Abstract
Psoriasis is a systemic disease with cutaneous manifestations. MicroRNAs (miRNAs) are small non-coding RNA molecules that are differentially expressed in psoriatic skin; however, only few cell- and region-specific miRNAs have been identified in psoriatic lesions. We used laser capture microdissection (LCM) and next-generation sequencing (NGS) to study the specific miRNA expression profiles in the epidermis (Epi) and dermal inflammatory infiltrates (RD) of psoriatic skin (N = 6). We identified 24 deregulated miRNAs in the Epi and 37 deregulated miRNAs in the RD of psoriatic plaque compared with normal psoriatic skin (FCH > 2, FDR < 0.05). Interestingly, 9 of the 37 miRNAs in RD, including miR-193b and miR-223, were recently described as deregulated in circulating peripheral blood mononuclear cells (PBMCs) from patients with psoriasis. Using flow cytometry and qRT-PCR, we found that miR-193b and miR-223 were expressed in Th17 cells. In conclusion, we demonstrate that LCM combined with NGS provides a robust approach to explore the global miRNA expression in the epidermal and dermal compartments of psoriatic skin. Furthermore, our results indicate that the altered local miRNA changes seen in the RD are reflected in the circulating immune cells, suggesting that miRNAs may contribute to the pathogenesis of psoriasis.
Keywords: laser capture microdissection, microRNAs, next-generation sequencing, psoriasis, skin
Introduction
Psoriasis is a systemic inflammatory skin disease characterized by intense proliferation, abnormal differentiation of keratinocytes (KCs) and dermal infiltration of activated immune cells. Several studies using whole-tissue extracts have established major differences in gene expression between lesional (PP) and non-lesional (PN) psoriatic skin (1,2). Using laser capture microdissection (LCM) enabling the study of cell- and region-specific alterations in the psoriatic transcriptome, Mitsui et al. (3) detected more than 1800 differentially expressed mRNA genes in the epidermis and dermis of psoriatic skin lesions. MicroRNAs (miRNAs) are small non-coding RNA molecules that modulate gene expression at the post-transcriptional level by binding to the 3′untranslated region (UTR) of their target mRNAs (4–6). MicroRNA detection in the skin is robust (7), and we have recently shown that a miRNA profile can be used to distinguish cutaneous T-cell lymphomas from benign skin diseases (8) indicating that miRNAs have an important role in both healthy and diseased skin. Additionally, we and others have previously identified a specific miRNA expression profile in psoriatic skin using whole-tissue extracts (9–11). However, the profiles reported in these three studies are only partially consistent. One explanation for this inconsistency could be the use of whole-tissue extracts, which, for example contain various degree of infiltrating immune cells in dermis and epidermis and do not allow the study of miRNA expression in specific cells and regions. Thus, the previously established differences in global miRNA expression in psoriatic skin represent the average miRNA changes from a mixture of cells including KCs, leucocytes and endothelial cells. Localizing disease-related miRNAs specifically to the KCs in the epidermis or immune cells in the dermal inflammatory infiltrates including T cells of systemic origin as well as resident T cells and dendritic cells is needed to increase our understanding of the role of miRNAs in psoriasis. To date, some deregulated miRNAs have been localized to specific cells or regions of psoriatic skin lesions, for example by in situ hybridization (ISH) (9,11–16). However, the global miRNA changes in specific cells and regions in PP versus PN as well as in T-cell subsets from patients with psoriasis have not been established.
In this study, we aimed to investigate whether certain disease-related miRNAs could be specifically confined to the epidermis or to immune cells in the dermal inflammatory infiltrates. By combining LCM and small RNA cDNA libraries coupled with next-generation sequencing (NGS), we investigated cell-/region-specific global miRNA changes in psoriatic skin. We identified significant differences in miRNA expression in the psoriatic plaque epidermis (PPEpi) and in the psoriatic plaque dermal inflammatory infiltrates (PPRD) compared with normal psoriatic epidermis (PNEpi) and reticular dermis (PNRD), respectively. To explore which T-cell subsets that likely contribute to the miRNA signature in the PPRD, we used flow cytometry to purify T-cell subsets from the peripheral blood mononuclear cells (PBMCs) from patients with psoriasis and healthy controls. We found that the Th17 cells possibly contribute to the altered miRNA expression seen in the PPRD.
Materials and methods
The detailed protocols and statistical analyses are described in Data S1.
Patients and sample preparation
From six patients diagnosed with chronic plaque-type psoriasis (psoriasis vulgaris), we obtained 4-mm paired punch biopsies from psoriatic plaque (PP) and normal psoriatic (PN) skin samples (Table S1a). All biopsies were embedded in Tissue-Tek (OCT) (Qiagen, Valencia, CA, USA) and stored at −80°C. Venous blood samples from another three patients diagnosed with moderate to severe chronic plaque-type psoriasis and four healthy controls were withdrawn (Table S1b) to purify different T-cell subsets. None of the patients had received any systemically immunosuppressive medications for 4 weeks before the study or used any local treatment at the site of biopsies for 2 weeks before study participation. Approvals from the Institutional review board [The Rockefeller University and the Danish National Committee on Biomedical Research Ethics (H-4–2010-097)] as well as written informed consent were obtained from patients before enrolment. The study was conducted in adherence with the Declaration of Helsinki Principles.
Slide preparation and laser capture microdissection
Laser capture microdissection (LCM) was performed to collect cells in the psoriatic plaque and normal epidermis (PPEpi and PNEpi), normal psoriatic reticular dermis (PNRD) or psoriatic plaque dermal inflammatory infiltrates (PPRD) following the manufacturer’s protocol for the CellCut system (Molecular Machines and Industries, Haslett, MI, USA).
Isolation and purification of PBMCs and T-cell subsets
Peripheral blood mononuclear cells (PBMCs) were isolated from blood collected in EDTA-treated tubes by standard Ficoll density-gradient centrifugation (VWR – Bie & Berntsen, Herlev, Denmark). T-cell subsets [naïve CD4+ (CD4+CCR7+CD45RA+CD 45RO−), Th1 (CD4+CXCR3+CCR6−CD161−), Th17 (CD4+CCR6+ CD161+CXCR3−) and Treg (CD4+CD127loCD25+)] were purified by fluorescence-activated cell sorting (FACSaria II; BD Biosciences, Albertslund, Denmark) using different combinations of established surface markers as described by Rossi et al. (17).
RNA extraction and quality control
Total RNA of the LCM samples and the whole-tissue samples was extracted using the miRNeasy Micro Kit (Qiagen). Total RNA of the different T-cell subsets was extracted using the miRNeasy Kit (Qiagen, Copenhagen, Denmark) in accordance with the manufacturer’s instructions.
Small RNA sequencing
Barcoded small RNA sequencing was performed as previously described by Hafner et al. (18,19). The microRNA expression profiles were deposited at the Gene Expression Omnibus database (GEO series accession number, GSE57012).
Quantitative real-time PCR
Individual quantitative real-time PCR (qRT-PCR) assay was used to validate the epidermal expression levels of miR-224, −378, −181a and −203 using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Naerum, Denmark) followed by PCR using TaqMan® MicroRNA assays (Applied Biosystems). Samples were analysed using the 7900HT sequence detection system (Applied Biosystems) according to the manufacturer’s directions. For assessment of miR-21, −99a, −125b, −126, −142–3p, −146a, −155, −193b, −223, −425 and let-7i in the different T-cell subsets, LNA™ miRNA primers (Exiqon, Vedbaek, Denmark) were used.
Bioinformatic and statistical analysis
The bioinformatic analysis of our small RNA sequence data was processed as described in details by Farazi et al. (20). FDR < 0.05 was considered as statistically significant. PCA was used to assess the variation in the data. Pearson’s correlation analysis was used to explore the association between the samples. For the qRT-PCR, data were exported and processed using the ΔΔCT method (21). The target prediction tool miRWalk was used to investigate potential target interactions for the deregulated miRNAs (22).
Results
MicroRNA signature in the epidermal and dermal compartments
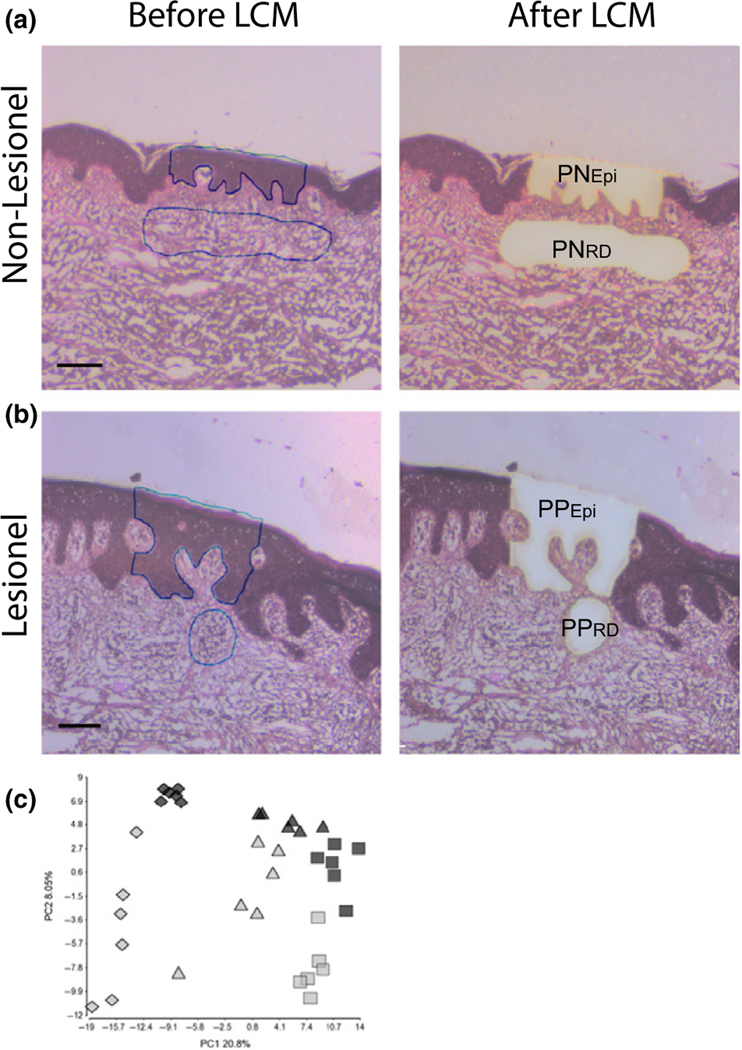
Previous studies of miRNA expression in psoriatic skin samples are based on punch biopsies including both epidermis and dermis (9–11). Using LCM combined with NGS, we investigated the global miRNA expression in the PPEpi and PPRD compared with PNEpi and PNRD, respectively (Fig. 1a,b, Table S1a). For comparison with previously established miRNA profiles, we also sequenced whole-tissue extracts from each patient (see Data S2). A principal component analysis (PCA) of the normalized expression values for all samples clearly demonstrates that the samples were separated according to the sample type (PNEpi/PPEpi, PNRD/PPRD and whole-tissue extracts) followed by the indication (PP vs PN) (Fig. 1c). The 10 most abundant miRNAs in the epidermal and dermal compartment as represented by most reads in each sample type (PNEpi + PPEpi and PNRD + PPRD) accounted for 52% and 47%, respectively, of all miRNA reads. This indicate that these abundantly expressed miRNAs to a great extend capture the biologically important changes and have a significant impact on gene expression (23,24). A comparison of the ten most abundant miRNAs in the PN/PPEpi and PN/PPRD showed a partial overlap of miRNAs (Figure S1) suggesting a common role in cutaneous biology. Of note, miR-203 and miR-205 were both highly abundant in both PNEpi and PPEpi supporting their role in skin differentiation as previously reported (25). To evaluate the specificity of LCM to detect biological relevant miRNAs, we compared the miRNA expression in PNEpi and PNRD (Table S2). We found 107 miRNAs to be differentially expressed between the two tissue compartments (55 upregulated, 52 downregulated). As expected, keratinocyte-related miRNAs such as miR-203 and miR-205 (9,26) were found to be highly abundant in Epi (Figure S1) and to be significantly upregulated in PNEpi compared with PNRD (miR-203: FCH = 44.5, miR-205: FCH = 15.7). Likewise, a vascular endothelial cell-related miRNA (miR-126) (27) was found to be abundant in the PNRD and PPRD (Figure S1) and significantly upregulated in PNRD compared with PNEpi (miR-126: FCH = 13.3).
Figure 1.
Combined use of laser capture microdissection (LCM) and small RNA sequencing identifies specific miRNA signatures in psoriatic plaque epidermis and dermis. LCM was performed on both normal psoriatic skin (a) and psoriatic plaque (b) samples. Cells were collected from psoriatic epidermis (PNEpi and PPEpi) and reticular dermis (PNRD) which included inflammatory cell infiltrates in lesional samples (PPRD). (a, b) Images are representative for 6 biopsies. Line equals 100 μm. A PCA plot based on the normalized read frequencies of all samples clearly showed a separation of the samples according to (c) the sample type (rhombus = PNEpi+PPEpi, triangle = whole-tissue extract, square = PNRD+PPRD) and indication (grey = psoriatic plaque, black = normal psoriatic skin).
MicroRNA signature in the epidermal and dermal compartments of plaque compared to normal psoriatic skin
The miRNA expression profile between PPEpi and PNEpi was compared in 6 patients with psoriasis. A total of 162 miRNAs fulfilled our detection criteria (see Data S1). Subsequent analysis identified 14 upregulated miRNAs and 10 downregulated miRNAs in PPEpi compared with PNEpi (FCH > 2, FDR < 0.05, Table 1). Of those, 54% (13 miRNAs) have not previously been described in psoriatic skin (marked with bold in Table 1). Hierarchical clustering based on these 24 deregulated miRNAs distinguished PPEpi from PNEpi (Figure S2). Potential biological relevant miRNAs in the epidermis include miR-21, −146a and −223 previously associated with inflammation (28,29). Consistent with previous work using whole-tissue samples, miR-125b and miR-99a were found to be significantly downregulated. From the lists of deregulated miRNAs in epidermis, we selected 3 miRNAs for validation with qRT-PCR: miR-378 previously described as aberrantly expressed in psoriatic skin (11) and miR-181a and miR-224 which not have been described in psoriatic skin. We confirmed that miR-224 and miR-378 were significantly upregulated (FCH = 4.99 and FCH = 4.07, respectively) in PPEpi compared with PNEpi (Figure S3a,b). Like-wise, we confirmed that miR-181a was significantly downregulated in PPEpi compared with PNEpi (FCH = −1.72) (Figure S3c).
Table 1.
Significantly deregulated miRNAs in the psoriatic plaque epidermis (PPEpi) compared with normal psoriatic epidermis (PNEpi) (LCM)1
| MicroRNA | FHC | P-value | FDR | Total RF |
|---|---|---|---|---|
|
| ||||
| hsa-miR-223 | 10.27 | 0.00 | 0.00 | 2781 |
| hsa-miR-1307–3p | 7.64 | 0.00 | 0.00 | 3186 |
| hsa-miR-21 | 7.44 | 0.00 | 0.00 | 423 060 |
| hsa-miR-146a | 7.11 | 0.00 | 0.00 | 12 450 |
| hsa-miR-130b-3p | 6.71 | 0.00 | 0.00 | 6359 |
| hsa-miR-142–3p | 5.20 | 0.00 | 0.00 | 4266 |
| hsa-miR-192 | 4.15 | 0.00 | 0.00 | 2130 |
| hsa-miR-18a | 4.09 | 0.00 | 0.00 | 1928 |
| hsa-miR-1307 | 3.43 | 0.00 | 0.00 | 4036 |
| hsa-miR-484-RNASEN | 3.11 | 0.00 | 0.00 | 4183 |
| hsa-miR-378 | 3.03 | 0.00 | 0.00 | 30 270 |
| hsa-miR-17–3p | 2.69 | 0.00 | 0.01 | 2140 |
| hsa-miR-224 | 2.35 | 0.00 | 0.01 | 30 986 |
| hsa-miR-20a | 2.14 | 0.01 | 0.04 | 11 803 |
| hsa-miR-99a | −2.17 | 0.00 | 0.01 | 99 704 |
| hsa-miR-26b | −2.27 | 0.00 | 0.00 | 37 166 |
| hsa-miR-181a-2 | −2.34 | 0.00 | 0.01 | 21 874 |
| hsa-miR-181a-1 | −2.35 | 0.00 | 0.01 | 21 890 |
| hsa-miR-29c | −2.42 | 0.00 | 0.01 | 10 765 |
| hsa-miR-125b-2 | −2.45 | 0.00 | 0.01 | 91 490 |
| hsa-miR-125b-1 | −2.46 | 0.00 | 0.01 | 92 345 |
| hsa-miR-26a-1 | −2.94 | 0.00 | 0.00 | 72 958 |
| hsa-miR-26a-2 | −2.95 | 0.00 | 0.00 | 72 650 |
| hsa-miR-181a-2-3p | −5.35 | 0.00 | 0.00 | 4813 |
FCH, fold change; FDR, false discovery rate; PNEpi, normal psoriatic epidermis, PPEpi, psoriatic plaque epidermis; RF, read frequencies.
The list include deregulated miRNAs in psoriatic plaque epidermis compared with normal psoriatic epidermis. FCH > 2, FDR < 0.05. MicroRNAs in bold are novel miRNAs that have not previously been identified in psoriatic skin
Next, we compared the reticular dermal inflammatory cell aggregate in PPRD with PNRD. A total of 226 miRNAs fulfilled our detection criteria. Subsequent analysis identified 17 upregulated miRNAs and 20 downregulated miRNAs in PPRD compared to PNRD. Of those, 17 miRNAs (46%) have not previously been described in psoriatic skin (Table 2). Upregulated miRNAs include miR-20a, miR-21, miR-142–3p, miR-146a, miR-155 and miR-223 which all previously have been associated with inflammation, autoimmunity, T-cell regulation and the innate immune response (28–30). Among downregulated miRNAs in PPRD are let-7c, miR-99a, miR-125b and miR-199a-5p. Hierarchical clustering based on the 37 deregulated miRNAs completely distinguished PPRD from PNRD (Figure S4). Unfortunately, the amount of RNA isolated from the PNRD was not sufficient to allow confirmation of the sequencing data by qRT-PCR.
Table 2.
Significantly deregulated miRNAs in psoriatic plaque dermal inflammatory infiltrates (PPRD) compared with normal psoriatic reticular dermis (PNRD) (LCM)1
| MicroRNA | FCH | P-value | FDR | Total RF |
|---|---|---|---|---|
|
| ||||
| hsa-miR-155 | 7.07 | 0.00 | 0.00 | 31 667 |
| hsa-miR-142-3p | 5.18 | 0.00 | 0.00 | 156 616 |
| hsa-miR-21-3p | 5.08 | 0.00 | 0.00 | 6316 |
| hsa-miR-142-5p | 5.07 | 0.00 | 0.00 | 57 553 |
| hsa-miR-150 | 4.97 | 0.00 | 0.00 | 78 173 |
| hsa-miR-21 | 3.98 | 0.00 | 0.00 | 4 403 103 |
| hsa-miR-150-3p | 3.60 | 0.00 | 0.00 | 1909 |
| hsa-miR-146b | 3.37 | 0.00 | 0.00 | 47 639 |
| hsa-miR-361-3p | 3.31 | 0.00 | 0.00 | 4190 |
| hsa-miR-363 | 3.31 | 0.00 | 0.00 | 2600 |
| hsa-miR-223 | 3.13 | 0.00 | 0.00 | 25 949 |
| hsa-miR-146a | 3.08 | 0.00 | 0.00 | 101 191 |
| hsa-miR-143-5p | 2.88 | 0.00 | 0.00 | 41 135 |
| hsa-miR-425 | 2.64 | 0.00 | 0.00 | 8515 |
| hsa-miR-1307 | 2.39 | 0.00 | 0.01 | 8253 |
| hsa-miR-342 | 2.24 | 0.00 | 0.00 | 29 477 |
| hsa-miR-20a | 2.09 | 0.00 | 0.02 | 20 289 |
| hsa-miR-193b | −2.08 | 0.00 | 0.02 | 7040 |
| hsa-miR-99a | −2.08 | 0.00 | 0.01 | 124 753 |
| hsa-miR-199a-1-5p | −2.16 | 0.00 | 0.00 | 255 334 |
| hsa-miR-199a-2-5p | −2.16 | 0.00 | 0.00 | 255 539 |
| hsa-miR-125b-2 | −2.32 | 0.00 | 0.00 | 251 720 |
| hsa-miR-125b-1 | −2.33 | 0.00 | 0.00 | 253 985 |
| hsa-miR-101-1-5p | −2.34 | 0.00 | 0.01 | 2895 |
| hsa-miR-365-2 | −2.39 | 0.01 | 0.05 | 1374 |
| hsa-miR-365-1 | −2.40 | 0.01 | 0.05 | 1372 |
| hsa-miR-100 | −2.43 | 0.00 | 0.00 | 132 066 |
| hsa-miR-125b-2-3p | −2.66 | 0.00 | 0.00 | 3189 |
| hsa-miR-139-3p | −2.75 | 0.00 | 0.00 | 4287 |
| hsa-miR-149 | −2.78 | 0.00 | 0.01 | 1651 |
| hsa-let-7c | −2.81 | 0.00 | 0.00 | 169 038 |
| hsa-miR-200a | −3.30 | 0.00 | 0.01 | 3486 |
| hsa-miR-141 | −3.41 | 0.00 | 0.02 | 9499 |
| hsa-miR-214-3p | −3.56 | 0.00 | 0.00 | 7468 |
| hsa-miR-200c | −4.40 | 0.00 | 0.00 | 8633 |
| hsa-miR-200b | −5.09 | 0.00 | 0.00 | 3639 |
| hsa-miR-205 | −8.08 | 0.00 | 0.00 | 30 020 |
FCH, fold change; FDR, false discovery rate; PNRD, normal psoriatic reticular dermis, PPRD, psoriatic plaque dermal inflammatory infiltrates; RF, read frequencies.
The list include deregulated miRNAs in psoriatic plaque dermal inflammatory infiltrates (PPRD) compared with normal psoriatic reticular dermis (PNRD). FCH > 2. FDR < 0.05. MicroRNAs in bold are novel miRNAs that have not previously been identified in psoriatic skin.
MicroRNA expression in T-cell subsets from patients with psoriasis
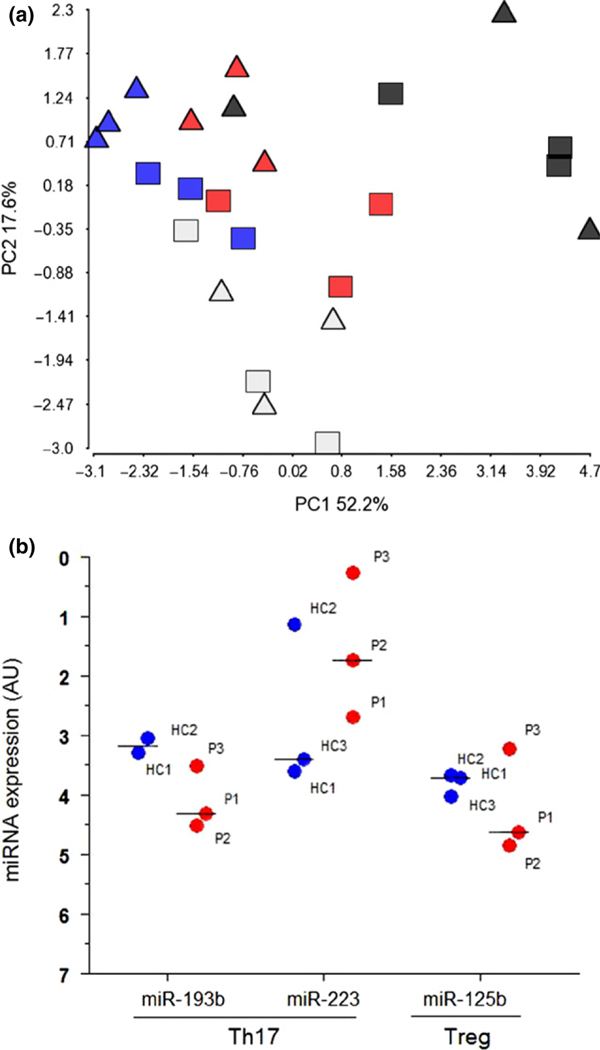
Given that psoriasis is a disease which also involves systemic changes, we sought to investigate if the altered miRNA profile in the PPRD was related to an altered miRNA expression in the circulating T cells from patients with psoriasis. Using qRT-PCR, we recently showed that PBMCs from six patients with moderate to severe psoriasis are characterized by a specific miRNA signature compared with six healthy controls (31). Among the deregulated miRNAs in PBMCs from patients with psoriasis, nine miRNAs were also found to be deregulated in the PPRD (Table S3) indicating that at least some of the deregulated miRNAs identified in the PPRD may reflect systemic miRNA changes in psoriasis. PBMCs consist of a mixture of different cell populations including lymphocytes and monocytes. As the Th17 cells play a critical role in psoriasis (2,32), we sought to investigate their contribution to the altered miRNA expression seen in the PBMCs from patients with psoriasis and thereby in relation to the PPRD. Using flow cytometry and qRT-PCR, we extracted RNA from Th17, naïve CD4+, Th1 and regulatory T-cell (Treg) subsets from three patients with psoriasis and three healthy controls (Table S1b). We measured the expression of seven of the nine overlapping miRNAs (miR-99a, miR-125b, miR-193b, miR-21, miR-223, miR-425 and let-7i) in the different T-cell subsets. In addition, we also included miR-142–3p, miR-146a and miR-155 that were identified as upregulated in PPRD as they are known to be immune-related (28,30). The expression of the vascular endothelial cell-related miR-126 (27) was measured as a negative control. As expected, miR-126 was not detected in any of the T-cell subsets (data not shown). A PCA based on the 10 miRNAs showed a clear separation of naïve CD4+ T cells from the remaining three subsets. Th1 and Th17 were also separated according to indication in the second component (Fig. 2a). MicroRNA-125b, MicroRNA-99a and MicroRNA-193b were highly expressed in the naïve CD4+ T cells compared with Th1, Th17 and Tregs (Figure S5). This was consistent with a previous report from Rossi et al. (17). Interestingly, similar to the results from both PPRD and PBMCs from patients with psoriasis, we found a tendency to miR-193b to be downregulated (FCH = −1.92) and miR-223 to be upregulated (FCH = 2.36) in the Th17 cells and miR-125b to be downregulated in the Treg cells (FCH = −1.35) (Fig. 2b). Altogether, this indicates that the Th17 cells and Treg cells may indeed contribute to the specific miRNA signature observed in the PPRD. An overview of the miRNA expression in the different T-cell subsets can be found in Table S4.
Figure 2.
MicroRNA expression in T-cell subsets. (a) A PCA based on the expression of 10 miRNAs (miR-99a, miR-125b, miR-193b, miR-21, miR-223, miR-425, miR-142–3p, miR-146a, miR-155 and let-7i) measured in four T-cell subset [naïve CD4+ (black), Th1 (blue), Th17 (red) and Treg (grey)]. Triangles = healthy controls, squares = patients with psoriasis. (b) Quantitative real-time PCR of miR-193b, −223 and −125b in T-cell subsets from patients with psoriasis (red dots) compared with healthy controls (blue dots). Median expression of each miRNA is depicted. MicroRNA expression was normalized to the global mean. No statistics were applied as n = 3 + 3. AU, arbitrary units; HC, healthy control; P, psoriasis; Th17, T helper 17 T cells; Treg, regulatory T cells.
Finally, given that miRNAs exert their effect by binding to the 3′UTR of their target mRNA thereby modulating the transcription (6), we performed target prediction using miRWalk (22) to investigate the potential importance of the identified miRNA signatures in PPEpi, PPRD and the T-cell subsets. Results and discussion from this analysis are presented in Data S2. In brief, for example miR-125b was predicted to target CCR7 [Chemokine (C–C motif) receptor 7] and FOXP3 (forkhead box P3) likely linking its role to Tregs.
Discussion
We demonstrate that the combination of LCM and barcoded small RNA sequencing provides a robust approach to explore the global miRNA expression in the psoriatic epidermis and dermal inflammatory infiltrates. Our findings revealed significant changes in the miRNA expression between PPEpi versus PNEpi and PPRD versus PNRD. Among the deregulated miRNAs, about half have not previously been described in psoriatic lesions and nine miR-NAs were recently described as deregulated in circulating immune cells from patients with psoriasis. Finally, our findings indicate that there is an association between the deregulated miRNAs in PPRD and the miRNAs identified in the T-cell subsets including the Th17 cells.
With the introduction of a barcoding approach to allow multi-plexing of several samples, the cDNA processing time and the sequencing costs were significantly reduced. Despite that it can be difficult to recover sufficient amounts of RNA for large-scale analyses; two studies recently showed that LCM can be used to investigate the global mRNA expression in the epidermal and dermal compartment of psoriatic and healthy skin, respectively (3,33). One prior study has used LCM to study miRNA expression in T-lymphocytes in dermis from psoriatic lesional skin and healthy skin; however, only a single miRNA (hsa-miR-21) was measured (16). Here, we show that we were able to perform global miRNA expression from nanogram quantities of input RNA. Hence, of the 21 deregulated miRNAs detected in our whole-tissue samples, 16 miRNAs were identified in at least one prior study using whole-tissue extracts, three of those (miR-21, miR-142–3p and miR-146a) being reported in all three studies (9–11) emphasizing their importance in psoriasis. In addition, our results support the ability of LCM to detect biological relevant miRNAs in epidermis and dermis. In psoriatic plaques, the inflammatory environment promotes extensive proliferation and premature maturation of the KCs. Several miRNAs have been reported to be involved in KC proliferation and differentiation, for example miR-203 (9,25,34–36), miR-205 (26), miR-99a (12), miR-125b (13) and miR-424 (15). MicroRNA-203 is expressed in the suprabasal layer of the epidermis (9) and has been established as an important regulator of KC differentiation (25). We measured miR-203 to be one of the most abundant miRNAs in the PNEpi and PPEpi supporting its role as a general important KC differentiation-related miRNA (Figure S1). However, we could not confirm the deregulation of miR-203 in psoriatic plaque as previously reported (9,10) (PPEpi, FCH = 1.13, P-value = 0.54, FDR = 0.68 and Figure S3d). Several of the deregulated miRNAs identified in psoriatic plaque epidermis has previously been associated with immune cells (e.g. miR-223, miR-146a and miR-21) suggesting that the signal from these miRNAs is a result of infiltrating immune cells into the epidermis which is a common pathological event in psoriasis. A recent study demonstrated that inhibition of miR-21 ameliorated disease pathology in patient-derived psoriatic skin xenotransplants in mice and in a psoriasis-like mouse model (37). Their findings suggested a causal role for miR-21 in the pathogenesis of epidermal hyperplasia. However, as we and others correspondingly have found miR-21 to be highly abundant and increased in the PPRD and dermal CD3+ T cells (16), the inhibition of miR-21 may actually be beyond the epidermis.
In this study, we found 13 deregulated miRNAs in PPEpi compared to PNEpi which have not previously been reported as differentially expressed in psoriatic skin (Table 1). Among those miRNAs were miR-181a, −26a, and −26b which notably also have been found to be downregulated in malignant melanoma (38,39), indicating a linking role for the hyperproliferation present in both pathologies. Given that these miRNAs were also highly abundant suggest that they indeed may play a significant regulatory function in psoriatic skin.
We also identified 11 epidermal miRNAs which previously have been identified in studies using whole-tissue extracts. Among those miR-378 (Table 1, Figure S3b) which also was found to be significantly upregulated in our lesional whole-tissue samples (Table S5) but not in the PPRD. These findings suggest that the signal arising from miR-378 in the whole-tissue samples is a result of an aberrant expression in the epidermal cells. This example highlights the benefit of using the LCM approach making it possible to localize which cells/regions in the skin that contributes to the altered miRNA expression in psoriasis.
A number of studies have explored the nature of the global miRNA expression profile across a panel of different human immune cell subsets (17,40). In the present study, we identified 18 miRNAs in the dermal inflammatory infiltrates (PPRD) which previously have been identified in psoriatic lesions using whole-tissue extracts. Several of these miRNAs seem to be active in multiple immune cells. For instance, the expression of miR-155 has been shown to increase upon T-cell activation and control the differentiation of CD4+ T cells into different T-cell subsets. Additionally, miR-155 was also reported to be involved in the development and function of Tregs (29) and DCs (41). An increased expression of miR-155 in lesions from patient with atopic dermatitis has been shown to be confined to infiltrating immune cells including CD4+ T cells and DCs (42). The cytotoxic T lymphocyte-associated protein 4 (CTLA-4) which is an important negative regulator of T-cell responses, was shown to be a direct target of miR-155, altogether suggesting that miR-155 contributes to the chronic skin inflammation characteristic for both atopic dermatitis and psoriasis by increasing the proliferative response of T cells through the inhibition of CTLA-4 (42). Interestingly, we also found 17 deregulated miRNAs in the PPRD which have not previously been reported as differentially expressed in psoriatic plaques (Table 2). One reason that we identified these miRNAs as deregulated in psoriatic skin could be that in previous studies using whole-tissue extracts, the signal from low-expressed miRNAs may be lost by ‘dilution’ with miRNAs from more dominant cell types such as the KCs. Among novel downregulated miRNAs were miR-200b/c, miR-205, miR-214 and miR-193b which also have been reported as downregulated in malignant melanoma, for example miR-193b which was shown to repress cell proliferation and regulate cyclin D1 expression (43). Together, our identified miRNA signature in the lesional dermal compartment is reliable relative to the nature of the immunologic functions of the miRNAs and furthermore to the inflammatory phenotype of psoriatic skin.
As the PPRD are a result of infiltration of immune cells and we at the same time observed an overlap of 9 deregulated miRNAs between PPRD and PBMCs from patients with psoriasis (Table S3), we sought to investigate which circulating T-cell subsets that likely contribute to the observed miRNA signature in PPRD. Interestingly, Rossi et al. (17) demonstrated that during activation of CD4+ naïve T cells, miR-193b decreases suggesting that the decrease we observed in miR-193b expression in particular in the Th17 cells could be a result of an activation of the Th17 cells. In addition, we found miR-223 to be upregulated in Th17 cells from patients with psoriasis. Notably, a prior study has demonstrated that miR-223 targets Roquin ubiquitin ligase which curtails IL-17A synthesis and thereby enhances the expression of IL-17A (44). Hence, it is possible that miR-223 contributes to the increased IL-17 production in the Th17 cells in the PPRD; however, as pure Th17 cell populations can be difficult to obtain further studies are warranted to investigate this hypothesis in more detail. Finally, miR-125b was found to be downregulated in the Tregs from patients with psoriasis. Besides also being decreased during activation of naïve CD4+ T cells (17), miR-125b was predicted to target among others CCR7, CD83 and CXCL13 (Table S8). As Tregs are present in the dermis and express CCR7 (45,46), we speculate that the decreased expression of miR-125b in Tregs from patients with psoriasis may contribute to the increased expression of the CCR7 in the PPRD as demonstrated by Mitsui et al. (3). Of notice, miR-125b was also predicted to target FOXP3 (forkhead box P3), a transcription factor important for the development and function of Tregs (47), potentially linking its role to Tregs. As normal, non-inflamed skin and resolved psoriatic skin have been shown to contain a number of skin resident effector memory T cells that provide local immune protection of the skin (48,49), it is conceivable that the specific miRNA signature that we observe in the circulating T cells arises as part of a psoriasis-associated immune response. Although we succeeded in measuring the global miRNA changes there are limitations to our study, such as a low number of patients in particular for the investigation of the miRNA expression in circulating T-cell subsets which therefore should be considered exploratory only. In addition, the low RNA yield obtained from the PNRD precluded qRT-PCR validation of the dermal miRNA profile, and in general the low input RNA may have caused a failure to identify deregulated miRNAs of extremely low abundance. Overall, our findings suggest that small RNA sequencing combined with LCM is a reliable method to localize which cells/regions in the skin that contributes to the miRNA expression in psoriasis. Despite that the microdissected epidermal and dermal samples each still contain different cell types, the results improve our understanding of which cells/regions that contribute to the aberrant miRNA expression seen in psoriatic skin and it could be interesting to apply this approach to other skin diseases but also to compare normal psoriatic skin to healthy skin. Although both LCM and flow cytometry are time consuming methods, we believe that the enrichment of specific cells and thereby the additional information which can be gained is favourable and we thereby suggest that especially LCM should be considered as a supplement to whole-tissue skin samples aiming to investigate miRNA expression. Even though we have localized which cell/regions that the miRNA signatures in psoriatic skin have emerged from, further studies are warranted to unravel the specific functions of the miRNAs in the pathogenesis of psoriasis.
Supplementary Material
Figure S1. Abundantly expressed miRNAs in psoriatic skin.
Figure S2. Epidermal psoriatic miRNA signature.
Figure S3. Validation of epidermal psoriatic deregulated miRNAs.
Figure S4. Dermal psoriatic miRNA signature.
Figure S5. MicroRNA expression in T cell subsets.
Figure S6. Overlap between deregulated miRNAs in psoriatic plaque epidermis (PPEpi), psoriatic plaque dermal inflammatory infiltrates (PPRD) and whole tissue extracts.
Table S1. (a) Patient Demographic. (b) Patient Demographics.
Table S2. Significant altered miRNAs between Epi and RD in normal psoriatic skin.
Table S3. Overlapping deregulated miRNAs between PPRD and PBMCs from patients with psoriasis.
Table S4. Deregulated miRNAs in T cell subsets.
Table S5. Significantly deregulated miRNAs in psoriatic plaque versus normal psoriatic skin (whole tissue extract).
Table S6. Experimental design.
Table S7. Target predictions for the epidermal miRNA signatures.
Table S8. Target predictions for the dermal miRNA signatures.
Table S9. Target prediction for the epidermal miRNA signature combined with deregulated transcription factors in epidermis.
Table S10. Characterization of phenotype of T cell subsets.
Data S1. Materials and method.
Data S2. Results and discussion.
Acknowledgements
We thank Israel Coats, The Laboratory for Investigative Dermatology, The Rockefeller University, New York, USA, Miguel Brown, Howard Hughes Medical Institute, Laboratory of RNA Molecular Biology, The Rockefeller University, New York, USA and Lisbeth Stolpe, Department of International Health, Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark, for their excellent technical assistance and William Levis, New York University School of Medicine, Department of Dermatology, New York, USA, for his interest and dedication in the study. Author Contributions: MBL, JRZ, MAR, MH, CMB, JGK and LS designed the study; MBL, HM, MH and BDA performed the research; MH, CMB and JGK contributed essential reagents and tools; MBL, HM, JRZ, MAR, MH, BDA, JGK and LS analysed the data; MBL, JRZ, MAR and LS wrote the manuscript.
MBL, JRZ and MAR are employed by LEO Pharma A ⁄ S. LS received consultancy and/or speaker honoraria from Abbott, Pfizer, Janssen-Cilag, MSD and LEO Pharma A/S and is a member of the Advisory Board of MDS, Novartis, Abbvie and Janssen-Cilag. Furthermore, grants for research projects are received from LEO Pharma A/S, Pfizer and Abbvie. JGK received consultant and/or sponsored research from Abbvie, Akros, Amgen, Astellas, Boehringer, Biogen-Idec, Centocor/Janssen, Dermira, Eli Lilly, Genzyme-Sanofi, LEO Pharma A/S, Merck, Novartis and Pfizer.
Funding
This study was supported by the Ministry of Science, Innovation and Higher education, Denmark.
Abbreviations:
- DC
dendritic cell
- Epi
epidermis
- FCH
fold change
- FDR
false discovery rate
- ISH
in situ hybridization
- LCM
laser capture microdissection
- PBMCs
peripheral blood mononuclear cells
- PN
normal psoriatic skin
- PNEpi
normal psoriatic epidermis
- PNRD
normal psoriatic reticular dermis
- PP
psoriasis plaque
- PPEpi
psoriatic plaque epidermis
- PPRD
psoriatic plaque dermal inflammatory infiltrate
- qRT-PCR
quantitative reverse-transcribed PCR
- RD
reticular dermis
- Treg
regulatory T cells
- UTR
untranslated region
Footnotes
Supporting Information
Additional supporting data may be found in the supplementary information of this article.
Conflict of interest
HM, MH, BDA and CMB declare no conflict of interests.
References
- 1.Tian S, Krueger JG, Li K et al. PLoS One 2012: 7: e44274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowes MA, Suárez-Fariñas M, Krueger JG. Annu Rev Immunol 2014: 32: 227–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitsui H, Suárez-Fariñas M, Belkin DA et al. J Invest Dermatol 2012: 132: 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambros V, Lee RC, Lavanway A et al. Curr Biol 2003: 13: 807–818. [DOI] [PubMed] [Google Scholar]
- 5.Bartel D P. Cell 2004: 116: 281–297. [DOI] [PubMed] [Google Scholar]
- 6.Bartel D P. Cell 2009: 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Løvendorf MB, Zibert JR, Hagedorn PH et al. Exp Dermatol 2012: 21: 299–301. [DOI] [PubMed] [Google Scholar]
- 8.Ralfkiaer U, Hagedorn PH, Bangsgaard N et al. Blood 2011: 118: 5891–5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonkoly E, Wei T, Janson PC et al. PLoS One 2007: 2: e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zibert JR, Løvendorf MB, Litman T et al. J Dermatol Sci 2010: 58: 177–185. [DOI] [PubMed] [Google Scholar]
- 11.Joyce CE, Zhou X, Xia J et al. Hum Mol Genet 2011: 20: 4025–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerman G, Avivi C, Mardoukh C et al. PLoS One 2011: 6: e20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu N, Brodin P, Wei T et al. J Invest Dermatol 2011: 131: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 14.Xu N, Meisgen F, Butler LM et al. J Immunol 2013: 190: 678–688. [DOI] [PubMed] [Google Scholar]
- 15.Ichihara A, Jinnin M, Yamane K et al. Br J Dermatol 2011: 165: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 16.Meisgen F, Xu N, Wei T et al. Exp Dermatol 2012: 21: 312–314. [DOI] [PubMed] [Google Scholar]
- 17.Rossi RL, Rossetti G, Wenandy L et al. Nat Immunol 2011: 12: 796–803. [DOI] [PubMed] [Google Scholar]
- 18.Hafner M, Landgraf P, Ludwig J et al. Methods 2008: 44: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafner M, Renwick N, Farazi TA et al. Methods 2012: 58: 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farazi TA, Brown M, Morozov P et al. Methods 2012: 58: 171–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen T D. Methods 2001: 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 22.Dweep H, Sticht C, Pandey P et al. J Biomed Inform 2011: 44: 839–847. [DOI] [PubMed] [Google Scholar]
- 23.Baek D, Villén J, Shin C et al. Nature 2008: 455: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selbach M, Schwanh€ausser B, Thierfelder N et al. Nature 2008: 455: 58–63. [DOI] [PubMed] [Google Scholar]
- 25.Yi R, Poy MN, Stoffel M et al. Nature 2008: 452: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory PA, Bracken CP, Bert AG et al. Cell Cycle 2008: 7: 3112–3118. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Olson EN. Curr Opin Genet Dev 2009: 19: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connell RM, Rao DS, Baltimore D. Annu Rev Immunol 2012: 30: 295–312. [DOI] [PubMed] [Google Scholar]
- 29.Curtale G, Citarella F. Int J Mol Sci 2013: 14: 17347–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomankova T, Petrek M, Gallo J et al. Scand J Immunol 2011: 75: 129–141. [DOI] [PubMed] [Google Scholar]
- 31.Løvendorf MB, Zibert JR, Gyldenløve M et al. J Dermatol Sci 2014: 75: 133–139. [DOI] [PubMed] [Google Scholar]
- 32.Lowes MA, Russel CB, Martin DA et al. Trends Immunol 2013: 34: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Percoco G, Bénard M, Ramdani Y et al. Exp Dermatol 2012: 21: 531–534. [DOI] [PubMed] [Google Scholar]
- 34.Sonkoly E, Wei T, Pavez Lorié E et al. J Invest Dermatol 2010: 130: 124–134. [DOI] [PubMed] [Google Scholar]
- 35.Lena AM, Shalom-Feuerstein R, Rivetti de val Cervo P et al. Cell Death Differ 2008: 15: 1187–1195. [DOI] [PubMed] [Google Scholar]
- 36.Wei T, Orfanidis K, Xu N et al. Exp Dermatol 2010: 19: 854–856. [DOI] [PubMed] [Google Scholar]
- 37.Guinea-Viniegra J, Jiménez M, Schonthaler HB et al. Sci Transl Med 2014: 6: 225re1. [DOI] [PubMed] [Google Scholar]
- 38.Poliseno L, Haimovic A, Segura MF et al. J Invest Dermatol 2012: 132: 1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozubek J, Ma Z, Fleming E et al. PLoS One 2013: 8: e72699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allantaz F, Cheng DT, Bergauer T et al. PLoS One 2012: 7: e29979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez AE, Vigorito E, Clare S et al. Science 2007: 316: 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonkoly E, Janson P, Majuri ML et al. J Allergy Clin Immunol 2010: 126: 581–589. [DOI] [PubMed] [Google Scholar]
- 43.Chen J, Feilotter HE, Pare GC et al. Am J Pathol 2010: 176: 2520–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaefer JS, Montufar-Solis D, Vigneswaran N et al. J Immunol 2011: 187: 5834–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattozzi C, Salvi M, D’Epiro S et al. Dermatology 2013: 227: 134–145. [DOI] [PubMed] [Google Scholar]
- 46.Bosé F, Petti L, Diani M et al. Am J Pathol 2013: 183: 413–421. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Zhao Y. J Cell Physiol 2007: 211: 590–597. [DOI] [PubMed] [Google Scholar]
- 48.Clark RA, Chong B, Mirchandani N et al. J Immunol 2006: 176: 4431–4439. [DOI] [PubMed] [Google Scholar]
- 49.Cheuk S, Wikén M, Blomqvist L et al. J Immunol 2014: 192: 3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Abundantly expressed miRNAs in psoriatic skin.
Figure S2. Epidermal psoriatic miRNA signature.
Figure S3. Validation of epidermal psoriatic deregulated miRNAs.
Figure S4. Dermal psoriatic miRNA signature.
Figure S5. MicroRNA expression in T cell subsets.
Figure S6. Overlap between deregulated miRNAs in psoriatic plaque epidermis (PPEpi), psoriatic plaque dermal inflammatory infiltrates (PPRD) and whole tissue extracts.
Table S1. (a) Patient Demographic. (b) Patient Demographics.
Table S2. Significant altered miRNAs between Epi and RD in normal psoriatic skin.
Table S3. Overlapping deregulated miRNAs between PPRD and PBMCs from patients with psoriasis.
Table S4. Deregulated miRNAs in T cell subsets.
Table S5. Significantly deregulated miRNAs in psoriatic plaque versus normal psoriatic skin (whole tissue extract).
Table S6. Experimental design.
Table S7. Target predictions for the epidermal miRNA signatures.
Table S8. Target predictions for the dermal miRNA signatures.
Table S9. Target prediction for the epidermal miRNA signature combined with deregulated transcription factors in epidermis.
Table S10. Characterization of phenotype of T cell subsets.
Data S1. Materials and method.
Data S2. Results and discussion.