SUMMARY
Mitochondria contain a specific translation machinery for the synthesis of mitochondria-encoded respiratory chain components. Mitochondrial tRNAs (mt-tRNAs) are also generated from the mitochondrial DNA and, similar to their cytoplasmic counterparts, are post-transcriptionally modified. Here, we find that the RNA methyltransferase METTL8 is a mitochondrial protein that facilitates 3-methyl-cytidine (m3C) methylation at position C32 of the mt-tRNASer(UCN) and mt-tRNAThr. METTL8 knockout cells show a reduction in respiratory chain activity, whereas overexpression increases activity. In pancreatic cancer, METTL8 levels are high, which correlates with lower patient survival and an enhanced respiratory chain activity. Mitochondrial ribosome profiling uncovered mitoribosome stalling on mt-tRNASer(UCN)- and mt-tRNAThr-dependent codons. Further analysis of the respiratory chain complexes using mass spectrometry revealed reduced incorporation of the mitochondrially encoded proteins ND6 and ND1 into complex I. The well-balanced translation of mt-tRNASer(UCN)- and mt-tRNAThr-dependent codons through METTL8-mediated m3C32 methylation might, therefore, facilitate the optimal composition and function of the mitochondrial respiratory chain.
In brief
The m3C methyltransferase METTL8 methylates C32 on mitochondrial tRNAs mt-tRNASer(UCN) and mt-tRNAThr. This modification affects mitochondrial translation efficiency and respiratory chain activity. Schöller et al. observe stalled ribosomes on specific mt-tRNASer(UCN) codons in the absence of METTL8, leading to misbalancing of respiratory-chain complexes potentially relevant for pancreatic cancer.
Graphical Abstract
INTRODUCTION
RNA modification is a conserved and very common phenomenon that is found on almost all RNA species. Non-coding RNAs, such as ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs), are heavily modified, leading to the characteristic RNA structures and functions. tRNAs are equipped with amino acids (aminoacylated) and the anticodon information to decode the genetic information on the mRNA. They interact with the ribosome as well as with the codons, and many modifications are important for these interactions. For example, the anticodon can carry many different chemical alterations at the wobble position. This allows for wobble pairing and an increase of the codon spectrum and, thus, the decoding potential of a particular tRNA. In addition, the anticodon arm is also heavily modified at regions flanking the anticodon, which is, for example, important for proper ribosome binding and progression through the translation cycle (Suzuki, 2021). Such modifications can lead to structural arrangements within the anticodon arm that are important for this process. Modifications in other parts of the tRNA are often required for correct aminoacylation or adapting of a specific secondary structure, which is needed for specific aspects of the tRNA life cycle. This is not only observed for human cytoplasmic tRNAs but also for mitochondrial tRNAs (mt-tRNAs), which, nevertheless, contain fewer modifications. The 22 human mt-tRNAs contain 18 types of chemical modifications, which have been mapped to 137 positions (Suzuki et al., 2020). All modifications are generated by specialized, nuclear-encoded enzymes, and many mitochondrial modification pathways are affected in human diseases, such as encephalopathy or the complex mitochondria-related diseases mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) and myoclonic epilepsy with ragged red fibers (MERRF) (Bohnsack and Sloan, 2018b; Kazuhito and Wei, 2020) (Suzuki et al., 2011).
Recently, RNA modifications have also been identified on human mRNAs. In addition to the well-known cap structure in which 7-methylguanosine (m7G) is added, N6-methyladenosine (m6A) is the most abundant modified nucleotide on mRNA (Desrosiers et al., 1974; Dominissini et al., 2012; Meyer et al., 2012) affecting alternative splicing; mRNA export, localization, and translation or mRNA stability (Balacco and Soller, 2019) (Ke et al., 2017; Knuckles et al., 2017; Lee et al., 2020; Slobodin et al., 2017; Zhou et al., 2019).
The m6A methylation mark is catalyzed by a specific writer complex composed of the enzyme methyltransferase-like 3 (METTL3) and its binding partner METTL14 (Liu et al., 2014). Within this tight heterodimeric complex, both proteins form the catalytic center that recognizes the well-known RRACH (R = G/A; H = A/C/U) motif for m6A methylation (Schöller et al., 2018; Śledz and Jinek, 2016; Wang et al., 2016a, 2016b).
METTL3 and METTL14 are members of a large family of methyltransferases and many of them have recently been associated with RNA or DNA methylation. The closest relative is METTL4, which has been implicated in m6A modification on DNA (m6dA) (Hao et al., 2020; Kweon et al., 2019), although quantitative liquid chromatography-mass spectrometry (LC-MS) experiments did not find m6dA, at least not in the DNA from mouse tissues (Schiffers et al., 2017). It also generates m6A on small nuclear RNAs (snRNAs), which are additionally methylated at the 2′ position of the ribose to generate m6Am (Chen et al., 2020) (Goh et al., 2020). METTL16 and METTL5 catalyze the formation of m6A as well. Although METTL5 generates m6A on the 18S rRNA at position A1832 (Ignatova et al., 2020b) (van Tran et al., 2019), METTL16 modifies one specific A of the U6 snRNA (Pendleton et al., 2017; Warda et al., 2017). Furthermore, METTL16 recognizes and modifies a distinct structural element in the S-adenosylmethionine (SAM) synthetase MAT2A mRNA (Doxtader et al., 2018) (Mendel et al., 2018).
Another methyltransferase acting on RNA is METTL1, which generates m7G modifications on a variety of different substrates, including tRNAs, microRNA precursors, and mRNAs (Alexandrov et al., 2002; Lin et al., 2018; Zhang et al., 2019). METTL15 localizes to mitochondria and introduces N4-methylcytidine (m4C) into mitochondrial 12S rRNA, which affects proper biogenesis of mitochondrial ribosomes (Van Haute et al., 2019).
METTL2, 6, and 8 catalyze the formation of 3-methylcytidine (m3C) in tRNAs and mRNAs (Xu et al., 2017). METTL2 and 6 target cytoplasmic tRNAs and modify them at distinct positions. For example, METTL6 methylates tRNAs at C32 and is important for pluripotency and tumor cell growth (Ignatova et al., 2020a). METTL8 may not affect tRNAs but has been suggested to add m3C modifications to cellular mRNAs. Furthermore, METTL8 seems to affect R-loop formation at ribosomal DNA (rDNA) loci (Zhang et al., 2020). R-loops are DNA-RNA hybrids at active transcription sites, but whether METTL8 directly modifies the nascent RNA within these structures remains unclear.
Because METTL3 and METTL14 form a tight complex, which is important for catalytic activity, it is possible that other METTL proteins are subunits of larger complexes as well. A comprehensive mass spectrometry study revealed, however, that most of the tested METTL proteins are most likely not organized in stable complexes but may function as monomeric subunits, with only METTL7, 8, and 9 seeming to engage in stable interactions with other proteins. It is, however, possible that interactions are restricted to specific compartments, tissues, or developmental stages that have not been investigatedso far (Ignatova et al., 2019).
Here, we have assessed the biochemical mechanisms and molecular functions of METTL8 in human cells. In contrast to recent reports, we did not find evidence for METTL8-mediated mRNA modification. Instead, we find that METTL8 is a mitochondrial protein that targets m3C32 modification of mt-tRNASer(UCN) and mt-tRNAThr. METTL8 knockout (KO) leads to unmodified tRNAs and compromised respiratory chain activity. Using ribosome profiling in METTL8 KO and METTL8-overexpressing cells, we find that ribosomes are stalled specifically at mt-tRNASer(UCN) and mt-tRNAThr codons, and mass spectrometry identifies that the mitochondrial proteins ND1 and ND6 are affected most by METTL8 activity. Finally, METTL8 overexpression is observed in highly aggressive pancreatic cancer cells, which are accompanied by a markedly enhanced respiratory chain activity. Such a METTL8 addiction of pancreatic cancer cells might be a valuable novel target for RNA therapeutics.
RESULTS
METTL8 is a mitochondrial protein
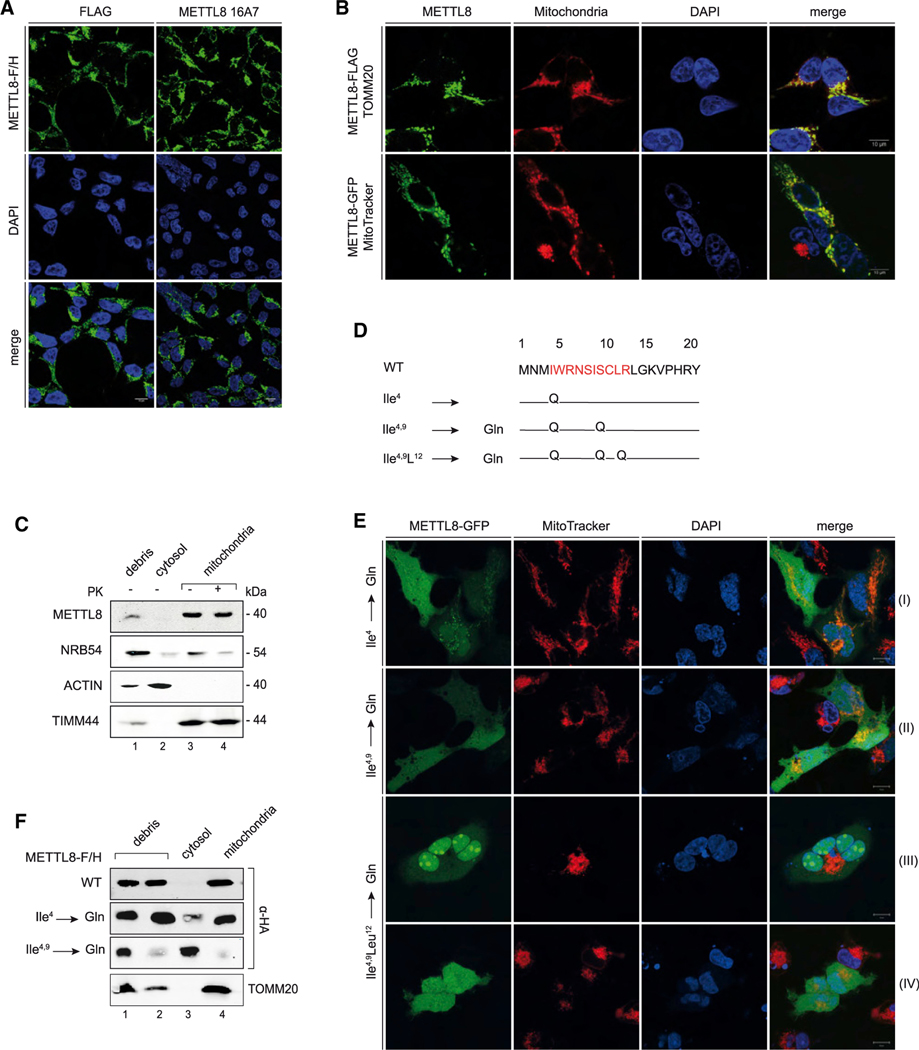
Previous research has suggested that human METTL8 modifies mRNAs or potentially R-loops, which would occur in the cytoplasm or in the nucleus (Xu et al., 2017) (Zhang et al., 2020). To better understand METTL8 function, we initiated our study by performing localization experiments (Figure 1). We fused FLAG/HA (F/H)-tags to the C terminus of METTL8 and performed immunofluorescence experiments (Figure 1A). We probed the METTL8-F/H-expressing cells with either antibodies against the FLAG tag (top panel, left) or a newly generated monoclonal antibody against METTL8 (top panel, right, Figure S1A, bottom; for METTL8 antibody validation). Unexpectedly, we did not observe nuclear staining but detected a granular cytoplasmic signal in both experiments. The characteristic cytoplasmic signal suggested a potential mitochondrial localization and thus, we co-stained METTL8-FLAG- or -GFP-expressing cells with either antibodies against the outer membrane protein TOMM20 (Figure 1B, top panels) or MitoTracker, a reagent that specifically localizes to mitochondria (bottom panels). In both experiments, METTL8 co-localized with mitochondrial markers supporting the mitochondrial localization of METTL8 in fluorescence experiments. Similar results were obtained with HeLa cells (Figure S1B). To further corroborate mitochondrial localization, we performed biochemical fractionation experiments (Figure 1C). We purified mitochondria through several steps and performed western blotting against the nuclear marker protein NRB54, the cytoplasmic marker ACTIN, and the mitochondrial inner membrane marker TIMM44. Endogenous METTL8 was enriched by immunoprecipitation before western blotting to increase signal intensities. Although METTL8 was not detectable in the debris (which also contains nuclei) or the cytoplasmic fraction, it was clearly detectable in the mitochondrial fraction (Figure 1C). To exclude that METTL8 associates specifically or unspecifically with the surface of mitochondria, we treated the purified mitochondria with proteinase K (PK). This treatment had no effect on the METTL8 signal in the mitochondrial fraction, demonstrating that METTL8 is a mitochondrial protein.
Figure 1. METTL8 is a mitochondrial protein.
(A) Immunofluorescence detection of METTL8-F/H by anti-FLAG (green, left panel) and anti-METTL8 16A7 (green, right panel) in Flp-In TREx293 cells. Nuclei were stained with DAPI (blue) (scale bars: 10 μm).
(B) Subcellular localization of METTL8-F/H (green, top panel) and METTL8-GFP (green, bottom panel) in Flp-In TREx293 cells. Top: immunofluorescence detection of METTL8-F/H (green), mitochondrial import receptor subunit TOMM20 (red), and DAPI (blue). Bottom: fluorescence imaging of METTL8-GFP (green), MitoTracker Deep Red (red), and Hoechst 33342 (blue) (scale bars: 10 μm).
(C) Fractionation of Flp-In TREx293 cell lysates into debris/nuclei (NRB54), cytosol (ACTIN) and mitochondria (TIMM44). Mitochondria were treated with Proteinase K. Endogenous METTL8 was detected after anti-METTL8 IP.
(D) Snapshot of the predicted MTS pre-sequence with the MPP (mitochondrial-processing peptidase) cleavage site (position 20) and mutation of amino acids Ile4 and 9 and Leu12 to glutamines.
(E) Live cell imaging of modified METTL8-GFP (green) by fluorescence detection of MitoTracker Deep Red (red) and Hoechst 33342 (blue). Fluorescence detection of the METTL8-GFP single (row I), double (row II), and triple MTS mutant (rows III and IV) (scale bars: 10 μm).
(F) Fractionation of Flp-In TREx293 cell lysates overexpressing METTL8-F/H constructs into debris/nuclei, cytosol, and mitochondria. METTL8-F/H was detected by anti-HA, and TOMM20 served as mitochondrial marker.
Mitochondrial localization is frequently mediated by mitochondrial targeting signals (MTS), and indeed, we found a potential motif at the very N terminus of METTL8 (Figure 1D). Of note, this motif is not present in the closely related proteins METTL2A, 2B, and 6 (Figure S1C). To test the functionality of this targeting motif directly, we mutated several key isoleucine (Ile) residues to glutamine (Gln) (Figure 1D). The GFP-tagged METTL8 mutants were transfected into HEK293 cells and co-stained with MitoTracker (Figure 1E). Mutation of the first Ile (Ile4) still resulted in mitochondrial localization, but a mild, diffuse cytoplasmic signal can also be detected (Figure 1E, panel I row). Double mutation of Ile4 and 9 (Ile4,9) led to a clear cytoplasmic signal (Figure 1E, panel II row) confirming that the targeting signal is required for mitochondrial localization. Biochemical fractionations fully confirmed our immunofluorescence observations (Figure 1F). Of note, a triple mutation, including Ile4 and 9 and Leu12, resulted in a complete mis-localization to the nucleoli (Figure 1E, panel III row), followed by rapid cell death (Figure 1E, panel IV row). Thus, it is possible that nucleolar localization, which has been reported before, could be due to N-terminal tagging of METTL8, which might interfere with mitochondrial targeting (Zhang et al., 2020). Moreover, it is also conceivable that METTL8 isoforms exist that lack the MTS and, thus, may have other cellular functions. However, scrutinizing known mouse isoforms, we detected a potential MTS in all isoforms, arguing against mitochondria-independent functions (Figure S1D). Taken together, we found that the RNA methyltransferase METTL8 is a mitochondrial protein.
METTL8 binds a subset of mitochondrial tRNAs but is dispensable for their aminoacylation
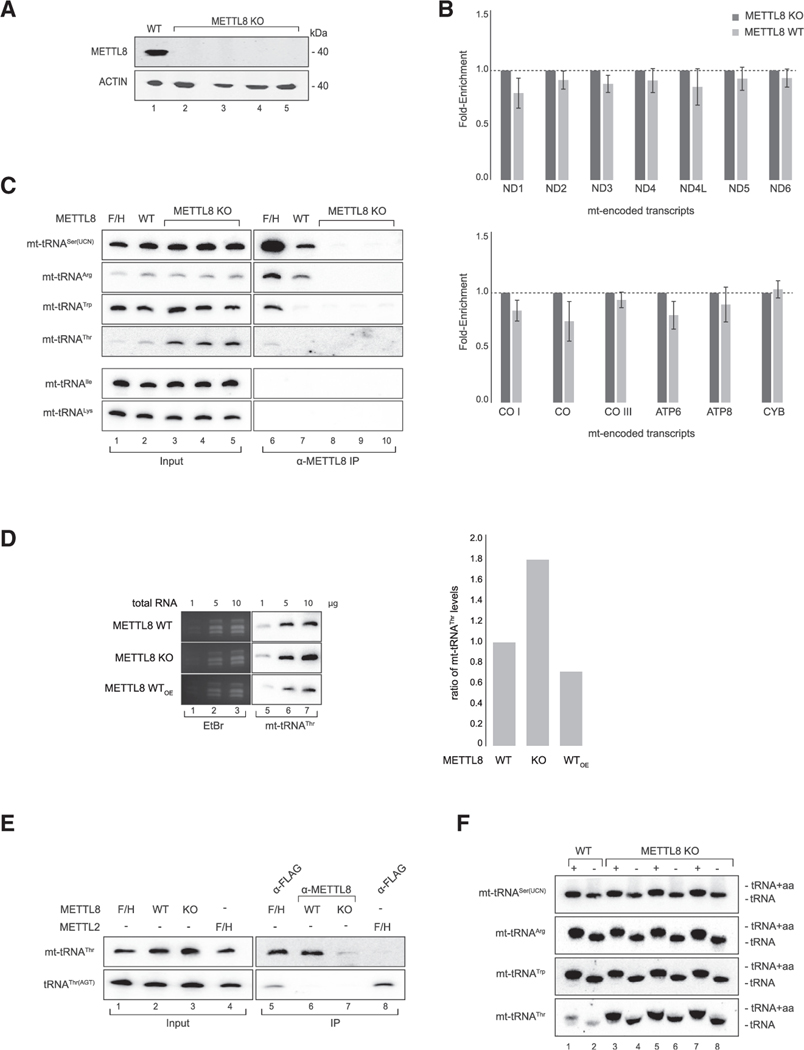
Because METTL8 localizes to the mitochondria, we examined whether METTL8 associates with mt-RNAs in human cells. First, we generated METTL8 KO HEK293 cells using CRISPR and confirmed successful KO by western blotting against endogenous METTL8 (Figure 2A). These cells served as the specificity control in our immunoprecipitations. We next immunoprecipitated METTL8 from HEK293 cells and analyzed co-isolated mt-mRNAs by qPCR (Figure 2B). None of the mt-mRNA transcripts were specifically enriched, indicating that METTL8 does not stably associate with mt-mRNAs. Because the closely related methyltransferases METTL6 and METTL2 act on tRNAs (Xu et al., 2017), we next investigated binding of METTL8 to mt-tRNAs. (Figure 2C). Using northern blotting, we observed that mt-tRNASer(UCN), mt-tRNAArg, mt-tRNAThr, and mt-tRNATrp associated with overexpressed METTL8-F/H, whereas no other mitochondrial tRNAs were associated (Figure 2C and data not shown). Furthermore, mt-tRNASer(UCN) and mt-tRNAArg were also efficiently co-immunoprecipitated with endogenous METTL8, whereas no signal was detected in METTL8 KO cells, indicating highly specific binding signals. Notably, mt-tRNAThr appears to be of low abundance, which is most likely the reason for the weak signal in METTL8 immunoprecipitates. Furthermore, it is the only mitochondrial tRNA that is upregulated in METTL8 KO cells, as evident from the northern blot input samples, suggesting additional METTL8 effects on this particular mt-tRNA (Figure 2C). To further corroborate these observations, we performed northern blots on increasing amounts of total RNA from METTL8 wild type (WT), KO, and overexpressing HEK293 cells (Figure 2D). Consistently, mt-tRNAThr levels depended on the expression status of METTL8. As a further control, we expressed F/H-METTL2, which is known to modify the cytoplasmic tRNAThr(AGT) (Figure 2E). Anti-FLAG-METTL2 immunoprecipitation revealed that tRNAThr(AGT) is efficiently enriched, whereas mt-tRNAThr does not bind to METTL2, indicating that the METTL8 interactions observed are highly specific.
Figure 2. METTL8 interacts with mitochondrial tRNAs.
(A) Detection of METTL8 in Flp-In TREx293 METTL8 WT and METTL8 KO cell lines after anti-METTL8 IP. ACTIN served as the control.
(B) METTL8 RNA immunoprecipitation (RIP)-qPCR of METTL8 WT, and METTL8 KO Flp-In TREx293 cell lines. METTL8 KO cell line was used as control. Means ± standard error of the difference (SED) of three independent measurements are plotted.
(C) Northern blot analysis of RNAs co-immunoprecipitated with METTL8. METTL8 KO cell lines and 0.5 μg total RNA for input were used as controls.
(D) Northern blot analysis of mt-tRNAThr levels in overexpressing METTL8 WT, METTL8 WT, and KO cells. EtBr staining served as the loading control. Right: corresponding phosphor imaging quantification is shown.
(E) Northern blot analysis of RNAs co-immunoprecipitated with METTL8 and METTL2. METTL8-F/H and F/H-METTL2 were immunoprecipitated using anti-FLAG. 0.5 μg total RNA was used as the input control.
(F) Mitochondrial tRNA aminoacylation (+) and deacylation (−) levels in METTL8 WT and METTL8 KO cell lines.
Under steady-state conditions, most cellular tRNAs are covalently bound to amino acids. We performed aminoacylation assays using acid gels to analyze whether METTL8 function is required for aminoacylation of its mt-tRNA targets (Figure 2F). Mitochondrial RNA was isolated from WT or METTL8 KO HEK293 cells, and the aminoacylation status was measured using acid gel electrophoresis (Varshney et al., 1991). Deacylated mt-tRNAs migrate faster in acid gels and can, thus, be easily detected. In both WT cells and three independent METTL8 KO clones, the METTL8-associated mt-tRNAs carry amino acids, indicating that METTL8 is dispensable for mt-tRNA aminoacylation (Figure 2)
Reduced m3C32 mt-tRNA modification in METTL8 KO cells
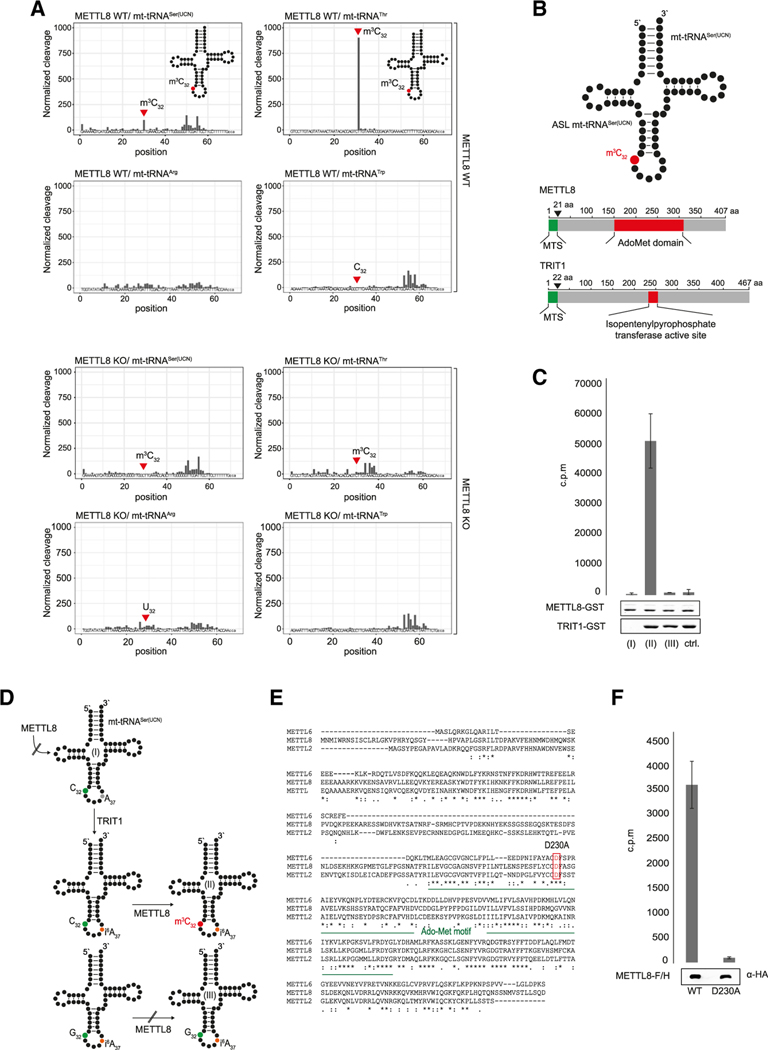
It has been reported that METTL8 generates m3C in mRNAs (Xu et al., 2017). Because our data pointed toward a mitochondrial function, we performed an unbiased m3C screening approach using AlkAniline sequencing (AlkAniline-seq), which allows for the genome-wide detection of m3C and m7G modifications (Figure S2A for schematic overview) (Marchand et al., 2018). Both mt-tRNASer(UCN) and mt-tRNAThr were reported to contain an m3C at position 32 (Suzuki et al., 2011, 2020; Suzuki and Suzuki, 2014). Indeed, AlkAniline-seq revealed that m3C32 is lost on both mt-tRNAs in METTL8 KO cells, whereas it can be readily detected in WT HEK293 cells (Figure 3A). m3C modification patterns of the cytoplasmic tRNASer clusters or the tRNAThr are unchanged in METTL8 KO cells, indicating that the effect is specific and restricted to mitochondrial tRNAs (Figure S2B). Notably, mt-tRNAArg, which was bound to METTL8 in our interaction assays (Figure 2C) does not contain a C at position 32 but, instead, carries a U. Consequently, we did not observe any changes in METTL8 KO cells (Figure 3A). Similarly, although mt-tRNATrp, which showed some binding to METTL8 (Figure 2C) carries a C at position 32, that C has not been reported to be at all methylated, (Suzuki et al., 2011; Suzuki and Suzuki, 2014) and correspondingly, we neither observed a modified C32 nor any changes between WT and METTL8 KO cells. We confirmed our AlkAniline-seq results in HAP-1 cells using classical primer extension experiments (Figure S2C and S2D Figures S3) Taken together, mt-tRNAs that contain m3C32 are unmodified in METTL8 KO cells, indicating that METTL8 modifies these two distinct sites within the mitochondrial tRNA population.
Figure 3. METTL8 methylates position C32 in mt-tRNASer(UCN) and mt-tRNAThr.
(A) AlkAniline-seq analysis of METTL8 WT and METTL8 KO cell lines. Top: m3C cleavage profiles of mt-tRNASer(UCN), mt-tRNAThr, mt-tRNAArg, and mt-tRNATrp in METTL8 WT. Bottom: the corresponding m3C cleavage profiles in METTL8 KO.
(B) The top schematic shows mt-tRNASer(UCN) with highlighted anticodon stem loop (ASL). The bottom cartoon illustrates METTL8 (UniProt:B3KW44) and TRIT1 (UniProt:Q9H3H1) protein domains. Catalytic domains (S-adenosyl-methionine binding site/isopentenylpyrophosphate transferase active site) are highlighted in red, and mitochondrial targeting signal (MTS) is in green.
(C) Methyltransferase activity assay of METTL8-GST. Full-length, unmodified mt-tRNASer(UCN) (column I); i6A37 pre-modified mt-tRNASer(UCN) (column II); and i6A37 pre-modified mt-tRNASer(UCN) mutant (C32G) (column III) were used as substrates. Mitochondrial i6A37 pre-modified tRNAs Phe, Trp, and Ser(AGY) were used as negative controls. Means ± SED of three to six independent measurements are plotted. As a loading control, 1 μg recombinant protein was separated on a 10 % SDS gel and stained by Coomassie Blue.
(D) Cartoon of the m3C formation on mt-tRNASer(UCN). Position 32 (green) is methylated (red) after position 37 (black) is isopentenylated (orange).
(E) Multiple sequence alignment of human METTL2B (GenBank:NM_018396.2), METTL6 (GenBank:NM_152396.3), and METTL8 (GenBank:NM_024770.4). Conserved S-adenosyl-methionine binding site is highlighted in green. Conserved predicted catalytically residue D230A is highlighted by a red box.
(F) Radioactive methylation assay of WT METTL8-F/H and METTL8-F/H D230A variant. Both constructs were enriched by an anti-FLAG immunoprecipitation (IP). Methylation activity was adjusted to western blot signals. I6A37 pre-modified mt-tRNASer(UCN) was used as substrate. Means ± SED of three independent measurements are plotted.
METTL8-mediated m3C32 methylation is linked to isopentenylation of A37
To unravel the biochemical principles underlying METTL8 activity, we set out to reconstitute mt-tRNA C32 methylation in vitro. We expressed METTL8 and purified it from bacterial lysates (Figures 3B and S2E). C32 is located in the anticodon arm of mt-tRNASer(UCN) and mt-tRNAThr, and thus, we designed an RNA substrate resembling the anticodon arm of mt-tRNASer(UCN) (Figure 3B). Recombinant METTL8 was subsequently incubated with the RNA substrate and radioactively labeled SAM as methyl donor. However, we did not observe METTL8 activity under those conditions (Figures 3C, column I, and 3D). Interestingly, mt-tRNASer(UCN) contains an isopentenylated A (i6A) at position 37 (Suzuki et al., 2011; Suzuki and Suzuki, 2014), and thus, we reasoned that METTL8 may require prior i6A37 modification, as has been observed for yeast cytoplasmic tRNAs (Arimbasseri et al., 2016) (Han and Phizicky, 2018). We expressed TRIT1, the human mitochondrial i6A-modifying enzyme (Lamichhane et al., 2013), in bacteria and purified the recombinant protein (Figures 3B and S2E). Strikingly, when the anticodon RNA substrate was pre-incubated with TRIT1 in an isopentenylation reaction, it became an efficient substrate for METTL8, and m3C32 was efficiently methylated (Figures 3C, column II, and 3D). In a control reaction, in which C32 was mutated to G32, no METTL8 activity was detected, even in the presence of i6A37 (Figures 3C, column III, and 3D). Similarly, a negative-control (ctrl) substrate did also not result in detectible activity. Therefore, our biochemical reconstitution revealed that METTL8 requires prior TRIT1 activity, presumably to generate the i6A37 modification to set the m3C32 mark on the mt-tRNASer(UCN).
To further define the catalytic activity of METTL8 and to control for specificity in our in vitro measurements, we mutated, and thus inactivated, METTL8. Because structural information for METTL8 is not available, we aligned METTL2, 6, and 8 and predicted D230 within the AdoMet domain as residue potentially relevant for catalysis (Figure 3B and 3E). We mutated D230 to Alanine (D230A), expressed it in HEK293 cells, and performed methylation assays with the immunoprecipitates. When the mutated METTL8 variant was used, m3C32 methylation was not detectable (Figure 3F). Therefore, the activity observed is METTL8 specific, and D230A is a critical amino acid, which is required for the enzymatic reaction.
Reduced respiratory chain activity in METTL8-deficient cells
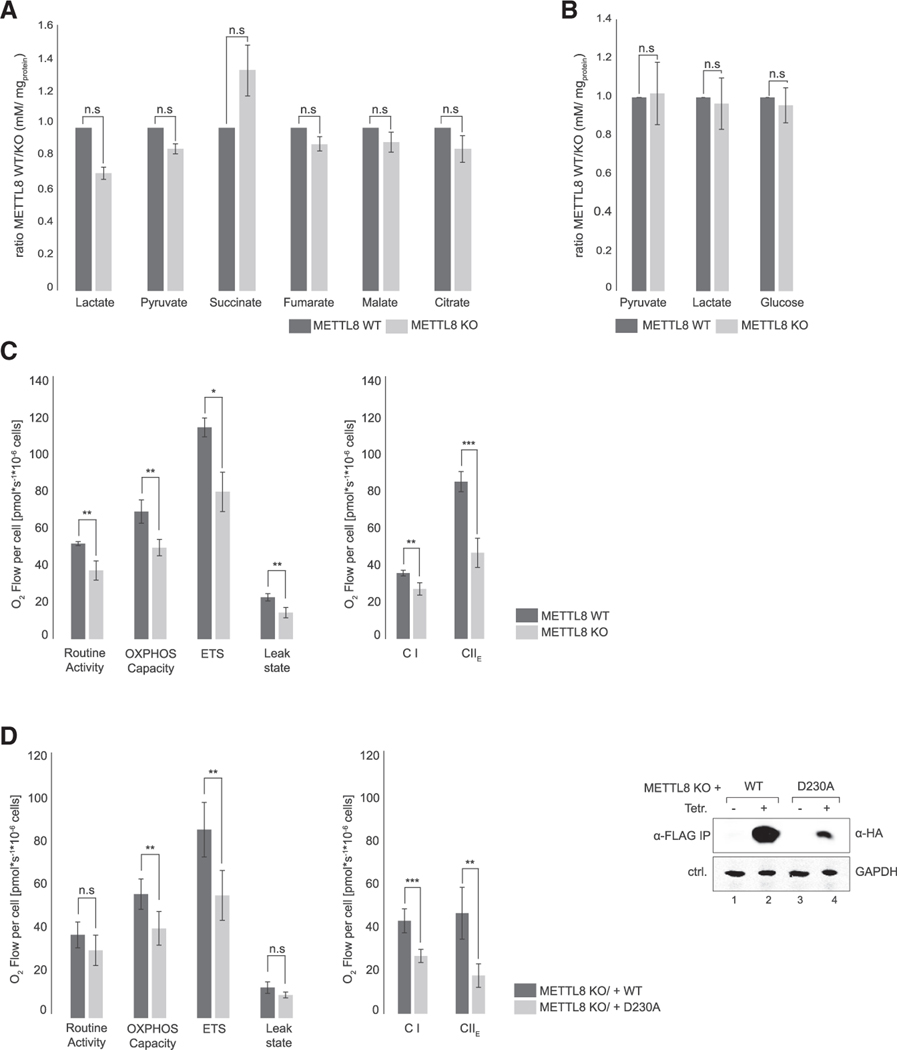
Mitochondria host the respiratory chain and provide most of the cellular energy under aerobic conditions. Because several components of the respiratory chain are translated from mitochondrial transcripts and METTL8 is important for mt-tRNA modification, we hypothesized that METTL8 deletion might affect respiratory chain activity. For a first glimpse on metabolism, we compared intra- (Figure 4A) and extracellular (Figure 4B) metabolite levels using gas chromatography-mass spectrometry. Although not statistically significant, we observed increased intracellular succinate levels in the METTL8 KO cells (Figure 4A). Extracellular pyruvate, lactate, and glucose levels, however, remained unchanged (Figure 4B). These data would be consistent with a model in which succinate accumulates, potentially because of an impaired respiratory chain function. Increased succinate levels might suggest compromised complex II (CII) activity of the respiratory chain downstream of the tricarboxylic acid (TCA) cycle. Negative feedback mechanisms may lead to an overall reduced glucose metabolism.
Figure 4. Loss of METTL8 disturbs metabolic balancing and reduces OXPHOS activity.
(A) Glycolysis and TCA-cycle intermediates in METTL8 WT (dark gray) and METTL8 KO (light gray) cell lines.Means ± SED of three independent measurements are plotted; n.s p > 0.05 in t test.
(B) Metabolic footprinting of the primary glycolysis carbon sources in METTL8 WT (dark gray) and METTL8 KO (light gray) cell lines. Means ± SED of three independent measurements are plotted; n.s p > 0.05 in t test.
(C) Oxygraph-2k measurements of WT METTL8 (dark gray) and METTL8 KO (light gray) Flp-In-TREx293 cell lines. Respiratory activity analysis was performed using the substrate-uncoupler-inhibitor-titration-protocol with Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP). Means ± SED of four independent measurements are plotted; * p < 0.05, ** p < 0.01, *** p < 0.0001 in t test.
(D) Left: Oxygraph-2k measurements of Flp-In-TREx293 METTL8 KO rescue variants. Respiratory activity analysis of METTL8 KO_WT-F/H (dark gray) and METTL8 KO_D230A-F/H (light gray) was performed as described in (C). Means ± SED of four independent measurements are plotted; n.s p > 0.05, ** p < 0.01, *** p < 0.0001 in t test. Western blot shows METTL8 KO_WT-F/H and METTL8 KO_D230A-F/H expression level after anti-FLAG IP. GAPDH was used as control.
To test respiratory chain activity directly, we performed high-resolution respirometry (Oroboros-2k) and assessed key features of respiratory chain activity (Figure 4C). Parameters such as routine activity, oxidative phosphorylation (OXPHOS) capacity, electron transport system (ETS), and the leak state were clearly reduced in METTL8 KO cells (Figure 4C). These general respiratory chain impairments can be linked to reduced CI and CII activities (Figure 4C).
We next investigated whether the reduced respiratory chain activity is caused by a general METTL8 loss or the lack of its enzymatic activity and, thus, loss of m3C32 modification of the mt-tRNAs. We rescued METTL8 KO HEK293 cells either with WT METTL8 or with its catalytic mutant (D230A) and repeated the high-resolution respiratory experiments (Figure 4D). We compared rescue activity of WT METTL8 and METTL8-D230A directly and found that WT METTL8 rescued routine activity, OXPHOS capacity, ETS, and the leak state efficiently, whereas the catalytic METTL8 mutant rescued OXPHOS capacity and ETS to a much lesser degree. Effects on routine activity and leak state did not reach statistical significance (Figure 4D). Differences between WT and mutant METTL8-rescue experiments were even more apparent when CI and CII activities were analyzed (Figure 4D). Therefore, we conclude that the loss of METTL8 activity and, thus, the loss of m3C32 modification of mt-tRNASer(UCN) and mt-tRNAThr affects specific activities of the respiratory chain.
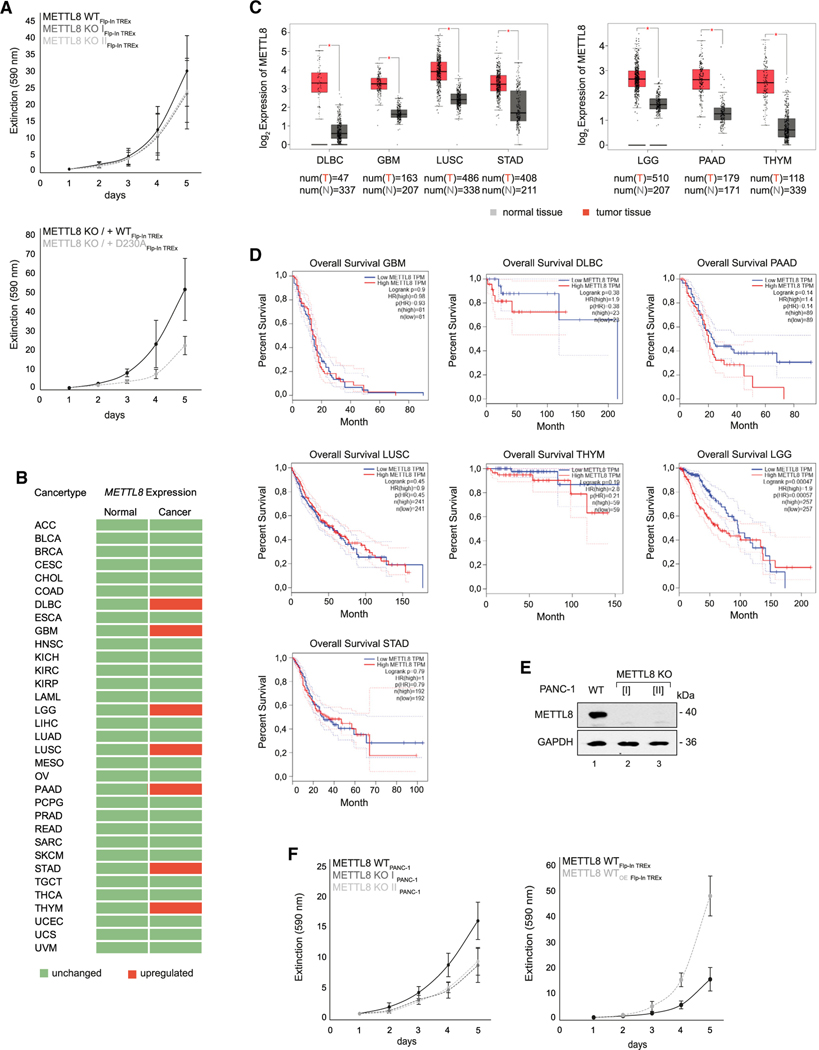
METTL8 expression correlates with cell growth and patient survival in pancreatic cancer
Our observations that METTL8 is important for proper respiratory chain function, together with previous findings that METTL8 is mis-expressed in several cancers (Begik et al., 2020; Zhang et al., 2020), prompted us to conduct a deeper investigation into a potential link between METTL8 and cancer. We first assessed whether METTL8 KO affects cell proliferation in HEK293 cells (Figure 5A). Although not statistically significant, a potential growth defect can be observed in two METTL8 KO HEK293 cell clones (Figure 5A, top panel). WT METTL8 rescued the growth phenotype more effectively than the catalytic mutant (D230A) did (Figure 5A, bottom panel) highlighting the importance of the catalytic activity for the phenotype. We next analyzed publicly available gene-expression data from cancer samples or normal tissues (Database: The Cancer Genome Atlas (TCGA); Figure 5B). In agreement with previous observations (Begik et al., 2020), METTL8 is upregulated in diffuse large B cell carcinoma (DLBC), glioblastoma (GBM), low-grade glioma (LGG), lung squamous cell carcinoma (LUSC), pancreatic adenocarcinoma (PAAD), stomach adenocarcinoma (STAD), and thyroid carcinoma (THYM) (Figures 5B and 5C). Because misregulation is not necessarily relevant for pathogenesis, we asked whether METTL8 upregulation also translates into patient survival rates and generated Kaplan-Meier curves based on TCGA data (Database: TCGA) (Figure 5D). In six of the seven cancer types, METTL8 upregulation is not correlated with overall patient survival. In contrast, in the highly aggressive pancreatic cancer, high METTL8 levels correlate with significantly lower survival rates, suggesting that increased METTL8 levels could be relevant for pancreatic cancer pathology (Figure 5D).
Figure 5. Upregulated METTL8 expression correlates with survival rate in PAAD cancer.
(A) The top diagram shows Flp-In TREx293 METTL8 WT cell proliferation in black and METTL8 KO in gray, and the bottom diagram shows growth curves of the Flp-In TREx293 METTL8 KO rescue variants METTL8 KO_WT-F/H (black) and METTL8 KO_D230A-F/H (gray). Cells were grown in presence of high glucose. Means ± SED of four independent measurements are plotted.
(B) List of cancer types from the Database: Gene Expression Profiling Interactive Analysis (GEPIA). Cancer types with upregulated METTL8 expression are highlighted in red.
(C) Boxplot of METTL8 expression in DLBC, GBM, LGG, LUSC, PAAD, STAD, and THYM (red) and normal tissue (gray) based on the Database: GEPIA dataset. p < 0.05.
(D) Correlation of METTL8 expression and patients’ overall survival in DLBC, GBM, LGG, LUSC, PAAD, STAD, and THYM. Correlation of overall survival with low METTL8 expression (blue) or high METTL8 expression level (red).
(E) Detection of METTL8 in PANC-1 METTL8 WT and METTL8 KO cell lines after anti-METTL8 IP. GAPDH was used as control.
(F) Left: growth curves of PANC-1 METTL8 WT in black and METTL8 KO in gray. Right: proliferation of tetracycline-treated METTL8 WT (black) and METTL8 overexpressing (WTOE) (gray) Flp-In TREx293 cells. Cells were grown in presence of high glucose. Means ± SED of four independent measurements are plotted.
To further corroborate that model, we knocked out METTL8 in the pancreatic cancer cell line PANC-1 (Figure 5E), which resulted in a much stronger delay in cell growth compared with HEK293 cells (Figure 5F, left panel). Furthermore, overexpression of METTL8 in HEK293 cells, which mirrors the conditions in PAAD and the PANC-1 cell line, strongly accelerated cell proliferation (Figure 5F, right panel). Our data, therefore, suggest that METTL8 may serve as bottleneck for cell proliferation, which might be specifically important for rapidly growing pancreatic cancer cells.
Increased mt-tRNASer(UCN) m3C32 methylation and respiratory chain activity in the pancreas carcinoma cell line PANC-1
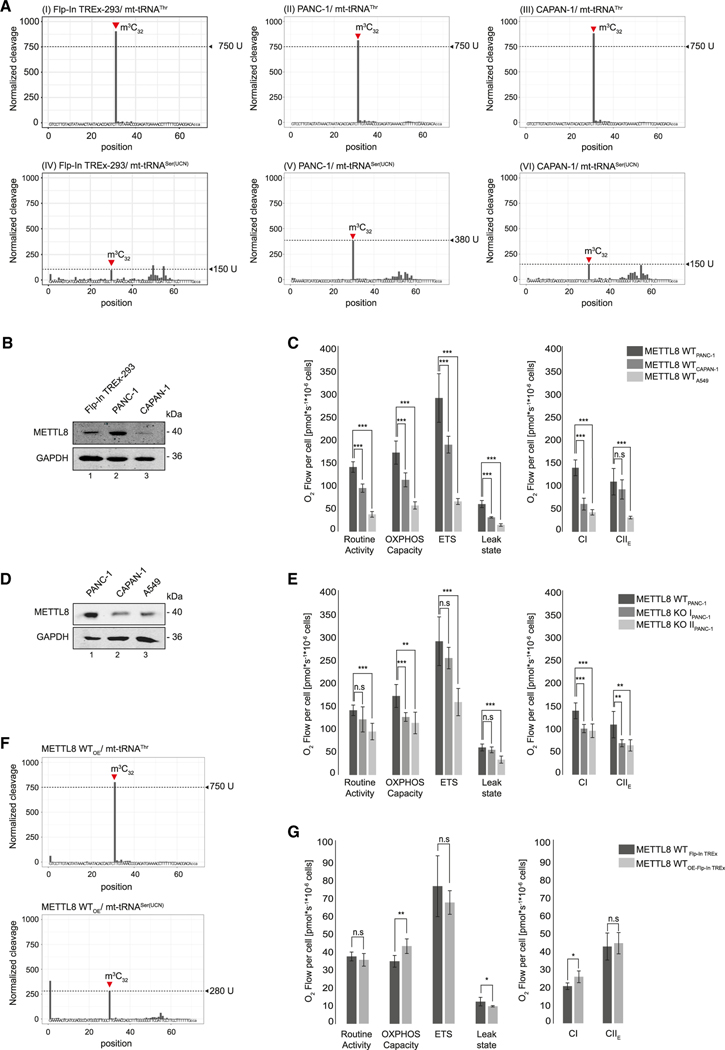
To further assess the role of METTL8 in pancreatic cancer, we analyzed the mt-tRNASer(UCN) methylation status in the PAAD-derived cell lines PANC-1 and CAPAN-1 and compared it with HEK293 cells using AlkAniline-seq (Figure 6A). The signal for the methylation status of mt-tRNAThr was >750 U, indicating that the mt-tRNAThr population is most likely fully methylated in all three cell lines (Figure 6A, graphs I–III). Interestingly, only a minor fraction (about 5%–10%) of the mt-tRNASer(UCN) population appears to be methylated in HEK293 cells, whereas the bulk of mt-tRNASer(UCN) remains unmethylated at position C32 (suggested by a signal of only about 150 U; Figure 6A, graph IV). Strikingly, mt-tRNASer(UCN) methylation is at least 2.5-fold greater in PANC-1 cells (380 U; please note that the calibration curve is non-linear for the AlkAniline-seq signals; Marchand et al., 2018), which would be in line with increased METTL8 activity in those cells (Figures 6A, graph V, and 6B). However, when assessing the second PAAD-derived cell line CAPAN-1, mt-tRNASer(UCN) C32 methylation was as low as in HEK293 cells (Figures 6A, graph VI). These results were puzzling because both PANC-1 and CAPAN-1 cells derive from PAAD. Because we reasoned that METTL8 levels correlate with the tRNA methylation status, we assessed METTL8 protein levels by western blotting (Figure 6B). In agreement with our model, METTL8 expression levels in CAPAN-1 cells are much lower compared with that in PANC-1 cells, which could readily explain the differential mt-tRNASer(UCN) C32 methylation status between the two pancreatic cancer cell lines (Figure 6A and B). Of note, CAPAN-1 cells are derived from liver metastasis of pancreas cancer and may have adjusted METTL8 levels to specific needs during liver colonization.
Figure 6. METTL8 expression regulates m3C32 level of mt-tRNASer(UCN).
(A) AlkAniline-seq analysis of mt-tRNAThr and mt-tRNASer(UCN) in Flp-In TREx293, PANC-1, and CAPAN-1 cell lines. Top: m3C cleavage profiles of mt-tRNAThr. Bottom: m3C cleavage profiles of mt-tRNASer(UCN).
(B) METTL8 expression level in Flp-In TREx293, PANC-1 and CAPAN-1 cell lines after anti-METTL8 IP. GAPDH was used as control.
(C) Oxygraph-2k measurements of PANC-1 (dark gray), CAPAN-1 (gray) and A549 (light gray) cell line using the substrate-uncoupler-inhibitor-titration-protocol with FCCP. Means ± SED of four independent measurements are plotted; n.s p > 0.05, ** p < 0.01, *** p < 0.0001 in t test.
(D) METTL8 expression level in PANC-1, CAPAN-1, and A549 cell line after anti-METTL8 IP. GAPDH was used as control.
(E) Oxygraph-2k measurements of WT METTL8 (dark gray) and METTL8 KO (light gray) PANC-1 cell lines. Respiratory activity analysis was performed as in (C). Means ± SED of four independent measurements are plotted; n.s p > 0.05, ** p < 0.01, *** p < 0.0001 in t test.
(F) m3C cleavage profiles of mt-tRNAThr (top panel) and mt-tRNASer(UCN) (bottom panel) in Flp-In TREx293 cells overexpressing METTL8 WT.
(G) Respiratory activity of METTL8 WT (dark gray) and METTL8 overexpressing (WTOE) (light gray) Flp-In TREx293 cell line analyzed by high-resolution respirometer Oxygraph-2k as described in (C). Means ± SED of four independent measurements are plotted; n.s p > 0.05, * p < 0.05, ** p < 0.01 in t test.
To examine whether the METTL8 levels in the three cell lines also affect their respiratory chain activities, we performed Oroboros measurements and again tested a number of respiratory chain parameters (Figure 6C). We found that routine activity, OXPHOS capacity, the ETS, and the leak state are increased in PANC-1 cells compared with that in CAPAN-1 and the non-cancer pancreas cell line A549 (Figure 6C). Moreover, PANC-1 cells possess much stronger CI activity, and CII activity is also moderately increased (Figure 6C), which is consistent with strong METTL8 upregulation in PANC-1 cells (Figure 6D). To further test the importance of METTL8 for PANC-1 cells, we assessed respiratory chain activity under METTL8 KO conditions (Figure 6E). Consistently, all respiratory chain parameters are reduced when METTL8 is deleted (Figure 6E). In summary, our data suggest a model in which PANC-1 cells, in particular, might be addicted to high METTL8 levels.
We next asked whether the METTL8 effects observed in PANC-1 cells can be reconstituted in other cell lines or whether there are unknown PANC-1-specific factors besides increased METTL8 that contribute to the enhanced activity of the respiratory chain. We, therefore, overexpressed METTL8 in HEK293 cells and assessed m3C modification using AlkAniline-seq. Overexpression of METTL8 leads to an at least 2-fold increase of m3C32 on mt-tRNASer(UCN) (Figure 6F, bottom panel), whereas mt-tRNAThr is not further modified because it is likely fully methylated already (Figure 6F, top panel). Furthermore, METTL8 overexpression results in a moderate increase of the OXPHOS capacity and CI activity. Other parameters are rather unaffected (Figure 6G). Thus, mimicking METTL8 levels of PANC-1 in HEK293 cells partially leads to similar phenotypes, which supports our hypothesis that METTL8 represents a general bottleneck for respiratory chain function through mt-tRNASer(UCN) methylation.
The mt-tRNASer(UCN) m3C32 status and respiratory chain composition depend on METTL8 levels
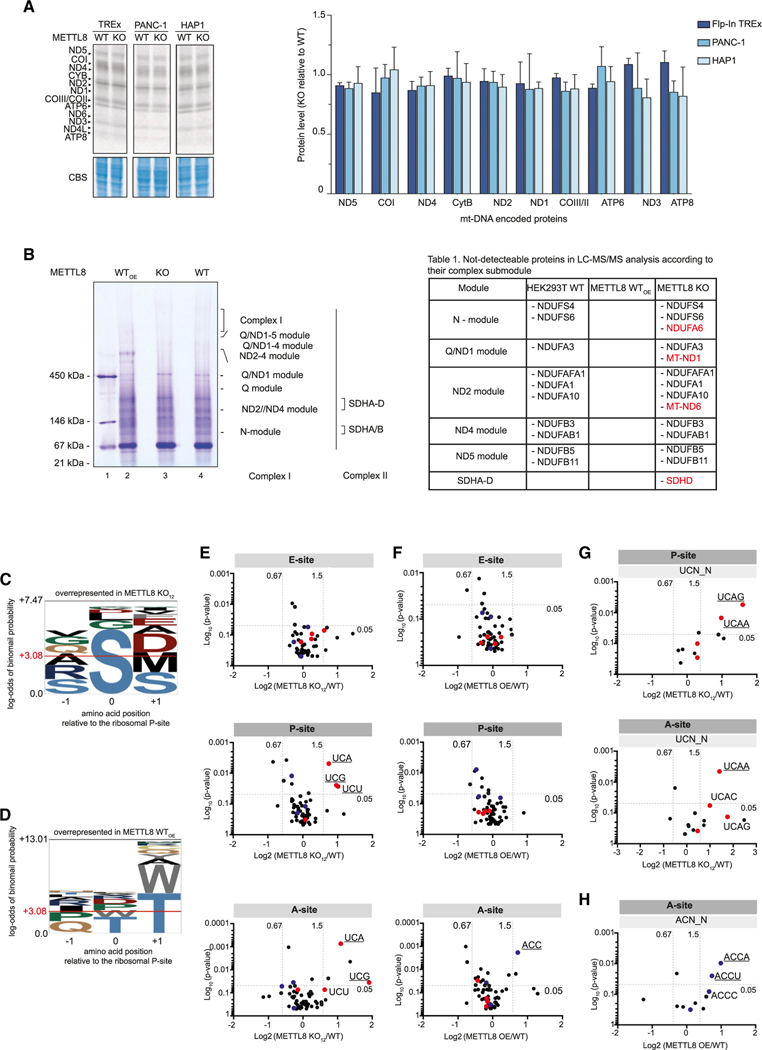
An obvious scenario would be that inefficient mt-tRNASer(UCN) methylation affects translation of transcripts containing mt-tRNASer(UCN) codons. Because those codons appear to be randomly distributed and found in almost all mitochondrial mRNAs (Figure S3A), we performed an unbiased metabolic labeling approach (Figure 7A). HEK293, PANC1, and HAP1 cells were grown on radiolabeled methionine in the presence of emetine dihydrochloride. Under those conditions, cytoplasmic translation is blocked, whereas mitochondrial translation is unaffected. The 13 mitochondrial proteins can be separated by gel electrophoresis and quantified. Using that approach, we did not find considerable differences between WT and METTL8 KO cells (Figure 7A). Of note, not all mitochondria-encoded proteins can be efficiently detected with this method. Because we did not observe a global effect on mitochondrial translation, we analyzed respiratory chain sub-complex composition by native gel electrophoresis, followed by mass spectrometry (Figure 7B). Mitochondrial proteins from WT, METTL8 KO, and METTL8-overexpressing HEK293 cells were loaded on a blue-native gel, separating the sub-complexes of the respiratory chain. CI and CII are assembled from distinct pre-formed modules (Figure S3B), which were excised from the gel, analyzed by mass spectrometry, and the identified proteins were compared between the investigated conditions (Figure 7B, left). A comprehensive detection of all components of the respiratory chain is challenging because of molecular weight and abundance in the native complexes, membrane association, or solubility (Barros and McStay, 2020). Accordingly, we did not reliably identify all components under normal conditions (Figure 7B, right; “HEK293 WT” represents factors that were not detectable). Interestingly, when METTL8 was overexpressed (Figure 7B; “METTL8 WTOE”), we readily detected all components of the respiratory chain in our analysis, suggesting that METTL8 levels may affect the abundance of specific components in respiratory chain assemblies. Moreover, in cells in which METTL8 was deleted (Figure 7B; “METTL8 KO”), four additional proteins were missing in our measurements (Figure 7B, highlighted in red). Although two of them are nuclear encoded (NDUFA6 and SDHD), ND1 and ND6 are expressed from the mitochondrial genome and are, therefore, candidates that could be directly affected by METTL8.
Figure 7. m3C32 unbalancing in mt-tRNASer(UCN) and mt-tRNAThr affects translation dynamics and respiratory complex assembly.
(A) Radioactive pulse labeling of de novo mitochondrial protein synthesis. Flp-In TREx293, PANC-1, HAP1 METTL8 WT and METTL8 KO cell lines were labeled with [35S] methionine after cytoplasmic protein synthesis was blocked by emetine dihydrochloride. Total lysates were separated by Tricine-SDS-PAGE and stained with Coomassie Blue as a loading control (left panel). Corresponding phosphorimaging quantification is shown (right panel).Means ± SED of two independent measurements are plotted.
(B) Mitochondrial lysates from METTL8 WT, METTL8 KO, and METTL8 overexpressing (WTOE) Flp-In TREx293 cell lines were separated by blue native (BN) page and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis (left panel). Assembly of the complex I and II submodules is shown to the side of the panel. Right: listed proteins were not detectable in LC-MS/MS analysis and are mapped to their related module. Additional proteins undetectable in Flp-In TREx METTL8 KO are highlighted in red.
(C) Amino acid overrepresentation at positions relative to the P site in Flp-In TREx293 METTL8 KO cells. Horizontal red line indicates significant overrepresentation.
(D) Amino acid overrepresentation at positions relative to the P site in Flp-In TREx293 cells overexpressing METTL8. Horizontal red line indicates significant overrepresentation.
(E) Ribosomal redistribution in E, P, and A site of Flp-In TREx293 METTL8 KO cells based on the codon. Red dots represent mt-tRNASer(UCN) codons, mt-tRNAThr codons are highlighted in blue. Significant overrepresented codons are underlined.
(F) Ribosomal redistribution in E, P, and A site of Flp-In TREx293 cells overexpressing METTL8 based on the codon. Red dots represent mt-tRNASer(UCN) codons, mt-tRNAThr codons are highlighted in blue. Significant overrepresented codons are underlined.
(G) Ribosome stalling in either the P site or A site of Flp-In TREx293 METTL8 KO cells based on the UCN codon and downstream nucleotides. Top: UCN codons with overrepresented downstream nucleotides in the P site. Bottom: UCN codons with overrepresented downstream stream nucleotides in the Asite. Red dots represent mt-tRNASer(UCN) codons. Significantly enriched codons are underlined.
(H) Ribosome stalling of Flp-In TREx293 cells overexpressing METTL8 based on the ACN codon and downstream nucleotides in the A site. Blue dots represent mt-tRNAThr codons. Significantly enriched codons are underlined.
Because mitochondrial translation is almost unchanged globally when METTL8 is lost (Figure 7A) and we did not find a bias for mt-tRNASer(UCN) or mt-tRNAThr codons on specific transcripts, we performed ribosome profiling to identify codons that are affected by METTL8 levels. Ribosomal complexes from total HEK293 extracts were separated by sucrose-gradient centrifugation (Figure S3C). After micrococcal nuclease (MNase) digestion, RNA fragments protected by the small mitochondrial ribosomal subunit were cloned and sequenced. This approach allows for precise mapping of codons in the A, P, and E sites of the mitochondrial ribosome. Strikingly, in METTL8 KO cells, codons coding for serine are clearly enriched in the P site, suggesting that the dwelling time of ribosomes at serine codons is increased. Threonine codons, however, are not enriched, suggesting that C32 methylation on the mt-tRNAThr is not essential for the translation cycle (Figure 7C; similar results were obtained using an independent METTL8 KO clone; Figures S3D and S3E). Curiously, under METTL8-overexpression conditions, we did not observe a specific codon enrichment in the P site but, in contrast, found a clear bias for threonine codons in the A site, indicating that the ribosome remain longer on those codons when METTL8 levels are high (Figure 7D). Finally, we asked which specific codons were affected in our analyses (Figures 7E and 7F). Our bioinformatic analyses revealed that the UCA and UCG/U codons of the mt-tRNASer(UCN) appears to be strongly enriched in the P site and UCA in the A site when METTL8 is knocked out (Figure 7E). When METTL8 is overexpressed, the ACC codon of the mt-tRNAThr is enriched in the A site (Figure 7F). To better define a potential sequence context of the affected codons, we investigated flanking nucleotides (Figures 7G and 7H). Although overrepresented in our sequencing, we find a mild enrichment of a 5′-C for the UCU, UCA, and UCG codons in either the P or A site (Figure S3F and data not shown). However, an A or G downstream of UCA in the P site and an A downstream of UCA in the A site are enriched and, thus, more severely affected (Figure 7G). A flanking A, either up or downstream of the mt-tRNAThr ACC codon, is also enriched in our data. However, that could also be a consequence of sequencing bias (Figures 7H, S3G, and S3J; data not shown). Taken together, our data suggest that specific codons of the mt-tRNA substrates of METTL8 are more affected than others, which may lead to changed protein levels and, therefore, better or worse incorporation into respiratory chain complexes.
DISCUSSION
tRNAs are extensively modified in all kingdoms of life, and such chemical alterations affect all aspects of tRNA function, including folding, loading with amino acids, binding to the ribosome, and recognition and pairing of the anticodon arm with the codon (Suzuki, 2021). Many of these modifications, as well as their modifying enzymes, have been associated with human diseases, including cancer (Bohnsack and Sloan, 2018a; Chujo and Tomizawa, 2021; Delaunay and Frye, 2019; Suzuki, 2021).
The tRNA modification m3C32 on mt-tRNASer(UCN) and mt-tRNAThr has long been known, but the responsible enzyme remained elusive (Bohnsack and Sloan, 2018a; Suzuki and Suzuki, 2014). Here, we have discovered that METTL8 is a mitochondrial protein that catalyzes C32 methylation of these mt-tRNAs. Our biochemical investigations further revealed that METTL8 requires the presence of i6A on A37, which most likely induces structural rearrangements, presenting C32 in a configuration that allows METTL8 to access the substrate (Cabello-Villegas et al., 2002; Ganichkin et al., 2011; Murphy et al., 2004; Weixlbaumer et al., 2007). It has been suggested before that METTL8 modifies mRNAs in the cytoplasm and specific R-loop structures in the nucleus (Xu et al., 2017; Zhang et al., 2020). Although our unbiased AlkAniline-seq experiments do not provide evidence for additional substrates, it cannot be excluded that isoforms lacking the N-terminal mitochondrial targeting signal are cell-type-specifically expressed, which might target non-mitochondrial RNAs. Interestingly, when we mutated residues in the mitochondrial targeting signal, we observe nucleolar mis-localization, and such mis-localization might also be caused by N-terminal tagging, which might interfere with mitochondrial translocation. Nevertheless, a thorough assessment of METTL8 isoforms will be needed to clarify whether METTL8 has additional functions.
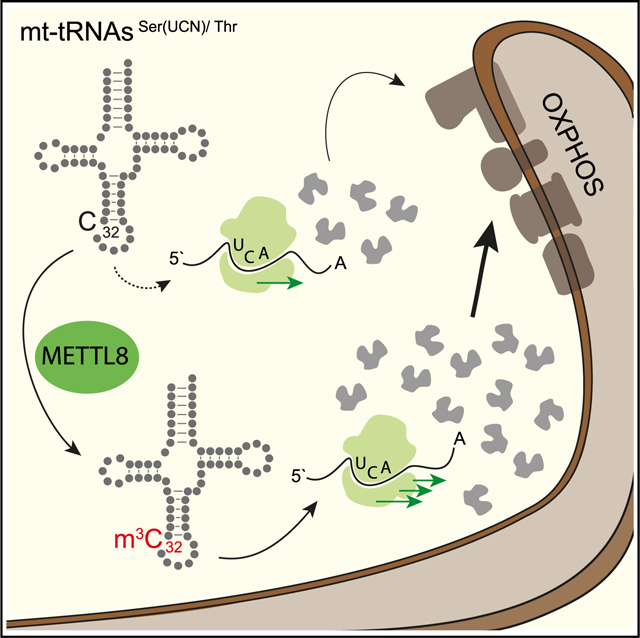
Thirteen genes code for respiratory chain components, and 22 tRNA genes are encoded on the mammalian mitochondrial genome. With the exception of leucine and serine, all amino acids are charged onto a single mt-tRNA. The two serine mt-tRNAs are characterized by specific structural features, but only mt-tRNASer(UCN) is methylated at position C32 (Anderson et al., 1981, 1982). Interestingly, our AlkAniline-seq experiments revealed that only a small portion of this tRNA is C32 methylated, at least under the analyzed conditions. Nevertheless, mt-tRNASer(UCN) is aminoacylated, indicating that the modification is not required for amino acid charging. When METTL8 is knocked out, mt-tRNASer(UCN) is not C32 methylated, and our ribosome profiling data revealed that ribosomes tend to dwell longer on UCA and UCG/C codons of the mt-tRNASer(UCN). Furthermore, the high resolution of our data allowed for mapping the stalled position to the A, P, and E sites of the mitoribosome. Here, we made several interesting observations. First, the cognate codon UCA of the mt-tRNASer(UCN) is stalled in the A and the P sites (Figure 7E). Closer examination revealed that stalling in the A site is preferentially flanked by a downstream A, whereas codons stalled in the P site by a downstream G (Figure 7G), which may suggest that a purine downstream of the cognate codon requires m3C32 methylation more than others do. Second, specific near cognate codons are preferentially stalled in the P site, independent of the flanking nucleotide. Near-cognate codons engage in wobble pairing at position three of the codon, whereas the cognate codon forms Watson-Crick base pairing between the codon and the anticodon. It is tempting to speculate that m3C32 is important for decoding fully complementary codons when flanked by G, whereas a flanking A, as well as several near-cognate codons require the modification for steps downstream of decoding. This model, however, needs to be further analyzed.
When investigating transcripts that might be important for the observed phenotypes, we find that ND6 has two of the three strongest stalling events, both involving potentially problematic mt-tRNASer(UCN) UCU codons, which are also stalled in the P site (Figure 7E and data not shown). At codon 20, ribosomes stall with a UCU codon positioned in both the P and A sites, and at position 24, a UCU codon is flanked by proline codons. A reduced ND6 production would also be consistent with our mass spectrometry results, in which ND6 was reproducibly missing from respiratory chain complexes. This, however, requires deeper and more comprehensive investigations.
METTL8 KO results in a complete loss of C32 methylation of mt-tRNAThr, which appears to be fully methylated within the mitochondrial tRNA pool. Under KO conditions, we did not observe stalled ribosomes, suggesting that the methylation is not essential for the translation cycle under tissue culture conditions. Curiously, we identified stalled ribosomes on the ACC mt-tRNAThr codon when METTL8 is overexpressed. In contrast to mt-tRNASer(UCN), ribosomes remain at the A site. By carefully analyzing our northern blotting experiments (Figure 2C), we observed that mt-tRNAThr is generally of very low abundance. Furthermore, when overexpressing METTL8-F/H, we observed further reduction, and METTL8 KO revealed stronger mt-tRNAThr signals (Figure 2C and D). Thus, METTL8 not only affects the methylation status but also the cellular levels of mt-tRNAThr. Thus, stalled ribosomes at the ACC codon in the A site are consistent with reduced mt-tRNAThr availability, followed by an extended dwelling time of the ribosome at this codon.
Although METTL8 is strongly expressed in a variety of cancers (Figure 5; Begik et al., 2020), patient survival is only correlated with high METTL8 levels in pancreatic cancer. Because an increase in METTL8 is accompanied with enhanced respiratory chain activity, an obvious benefit for cancer cells could be a better energy supply, allowing fast and aggressive progression (Tataranni et al., 2017). However, given the complexity of the underlying molecular principles, i.e., only a minor portion of mt-tRNASer(UCN) is methylated and METTL8 also has an effect on mt-tRNAThr abundance, such a model might be too greatly simplified. An intriguing question that still remains is why only a very specific form of pancreatic cancer uses such an apparent advantage. It is more likely that cell-type-specific effects or micro-environmental cues influence METTL8 activity in these cells. It is also possible that glycolysis is increased for the generation of building blocks (Warburg effect), and the generated NADHs could, in addition to lactate production, also be regenerated by an enhanced respiratory chain activity. Although this remains speculation, such a scenario could be supported by our finding that PANC-1 cells show greater proton leakage compared with that of control cells (Figure 6C). Nevertheless, METTL8 appears to be a very attractive target for treating the so-far incurable pancreas carcinoma. RNA therapeutics, e.g., small interfering RNAs (siRNAs) or target mimics, could be used for specific inhibition of METTL8, and because such cancer cells appear to be more addicted to METTL8 than other cells, such a treatment could be specific and unproblematic for healthy cells.
Taken together, we provide evidence for a sophisticated balancing system of mitochondrial translation fueled by METTL8-mediated C32 methylation of the mt-tRNASer(UCN) and mt-tRNAThr, which is important for synthesizing optimal respiratory chain compositions. Although not vital under cell culture conditions, a mis-balancing of this sophisticated gene expression program leads to partially incomplete or functionally compromised respiratory chains.
Limitations of the study
Our work uncovers the enzyme that generates m3C32 on the mitochondrial tRNAs mt-tRNASer(UCN) and mt-tRNAThr, and we find that, in particular, the loss or increase of mt-tRNASer(UCN) methylation affects respiratory chain activity. However, we have measured respiratory chain activity in cultured cell lines, which are not in a physiological environment. Many such cell lines do not rely on oxidative phosphorylation and effects might be different in tissues. Furthermore, we find that mitoribosomes are stalled at codons used by the mt-tRNASer(UCN) when the m3C32 is missing. Although we observe effects on the abundance of several mitochondria-encoded proteins in respiratory chain complexes using mass spectrometry, the affected codons are widespread and found on almost all mitochondrial transcripts. We hypothesize that the protein ND6 could be the main affected protein, but more work is needed to strengthen this model. Finally, we find that METTL8 is overexpressed in a number of cancers, which has also been observed by others. Based on TCGA and GTex data (Database:TCGA and Database:GTex), overexpression seems to correlate with overall patient survival, mainly in pancreatic cancer. An obvious scenario would be that pancreatic cancer cells tune respiratory chain activity via METTL8 overexpression. However, such an obvious advantage appears not to be selected by other types of cancer, and thus, it is conceivable that other, potentially even indirect, effects could be involved. This needs to be further unraveled.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Gunter Meister (gunter.meister@ur.de).
Materials availability
Plasmids and cell lines generated in this study are shared by the lead contact upon request.
Data and code availability
MitoRibosome profiling and AlkAnilineSeq data have been deposited at GEO and ENA and are publicly available as of the date of publication. Accession numbers are listed in the Key resource table. Original western blot images and microscopy data have been deposited at Mendeley and are publicly available as of the date of publication.
All original code is publicly available as of the date of publication and shared by James Marks (MitoRibosome Profiling) and Yuri Motorin (AlkAnilineSeq) upon request.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-HA.11 antibody (clone 16B2) | Covance | Cat# MMS-101P, RRID: AB_2314672 |
| Mouse monoclonal anti-beta actin antibody (ab6276) | Abcam | Cat# ab6276, RRID: AB_2223210 |
| Mouse monoclonal anti-GAPDH (GT239) | GeneTEx | Cat# GTX627408, RRID: AB_11174761 |
| Mouse monoclonal anti-p54[nrb] | BD Transduction Laboratories | Cat# 611278, RRID: AB_398806 |
| Mouse monoclonal anti-FLAGM2 (F3165) | Sigma | Cat# F3165, RRID: AB_259529 |
| Rabbit polyclonal anti-TOM20 (FL-145) | Santa Cruz Biotechnologies | Cat# sc-11415, RRID: AB_2207533 |
| Goat polyclonal anti-mouse IgG, IRDye 800CW conjugated antibody |
LI-COR Bioscience | Cat#925–32210; RRID: AB_2687825 |
| Goat polyclonal anti-rabbit IgG, IRDye 800CW conjugated antibody |
LI-COR Bioscience | Cat#926–32211; RRID: AB_621843 |
| Rat monoclonal anti-METTL8 antibody (clone 16A7) | this paper | N/A |
| Rat monoclonal anti-METTL8 antibody (clone 19A10) | this paper | N/A |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| Escherichia coli Rosetta (DE3) competent cells | Novagen | Cat#70954–3 |
| XL1 blue competent cells | this paper | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| METTL8 (22–407 aa.) – GST (human) | this paper | N/A |
| TRIT1 (22–467 aa.) – GST (human) | this paper | N/A |
| OVA-METTL8 peptide | Peps4LS, Heidelberg | N/A |
| Thermostable inorganic pyrophosphatase | New England BioLabs | Cat#M0296 |
| RNA T7 Polymerase | this paper | N/A |
| T4 Polynucleotide Kinase (T4 PNK) | Thermo Fisher Scientific | Cat#EK0031 |
| Antarctic phosphatase | New England BioLabs | Cat#M0289S |
| RiboLock RNase Inhibitor | Thermo Fisher Scientific | Cat#EO0384 |
| ProteinaseK | AppliChem | Cat#A3830 |
| ProteinaseK | Thermo Fisher Scientific | Cat#AM2546 |
| Blasticidin | GIBCO | Cat#A1113903 |
| Zeocin | Invitrogen | Cat#R25001 |
| Hygromycin B | Invitrogen | Cat#10687010 |
| Tetracycline | AppliChem | Cat#A2228.0025 |
| Penicillin/ Streptomycin | Sigma | Cat#P0781 |
| AEBSF | Sigma Aldrich | Cat#A8456 |
| Digitonin | Merck | Cat#300410 |
| Glutamate | Sigma Aldrich | Cat#G1626 |
| Malate | Sigma Aldrich | Cat#M1000 |
| ADP | Calbiochem | Cat#11705 |
| Cytochrome c | Sigma Aldrich | Cat#C7752 |
| Succinate | Sigma Aldrich | Cat#S2378 |
| Oligomycin | Sigma Aldrich | Cat#O4876 |
| FCCP | Sigma Aldrich | Cat#C2920 |
| Rotenone | Sigma Aldrich | Cat#8875 |
| Antimycin | Sigma Aldrich | Cat#A6874 |
| Aprotinin | Roche | N/A |
| Emetine dihydrochloride | Calbiochem | Cat#324693 |
| Incomplete Freund”s adjuvant (IFA) | Helmholtz Center Munich | N/A |
| Lipofectamin 2000 | Invitrogen | Cat#11668500 |
| Fibronectin Solution (Bovine) | Sigma Aldrich | Cat#F1141 |
| Collagen solution from calf skin | Sigma Aldrich | Cat#C8918 |
| Ham”s F12 Nutrient Mixture | Thermo Fisher Scientific | Cat#11765054 |
| Poly-D lysine | Sigma Aldrich | Cat#P6407 |
| Crystal Violet | Sigma Aldrich | Cat#HT90132 |
| MitoTracker Deep Red FM | Invitrogen | Cat#M22426 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat#H1399 |
| Coomassie Brilliant Blue R250 | BioRad | Cat#1610436 |
| γ,γ-Dimethylallyl pyrophosphate (DMAPP) | Sigma Aldrich | Cat#38426 |
| EDC (1-ethyl-3-(3-dimethylaminopropyl) - carbodiimide hydrochloride) | Sigma Aldrich | Cat#E7750; CAS: 25952–53-8 |
| 1-Methylimidazole | Sigma-Aldrich | Cat#M50834; CAS: 616–47-7 |
| CPG oligonucleotide | Tib Molbiol, Berlin | N/A |
| γ−32P-ATP | Hartmann Analytic | Cat#FP-501 |
| [3H] S-Adenosyl-L-Methionine | Hartmann Analytic | Cat#ART2008 |
| [35S]-methionine | Perkin Elmer | Cat# NEG009A005MC |
| Scintillations Cocktail | Zinsser Analytic | N/A |
| illustra™ MicroSpin G-25 column | GE Healthcare | Cat#27532501 |
| Glutathione Sepharose™ 4 FastFlow | GE Healthcare | Cat#17–5132-02 |
| Superdex™ 200 10/30 GL | GE Healthcare | Cat#17–5175-01 |
| Protein G Sepharose slurry | GE Healthcare | Cat#17061801 |
| ANTI-FLAG M2 Affinity Gel | Sigma Aldrich | Cat#A2220 |
| Phenol/Chloroform/Isoamylalcohol | Roth | Cat#A156.1 |
| TRIzol | Invitrogen | Cat#15596018 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Phusion High-Fidelity DNA Polymerase | New England BioLabs | Cat#M0530L |
| NucleoSpin Gel and PCR Clean-up kit | Macherey-Nagel | Cat#740609.50 |
| NucleoSpin Mini Plasmid, Mini kit | Macherey-Nagel | Cat#740588.50 |
| NucleoBond Xtra Midi EF, Midi kit | Macherey-Nagel | Cat#740422.50 |
| MiSeq reagent Kit V3 (150 cycles) | Illumina | Cat#MS-102–3001 |
| SuperScript III First-Strand Synthesis System | Invitrogen | Cat#18080400 |
| T4 DNA Ligase kit | Thermo Fisher Scientific | Cat#EL0011 |
| First-strand cDNA synthesis kit | Thermo Fisher Scientific | Cat#K1612 |
| Takyon No ROX SYBR 2x MasterMix blue dTTP | Eurogentec | Cat#NA.55 |
| T4 Polynucleotide Kinase (T4 PNK) kit – Buffer B | Thermo Fisher Scientific | Cat#EK0031 |
| Next Small RNA kit | New England BioLabs | Cat#E7330S/L |
| SERVAGel Native Gel Starter Kit | Serva | Cat#43204.1 |
|
| ||
| Deposited data | ||
|
| ||
| Alk-Aniline-Seq | this paper | ENA: PRJEB45091 |
| mitoRibosome Profiling | this paper | NCBI GEO:GSE180400 |
| original Western/Northern blot images and microscopy data | this paper | Mendeley Data: https://data.mendeley.com/datasets/47kvzxmgkg/draft?a=360446dc-9a9d-46e1-a5a6–1dfcc6d90234 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| Human: Flp-InTREx −293 | ATCC | Cat# CRL-1573, RRID: CVCL_0045 |
| Human: Flp-InTREx 293 METTL8 −/− clone C12_05 | this paper | N/A |
| Human: Flp-InTREx 293 METTL8 −/− clone C07_05 | this paper | N/A |
| Human: Flp-InTREx 293 METTL8-F/H | this paper | N/A |
| Human: Flp-InTREx −293 METTL8-F/H D230A | this paper | N/A |
| Human: Flp-InTREx −293 F7H-METTL2 | this paper | N/A |
| Human: PANC-1 | ATCC | Cat# 300228/p635_Panc-1, RRID: CVCL_0480 |
| Human: PANC-1 METTL8 −/− clone C13_13 | this paper | N/A |
| Human: PANC-1 METTL8 −/− clone C13–24 | this paper | N/A |
| Human: CAPAN-1 | ATCC | Cat# 300143/p663_Capan-1, RRID: CVCL_0237 |
| Human: A-549 | ATCC | Cat# A549, RRID: CVCL_0023 |
| Human: HAP1 | Horizon Discovery | RRID: CVCL_Y019 |
| Human: HAP1 METTL8 −/− | Horizon Discovery | RRID: CVCL_SY13 |
| Human: HeLa | ATCC | Cat# CCL-2, RRID: CVCL_0030 |
| Human: HeLa S3 | ATCC | Cat# 300384/p699_HeLa_S3, RRID: CVCL_0058 |
| P3X63Ag8.653 myeloma cells | ATCC | ATCC Cat# CRL-8008, RRID: CVCL_4032 |
|
| ||
| Oligonucleotides | ||
|
| ||
| DNA oligonucleotide for strand-specific cDNA-synthesis, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| DNA oligonucleotide for mt-mRNA RIP by qPCR, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| Northern blot probe sequences, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| DNA oligonucleotide sequences for cloning and mutagenesis, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| DNA oligonucleotide sequences for mt-tRNA in vitro transcription, see Table S1. Table of used oligonucleotides, related to STAR Methods |
Metabion | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| pcDNA5/FRT/TO modified with N-terminal F/H-tag | Invitrogen | Cat#V652020 |
| pOG44 | Invitrogen | Cat#V600520 |
| pcDNA5/FRT/TO METTL8-F/H | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H D230A | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H I4Q | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H I4/9Q | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H I4/9 L12Q | this paper | N/A |
| pcDNA5/FRT/TO F/H-METTL2 | this paper | N/A |
| pX459 sgRNA METTL8 | this paper | N/A |
| pcDNA5/FRT/TO METTL8 FLAG.STREP2-tag |
this paper | N/A |
| pIRES-VP5 modified | (Meister et al., 2004) | N/A |
| pIRES-VP5 METTL8-EGFP | this paper | N/A |
| pIRES-VP5 METTL8-EGFP I4Q | this paper | N/A |
| pIRES-VP5 METTL8-EGFP I4/6Q | this paper | N/A |
| pIRES-VP5 METTL8-EGFP I4/9 L12Q | this paper | N/A |
| pIRES-VP5 F/H-METTL2 | this paper | N/A |
| pIRES-VP5 F/H-METTL8-F/H | this paper | N/A |
| pETM14 TRIT1-GST | this paper | N/A |
| pETM14 METTL8-GST | this paper | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| Quantity One Software | BioRad | Quantity One 1-D Analysis Software, RRID: SCR_014280 |
| Data Analysis 4.2 | Bruker Daltonics | N/A |
| Protein Scape 3.1.3 | Bruker Daltonics | N/A |
| Mascot 2.5.1 | Matrix Science | RRID: SCR_014322 |
| Protein Scape | Bruker Daltonics | N/A |
| Uniprot | UniProtKB, RRID: SCR_004426 | |
| PyMOL | PyMOL, RRID: SCR_000305 | |
| Odyssey | LI-CORE | Odyssey CLx, RRID: SCR_014579 |
| Zen10 Microscope Software | Zeiss | N/A |
| DatLab | Oroboros Instruments | Product ID: 20700 |
| RCSB Protein Data Bank (PDB) | RCSB PDB), RRID: SCR_012820 | |
| MitoFates | http://mitf.cbrc.jp/MitoFates/cgi-bin/top.cgi | N/A |
| Clustal Omega | https://www.ebi.ac.uk/Tools/msa/clustalo/ | N/A |
| PrimerX | http://www.bioinformatics.org/primerx/ | N/A |
| NCBI database | NCBI, RRID: SCR_006472 | |
| pLOGO | pLogo, RRID: SCR_018185 | |
|
| ||
| Other | ||
|
| ||
| LSM 710, AxioObserver microscope with a C-Apochromat 63x/1.20 W Korr M27 objective | Zeiss | N/A |
| Zeiss Axiovert200M microscope | Zeiss | N/A |
| Multimode-Microplate reader Mithras LB 940 | Berthold Technologies | N/A |
| CFX96real-Time System BioRad | BioRad | N/A |
| Personal Molecular Imager System | BioRad | N/A |
| Multipurpose Scintillation Counter | Beckmann Coulter LS6500 | N/A |
| Potter S Homogenizer | B. Braun Biotech International | N/A |
| UltiMate 3000 RSLCnano System | Thermo Fisher Scientific | N/A |
| C18 Acclaim Pepmap100 preconcentration column | Thermo Fisher Scientific | N/A |
| Acclaim Pepmap100 C18 nano column | Thermo Fisher Scientific | N/A |
| maXis plus UHR-QTOF System | Bruker Daltonics | N/A |
| CaptiveSpray nanoflow electrospray source | Bruker Daltonics | N/A |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Generation of inducible Flp-In T-REx –293 cell stable expression cell lines
Inducible Flp-In T-REx 293 cell lines stably expressing F/H-METTL2B// METTL8-F/H constructs were generated by co-transfection of pcDNA5/FRT/TO harboring F/H-METTL2B //METTL8-F/H constructs and pOG44 plasmid. Transfection was carried out by Lipofectamin 2000 (Invitrogen) reagent according to the manufacture’s protocol. Cells grown in 24-wells were transfected at 80%–90% confluence using 100 ng pcDNA5/FRT/TO and 900 ng pOG44 plasmid. To select transfectants, cells were cultured in medium supplemented with 15 μg/ml blasticidin and 150 μg/ml hygromycin B.
Generation of METTL8 knockout cell lines
METTL8 knockout (KO) was generated in Flp-In T-REx293 and PANC-1 cell lines by CRISPR/Cas9-directed genome editing. The site-directed frameshift in METTL8 was guided by the METTL8 complementary sgRNA (5’-CACCGAGAGAAGCTAGTAAATACT-’3) embedded in a pX459 backbone. Cells were transfected twice by Lipofectamin 2000 (Invitrogen) with an intermediary puromycin selection round of 12–16 hours, which was skipped for PANC-1 cells. After transfection and recovering step, cells were separated into single clones. Flp-In T-REx 293 METTL8 KO clones were analyzed by sequencing strategy and validated by western blotting. For sequencing, genomic DNA of clonal cell lines was extracted by 0.2 mg/ml ProteinaseK (AppliChem) in 500 μl ProteinaseK buffer (200 mM Tris/HCl pH 7.5, 300 mM NaCl, 25 mM EDTA, 2% [w/v] SDS) at 50°C overnight. DNA was precipitated by addition of 400 μl 2-propanol and centrifugation at 20 000 g and 4°C for at least 30 minutes. Pellet was washed twice with 70% EtOH [v/v], dried 10 minutes at 50°C and dissolved in 50 ml water. 150 bp region comprising sgRNA annealing site was amplified in a 50 μl PCR reaction (1x Phusion HF Buffer, 200 μM dNTPs, 2× 0.5 μM specific primer, 3%[v/v] DMSO, 3 μl purified DNA, 1 U Phusion High-Fidelity DNA Polymerase) (New England BioLabs). DNA region was amplified by thermocycling and amplicons were purified by a 2% [w/v] agarose gel, followed by a NucleoSpin Gel and PCR Clean-up Kit (Macherey-Nagel). In a second PCR round, purified amplicons were attached to barcodes of the Illumina TrueSeq-System and sequenced by a MiSeq-sequencing platform. Genotypes of the utilized METTL8 KO cell lines are given below.
| METTL8 KO clone | Allele | Genotype |
|---|---|---|
| C12_05 | 1 | Insertion 1 nt |
| 2 | Insertion 1 nt | |
| C07_05 | 1 | Deletion 5 nt |
| 2 | Deletion 1 nt |
PANC-1 METTL8 KO clones were tested directly by western blotting.
General cell culture conditions
Cell lines and adherent HeLa S3 were grown in Dulbecco”s modified Eagle”s medium (DMEM; Sigma Aldrich) supplemented with 10% fetal bovine serum (FBS; GIBCO) under standard conditions at 37°C in 5% CO2 atmosphere. Medium for Flp-In T-REx 293 cells was completed with 15 μg/ml blasticidin (GIBCO) and 100 μg/ml zeocin (Invitrogen), medium for Flp-In T-REx 293 cell stably expressing METTL2B or METTL8 constructs with 15 μg/ml blasticidin (GIBCO) and 150 μg/ml hygromycin B (Invitrogen). HeLa and HAP1 cell lines were treated with antibiotics penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma). HeLa S3 suspension cells were expanded in spinner flasks and Joklik’s medium (Sigma Aldrich) supplemented with 10% fetal bovine serum (FBS; GIBCO) at 37°C.
| Name | Species | Tissue | Sex | Disease | Identifier |
|---|---|---|---|---|---|
| Flp-In T-REx 293 | human | embryonic kidney | Fetus | ATCC CRL-1573 | |
| PANC-1 | human | pancreas/duct | Male | epithelioid carcinoma | ATCC CRL-1649 |
| CAPAN-1 | human | pancreas/derived from metastatic site: liver | Male | adenocarcinoma | ATCC HTB-79 |
| A549 | human | lung | male | carcinoma | ATCC CCL-185 |
| HAP1 | human | male | chronic myelogenous leukemia | HZGHC005033c009 | |
| HeLa | human | cervix | female | adenocarcinoma | ATCC CCL-2 |
| HeLa S3 | human | cervix | female | adenocarcinoma | ATCC CCL-2.2 |
METHOD DETAILS
Immunofluorescence
HeLa cells for immunofluorescence analysis were grown on coverslips and transiently transfected with METTL8.FLAG.STREP2 using Lipofectamin 2000 (Invitrogen) reagent. After 24 h HeLa cells were fixed with 4% formaldehyde solution and permeabilized with 1% Triton X-100.
Flp-In T-REx 293 cells stably expressing METTL8-F/H were grown on coverslips without blasticidin and hygromycinB. Expression was induced by 1 μg/ml tetracycline 24 h before fixation with ice cold acetone at −20°C for 7 minutes (Alshammari et al., 2016).
Glass slides were incubated in blocking solution (1x TBS, 6% [w/v] BSA, 0.1% [v/v] Tween-20) at RT for 1 h, followed by overnight incubation at 4°C with primary antibody (1x PBS, 3% [w/v] BSA, 0.1% [v/v] Tween-20). In the next step glass slides incubated with secondary antibody (1x PBS, 3% [w/v] BSA, 0.1% [v/v] Tween-20) at RT for 1 h. Between fixation and antibody treatments five 1x PBS washing steps of 10 minutes were performed. Terminally coverslips were rinsed with water and mounted by using ProLong Gold Anti-fade Mountant with DAPI (Life Technologies). Images were recorded on a Zeiss Axiovert200M microscope and analyzed using Fiji. Used antibodies were listed below.
| Primary Antibody | Company | Dilution | Source | Secondary Antibody (Invitrogen) |
|---|---|---|---|---|
| α-FLAGM2 | Sigma Aldrich (F3165) | 1:100–200 | mouse, mAb | Alexa Fluor 488 goat-α-mouse IgG (H+L) |
| α-TOM20 | Santa Cruz Biotechnologies (FL-145) | 1:100–200 | rabbit, pAb | Alexa Fluor 555 goat-α-rabbit IgG (H+L) |
| α-METTL8 | Helmholtz Center Munich (MEL8 16A7; 19A10) | 1:50–100 | rat, mAb | Alexa Fluor 488 goat-α-rat IgG (H+L) |
Live cell Imaging
To analyze the subcellular localization of METTL8-EGFP constructs containing MTS mutations, live cell imaging was performed. Flp-In T-REx 293 cells were grown in 15 cm dishes and normal DMEM medium on coated coverslips (97% [v/v] Ham’s F12 Nutrient Mixture, 1% [w/v] Fibronectin Solution (Bovine),1% [w/v] Collagen solution from calf skin, 0.05% [w/v] BSA, 1% [v/v] Penicillin-Streptomycin). Cells at 60%–70% confluence were transfected by calcium phosphate (2x HEPES-buffered saline, 2.5 M CaCl2, 10 μg DNA) and grown for additional 48 h under standard conditions. Ahead visualization, cells were washed trice with warm 1x PBS, stained with 1 μg/ml Hoechst 33342 and 133 nM MitoTracker Deep Red FM (Invitrogen) in normal growth medium for 20 minutes under standard conditions. Cells were rinsed twice in warm 1x PBS and analyzed on a LSM 710, AxioObserver microscope with a C-Apochromat 63x/1.20 W Korr M27 objective. For detection following filter were used: 450–517 nm (Hoechst 33342), 493–549 nm (EGFP), 638–755 nm (Red FM). Images were analyzed by the software Zen 2011 (Carl Zeiss).
High-resolution respirometry by Oxygraph-2k
Oxygen consumptions rate was recorded by Oxygraph-2k (O2k; Oroboros Instruments) at 37°C. Before analysis start, O2k was cleaned and equilibrated with air saturated MIR05 growth medium (20 mM HEPES, 10 mM KH2PO4, 20 mM Taurine, 60 mM Lacto-bionic acid, 3 mM MgCl2, 0.5 mM EGTA, 0.1% [w/v] BSA) under stirring at 700 rpm. Cells at 70%–80% confluence were washed with warm PBS and detached by Trypsin, which was inactivated after desired incubation time by adding DMEM supplemented with 10% FBS. Cells were centrifuged at 400 g and RT for 10 minutes, resuspended in MIR05 growth medium and adjusted to a concentration of 0.5× 106 cell/ml. For high resolution respirometry analysis 2 mL cell suspension was used and applied to the adapted Substrate-Uncoupler-Inhibitor-Titration Protocol - 001 O2 ce-pce D004 (SUIT; SUIT-001 O2 ce-pce D004). After monitoring routine respiration, plasma membrane was permeabilized by an optimal Digitonin (Merck) concentration depending on cell line.
| Cell line | Digitonin [nM] |
|---|---|
| Flp-In T-REx 293 METTL8 WT | 8.1 |
| Flp-In T-REx 293 METTL8 KO | 8.1 |
| Flp-In T-REx 293 METTL8 WTOE | 8.1 |
| Flp-In T-REx 293 METTL8 D230A | 8.1 |
| PANC-1 METTL8 WT | 6.1 |
| PANC-1 METTL8 KO | 4.1 |
LEAK respiration with NADH-linked substrates Glutamate (10 mM; Sigma Aldrich) and Malate (2 mM; Sigma Aldrich) was detected first before stimulating OXPHOS capacity with saturating ADP (5 mM; Calbiochem). Cytochrome c (10 μM; Sigma Aldrich) evaluated the integrity of the mitochondrial outer membrane and supported the validity of the respiratory activity. By addition of succinate (10 mM; Sigma Aldrich) the TCA cycle function was reconstituted. After monitoring LEAK respiration in present of ADP by oligomycin (2 mM; Sigma Aldrich), the electron transfer (ET) capacity was stimulated by titration of the uncoupler FCCP (Sigma Aldrich). Rotenone (2 mM; Sigma Aldrich) inhibited complex I and set apart the succinate pathway control state. Terminally complex III was blocked by antimycin (1 mM; Sigma Aldrich) to detect the residual oxygen consumption due to oxidative side reactions.
Proliferation assay
For proliferation assay 1× 103 Flp-In T-REx 293 cells or 2.5 × 103 PANC-1 cells were seeded without any antibiotics in 96-well plates in DMEM without pyruvate and monitored five days under standard conditions. 96-well plates used for Flp-In T-REx 293 cells were coated with 50 μg/ml Poly-D lysine (Sigma Aldrich) in PBS before. METTL8-F/H expression in Flp-In T-REx –293 cells was induced by 1 μg/ml tetracycline (AppliChem). After desired incubation time, cells were fixed with 50 μl crystal violet solution (0.5% [w/v] crystal violet in 20% [v/v] MeOH) at RT for 10 minutes. Fixed cells were washed twice with 100 μl warm water followed by two further PBS washing steps of two minutes. Lastly cells were rinsed with water and air-dried. Cells were quantified by crystal violet intensity detected at 590 nm using a Multimode-Microplate reader Mithras LB 940 (Berthold Technologies). Crystal violet was eluted from the cells before by 50 μl 0.1 M sodium citrate in 50% [v/v] EtOH.
Plasmids
Open reading frame (ORF) of METTL2B (GenBank: NM_018396.2), METTL8 (GenBank: NM_024770.4) and TRIT1 (GenBank: NM_017646.5) were amplified from Flp-In T-REx 293 cDNA (SuperScript III First-Strand Synthesis System - Invitrogen) using target specific primer with appropriate restriction sites (Table S1) in a 50 μl PCR reaction (1x Phusion HF Buffer, 200 μM dNTPs, 2× 0.5 μM specific primer, 3% [v/v] DMSO, 0.5 μl cDNA, 1 U Phusion High-Fidelity DNA Polymerase) (New England BioLabs). METTL2B amplicon was cloned into a modified pIRES-VP5 backbone encoding a N-terminal FLAG/HA tag using the restrictions enzymes NheI/BglII. METTL8 cDNA was either cloned into a pcDNA5/FRT/TO plasmid encoding FLAG.STREP2-tag (Minczuk et al., 2011) or into a modified pIRES-VP5 backbone using the restriction enzymes NheI/EcoRI. METTL8 cDNA used for pIRES-VP5 cloning was linked to FLAG/HA tag C-terminally by PCR amplification before cloning into the modified pIRES-VP5 plasmid encoding a N-terminal FLAG/HA tag. Mutations in METTL8 sequence were introduced by site-directed mutagenesis. DpnI digestion at 37°C for at least 3 h extracted plasmids with incorporated mutation/s. METTL8-EGFP constructs were set up in two consecutive steps. EGFP amplicon, amplified from a random EGFP vector, was imbedded into a modified pIRES-VP5 backbone by using the restriction enzymes NheI/EcoRI. Thereupon METTL8 DNA, amplified from the corresponding VP5 plasmid, were inserted N-terminally by FseI/AscI. For generating tetracycline inducible Flp-In T-REx-293 expression cell lines, METTL8-F/H was inserted into a modified pCDNA5/FRT/TO vector by AscI/FseI. Recombinant proteins fused to a GST tag at their C terminus were expressed from a modified pETM14 backbone without a N-terminal 6x His-tag and 3C-site. METTL8 was combined to GST C-terminally by using XbaI/NheI. TRIT1-GST fusion protein was generated by NcoI/NheI. For CRISPR/Cas9-directed genome editing, chimeric guideRNA (sgRNA) complementary to the nearer METTL8 N terminus was embedded into the backbone vector pX459. DNA oligonucleotides were phosphorylated in a 10 μl PNK reaction (2× 100 μM DNA oligonucleotide,1x T4 DNA Ligation Buffer, T4 PNK) (Thermo Fisher Scientific) at 37°C for 30 minutes, boiled up to 95°C and annealed during cooling down to 25°C. BbsI digested pX459 backbone and annealed DNA oligonucleotides were ligated at room temperature (RT) for 1 h.
All plasmids and their target sequence were verified by sanger sequencing.
cDNA Synthesis and RNA analysis by Quantitative Real Time RT-PCR
Total volume of RNA isolated from METTL8 immunoprecipitations were reversed transcripted by First-strand cDNA synthesis kit (Thermo Fisher Scientific) using random and mt-tRNASer(UCN) specific primer. Fluorometric amplification was performed by Takyon No ROX SYBR 2x MasterMix blue dTTP (Eurogentec) with mt-mRNA and mt-tRNASer(UCN) specific primer and CFX96real-Time System (BioRad). Primer sequences are listed in Table S1.
RNA Immunoprecipitation
METTL8-RNA interaction was analyzed by RNA-immunoprecipitation (RIP). Verification of METTL8-RNA interaction was done by either qPCR or Northern Blotting. Flp-In T-REx 293 METTL8 WT and KO cells were expanded in normal growth medium (DMEM supplemented with 10% FBS), Flp-In T-REx 293 cells stably expressing METTL8-F/H were grown overnight in normal growth medium supplemented with 1 μg/ml tetracycline. Cells of 5× 15 cm dishes were harvested and lysed in 1 mL NP-40 lysis buffer (20 mM Tris/HCl pH 8, 137 mM NaCl, 10% glycerol, 1% [v/v] NP-40, 2 mM EDTA, 1 mM AEBSF and 1 mM DTT) on ice for 30 minutes. For Northern Blot analysis, living cells were irritated by 280 nm UV light before (120 mJ/cm2)(Strategene). Cell lysates were cleared at 20 000 g and 4°C for 30 minutes. Afterward, samples were adjusted to the same concentration and 10% of the lysate was used as input sample. For immunoprecipitation 50 μl Protein G Sepharose slurry (GE Healthcare) were used and equilibrated in lysis buffer (20 mM Tris/HCl pH 8, 137 mM NaCl, 10% glycerol, 1% [v/v] NP-40, 2 mM EDTA, 1 mM AEBSF and 1 mM DTT). Beads were incubated with 1 mL protein lysate and 5 μg mAb α-METTL8 at 4°C overnight with rotation. Antibody coupling to protein G was carried out in parallel. FLAG/HA tagged proteins were immunoprecipitated by ANTI-FLAG M2 Affinity Gel (Sigma Aldrich). Next day beads were washed trice with washing buffer (50 mM Tris/HCl pH 8, 300 – 1000 mM NaCl, 0.1% [v/v] NP-40, 1.5 mM MgCl2, 1 mM AEBSF,1 mM DTT). After transferring beads into a new reaction tube, beads were washed twice again and terminally cleansed in PBS. To isolate the METTL8 bound RNA, proteins were digested first by 6 U/ml ProteinaseK (Thermo Fisher Scientific) in 200 μl ProteinaseK buffer (200 mM Tris/HCl pH 7.5, 300 mM NaCl, 25 mM EDTA, 2% [w/v] SDS) at 50°C for 30 minutes while mild shaking. Subsequently RNA was extracted by Roti Phenol/Chloroform/Isoamylalcohol (Roth) and ethanol precipitation. Total RNA of input samples was purified by TRIzol (Invitrogen).
Northern Blotting including 32P –oligonucleotide labeling
0.5 μg total RNA and RNA derived from METTL8 IP were separated on a 6% urea-PAGE running at 400 V in 1x TBE buffer. After electrophoresis, RNA quality was checked by EtBr staining first and transferred onto a water equilibrated Hybond -N membrane (GE Healthcare) at 20 V for 45 minutes by semi dry blotting (SD. Semi-Dry Transfer Cell, Bio-RAD). EDC (1-ethyl-3-(3-dimethylaminopropyl) - carbodiimide hydrochloride) (160 mM EDC, 130 mM 1-methylimidazol, adjusted to pH 8 by HCl) crosslinked RNA to the membrane at 50°C in 1 h. Crosslink was completed with UV light at 254 nm (120 mJ/cm2) (Stratagene). Membrane was rinsed with water and air-dried. Meanwhile 20 pmol of DNA oligonucleotide (Table S1) were 5’ end labeled by using 0.5 U/ml Polynucleotide Kinase (PNK)(Thermo Fisher Scientific) and 20 mCi γ−32P-ATP (Hartmann Analytic). PNK reaction was carried out in PNK Buffer A (Thermo Fisher Scientific) 30 minutes at 37°C and stopped by addition of 18 mM EDTA. [32P] radiolabelled DNA was purified by illustra MicroSpin G-25 column (GE Healthcare) and mixed with hybridization solution (20 mM sodium phosphate buffer pH 7.2, 5x SSC, 1% [w/v] SDS, 2% [v/v] Denhardt”s solution). Hybridization between RNA and radioactive labeled DNA was carrying out overnight at 50°C. After 12–15 h membrane was washed twice with washing solution I (5x SSC, 1% [w/v] SDS) and once with washing solution II (1x SSC, 1% [w/v] SDS) at 50°C for 10 minutes. Radioactive signals were detected by storage phosphor screens and Personal Molecular Imager System (BioRad) with Quantity One Software (version 4.6.9, Bio-Rad). Membrane was interrogated several times with different probes by consecutive stripping and re-probing. For stripping, membrane was washed twice in hot 0.1% [w/v] SDS solution for 10 minutes with agitation and cleaned with hot water for another 10 minutes.
Aminoacylation assay
RNA for aminoacylation assay was extracted by TRIzol (Invitrogen). To keep charged tRNAs, all steps were performed on ice and RNA was treated under acid conditions. Aminoacylated RNA was dissolved in 10 mM NaOAc/ HOAc (pH 4.8) at 37°C for 5 minutes. Deacylated RNA was resuspended in 0.2 M Tris/HCl pH 9.5 and dissolved 1 h at 37°C. 10 μg of either charged or uncharged RNA was mixed with acid denaturing sample buffer (90% [v/v] formamide, 0.1 M NaOAc/ HOAc (pH 4.8), 0.05% [w/v] bromophenol blue and 0.05% [w/v] xylene cyanol) and separated on a 6% urea-PAGE containing 0.1 M NaOAc/ OHAc (pH 4.8). Electrophoresis was running at 100 V and 4°C in 0.1 M NaOAc/ OHAc (pH 4.8) overnight. Separated RNA was transferred onto a water equilibrated Hybond -N membrane (GE Healthcare) at 20 V for 30 minutes by semi dry blotting (SD. Semi-Dry Transfer Cell, Bio-RAD). RNA crosslinking and visualization were performed according to Northern blotting.
Primer extension analysis of m3C32
Total RNA from WT and METTL8 KO cells was isolated using TRIzol (ThermoFisher) according to the manufacturer”s instructions. The subsequent primer extension assay was performed essentially as previously (Rorbach et al., 2014), with the exception that dTTP was excluded from the reaction in order to cause primer stalling downstream of C32. The following primers were used:
| Primer | |
|---|---|
| m3C32_mt-tRNASer(UCN) | 5′ aatcgaaccccccaaagctggtttc 3′ |
| m3C32_mt-tRNAThr | 5’ aaaaaggttttcatctccggtttac’3 |
Alkanilineseq
Analysis of m3C residues in human cytoplasmic and mitochondrial tRNAs was performed as described previously (Marchand et al., 2018). In brief, total RNA sample was subjected to partial alkaline hydrolysis in bicarbonate buffer (96°C, pH 9.2), the reaction was stopped by ethanol precipitation. RNA fragments were extensively de-phosphorylated by Antarctic phosphatase and subjected to aniline cleavage (1M pH 4.5, 15 min in the dark). Resulting fragments having a 5′-phosphate at N+1 nucleotide were converted to a sequencing library using NEBNext Small RNA kit. Sequencing was performed in single-read 50 nt (SR50) mode on HiSeq1000 (Illumina). Sequencing reads were trimmed to remove adaptor sequence and aligned to human tRNA reference sequence (both cytoplasmic and mitochondrial tRNAs). Normalized cleavage is calculated as a 1000x ratio of reads starting at a given position to total number of sequencing reads mapped to a given RNA. Please notice that the calibration curve for modification rate to Normalized cleavage is non-linear and shows very high sensitivity for very low modification rates.
In vitro transcription of mt-tRNAs
tRNAs were transcribed by T7 RNA polymerase. 100 μM 5’ and 3’ DNA oligonucleotides linked to T7 promotor sequence (Table S1) (Metabion) were boiled up at 95°C for 30 s in 1x T4 DNA Ligation Buffer (Thermo Fisher Scientific) and annealed by cooling down to 25°C. Annealed DNA oligonucleotides were transcribed in a 1 mL transcription reaction (30 mM Tris pH 8.0, 10 mM DTT, 0.01% [v/v] Triton X-100, 25 mM MgCl2, 2 mM spermidine, 30% [v/v] DMSO, 5 mM ADP, 5 mM CTP, 5 mM UTP, 5 mM GTP, 0.4 U/ml thermostable inorganic pyrophosphatase (NEB) and 0.1 mg/ml T7-polymerase) overnight at 37°C. After 12–14 h transcription reaction was mixed with 2x RNA denaturing sample buffer and purified by a 6% urea-PAGE. RNA was visualized by UV shadowing, cut out and extracted by RNA elution buffer (300 mM NaCl, 2 mM EDTA) overnight with agitation at 4°C. By adding 0.7 volume 2-propanol RNA precipitated at −20°C overnight. RNA was pelleted at 20 000 g and 4°C for at least 30 minutes, washed twice with 80% [v/v] ethanol, dried at 37°C for 5 minutes and dissolved in water at 65°C for 5 minutes.
In vitro m3C design on mt-tRNASer(UCN) (Isopentenylation// Methylation Assay)
m3C incorporation on mt-tRNASer(UCN) catalyzed by METTL8 depends on an isopentenylation reaction at A37 (i6A37). N6-isopenteny-ladenosine was introduce in 1 μM mt-tRNA by 250 nM TRIT1 and 10 μM γ,γ-Dimethylallyl pyrophosphate (DMAPP)(Sigma Aldrich). Reaction was carried out in isopentenylation buffer (50 mM Tris/HCl pH 7.5, 5 mM MgCl2, 100 μM β-mercaptoethanol, 0.2 U/ml RiboLock RNase Inhibitor (Thermo Fisher Scientific)) at 37°C for 1 h (Lamichhane et al., 2013). Modified RNA was isolated by TRIzol extraction (Invitrogen) according to the manufacture’s protocol. RNA pellet was resuspended in 10 μl water and used for the consecutive methylation assay. m3C incorporation was performed by 250 nM METTL8 in methylation buffer (10 mM Tris/HCl pH 7.5, 100 mM KCl, 1.8 mM MgCl2, 5% [v/v] Glycerol, 0.1 mg/ml BSA, 1 mM DTT, 0.2 U/mL RiboLock RNase Inhibitor (Thermo Fisher Scientific), 10 nCi/μl SAM[3H] (Hartmann Analytic)) at 37°C for 1 h. m3C modified tRNA was purified by TRIzol extraction (Invitrogen) according to the manufacture’s protocol and resuspended in 25 μl water (65°C for 5 minutes). RNA was mixed with 10 mL scintillations cocktail (Zinsser Analytic) and measured by a multipurpose scintillation counter (Beckmann Coulter LS6500). Activity was counted per minute (c.p.m.).
Generation of monoclonal antibodies
A peptide comprising amino acids 149VPDEKNHYEKSSG 161 from human METTL8 protein was synthesized and coupled to ovalbumin (Peps4LS, Heidelberg, Germany). Lou/c rats were immunized subcutaneously and intraperitoneally with a mixture of 50 μg OVA-peptide, 5 nmol CPG oligonucleotide (Tib Molbiol, Berlin), 500 μl PBS and 500 μl Incomplete Freund”s adjuvant (IFA). Eight week later, a boost injection without IFA was given three days before fusion of rat spleen cells with P3X63Ag8.653 myeloma cells using standard procedures. Hybridoma supernatants were screened by ELISA for binding to biotinylated METTL8 peptide on avidin-coated ELISA plates. Positive supernatants were further validated in immunoprecipitations and western blot analysis. Hybridoma cells from selected supernatants were subcloned twice by limiting dilution to obtain stable monoclonal cell lines. Experiments in this work were performed with supernatants from monoclonal antibody clones MEL8 19A10 and 16A7(both rat IgG2c/k).
Cell lysis – immunoprecipitation – western blotting
Cells for immunoprecipitation (IP) were generally broken up by 1 mL NP-40 lysis buffer (20 mM Tris/HCl pH 8, 137 mM NaCl, 10% glycerol, 1% [v/v] NP-40, 2 mM EDTA, 1 mM AEBSF and 1 mM DTT). PANC-1, CAPAN-1 and A459 cells were disrupted by glass beads additionally. Cells were lysed 30 minutes on ice and centrifuged at 20 000 g at 4°C for 30 minutes.
Lysates were adjusted to the same protein concentration by Bradford assay. For immunoprecipitation 50 μl Protein G Sepharose slurry (GE Healthcare) was used and equilibrated in NP-40 lysis buffer. Protein lysates adjusted to the same protein concentration by Bradford were incubated with equilibrated Protein G Sepharose and 5 μg mAb α-METTL8 at 4°C overnight with agitation. 10% of the protein lysate was used as input control. F/H tagged proteins were immunoprecipitated by ANTI-FLAG M2 Affinity Gel (Sigma Aldrich). In the next step beads were washed trice with washing buffer (50 mM Tris/HCl pH 8, 300 mM NaCl, 0.1% [v/v] NP-40, 1.5 mM MgCl2, 1 mM AEBSF,1 mM DTT). After the third washing step beads were transferred into a new reaction tube and washed twice again. Immunoprecipitated proteins were eluted by 5x SDS loading buffer. Input samples were mixed with 1x SDS loading buffer. Samples were boiled at 95°C for 5 minutes and separated by a 10% SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane (0.45 μm, GE Healthcare) by semi dry blotting (SD. Semi-Dry Transfer Cell, Bio-RAD). Nitrocellulose membrane was blocked in 5% [w/v] milk at RT for 15–60 minutes and subsequently incubated with primary antibody overnight at 4°C with agitation. Secondary antibody was applied afterward for 1 h at RT. Between and after antibody application membrane was washed 3x with 1x TBST for 10 minutes with agitation. Used antibodies were listed below. Signals were detected by Odyssey Infrared Imaging System (LI-COR Biosciences). Gels for LC-MS/MS analysis were stained by Coomassie Blue (0.25% [w/v] Coomassie Brilliant Blue R250 (#1610400, Bio-Rad),10% [v/v] acetic acid, 30% [v/v] ethanol) and cleansed afterward with destainer (10% [v/v] acetic acid, 30% [v/v] ethanol).
| Primary Antibody | Company | Dilution 5% [w/v] | Source | Incubation | Secondary Antibody (LI-COR) |
|---|---|---|---|---|---|
| α-HA.11 | Covance (clone 16B12) | 1:1000 / milk | mouse, mAb | o/n // 4°C | IRDye 800CW Goat α-mouse IgG |
| α- β Actin | Abcam (ab6276) | 1:10000 / milk | mouse, mAb | o/n // 4°C | IRDye 800CW Goat α-mouse IgG |
| α-METTL8 | Helmholtz Center Munich (MEL8 16A7; 19A10) | 1:500 / milk | rat, mAb | o/n // 4°C | IRDye 800CW Goat α-rat IgG |
| α-TIMM44 | ProteinTech | 1:3000 / milk | rabbit, pAb | o/n // 4°C | IRDye 800CW Goat α-rabbit IgG |
| α-GAPDH | GeneTEx (GT239) | 1:1000 / milk | mouse, mAb | o/n // 4°C | IRDye 800CW Goat α-mouse IgG |
| α-p54[nrb] | BD Transduction Laboratories | 1:1000 / milk | mouse, mAb | o/n // 4°C | IRDye 800CW Goat α-mouse IgG |
Protein expression and purification
METTL8 (aa. 22–407) and TRIT1 (aa. 23–467) were expressed as GST-fusion protein from pETM-14 vector in E.coli Rosetta without mitochondrial targeting sequence (MTS). Bacteria were grown to an OD600 of 0.5 and cool down at 4°C until OD600 of 0.6. Protein expression was subsequently induced by 100 μM IPTG. TRIT1-GST was expressed 6 hours at 37°C, METTL8-GST overnight at 23°C. Bacteria pellet was resuspended in lysis buffer (20 mM Tris/HCl pH 8, 1 M NaCl, 0.2 mM EDTA, 1 mM DTT) and sonicated 3× 5 minutes (duty cycle 50%, output control 5) with a consecutive break of 5 minutes on ice. Lysate was cleared by centrifugation at 50 000 g and 4°C for 45 minutes. Before performing GST-pulldown by using a 6 mL GST-column (GE Healthcare), supernatant was passed through a filter with a diameter of 45 μm (Roth). To remove proteins bound unspecifically, column was washed industriously with lysis buffer and protein of interest was finally eluted with 50 mM Tris/HCl pH 8 and 10 mM Glutathion. The eluate was concentrated to a volume of 0.5–1 mL by Vivaspin 20 ultrafiltration device (MWCO 30 000, Sartorius) and loaded on a Superdex 200 10/30 GL (GE Healthcare) equilibrated with 25 mM Tris/HCl pH 8 and 150 mM NaCl. Peak fractions were analyzed by a 10% SDS-PAGE, pooled, adjusted to 50% [v/v] glycerol and stored at −80°C.
Mitochondrial preparation
Flp-In T-REx-293 cells from a confluent 15 cm dish were resuspended in 1 mL trehalose buffer (10 mM HEPES/KO pH 6.9, 10 mM KCl, 300 mM trehalose, 0.1% [w/v] BSA, 0.2% [w/v] Digitonin) and gently disrupted by using a Potter S Homogenizer (B. Braun Biotech International – sartorius group). Up down movement of the PTFE plungers at 700 rpm ruptured the cell membrane, which was subsequently pelletized at 800 g for 7 minutes at 4°C. In this centrifugation step nuclei were sediment as well. To separate mitochondria from the cytosol, supernatant was centrifuged at 10 000 g and 4°C for 10 minutes. Mitochondria were washed trice with trehalose buffer and pelletized at 10 000 g and 4°C for 5 minutes. To reduce contaminants, mitochondria were resuspended in 1 mL trehalose buffer containing 3 μU/ml ProteinaseK (Thermo Fisher Scientific) and incubated 20 minutes on ice. The reaction was stopped by 2 μg/ ml Aprotinin (Roche), 1 mM AEBSF and centrifugation (10 000 g at 4°C, 5 minutes). After two washing steps with trehalose buffer, mitochondria were used in BN experiments.
Blue native gel electrophoresis (BN)
120 μg mitochondria were resuspended in 100 μl non-denaturing buffer (20 mM Tris/HCl pH 8, 1 mM sucrose, 1%, [w/v] Digitonin) and solubilized 30 minutes on ice. Insoluble proteins were pelletized at 20 000 g and 4°C for 30 minutes. Native protein migration was performed by using SERVAGel Native Gel Starter Kit. Solubilized mitochondrial proteins were mixed with 2x Sample buffer for BN and separated on a SERVAGel N 3–12 (vertical native gel 3%–12%). Electrophoresis was running 120 minutes at 50–200 V in anode and blue cathode buffer. Blue cathode buffer was exchanged after 2/3 of the electrophoresis. For LC-MS/MS analysis, proteins were fixed in 20% [w/v] trichloroacetic acid at RT for 30 minutes and stained with Coomassie blue (0.1% [w/v] Coomassie Brilliant Blue R250 (# 1610400, Bio-Rad), 10% [v/v] acetic acid, 45% [v/v] ethanol). After 30 minutes, gel was cleansed 2× 60 minutes with destainer (20% [v/v] ethanol, 5% [v/v] acetic acid, 1% [w/v] glycerol) and prepared for LC-MS/MS analysis.
Protein analysis by mass spectrometry
For mass spectrometric analysis of proteins gel lanes were cut into consecutive slices. The gel slices were then transferred into 2ml micro tubes (Eppendorf) and washed with 50 mM NH4HCO3, 50mM NH4HCO3/acetonitrile (3/1) and 50mM NH4HCO3/acetonitrile (1/1) while shaking gently in an orbital shaker (VXR basic Vibrax, IKA). Gel pieces were lyophilized after shrinking by 100% acetonitrile. To block cysteines, reduction with DTT was carried out for 30 min at 57°C followed by an alkylation step with iodoacetamide for 30 min at room temperature in the dark. Subsequently, gel slices were washed and lyophilized again as described above. Proteins were subjected to in gel tryptic digest overnight at 37°C with approximately 2 μg trypsin per 100 μl gel volume (Trypsin Gold, mass spectrometry grade, Promega). Peptides were eluted twice with 100 mM NH4HCO3 followed by an additional extraction with 50 mM NH4HCO3 in 50% acetonitrile. Prior to LC-MS/MS analysis, combined eluates were lyophilized and reconstituted in 20 μl of 1% formic acid. Separation of peptides by reversed-phase chromatography was carried out on an UltiMate 3000 RSLCnano System (Thermo Scientific, Dreieich) which was equipped with a C18 Acclaim Pepmap100 preconcentration column (100μm i.D.x20mm, Thermo Fisher) in front of an Acclaim Pepmap100 C18 nano column (75 μm i.d. × 150 mm, Thermo Fisher). A linear gradient of 4% to 40% acetonitrile in 0.1% formic acid over 90 min was used to separate peptides at a flow rate of 300 nl/min. The LC-system was coupled on-line to a maXis plus UHR-QTOF System (Bruker Daltonics, Bremen) via a CaptiveSpray nanoflow electrospray source (Bruker Daltonics). Data-dependent acquisition of MS/MS spectra by CID fragmentation was performed at a resolution of minimum 60000 for MS and MS/MS scans, respectively. The MS spectra rate of the precursor scan was 2 Hz processing a mass range between m/z 175 and m/z 2000. Via the Compass 1.7 acquisition and processing software (Bruker Daltonics) a dynamic method with a fixed cycle time of 3 s and a m/z dependent collision energy adjustment between 34 and 55 eV was applied. Raw data processing was performed in Data Analysis 4.2 (Bruker Daltonics), and Protein Scape 3.1.3 (Bruker Daltonics) in connection with Mascot 2.5.1 (Matrix Science) facilitated database searching of the Swiss-Prot Homo sapiens database (release-2020_01, 220420 entries). Search parameters were as follows: enzyme specificity trypsin with 1 missed cleavage allowed, precursor tolerance 0.02 Da, MS/MS tolerance 0.04 Da, carbamidomethylation or propionamide modification of cysteine, oxidation of methionine, deamidation of asparagine and glutamine were set as variable modifications. Mascot peptide ion-score cut-off was set 15. If necessary, fragment spectra were validated manually. Protein list compilation was done using the Protein Extractor function of Protein Scape.
Metabolite analysis by gas chromatography – mass spectrometry
Intermediates of glycolysis and TCA cycle were analyzed by gas chromatography coupled to mass spectrometry (GC-MS). To harvest the cells for analysis, the cell culture supernatant was removed and cells were washed three times with phosphate-buffered saline (PBS) and then scrapped with cold 80% methanol. The suspension was collected, the cell culture dish was washed with 80% methanol and the combined sample was stored at −80°C.
For further sample preparation, the sample suspension was thawed and 10 μL of an aqueous internal standard mix containing stable isotope labeled analogs of the analytes was added. The sample was vortexed, and centrifuged (9560 g, 5min, 4°C). The supernatant was collected and the pellet was resuspended twice in 200 μL 80% methanol with in-between supernatant collection. The last wash was centrifuged at a higher speed (13800 g). All supernatants were combined and dried in a vacuum evaporator (CombiDancer, Hettich AG, Bach, Switzerland). Sample residues were dissolved in 100 μL pure water and an aliquot was transferred into a flat bottom insert in a 1.5-mL glass vial. The aliquot was evaporated to dryness and then subjected to methoximation and silylation for GC-MS analysis using the derivatization protocol and instrumental setup previously described (Dettmer et al., 2011). A Rxi-5ms column (30 m, 0.25μm ID, 0.25mm film thickness, Restek, Bad Homburg Germany) with a 2-m guard column was used. The temperature program started at 50°C (0.5 min), followed by a ramp of 5°C/min to 120°C, then 8°C/min to 300°C, and the final temperature was held for 5 min. The carrier gas was helium with a flow rate of 0.7 mL/min. The mass spectrometer was operated in full scan mode with a scan range of 50 to 550 m/z. An injection volume of 1 μL with splitless injection at 280°C was used.
For analysis of cell culture supernatants, 10 μL of the supernatant and 10 μL of the aqueous internal standard mix were transferred into a flat bottom insert in a 1.5-mL glass vial, dried and subjected to derivatization and GC-MS analysis as described.
Quantification was performed using calibration curves based on the area ratio of the endogenous compound to the stable isotope labeled standard (Mass Hunter Quantitative Analysis, version B.07.01/build 7.1.524.0, Agilent Technologies). Data were normalized to total protein. Protein amount was determined using a fluorescence assay as recently described (Berger et al., 2021).
35S-methionine metabolic labeling of mitochondrial proteins
In order to label newly synthesized mitochondrially expressed proteins, the previously published protocol was used (Pearce et al., 2017). Briefly, cells at approximately 80% confluency were incubated in methionine/cysteine-free medium for 10 min before the medium was replaced with methionine/cysteine-free medium containing 10% dialyzed FCS and emetine dihydrochloride (100 μg/ml) to inhibit cytosolic translation. Following a 20 min incubation, 120 μCi/ml of [35S]-methionine (Perkin Elmer) was added and the cells were incubated for 30 min. After washing with PBS, cells were lysed, and 30 μg of protein was loaded on 10%–20% Tris- glycine SDS-PAGE gels. Dried gels were visualized with a PhosphorImager system.
Ribosome profiling of mitochondria
Cells were grown in T-75 flasks to 70% confluence in DMEM media and METTL8 overexpression was induced with 2 μg/mL of doxycycline 24 hours prior to collection. Cells were washed once with ice cold PBS, flash frozen on liquid nitrogen and stored at −80°C until lysis. 667 μL of 1.5x Lysis buffer (30 mM Tris–HCl pH 7.8, 150 mM KCl, 15 mM MgCl2, 1.5 mM DTT, 1.5% Triton X-100, 0.15% NP40, 1 3 complete phosphatase and protease inhibitors) was added to each flask and cells were collected with a cell scraper while flasks thawed on an ice slurry. ~1 mL of lysate was transferred to a chilled 1.5 mL tube, gently passed through a 27-gauge needle 10 times, and spun at 5,000G for 10 minutes at 4°C. Digestion was carried out by adding 1,500 U of MNase, 10 μL of SUPErase●In, and CaCl2 to a final concentration of 5mM to 400 μL of lysate and incubating at 22°C for 1 hour. Digestion was stopped by adding EGTA to a final concentration of 6 mM. Lysate was loaded onto a 5%–30% sucrose gradient (20mM Tris–HCl pH 7.8, 100 mM KCl, 10 mM MgCl2, and 1 mM DTT and spun for 2.5 hr at 40,000 rpm in an SW40 rotor. The 55S mitoribosome fraction was collected using a BioComp Fractionator. Footprints were isolated through SDS/hot phenol/chloroform extraction.
Following each step, footprints or PCR products were cleaned by either Zymo”s Oligo Clean and Concentrator or DNA Clean and Concentrator, respectively. Small RNAs were size selected using the Purelink miRNA isolation kit. 3′ ends were dephosphorylated with NEB Quick CIP and 3′ adaptor ligation was carried out with Rnl2(1–249)K227Q ligase over night at 4°C. 3′ adaptor: 5′-rAppNNT-GACTGTGGAATTCTCGGGTGCCAAGG-L, where the underlined sequence is the library barcode. Barcoded footprint libraries were then combined and phosphorylated by PNK. Footprints were then ligated to a 5′ DNA-RNA adaptor with RNA Ligase. 5′(aminolinker) GTTCAGAGTTCTACAGTCCGACGATCrNrNrNrN. Reverse transcription was carried out with SuperScript IV. RT Primer GCCTTGGCACCCGAGAATTCCA. Libraries were initially amplified by PCR using the RT primer and a short 5′ primer, CTTCA-GAGTTCTACAGTCCGACGA. Footprints were size selected by a Pippen Prep, 3% C gel. Sizes of 74–94 were selected, corresponding to footprint sizes of 15–35. Libraries were then amplified with long indexing primers and sequenced.
Ribosome profiling analysis
Deduplicated reads were mapped to a chrM transcriptome based on the hg38 genome. P site positioning was determined by Plastid, measuring from the start codons of ND4 and ATP6. Changes in relative ribosomal occupancy was measured by first determining the proportion of ribosome density at each codon relative to the total density in the encompassing gene. Then fold change is then calculated dividing the relative ribosomal density at each position in the experimental dataset by the WT control.
pLOGO analysis
pLOGO (O”Shea et al., 2013) backgrounds were the E, P, and A site sequences of all codons that had ribosomal density in both the experimental and control datasets. Foregrounds were defined as the E, P, and A sites that had a fold change greater than 2.
QUANTIFICATION AND STATISTICAL ANALYSIS
Please refer to the Figure Legends or the Experimental model and subject details for description of sample size and statistical analyses.
Supplementary Material
Highlights.
METTL8 catalyzes m3C32 modification in mt-tRNASer(UCN) and mt-tRNAThr
Respiratory activity and cell proliferation correlate with METTL8 levels
High METTL8 levels correlate with patient survival in pancreatic cancer
METTL8 depletion triggers ribosome stalling on specific mt-tRNASer(UCN) codons
ACKNOWLEDGMENTS
We thank S. Ammon, C. Friederich and the mass spectrometry group for excellent support and Joe Carroll for providing the HAP1 METTL8 knockout cells. This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grants SPP 1784/1 and 2, the Bavarian Systems-Biology Network (Bio-SysNet), Grand Est Region (France) FRCR grant EpiARN, and the European Research Council (ERC grant 682291, “moreRNA”). M.M., C.A.P., and C.D.M. were supported by the Medical Research Council core funding (MC_UU_00015/4).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.molcel.2021.10.018.
REFERENCES
- Alexandrov A, Martzen MR, and Phizicky EM (2002). Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA 8, 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari MA, Alshammari TK, and Laezza F. (2016). Improved methods for fluorescence microscopy detection of macromolecules at the axon initial segment. Front. Cell. Neurosci. 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. (1981). Sequence and organization of the human mitochondrial genome. Nature 290, 457–465. [DOI] [PubMed] [Google Scholar]
- Anderson S, de Bruijn MH, Coulson AR, Eperon IC, Sanger F, and Young IG (1982). Complete sequence of bovine mitochondrial DNA: Conserved features of the mammalian mitochondrial genome. J. Mol. Biol. 156, 683–717. [DOI] [PubMed] [Google Scholar]
- Arimbasseri AG, Iben J, Wei FY, Rijal K, Tomizawa K, Hafner M, and Maraia RJ (2016). Evolving specificity of tRNA 3-methyl-cytidine-32 (m3C32) modification: A subset of tRNAsSer requires N6-isopentenylation of A37. RNA 22, 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balacco DL, and Soller M. (2019). The m6A writer: Rise of a machine for growing tasks. Biochemistry 58, 363–378. [DOI] [PubMed] [Google Scholar]
- Barros MH, and McStay GP (2020). Modular biogenesis of mitochondrial respiratory complexes. Mitochondrion 50, 94–114. [DOI] [PubMed] [Google Scholar]
- Begik O, Lucas MC, Liu H, Ramirez JM, Mattick JS, and Novoa EM (2020). Integrative analyses of the RNA modification machinery reveal tissue-and cancer-specific signatures. Genome Biol. 21, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RS, Wachsmuth CJ, Waldhier MC, Renner-Sattler K, Thomas S, Chaturvedi A, Niller HH, Bumes E, Hau P, Proescholdt M, et al. (2021). Lactonization of the oncometabolite D-2-hydroxyglutarate produces a novel endogenous metabolite. Cancers (Basel) 13, 1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack MT, and Sloan KE (2018a). The mitochondrial epitranscriptome: the roles of RNA modifications in mitochondrial translation and human disease. Cell. Mol. Life Sci. 75, 241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack MT, and Sloan KE (2018b). Modifications in small nuclear RNAs and their roles in spliceosome assembly and function. Biol. Chem. 399, 1265–1276. [DOI] [PubMed] [Google Scholar]
- Cabello-Villegas J, Winkler ME, and Nikonowicz EP (2002). Solution conformations of unmodified and A(37)N(6)-dimethylallyl modified anticodon stem-loops of Escherichia coli tRNA(Phe). J. Mol. Biol. 319, 1015–1034. [DOI] [PubMed] [Google Scholar]
- Chen H, Gu L, Orellana EA, Wang Y, Guo J, Liu Q, Wang L, Shen Z, Wu H, Gregory RI, et al. (2020). METTL4 is an snRNA m(6)Am methyltransferase that regulates RNA splicing. Cell Res. 30, 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo T, and Tomizawa K. (2021). Human transfer RNA modopathies: diseases caused by aberrations in transfer RNA modifications. FEBS J. Published online ahead of print January 29, 2021. 10.1111/febs.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay S, and Frye M. (2019). RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 21, 552–559. [DOI] [PubMed] [Google Scholar]
- Desrosiers R, Friderici K, and Rottman F. (1974). Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl. Acad. Sci. USA 71, 3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer K, Nürnberger N, Kaspar H, Gruber MA, Almstetter MF, and Oefner PJ (2011). Metabolite extraction from adherently growing mammalian cells for metabolomics studies: optimization of harvesting and extraction protocols. Anal. Bioanal. Chem. 399, 1127–1139. [DOI] [PubMed] [Google Scholar]
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. [DOI] [PubMed] [Google Scholar]
- Doxtader KA, Wang P, Scarborough AM, Seo D, Conrad NK, and Nam Y. (2018). Structural basis for regulation of METTL16, an S-adenosylme-thionine homeostasis factor. Mol. Cell 71, 1001–1011.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganichkin OM, Anedchenko EA, and Wahl MC (2011). Crystal structure analysis reveals functional flexibility in the selenocysteine-specific tRNA from mouse. PLoS ONE 6, e20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YT, Koh CWQ, Sim DY, Roca X, and Goh WSS (2020). METTL4 catalyzes m6Am methylation in U2 snRNA to regulate pre-mRNA splicing. Nucleic Acids Res. 48, 9250–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, and Phizicky EM (2018). A rationale for tRNA modification circuits in the anticodon loop. RNA 24, 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Wu T, Cui X, Zhu P, Tan C, Dou X, Hsu KW, Lin YT, Peng PH, Zhang LS, et al. (2020). N6-deoxyadenosine methylation in mammalian mitochondrial DNA. Mol. Cell 78, 382–395.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova VV, Jansen PWTC, Baltissen MP, Vermeulen M, and Schneider R. (2019). The interactome of a family of potential methyltransferases in HeLa cells. Sci. Rep. 9, 6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova VV, Kaiser S, Ho JSY, Bing X, Stolz P, Tan YX, Lee CL, Gay FPH, Lastres PR, Gerlini R, et al. (2020a). METTL6 is a tRNA m3C methyltransferase that regulates pluripotency and tumor cell growth. Sci. Adv. 6, eaaz4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova VV, Stolz P, Kaiser S, Gustafsson TH, Lastres PR, SanzMoreno A, Cho YL, Amarie OV, Aguilar-Pimentel A, Klein-Rodewald T, et al. (2020b). The rRNA m6A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 34, 715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazuhito T, and Wei FY (2020). Posttranscriptional modifications in mitochondrial tRNA and its implication in mitochondrial translation and disease. J. Biochem. 168, 435–444. [DOI] [PubMed] [Google Scholar]
- Ke S, Pandya-Jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Darnell JE Jr., and Darnell RB (2017). m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 31, 990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuckles P, Carl SH, Musheev M, Niehrs C, Wenger A, and Bühler M. (2017). RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat. Struct. Mol. Biol. 24, 561–569. [DOI] [PubMed] [Google Scholar]
- Kweon SM, Chen Y, Moon E, Kvederaviciutė K, Klimasauskas S, and Feldman DE (2019). An Adversarial DNA N6-methyladenine-sensor network preserves polycomb silencing. Mol. Cell 74, 1138–1147.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane TN, Mattijssen S, and Maraia RJ (2013). Human cells have a limited set of tRNA anticodon loop substrates of the tRNA isopentenyltransferase TRIT1 tumor suppressor. Mol. Cell. Biol. 33, 4900–4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Choe J, Park OH, and Kim YK (2020). Molecular mechanisms driving mRNA degradation by m6A modification. Trends Genet. 36, 177–188. [DOI] [PubMed] [Google Scholar]
- Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, and Gregory RI (2018). Mettl1/Wdr4-mediated m7G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell 71, 244–255.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. (2014). A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand V, Ayadi L, Ernst FGM, Hertler J, Bourguignon-Igel V, Galvanin A, Kotter A, Helm M, Lafontaine DLJ, and Motorin Y. (2018). AlkAniline-seq: Profiling of m7 G and m3 C RNA modifications at single nucleotide resolution. Angew. Chem. Int. Ed. Engl. 57, 16785–16790. [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, and Tuschl T. (2004). Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15, 185–197. [DOI] [PubMed] [Google Scholar]
- Mendel M, Chen KM, Homolka D, Gos P, Pandey RR, McCarthy AA, and Pillai RS (2018). Methylation of structured RNA by the m6A writer METTL16 is essential for mouse embryonic development. Mol. Cell 71, 986–1000.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, and Jaffrey SR (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3° UTRs and near stop codons. Cell 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minczuk M, He J, Duch AM, Ettema TJ, Chlebowski A, Dzionek K, Nijtmans LG, Huynen MA, and Holt IJ (2011). TEFM (c17orf42) is necessary for transcription of human mtDNA. Nucleic Acids Res. 39, 4284–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FV 4th, Ramakrishnan V, Malkiewicz A, and Agris PF (2004). The role of modifications in codon discrimination by tRNA(Lys)UUU. Nat. Struct. Mol. Biol. 11, 1186–1191. [DOI] [PubMed] [Google Scholar]
- O”Shea JP, Chou MF, Quader SA, Ryan JK, Church GM, and Schwartz D. (2013). pLogo: a probabilistic approach to visualizing sequence motifs. Nat. Methods 10, 1211–1212. [DOI] [PubMed] [Google Scholar]
- Pearce SF, Rorbach J, Van Haute L, D”Souza AR, Rebelo-Guiomar P, Powell CA, Brierley I, Firth AE, and Minczuk M. (2017). Maturation of selected human mitochondrial tRNAs requires deadenylation. eLife 6, e27596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, and Conrad NK (2017). The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169, 824–835.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorbach J, Bobrowicz A, Pearce S, and Minczuk M. (2014). Polyadenylation in bacteria and organelles. Methods Mol. Biol. 1125, 211–227. [DOI] [PubMed] [Google Scholar]
- Schiffers S, Ebert C, Rahimoff R, Kosmatchev O, Steinbacher J, Bohne AV, Spada F, Michalakis S, Nickelsen J, Müller M, and Carell T. (2017). [DOI] [PubMed] [Google Scholar]
- Quantitative LC-MS provides no evidence for m6 dA or m4 dC in the genome of mouse embryonic stem cells and tissues. Angew. Chem. Int. Ed. Engl. 56, 11268–11271. [DOI] [PubMed] [Google Scholar]
- Schöller E, Weichmann F, Treiber T, Ringle S, Treiber N, Flatley A, Feederle R, Bruckmann A, and Meister G. (2018). Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA 24, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Śledź P, and Jinek M. (2016). Structural insights into the molecular mecha-nism of the m(6)A writer complex. eLife 5, e18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodin B, Han R, Calderone V, Vrielink JAFO, Loayza-Puch F, Elkon R, and Agami R. (2017). Transcription impacts the efficiency of mRNA translation via co-transcriptional N6-adenosine methylation. Cell 169, 326–337.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. (2021). The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol. 22, 375–392. [DOI] [PubMed] [Google Scholar]
- Suzuki T, and Suzuki T. (2014). A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 42, 7346–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nagao A, and Suzuki T. (2011). Human mitochondrial tRNAs: Biogenesis, function, structural aspects, and diseases. Annu. Rev. Genet. 45, 299–329. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yashiro Y, Kikuchi I, Ishigami Y, Saito H, Matsuzawa I, Okada S, Mito M, Iwasaki S, Ma D, et al. (2020). Complete chemical structures of human mitochondrial tRNAs. Nat. Commun. 11, 4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni T, Agriesti F, Ruggieri V, Mazzoccoli C, Simeon V, Laurenzana I, Scrima R, Pazienza V, Capitanio N, and Piccoli C. (2017). Rewiring carbohydrate catabolism differentially affects survival of pancreatic cancer cell lines with diverse metabolic profiles. Oncotarget 8, 41265–41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute L, Hendrick AG, D”Souza AR, Powell CA, Rebelo-Guiomar P, Harbour ME, Ding S, Fearnley IM, Andrews B, and Minczuk M. (2019). METTL15 introduces N4-methylcytidine into human mitochondrial 12S rRNA and is required for mitoribosome biogenesis. Nucleic Acids Res. 47, 10267–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tran N, Ernst FGM, Hawley BR, Zorbas C, Ulryck N, Hackert P, Bohnsack KE, Bohnsack MT, Jaffrey SR, Graille M, and Lafontaine DLJ (2019). The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 47, 7719–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney U, Lee CP, and RajBhandary UL (1991). Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J. Biol. Chem. 266, 24712–24718. [PubMed] [Google Scholar]
- Wang P, Doxtader KA, and Nam Y. (2016a). Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 63, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al. (2016b). Structural basis of N6-adenosine methylation by the METTL3-METTL14 complex. Nature 534, 575–578. [DOI] [PubMed] [Google Scholar]
- Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Hoöbartner C, Sloan KE, and Bohnsack MT (2017). Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 18, 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weixlbaumer A, Murphy FV 4th, Dziergowska A, Malkiewicz A, Vendeix FA, Agris PF, and Ramakrishnan V. (2007). Mechanism for expanding the decoding capacity of transfer RNAs by modification of uridines. Nat. Struct. Mol. Biol. 14, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Liu X, Sheng N, Oo KS, Liang J, Chionh YH, Xu J, Ye F, Gao YG, Dedon PC, and Fu XY (2017). Three distinct 3-methylcytidine (m3C) methyltransferases modify tRNA and mRNA in mice and humans. J. Biol. Chem. 292, 14695–14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LS, Liu C, Ma H, Dai Q, Sun HL, Luo G, Zhang Z, Zhang L, Hu L, Dong X, and He C. (2019). Transcriptome-wide mapping of internal N7-methylguanosine methylome in mammalian mRNA. Mol. Cell 74, 1304–1316.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Zhang XY, Hu T, Chen XY, Li JJ, Raida M, Sun N, Luo Y, and Gao X. (2020). The SUMOylated METTL8 induces R-loop and tumor-igenesis via m3C. iScience 23, 100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Ż, Pan JN, He C, Parisien M, and Pan T. (2019). Regulation of co-transcriptional pre-mRNA splicing by m6A through the low-complexity protein hnRNPG. Mol. Cell 76, 70–81.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MitoRibosome profiling and AlkAnilineSeq data have been deposited at GEO and ENA and are publicly available as of the date of publication. Accession numbers are listed in the Key resource table. Original western blot images and microscopy data have been deposited at Mendeley and are publicly available as of the date of publication.
All original code is publicly available as of the date of publication and shared by James Marks (MitoRibosome Profiling) and Yuri Motorin (AlkAnilineSeq) upon request.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-HA.11 antibody (clone 16B2) | Covance | Cat# MMS-101P, RRID: AB_2314672 |
| Mouse monoclonal anti-beta actin antibody (ab6276) | Abcam | Cat# ab6276, RRID: AB_2223210 |
| Mouse monoclonal anti-GAPDH (GT239) | GeneTEx | Cat# GTX627408, RRID: AB_11174761 |
| Mouse monoclonal anti-p54[nrb] | BD Transduction Laboratories | Cat# 611278, RRID: AB_398806 |
| Mouse monoclonal anti-FLAGM2 (F3165) | Sigma | Cat# F3165, RRID: AB_259529 |
| Rabbit polyclonal anti-TOM20 (FL-145) | Santa Cruz Biotechnologies | Cat# sc-11415, RRID: AB_2207533 |
| Goat polyclonal anti-mouse IgG, IRDye 800CW conjugated antibody |
LI-COR Bioscience | Cat#925–32210; RRID: AB_2687825 |
| Goat polyclonal anti-rabbit IgG, IRDye 800CW conjugated antibody |
LI-COR Bioscience | Cat#926–32211; RRID: AB_621843 |
| Rat monoclonal anti-METTL8 antibody (clone 16A7) | this paper | N/A |
| Rat monoclonal anti-METTL8 antibody (clone 19A10) | this paper | N/A |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| Escherichia coli Rosetta (DE3) competent cells | Novagen | Cat#70954–3 |
| XL1 blue competent cells | this paper | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| METTL8 (22–407 aa.) – GST (human) | this paper | N/A |
| TRIT1 (22–467 aa.) – GST (human) | this paper | N/A |
| OVA-METTL8 peptide | Peps4LS, Heidelberg | N/A |
| Thermostable inorganic pyrophosphatase | New England BioLabs | Cat#M0296 |
| RNA T7 Polymerase | this paper | N/A |
| T4 Polynucleotide Kinase (T4 PNK) | Thermo Fisher Scientific | Cat#EK0031 |
| Antarctic phosphatase | New England BioLabs | Cat#M0289S |
| RiboLock RNase Inhibitor | Thermo Fisher Scientific | Cat#EO0384 |
| ProteinaseK | AppliChem | Cat#A3830 |
| ProteinaseK | Thermo Fisher Scientific | Cat#AM2546 |
| Blasticidin | GIBCO | Cat#A1113903 |
| Zeocin | Invitrogen | Cat#R25001 |
| Hygromycin B | Invitrogen | Cat#10687010 |
| Tetracycline | AppliChem | Cat#A2228.0025 |
| Penicillin/ Streptomycin | Sigma | Cat#P0781 |
| AEBSF | Sigma Aldrich | Cat#A8456 |
| Digitonin | Merck | Cat#300410 |
| Glutamate | Sigma Aldrich | Cat#G1626 |
| Malate | Sigma Aldrich | Cat#M1000 |
| ADP | Calbiochem | Cat#11705 |
| Cytochrome c | Sigma Aldrich | Cat#C7752 |
| Succinate | Sigma Aldrich | Cat#S2378 |
| Oligomycin | Sigma Aldrich | Cat#O4876 |
| FCCP | Sigma Aldrich | Cat#C2920 |
| Rotenone | Sigma Aldrich | Cat#8875 |
| Antimycin | Sigma Aldrich | Cat#A6874 |
| Aprotinin | Roche | N/A |
| Emetine dihydrochloride | Calbiochem | Cat#324693 |
| Incomplete Freund”s adjuvant (IFA) | Helmholtz Center Munich | N/A |
| Lipofectamin 2000 | Invitrogen | Cat#11668500 |
| Fibronectin Solution (Bovine) | Sigma Aldrich | Cat#F1141 |
| Collagen solution from calf skin | Sigma Aldrich | Cat#C8918 |
| Ham”s F12 Nutrient Mixture | Thermo Fisher Scientific | Cat#11765054 |
| Poly-D lysine | Sigma Aldrich | Cat#P6407 |
| Crystal Violet | Sigma Aldrich | Cat#HT90132 |
| MitoTracker Deep Red FM | Invitrogen | Cat#M22426 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat#H1399 |
| Coomassie Brilliant Blue R250 | BioRad | Cat#1610436 |
| γ,γ-Dimethylallyl pyrophosphate (DMAPP) | Sigma Aldrich | Cat#38426 |
| EDC (1-ethyl-3-(3-dimethylaminopropyl) - carbodiimide hydrochloride) | Sigma Aldrich | Cat#E7750; CAS: 25952–53-8 |
| 1-Methylimidazole | Sigma-Aldrich | Cat#M50834; CAS: 616–47-7 |
| CPG oligonucleotide | Tib Molbiol, Berlin | N/A |
| γ−32P-ATP | Hartmann Analytic | Cat#FP-501 |
| [3H] S-Adenosyl-L-Methionine | Hartmann Analytic | Cat#ART2008 |
| [35S]-methionine | Perkin Elmer | Cat# NEG009A005MC |
| Scintillations Cocktail | Zinsser Analytic | N/A |
| illustra™ MicroSpin G-25 column | GE Healthcare | Cat#27532501 |
| Glutathione Sepharose™ 4 FastFlow | GE Healthcare | Cat#17–5132-02 |
| Superdex™ 200 10/30 GL | GE Healthcare | Cat#17–5175-01 |
| Protein G Sepharose slurry | GE Healthcare | Cat#17061801 |
| ANTI-FLAG M2 Affinity Gel | Sigma Aldrich | Cat#A2220 |
| Phenol/Chloroform/Isoamylalcohol | Roth | Cat#A156.1 |
| TRIzol | Invitrogen | Cat#15596018 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Phusion High-Fidelity DNA Polymerase | New England BioLabs | Cat#M0530L |
| NucleoSpin Gel and PCR Clean-up kit | Macherey-Nagel | Cat#740609.50 |
| NucleoSpin Mini Plasmid, Mini kit | Macherey-Nagel | Cat#740588.50 |
| NucleoBond Xtra Midi EF, Midi kit | Macherey-Nagel | Cat#740422.50 |
| MiSeq reagent Kit V3 (150 cycles) | Illumina | Cat#MS-102–3001 |
| SuperScript III First-Strand Synthesis System | Invitrogen | Cat#18080400 |
| T4 DNA Ligase kit | Thermo Fisher Scientific | Cat#EL0011 |
| First-strand cDNA synthesis kit | Thermo Fisher Scientific | Cat#K1612 |
| Takyon No ROX SYBR 2x MasterMix blue dTTP | Eurogentec | Cat#NA.55 |
| T4 Polynucleotide Kinase (T4 PNK) kit – Buffer B | Thermo Fisher Scientific | Cat#EK0031 |
| Next Small RNA kit | New England BioLabs | Cat#E7330S/L |
| SERVAGel Native Gel Starter Kit | Serva | Cat#43204.1 |
|
| ||
| Deposited data | ||
|
| ||
| Alk-Aniline-Seq | this paper | ENA: PRJEB45091 |
| mitoRibosome Profiling | this paper | NCBI GEO:GSE180400 |
| original Western/Northern blot images and microscopy data | this paper | Mendeley Data: https://data.mendeley.com/datasets/47kvzxmgkg/draft?a=360446dc-9a9d-46e1-a5a6–1dfcc6d90234 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| Human: Flp-InTREx −293 | ATCC | Cat# CRL-1573, RRID: CVCL_0045 |
| Human: Flp-InTREx 293 METTL8 −/− clone C12_05 | this paper | N/A |
| Human: Flp-InTREx 293 METTL8 −/− clone C07_05 | this paper | N/A |
| Human: Flp-InTREx 293 METTL8-F/H | this paper | N/A |
| Human: Flp-InTREx −293 METTL8-F/H D230A | this paper | N/A |
| Human: Flp-InTREx −293 F7H-METTL2 | this paper | N/A |
| Human: PANC-1 | ATCC | Cat# 300228/p635_Panc-1, RRID: CVCL_0480 |
| Human: PANC-1 METTL8 −/− clone C13_13 | this paper | N/A |
| Human: PANC-1 METTL8 −/− clone C13–24 | this paper | N/A |
| Human: CAPAN-1 | ATCC | Cat# 300143/p663_Capan-1, RRID: CVCL_0237 |
| Human: A-549 | ATCC | Cat# A549, RRID: CVCL_0023 |
| Human: HAP1 | Horizon Discovery | RRID: CVCL_Y019 |
| Human: HAP1 METTL8 −/− | Horizon Discovery | RRID: CVCL_SY13 |
| Human: HeLa | ATCC | Cat# CCL-2, RRID: CVCL_0030 |
| Human: HeLa S3 | ATCC | Cat# 300384/p699_HeLa_S3, RRID: CVCL_0058 |
| P3X63Ag8.653 myeloma cells | ATCC | ATCC Cat# CRL-8008, RRID: CVCL_4032 |
|
| ||
| Oligonucleotides | ||
|
| ||
| DNA oligonucleotide for strand-specific cDNA-synthesis, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| DNA oligonucleotide for mt-mRNA RIP by qPCR, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| Northern blot probe sequences, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| DNA oligonucleotide sequences for cloning and mutagenesis, see Table S1. Table of used oligonucleotides, related to STAR Methods | Metabion | N/A |
| DNA oligonucleotide sequences for mt-tRNA in vitro transcription, see Table S1. Table of used oligonucleotides, related to STAR Methods |
Metabion | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| pcDNA5/FRT/TO modified with N-terminal F/H-tag | Invitrogen | Cat#V652020 |
| pOG44 | Invitrogen | Cat#V600520 |
| pcDNA5/FRT/TO METTL8-F/H | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H D230A | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H I4Q | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H I4/9Q | this paper | N/A |
| pcDNA5/FRT/TO METTL8-F/H I4/9 L12Q | this paper | N/A |
| pcDNA5/FRT/TO F/H-METTL2 | this paper | N/A |
| pX459 sgRNA METTL8 | this paper | N/A |
| pcDNA5/FRT/TO METTL8 FLAG.STREP2-tag |
this paper | N/A |
| pIRES-VP5 modified | (Meister et al., 2004) | N/A |
| pIRES-VP5 METTL8-EGFP | this paper | N/A |
| pIRES-VP5 METTL8-EGFP I4Q | this paper | N/A |
| pIRES-VP5 METTL8-EGFP I4/6Q | this paper | N/A |
| pIRES-VP5 METTL8-EGFP I4/9 L12Q | this paper | N/A |
| pIRES-VP5 F/H-METTL2 | this paper | N/A |
| pIRES-VP5 F/H-METTL8-F/H | this paper | N/A |
| pETM14 TRIT1-GST | this paper | N/A |
| pETM14 METTL8-GST | this paper | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| Quantity One Software | BioRad | Quantity One 1-D Analysis Software, RRID: SCR_014280 |
| Data Analysis 4.2 | Bruker Daltonics | N/A |
| Protein Scape 3.1.3 | Bruker Daltonics | N/A |
| Mascot 2.5.1 | Matrix Science | RRID: SCR_014322 |
| Protein Scape | Bruker Daltonics | N/A |
| Uniprot | UniProtKB, RRID: SCR_004426 | |
| PyMOL | PyMOL, RRID: SCR_000305 | |
| Odyssey | LI-CORE | Odyssey CLx, RRID: SCR_014579 |
| Zen10 Microscope Software | Zeiss | N/A |
| DatLab | Oroboros Instruments | Product ID: 20700 |
| RCSB Protein Data Bank (PDB) | RCSB PDB), RRID: SCR_012820 | |
| MitoFates | http://mitf.cbrc.jp/MitoFates/cgi-bin/top.cgi | N/A |
| Clustal Omega | https://www.ebi.ac.uk/Tools/msa/clustalo/ | N/A |
| PrimerX | http://www.bioinformatics.org/primerx/ | N/A |
| NCBI database | NCBI, RRID: SCR_006472 | |
| pLOGO | pLogo, RRID: SCR_018185 | |
|
| ||
| Other | ||
|
| ||
| LSM 710, AxioObserver microscope with a C-Apochromat 63x/1.20 W Korr M27 objective | Zeiss | N/A |
| Zeiss Axiovert200M microscope | Zeiss | N/A |
| Multimode-Microplate reader Mithras LB 940 | Berthold Technologies | N/A |
| CFX96real-Time System BioRad | BioRad | N/A |
| Personal Molecular Imager System | BioRad | N/A |
| Multipurpose Scintillation Counter | Beckmann Coulter LS6500 | N/A |
| Potter S Homogenizer | B. Braun Biotech International | N/A |
| UltiMate 3000 RSLCnano System | Thermo Fisher Scientific | N/A |
| C18 Acclaim Pepmap100 preconcentration column | Thermo Fisher Scientific | N/A |
| Acclaim Pepmap100 C18 nano column | Thermo Fisher Scientific | N/A |
| maXis plus UHR-QTOF System | Bruker Daltonics | N/A |
| CaptiveSpray nanoflow electrospray source | Bruker Daltonics | N/A |