Abstract
Topic Importance
COVID-19 has caused > 7 million deaths worldwide since its onset in 2019. Although the severity of illness has varied throughout the pandemic, critical illness related to COVID-19 persists. Survivors of COVID-19 critical illness can be left with sequelae of both the SARS-CoV-2 virus and long-term effects of critical illness included within post-intensive care syndrome. Given the complexity and heterogeneity of COVID-19 critical illness, the biopsychosocial-ecological model can aid in evaluation and treatment of survivors, integrating interactions among physical, cognitive, and psychological domains, as well as social systems and environments.
Review Findings
Prolonged illness after COVID-19 critical illness generally can be classified into effects on physical, cognitive, and psychosocial function, with much interaction among the various effects, and includes a wide range of symptoms such as ICU-acquired weakness, prolonged respiratory symptoms, cognitive changes, post-traumatic stress disorder post-traumatic stress disorder, anxiety, and depression. Risk factors for COVID-19 critical illness developing are complex and include preexisting factors, disease course, and specifics of hospitalization in addition to psychological comorbidities and socioenvironmental factors. Recovery trajectories are not well defined, and management requires a comprehensive, interdisciplinary, and individualized approach to care.
Summary
The onset of vaccinations, new therapeutics, and new strains of SARS-CoV-2 virus have decreased COVID-19 mortality; however, the number of survivors of COVID-19 critical illness remains high. A biopsychosocial-ecological approach is recommended to guide care of COVID-19 critical illness survivors.
Key Words: COVID-19, ICU survivorship, post-ICU
COVID-19 has strained health care systems worldwide, causing staggering volumes of severe illness and > 7 million deaths.1 Evolving evidence sheds light on the dynamic patterns of hospitalizations and outcomes of this viral infection.2,3 Although the number of patients requiring ICU care has decreased with vaccinations, new therapeutics, and newer strains of the virus, a significant burden of critical illness persists. In the United States alone, approximately 1,000 people are still hospitalized in an ICU for COVID-19-related illness in as recently as August 2023.4
Although mortality has improved,5 the persistent sequelae among the growing number of survivors of COVID-19 presents another challenge to health care systems navigating a global pandemic.5 Long-term consequences of critical illness are well described and included in post-intensive care syndrome (PICS). PICS is defined as new or worsening physical, cognitive, or psychological deficits, or a combination thereof, after an ICU admission.6 This clinical syndrome encompasses a heterogeneous group of patients that includes survivors of COVID-19 critical illness. Recently, the PICS framework has been used to organize deficits in survivors of an ICU stay with post-COVID conditions to help provide a patient-centered approach to recovery.7 Concurrently, SARS-CoV-2 may have unique and lasting consequences in those who do not experience severe disease. The sequelae of non-critically ill patients are beyond the scope of this review.
Together with the PICS framework, and given the multidimensional symptoms after COVID-19 critical illness, the biopsychosocial-ecological model can aid in evaluation and treatment of survivors, recognizing the key interactions among physical, cognitive, and psychological domains, as well as social systems and environments. The biopsychosocial model was introduced in 19778 as a model for holistic medical care and since has been updated to include systems theory, viewing health conditions through a framework that considers interactions among individual characteristics, interpersonal relationships, community settings, and wider societal policies, with applications for prevention, public health, and clinical care.9, 10, 11, 12 Reference to these multidimensional frameworks is included throughout this review, which describes the current understanding and approach to survivors of COVID-19 critical illness with persistent symptoms and dysfunction.
Literature Review
Our team included experts in pulmonary and critical care medicine, PICS, rehabilitation, and neuropsychology. We developed areas of emphasis in this review through discussion and iterative revision. We completed a literature search in PubMed and PsycINFO to identify relevant literature using the following search terms: critical illness, intensive care unit, follow-up, and post-intensive care syndrome AND COVID-19, prioritizing literature that addressed physical, cognitive, psychosocial issues, or a combination thereof, in COVID-19 critical illness. However, because of the limited number of articles available that focused on COVID-19 critical illness, articles that included a mixed severity of COVID-19 illness also were reviewed.
Evidence Review
Epidemiology
The number of ICU admissions for COVID-19-related illness has varied throughout the pandemic, peaking in the United States in January 2021, with > 28,000 patients in an ICU. This number decreased to fewer than 1,000 patients in June and July 2023.4 Survival to hospital discharge of patients with COVID-19 critical illness also has varied throughout the pandemic. A systematic review completed in 2020 found that the overall ICU mortality rate was 25.7%, although 56% of patients were still in the ICU at the time of the study completion.13 A retrospective cohort review from February 2020 through March 2021 showed mortality rates of ICU admissions for COVID-19 at 30.7%.14 These results reveal large number of survivors of COVID-19 critical illness. Among these survivors, approximately 15% experience post-COVID-19 conditions, which refer to symptoms after COVID-19 that linger after recovery from acute infection.15 In addition to post-COVID-19 conditions, small cohort studies among survivors of COVID-19 critical illness found rates of PICS as high as 69%.16, 17, 18, 19, 20 Figure 1, adapted from Mikkelson et al,21 demonstrates COVID-19 and pandemic-specific factors superimposed on a model of PICS. This highlights acute illness and hospitalization factors that can contribute to poor outcomes as well as pandemic-specific factors that exacerbate PICS. These factors include21 a strained health care system, decreased access to care, increased health care provider burnout, increased isolation, and increased uncertainty on both the part of the patient and the provider related to the novel nature of the disease.22,23
Figure 1.
Diagram showing COVID-19 pandemic and post-intensive care syndrome factors impacting post-ICU outcomes. (Adapted with permission from Mikkelson and Iwashyna.21 Persistent problems and recovery after critical illness. In: Warrell DA, Cox TM, Firth JD, eds. Textbook of Medicine. Oxford Univeristy Press; 2020:3925-3930.21. Copyright Oxford University Press, 2021.Reproduced with permission of the Licensor through PLSclear.)
Pathophysiology
The mechanisms underlying symptoms after COVID-19 critical illness remain uncertain and are likely multifactorial, possibly in part because of direct tissue damage in the setting of the viral infection and the immune response of the host.24 A pronounced stress response and overactivation of the hypothalamic paraventricular nucleus may contribute to psychological factors.25 Similar to the cause of PICS, which also is poorly defined, long-lasting effects likely are related to common ICU interventions such as sedation, prolonged immobility, and delirium, as well as social-ecological factors such as presence of interpersonal and systemic support.26
General Risk Factors
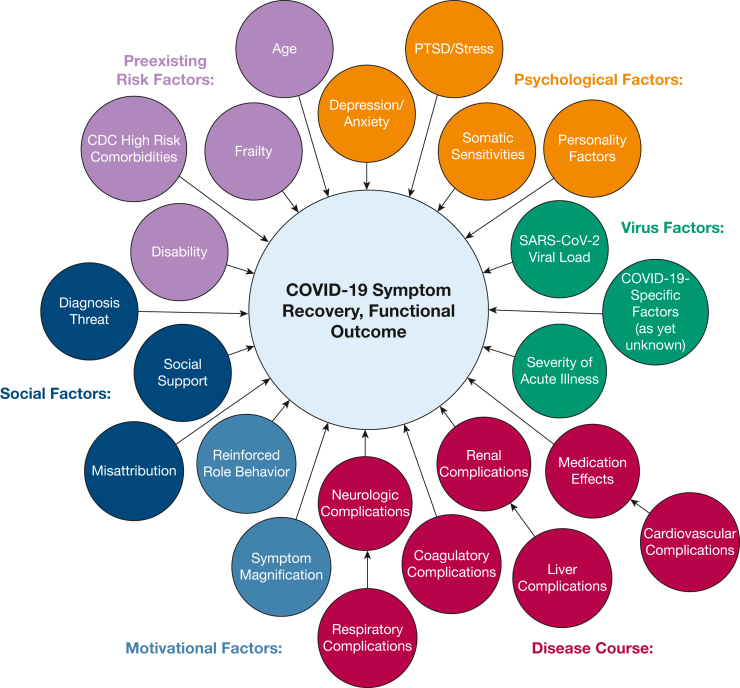
Risk of prolonged illness after COVID-19 critical illness is complex, and risk factors include preexisting factors (age, comorbidities, frailty), disease course (organ system insults), COVID-19-specific factors (viral load, variant), as well as psychological comorbidities and social factors (social support). Vance et al27 (Fig 2) proposes a model highlighting the biopsychosocial-ecological framework that encompasses these complex factors.
Figure 2.
Diagram showing possible factors influencing symptom reporting acutely, postacutely, and long after a COVID-19 infection. CDC = Centers for Disease Control and Prevention; PTSD = post-traumatic stress disorder.
Physical Function
Risk Factors
Increased age and frailty are risk factors for COVID-19 critical illness developing,28,29 as well as risk factors for development of ICU-acquired weakness, defined as multidimensional functional disability including impairments such as compromised ambulation, impaired activities of daily living, swallowing difficulties, and prolonged mechanical ventilation.30 Additional risk factors for physical impairment include longer duration of bed restriction, hyperglycemia, and use of neuromuscular blockade or corticosteroids, which became standard of care for COVID-19 critical illness.30, 31, 32, 33, 34 Additionally, multiple organ dysfunction or prolonged duration of illness also can contribute to development of ICU-acquired weakness.35 Although pulmonary manifestations of COVID-19 are not limited to critically ill patients, one of the strongest predictors for post-COVID-19 pulmonary fibrosis developing is invasive ventilation during inpatient admission.36
Prevalence and Clinical Manifestations
Physical impairments are reported frequently among survivors of COVID-19 critical illness and encompass a variety of conditions, often overlapping, with ICU-acquired weakness being the most common. Among a cohort of 92 survivors of COVID-19 critical illness in Belgium, 87.5% did not return to their previous level of activity at 3 months after discharge.37 Among a cohort of 486 patients hospitalized with COVID-19, 52% of patients treated with mechanical ventilation had ICU-acquired weakness at ICU discharge.38 In addition to ICU-acquired weakness, survivors of CCI are at risk for development of diaphragmatic dysfunction, laryngeal injury, dysphagia, and dysphonia primary due to prolonged invasive mechanical ventilation.33,39
Pulmonary manifestations (eg, dyspnea, cough) after both severe and nonsevere COVID-19 infection are among the most commonly described sequelae. Multiple studies of survivors of COVID-19 critical illness have reported impairment of pulmonary function test results at 3 months after hospital discharge, including reduced diffusion capacity of lungs for carbon monoxide in 52% to 75% of patients and decreased total lung capacity (TLC) in 37% to 48% of patients.40, 41, 42, 43
Radiographic evidence of interstitial lung disease has been observed after varying acuity of illness with COVID-19 pneumonia.44 Residual lung abnormalities are common and can be seen in up to 11% of patients after hospitalizations for COVID-19-related illness; greater risk of these abnormalities can be seen with severe illness including invasive mechanical ventilation or extracorporeal membrane oxygenation. Organizing pneumonia is the most common persistent interstitial lung disease among survivors of COVID-19, accounting for 59% of interstitial changes in a cohort of 59 patients.44 A meta-analysis found that 44.9% of survivors of COVID-19 demonstrate fibrotic changes on imaging.45
Approach
The diagnosis and treatment of post-COVID-19 conditions can be difficult given the novelty, heterogeneity in presentation, and lack of standardized screening or diagnostic criteria.46,47 The 2020 Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments after Critical Illness recommended that an assessment of patients for PICS should occur between 2 and 4 weeks after discharge.48 Given the significant overlap of PICS and post-COVID-19 conditions in those who were critically ill and the evolving understanding of post-COVID-19 critical illness, currently a similar model can be used for evaluation of post-COVID-19 conditions. The 6-min walk test and EQ-5D-5L are recommended screenings for physical impairments seen in PICS.49 The World Health Organization has developed a scale for assessing functional status across multiple domains (World Health Organization Disability Assessment Schedule 2.0), including physical functioning, and post-COVID-19-specific measures are beginning to emerge, with the Post-COVID-19 Functional Status Scale being particularly useful in assessing level of participation in usual daily activities with a physical function component; see Table 150, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63 for a comprehensive list of measures and references.
Table 1.
Suggested Assessment Methods After COVID-19
| Domain | Assessment | Description | Considerations |
|---|---|---|---|
| Physical or general functional status | 6-min walk test50 |
|
|
| EQ-5D-5L51 |
|
|
|
| World Health Organization Disability Assessment Schedule 2.052 |
|
|
|
| Post-COVID-19 Functional Status Scale53 |
|
|
|
| Psychological functioning | Impact of Events Scale-Reviseda54 |
|
|
| Patient Health Questionnaire-955 |
|
|
|
| General Anxiety Disorder-756 |
|
|
|
| Health Anxiety and Depression Scale57a |
|
|
|
| Posttraumatic Stress Symptoms-1458 |
|
|
|
| PTSD Checklist for DSM-559 |
|
|
|
| Cognitive functioning | Montreal Cognitive Assessmenta60 |
|
|
| Montreal Cognitive Assessment-Blind61 |
|
|
|
| 5-min Montreal Cognitive Assessment62 |
|
|
|
| Repeatable Battery for Neuropsychological Status63 |
|
|
ADLs = activities of daily living; iADLs = instrumental activities of daily living; DSM-4 = Diagnostic and Statistical Manual of Mental Disorders; DSM-5 = Diagnostic and Statistical Manual of Mental Disorders; MDD = major depressive disorder; MoCA = Montreal Cognitive Assessment; PICS = post-intensive care syndrome; PTSD = post-traumatic stress disorder.
Recommended by the Society of Critical Care Medicine.
Recommendations for primary care providers evaluating patients after COVID-19 illness focus on identifying those with more serious complications, including physical manifestations of pulmonary embolism, heart failure, stroke, myocardial infarction, and lung fibrosis,64 for referral to specialists while managing mild to moderate symptoms in the general medicine setting in a collaborative fashion with the patient and family. Providing a comprehensive and user-friendly workup for primary care provider reference (eg, recommended evaluation and treatment by system and symptom, what can be treated by the primary care provider and when to refer to a specialist) may be useful for decreasing the need for multiple coordinated specialists.27 For example, dyspnea and cough, common after varying acuity of COVID-19 illness, may require additional testing, including chest imaging or pulmonary function testing and consideration for pulmonary referral, particularly if the patient has progressive dyspnea, crackles on examination, symptoms of > 12 weeks’ duration, a sit-to-stand test with ≥ 4% oxygen desaturation, concerning findings on chest radiograph or spirometry, or a combination thereof.65
Pulmonary rehabilitation and physical therapy also are important tools in COVID-19 critical illness recovery. Prospective studies have found that patients across the spectrum of COVID-19 illness who completed both inpatient and outpatient pulmonary rehabilitation programs showed improvement in objective measures of both functional and mental status on completion.66,67 It is recommended to consider pulmonary rehabilitation for all patients who have been hospitalized for COVID-19.68 As a result of the pandemic, use of telerehabilitation programs increased, allowing the patient to complete pulmonary rehabilitation at home; early studies of have shown that these virtual programs can provide a safe alternative to in-person sessions.69
Cognitive Function
Risk Factors
Cognitive status before illness and during the ICU stay both are risk factors for development of prolonged post-COVID-19 cognitive symptoms. Preexisting dementia and other cognitive vulnerabilities, such as risk factors for vascular disease or mild cognitive impairment, increase the risk of delirium during acute COVID-19 infection, which subsequently may increase the risk of long-term cognitive impairment.70 Similar to physical impairments, risk of cognitive impairment, particularly for those with the aforementioned vulnerabilities, is compounded by longer duration of mechanical ventilation, bed restriction, and use of neuromuscular blockade or corticosteroids, although these factors are likely important because they contribute directly to length of delirium.30, 31, 32, 33, 34
Rates of ICU delirium for patients with severe COVID-19 range from 40% to 70%, making it one of the most common complications.71, 72 Limited data demonstrate variable rates of cognitive dysfunction after acute delirium among patients with COVID-19. Some studies show similar rates of cognitive dysfunction in those with and without delirium, although with much greater physical decline in those with delirium at the 4-week follow-up, whereas others show significant increase in cognitive dysfunction after COVID-19 with acute delirium.71,73
Prevalence and Clinical Manifestations
Observed rates of cognitive difficulties have a wide degree of variability because of the heterogeneity in assessment methods (eg, self-report vs cognitive testing, different measurement tools) and timing of measurement. In an observational series of 45 of 51 consecutive patients with ARDS resulting from COVID-19 who were discharged from an ICU during the first epidemic wave, Daste et al74 found that all patients experienced some level of cognitive dysfunction in the short term. The most common cognitive difficulties seen in the short term are in the domains of processing speed, working memory, and executive functions such as set shifting and divided attention, although significant improvement tends to occur by 3 months after discharge, which is consistent with other causes of PICS.75 Longer-term cognitive manifestations after an ICU stay with COVID-19 can range from nonexistent to prolonged disorders of consciousness.18,76 Many survivors of ICU stays for COVID-19 will not sustain clinically significant, long-term cognitive impairment when cognition is assessed objectively and appropriate comparison groups (norms) are used, although objective cognitive changes in the short term seem to be much more common.75,77 Self-report of cognitive concerns tends to be higher, indicating that factors other than neurocognitive impairment, such as psychological distress, sleep disturbance, and medication effects, may contribute to cognitive difficulties after COVID-19.75,78 Neurologic complications such as stroke increase the likelihood of long-term cognitive sequelae after COVID-19, again with similar presentation to strokes of other causes.71
Approach
Recommended screening tools for assessment of cognitive status include the Montreal Cognitive Assessment, or Montreal Cognitive Assessment-Blind if administering to a patient with visual difficulties or via telemedicine platforms. The 5-min Montreal Cognitive Assessment is an even briefer, although well validated, screening tool that may be useful for providers who have little time to assess general cognitive status. These tools now require that the test administrator be certified in the administration and interpretation of the instrument to maintain validity. Certification takes approximately 1 h.
The Repeatable Battery for Neuropsychological Status is a much more comprehensive cognitive screening measure that takes approximately 45 to 60 min to complete. The resultant data provide index scores for various cognitive domains, including attention, language, visuospatial and constructional abilities, and immediate and delayed memory. Indexes also exist that are fairly accurate in determining whether the patient’s presentation is most consistent with a cortical or subcortical process and may help to differentiate between something like Alzheimer disease and new-onset subcortical dysfunction related to hypoxia. This measure requires significant training before administration and interpretation, although typically is administered by a properly trained professional who is not a neuropsychologist. See Table 1 for a comprehensive list of measures and references.
Similar to the assessment and treatment of physical symptoms, nonspecialists may manage cognitive symptoms for many patients. Referral to a neuropsychologist is recommended if performance is impaired on a cognitive screener and cognition is preventing the patient from functioning as they would like.79 Treatment of objectively confirmed cognitive impairment may include cognitive rehabilitation with a rehabilitation psychologist or speech language pathologist. Although randomized controlled trials are just being started to determine specific cognitive rehabilitation techniques for post-COVID-19 conditions, it is likely that findings will be similar to what is already known to be effective in other similar conditions.79 Cognitive rehabilitation should include an emphasis on compensatory strategies and environmental supports to optimize independence because these strategies are more evidence-based than most remediation approaches.80 Mass-marketed computer-based cognitive games have not been proven efficacious.80 For subjective cognitive concerns that are not supported with objective measures, as well as objective cognitive impairment, assessment of and education on noncognitive factors that may be contributing to the experience of cognitive impairment, such as mood, anxiety, pain, and medication effects, should be provided, and these factors should be addressed with evidence-based treatment.
Psychosocial Function
Risk Factors
Psychosocial factors confer significant risk for development of COVID-19-related post-ICU conditions, and impairments in psychosocial function can result from COVID-19 critical illness. Risk factors can include psychological symptoms during acute illness, impaired adjustment or coping in the aftermath, and level of social support, both at the personal and the systems level.16,17 Notably, psychological status impacts health behaviors and is a key factor in adherence with health care recommendations that will optimize outcomes.81 Improvements in depression and anxiety are associated with better adherence in recently hospitalized patients.82 Preexisting psychological conditions also may be a risk factor for development of severe disease itself. In a large systematic review and meta-analysis comprising 1,469,731 patients with COVID-19, of whom 43,938 showed preexisting psychological disorders, Vai et al83 found that the presence of any psychological disorder was associated with an increased risk of COVID-19 mortality (OR, 2.00). This association also was observed for psychotic disorders (OR, 2.05), mood disorders (OR, 1.99), substance use disorders (OR, 1.76), and intellectual disabilities or developmental disorders (OR, 1.73), but not for anxiety disorders. Exposure to antipsychotics (OR, 3.71), anxiolytics (OR, 2.58), and antidepressants (OR, 2.23) also was associated with COVID-19 mortality.
Prevalence and Clinical Manifestations
Psychological manifestations after an ICU stay for COVID-19 span a wide range, from healthy adjustment to debilitating mental illness. Literature examining psychological manifestations specific to critically ill patients with COVID-19 is limited and has shown variable results. Some studies have found that patients meet post-traumatic stress disorder criteria less frequently and reported less severe symptoms of anxiety and depression than a historical critical illness cohort, whereas other studies indicate acute stress disorder occurs in about 40% of patients with COVID-19 in the ICU.84,85 Taken together, the existing research indicates that psychological distress may be more pronounced early on in COVID-19 critical illness, but may dissipate more quickly than in other critical illnesses. In a qualitative study conducted early in the pandemic, researchers followed up patients through their hospitalizations, identifying COVID-19-specific attitudes and stressors, including high levels of fear, denial, and stigma associated with COVID-19 infection. The major sources of stress included the contagious nature of the disease, quarantine measures, and concerns regarding the health of family members. Manifestations of stress included excessive attention to symptoms, rumination, and changes in diet and sleep. Supportive factors included psychological flexibility in adjustment, perception of good medical care, and family and social support. For some, the disease resulted in psychological growth because patients viewed their experience with gratitude through the cherishing of life, family, bravery, and tenacity.86
Approach
Recommended screening tools for assessment of psychosocial status include the Hospital Anxiety and Depression Scale for anxiety and depression and the Impact of Events Scale for post-traumatic stress disorder (see Table 1 for a more comprehensive list of measures).87 Similar to the assessment and treatment of physical and cognitive symptoms, nonspecialists may manage psychosocial concerns for some patients, although a low bar should be in effect for referrals to social work for management of risk factors relating to social determinants of health and to a psychologist or other qualified mental care professional for psychological concerns that prevent the patient from functioning as they would like. Referral to a rehabilitation psychologist may be particularly beneficial because they have expertise in helping patients adjust to illness or disability. It is important to ensure that evidence-based techniques are used, such as cognitive-behavior therapy or acceptance and commitment therapy for anxiety and depression and cognitive processing therapy or prolonged exposure therapy for post-traumatic stress.88 The referring provider can use motivational interviewing skills to encourage psychotherapy if a patient expresses ambivalence.89
Encouraging and educating patients in self-management of chronic symptoms also is important for patient self-efficacy and optimal adjustment and to reduce unnecessary use of the medical system. PASCguide.com offers patient resources for education, self-management strategies by symptom, and methods for tracking symptom management that may aid with understanding what works or does not work for a particular patient.90
Clinical Outcomes
Prospective research to inform understanding of recovery trajectories for this population is limited, and for the most part, long-term studies are limited to 6-month and 1-year follow-up periods. Marked variability in outcomes suggests that factors such as case mix (eg, age, disease burden, comorbidities) and other variables that likely impact findings (eg, selection or survival biases, assessment methods) should be considered, although this also highlights underlying variability in outcomes. One exploratory prospective multicenter cohort study of ICU survivors with COVID-19 found that at 1 year, 74.3% of patients reported physical symptoms (eg, overall weakness, joint stiffness and pain, muscle weakness, and myalgias), 26.2% reported psychological symptoms, and 16.2% reported cognitive symptoms.91 However, another study found that approximately 50% of patients with COVID-19 who were treated in the ICU experience full recovery of symptoms from 3 months to 1 year, although for some, symptoms of depression and anxiety remain largely unchanged despite improvements in physical functioning.18
Prior to the COVID-19 pandemic, studies evaluating pulmonary function after ARDS demonstrated normalization of spirometry values and lung volumes at 6 months for most patients with a more prolonged impairment in diffusion capacity of lungs for carbon monoxide.43,92 A study comparing patients requiring mechanical ventilator for acute respiratory failure resulting from COVID-19 compared with those requiring mechanical ventilator for causes other than COVID-19 did not find a difference in rates of new disability at 6 months, suggesting that the overall trajectory and recovery of physical impairments are similar for ARDS resulting from both COVID-19 and all other causes.93 A recent multicenter study of patients with severe to critical COVID-19 showed a high prevalence of radiologic and pulmonary function abnormalities at the 3-month follow-up, but continued improvement at the 6-month and 12-month follow-up.94
Persistent cognitive dysfunction after COVID-19 critical illness is another commonly reported concern and likely is associated with acute factors discussed previously (eg, delirium and psychosocial complexity). Some degree of cognitive dysfunction based on screening measure results is present in 33% to 42% of survivors of ICU stays for COVID-19 6 months after discharge.95,96 However, comprehensive studies assessing long-term cognitive outcomes in survivors of ICU stays for COVID-19 are limited. Another study examined patients with COVID-19 who were and were not treated in the ICU with comprehensive neuropsychological testing. Attention and executive functioning domains were impacted the most, and in contrast to studies suggesting no differences in cognitive functioning between hospitalized and not-hospitalized patients with COVID-19,97 ICU-treated patients demonstrated more severe impairments. Studies with longer follow-up periods currently are lacking, although one survey suggests that clinically significant cognitive dysfunction is reported by 16% of patients 1 year after discharge.91
Clinically significant depression and anxiety symptoms are more prevalent than post-traumatic stress disorder both at the 6-month95 and 1-year91 follow-up, with rates of depression, anxiety, and post-traumatic stress disorder of 42%, 38%, and 33% at 6 months and 18%, 18%, and 10% at 1 year, respectively. These rates are similar to those of survivors of ICU stays who do not have COVID-19.98 Within patient populations with ARDS, psychological dysfunction has been documented up to 5 years after ICU discharge.99 Taken together, these findings highlight the importance of understanding who is most at risk for persistent symptoms to provide early intervention.
Future Directions
A complex relationship exists between disease-specific and patient-specific factors in post-COVID-19 critical illness, with important implications for optimizing outcomes. Findings of the limited studies thus far often are in disagreement with one another. For example, some teams have reported unexpectedly similar cognitive performances among survivors of COVID-19 who required ICU care and those who did not, whereas others reported differing results commensurate with severity of acute illness.100 In some studies, among those with impaired neuropsychological scores, no relationship was found between disease severity and cognitive functioning, suggesting that other key negative prognostic variables (eg, premorbid factors and other psychosocial-ecological complications, as well as secondary medical complications) should be examined carefully. These variable findings suggest that interpretation of any one test result should not be overgeneralized because many different causes underlying a patient’s experience of physical, cognitive, or psychological dysfunction are likely across the stages of severe acute and post-acute COVID-19. Additional studies examining these nuanced relationships, accounting for biopsychosocial-ecological factors, are required to tailor treatments better and to predict and optimize outcomes.
Understanding recovery trajectories with longer follow-up periods is another valuable area of future research, and more opportunities to accomplish this will be available as time elapses since the outset of pandemic. Given what has been observed with other patient populations, we know that post-traumatic stress symptoms can have a delayed onset of up to 1 year101 and that psychological symptoms can persist more generally,99 indicating that this likely will be a patient population with ongoing follow-up and intervention needs. However, just as future research will be tasked with fully understanding negative prognostic variables, defining what constitutes and predicts a good recovery will further our understanding of post-COVID-19 trajectories and will inform possible intervention targets. For example, Sun et al86 provide insight into protective factors associated with improved emotional adjustment, including psychological flexibility and perception of good medical care. Post-COVID-19 recovery clinics in addition to post-ICU recovery clinics have increased since the onset of the pandemic, with interdisciplinary care, but have variable approaches to structure, patient evaluation and assessment, and treatment.102 Continued investigation on the impact of these clinics on patients who have undergone an ICU stay and who have survived COVID-19 is warranted.
Limitations with study design also point to future areas of needed research. Some studies have strict exclusion criteria, including excluding those with preexisting psychiatric diagnoses or neurologic disease,97,103, 104, 105 limiting not only the generalizability of findings, but also our understanding of recovery trajectories in those who may be at highest risk of poor outcomes. Similarly, fully accounting for sample characteristics is necessary to avoid making misleading interpretations. For example, different conclusions may be drawn from studies with samples comprising mainly young, previously fit patients as opposed to studies including older patients with a high comorbidity burden. Additionally, many ICU-specific studies are limited by smaller sample sizes, which prohibits more nuanced analysis of data.75,84,106
Summary
The advent of vaccinations and new therapeutics has lowered COVID-19 mortality, but the number of survivors of ICU stays for COVID-19 remains high. Research that is specific to caring for these patients is sparse, and additional methodologically sound research, such as prospective studies accounting for the nuanced interactions of disease-specific and patient-specific factors, as well as intervention randomized controlled trials, are needed. In the interim, early findings suggest that a model similar to critical illness recovery care may be helpful. A biopsychosocial-ecological approach is recommended to address the many factors that may contribute to prolonged symptom experience after COVID-19 critical illness.107 Helping patients move toward self-management, when possible, will be an important part of the care model to decrease burden on the health care system and prevent provider burnout.
Funding/Support
This study was supported in part by the US Department of Veterans Affairs, Health Services Research and Development Service [Grant VA IIR 20-313]. T. E. is supported by the VA Office of Academic Affiliations through the VA/National Clinician Scholars Program (NCSP) and the University of Michigan Medicine at University of Michigan. T. V. is supported by the National Heart, Lung, and Blood Institute [Grants K23 140165 and R01 HL157361 and the Agency for Healthcare Research and Quality [Grant R01 HS028038].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: H. P. receives support from Agency for Healthcare Research and Quality (unrelated to this manuscript) and serves on the Surviving Sepsis Campaign Guidelines for Sepsis and Severe COVID. None declared (L. C., K. S., E. N., T. V., T. E., J. I. M.).
Acknowledgments
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Disclaimer: This manuscript represents the views of the authors and does not necessarily represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Drs Cagino and Seagly contributed equally to this manuscript.
References
- 1.Ahmed O.F., Amin B.J.H., Abdullah B.A., et al. Post COVID-19 pulmonary complications; a single center experience. Ann Med Surg (Lond) 2021;72 doi: 10.1016/j.amsu.2021.103052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan E., Song J., Deane A.M., Plummer M.P. Global impact of coronavirus disease 2019 infection requiring admission to the ICU: a systematic review and meta-analysis. Chest. 2021;159(2):524–536. doi: 10.1016/j.chest.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S., Patel K., Pham H., et al. Clinical trends among U.S. adults hospitalized with COVID-19, March to December 2020: a cross-sectional study. Ann Intern Med. 2021;174(10):1409–1419. doi: 10.7326/M21-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Our World in Data. Coronavirus (COVID-19) hospitalizations Secondary Our World in Data: coronavirus (COVID-19) hospitalizations. Our World in Data website. https://ourworldindata.org/COVID-hospitalizations#what-is-the-weekly-number-of-new-admissions-to-icu-due-to-covid-19
- 5.Anesi G.L., Jablonski J., Harhay M.O., et al. Characteristics, outcomes, and trends of patients with COVID-19-related critical illness at a learning health system in the United States. Ann Intern Med. 2021;174(5):613–621. doi: 10.7326/M20-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 7.Schwab K., Schwitzer E., Qadir N. Postacute sequelae of COVID-19 critical illness. Crit Care Clin. 2022;38(3):455–472. doi: 10.1016/j.ccc.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel G.L. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 9.Karriker-Jaffe K.J., Witbrodt J., Mericle A.A., Polcin D.L., Kaskutas L.A. Testing a socioecological model of relapse and recovery from alcohol problems. Subst Abuse. 2020;14 doi: 10.1177/1178221820933631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Register-Mihalik J.K., DeFreese J.D., Callahan C.E., Carneiro K. Utilizing the biopsychosocial model in concussion treatment: post-traumatic headache and beyond. Curr Pain Headache Rep. 2020;24(8):1–7. doi: 10.1007/s11916-020-00870-y. [DOI] [PubMed] [Google Scholar]
- 11.van Erp R.M.A., Huijnen I.P.J., Jakobs M.L.G., Kleijnen J., Smeets R. Effectiveness of primary care interventions using a biopsychosocial approach in chronic low back pain: a systematic review. Pain Pract. 2019;19(2):224–241. doi: 10.1111/papr.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matney C, Bowman K. In: Traumatic Brain Injury: A Roadmap for Accelerating Progress. Berwick D, editor. National Academies Press; 2022. [PubMed] [Google Scholar]
- 13.Quah P., Li A., Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbonell R., Urgelés S., Rodríguez A., et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: a multicentre retrospective cohort study. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Secondary long COVID or post-COVID conditions. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html?s_cid=11840:long%20covid:sem.ga
- 16.Beck K., Vincent A., Becker C., et al. Prevalence and factors associated with psychological burden in COVID-19 patients and their relatives: a prospective observational cohort study. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0250590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey S., Biswas P., Ghosh R., et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14(5):779–788. doi: 10.1016/j.dsx.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamberini L., Mazzoli C.A., Sintonen H., et al. Quality of life of COVID-19 critically ill survivors after ICU discharge: 90 days follow-up. Qual Life Res. 2021;30(10):2805–2817. doi: 10.1007/s11136-021-02865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardashkhani S., Ajri-Khameslou M., Heidarzadeh M., Sedigh S.R. Post–intensive care syndrome in Covid-19 patients discharged from the intensive care unit. J Hosp Palliat Nurs. 2021;23(6):530. doi: 10.1097/NJH.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hameed F., Palatulan E., Jaywant A., et al. Outcomes of a COVID-19 recovery program for patients hospitalized with SARS-CoV-2 infection in New York City: a prospective cohort study. PM R. 2021;13(6):609–617. doi: 10.1002/pmrj.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikkelsen ME, Iwashyna TJ. In: Textbook of Medicine. Warrell DA, Cox TM, Firth JD, editors. Oxford Univeristy Press; 2020. Persistent problems and recovery after critical illness; pp. 3925–3930. [Google Scholar]
- 22.Eaton T.L., Sevin C.M., Hope A.A., et al. Evolution in care delivery within critical illness recovery programs during the COVID-19 pandemic: a qualitative study. Ann Am Thorac Soc. 2022;19(11):1900–1906. doi: 10.1513/AnnalsATS.202203-255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker A.M., Brigham E., Connolly B., et al. Addressing the post-acute sequelae of SARS-CoV-2 infection: a multidisciplinary model of care. Lancet Respir Med. 2021;9(11):1328–1341. doi: 10.1016/S2213-2600(21)00385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 25.Mackay A. A paradigm for post-Covid-19 fatigue syndrome analogous to ME/CFS. Front Neurol. 2021;12:701419. doi: 10.3389/fneur.2021.701419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voiriot G., Oualha M., Pierre A., et al. Chronic critical illness and post-intensive care syndrome: from pathophysiology to clinical challenges. Ann Intensive Care. 2022;12(1):58. doi: 10.1186/s13613-022-01038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vance H., Maslach A., Stoneman E., et al. Addressing post-COVID symptoms: a guide for primary care physicians. J Am Board Fam Med. 2021;34(6):1229–1242. doi: 10.3122/jabfm.2021.06.210254. [DOI] [PubMed] [Google Scholar]
- 28.Lim J.P., Low K.Y.H., Lin N.J.J., et al. Predictors for development of critical illness amongst older adults with COVID-19: beyond age to age-associated factors. Arch Gerontol Geriatr. 2021;94 doi: 10.1016/j.archger.2020.104331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L., Mao Y., Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging (Albany NY) 2020;12(12) doi: 10.18632/aging.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas J.S., Teixeira C., Cabral C.R., et al. Factors influencing physical functional status in intensive care unit survivors two years after discharge. BMC Anesthesiol. 2013;13(1):1–9. doi: 10.1186/1471-2253-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes L.F., Murthy S., Garcia-Gallo E., et al. Clinical characteristics, risk factors and outcomes in patients with severe COVID-19 registered in the International Severe Acute Respiratory and Emerging Infection Consortium WHO clinical characterisation protocol: a prospective, multinational, multicentre, observational study. ERJ Open Res. 2022;8(1):00552–2021. doi: 10.1183/23120541.00552-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang R., Elhusseiny K.M., Yeh Y.-C., Sun W.-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—a systematic review and meta-analysis. PloS One. 2021;16(2) doi: 10.1371/journal.pone.0246318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosey M.M., Needham D.M. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):1–2. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Jonghe B., Lacherade J.-C., Sharshar T., Outin H. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37(10):S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 35.Herridge M.S., Azoulay É. Outcomes after critical illness. N Engl J Med. 2023;388(10):913–924. doi: 10.1056/NEJMra2104669. [DOI] [PubMed] [Google Scholar]
- 36.Aul R., Gates J., Draper A., et al. Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir Med. 2021;188 doi: 10.1016/j.rmed.2021.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rousseau A.-F., Minguet P., Colson C., et al. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann Intensive Care. 2021;11(1):118. doi: 10.1186/s13613-021-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Aerde N, Van den Berghe G, Wilmer A, et al. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med. 2020;46(11):2083–2085. doi: 10.1007/s00134-020-06244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodsky M.B., Levy M.J., Jedlanek E., et al. Laryngeal injury and upper airway symptoms after oral endotracheal intubation with mechanical ventilation during critical care: a systematic review. Crit Care Med. 2018;46(12):2010. doi: 10.1097/CCM.0000000000003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Gassel R.J., Bels J.L., Raafs A., et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203(3):371–374. doi: 10.1164/rccm.202010-3823LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Compagnone N., Palumbo D., Cremona G., et al. Residual lung damage following ARDS in COVID-19 ICU survivors. Acta Anaesthesiol Scand. 2022;66(2):223–231. doi: 10.1111/aas.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.González J., Benítez I.D., Carmona P., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27(4):328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myall K.J., Mukherjee B., Castanheira A.M., et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hama Amin B.J., Kakamad F.H., Ahmed G.S., et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond) 2022;77 doi: 10.1016/j.amsu.2022.103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raveendran A.V. Long COVID-19: challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr. 2021;15(1):145–146. doi: 10.1016/j.dsx.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munblit D., O’Hara M.E., Akrami A., Perego E., Olliaro P., Needham D.M. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632–634. doi: 10.1016/S2213-2600(22)00135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikkelsen M.E., Still M., Anderson B.J., et al. Society of Critical Care Medicine’s international consensus conference on prediction and identification of long-term impairments after critical illness. Crit Care Med. 2020;48(11):1670–1679. doi: 10.1097/CCM.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen M.E., Still M., Anderson B.J., et al. Society of Critical Care Medicine’s International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness. Crit Care Med. 2020;48(11):1670–1679. doi: 10.1097/CCM.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 50.Enright P.L. The six-minute walk test. Respir Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 51.Group T.E. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 52.Gold L.H. DSM-5 and the assessment of functioning: the World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) J Am Acad Psychiatry Law. 2014;42(2):173–181. [PubMed] [Google Scholar]
- 53.Klok F.A., Boon G.J., Barco S., et al. The Post-COVID-19 Functional Status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss D., Marmar C. Guilford Press; 1997. Assessing Psychological Trauma and PTSD; pp. 399–411. [Google Scholar]
- 55.Kroenke K., Spitzer R.L. SLACK Incorporated; 2002. The PHQ-9: A New Depression Diagnostic and Severity Measure; pp. 509–515. [Google Scholar]
- 56.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Internal Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 57.Montgomery S.A., Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 58.Twigg E., Humphris G., Jones C., Bramwell R., Griffiths R.D. Use of a screening questionnaire for post-traumatic stress disorder (PTSD) on a sample of UK ICU patients. Acta Anaesthesiol Scand. 2008;52(2):202–208. doi: 10.1111/j.1399-6576.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 59.Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 60.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Am J Geriatr Psychiatry. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 61.Wittich W., Phillips N., Nasreddine Z.S., Chertkow H. Sensitivity and specificity of the Montreal Cognitive Assessment modified for individuals who are visually impaired. J Vis Impair Blind. 2010;104(6):360–368. [Google Scholar]
- 62.Wong A., Nyenhuis D., Black S.E., et al. Montreal Cognitive Assessment 5-minute protocol is a brief, valid, reliable, and feasible cognitive screen for telephone administration. Stroke. 2015;46(4):1059–1064. doi: 10.1161/STROKEAHA.114.007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Randolph C., Tierney M.C., Mohr E., Chase T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exper Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 64.Greenhalgh T., Knight M. Long COVID: a primer for family physicians. Am Fam Physician. 2020;102(12):716–717. [PubMed] [Google Scholar]
- 65.Greenhalgh T., Knight M., Buxton M., Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 66.Gloeckl R., Leitl D., Jarosch I., et al. Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ Open Res. 2021;7(2):00108–2021. doi: 10.1183/23120541.00108-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zampogna E., Paneroni M., Belli S., et al. Pulmonary rehabilitation in patients recovering from COVID-19. Respiration. 2021;100(5):416–422. doi: 10.1159/000514387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang T.J., Chau B., Lui M., Lam G.-T., Lin N., Humbert S. PM&R and pulmonary rehabilitation for COVID-19. Am J Phys Med Rehabil. 2020;9:769–774. doi: 10.1097/PHM.0000000000001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinnock H., Murphie P., Vogiatzis I., Poberezhets V. Telemedicine and virtual respiratory care in the era of COVID-19. ERJ Open Res. 2022;8(3):00111–2022. doi: 10.1183/23120541.00111-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotfis K., van Diem-Zaal I., Roberson S.W., et al. The future of intensive care: delirium should no longer be an issue. Crit Care. 2022;26(1):1–11. doi: 10.1186/s13054-022-04077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mcloughlin B.C., Miles A., Webb T.E., et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020;11(5):857–862. doi: 10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmes E.A., O’Connor R.C., Perry V.H., et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beaud V., Crottaz-Herbette S., Dunet V., et al. Pattern of cognitive deficits in severe COVID-19. J Neurol Neurosurg Psychiatry. 2021;92(5):567–568. doi: 10.1136/jnnp-2020-325173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daste C, Ficarra S, Dumitrache A, et al. Post-intensive care syndrome in patients surviving COVID-19. Ann Phys Rehabil Med. 2021;64(6):101549. doi: 10.1016/j.rehab.2021.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Pémille C.V., Ray A., Michel A., et al. Prevalence and prospective evaluation of cognitive dysfunctions after SARS due to SARS-CoV-2 virus. The COgnitiVID study. Rev Neurol (Paris) 2022;8:802–807. doi: 10.1016/j.neurol.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nersesjan V., Fonsmark L., Christensen R.H., et al. Neuropsychiatric and cognitive outcomes in patients 6 months after COVID-19 requiring hospitalization compared with matched control patients hospitalized for non–COVID-19 illness. JAMA Psychiatry. 2022;79(5):486–497. doi: 10.1001/jamapsychiatry.2022.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46(13):2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaseda E.T., Levine A.J. Post-traumatic stress disorder: a differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol. 2020;34(7-8):1498–1514. doi: 10.1080/13854046.2020.1811894. [DOI] [PubMed] [Google Scholar]
- 79.Hagen B.I., Lerdal A., Soraas A., et al. Cognitive rehabilitation in post-COVID-19 condition: a study protocol for a randomized controlled trial. Contemp Clin Trials. 2022;122 doi: 10.1016/j.cct.2022.106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waldron-Perrine B., Mudar R., Mashima P., et al. Interprofessional collaboration and communication to facilitate implementation of cognitive rehabilitation in persons with brain injury. J Interprof Care. 2022;36(4):529–537. doi: 10.1080/13561820.2021.1971956. [DOI] [PubMed] [Google Scholar]
- 81.Arora T., Grey I. Health behaviour changes during COVID-19 and the potential consequences: a mini-review. J Health Psychol. 2020;25(9):1155–1163. doi: 10.1177/1359105320937053. [DOI] [PubMed] [Google Scholar]
- 82.Bauer L.K., Caro M.A., Beach S.R., et al. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. Am J Cardiol. 2012;109(9):1266–1271. doi: 10.1016/j.amjcard.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 83.Vai B., Mazza M.G., Colli C.D., et al. Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry. 2021;8(9):797–812. doi: 10.1016/S2215-0366(21)00232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mongodi S., Salve G., Tavazzi G., Politi P., Mojoli F. High prevalence of acute stress disorder and persisting symptoms in ICU survivors after COVID-19. Intensive Care Med. 2021;47(5):616–618. doi: 10.1007/s00134-021-06349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vlake J.H., Wesselius S., van Genderen M.E., van Bommel J., Boxma-de Klerk B., Wils E.J. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: a single-center, observational study. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun N., Wei L., Shi S., et al. A qualitative study on the psychological experience of caregivers of COVID-19 patients. Am J Infect Control. 2020;48(6):592–598. doi: 10.1016/j.ajic.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biehl M, Sese D. Post-intensive care syndrome and COVID-19: implications post pandemic [published online ahead of print August 8, 2020]. Cleve Clin J Med. https://doi.org/10.3949/ccjm.87a.ccc055 [DOI] [PubMed]
- 88.David D., Lynn S.J., Montgomery G.H., editors. Evidence-Based Psychotherapy: the State of the Science and Practice. Wiley Blackwell; 2018. [Google Scholar]
- 89.Anstiss T. Motivational interviewing in primary care. J Clin Psychol Med Settings. 2009;16:87–93. doi: 10.1007/s10880-009-9155-x. [DOI] [PubMed] [Google Scholar]
- 90.Michigan Medicine, University of Michigan. Post-Acute Care Services Patient Guide. Last revised March 2021. https://www.med.umich.edu/1libr/PACS/PACSGuide.pdf
- 91.Heesakkers H., van der Hoeven J.G., Corsten S., et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herridge M.S., Tansey C.M., Matte A., et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 93.Hodgson C.L., Higgins A.M., Bailey M.J., et al. Comparison of 6-month outcomes of survivors of COVID-19 versus non–COVID-19 critical illness. Am J Respir Crit Care Med. 2022;205(10):1159–1168. doi: 10.1164/rccm.202110-2335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlemmer F., Valentin S., Boyer L., et al. Respiratory recovery trajectories after severe-to-critical COVID-19: a 1-year prospective multicentre study. Eur Respir J. 2023;61(4) doi: 10.1183/13993003.01532-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maley J.H., Sandsmark D.K., Trainor A., et al. Six-month impairment in cognition, mental health, and physical function following COVID-19–associated respiratory failure. Crit Care Explor. 2022;4(4):e0673. doi: 10.1097/CCE.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hodgson C.L., Higgins A.M., Bailey M.J., et al. The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care. 2021;25(1):1–12. doi: 10.1186/s13054-021-03794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnan K., Miller A.K., Reiter K., Bonner-Jackson A. Neurocognitive profiles in patients with persisting cognitive symptoms associated with COVID-19. Arch Clin Neuropsychol. 2022;37(4):729–737. doi: 10.1093/arclin/acac004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hatch R., Young D., Barber V., Griffiths J., Harrison D.A., Watkinson P. Anxiety, depression and post traumatic stress disorder after critical illness: a UK-wide prospective cohort study. Crit Care. 2018;22(1):1–13. doi: 10.1186/s13054-018-2223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bienvenu O.J., Friedman L.A., Colantuoni E., et al. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med. 2018;44(1):38–47. doi: 10.1007/s00134-017-5009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Whiteside D.M., Basso M.R., Naini S.M., et al. Outcomes in post-acute sequelae of COVID-19 (PASC) at 6 months post-infection part 1: cognitive functioning. Clin Neuropsychol. 2022;36(4):806–828. doi: 10.1080/13854046.2022.2030412. [DOI] [PubMed] [Google Scholar]
- 101.Myhren H., Ekeberg Ø., Tøien K., Karlsson S., Stokland O. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14(1):1–10. doi: 10.1186/cc8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Danesh V., Boehm L.M., Eaton T.L., et al. Characteristics of post-ICU and post-COVID recovery clinics in 29 U.S. health systems. Crit Care Explor. 2022;4(3) doi: 10.1097/CCE.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9 doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.García-Sánchez C., Calabria M., Grunden N., et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022;12(3) doi: 10.1002/brb3.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ollila H., Pihlaja R., Koskinen S., et al. Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care. 2022;26(1):1–11. doi: 10.1186/s13054-022-04092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zante B., Erne K., Grossenbacher J., Camenisch S.A., Schefold J.C., Jeitziner M.-M. Symptoms of post-traumatic stress disorder (PTSD) in next of kin during suspension of ICU visits during the COVID-19 pandemic: a prospective observational study. BMC Psychiatry. 2021;21(1):1–9. doi: 10.1186/s12888-021-03468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeng B., Chen D., Qiu Z., et al. Expert consensus on protocol of rehabilitation for COVID-19 patients using framework and approaches of WHO International Family Classifications. Aging Med (Milton) 2020;3(2):82–94. doi: 10.1002/agm2.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]