Abstract
Background
The cerebellopontine angle (CPA) cistern houses vital neurovascular structures such as cranial nerves V, VII, and VIII and the anterior inferior cerebellar artery (AICA), often leading to neurovascular compression syndromes due to its complex anatomy. Although vascular compression is a recognized cause of certain neuralgias, its association with otologic symptoms such as tinnitus, hearing loss, and dizziness remains uncertain. Hence, this study aims to determine the prevalence of the AICA vascular loop in the CPA cistern on MRI in patients with asymptomatic audiovestibular symptoms.
Methodology
Adult patients who underwent MRI, including the posterior fossa’s high-resolution volumetric T2 sequence (three-dimensional constructive interference in steady state (3D-CISS)), were assessed. Patients with a history of audiovestibular symptoms (tinnitus/dizziness/vertigo/sensorineural hearing loss), intracranial tumor, vascular lesions, intracranial surgery, brain radiation therapy, traumatic brain injury, poor image quality, and MRI scans without 3D-CISS sequences were excluded. Two radiologists independently reviewed 114 (228 sides) MRI studies for the vascular loop of AICA in the CPA cistern and the extension of the AICA loop into the ipsilateral internal acoustic meatus which was graded by Chavda’s classification.
Results
The prevalence of vascular loop of AICA in the CPA cistern was as high as 47.6% in asymptomatic patients. Grade I Chavda vascular loop was the most common type followed by type II, with type III being the least common type.
Conclusions
Knowledge regarding the high prevalence of the AICA loop in the asymptomatic population and the lack of significant correlation between the presence of the AICA loop and otovestibular symptoms should be considered in preoperative planning for decompression procedures.
Keywords: 3d-ciss mri, asymptomatic, vascular loop, internal auditory canal, cerebellopontine angle cistern, anterior inferior cerebellar artery
Introduction
The cerebellopontine angle (CPA) cistern is a paired basal cistern at the CPA in which the vascular and neural structures highly interact. The neurovascular structures in CPA include cranial nerves V, VII, and VIII; anterior inferior cerebellar artery (AICA); auditory artery; branches of the petrosal vein; vein of the middle cerebellar peduncle; vein of the lateral recess of the fourth ventricle; and transverse pontine vein [1]. Anatomical interactions between these elements may result in neurovascular compression syndromes.
AICA usually originates from the basilar artery about 1 cm above the junction of the two vertebral arteries and courses laterally, crossing the cranial nerve VIII within the CPA cistern [2]. A loop of AICA also enters the internal auditory canal (IAC) along with VII and VIII cranial nerves in about 20-40% of cases. This anatomical course of AICA makes it the culprit vessel implicated in various compression syndromes [3]. The term “vascular compression syndrome” was first described by McKenzie in 1936 and made popular by Jannetta in 1975. It refers to a group of diseases caused by direct contact between a blood vessel and a cranial nerve [4].
Although vascular contacts at the root exit zone of the trigeminal, facial, and glossopharyngeal nerves have been widely accepted as a cause of trigeminal neuralgia, hemifacial spasm (HFS), and glossopharyngeal neuralgia, respectively, their relationship to otologic symptoms such as tinnitus, hearing loss, and dizziness remains unclear [5]. The prevalence of otological symptoms is 2% for tinnitus, 66% for sensorineural hearing loss, and 11% for dizziness in the South Indian population [1]. While various diseases are linked to otologic symptoms, the exact etiology is not always known [6]. MRI is considered the modality of choice for unexplained otological symptoms and is used to exclude retro-cochlear pathologies, especially in patients with asymmetrical sensorineural hearing loss, unilateral tinnitus, or vestibular findings [7].
The World Health Organization estimates that by 2050, hearing loss will approach approximately 2.5 billion, with 700 million people requiring intervention [8]. Although various variables, including infections, vascular, immunological, metabolic, neoplastic, ototoxic, and traumatic events, have been linked to cochlear damage, precise etiology is typically unknown [5].
There is insufficient information to address the frequency with which a vessel is contacting or compressing the vestibulocochlear nerve (cranial nerve VIII) as an incidental finding in the absence of any otological history, despite investigators having studied the accuracy of MRI for identifying vascular loop compression preoperatively compared with the gold standard of intraoperative findings during microvascular decompression for HFS. Vascular loops have long been attributed as a potential cause of unexplained tinnitus/idiopathic sensorineural hearing loss. Few studies have tried to find a correlation between otological symptoms and attribute these to the vascular loops in the CPA cistern/IAC. However, no study has shown the prevalence of such vascular loops contacting/compressing the VII and VIII nerve complex as an incidental finding in asymptomatic patients. Hence, this study aims to determine the prevalence of vascular loops of AICA in the CPA cistern and IAC on MRI in patients asymptomatic for audiovestibular symptoms.
Materials and methods
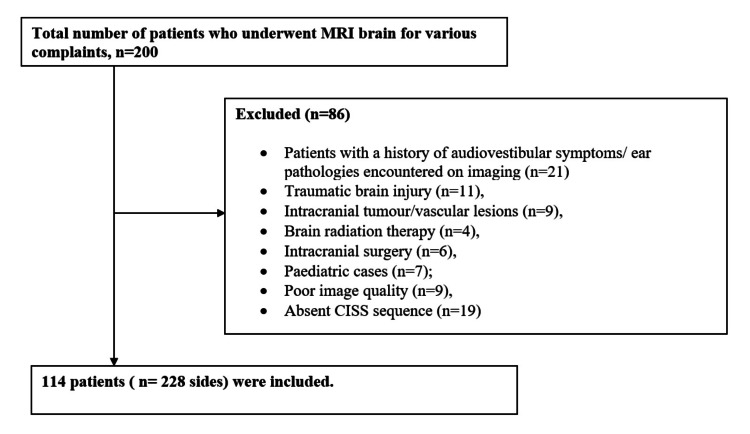
A retrospective study was conducted by accessing the radiology database and the need for informed consent was waived. MRI studies of adult patients (age >18 years) that included high-resolution volumetric T2 sequence, that is, three-dimensional-constructive interference in steady state (3D-CISS) sequence of the posterior fossa were assessed. Patients with audiovestibular symptoms (tinnitus/dizziness/vertigo/sensorineural hearing loss), a history of specific conditions (intracranial tumor, vascular lesions, intracranial surgery, brain radiation therapy, traumatic brain injury), or poor image quality and MRI scans without 3D- CISS sequences were excluded. The predominant indications for the MRI scan were headache, seizures, and visual symptoms. A total of 200 consecutive MRI brain studies of all patients were evaluated. Patients with a history of audiovestibular symptoms/ear pathologies encountered on imaging (n = 21), traumatic brain injury (n = 11), intracranial tumor/vascular lesions (n = 9), brain radiation therapy (n = 4), intracranial surgery (n = 6), pediatric cases (n = 7), poor image quality (n = 9), and absent CISS sequence (n = 19) were excluded. A total of 114 patients (228 sides) who met the eligibility criteria were independently reviewed by two radiologists (Figure 1).
Figure 1. Flowchart of the study population.
CISS = constructive interference in steady state
The volumetric high-resolution MRI sequence (3D-CISS) of the posterior fossa was acquired with one of the following two MRI scanners: (1) 1.5 Tesla Seimens Magnetom Avanto MR system (Siemens AG, Berlin/Munich, Germany) - axial CISS sequence (images through the IACs and brainstem: repetition time (TR) = 1,200 ms, time to echo (TE) = 268 ms, flip angle = 150 degrees, matrix = 320 × 256, field of view (FOV) = 240 mm, no saturation, 0.7 mm axial slice thickness with coronal and sagittal reformatted images); (2) 1.5 Tesla United Imaging uMR-570LH MR system (United Imaging, Houston, Texas, USA) - T2 axial SPAIR_CISS sequence (spectral attenuated inversion recovery constructive interference in steady state images through the internal auditory canals and brainstem: TR = 1,300 ms, TE = 240 ms, flip angle = 150 degrees, matrix = 352 × 317, FOV = 180 × 200 mm, no saturation, 0.68 mm axial slice thickness with sagittal and coronal reformatted images).
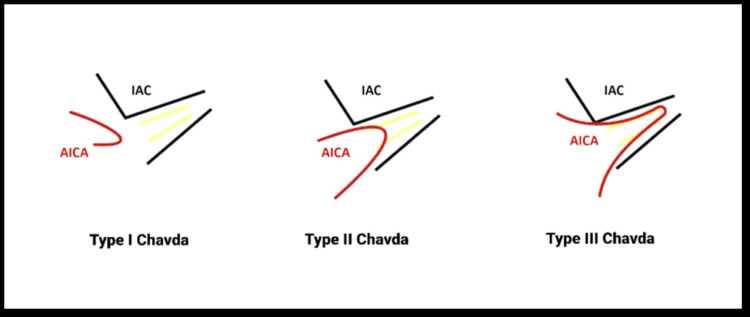
3D-CISS sequence greatly improves the contrast between the cerebrospinal fluid, cranial nerves, and vessels in the CPA cistern. The reviewing radiologists were aware of the study objectives but blinded to the clinical history of audiovestibular symptoms. 3D-CISS MRI pulse sequence and multiplanar reconstructions were assessed for the vascular loop of AICA in the CPA cistern and the extension of the AICA loop into the ipsilateral internal acoustic meatus. The AICA loop was graded according to the Chavda classification [9] (Figure 2, Table 1).
Table 1. Chavda classification of the AICA vascular loop in the CPA cistern and IAC.
AICA = anterior inferior cerebellar artery; CPA = cerebellopontine angle; IAC = internal auditory canal
| Type | Description |
| Type I | When the AICA loop is lying within CPA but not entering the IAC |
| Type II | When the AICA loop enters the IAC but does not extend >50% of the length of the IAC |
| Type III | When the AICA loop extends >50% of the IAC |
Figure 2. Chavda classification of the AICA vascular loop in the CPA cistern and IAC.
This figure is the authors’ creation. However, the idea for the image has been taken from the author Aqeel Alameer and the website https://radiopaedia.org/cases/aica-loop-classification.
CPA = cerebellopontine angle; AICA = anterior inferior cerebellar artery; IAC = internal auditory canal
Patient demographics were described using descriptive statistics. The Kolmogorov-Smirnov test was used to check the normal distribution of the data. Continuous variables were expressed as mean, standard deviation (SD), median, and interquartile range (IQR). Ordinal or categorical variables were expressed as frequency (n) and percentage (%). The kappa value was used to assess interobserver variation/agreement levels. The kappa result was interpreted as follows: values ≤0 indicating no agreement, 0.01-0.20 indicating none to slight, 0.21-0.40 indicating fair, 0.41-0.60 indicating moderate, 0.61-0.80 indicating substantial, and 0.81-1.00 indicating almost perfect agreement [10]. A p-value <0.05 was considered statistically significant.
Results
The mean age of the participants was 42.5 years (range = 18-81 years). There were 43 (37.7%) men and 71 (62.3%) women. Results of the assessment of the vascular loop of AICA in CPA and IAC are reported in Table 2.
Table 2. Results of the assessment of the vascular loop of AICA in CPA and IAC.
The data are represented as frequency (n), percentage (%), and mean. Sample size of the study, n = 228.
AICA = anterior inferior cerebellar artery; CPA = cerebellopontine angle; IAC = internal auditory canal
| Variable | Radiologist 1, n (%) | Radiologist 2, n (%) | Mean, n (%) | |
| Presence of the AICA loop | No | 120 (52.6%) | 119 (52.2%) | 119.5 (52.4%) |
| Yes | 108 (47.4%) | 109 (47.8%) | 108.5 (47.6%) | |
| Chavda classification of the AICA loop | Type 1 | 59 (54.6%) | 62 (56.9%) | 60.5 (55.8%) |
| Type 2 | 45 (41.7%) | 45 (41.3%) | 45 (41.5%) | |
| Type 3 | 4 (3.7%) | 2 (1.8%) | 3 (2.7%) | |
The first radiologist identified vascular loops of AICA in 108 (47.4%) of 228 sides, with 59 (54.6%) sides showing type I Chavda vascular loop (Figure 3), which was the most common type, followed by type II (Figure 4) Chavda vascular loop (Figure 2) seen in 45 sides (41.7%), and, lastly, four (3.7%) sides showing type III Chavda vascular loop (Figure 5). The second radiologist identified vascular loops of AICA in 109 (47.8%) of 228 sides. In total, 62 (56.9%) sides showed type I Chavda vascular loop, 45 (41.3%) sides presented as type II Chavda vascular loop, and two (1.8%) sides showed type III Chavda vascular loop, as depicted in Table 3.
Table 3. Results of the assessment of the vascular loop of AICA in bilateral CPA and IAC.
The data are represented as frequency (n), percentage (%), and mean. Sample size of the study, n = 228.
AICA = anterior inferior cerebellar artery; CPA = cerebellopontine angle; IAC = internal auditory canal
| Variable | Radiologist 1, n | Radiologist 2, n | |
| Presence of AICA loop - left | No | 61 | 60 |
| Yes | 53 | 54 | |
| Presence of AICA loop - right | No | 59 | 59 |
| Yes | 55 | 55 | |
| Chavda classification of AICA loop - left | Type 1 | 29 | 30 |
| Type 2 | 22 | 22 | |
| Type 3 | 2 | 2 | |
| Chavda classification of AICA loop - tight | Type 1 | 30 | 32 |
| Type 2 | 23 | 23 | |
| Type 3 | 2 | 0 | |
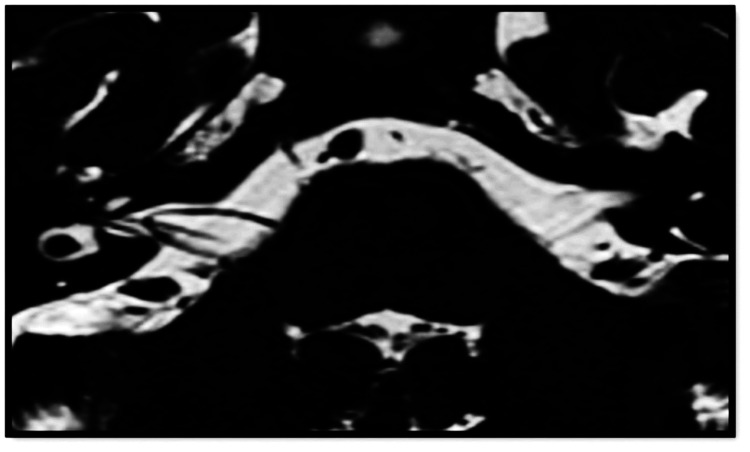
Figure 3. Chavda type I vascular loop.
Axial high-resolution T2-weighted 3D-CISS sequence demonstrates bilateral type I Chavda vascular loop in the CPA cisterns. AICA vascular loop borders the internal auditory meatus without entering the IAC.
CISS = constructive interference in steady state; CPA = cerebellopontine angle; AICA = anterior inferior cerebellar artery; IAC = internal auditory canal
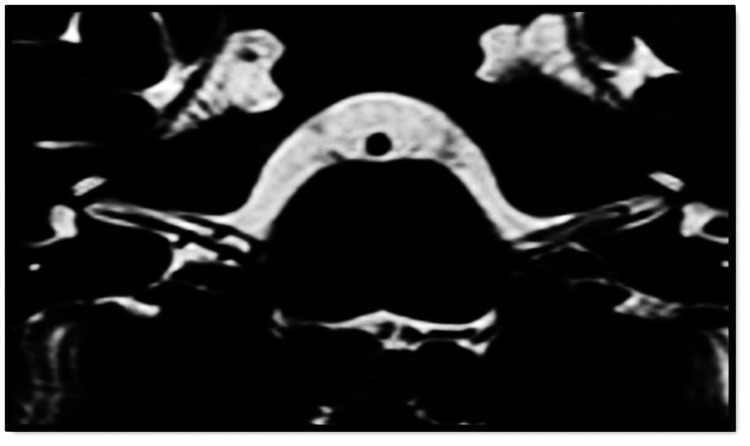
Figure 4. Chavda type II vascular loop.
Axial high-resolution T2-weighted 3D-CISS sequence demonstrates the right AICA loop extending into the IAC (<50%).
CISS = constructive interference in steady state; AICA = anterior inferior cerebellar artery; IAC = internal auditory canal
Figure 5. Chavda type III vascular loop.
Axial high-resolution T2-weighted 3D-CISS sequence demonstrates the left AICA loop extending into the IAC (>50%).
CISS = constructive interference in steady state; AICA = anterior inferior cerebellar artery; IAC = internal auditory canal
In 119 (52.2%) of 228 sides, the absent vascular loop was agreed upon by both radiologists. Therefore, the prevalence of vascular loop in asymptomatic patients may range from 47.4% to 47.8% with a mean of 47.6%. Table 4 shows the interobserver agreement between the two radiologists. The kappa coefficient of agreement for the presence/absence of vascular loop of AICA was 0.97 (95% confidence interval = 0.94-1.00), indicating almost perfect agreement between the two radiologists. The differences in opinion between the two radiologists varied by only one grade of severity (i.e., Chavda type 1 or 2, or type 2 or 3).
Table 4. Interobserver agreement.
The data are represented as frequency (n) and percentage (%). Sample size of the study, n = 228.
CI = confidence interval
| Presence of the AICA loop | Radiologist 2 | Total | ||
| Yes (n) | No (n) | |||
| Radiologist 1 | Yes (n) | 106 (46.5%) | 2 (0.9%) | 108 (47.4%) |
| No (n) | 1 (0.4%) | 119 (52.2%) | 120 (52.6%) | |
| Total | 107 (46.9%) | 121 (53.1%) | 228 (100.0%) | |
| Kappa coefficient: 0.97, 95% CI = 0.94-1.00, standard error = 0.01), percentage of agreement = 98.68% | ||||
Discussion
The CPA cistern houses important structures such as the trigeminal nerve, facial nerve, and vestibulocochlear nerve after they exit the brainstem. After the vestibular and cochlear roots merge, the trunk of the vestibulocochlear nerve extends anterolaterally with the VII nerve and the nervus intermedius and enters the IAC. Vestibular and cochlear roots of the VIII nerve split off at the fundus of the IAC [11].
AICA can originate from the basilar artery (98.1%) or the vertebral artery (1.9%) and as a single (92.3%) or duplicate (7.7%) artery [12]. AICA traverses closer to the abducens, facial, and vestibulocochlear nerves. In the CPA cistern, AICA lies outside the internal acoustic meatus (~19%-40%), at the meatus (33%-56%), or within the IAC (25%-27%) [13]. Vascular loops of AICA are anatomical variations that usually originate from the basilar artery (98%) or less frequently from the vertebral artery (2%) [14]. The internal auditory artery, a branch of AICA given in CPA, passes immediately into the IAC. After the internal auditory branch, AICA divides into two major branches within the CPA cistern. The lateral branch courses laterally toward the semilunar lobules and the medial branch courses toward the biventral lobule of the cerebellum [15].
The root entry/exit zone (REZ) is the transition point between the peripheral nervous system (PNS) and central nervous system (CNS), segments of a cranial nerve. Histological differences between the CNS and PNS segments are that the peripheral segment is more resilient to compression than the central segment [16]. Due to the proximity of the REZ to the IAC, the vestibulocochlear nerve (VIII nerve) has the longest CNS section. This suggests that the entire cisternal segment of the VIII nerve, extending from the brainstem to the IAC, could be especially susceptible to compression by blood vessels.
Vascular compression syndrome describes a clinical entity characterized by compression of one of the cranial nerves by a vessel. Microvascular decompression for intractable vertigo was originally performed by Janetta et al. [17]. According to one theory, at the intersection of the central glial and peripheral non-glial junctions, continuous or pulsatile compression may result in localized demyelination, reorganization, and axonal hyperactivity [18-20]. The other theory is that decreased vascular perfusion could be the outcome of diminished blood flow caused by neurovascular compression [21]. The prevalence of vascular loops affecting the VIII nerve in the REZ, where the nerve leaves the CPA without a myelin sheath, ranges from 7% to 23% [22].
Microvascular decompression is a surgical technique wherein the culprit vessel compressing the nerve root entry zone is identified and mobilized away from the nerve. Small pads of woven Teflon (pledgets) are introduced between the nerve and the vessel [23,24]. Decompression of the vestibulocochlear nerve with the opening of the IAC and transposition of the vascular loop of AICA has shown to be an effective treatment modality for the intrameatal vascular compression of the cranial nerve VIII causing tinnitus, vertigo, and hearing loss [25].
Few studies have shown a correlation between the vascular loop and audiological symptoms, albeit the majority did not show a correlation [26,27]. Mejía-Quiñones et al. observed an association between vascular loops and tinnitus, but not with sensorineural hearing loss, nystagmus, or vertigo [26]. In contrast to the non-pulsatile tinnitus group, Nowé et al. demonstrated that patients with arterial pulsatile tinnitus had a notably greater quantity of vascular loops in the IAC [27].
According to Zhang et al., about 45.5% of contralateral unaffected ears of unilateral idiopathic sudden sensorineural hearing loss (ISSNHL) patients had the AICA entering the IAC [28]. When the AICA enters the IAC (Chavda type II vascular loop) or crosses between the seventh and eighth cranial nerves (Gorrie type C), the severity and frequency of hearing impairment in ISSNHL will be affected but not the occurrence of ISSNHL [28]. According to Papadopoulou et al., a larger group of patients (70%) did not exhibit any correlation between otovestibular symptoms and vascular loops, suggesting that vascular loops of AICA are anatomical variations in a substantial majority of cases with a less common subset causing some audiovestibular symptoms [29]. Kim et al. also observed an insignificant difference in the position and neurovascular contact of the AICA loop between ears with and without ISSNHL based on the Chavda and Gorrie classification [30]. Although few studies showed some correlation between vascular loops and audiovestibular symptoms, most did not show a significant correlation.
In concordance with most studies done in symptomatic patients, our study also showed a high prevalence (47.8%) of the AICA loop, but in asymptomatic individuals. Thus, the AICA loop in the CPA cistern and IAC likely represents an anatomical variation.
The limitations of our study are the lack of correlation with a cohort of patients presenting with otovestibular symptoms and the lack of comparison between other existing classification systems for the vascular loop in the CPA cistern.
Conclusions
The prevalence of AICA vascular loop in the CPA cistern is as high as 47.6%. This high incidence in asymptomatic patients is likely to be an anatomical variant. Therefore, just the radiological evidence of the vascular loop should not amount to pathology but should be further evaluated only to consider the AICA loop as an etiology of exclusion. Knowledge regarding the high prevalence of the AICA loop in the asymptomatic population and the lack of significant correlation between the presence of the AICA loop and otovestibular symptoms should be considered in preoperative planning for decompression procedures. Hence, it is pivotal to consider the MRI findings along with clinical features in evaluating the candidacy of symptomatic individuals for microvascular decompression to avoid unnecessary or futile interventions.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Samanvitha H, Nithish G, Harsha M. T, Shantkumar S. Sajjan
Acquisition, analysis, or interpretation of data: Samanvitha H, Nithish G, Monika S
Drafting of the manuscript: Samanvitha H, Nithish G
Critical review of the manuscript for important intellectual content: Samanvitha H, Nithish G, Harsha M. T, Shantkumar S. Sajjan, Monika S
Supervision: Samanvitha H, Nithish G, Harsha M. T, Monika S
References
- 1.Vascular loops at the cerebellopontine angle and their correlation with otological symptoms. Padma Reka D, Anand A, Kulasekaran N, Balachandran G, Elamparidhi P, Sibhithran R. https://www.ijars.net/articles/PDF/2493/41335_CE[Ra1]_F(SHU)_PF1(AG_SHU)_PFA(SHU)_PBNC(AG_SHU)_PN(SHU).pdf Int J Anat Radiol Surg. 2019;8:0–4. [Google Scholar]
- 2.The anterior inferior cerebellar artery: its radiographic anatomy and significance in the diagnosis of extra-axial tumors of the posterior fossa. Takahashi M, Wilson G, Hanafee W. https://pubs.rsna.org/doi/abs/10.1148/90.2.281 Radiology. 1968;90:281–287. [Google Scholar]
- 3.Anterior inferior cerebellar artery loop and tinnitus—is there any association between them? Saraf A, Gupta N, Manhas M, Kalsotra P. https://ejo.springeropen.com/articles/10.1186/s43163-022-00369-w Egypt J Otolaryngol. 2022;38:171. [Google Scholar]
- 4.Neurovascular cross-compression in patients with hyperactive dysfunction symptoms of the eighth cranial nerve. Jannetta PJ. https://pubmed.ncbi.nlm.nih.gov/1216194/ Surg Forum. 1975;26:467–469. [PubMed] [Google Scholar]
- 5.The impact of vascular loops in the cerebellopontine angle on audio-vestibular symptoms: a systematic review. Papadopoulou AM, Bakogiannis N, Sofokleous V, Skrapari I, Bakoyiannis C. Audiol Neurootol. 2022:200–207. doi: 10.1159/000521792. [DOI] [PubMed] [Google Scholar]
- 6.Vascular loops in the anterior inferior cerebellar artery, as identified by magnetic resonance imaging, and their relationship with otologic symptoms. de Abreu Junior L, Kuniyoshi CH, Wolosker AB, Borri ML, Antunes A, Ota VK, Uchida D. Radiol Bras. 2016;49:300–304. doi: 10.1590/0100-3984.2015.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnetic resonance imaging assessment of vascular contact of the facial nerve in the asymptomatic patient. Deep NL, Fletcher GP, Nelson KD, Patel AC, Barrs DM, Bendok BR, Hoxworth JM. J Neurol Surg B Skull Base. 2016;77:503–509. doi: 10.1055/s-0036-1584196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Deafness and hearing loss. [ Dec; 2022 ]. 2022. https://www.who.int/health-topics/hearing-loss https://www.who.int/health-topics/hearing-loss
- 9.Anterior inferior cerebellar artery syndrome: fact or fiction. McDermott AL, Dutt SN, Irving RM, Pahor AL, Chavda SV. Clin Otolaryngol Allied Sci. 2003;28:75–80. doi: 10.1046/j.1365-2273.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 10.Interrater reliability: the kappa statistic. McHugh ML. https://pubmed.ncbi.nlm.nih.gov/23092060/ Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 11.Vascular loops at the cerebellopontine angle: is there a correlation with tinnitus? Gultekin S, Celik H, Akpek S, Oner Y, Gumus T, Tokgoz N. AJNR Am J Neuroradiol. 2008;29:1746–1749. doi: 10.3174/ajnr.A1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnetic resonance imaging based classification of anatomic relationship between the cochleovestibular nerve and anterior inferior cerebellar artery in patients with non-specific neuro-otologic symptoms. Sirikci A, Bayazit Y, Ozer E, Ozkur A, Adaletli I, Cüce MA, Bayram M. Surg Radiol Anat. 2005;27:531–535. doi: 10.1007/s00276-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 13.Is microvascular compression of the vestibulocochlear nerve a cause of unilateral hearing loss? Hornibrook J, MacFarlane M. Ann Otol Rhinol Laryngol. 2008;117:395–396. doi: 10.1177/000348940811700511. [DOI] [PubMed] [Google Scholar]
- 14.Is presence of vascular loop in magnetic resonance imaging always related to tinnitus? Ensari N, Gür ÖE, Selçuk ÖT, Renda L, Osma Ü, Eyigör H, Çekiç B. J Craniofac Surg. 2017;28:0–8. doi: 10.1097/SCS.0000000000003546. [DOI] [PubMed] [Google Scholar]
- 15.The anterior inferior cerebellar artery: its radiographic anatomy and significance in the diagnosis of extra-axial tumours of the posterior fossa. Takahashi M, Wilson G, Hanafee W. https://pubs.rsna.org/doi/abs/10.1148/90.2.281 Radiology. 1968;90:281–287. [Google Scholar]
- 16.Is the root entry/exit zone important in microvascular compression syndromes? De Ridder D, Møller A, Verlooy J, Cornelissen M, De Ridder L. Neurosurgery. 2002;51:427–433. doi: 10.1097/00006123-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Disabling positional vertigo. Jannetta PJ, Møller MB, Møller AR. N Engl J Med. 1984;310:1700–1705. doi: 10.1056/NEJM198406283102604. [DOI] [PubMed] [Google Scholar]
- 18.Three-dimensional MRI of hemifacial spasm with surgical correlation. Girard N, Poncet M, Caces F, et al. Neuroradiology. 1997;39:46–51. doi: 10.1007/s002340050366. [DOI] [PubMed] [Google Scholar]
- 19.Vascular loop as a cause of incapacitating dizziness. McCabe BF, Gantz BJ. https://pubmed.ncbi.nlm.nih.gov/2735382/ Am J Otol. 1989;10:117–120. [PubMed] [Google Scholar]
- 20.Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Nielsen VK. Neurology. 1984;34:418–426. doi: 10.1212/wnl.34.4.418. [DOI] [PubMed] [Google Scholar]
- 21.Internal auditory canal vascular loops: audiometric and vestibular system findings. Applebaum EL, Valvasorri G. https://pubmed.ncbi.nlm.nih.gov/3878086/ Am J Otol. 1985;Suppl:110–113. [PubMed] [Google Scholar]
- 22.Loop characteristics and audio-vestibular symptoms or hemifacial spasm: is there a correlation? A multiplanar MRI study. Di Stadio A, Dipietro L, Ralli M, Faralli M, Della Volpe A, Ricci G, Messineo D. Eur Radiol. 2020;30:99–109. doi: 10.1007/s00330-019-06309-2. [DOI] [PubMed] [Google Scholar]
- 23.Microvascular decompression for the treatment of neurogenic hypertension with trigeminal neuralgia. Lu W, Wang H, Yan Z, Wang Y, Che H. BMC Neurol. 2019;19:341. doi: 10.1186/s12883-019-1569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vascular complications in microvascular decompression: a survey of 4000 operations. Lee S, Park SK, Joo BE, Lee JA, Park K. World Neurosurg. 2019;130:0–82. doi: 10.1016/j.wneu.2019.06.155. [DOI] [PubMed] [Google Scholar]
- 25.Vascular loop of anterior inferior cerebellar artery causing disabling tinnitus, vertigo, and hearing loss - a review. Swain SK. Matrix Sci Medica. 2022;6:29–33. [Google Scholar]
- 26.Vascular loop in the cerebellopontine angle: clinical-radiological correlation. Mejía-Quiñones V, Valderrama-Chaparro JA, Paredes-Padilla S, Orejuela-Zapata JF, Granados-Sánchez AM. Radiologia (Engl Ed) 2022;64:407–414. doi: 10.1016/j.rxeng.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Does the location of a vascular loop in the cerebellopontine angle explain pulsatile and non-pulsatile tinnitus? Nowé V, De Ridder D, Van de Heyning PH, et al. Eur Radiol. 2004;14:2282–2289. doi: 10.1007/s00330-004-2450-x. [DOI] [PubMed] [Google Scholar]
- 28.Location of the AICA influences the severity but not occurrence of ISSNHL: a reappraisal using high-resolution 3 T MRI. Zhang G, Li H, Zhao Z, Zhang M, Zou J. J Otol. 2023;18:193–198. doi: 10.1016/j.joto.2023.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The impact of vascular loops in the cerebellopontine angle on audio-vestibular symptoms: a systematic review. Papadopoulou AM, Bakogiannis N, Sofokleous V, Skrapari I, Bakoyiannis C. Audiol Neurootol. 2022;27:200–207. doi: 10.1159/000521792. [DOI] [PubMed] [Google Scholar]
- 30.Association of idiopathic sudden sensorineural hearing loss with affective disorders. Kim JY, Lee JW, Kim M, Kim MJ, Kim DK. JAMA Otolaryngol Head Neck Surg. 2018;144:614–621. doi: 10.1001/jamaoto.2018.0658. [DOI] [PMC free article] [PubMed] [Google Scholar]