous outcomes for effect in terms of risk ratio (RR) with their 95% confidence intervals. We calculated summary statistics using meta‐analytic methods and presented findings in GRADE Summary of Findings tables and GRADE evidence profiles for the main outcomes mortality (as an indicator of quality) and loss to care (an indicator of retention).
Dealing with missing data
Study authors were contacted when missing data was an issue, for example, we sought additional data from the contact authors for Assefa 2012; Bedelu 2007, Fairall 2012 and Sanne 2010.
Assessment of heterogeneity
Where clinical or methodological heterogeneity was minimal, we examined statistical heterogeneity by using the χ2 statistic with a significance level of 0.10, and the I2 statistic. We interpret an I2 estimate greater than 50% as indicating moderate or high levels of heterogeneity and investigated its causes where possible (Deeks 2008).
Assessment of reporting biases
There were not sufficient studies available to use a funnel plot to provide a visual indication of whether reporting bias was present or not.
Data synthesis
Data were grouped by the level of health care worker providing care, compared to doctors as outlined in Table 4. When interventions and study populations were sufficiently similar across the different studies, we pooled the data across studies and estimated summary effect sizes using random‐effects models. We used the inverse variance method for analysis of cluster randomised designs.The inverse variance method assumes that the variance for each study is inversely proportional to its importance, therefore more weight is given to studies with less variance than studies with greater variance. We summarised the quality of evidence for the main outcomes reporting the randomised controlled trial data in GRADE Summary of Findings tables and GRADE evidence profiles (Guyatt 2011)
Subgroup analysis and investigation of heterogeneity
Data were grouped by the level of health care provider involved as outlined in Table 4, or additional health care workers as described in the various studies, degree of task shifting (initiation and maintenance of ART or maintenance of ART) and study design (RCT or cohort studies).
Sensitivity analysis
As the trials included were based on different approaches (superiority vs non‐inferiority vs equivalence) we would have liked to conduct a sensitivity analysis to see if this had any effect on the result. However, there were too few trials for us to conduct this analysis. We would also have conducted sensitivity analysis in the event of significant risk of bias, however, the few trials, were generally of high quality. Subsequent updates of this review will attempt to conduct sensitivity analyses where possible.
Results
Description of studies
Results of the search
Searches of all mentioned databases were originally conducted in April 2013, and a repeat search was conducted in Pubmed, Embase, The Cochrane Library, ICTRP and clinicaltrials.gov in March 2014. We searched the Conference on Retroviruses and Opportunistic Infections 2014 and International AIDS Society conference 2013 in May 2014. These search outputs produced 3694 titles after 257 duplicates were removed (Figure 1). TK and FBA independently selected potentially relevant studies by scanning the titles, abstracts, and descriptor terms of all downloaded material from the electronic searches. Irrelevant reports were discarded and full text articles were obtained for 39 potentially relevant reports. TK and FBA independently applied the inclusion criteria. Studies were reviewed for relevance based on: study design, types of participants, exposures and outcomes measures. Disagreements were resolved by discussion. An additional study, suggested through contact with the technical team at the World Health Organization was also reviewed. We thus identified four randomised controlled trials and six cohort studies that met our inclusion criteria for data extraction, coding and potential meta‐analysis. TK, FBA, EP and MB independently extracted data for the included studies.
1.
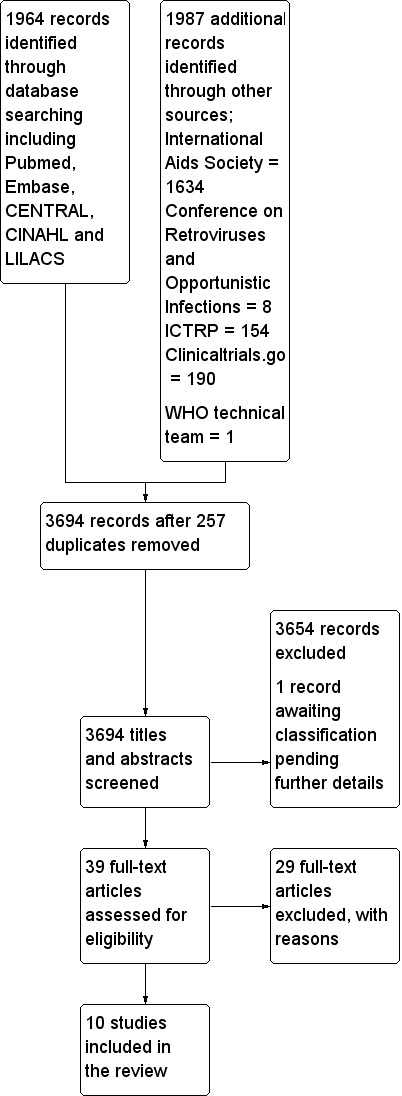
Study flow diagram.
Included studies
See Characteristics of included studies and a summary of the models of care reported in the included studies in Table 5. Ten studies, all conducted in adults, are included in this review. Of these, four are randomised controlled trials (Fairall 2012; Jaffar 2009; Sanne 2010, Kiweewa 2013), two are prospective cohorts (Humphreys 2010; Kipp 2010), while four are retrospective cohort studies (Assefa 2012; Bedelu 2007; Brennan 2011; Sherr 2010). One study, was conducted in urban, peri‐urban and rural settings in Ethiopia (Assefa 2012) , two studies were conducted in urban and rural Uganda (Jaffar 2009;Kipp 2010;), one in urban Uganda ( Kiweewa 2013), one in urban Mozambique (Sherr 2010), one in rural Swaziland (Humphreys 2010) while the remaining four studies were conducted in various urban, peri‐urban and rural settings in South Africa (Bedelu 2007; Brennan 2011; Fairall 2012; Sanne 2010). The ten studies contributed a total of 66,606 participants to this review with median baseline CD4 cell count ranging from 100 to 450 cells/mm3. Seven studies compared nurse‐led care with doctor‐led care (Assefa 2012; Bedelu 2007; Brennan 2011; Fairall 2012; Humphreys 2010; Kiweewa 2013; Sanne 2010), one study compared clinical officer (or non‐physician clinician) led care with doctor‐led care (Sherr 2010), while two studies compared care by trained community health workers to doctor‐led care (Jaffar 2009; Kipp 2010). In six of the studies, the model of care also involved decentralisation to a more basic level of healthcare facility or to the community (Assefa 2012; Bedelu 2007; Brennan 2011; Humphreys 2010; Jaffar 2009; Kipp 2010).
2. Description of Model of care in included studies.
| Model of care | ||||
| Study ID | Study design | intervention | control | Co‐interventions |
| Doctor versus nurse or clinical officer for initiation and maintenance of ART | ||||
| Assefa 2011 | Retrospective cohort | Nurses and clinical officers initiate and maintain ART in health centres | Doctors initiate and maintain ART in health centres | |
| Bedelu 2007 | Retrospective cohort | Nurses and clinical officers initiate and maintain ART in health centre | Doctors initiate and maintain ART in hospitals | |
| Fairall 2012 (Cohort 1) | Cluster randomised controlled trial | Nurses initiate and maintain ART (in health centres) in addition to training, as well as educational and managerial support | Doctors initiate and maintain ART in health centres | Model of care includes specific training package, mentoring and supervision and organisational changes to ensure support from clinical managers |
| Sherr 2010 | Retrospective cohort | Clinical officers initiate and maintain ART in hospitals | Doctor initiate and maintain ART in hospitals | |
| Doctor versus nurse or clinical officer for maintenance of ART | ||||
| Fairall 2012 (Cohort 2) | Cluster randomised controlled trial | Nurses follow up patients previously initiated on ART by doctors, for maintenance care of ART in health centres | Doctors follow up patients who have previously been initiated on ART by doctors for ART maintenance care in health centres | Model of care includes specific training package, mentoring and supervision and organisational changes to ensure support from clinical managers |
| Humphreys 2010 | Prospective cohort study | Nurses follow up patients at the health centre after initiation by doctors at a hospital | Doctors follow up patients who have previously been initiated on ART by doctors for ART maintenance care in hospitals | |
| Matovu 2013 | Randomised controlled trial | Nurses follow up patients at the specialised clinic at a hospital, with support from peer counsellors | Doctors follow up patients monthly at the specialised clinic within the hospital | Peer counsellors do home visits for patients who miss appointments in the intervention arm |
| Sanne 2010 | Randomised controlled trial | Nurses follow up patients already initiated on ART by doctors at the health centre | Doctors follow up patients who have previously been initiated on ART by doctors for ART maintenance care in health centres | Intervention includes didactic and clinical training, and there is available clinical supervision as needed. |
| Doctor versus community health worker for maintenance of ART | ||||
| Jaffar 2009 | Cluster randomised controlled trial | Field officers follow up patients who had previously been initiated by doctors on ART in the community for ART maintenance care | Doctors follow up patients who have previously been initiated on ART by doctors for ART maintenance care in hospitals | |
| Kipp 2010 | Prospective cohort study | Community volunteers follow up patients previously initiated on ART by clinical officers in the community for ART maintenance car | Doctors follow up patients who have previously been initiated on ART by doctors for ART maintenance care in hospitals | |
There are three main comparisons for this review:
‐ doctor versus nurse or clinical officer care for initiation and maintenance of antiretrovirals
‐ doctor versus nurse or clinical officer care for maintenance of antiretroviral therapy
‐ doctor versus community health workers for maintenance antiretroviral therapy Four studies compared doctor‐led care versus other cadres for initiation and subsequent maintenance of patients on ART (Assefa 2012; Bedelu 2007; Fairall 2012; Sherr 2010), while seven studies evaluated patients who had previously been initiated on ART comparing doctor‐led care versus other cadre (Brennan 2011; Fairall 2012; Humphreys 2010; Jaffar 2009; Kipp 2010;Kiweewa 2013; Sanne 2010), for maintenance of ART. One trial (Fairall 2012) recruited two patient cohorts: one for initiation and maintenance and the second for maintenance of ART. In the included studies, nurses or clinical officers were generally responsible for the delivery of ART‐related tasks including ART initiation or re‐prescription, clinical staging and managing opportunistic infections. Clinical officers were usually trained for two to three years (Sherr 2009, Mullan 2007). Detailed descriptions of these roles are provided in some of the included studies (Bedelu 2007; Sherr 2010). Severe cases and treatment failures were usually managed by doctors. Community health workers whose ART specific training ranged from two days (Kipp 2010) to four weeks (Jaffar 2009) were not responsible for prescribing ART but rather delivered ART drugs to patients at home, performed adherence monitoring, monitored clinical symptoms and signs of drug toxicity and reported back or referred patients when necessary to the clinical officer at the facility. All the studies reported some form of adherence support although this was more prominently reported in the intervention group in some studies (Bedelu 2007; Humphreys 2010; Kipp 2010; Kiweewa 2013). All studies reported access to doctor support when indicated however, in Bedelu 2007, intervention groups had regular support visit by a mobile team of experienced doctors.
Excluded studies
See Characteristics of excluded studies
After review of all records found through the search, we evaluated the full texts of 39 potentially eligible studies. We excluded 29 studies from this review for different reasons. Most of the studies were excluded based on study design; some were purely descriptive or cross‐sectional study designs and or studies with non‐contemporaneous comparisons. In one study (Selke 2010), the control arm had intermittent, poorly quantified, access to a doctor.
Risk of bias in included studies
See Figure 2
2.
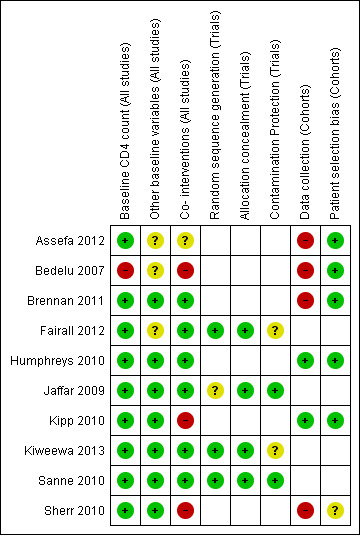
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We adapted the EPOC and Newcastle Ottawa risk of bias criteria to assess the risk of bias in the included studies in order to reflect the two study designs included in this review: cohort studies and randomised controlled trials (including cluster randomised controlled trials). See summary of risk of bias by study in Figure 2. Risk of bias in randomised controlled trials: Of the four RCTs included in this review, two studies randomised participants at cluster level (Fairall 2012; Jaffar 2009) while in the other two studies, randomisation was done at individual level (Kiweewa 2013;Sanne 2010). All four studies reported comparable baseline CD4 cell counts (as an indicator of baseline morbidity) in both intervention and control groups and were rated as low risk of bias. Three of the studies were balanced on baseline characteristics such as sex and age and were rated as having a low risk of bias (Jaffar 2009;Kiweewa 2013; Sanne 2010) , however, Fairall 2012 had a higher proportion of patients with lower WHO clinical stage in the intervention group and was therefore rated as having an unclear risk of bias. Co‐interventions provided to participants or staff were similar for both intervention and control groups in Jaffar 2009 and Sanne 2010 and were thus rated as being at low risk of performance bias. Furthermore, Fairall 2012 was also rated as being at low risk of performance bias because the additional training and support provided to the intervention group was part of the model of care being tested. Sequence generation was well described and adequate for Fairall 2012, Kiweewa 2013 and Sanne 2010 and hence judged as having a low risk of bias, however sequence generation was not described for Jaffar 2009 thus presenting an unclear risk of bias. Allocation concealment was well described and adequate for all four RCTsand were rated as being at low risk of bias. Fairall 2012 was judged to be at unclear risk of contamination bias because additional doctors were assigned to intervention clinics by the government. No information was provided regarding the measures to avoid contamination in Kiweewa 2013, therefore rated as having an unclear risk of contamination bias. For the other two studies, the risk of contamination was low. Overall the risk of bias was low for Jaffar 2009, Sanne 2010, Fairall 2012, and Kiweewa 2013 Risk of bias in cohort studies Six cohort studies were included in this review, four of which were retrospective cohorts and were therefore judged to be at high risk of bias from the method of data collection. On the indicator of baseline CD4 cell count, a potential confounder, five studies had comparable CD4 cell counts in both intervention and control groups and were judged to be at low risk of bias, except for Bedelu 2007 which was assessed to be at high risk of bias on account of higher CD4 cell counts in intervention group. In two studies, (Brennan 2011; Sherr 2010) both intervention and control groups were similar for the indicator evaluating other baseline characteristics (which included age, sex, WHO clinical stage, amongst others) and were thus assessed as being at low risk of bias. The other four studies did not present adequate data to assess this domain and therefore had an unclear risk of bias for this indicator. As regards co‐interventions, apart from Brennan 2011 and Humphreys 2010 which were rated as being at low risk of bias, all other studies had a high or unclear risk of performance bias. In Bedelu 2007, the intervention group received regular support visits by experienced clinicians, while Kipp 2010 required a treatment supporter in the intervention group to support adherence. In Sherr 2010, both groups saw multiple care providers during the period of analysis ‐ these three studies were rated as being at high risk for performance bias. Information presented by Assefa 2012 was inadequate and was thus judged to have an unclear risk of performance bias. Except for Sherr 2010 which was rated as having an unclear risk of selection bias because assignment to initial provider was said to be at "clinic discretion", we did not identify other additional risks of bias in patient selection for the other five studies and thus judged this indicator as low risk.
Effects of interventions
See: Table 1; Table 2; Table 3
Nurses or clinical officers versus doctors for initiation and maintenance of antiretroviral therapy
See Table 1
Four studies, including one randomised controlled trial (Fairall 2012) and three retrospective cohorts (Assefa 2012; Bedelu 2007; Sherr 2010) examined this comparison. Data was grouped by study design. Sherr 2010 was not included in the data analysis as there was substantial contamination and patients could choose whether they saw a doctor or clinical officer at their follow‐up visits. 1. Death (12 months)
Overall there is high quality evidence of no difference in mortality whether nurses or doctors initiate antiretroviral therapy. This is true provided that the model of care includes specific training and organisational support for professional nurse practitioners prescribing and following up antiretroviral therapy for newly initiated patients. Data from two retrospective cohorts with methodological limitations due to selection bias provided low quality evidence, however they were not downgraded further as the direction of the bias was likely to favour the intervention (Analysis 1.1, Figure 3).
1.1. Analysis.
Comparison 1 Doctor versus nurse or clinical officer (initiation and Maintenance of ART) , Outcome 1 Death (12 months).
3.
Forest plot of comparison: 1 Doctor versus nurse or clinical officer (initiation and Maintenance of ART) , outcome: 1.1 Death (12 months).
Evidence from clinical trials One cluster randomised trial (Fairall 2012), conducted in 31 peri‐urban and rural clinics in South Africa, enrolled patients between January 2008 and June 2009. The specific group (cohort 1) within this trial included patients who were eligible for ART or were likely to become eligible during the trial. A total of 9252 patients were enrolled, however, adjusting for the design effect introduced by the clustering, an effective sample size of 2,770 adults provided data for this outcome. Participant characteristics were similar at baseline (sex, age, CD4 cell count), except there were more WHO clinical stage I (52% vs. 32%) and fewer WHO clinical stage III (24% vs. 38%) patients in the intervention group. The trial had methodological limitations due to unclear risk of contamination from additional doctors being drafted to intervention clinics by the provincial department of health. In addition, 965/3712 (26%) patients were initiated on therapy by a nurse in the intervention group compared to none in the control group. The results reported no difference in mortality between nurse‐led and doctor‐led initiation of ART and maintenance care with the adjusted HR 0.92 (95% CI 0.76 to 1.12), P = 0.4, adjusted for age, sex, CD4 cell count and available identify number. The unadjusted risk ratio was 0.96 (95% CI 0.82 to 1.12), P = 0.57 (Analysis 1.1). Overall there is high quality evidence from the clinical trial that there is no difference in mortality outcomes between those patients initiated and followed up by nurses compared to those initiated and followed up by doctors.
Evidence from observational studies Three retrospective cohort studies examined this outcome (Assefa 2012; Bedelu 2007; Sherr 2010), but only the first two including 39,160 adults contributed data for this analysis. For the two included cohorts, in addition to the inherent bias and confounding associated with cohort studies, Bedelu 2007, set in rural South Africa, included patients with higher CD4 cell counts in the intervention group presenting a high risk of selection bias, furthermore, the intervention group also received regular support from experienced clinical staff with resultant further risk of performance bias. On the other hand, although Assefa 2012 , set in rural and peri‐urban Ethiopia, had similar baseline CD4 cell counts in both groups, other characteristics such as sex, age and clinical stage at baseline were not described. Analysis of mortality at 12 months reported an increased risk of death in the task shifting group; the RR was 1.23 (95% CI 1.14 to 1.33) with no statistical heterogeneity detected. There was low quality evidence of a 23% increased risk of death in the task shifting group in spite of the fact that the baseline imbalance in morbidity should bias the result in favour of task shifting.
The use of crude proportions extracted from the study reports does not adjust for important potential confounding or take into account the losses of patients over time. The quality of the data was not further downgraded for risk of bias, as the direction of the bias was expected to favour the intervention and yet the control was favoured in the results. This cohort provided low quality evidence that there may be an increased risk of death in models of care that include both decentralisation and task shifting. 2. Loss to follow‐up (12 months)
We extracted data on patients lost to follow‐up, as it is more consistently reported and defined across studies, as a surrogate for the outcome retention. Overall there is moderate quality evidence from one cluster randomised trial that supports lower lossto follow‐up when nurses initiated and maintained patients on antiretroviral therapy, compared to doctors. The quality of the data was downgraded for imprecision due to the relatively low number of events. The trial describes specific methods to support nurse practitioners to initiate treatment and also ensures adequate adherence support for patients (Analysis 1.2, Figure 4) Evidence from clinical trials The Fairall 2012 trial including a cohort of patients eligible to initiate antiretroviral therapy is described in detail above. Losses to follow‐up were defined as patients not having reported to the clinic in previous three months and whose vital status was not known at the end of the trial. The trial reported a slightly lower rate of loss to follow‐up in the task shifting group (5.5%) compared with the doctor group (7.7%) with a RR of 0.73 (95% CI 0.55 to 0.97), P = 0.03 (Analysis 1.2). In this trial, retention was defined as patients who are alive and still enrolled in the programme after 12 months, were not known to have withdrawn or relocated, and had a documented clinic visit or laboratory test in the previous six months if started on ART or last known CD4 cell count was below 200 cells/mm3 or in the past nine months if they had not yet started ART and had a CD4 cell count > 200 cells/mm3. The adjusted risk ratio for participant retention in this trial was 1.10 (95% CI 1.04 to 1.16), P = 0.001, adjusted for randomisation strata and intra‐cluster correlation of outcomes. Evidence from observational studies
1.2. Analysis.
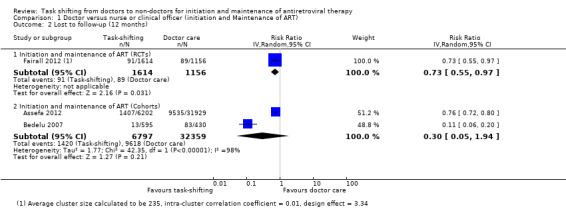
Comparison 1 Doctor versus nurse or clinical officer (initiation and Maintenance of ART) , Outcome 2 Lost to follow‐up (12 months).
4.
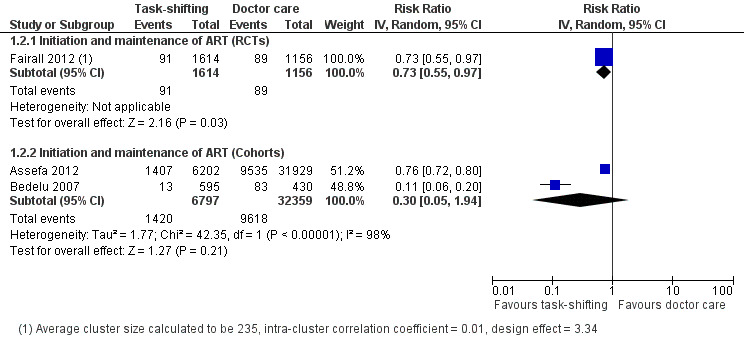
Forest plot of comparison: 1 Doctor versus nurse or clinical officer (initiation and Maintenance of ART) , outcome: 1.2 Lost to follow‐up (12 months).
Two retrospective cohort studies, Bedelu 2007 and Assefa 2012, contributed data for this outcome. There were no adjusted results available and as such the crude data is reported. There was no difference in patients lost to follow‐up between the task shifting group and doctor‐led care, the relative risk was 0.30 (95% CI 0.05 to 1.94), P = 0.21 (Analysis 1.2). There was substantial statistical heterogeneity between the studies (I2= 98%). In addition, we found potential clinical heterogeneity in patients' baseline characteristics (e.g. CD4 cell counts), and methodological heterogeneity of the models of care provided. Although both studies are consistent in favouring lower numbers of patients lost to follow‐up in the intervention groups, due to imprecision indicated by the wide confidence interval, we have downgraded the quality of the data. These data provide very low quality evidence that there may be no difference in the patients lost to follow‐up.
3. Attrition (death or lost to follow‐up at 12 months):
Evidence from trials
Analysis of the composite outcome of attrition from the single trial above shows that there is high quality evidence that overall attrition is similar in both task‐shifted and doctor‐led initiation and maintenance of antiretroviral therapy, at 12 months follow up, RR = 0.89 (95% CI 0.0.79 to 1.01), P = 0.08 (Analysis 1.3).
1.3. Analysis.
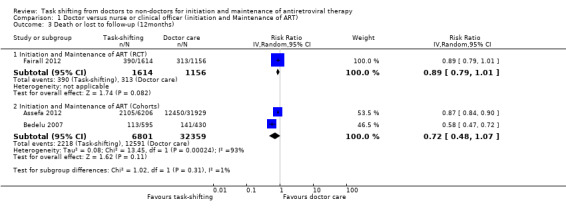
Comparison 1 Doctor versus nurse or clinical officer (initiation and Maintenance of ART) , Outcome 3 Death or lost to follow‐up (12months).
Evidence from observational studies
Overall, there is very low quality evidence that attrition in patients task shifted to nurses or clinical officers compared with doctor‐led initiation and maintenance of care were similar, RR = 0.72 (95% CI 0.48 to 1.07), P = 0.11. Despite quantitative heterogeneity, both studies consistently showed that attrition was decreased with decentralisation and task shifting. The effect sizes varied however, with Assefa 2012 showing more modest effects, 3%, while Bedelu 2007 reports a 42% decrease. The quality of the evidence was downgraded due to imprecision. Nurses or clinical officers versus doctors for maintenance of antiretroviral therapy
See Table 2 Three studies including two randomised controlled trials (Fairall 2012; Sanne 2010) and one retrospective cohort study (Brennan 2011) examined this comparison. Data was grouped by study design and describes the two critical outcomes, death and those patients lost to care. 1. Death (12months)
Overall, there is moderate quality evidence that there is probably no difference in death whether antiretroviral maintenance care is delivered by a nurse or by a doctor. The data was downgraded for imprecision due to a wide confidence interval and relatively low event rate. (Analysis 2.1, Figure 5) Evidence from trials Two trials contributed data to this analysis (Fairall 2012; Sanne 2010). Both trials took place in rural or peri‐urban settings in South Africa, shifting care to nurses.. Both had a low risk of bias for all the parameters in our modified criteria for risk of bias, except that Fairall 2012 had an unclear risk of contamination from doctors being drafted to intervention sites by the provincial government. Fairall 2012 recruited a different cohort (cohort 2 in the trial) from that described above, as patients were already on antiretroviral therapy and were then referred to care by a nurse, rather than the standard care which included a doctor. The professional nurses received a specific package of training to enable them to monitor clinical and drug effects for these patients and received specific support through supervision and involvement of clinic management in the model of care. In the Sanne 2010 trial, primary care nurses and doctors, not experienced with ART delivery were provided with specific didactic and clinical training in line with National guidelines. Contamination was prevented in this trial by scheduling patients for the respective cadre of health workers on different days. The trial aimed to provide continuity of care, and patients were able to see the same practitioner at each follow up visit. Additional adherence support was provided by lay counsellors. Prior to meta‐analysis, data from the cluster randomised trial Fairall 2012, was adjusted for the design effect using the intra‐cluster correlation coefficient reported in the study before being combined in a meta‐analysis. The result showed no difference in mortality between task shifting and doctor‐led ART maintenance care when ART had previously been initiated by a doctor, with a RR of 0.89 (95% CI 0.59 to 1.32), P= 0.55. There was no statistical heterogeneity (I2= 0%). Evidence from observational studies There is very low quality data from one retrospective cohort study (Brennan 2011) that mortality may be lower in the nurse‐led care arm, The study used propensity scoring to match patients in intervention and control groups. In this study, task shifting also involved decentralization from hospital to health centre care. In addition to the propensity scoring, Brennan 2011 reported adjusted rates of mortality at 12 months, adjusted hazard ratio was 0.2 (95% CI 0.04 to 0.8)/ 100 person years. This was adjusted for baseline characteristics including sex, age, CD4+ cell count. The crude result from the proportions data we extracted provides similar results, a statistically significantly reduced risk of death in task shifting group compared to doctor‐led care; RR of 0.19 (95% CI 0.05 to 0.78), P = 0.02. 2. Loss to follow‐up (12 months)
2.1. Analysis.
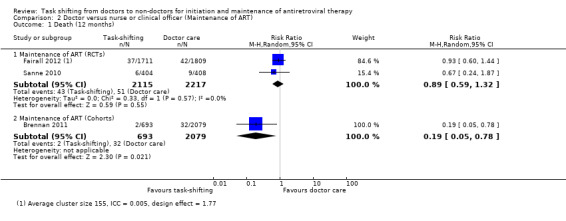
Comparison 2 Doctor versus nurse or clinical officer (Maintenance of ART), Outcome 1 Death (12 months).
5.
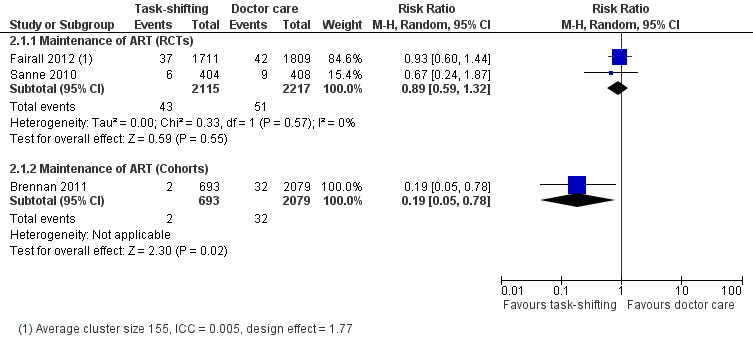
Forest plot of comparison: 2 Doctor versus nurse or clinical officer (Maintenance of ART), outcome: 2.1 Death (12 months).
Overall there is moderate quality evidence from two trials that there is probably no difference in the numbers of patients lost to follow‐up whether nurses or doctors provide follow up and maintenance antiretroviral care after 1 year of follow up.(Analysis 2.2, Figure 6) Evidence from clinical trials Analysis of data from the two RCTs which reported on death also showed no difference in the numbers of patients lost to care in task shifted care compared to doctor‐led care with a relative risk of 1.27 (95% CI 0.92 to 1.77), P = 0.15. There was no statistical heterogeneity between the results of studies included in this analysis. The quality of the data was downgraded for imprecision, and we therefore report moderate quality data that there is probably no difference in patients lost to care whether doctors or nurses provide HIV care at similar primary care health centres. Evidence from observational studies The single cohort (Brennan 2011), showed significantly less loss to follow‐up in the task shifting group compared to doctor led ART maintenance care. There was a relative risk reduction of 66% ‐ RR = 0.34 (95% CI 0.18 to 0.66), P = 0.001. This data is considered very low quality due to the nature of the study design and the imprecision of the results with the low event rates reported.
2.2. Analysis.
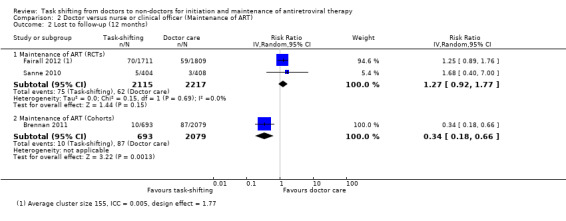
Comparison 2 Doctor versus nurse or clinical officer (Maintenance of ART), Outcome 2 Lost to follow‐up (12 months).
6.
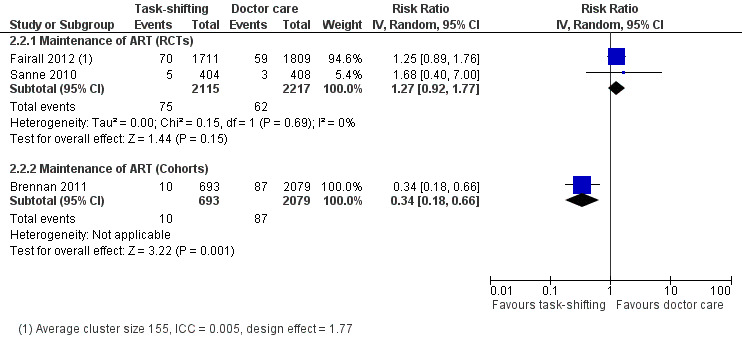
Forest plot of comparison: 2 Doctor versus nurse or clinical officer (Maintenance of ART), outcome: 2.2 Lost to follow‐up (12 months).
3. Attrition (death or lost to follow‐up at 12 months):
Evidence from clinical trials
Analysis of data on this composite outcome of death or loss to follow‐up from the two RCTs (Fairall 2012; Sanne 2010) also showed no difference in overall attrition in task shifted care compared to doctor‐led care with a relative risk of 1.1 (95% CI 0.86 to 1.41), P = 0.46. There was no statistical heterogeneity between the results of studies included in this analysis. The quality of the data was downgraded for imprecision , and we therefore report moderate quality evidence that there is probably little or no difference in overall patient attrition whether doctors or nurses provide HIV maintenance care at similar primary care health centres.
Evidence from observational studies
The single cohort (Brennan 2011), showed that overall patient attrition was significantly less in the task shifting group compared to doctor led ART maintenance care. There was a relative risk reduction of 70% ‐ RR = 0.3 (95% CI 0.17 to 0.54), P < 0.0001. This data is considered very low quality due to the imprecision of the results with the low event rates reported and should be cautiously interpreted on account of the bias and confounding inherent in the observational study design.
Doctor versus community health worker for maintenance of antiretroviral treatment
See Table 3
One randomised controlled cluster trial (Jaffar 2009) contributed data for the critical outcomes death and patients lost to follow‐up at 12 months.
1. Death (12 months)
This analysis provides moderate quality evidence that there is probably no difference in mortality when doctors deliver care in the hospital or specially trained field workers provide home‐based maintenance care and ART delivery. The quality of the data was downgraded for imprecision due to the small effective sample size and event rate, resulting in a wide confidence interval. (Analysis 3.1, Figure 7).
3.1. Analysis.
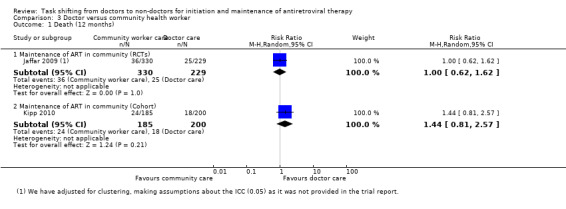
Comparison 3 Doctor versus community health worker, Outcome 1 Death (12 months).
7.
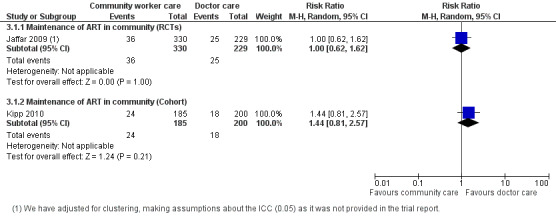
Forest plot of comparison: 3 Doctor versus community health worker, outcome: 3.1 Death (12 months).
Evidence from clinical trial
One trial investigated task shifting from doctor to specifically trained community worker for ART maintenance care (Jaffar 2009). This trial took place in various parts of urban, peri‐urban and rural Uganda and included adults patients, who were initiated on treatment in hospital by doctors, and then down referred only once stable on their treatment and consented to down referral. The intervention group received home based community worker support and monitoring with a field officer monthly using a checklist and mobile phone, with daily review of the field officers notes by the designated medical officer. The patients were still seen at the hospital six monthly (Jaffar 2009). Overall, this trial had a low risk of poor internal validity (Figure 2), however it was underpowered (small sample sizes and event rates) to address the question. The adjusted rate ratio for mortality was 0.95 (95% CI 0.71 to 1.28)/100 person years, adjusted for study stratum and CD4 cell count category. In order to adjust for the design effect, we required an intra‐cluster co‐efficient (ICC). As this was not provided for this trial, we made a statistical assumption and used a liberal ICC of 0.05. After adjusting for clustering with an assumed intra‐cluster coefficient of 0.5, the effective sample size was 559, and the calculated risk ratio is 1.0 (95% CI 0.62 to 1.62).
Evidence from observational studies
None provided 12 month data. Kipp 2010 provided data for 6 months of follow up, shown in the data and analysis table (Analysis 3.1). This cohort evidence was not included in the summary of finding tables for 12 month follow up.
2. Loss to follow‐up (12 months)
There is moderate quality evidence from this trial that loss to follow‐up is probably not different whether doctor or home‐based care is provided in this manner. The quality of the data was downgraded for the small sample size, low number of events and wide confidence interval.
Evidence from clinical trial
The Jaffar 2009 trial also reported on this outcome. No adjusted result was found in the published report, however, we adjusted for clustering (using the liberal ICC of 0.05). The relative risk for patients lost to follow‐up was 0.52 (0.12 to 2.3), P = 0.39. There is moderate quality evidence from this trial that losses to follow‐up are probably no different whether doctor or home‐based care is provided in this decentralised manner. The quality of the data was downgraded for the small sample size, low number of events and wide confidence interval.(Analysis 3.2, Figure 8)
3.2. Analysis.
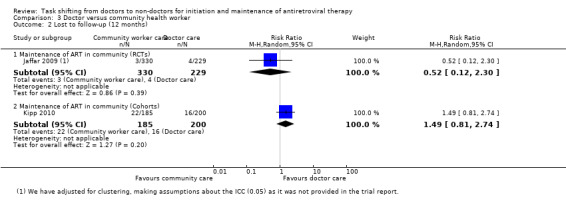
Comparison 3 Doctor versus community health worker, Outcome 2 Lost to follow‐up (12 months).
8.
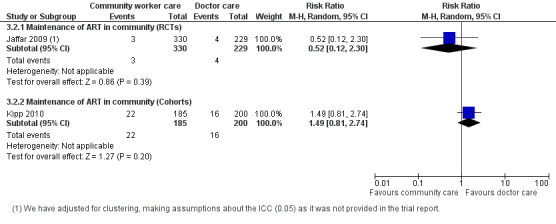
Forest plot of comparison: 3 Doctor versus community health worker, outcome: 3.2 Lost to follow‐up (12 months).
Evidence from observational studies
None provided 12 month data. Kipp 2010 provided data for 6 months of follow up, shown in the data and analysis table (Analysis 3.2). This cohort evidence was not included in the summary of finding tables for 12 month follow up.
3. Attrition (death or lost to follow‐up at 12 months):
Evidence from clinical trials
Similarly the single RCT providing data for this composite outcome shows similar overall attrition for doctor‐led as well as community officer‐led (home based) maintenance care of HIV. The relative risk for attrition was 0.93 (0.6 to 1.46), P = 0.76, this evidence was of moderate quality as we downgraded for imprecision.
Evidence from observational studies None provided 12 month data. Kipp 2010 provided data for 6 months of follow up, shown in the data and analysis table (Analysis 3.3). This cohort evidence was not included in the summary of finding tables for 12 month follow up.
3.3. Analysis.
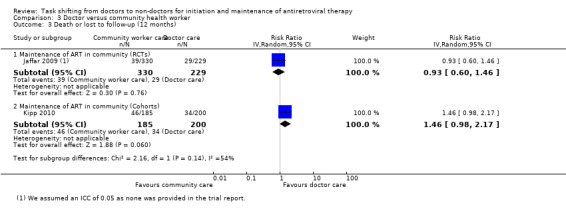
Comparison 3 Doctor versus community health worker, Outcome 3 Death or lost to follow‐up (12 months).
Other outcomes
Immunological changes ‐ CD4+ count
Seven of the ten included studies report on CD4 cell count. As they all report the CD4 cell count variably with some reporting means and others medians, this data was not pooled.
For initiation and maintenance, Fairall 2012 (cohort 1) reports a CD4 cell count mean (SD) at follow up by nurses 161 cells/ mm3 (175) compared to doctor 141 cells/ mm3 (161), with the difference in the means of 22.3 cells/ mm3 (3.6 to 40.9), P = 0.02, favouring nurse led care. This was adjusted for randomisation strata and intra‐cluster correlation. Bedelu 2007 reports the proportion of patients with a CD4 cell count above 200 cells/m3. The crude proportion indicated that 303/348 tests done in the health centre under nurse care were above this threshold compared to 61/81 of those conducted at the hospital by doctors. Assefa 2012 does not report on this outcome.
For maintenance of care, Fairall 2012 (cohort 2) reports that the mean (standard deviation) for CD4 cell count at follow up by nurses was 438 (SD 219) compared to doctors 418 (SD 201), with the difference in the means reported as 24.2 (7.2 to 41.3), P = 0.007 favouring nurse care. This was adjusted for randomisation strata and intra‐cluster correlation. Sanne 2010 reported the median increase in CD4 cell count. At 1 year, patients in nurse‐led care had a median increase of 155 cells/mm3 (IQR 119 to 193) compared to doctor ‐led care with a median increase of 158 cells/mm3 (IQR 125 to 169). This was sustained to 2 years, where patients being cared for by nurses has a median increase of 239 cells/mm3 (IQR 217 to 290) compared to doctor‐led care of 220 cells/mm3 (IQR 174 to 274). These results are not clinically significantly different. Humphreys 2010 reports mean change at six months only, where patients in nurse care have a CD4 cell count of 103 cells/mm3 compared to those in doctor care with a mean change of 103 cells/mm3 (P = 0.7). Kiweewa 2013 reports similar mean increases in CD4 count between groups around 200 cells/mm3 after 6 to 12 months. Brennan 2011 did not report on this outcome.
For the comparison of community field worker follow up compared to doctor care, the 12 month median (IQR) is reported, where patients in the community had a CD4 cell count of 250 cells/ mm3 (IQR 180 to 350) compared to 260 cells/mm3 (IQR 190 to 375) which was not statistically significant.
Viral load suppression
Five of the included studies report variably on this outcome. Bedelu 2007 and Kiweewa 2013 reported on the proportion of patients with viral loads below 400 copies/mL. The nurses group in Bedelu 2007 was 89.5% below this threshold compared to 78% in the doctors group, but of note, there was a substantial difference in the numbers of viral load tests conducted between the groups, 50% compared to 10% of patients tested in the nurse and doctor group respectively. In Kiweewa 2013 the proportion with a viral load <400 copies/mL at 6 to 12 months was similar between groups.
Fairall 2012 found no difference in either cohort for virological suppression. The trial reports an adjusted risk ratio for those patients with a viral load below 400 copies/mL in cohort 1 (initiation and maintenance) with an adjusted RR of 0.97 (95% CI 0.9 to 1.03), P = 0.324. In cohort 2 (maintenance of care), the risk difference of those with suppressed viral load is reported as RD 1.1% (‐2.3 to 4.6), P = 0.534. Sanne 2010 reports the hazard ratio for all virological failure (including early failure which is less than 1.5 log decrease in viral load from baseline to 12 weeks of treatment; and late virological failure which is two consecutive viral load four weeks apart of more than 1000 copies/mL. The hazard ratio was 1.15 (95% CI 0.75 to 1.76) indicating no statistical difference between the groups.
Jaffar 2009 was the only study reporting this outcome for the comparison of community field officer care with doctor care. They reported an adjusted rate ratio for having a viral load above 500 copies/mL of 1.04 (95% CI 0.78 to 1.4), thus, there was no difference between the groups.
Overall, the reported virological suppression or failure rates were similar between the intervention and control groups, except in Bedelu 2007 which had a relative imbalance in the numbers of patients being tested for viral loads between groups and being a retrospective cohort, may not have been adequately powered for this outcome.
Cost to providers and patients
One prospective cohort, Humphreys 2010, reports specifically on the cost of travel for patients. The average cost for a patient seen by a nurse was USD 0.74 compared to USD 1.5 for a patient seen at the hospital by a doctor (P = 0.001) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Doctor versus nurse or clinical officer (initiation and Maintenance of ART) , Outcome 4 Cost of travel.
| Cost of travel | |||
|---|---|---|---|
| Study | Down referred patient | Hospital care patient | P‐value |
| cost of travel | |||
| Humphreys 2010 | average cost for follow up care ‐ USD 0.74 | average cost for follow up care USD 1.5 | P = 0.001 |
Two studies provided data for the outcome on overall cost to patients. Both reports come from community based treatment by a field worker compared to standard doctor‐led hospital based treatment (Jaffar 2009; Kipp 2010). Both studies indicate substantial increases in the cost to patients when they are required to travel to the hospital, which is usually further from their homes. Kipp 2010 reports a doubling of cost to patients when accounting for transport only. Jaffar 2009, in the cluster trial, reports a three times increase in costs, including transport, lost work time, child‐care costs and food (Analysis 3.4).
3.4. Analysis.
Comparison 3 Doctor versus community health worker, Outcome 4 Cost to patient.
| Cost to patient | ||
|---|---|---|
| Study | Home based care | Hospital based care |
| Jaffar 2009 | total cost per year for transport, lunch, childcare costs, lost work time: $18/year (after first year) | total cost per year for transport, lunch, childcare costs, lost work time: $54/ year (after the first year) |
| Kipp 2010 | Transport cost $0.74/ visit for home based care | Transport cost $1.5/ visit for facility based care |
Costs to the health service are also reported. Jaffar 2009 reported costs to health service for community care versus doctor‐led hospital based groups. These costs included staff, transport, drugs, laboratory, training, supervision, capital and utilities costs and was a mean of USD 793 / year for each patient in the home based group compared to USD 838 / year / patient in the hospital based group.
A sub‐study of the Fairall 2012 included an evaluation of cost‐effectiveness of the interventions related to doctor‐ versus nurse‐led care. In particular, the study evaluated the incremental cost effectiveness ratio (ICER). They concluded that nurse‐led care (including training, supervision, and treatment for TB and HIV) was associated with higher mean health service costs compared to doctor led care without substantial effects on the health outcomes of patients.
Time to initiation of ART,
For initiation and maintenance Fairall 2012 (cohort 1) reported the adjusted hazard ratio for time to initiation of ART as 1.14 (95% CI 0.92 to 1.43), P = 0.232, adjusted for randomisation strata and intra‐cluster correlation of outcomes.
New tuberculosis diagnosis
For initiation and maintenance, Fairall 2012 (cohort 1) reports on new tuberculosis diagnoses being made, the risk ratio was 1.46 (95% CI 1.18 to 1.81), P = 0.001, supporting superiority of nurses compared to doctors in this model of care for diagnosing tuberculosis. For maintenance of care Fairall 2012 (cohort 2) report the risk difference between groups for new tuberculosis diagnosis of 0.21% (95% CI ‐2.1 to 1.54), P = 0.758, indicating equivalence of the diagnosis rate regardless of whether a doctor or nurses delivered the care.
Patient satisfaction with care
Assefa 2012 and Humphreys 2010 included a qualitative component to their studies which reports on patient satisfaction with the model of care by group.
Assefa 2012 evaluated patient satisfaction with care by conducting two hour long focused group discussions (57 patients, in 7 groups). They looked predominantly at the issue of task shifting and its acceptability amongst patients and healthcare providers. Patients reported that nurse and health officer (clinical officer) services were 'generally well accepted, and reduced waiting time', they also revealed that they were 'more comfortable with nurses than with physicians because nurses were friendlier and more supportive'. Patients emphasised that nurses and health officers spent more time with them discussing their medical problems and took enough time examining them. Patients, who assisted with care provision, identified three additional benefits of being involved in ART delivery: their life experience helped them to provide appropriate counselling; it helped combat stigma and discrimination in society; and it provided them with an opportunity for employment. In the same study, focused group discussions were held with programme managers and health care providers who agreed that the model including task shifting provided a timely solution for Ethiopias needs. They also agreed that nurses and health officers can provide high quality care given adequate training and supervision.
Humphreys 2010 was a prospective cohort that used the model of task shifting from doctors to nurses and decentralisation from hospital to health centre. This study included a qualitative assessment of patient satisfaction as a primary outcome. Those attending the intervention clinic were asked about their level of satisfaction, 25/31 of those who responded said that they were very satisfied with the care received. Reasons provided included the reduced cost of transport, being nearer to home, shorter queue, being treated better by staff, receiving better care and that they would not be talked about. The two respondents who were not satisfied with the care complained about the lack of a doctor, saying they did not have money to get to the main clinic, and that there was a delay because staff from the hospital arrived late at the health centre.
Time to initiation of antiretroviral therapy
Fairall 2012 (cohort 1) reports on initiation and maintenance, and includes time to initiation of ART as one of the secondary outcomes. The reported hazard ratio is 1.14 (0.92 to 1.43), P = 0.2, comparing nurses to doctor‐led care. This was adjusted for the competing risk, death.
New AIDS defining illness, any negative impact on the health delivery
None of the included studies specifically reported on new AIDS defining illness or on general negative impacts on health delivery.
Discussion
Summary of main results
This review found high quality evidence that there is no difference in the mortality whether nurses or doctors initiate antiretroviral therapy provided that the model of care includes specific training and organisational support for professional nurse practitioners prescribing and following up antiretroviral therapy for newly initiated patients. Overall there is moderate quality evidence from one cluster trial that supports lower loss to follow‐up when nurses initiated and maintained patients on antiretroviral therapy, compared to doctors. The quality of the data was downgraded for imprecision due to the relatively low number of events. Cohort data with methodological limitations and low precision conflicted with the trial evidence, as we found low quality evidence of increased deaths in the task shifted group, and very low quality evidence of no difference in patients lost to follow‐up.
We found moderate quality evidence that task shifting of antiretroviral maintenance care from doctors to nurses probably results in no difference in death at one year. Overall there is moderate quality evidence from two trials that there is probably no difference in the numbers of patients lost to follow‐up whether nurses or doctors provide maintenance antiretroviral therapy at one year. The data was downgraded for imprecision due to a wide confidence interval and relatively low event rate. Cohort data conflicted with the evidence from the trials and reported very low quality evidence of lower deaths and losses to follow up at one year.
We found moderate quality evidence that there is probably no difference in the mortality when doctors deliver care in the hospital or specially trained field workers provide home‐based maintenance care and ART delivery in the community. There is moderate quality evidence from this trial that loss to follow‐up is probably no different whether a doctor or a field worker deliver care in this decentralised manner. The quality of the data was downgraded for imprecision due to the small effective sample size and event rate, resulting in a wide confidence interval. There was no data from the cohorts for these outcomes at one year.
With respect to other relevant outcomes, the cohorts reporting CD4 cell counts showed increases in immunological status, with no difference between models of care. Similar results were found for changes in viral load, a marker of the effectiveness of antiretroviral therapy, with studies reporting comparable virological suppression regardless of the model of care employed.
Costs, reported by three studies are considerably reduced for both the patient and provider, when issues of travel and staffing are considered. However, when the cost of implementing the intervention are considered, including training and supervision, the costs for task shifted care may be increased for the health system.
There was a high level of acceptability to patients reported by the two studies in which this was assessed when therapy is delivered by nurses rather than doctors.
Overall completeness and applicability of evidence
The clinical trials that are included in this review provide moderate quality evidence that substitution of nurses or community workers to initiate and maintain ART for people receiving HIV services in rural and peri‐urban settings in South Africa and Uganda is feasible and are likely to be representative of lower‐ and middle‐income countries.
Task shifting did not impact on the quality of the therapy delivered, however, the research conducted in South Africa included nurses, rather than clinical officers, and this is likely to differ from treatment delivered in other country settings.
Pragmatic trials provided most of the data for this review, including both task shifting within facilities and to the community. These trials are designed to include real‐life settings and be applicable to general clinical care.
The observational studies included in the review represented large cohorts of task shifting care, however, there was substantial heterogeneity in the settings and models of care delivered. This may introduce bias, particularly in the selection of the populations, and reporting of outcomes. A key concern for the interpretation of these studies is that individuals could choose whether to be treated by a physician or not and whether to be down referred, by decentralised services, but the methods for this decision were not clearly reported. In addition, the quality of data collection in these studies is variable, and generally based on a secondary analysis of routinely collected programme data. Of concern is that the models of care, including health care provider training, supervision and mentoring and the necessary organisational planning that is required, were likely to differ across the studies. Even within the cohorts, there is a possibility that task shifting did not occur in a systematic way.
Quality of the evidence
In the GRADE system, well‐conducted randomised controlled trials (without additional limitations) provide high quality evidence, and observational studies without any special strengths (and without additional limitations) provide low‐quality evidence. The quality of evidence provided by a body of literature comprised exclusively of observational studies would thus generally be graded as low, except in circumstances where observational studies are upgraded. For this review randomised controlled trials were available to address the critical outcomes including death, loss to follow‐up and the composite outcome, attrition (death plus loss to follow‐up).
The results of all key outcomes are provided by randomised controlled trials.The trial evaluating doctors compared to nurses for initiation and maintenance of therapy, provided high quality evidence that there is likely no difference in the rates of death and attrition, and moderate quality evidence of fewer losses to follow‐up with nurse‐led care. The trials were methodologically sound and rated to have low risk of bias regarding their conduct, however, the issue of contamination could not be excluded. In addition, adjusting the sample size to account for the cluster design, introduced a degree of imprecision which required that we downgrade the evidence quality some of the outcomes.
The trials evaluating doctors compared to nurses for maintenance of therapy, provided moderate quality evidence that there is likely no difference in the rates of death, loss to follow‐up and attrition with nurse‐led care. The evidence was downgraded for imprecision, due to the low number of event rates and wide confidence intervals including appreciable harm or benefit.
The cohorts included in the review provided low or very low quality evidence, due to their inherent biases with cohort study design and at times imprecision despite the large sample sizes.
Potential biases in the review process
Biases in the review process were minimised by performing a comprehensive search of databases and conference proceedings, not limiting for language or time. In addition, we contacted expert researchers in the field and other experts associated with relevant organisations for unpublished and ongoing studies. We did not explore publication bias by using funnel plots as there were too few studies to draw conclusions from this analysis.
Agreements and disagreements with other studies or reviews
The high levels of healthcare worker shortage is recognised as a severe impediment to increasing expansion of patients access to antiretroviral therapy. In response there has been substantial research published regarding the issue of task shifting in HIV care. A prior narrative review of task shifting found similar outcomes comparing physician and non‐physician led care (Callaghan 2010), however, there was not a systematic approach to interrogating the quality of the included studies. A systematic review evaluating community‐based antiretroviral therapy programs was published in 2013 (Decroo 2013), however, their question differed from ours as their comparator was not specifically doctor led treatment. They also included a variety of study designs to answer their review question, however, they did not evaluate the quality of included studies which may result in bias in the reporting of their results.Another systematic review, including a meta‐analysis of death, loss to follow‐up and immunological and virological outcomes had similar findings to our review (Emdin 2013). The researchers found that death was no different and loss to follow‐up possibly improved when nurses initiated antiretroviral management. However, it should be noted that their approach differed from Cochrane standard methods and their review pooled a variety of study designs which may introduce bias in the meta‐analysis.
In addition, decentralisation of care often includes task shifting as part of the model of care, whereas task shifting may or may not occur with decentralisation. A recent Cochrane review of decentralisation reports similar evidence of the relative feasibility of decentralisation care, including at times task shifting (Kredo 2013).
In June 2013, the WHO launched their consolidated guidelines for managing HIV using a public health approach. It includes reference to operational changes that are recommended to improve access to and retention in care, including task shifting of care to non‐physicians (Hirnschall 2013).
Authors' conclusions
Implications for practice.
There is moderate quality evidence that substitution of nurses or community workers for doctors to initiate and maintain ART in adults in lower‐ and middle‐income countries is feasible and probably does not compromise the quality of care provided. We did not find studies that included special risk groups (e.g. pregnant women, children) to inform practice for these populations.
For programme managers and policy makers considering the application of the results, implementation strategies should carefully consider the models of care that were used. The pragmatic trial designs are more likely than typical trials to reflect real life treatment programmes and settings that roll‐out treatment. However, the researchers stress the importance of adequate training and the need for organisational structures to support the shifting of tasks to ensure high quality clinical care. This approach of supporting and mentoring staff to take on additional tasks is aligned with broader issues of strengthening of health services and reducing work load for the sparse numbers of doctors.
Successful application of task shifting is likely to require leadership from national governments in harnessing multiple stakeholders to buy‐in to these concepts and create enabling regulatory frameworks to support sustainable, cost‐effective, equitable models relevant for each country setting. This must crucially include clarifying roles of different cadres of health workers, changed scopes of practice and related regulatory frameworks. This reconfiguration of health teams, needs to avoid fragmentation and consider long term views of health system strengthening.
Implications for research.
All of the critical outcomes for this review have been addressed by randomised controlled trial data. However, two of the trials that contributed most of the data were set in South Africa, where management was shifted from doctors to nurses. Thus data on the inclusion on task shifting to clinical officers is limited to cohort data. The cohort data included in the review does provide evidence of the feasibility of running HIV programmes incorporating non‐physician based care.
Further research, using pragmatic clinical trial designs, should explore the specific models of care and the health care workers that are best suited to various settings in lower and middle‐income country settings. In addition, where appropriate, special risk groups (e.g. children) should be investigated, and the programmatic requirements specific for them clearly described.
What's new
| Date | Event | Description |
|---|---|---|
| 1 July 2014 | Amended | Correct affiliation. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 7, 2014
| Date | Event | Description |
|---|---|---|
| 8 June 2012 | New citation required and major changes | New authors taking forward this review |
Acknowledgements
We acknowledge the immeasurable support from staff of the South African Cochrane Centre and the HIV/AIDS review group and Idowu Araoyinbo who initiated the review protocol. The model of care outlined in Table 1 was developed by Professor Paul Garner of the Liverpool School of Tropical Medicine.
Appendices
Appendix 1. MEDLINE Search strategy
Database: PubMed 1996 to 2014
| Search | Query |
| #7 | Search #3 AND #4 AND #5 Limits: Publication Date from 1996/01/01 to 2013/03/25 |
| #6 | Search #3 AND #4 AND #5 |
| #5 | Search task* OR task‐shifting OR referr* OR referral and consultation[mh] OR role* |
| #4 | Search health personnel[mh] OR doctor OR doctors OR clinician OR clinicians OR physician OR physicians OR "healthcare provider" OR "healthcare providers" OR "health care provider" OR "health care providers" |
| #3 | Search #1 AND #2 |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] |
Appendix 2. CENTRAL search strategy
Database:CLIB(1996 ‐ 2014)
| ID | Search |
| #1 | MeSH descriptor HIV Infections explode all trees |
| #2 | MeSH descriptor HIV explode all trees |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) |
| #7 | MeSH descriptor Antiretroviral Therapy, Highly Active, this term only |
| #8 | MeSH descriptor Anti‐HIV Agents explode all trees |
| #9 | MeSH descriptor Antiviral Agents, this term only |
| #10 | MeSH descriptor AIDS Vaccines, this term only |
| #11 | ANTI HIV OR ANTIRETROVIRAL* OR ANTI RETROVIRAL* OR AIDS VACCIN* |
| #12 | (#7 OR #8 OR #9 OR #10 OR #11) |
| #13 | (#6 AND #12) |
| #14 | MeSH descriptor Health Personnel explode all trees |
| #15 | doctor* OR clinician* OR physician* OR "healthcare provider*" OR "health care provider*" |
| #16 | (#14 OR #15) |
| #17 | MeSH descriptor Referral and Consultation explode all trees |
| #18 | task* OR task‐shifting OR referr* OR role* |
| #19 | (#17 OR #18) |
| #20 | (#13 AND #16 AND #19) |
| #21 | (#13 AND #16 AND #19), from 1996 to 2013 |
Appendix 3. EMBASE search strategy
Database:EMBASE (1996 ‐ 2014)
| No. | Query |
| #7 | #3 AND #4 AND #5 AND [humans]/lim AND [embase]/lim AND [1996‐2013]/py |
| #6 | #3 AND #4 AND #5 |
| #5 | task* OR 'task‐shifting' OR referr* OR role* OR 'task performance'/syn OR 'patient referral'/syn |
| #4 | 'health care personnel'/syn OR doctor* OR clinician* OR physician* OR 'healthcare provider'/syn OR 'healthcare providers' OR 'health care provider'/syn OR 'health care providers' OR 'health auxiliary'/syn |
| #3 | #1 AND #2 |
| #2 | 'human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine' OR 'anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab OR 'anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab OR 'anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab OR 'anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab OR 'anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab OR 'anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab OR 'anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab OR 'anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab OR 'anti hiv':ti OR 'anti hiv':ab OR antiretrovir*:ti OR antiretrovir*:ab OR 'anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab OR haart:ti OR haart:ab OR 'aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab OR 'anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent' OR 'antiretrovirus agent'/de OR 'antiretrovirus agent' OR 'antivirus agent'/de OR 'antivirus agent' OR 'highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy' |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'b cell lymphoma'/de OR 'b cell lymphoma' OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab |
Appendix 4. Clinicaltrials.gov search strategy
Database: Clinicaltrials.gov (1996 ‐ 2014) Search strategy: HIV | doctor OR nurse OR physician OR "health personnel" OR "task shifting" OR referral | received from 01/01/1996 to 04/25/2013
Appendix 5. ICTRP search strategy
Database: ICTRP (1996 ‐ 2014) Search strategy: HIV | doctor% OR nurse% OR healthcare personnel OR physician% OR task& OR referral& OR role% | received from 01/01/1996 to 04/25/2013
Data and analyses
Comparison 1. Doctor versus nurse or clinical officer (initiation and Maintenance of ART) .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (12 months) | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Initiation and maintenance of ART (RCTs) | 1 | 2770 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.82, 1.12] |
| 1.2 Initiation and maintenance of ART (Cohort) | 2 | 39160 | Risk Ratio (IV, Random, 95% CI) | 1.23 [1.14, 1.33] |
| 2 Lost to follow‐up (12 months) | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Initiation and maintenance of ART (RCTs) | 1 | 2770 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.55, 0.97] |
| 2.2 Initiation and maintenance of ART (Cohorts) | 2 | 39156 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.05, 1.94] |
| 3 Death or lost to follow‐up (12months) | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Initiation and Maintenance of ART (RCT) | 1 | 2770 | Risk Ratio (IV, Random, 95% CI) | 0.89 [0.79, 1.01] |
| 3.2 Initiation and Maintenance of ART (Cohorts) | 2 | 39160 | Risk Ratio (IV, Random, 95% CI) | 0.72 [0.48, 1.07] |
| 4 Cost of travel | Other data | No numeric data | ||
| 4.1 cost of travel | Other data | No numeric data |
Comparison 2. Doctor versus nurse or clinical officer (Maintenance of ART).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (12 months) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Maintenance of ART (RCTs) | 2 | 4332 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.32] |
| 1.2 Maintenance of ART (Cohorts) | 1 | 2772 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.78] |
| 2 Lost to follow‐up (12 months) | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Maintenance of ART (RCTs) | 2 | 4332 | Risk Ratio (IV, Random, 95% CI) | 1.27 [0.92, 1.77] |
| 2.2 Maintenance of ART (Cohorts) | 1 | 2772 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.18, 0.66] |
| 3 Death or lost to follow‐up (12 months) | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Maintenance of ART (RCT) | 2 | 4332 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.86, 1.41] |
| 3.2 Maintenance of ART (Cohorts) | 1 | 2772 | Risk Ratio (IV, Random, 95% CI) | 0.30 [0.17, 0.54] |
2.3. Analysis.
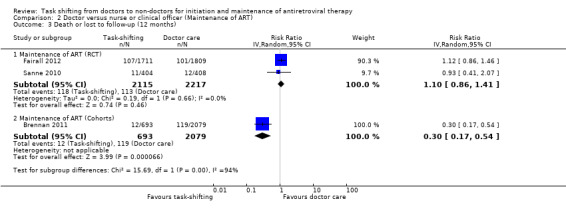
Comparison 2 Doctor versus nurse or clinical officer (Maintenance of ART), Outcome 3 Death or lost to follow‐up (12 months).
Comparison 3. Doctor versus community health worker.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (12 months) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Maintenance of ART in community (RCTs) | 1 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.62, 1.62] |
| 1.2 Maintenance of ART in community (Cohort) | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.81, 2.57] |
| 2 Lost to follow‐up (12 months) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Maintenance of ART in community (RCTs) | 1 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.12, 2.30] |
| 2.2 Maintenance of ART in community (Cohorts) | 1 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.81, 2.74] |
| 3 Death or lost to follow‐up (12 months) | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Maintenance of ART in community (RCTs) | 1 | 559 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.60, 1.46] |
| 3.2 Maintenance of ART in community (Cohorts) | 1 | 385 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.98, 2.17] |
| 4 Cost to patient | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Assefa 2012.
| Methods | Design: retrospective cohort Duration of study: recruitment September 2006 ‐ 2008, censored March 2009 (minimum 6 months, maximum 24 months follow‐up) | |
| Participants | Country: Ethiopia Setting: nationwide, 30 hospitals, 25 health centres Inclusion and exclusion criteria: nil described Comparable CD4 count or clinical stage at baseline: similar CD4 count | |
| Interventions | Intervention: patients initiated and maintained at health centres by nurses and health officers. Severe manifestations, treatment failures were referred to hospital. Control: initiated and followed up at hospital with physicians. Co‐interventions: community health workers performed counselling, referrals and linkage between facilities and defaulter tracing in both groups | |
| Outcomes | Mortality, loss to follow‐up, retention, and median CD4 Count. Assessed at 6, 12 and 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar median CD4 count in both groups |
| Other baseline variables (All studies) | Unclear risk | Not described |
| Co‐ interventions (All studies) | Unclear risk | Community health workers delivered adherence and referral services from health centres to hospitals, unclear whether this was for both groups or for the health centre group only |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | Randomly selected folders in all included sites in both groups |
Bedelu 2007.
| Methods | Design: retrospective cohort Duration: January 2004 ‐ June 2005, completed 12 months follow‐up by July 2006 | |
| Participants | Country: South Africa Setting: rural, 12 health centres, 1 hospital inclusion criteria: adults eligible for ART CD4 count <200cells/mm3, WHO clinical stage 4 exclusion criteria: nil described Comparable CD4 count or clinical stage at baseline: CD4 counts differed at baseline | |
| Interventions | Intervention: initiated and maintained antiretroviral therapy at health centre by nurses, physician support with mobile team, adherence counsellors and patient support groups available. Control: initiated and maintained antiretroviral therapy at hospital by doctors | |
| Outcomes | Mortality, loss to follow‐up, CD4 count, viral load Assessed at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | High risk | CD4 counts differed between groups at baseline. |
| Other baseline variables (All studies) | Unclear risk | Only reported on sex |
| Co‐ interventions (All studies) | High risk | Model differed by group, Nurse (health centre) group received additional adherence support and visits from a mobile support team of experienced clinicians |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
Brennan 2011.
| Methods | Design: retrospective matched cohort analysis Duration: April 2004 ‐ January 2009 | |
| Participants | Country: South Africa. Setting: peri‐urban, urban, 1 hospital, 1 clinic. Inclusion criteria: stable on antiretroviral treatment for at least 11 months, no opportunistic infections, CD4 count > 200cells/mm3, stable weight and virologically suppressed <400 copies/mL. Considered good candidates by doctors and agree to down‐refer. Exclusion criteria: refuse down‐referral Comparable CD4 count or clinical stage at baseline: control matched on sex, age, months on Rx, regimen, BMI, HB and CD4 count (propensity scoring) | |
| Interventions | Intervention: initiated at advanced hospital by doctors, maintained at health centre by nurses, seen every 2 months for medicine pick up. "Up referred" if default (>7 days), toxicity, detectable viral load Control: initiated and maintained by doctor at advanced hospital, seen 6 monthly, pick up medicines every 2 months. Co‐interventions: adherence counselling provided at both facilities | |
| Outcomes | Death, loss to follow‐up, mean CD4 count, viral load rebound. Assessed at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar CD4 count in both groups. |
| Other baseline variables (All studies) | Low risk | Matched by Propensity scores on all baseline characteristics. |
| Co‐ interventions (All studies) | Low risk | Both groups received adherence counselling. |
| Data collection (Cohorts) | High risk | Retrospective cohort. |
| Patient selection bias (Cohorts) | Low risk | All participants were equally eligible for down referral, and were matched using propensity scores on baseline characteristics. |
Fairall 2012.
| Methods | Design: pragmatic Cluster randomised trial. Duration of study: recruitment and enrolment January 2008‐ June 2009. Each patient’s follow‐up was censored between 12 and 18 months after enrolment | |
| Participants | Country: Free state, South Africa. Setting: rural, Periurban, 30 nurse led primary care clinics. Inclusion criteria: the study enrolled two different cohorts. 1) Adult patients (≥16 years) with CD4 counts between 200 and 350 cells and not yet started on ART 2) adults already on ART for at least six months, still on treatment at the time of enrolment. Exclusion Criteria: eligible patients who did not return for a follow up clinic appointment after enrolment on ART | |
| Interventions | Intervention 1: patients in Cohort 1 were monitored by nurses till they became eligible for ART which was then initiated by designated nurses and subsequently monitoring as well as re‐prescriptions done by the nurses. Nurses did not initiate ART in patients meeting the following criteria but referred them to the doctor; (CD4 < 50, Stage 4 AIDS, previous ART, bed‐ or wheelchair‐bound, using drugs other than cotrimoxazole or vitamins, pregnant, weight < 40 kg or body mass index >28). Intervention 2: patients in cohort 2 were followed up by nurses for monitoring as well as re‐prescription of ART. Control: Patients eligible for ART were referred to a doctor to initiate and repeat prescriptions for ARTand review patients every six months. Between visits to doctors, patients were seen monthly by nurses. Co‐interventions: as part of the STRETCH intervention, nurses received four educational outreach sessions using PALSA PLUS guidelines. The training related to; ART prescribing and side effects, practice guidelines/ algorithms as well as identifying patients needing referral to a doctor. Managerial support was also provided by STRETCH coordinators. |
|
| Outcomes | Cohort 1: Primary: time to death. Secondary: change in weight, CD4, viral loads; hospital admissions, and inpatient days; ART initiation, time from enrolment to starting ART, detection of tuberculosis, cotrimoxazole provision, program retention one year after enrolment, baseline CD4 count among those who started ART, and clinic consultations with nurses and doctors Cohort 2: Primary: viral load suppression 12 months after enrolment Secondary: time to death censored between 12 and 18 months after enrolment, changes in weight and CD4 counts, hospital admissions, and inpatients days; programme retention, diagnosis of tuberculosis, cotrimoxazole provision, ART regimen switching, and clinic consultations with nurses and doctors |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Both groups had similar CD4 counts in cohort 1. |
| Other baseline variables (All studies) | Unclear risk | STRETCH group had higher proportion of WHO clinical stage I and fewer patients with WHO clinical stage III in Cohort 1 |
| Co‐ interventions (All studies) | Low risk | The intervention groups received additional training and support as part of the implementation of the guideline, compared to provision of the guideline to the control groups. However this was part of the model of care being tested. |
| Random sequence generation (Trials) | Low risk | Randomisation was carried out by the trial statistician before the trial started, using N‐Query Advisor |
| Allocation concealment (Trials) | Low risk | Randomisation conducted centrally by statistician prior to the start of the trial. |
| Contamination Protection (Trials) | Unclear risk | During the trial, some of the control clinics received input from additional doctors sent by the provincial administration, with potential contamination bias |
Humphreys 2010.
| Methods | Design: prospective cohort. Duration: started recruitment January 2007 ‐ June 2007, followed up until November 2007, minimum 6 months follow up | |
| Participants | Country: Swaziland Setting: rural setting, one district hospital, 30 nurse led health centres. Inclusion criteria: adults >14 years on antiretroviral therapy for at least 4 weeks, CD4 count >100 cells/mm3. Exclusion criteria: refused to be down referred. Comparable CD4 count or clinical stage at baseline: CD4 count and clinical stage similar at baseline | |
| Interventions | Intervention: initiated at hospital by doctor and maintained at health centre by nurses. Control: initiated and maintained at hospital by doctors. Co‐interventions: training for primary care centre nurses, monthly outreach support visit by at least one counsellor and nurse | |
| Outcomes | Clinic attendance, patient experience, loss to follow up, change in CD4 count, weight, death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar mean CD4 between groups. |
| Other baseline variables (All studies) | Low risk | Age, sex, and weight were similar at baseline, duration on antiretrovirals (longer for the control group) and and length of follow up differed by group which may favour outcomes in the control group |
| Co‐ interventions (All studies) | Low risk | No additional intervention described, other than monthly mobile support team visits which are part of the model of care |
| Data collection (Cohorts) | Low risk | Prospective cohort |
| Patient selection bias (Cohorts) | Low risk | Assignment was based on catchment areas (intervention clinics/control clinics) |
Jaffar 2009.
| Methods | Design: cluster randomised trial, 22 clusters for each arm, median size 25 ‐ 36, inter‐cluster co‐efficient 0.2 Duration: February 2005 ‐ December 2006, follow‐up until 31 January 2009, median follow‐up 27 ‐ 28 months | |
| Participants | Country: Uganda. Setting: urban, peri‐urban and rural, varying distance from the hospital. Inclusion criteria: adults >18 years old, CD4 count <200cells/mm3, WHO clinical stage 3 or 4. Exclusion criteria: living >100 km from facility. Comparable CD4 count or clinical stage at baseline: similar, slightly lower CD4 count for intervention arm. | |
| Interventions | Intervention: initiated at hospital by doctors, maintained in community by field officers who delivered treatments every month on motorcycles, monitored adherence, drug toxicity and disease, they referred patients; had access to mobile phones for on‐site call to doctor. If patients was absent, followed up. Reviewed at hospital 6 monthly. Control: initiated and maintained at hospital. Monthly clinic visits to collect medicine, reviewed by medical officer 3 monthly, drop in clinic; if defaulted, followed up at home; household vouchers for counselling. | |
| Outcomes | Rate of virological failure, time to detectable viral load >500 copies/mL, time to detectable viral load >500 copies/mL at any visit from 12 months if it was <500 copies/mL at 6 months or increase in 1000 copies/mL between two consecutive tests in those who did not have viral load <500 copies/ mL at 6 months, all cause mortality, admission, change to second line antiretrovirals, outpatient attendance, adherence in previous 28 days, cost incurred by health services and patients, patient diagnosed with TB at first admission, proportion of those with CD4 count > 200cells/mm3. Timepoints of outcome assessment not clear. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar, slightly lower CD4 count for intervention arm. |
| Other baseline variables (All studies) | Low risk | Similar baseline characteristics for both groups. |
| Co‐ interventions (All studies) | Low risk | model of care in both groups differed by facility. |
| Random sequence generation (Trials) | Unclear risk | Not described. |
| Allocation concealment (Trials) | Low risk | Allocation cards labelled with stratum number and sealed in advance was drawn from a concealed box in the presence of all stakeholders. |
| Contamination Protection (Trials) | Low risk | No evidence of contamination. |
Kipp 2010.
| Methods | Design: prospective cohort. Duration: six month results available (follow up for 2 years) | |
| Participants | Country: Rwimi, Uganda. Setting: intervention in rural setting, control in urban setting. Inclusion criteria: adults >18 years, eligible for antiretroviral therapy, antiretroviral therapy naive, resident in the sub‐county. Exclusion criteria: nil described. Comparable CD4 count or clinical stage at baseline: similar CD4 count at baseline. | |
| Interventions | Intervention: initiated at the health centre, maintained in community volunteer community health workers who did weekly home visits ‐ anti‐retrovirals delivered monthly, adherence monitored and supported, monitored adverse effects and clinical symptoms. Control: initiated and maintained in hospital, by doctors. Co‐intervention: an additional treatment support was required by those in the home‐based group to support adherence and disclosure. | |
| Outcomes | Mortality, viral load, increase in CD4 count, cost to provider. Assessed at 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar mean CD4 count in both groups |
| Other baseline variables (All studies) | Low risk | Age and sex similar at baseline, although occupations different |
| Co‐ interventions (All studies) | High risk | Treatment supporter was required by home based care (intervention) group |
| Data collection (Cohorts) | Low risk | Prospective |
| Patient selection bias (Cohorts) | Low risk | No selection bias identified |
Kiweewa 2013.
| Methods | Design: open label randomised non inferiority trial
Duration: mean duration of follow‐up was 10 months. 57% were followed up for 12 months following ART initiation while 43% were followed up for only 6 months. |
|
| Participants | Country: Kampala, Uganda Setting: urban, Mulago National Referral Hospital at comprehensive ART clinic attached to the PMTCT unit within the reproductive child health clinic. Inclusion criteria: HIV infected pregnant women referred from PMTCT programme before or after delivery initiated on ART, 18 years or older, written informed consent, residence in a stable home within 15km of Mulago hospital, willingness to be home visited,eligibility to start ART based on Ugandan ministry of health guidelines at that time (WHO clinical stage 3 or 4 or CD4 count <200cells/mm3 Exclusion criteria: lack of willingness to be home visited, lack of interest or busy schedule, working or staying upcountry, ineligibility for ART. Comparable CD4 count or clinical stage at baseline: CD4 count and clinical stage similar at baseline |
|
| Interventions | Intervention: initiated by doctor, then seen at 2 months and 12 months by doctor ‐ but at 2 weeks, 1, 3, 6, 9 months seen by nurses and peer counsellors. Home visits made if missed appointment; counsellors were PLWH with 10 day training, nurses had special training on HIV ART management. Control: initiated in hospital by doctors, maintained by doctors and certified adherence counsellors (standard of care; seen monthly at the hospital) |
|
| Outcomes | Primary outcome: virological success (viral load ,400 copies/mL at 6 ‐12 months after initiation) Secondary outcomes: mean increase in CD4 count; drug adherence through pill counts at 6 ‐ 12 months after initiation of ART |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar mean CD4 count in both groups |
| Other baseline variables (All studies) | Low risk | Similar baseline characteristics for both groups |
| Co‐ interventions (All studies) | Low risk | The intervention group received home visits for those who missed clinic appointments as part of the model of care. However, since there was no loss to follow up in either group, this was not considered a significant source of bias which would impact an outcome |
| Random sequence generation (Trials) | Low risk | Randomisation was done using a table of random numbers |
| Allocation concealment (Trials) | Low risk | The allocation codes for a particular site were sealed in sequentially numbered envelopes |
| Contamination Protection (Trials) | Unclear risk | No information was provided on measures to avoid contamination |
Sanne 2010.
| Methods | Design: open label randomised non inferiority trial Duration of study: median follow‐up 120 weeks |
|
| Participants | Country: South Africa. Setting: peri‐urban in 2 Primary health clinics in Cape town and Johannesburg. Inclusion criteria: adults (>16 years), with a CD4 count < 350cells/mm3 or with a previous AIDS Defining illness, who had taken ART for < 6 weeks. Exclusion Criteria: pregnant and in the 1st trimester. Renal, liver and hematology values >3 times the ULN; concomitant treatment with systemic myelosuppressive, neurotoxic, pancreatotoxic, hepatotoxic or cytotoxic treatment within 30 days of randomisation; acute hepatitis, intractable diarrhoea lasting >6 weeks; Bilateral peripheral neuropathy grade 2 or higher, Current alcohol or substance abuse that, in the opinion of the investigators, would interfere with the study, patients with an active OI <7 days treatment, TB if treatment was less than 8 weeks or <2 weeks if recruited after Oct 2005. Comparable CD4 count or clinical stage at baseline: similar CD4 count at baseline | |
| Interventions | Intervention: ART maintenance care by primary health care nurses (With additional 1year training in primary care) in Primary health clinics. Control: ART maintenance care from doctors ( medical officers with no previous HIV‐care experience) in Primary health clinics. Both groups had ART initiated by doctors in Primary health clinics. Co‐interventions: both groups received adherence counselling by a team of lay community counsellors who were trained in treatment adherence counselling. | |
| Outcomes | Cumulative treatment failure, defined as a composite endpoint consisting of virologic failure, toxicity failure, withdrawn consent, defaulting clinic schedule, loss to follow‐up, disease progression, and death. Cumulative virologic failure, defined by viral load decline of less than 1.5 log after 12 weeks of treatment OR 2 viral load measures of greater than 1,000 copies/ml on 2 consecutive occasions more than 4 weeks apart after 24 weeks of treatment. CD4 count increase. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | CD4 count was similar in both groups. |
| Other baseline variables (All studies) | Low risk | Both groups had similar baseline characteristics |
| Co‐ interventions (All studies) | Low risk | Both groups received adherence counselling from lay community counsellors. |
| Random sequence generation (Trials) | Low risk | Randomisation lists were generated centrally with a stratified permuted block randomisation. |
| Allocation concealment (Trials) | Low risk | The allocation codes for a particular site were sealed in sequentially numbered envelopes. |
| Contamination Protection (Trials) | Low risk | To limit contamination between randomised groups, work activity and monitoring schedules were separated with routine visits scheduled on different days of the week. |
Sherr 2010.
| Methods | Design: retrospective cohort. Duration of study: Enrolled for ART between July 2004 and October 2007. | |
| Participants | Country: Mozambique. Setting: two public sector ambulatory HIV clinics in urban central Mozambique (both clinics situated in Central and Provincial Hospitals). Inclusion criteria: ART‐naive adult patients initiating ART during study period. Exclusion Criteria: <15 years of age, pregnant, patients whose initial physician was an expatriate physician. Comparable CD4 count or clinical stage at baseline: similar CD4 count at baseline | |
| Interventions | Intervention: patients whose initial provider at clinic enrolment was a clinical officer (Non physician clinician). Control:patients whose initial provider at clinic enrolment was a Doctor (Specialist or Generalist). Co‐intervention: both groups had free access to preferred provider (doctor or clinical officer) during the study period. | |
| Outcomes | Proportion with CD4 evaluated, (90 to 210 days and 330 to 390 days post ART initiation), Loss to follow up, Adherence to ART, a clinical visit at least once per quarter for three of the first four quarters post‐ART initiation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Baseline CD4 count (All studies) | Low risk | Similar CD4 count at baseline. |
| Other baseline variables (All studies) | Low risk | Similar baseline characteristics except control patients were more likely to be of higher socioeconomic status |
| Co‐ interventions (All studies) | High risk | Patient in both groups had free access to provider of choice. 27% clinical officer group switched to the Doctor group while 39.4% in Doctor group switched to clinical officer group |
| Data collection (Cohorts) | High risk | Retrospective cohort |
| Patient selection bias (Cohorts) | Unclear risk | Assignment of patients to clinical provider was at "clinic discretion" |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Babigumira 2009 | Modeling study |
| Bemelmans 2010 | Descriptive. No clear comparison between doctor‐led management and non‐doctor led management of ART. |
| Boulle 2013 | Both groups were seen by doctors and nurses. Outcome analysed was consultant ratio. |
| Celletti 2010 | Analysis of regulatory framework |
| Chang 2010 | Not comparing doctor led care versus non‐doctor led care |
| Chang 2011 | Mixed methods study, not comparing doctor led care versus non‐doctor led care |
| Colvin 2010 | Descriptive study for STRETCH (Fairall 2012) |
| Connolly 2013 | Comparison groups not clearly doctor vs. nurse care. |
| de Wet 2011 | Purely qualitative study. |
| Dewo 2012 | No control arm evident. |
| Georgeu 2012 | Purely qualitative process evaluation of the STRETCH trial. No results by arm. |
| Ivers 2011 | Mapping descriptive study in Haiti. |
| Labhardt 2012 | decentralisation study, not evident if task shifting included in the model |
| Matovuu 2013 | Duplicate of Kiweewa trial, already included |
| McGuire 2013 | Mixed care groups, not doctor vs. nurse. |
| Morris 2009 | Descriptive study of programme in Zambia. |
| Nyasulu 2013 | Interrupted time series design. No contemporary arms |
| O'Malley 2014 | Mixed methods study. |
| Rasschaert 2011 | Descriptive study in Malawi and Ethiopia. |
| Selke 2010 | Limited access to doctor in control arm. |
| Sherr 2009 | Descriptive. Sub study of Sherr 2010. |
| Simoni 2011 | Adherence support, not comparing doctor led care versus other carer. |
| Torpey 2008 | Cross‐sectional study. |
| Uebel 2011 | Descriptive. Substudy to Fairall 2012. |
| Van Rie 2014 | Historical control group. |
| Vasan 2009 | Qualitative study of agreement between health workers. |
| Venkatesh 2010 | Case control study design. |
| Zachariah 2009 | Descriptive study. |
Differences between protocol and review
We added attrition as an outcome, which is the composite of two of the outcomes: death and loss to follow‐up. This was done as there is published evidence that of those patients lost to follow‐up in HIV programmes, approximately 40% may have died.
There were changes made to the original framework in the protocol describing the health service nomenclature for lower and middle income settings. We added the concepts 'health centre (advanced)' and 'hospital (advanced)'. Through our exploration of the trial and programmatic reports it became clearer that in addition to services we had described, some of the facilities had additional assistance from mobile or visiting doctors and specialists.
In the protocol, we planned to evaluated the risk of bias in included studies using the Newcastle ‐ Ottawa approach, and EPOC criteria for evaluating trials. We adapted these criteria to allow all components of the risk of bias to be displayed in the Cochrane Tool, although specific criteria were adapted for the different included study designs.
In the protocol, we planned to conduct sensitivity analyses excluding trials with high risk of bias to evaluate the effects on the outcomes. During the review process we identified another methodological challenge, which is the inclusion of both superiority and non‐inferiority study designs. We have amended our methods to include this possible sensitivity analysis of these trials in future reviews.
Contributions of authors
TK updated the protocol with the assistance of FBA, EP, MB.
All authors contributed to data extraction. TK and FBA undertook data entry and data analysis. TK and FBA conducted the GRADE evaluation. TK wrote the first draft of the review, with the assistance of FBA. This was assessed by all authors prior to finalisation and submission for peer review.
Sources of support
Internal sources
South Africa Cochrane Centre HIV/AIDS Mentoring programme; Medical Research Council, South Africa.
External sources
-
World Health Organization, Department of HIV/AIDS, Switzerland.
Provided part funding support towards completing this review, as a subcontract through the University of California, San Francisco (UCSF)
-
UKaid from the UK Government for the benefit of developing countries (DFiD), UK.
Funding received through a grant from DFiD to the Effective Health Care Research Consortium, Liverpool School of Tropical Medicine
Declarations of interest
We declare we have no affiliation with any organization or interest group that is involved in the topic of this review
Edited (no change to conclusions)
References
References to studies included in this review
Assefa 2012 {published data only}
- Assefa Y, Kiflie A, Tekle B, Mariam DH, Laga M, Damme W. Effectiveness and acceptability of delivery of antiretroviral treatment in health centres by health officers and nurses in Ethiopia. Journal of health services research & policy 2012;17(1):24‐9. [PUBMED: 22096081] [DOI] [PubMed] [Google Scholar]
Bedelu 2007 {published data only}
- Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. The Journal of infectious diseases 2007;196 Suppl 3:S464‐8. [PUBMED: 18181695] [DOI] [PubMed] [Google Scholar]
Brennan 2011 {published data only}
- Brennan AT, Long L, Maskew M, Sanne I, Jaffray I, MacPhail P, et al. Outcomes of stable HIV‐positive patients down‐referred from a doctor‐managed antiretroviral therapy clinic to a nurse‐managed primary health clinic for monitoring and treatment. AIDS (London, England) 2011;25(16):2027‐36. [PUBMED: 21997488] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L, Brennan A, Fox MP, Ndibongo B, Jaffray I, Sanne I, et al. Treatment outcomes and cost‐effectiveness of shifting management of stable ART patients to nurses in South Africa: an observational cohort. PLoS medicine 2011;8(7):e1001055. [PUBMED: 21811402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fairall 2012 {published data only}
- Barton, G. R, Fairall, L, Bachmann, M. O, et al. Cost‐effectiveness of nurse‐led versus doctor‐led antiretroviral treatment in South Africa: Pragmatic cluster randomised trial. Tropical Medicine and International Health 2013;18(6):1‐9. [DOI: 10.1111/tmi.12093] [DOI] [PubMed] [Google Scholar]
- Fairall L, Bachmann M, Lombard C, Timmerman V, Uebel K, et al. Task‐shifting of antiretroviral treatment from doctors to primary care nurses in South Africa (STRETCH): pragmatic cluster randomised trial. Lancet 2012;380(9845):889‐98. [DOI: 10.1016/S0140-6736(12)60730-2; PUBMED: 22901955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphreys 2010 {published data only}
- Humphreys CP, Wright J, Walley J, Mamvura CT, Bailey KA, Ntshalintshali SN, et al. Nurse led, primary care based antiretroviral treatment versus hospital care: a controlled prospective study in Swaziland. BMC health services research 2010;10:229. [PUBMED: 20687955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffar 2009 {published data only}
- Amuron B, Coutinho A, Grosskurth H, Nabiryo C, Birungi J, Namara G, et al. A cluster‐randomised trial to compare home‐based with health facility‐based antiretroviral treatment in Uganda: study design and baseline findings. The open AIDS journal 2007;1:21‐7. [PUBMED: 18923692] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuron B, Levin J, Birunghi J, Namara G, Coutinho A, Grosskurth H, et al. Mortality in an antiretroviral therapy programme in Jinja, south‐east Uganda: a prospective cohort study. AIDS research and therapy 2011;8:39. [PUBMED: 22018282] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar S, Amuron B, Foster S, Birungi J, Levin J, Namara G, et al. Rates of virological failure in patients treated in a home‐based versus a facility‐based HIV‐care model in Jinja, southeast Uganda: a cluster‐randomised equivalence trial. Lancet 2009;374(9707):2080‐9. [PUBMED: 19939445] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kipp 2010 {published data only}
- Kipp W, Konde‐Lule J, Rubaale T, Okech‐Ojony J, Alibhai A, Saunders DL. Comparing antiretroviral treatment outcomes between a prospective community‐based and hospital‐based cohort of HIV patients in rural Uganda. BMC international health and human rights 2011;11 Suppl 2:S12. [PUBMED: 22166168] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipp W, Konde‐Lule J, Saunders LD, Alibhai A, Houston S, Rubaale T, et al. Results of a community‐based antiretroviral treatment program for HIV‐1 infection in Western Uganda. Current HIV research 2010;8(2):179‐85. [PUBMED: 20163349] [DOI] [PubMed] [Google Scholar]
Kiweewa 2013 {published data only}
- Kiweewa F, Wabwire, D, Nakibuuka, J. Non‐inferiority of a task‐shifting HIV care and treatment model using peer counsellors and nurses among Ugandan women initiated on HAART: Evidence from a randomized trial. Journal of Acquired Immune Deficiency Syndrome 2013;63(4):Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Sanne 2010 {published data only}
- Sanne I, Orrell C, Fox MP, Conradie F, Ive P, Zeinecker J, et al. Nurse versus doctor management of HIV‐infected patients receiving antiretroviral therapy (CIPRA‐SA): a randomised non‐inferiority trial. Lancet 2010;376(9734):33‐40. [PUBMED: 20557927] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherr 2010 {published data only}
- Sherr KH, Micek MA, Gimbel SO, Gloyd SS, Hughes JP, John‐Stewart GCetal. Quality of HIV care provided by non‐physician clinicians and physicians in Mozambique: A retrospective cohort study. AIDS 2010;24(SUPPL. 1):S59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Babigumira 2009 {published data only}
- Babigumira JB, Sethi AK, Smyth KA, Singer ME. Cost effectiveness of facility‐based care, home‐based care and mobile clinics for provision of antiretroviral therapy in Uganda. PharmacoEconomics 2009;27(11):963‐73. [PUBMED: 19888795] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bemelmans 2010 {published data only}
- Bemelmans M, Akker T, Ford N, Philips M, Zachariah R, Harries A, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Tropical medicine & international health : TM & IH 2010;15(12):1413‐20. [PUBMED: 20958897] [DOI] [PubMed] [Google Scholar]
Boulle 2013 {published data only}
- Boulle, C, Kouanfack, C, Laborde‐Balen, G, et al. Task shifting HIV care in rural district hospitals in Cameroon: Evidence of comparable antiretroviral treatment related outcomes between nurses and physicians in the Stratall ANRS/ESTHER trial. Journal of Acquired Immune Deficiency Syndrome 2013; Vol. 62, issue 5:569‐76. [DOI] [PubMed]
Celletti 2010 {published data only}
- Celletti F, Wright A, Palen J, Frehywot S, Markus A, Greenberg A, et al. Can the deployment of community health workers for the delivery of HIV services represent an effective and sustainable response to health workforce shortages? Results of a multicountry study. AIDS (London, England) 2010;24 Suppl 1:S45‐57. [PUBMED: 20023439] [DOI] [PubMed] [Google Scholar]
Chang 2010 {published data only}
- Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster‐randomized trial. PloS one 2010;5(6):e10923. [PUBMED: 20532194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang LW, Kagaayi J, Arem H, Nakigozi G, Ssempijja V, Serwadda D, et al. Impact of a mHealth intervention for peer health workers on AIDS care in rural Uganda: a mixed methods evaluation of a cluster‐randomized trial. AIDS and behavior 2011;15(8):1776‐84. [PUBMED: 21739286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colvin 2010 {published data only}
- Colvin CJ, Fairall L, Lewin S, Georgeu D, Zwarenstein M, Bachmann MO, et al. Expanding access to ART in South Africa: the role of nurse‐initiated treatment. South African medical journal = Suid‐Afrikaanse tydskrif vir geneeskunde 2010;100(4):210‐2. [PUBMED: 20459957] [DOI] [PubMed] [Google Scholar]
Connolly 2013 {published data only}
- Connolly, N. B, Mellor, J, Fothergill, H, et al. Retaining people living with HIV (PWH) in high‐quality specialist care by means of a hub‐and‐spoke outreach clinic. HIV Medicine 2013; Vol. 14.
de Wet 2011 {published data only}
- Wet K, Wouters E, Engelbrecht M. Exploring task‐shifting practices in antiretroviral treatment facilities in the Free State Province, South Africa. Journal of public health policy 2011;32 Suppl 1:S94‐101. [PUBMED: 21730997] [DOI] [PubMed] [Google Scholar]
Dewo 2012 {published data only}
- Dewo, Z, Tollera, G, Trivelli, M, et al. Strengthening treatment, care and support to people living with HIV through community‐based treatment services. Journal of the International AIDS Society 2012; Vol. 15.
Georgeu 2012 {published data only}
- Georgeu, D, Colvin, C. J, Lewin, S, et al. Implementing nurse‐initiated and managed antiretroviral treatment (NIMART) in South Africa: a qualitative process evaluation of the STRETCH trial. Implementation Science 2012; Vol. 7:66. [DOI] [PMC free article] [PubMed]
Ivers 2011 {published data only}
- Ivers LC, Jerome JG, Cullen KA, Lambert W, Celletti F, Samb B. Task‐shifting in HIV care: a case study of nurse‐centered community‐based care in Rural Haiti. PloS one 2011;6(5):e19276. [PUBMED: 21573152] [DOI] [PMC free article] [PubMed] [Google Scholar]
Labhardt 2012 {published data only}
- Labhardt, N. D, Sello, M, Mohlaba, M, et al. Outcomes of antiretroviral treatment programs in rural Lesotho: Health centers and hospitals compared. Journal of International AIDS Society 2012; Vol. 15. [DOI] [PMC free article] [PubMed]
Matovuu 2013 {published data only}
- Matovu F, Wabwire, D, Nakibuuka, J. Non‐inferiority of a task‐shifting HIV care and treatment model using peer counsellors and nurses among Ugandan women initiated on HAART: Evidence from a randomized trial. Journal of Acquired Immune Deficiency Syndrome 2013;63(4):Epub ahead of print. [DOI] [PubMed] [Google Scholar]
McGuire 2013 {published data only}
- McGuire, M, Ben Farhat, J, Pedrono, G, et al. Task‐sharing of HIV care and ART initiation: evaluation of a mixed‐care non‐physician provider model for ART delivery in rural Malawi. PLoS ONE 2013; Vol. 8, issue 9. [DOI] [PMC free article] [PubMed]
Morris 2009 {published data only}
- Morris MB, Chapula BT, Chi BH, Mwango A, Chi HF, Mwanza J, et al. Use of task‐shifting to rapidly scale‐up HIV treatment services: experiences from Lusaka, Zambia. BMC health services research 2009;9:5. [PUBMED: 19134202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyasulu 2013 {published data only}
O'Malley 2014 {published data only}
- O'Malley, G, Asrat, L, Sharma, A, et al. Nurse task shifting for antiretroviral treatment services in namibia: implementation research to move evidence into action. PLoS ONE 2014; Vol. 9, issue 3. [DOI] [PMC free article] [PubMed]
Rasschaert 2011 {published data only}
- Rasschaert F, Philips M, Leemput L, Assefa Y, Schouten E, Damme W. Tackling health workforce shortages during antiretroviral treatment scale‐up‐‐experiences from Ethiopia and Malawi. Journal of acquired immune deficiency syndromes (1999) 2011;57 Suppl 2:S109‐12. [PUBMED: 21857292] [DOI] [PubMed] [Google Scholar]
Selke 2010 {published data only}
- Selke HM, Kimaiyo S, Sidle JE, Vedanthan R, Tierney WM, Shen C, et al. Task‐shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community‐based program in Kenya. Journal of acquired immune deficiency syndromes (1999) 2010;55(4):483‐90. [PUBMED: 20683336] [DOI] [PubMed] [Google Scholar]
Sherr 2009 {published data only}
- Sherr K, Pfeiffer J, Mussa A, Vio F, Gimbel S, Micek M, et al. The role of nonphysician clinicians in the rapid expansion of HIV care in Mozambique. Journal of acquired immune deficiency syndromes (1999) 2009;52 Suppl 1:S20‐3. [PUBMED: 19858931] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simoni 2011 {published data only}
- Simoni JM, Chen WT, Huh D, Fredriksen‐Goldsen KI, Pearson C, Zhao H, et al. A preliminary randomized controlled trial of a nurse‐delivered medication adherence intervention among HIV‐positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS and behavior 2011;15(5):919‐29. [PUBMED: 20957423] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torpey 2008 {published data only}
- Torpey KE, Kabaso ME, Mutale LN, Kamanga MK, Mwango AJ, Simpungwe J, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PloS one 2008;3(5):e2204. [PUBMED: 18493615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uebel 2011 {published data only}
- Uebel KE, Fairall LR, Rensburg DH, Mollentze WF, Bachmann MO, Lewin S, et al. Task shifting and integration of HIV care into primary care in South Africa: The development and content of the streamlining tasks and roles to expand treatment and care for HIV (STRETCH) intervention. Implementation science : IS 2011;6:86. [PUBMED: 21810242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rie 2014 {published data only}
Vasan 2009 {published data only}
- Vasan A, Kenya‐Mugisha N, Seung KJ, Achieng M, Banura P, Lule F, et al. Agreement between physicians and non‐physician clinicians in starting antiretroviral therapy in rural Uganda. Human resources for health 2009;7:75. [PUBMED: 19695083] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venkatesh 2010 {published data only}
- Venkatesh KK, Bruyn G, Lurie MN, Lentle K, Tshabangu N, Moshabela M, et al. Patient referral from nurses to doctors in a nurse‐led HIV primary care clinic in South Africa: implications for training and support. AIDS care 2010;22(11):1332‐9. [PUBMED: 20711891] [DOI] [PubMed] [Google Scholar]
Zachariah 2009 {published data only}
- Zachariah R, Ford N, Philips M, Lynch S, Massaquoi M, Janssens V, et al. Task shifting in HIV/AIDS: opportunities, challenges and proposed actions for sub‐Saharan Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(6):549‐58. [PUBMED: 18992905] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Walter 2014 {unpublished data only}
- Walter J, Tombo M.L, Edwards C.G, Lujan J, Antierens A, Mofino L. Treatment outcomes before and after the decentralisation of ART in an urban setting in Mozambique. Conference on Retroviruses and Opportunistic Infections. 2014; Vol. 1046.
Additional references
Anglemyer 2013
- Anglemyer A, Rutherford GW, Horvath T, Baggaley RC, Egger M, Siegfried N. Antiretroviral therapy for prevention of HIV transmission in HIV‐discordant couples. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2013, issue 4. [DOI: 10.1002/14651858.CD009153.pub2; CD009153] [DOI] [PMC free article] [PubMed]
Barnighausen 2007
- Barnighausen T, Bloom DE, Humair S. Human resource for treating HIV/AIDS: needs, capacities and gaps. AIDS patient care and sexually transmitted diseases 2007;21:799‐812. [DOI] [PubMed] [Google Scholar]
Callaghan 2010
- Callaghan M, Ford N, Schneider H. A systematic review of task‐ shifting for HIV treatment and care in Africa. Human resources for health 2010;8:8. [PUBMED: 20356363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2011
- Cohen MD, Chen YQ, McCauley M, Gamble T, Hosseinipour MC. Prevention of HIV‐1 Infection with Early Antiretroviral Therapy. N Engl J Med 2011;365(6):493‐505. [DOI: 10.1056/NEJMoa1105243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Decroo 2013
- Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community‐based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub‐ saharan africa: A systematic review. International Health 2013;5:169‐79. [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:276‐82. [Google Scholar]
Emdin 2013
- Emdin CA, Chong NJ, Millson PE. Non‐physician clinician provided HIV treatment results in equivalent outcomes as physician‐provided care: a meta‐analysis. Journal of the International AIDS Society 2013;16:18445. [PUBMED: 23827470] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2011
- Ford N, Calmy A, Mills EJ. The first decade of antiretroviral therapy in Africa. Globalization and health 2011; Vol. 7:33. [PUBMED: 21958478] [DOI] [PMC free article] [PubMed]
GRADEpro 2008 [Computer program]
- Schünemann H, Brozek J, Oxman A. GRADEpro. GRADE Working Group, 2008.
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of clinical epidemiology 2011;64(4):380‐2. [PUBMED: 21185693] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Chichester: Wiley Publishers, 2008. [Google Scholar]
Higgins 2008A
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:187‐241. [Google Scholar]
Hirnschall 2013
- Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. Journal of the International AIDS Society 2013;16:18757. [PUBMED: 23819908] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollinghurst 2006
- Hollinghurst S, Horrocks S, Anderson E, Salisbury C. Comparing the cost of nurse practitioners and GPs in primary care: modelling economic data from randomised trials. British Journal of General Practice 2006;56(528):530‐5. [PMC free article] [PubMed] [Google Scholar]
Kitahata 1996
- Kitahata MM, Koepsell TD, Deyo RA, Maxwell CL, Dodge WT, Wagner EH. Physicians' experience with the acquired immunodeficiency syndrome as a factor in patients' survival. The New England journal of medicine 1996;334(11):701‐6. [PUBMED: 8594430] [DOI] [PubMed] [Google Scholar]
Kredo 2013
- Kredo T, Ford N, Adeniyi FB, Garner P. Decentralising HIV treatment in lower‐ and middle‐income countries. The Cochrane database of systematic reviews 2013;6:CD009987. [PUBMED: 23807693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landon 2005
- Landon BE, Wilson IB, McInnes K, Landrum MB, Hirschhorn LR, Marsden PV, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Archives of internal medicine 2005;165(10):1133‐9. [PUBMED: 15911726] [DOI] [PubMed] [Google Scholar]
Laurant 2004
Mullan 2007
- Mullan F, Frehywot S. Non‐physician clinicians in 47 sub‐Saharan African countries. Lancet 2007;370(9605):2158‐63. [PUBMED: 17574662] [DOI] [PubMed] [Google Scholar]
Newcastle‐Ottawa Scale
- Wells GA, Shea B, O'Connell D, Petersen J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 20 February 2012).
Palella 1998
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine 1998;338(13):853‐60. [PUBMED: 9516219] [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schneider 2006
- Schneider H, Blaauw D, Gilson L, Chabikuli N, Goudge J. Health systems and access to Antiretroveral Drugs for HIV in Southern Africa: service delivery and human resources challenges. Reproductive health matters 2006;14(27):12‐23. [DOI] [PubMed] [Google Scholar]
Siegfried 2010
- Siegfried N, Uthman Olalekan A, Rutherford George W. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV‐infected, treatment‐naive adults. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2010, issue 3. [DOI: 10.1002/14651858.CD008272.pub2; CD008272] [DOI] [PMC free article] [PubMed]
UNAIDS 2011
- UNAIDS Report on the Global AIDS Epidemic. http://www.unaids.org/documents/20101123_GlobalReport_em.pdf 2010 (accessed 21 November 2011).
WHO 2006
- World Health Organization. World Health Statistics, 2006. available from:http://www.who.int/whosis/whostat2006/en/index.html (accessed 5 July 2012). Geneva Switzerland: WHO Press. [ISBN 9241563214]
WHO 2008
- World Health Organization. Task shifting : rational redistribution of tasks among health workforce teams : global recommendations and guidelines. Accessed: http://www.who.int/healthsystems/task_shifting/en/. WHO Press, 2008. [ISBN 978 92 4 159631 2]
WHO 2013
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html (accessed 30 June 2013). Geneva Switzerland: WHO Press, 2013. [PubMed]


