Abstract
Multisystem inflammatory syndrome of childhood (MIS-C) is a recently described entity in pediatrics post-COVID-19 pandemic. Hemophagocytic lymphohistiocytosis (HLH) is a clinical syndrome caused by an unregulated proliferation of macrophages as well as T lymphocytes. Both entities can be considered overlapping, although distinct criteria for each can be found in the literature. Herein, we report a patient with MIS-C post-COVID-19 infection, complicated with HLH secondary to Plasmodium falciparum malaria from a blood transfusion.
Keywords: MIS-C, HLH, Malaria, Plasmodium falciparum, COVID-19
INTRODUCTION
Multisystem inflammatory syndrome in children (MIS-C) is a rare yet severe and potentially life-threatening complication of SARS-CoV-2 infection. According to the Centers for Disease Control and Prevention (CDC), the criteria of MIS-C include any individual aged below 21 years presenting with a prolonged febrile illness, with laboratory evidence of hyper-inflammation, multisystem (>2) organ involvement, with a positive current or recent SARS-CoV-2 infection or exposure within the 4 weeks before the onset of symptoms [1].
Although MIS-C is a newly described condition, it shows overlapping features with other inflammatory disorders such as secondary hemophagocytic lymphohistiocytosis (HLH, Table 1). HLH is typically characterised by high-grade fever, lymphadenopathy, hepatosplenomegaly, laboratory signs of a high inflammatory status along with signs of liver, central nervous system (CNS), and kidney involvement, which may lead to multiple organ failure (Table 2) [2]. Both entities are elicited by an exaggerated immunological response leading to a cytokine storm and subsequently a systemic hyper-inflammation [1,2].
Table 1.
Difference in MIS-C diagnostics criteria between the Royal College of Paediatrics and Child Health (RCPCH), CDC, and the World Health Organization (WHO).
| Differences | RCPCH | CDC | WHO |
|---|---|---|---|
| Name | PIMS-temporally associated with COVID-19 | MIS-C | MIS-C |
| Length of fever | Not specified | ≥24 hours | ≥3 days |
| Age | Child | <21 years | 0 to 19 years |
| Evidence of inflammation | Yes | Yes | Yes |
| Multisystem involvement | Single organ or multisystem | ≥2 systems involved | ≥2 systems involved |
| Exclude other causes | Yes | Yes | Yes |
| SARS-CoV-2-PCR or antibody or exposure | Not necessary | Necessary | Necessary |
MIS-C, multisystem inflammatory syndrome in children; PIMS, Paediatric inflammatory multisystem syndrome; SARS-CoV-2, COVID19; PCR, Polymerase Chain Reaction.
Table 2.
HLH diagnostic criteria.
| HLH diagnostic criteria | |
|---|---|
| Clinical | Laboratory |
| Unremitting fever | Fall in ESR, but high CRP |
| Bruising, purpura and mucosal bleeding | Pancytopenia (early stages neutrophelia) |
| Hepatosplenomegaly | Hyperferritinemia (>10,000) |
| Jaundice | High liver enzymes and LDH |
| CNS involvement (seizures, disorientation) | High triglycerides |
| Multiple organ failure | Low albumin |
| Lymphadenopathy | Low fibrinogen and elevated D-dimer, prolonged PT and PTT. |
| Bone marrow hemophagocytosis (negative in 15%) | |
CNS, Central nervous system; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; LDH, Lactate dehydrogenase; PT, Prothrombin time; PTT, Partial thromboplastin time.
In both entities, an infectious contributor is responsible for dysregulating the immune system and driving the symptoms of inflammation [3]. Among infections associated with HLH, viral infections, particularly Epstein-Barr virus, were found to be the most common contributor as described in the literature [4]. Other reports of atypical organisms, such as Palsimidum falciparum, as an underlying cause of the development of secondary HLH [4,5].
We hereby report a series of unusual events in a 17-month-old boy who developed MIS-C post-COVID-19 infection, required blood transfusion during treatment, and consequently acquired transfusion-transmitted P. falciparum malaria which triggered secondary HLH. To the best of our knowledge, this is the first report of HLH secondary to transfusion-related malaria complicated with MIS-C.
CASE REPORT
A 17-month-old previously healthy boy of Arab descent, with no history of travel, was diagnosed with COVID-19 infection. Two weeks later, the child presented with high-grade prolonged fever, up to 40°C, with vomiting, diarrhea, and labored breathing. On physical examination, he had red cracked lips and hepatosplenomegaly. The child was admitted to an outside hospital for further evaluation and his blood tests revealed pancytopenia and elevated inflammatory markers, hence favoring the diagnosis of MIS-C. He was initially treated with 2g/kg Intravenous (IV) Immunoglobulin (IVIG) therapy and 4 mg/kg pulse methylprednisolone. He also received blood and platelet transfusions due to his severe anemia and thrombocytopenia.
Despite treatment, his clinical condition worsened with unremitting high-grade fever and rising inflammatory markers. He had persistent anemia and thrombocytopenia, despite multiple transfusions. On day 5 of admission, he exhibited a generalised tonic-clonic status epilepticus. As his clinical condition worsened, he was started on Anakinra® (recombinant IL-1β antagonist) and broad-spectrum antibiotics. Although the child was diagnosed and treated as a case of MIS- C, other differential diagnoses including CNS infection, malignancies, and autoimmune/inflammatory conditions could not be overlooked; thus, the patient was covered with IV piperacillin/tazobactam at meningitis dosing. He was subsequently transferred to our facility, as a case of MIS-C refractory to IVIG to rule out malignancy. Upon arrival, he was pale, edematous, with marked abdominal distension with hepatosplenomegaly. His blood tests showed pancytopenia, high D-dimer, hypoalbuminemia, deranged inflammatory markers, and elevated liver enzymes (Table 3). A peripheral smear was done, which surprisingly revealed heavy P. falciparum parasitemia with a parasite index of 14%, both ring forms and gametocytes. As part of the workup, a bone marrow biopsy was performed; which demonstrated prominent macrophages with haemophagocytic activity, a large number of ring forms, and male and female gametocytes of P. falciparum. Blood and cerebrospinal fluid cultures did not grow any organisms at 5 days.
Table 3.
Laboratory values on admission, post treatment, and on follow-up.
| Days after imitation of treatment | Normal value | On admission | Day 3 (Post pulse steroids) | Day 7 | Day 14 (Before discharge) | Day 43 (Last outpatient visit) | |
|---|---|---|---|---|---|---|---|
| Lab value | |||||||
| Complete blood count | White blood cells (x109/l) | 5–15 | 4.99 | 17.56 | 10.29 | 11.9 | 7.8 |
| Hemoglobin (g/dl) | 11–14 | 5.1 | 8.6 | 7.2 | 8 | 11 | |
| Platelets (x103/ul) | 200–490 | 103 | 264 | 155 | 305 | 399 | |
| Inflammatory markers | Ferritin (ng/ml) | 6–67 | 3,359 | 2,073 | 2,246 | 1,749 | 585 |
| Fibrinogen (mg/dl) | 162–400 | 135 | 96 | 198 | 227 | - | |
| Triglycerides (mg/dl) | 0–200 | 830 | 766 | 599 | - | - | |
| Lactate dehydrogenase (U/l) | 0–300 | 1,665 | - | 994 | 967 | 319 | |
| Coagulation profile | D-dimer (ug/ml) | 0–0.5 | 13.53 | 1.94 | 0.63 | 0.57 | 0.27 |
There was also enhancement and dilatation of the coronary arteries on echocardiogram, hepatosplenomegaly with ascites, and minimal bilateral pleural effusions, indicating multisystem involvement. Furthermore, his brain magnetic resonance imaging showed meningeal enhancement seen in bilateral parieto-occipital regions representing inflammatory changes.
The combination of his clinical presentation along with his laboratory values led to the suspicion of HLH secondary to P. falciparum malaria. Thereafter, anti-malarial therapy was commenced. Given the fact that the origin of P. falciparum is unknown and thus the regional resistance patterns could not be established, the case was considered as resistant P. falciparum malaria. He was treated with a 5-day course of oral artemether/lumefantrine.
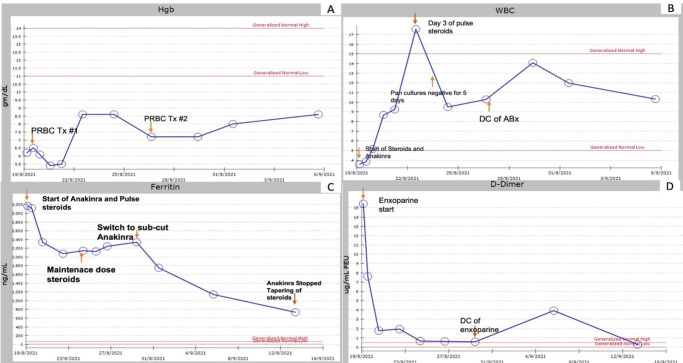
As for the treatment of HLH, the child’s presentation fulfilled six out of eight of the diagnostic criteria of HLH, and received a 3-day course of pulse methylprednisolone (20 mg/kg/day IV), followed by a maintenance course of prednisolone (1 mg/kg/day PO) which was tapered down and then discontinued over the span of 4 weeks (Figure 1).
Figure 1.
Trend of hemoglobin (A), white blood count (B), ferritin (C), and D-Dimer since patient transfer to our facility till most recent follow up after discharge. ABx, Antibiotics; DC, Discontinue; PRBC, Packed red blood cell; Tx, treatment.
The child also received Anakinra® (4 mg/kg/day) for 5 days, then decreased to (2 mg/kg/day), with marked improvement in inflammatory status. Moreover, his high values of D-dimer reflected a hypercoagulable state and a high risk of thromboembolic events which warranted anticoagulation therapy and treatment with enoxaparin.
As he was in a hypercoagulable status, enoxaparin was given for 4 days with close monitoring of platelet counts and coagulation profile, and ultimately switched to low dose Aspirin® as D-dimer levels normalised. Low dose Aspirin® was continued after discharge considering coronary artery changes found on the echocardiogram.
The child’s general condition improved significantly after initiation of treatment with resolution of fever, normalization of hematological abnormalities, and regression of inflammatory markers. Table 3 demonstrates the trend of his laboratory values throughout admission. The patient was eventually discharged and followed up weekly in the outpatient department until his inflammatory markers normalised.
DISCUSSION
This case demonstrates an extensive overlap between two hyper-inflammatory syndromes that occur in children, both severe and require a cautious approach to management. Although MIS-C and secondary HLH have been described as separate entities in literature, similarities in the presentation and management reflect an overlapping pathophysiology [5,6]. The pathogenesis in both disorders suggests a post-infectious immune dysregulation [6].
In MIS-C, a delayed immunological phenomenon is observed, characterised by profound depletion of CD4 lymphocytes, CD19 lymphocytes, and natural killer cells, besides a cytokine storm [tumor necrosis factor (TNF), IL-6, and interferon gamma (IFN-γ)] [5,6].
Similarly, in patients with secondary HLH, an inflammatory stimulus (infections metabolic, malignancy) triggers excessive production of IFN-γ which leads to uncontrolled macrophage and cytotoxic T cell activation and the onset of hemophagocytosis [7]. Given a similar pathological mechanism, the goal of treatment in both entities consists of the use of immunomodulatory therapy to suppress systemic inflammation [3,8].
A variety of infective pathogens, particularly viruses, have been frequently linked to the onset of secondary HLH [7]. Few cases reported HLH secondary to P. falciparum malaria, similar to the case at hand. Fuchs et al. [9] described the case of a healthy male who acquired secondary HLH post P. falciparum, due to a direct infection by the vector, and the patient was treated with ruxolitinib when he failed to respond to the standard treatment [9]. Trapani et al. [10] in 2013, reported a case of a 6-year-old boy with HLH secondary to malaria infection, treated with 2 days of pulse methylprednisolone followed by oral prednisolone [10]. Both reports highlight the importance of early recognition and management of secondary HLH in patients with malaria.
In our case, the patient fulfilled the diagnostic criteria of MIS-C, as per CDC definition, and was managed as such. His clinical condition deteriorated further following the blood transfusion he received before his transfer to our facility. Although the source of malaria could not be confirmed, it was attributed to contaminated blood transfusion as there was no history of travel and no other risk factors supporting an alternative source. Evidence of HLH developed simultaneously with the malarial infection, and the laboratory abnormalities observed (pancytopenia, hyperferritinemia, hypofibrinogenemia, high D-dimer) were initially justified by MIS-C which was thought to be refractory to standard therapy.
Although the diagnosis of HLH was challenging since it is difficult to distinguish from MIS-C, the presence of hyperferritinemia (> 684 ng/ml) in the context of an infectious insult was alarming and the diagnosis was ultimately confirmed by the bone marrow biopsy findings.
It is reasonable to hypothesize that several factors, acting at different stages, contributed to the development of HLH in this patient. We speculate that the cytokine-storm seen in MIS-C along with P. falciparum malaria triggered the immune system of a previously healthy child towards a severe immune reaction. Transfusion-transmitted malaria (TTM) although relatively rare in resource-rich countries, is still reported. Contaminated blood releases P. falciparum directly into the bloodstream, which contributes to the development of high-risk and life-threatening complications [11,12]. Fatal outcomes are observed especially in those with no previous exposure, or in immuno-compromised individuals due to other coexisting diseases as the case at hand [11,12].
Verra et al. [13] described how TTM carries a more serious risk than a natural malarial infection acquired by a mosquito bite, an initial asymptomatic phase in seen in the later which allows the innate immune system to develop a specific protective immunity, which is an advantage not observed in TTM [13].
CONCLUSION
As MIS-C is a newly recognised disorder, data regarding its clinical features and complications remain to be limited. Clinicians should be aware of the fine line that distinguishes MIS-C from secondary HLH in patients who are not responding to first-line therapy, to ensure early recognition and timely management. HLH is an under-recognised diagnostically challenging syndrome, hence should be considered in cases with overt inflammatory response. This case also serves as a prime example of transfusion-related infections, emphasizing the importance of blood screening programs and the identification of donors at risk of Plasmodium infection in non-endemic countries. Although there are reports of HLH secondary to P. falciparum, to the best of our knowledge, this is the first report of a patient developing MIS-C complicated by HLH secondary to transfusion-transmitted P. falciparum malaria.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
FUNDING
None.
ETHICAL APPROVAL
Signed informed consent for participation and publication of medical details was obtained from the parents. Confidentiality of patient data was ensured at all stages. The authors declare that ethics committee approval was not required for this case report.
REFERENCES
- 1.Multisystem Inflammatory Syndrome (MIS) Centers for Disease Control and Prevention. 2021. [2021 Dec 24]. Available from: https://www.cdc.gov/mis/mis-c/hcp/provider-resources/index.html.
- 2.Bracaglia C, Prencipe G, De Benedetti F. Macrophage activation syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017 Jan;15(1):5. doi: 10.1186/s12969-016-0130-4. https://doi.org/10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020 Jul;7(7):69. doi: 10.3390/children7070069. https://doi.org/10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi-Sadraei N, Vejpongsa P, Baljevic M, Chen L, Idowu M. Epstein-barr virus-related hemophagocytic lymphohistiocytosis: hematologic emergency in the critical care setting. Case Rep Hematol. 20152015:491567. doi: 10.1155/2015/491567. https://doi.org/10.1155/2015/491567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 Jun;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. https://doi.org/10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugud AA, Nugud A, Wafadari D, Abuhammour W. Kawasaki shock syndrome in an Arab female: case report of a rare manifestation and review of literature. BMC Pediatr. 2019 Aug;19(1):295. doi: 10.1186/s12887-019-1662-9. https://doi.org/10.1186/s12887-019-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brisse E, Wouters CH, Andrei G, Matthys P. How viruses contribute to the pathogenesis of hemophagocytic lymphohistiocytosis. Front Immunol. 2017 Sep;8:1102. doi: 10.3389/fimmu.2017.01102. https://doi.org/10.3389/fimmu.2017.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McArdle AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C, et al. BATS consortium. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021 Jul;385(1):11–22. doi: 10.1056/NEJMoa2102968. https://doi.org/10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs A, Orth HM, Germing U, Kondakci M, Holtfreter M, Feldt T, et al. Falciparum malaria-induced secondary hemophagocytic lymphohistiocytosis successfully treated with ruxolitinib. Int J Infect Dis. 2020 Nov;100:382–5. doi: 10.1016/j.ijid.2020.07.062. https://doi.org/10.1016/j.ijid.2020.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Trapani S, Canessa C, Fedi A, Giusti G, Barni S, Montagnani C, et al. Macrophage activation syndrome in a child affected by malaria: the choice of steroid. Int J Immunopathol Pharmacol. 2013;26(2):535–9. doi: 10.1177/039463201302600229. https://doi.org/10.1177/039463201302600229. [DOI] [PubMed] [Google Scholar]
- 11.Aydın F, Çelikel E, Ekici Tekin Z, Coşkun S, Sezer M, Karagöl C, et al. Comparison of baseline laboratory findings of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis and multisystem inflammatory syndrome in children. Int J Rheum Dis. 2021 Apr;24(4):542–7. doi: 10.1111/1756-185X.14078. https://doi.org/10.1111/1756-185X.14078. [DOI] [PubMed] [Google Scholar]
- 12.Singh G, Sehgal R. Transfusion-transmitted parasitic infections. Asian J Transfus Sci. 2010 Jul;4(2):73–7. doi: 10.4103/0973-6247.67018. https://doi.org/10.4103/0973-6247.67018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verra F, Angheben A, Martello E, Giorli G, Perandin F, Bisoffi Z. A systematic review of transfusion-transmitted malaria in non-endemic areas. Malar J. 2018 Jan;17(1):36. doi: 10.1186/s12936-018-2181-0. https://doi.org/10.1186/s12936-018-2181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]