Abstract
Objective
To evaluate the role of visual and quantitative chest CT parameters in assessing treatment response in patients with severe asthma.
Materials and Methods
Korean participants enrolled in a prospective multicenter study, named the Precision Medicine Intervention in Severe Asthma study, from May 2020 to August 2021, underwent baseline and follow-up chest CT scans (inspiration/expiration) 10–12 months apart, before and after biologic treatment. Two radiologists scored bronchiectasis severity and mucus plugging extent. Quantitative parameters were obtained from each CT scan as follows: normal lung area (normal), air trapping without emphysema (AT without emph), air trapping with emphysema (AT with emph), and airway (total branch count, Pi10). Clinical parameters, including pulmonary function tests (forced expiratory volume in 1 s [FEV1] and FEV1/forced vital capacity [FVC]), sputum and blood eosinophil count, were assessed at initial and follow-up stages. Changes in CT parameters were correlated with changes in clinical parameters using Pearson or Spearman correlation.
Results
Thirty-four participants (female:male, 20:14; median age, 50.5 years) diagnosed with severe asthma from three centers were included. Changes in the bronchiectasis and mucus plugging extent scores were negatively correlated with changes in FEV1 and FEV1/FVC (ρ = from -0.544 to -0.368, all P < 0.05). Changes in quantitative CT parameters were correlated with changes in FEV1 (normal, r = 0.373 [P = 0.030], AT without emph, r = -0.351 [P = 0.042]), FEV1/FVC (normal, r = 0.390 [P = 0.022], AT without emph, r = -0.370 [P = 0.031]). Changes in total branch count were positively correlated with changes in FEV1 (r = 0.349 [P = 0.043]). There was no correlation between changes in Pi10 and the clinical parameters (P > 0.05).
Conclusion
Visual and quantitative CT parameters of normal, AT without emph, and total branch count may be effective for evaluating treatment response in patients with severe asthma.
Keywords: Asthma, Air trapping, Airway, Quantitative imaging, Computed tomography
INTRODUCTION
Severe asthma treatment consists of high-dose inhaled corticosteroids, along with a second controller to manage symptoms. Nevertheless, this condition can be persistent and uncontrolled despite the use of these medications [1]. Severe asthma accounts for 3%–5% of cases of asthma, with individuals often presenting daily symptoms and multiple exacerbations each year, requiring substantial corticosteroid treatment [2,3]. Recent advancements in understanding of asthma pathophysiology have led to the development of biologic therapies that target type 2 (T2) inflammatory pathways, resulting in a paradigm shift in the treatment of severe asthma [4,5,6].
Eosinophilic airway inflammation, mucus plugging, goblet cell hyperplasia, smooth muscle hypertrophy, and remodeling of the airway wall constitute important pathophysiologic features in T2-high asthma phenotype [7]. Notably, mucus plugging can be observed in persistent eosinophilic asthma, and it is a plausible mechanism of chronic airflow obstruction in severe asthma [8,9].
Recently, quantitative chest CT studies have been conducted in patients with asthma to explore the pulmonary structure-function correlation [10,11]. Mucus plugging was predominantly observed in subsegmental airways, whereas the number of lung segments with mucus plugs on chest CT was correlated with ventilation defect and reduced lung function in patients with asthma [8]. Eddy et al. [12] studied the total airway count in patients with asthma and observed an increase in the number of missing airways on CT corresponding to a decrease in forced expiratory volume in 1 s (FEV1).
A recent study by McIntosh et al. [13] demonstrated that 129Xe ventilation significantly improved after a single dose of benralizumab in nine patients with severe asthma presenting five or more mucus plugs. Based on this finding, we hypothesized that changes in CT mucus plugging and quantitative CT parameters after treatment may be associated with improved lung function. Therefore, visual and quantitative CT parameters could serve as indicators of treatment response in patients with severe asthma. However, CT analysis before and after treatment for severe asthma remains unexplored. Therefore, this study aimed to assess whether visual and quantitative CT parameters could be correlated with changes in clinical parameters, including pulmonary function, following biologic treatment. Additionally, we examined the correlation between changes in CT mucus plugging and changes in CT quantitative analysis.
MATERIALS AND METHODS
Study Design and Participants
Our study included consecutive Korean participants who were enrolled in the Precision Medicine Intervention in Severe Asthma (PRISM) cohort from May 2020 to August 2021 and underwent chest CT scans before and after therapy for asthma with biologic agents. The PRISM is a prospective, observational, multicenter study including patients with severe asthma. The Institutional Review Board of the participating centers approved the study, and all patients completed a written informed consent form (IRB No. 2019-1676). The study enrolled patients with asthma aged 18–80 years who were diagnosed with severe asthma according to the 2014 American Thoracic Society/European Respiratory Society guidelines II [14]. Patients assessment was performed by respiratory experts. Exclusion criteria were a history of previous administration of other biological agents within 3 months prior to study registration, acute exacerbation requiring the use of systemic steroids, clinically serious respiratory diseases other than severe asthma, concurrent hypereosinophilic syndrome, and difficulty in evaluating asthma alone due to severe respiratory disease. The study design of the PRISM is presented in Supplementary Figure 1. During the initial 4-month period, demographic and clinical information and history of asthma was collected, and participants were screened based on the results of blood tests, sputum analysis and fractional exhaled nitric oxide levels and were assigned different phenotypes of asthma. Patients with severe asthma characterized by the T2 inflammation phenotype were eligible for biologic agents, including mepolizumab, reslizumab, benralizumab, dupilumab, or omalizumab. However, those who did not present this phenotype or those who preferred to continue conventional treatment were administered the standard-of-care treatment for asthma. Chest CT scans were performed at baseline and follow-up, at a 10–12 month interval. Additionally, clinical parameters, including pulmonary function tests, blood eosinophil counts, induced sputum analysis, and asthma control test scores [15], were assessed during these periods. Details regarding the study protocol are published elsewhere [16].
Protocols and Visual Analysis of Chest CT
Patients underwent a non-contrast chest CT scan in the supine position, at full inspiration and expiration, using CT systems from three different vendors at each center (Definition AS, Definition Flash, Siemens Healthcare, Forchheium, Germany; Revolution GE, GE Healthcare, Milwaukee, WI, USA). All parameters used were the same for both the inspiration and expiration scans, which were as follows: 120 kV; 100 effective mAs without dose modulation; pitch, 1; collimation, 0.6; kernel, B30f or standard; slice thickness and reconstruction interval, 0.6 or 0.625/0.5 mm.
Two thoracic radiologists, M.J.L. and H.J.H., with 1 and 14 years of experience in chest imaging, respectively, independently assessed bronchiectasis and mucus plugging extent scores using the Modified Bhalla scoring system [17,18]. This evaluation was performed on both the initial and follow-up chest CT scans. Radiologists were blinded to information such as the types of drugs administered and clinical parameters, as well as quantitative CT parameters. The Modified Bhalla scoring system was used to evaluate the severity and extent of bronchiectasis, mucus plugging, and mosaic perfusion on inspiration CT (Supplementary Table 1). For example, the extent of mucus plugging was based on the number of affected bronchopulmonary segments. In this calculation, if more than one distal subsegmental bronchus was involved, the count of affected segmental bronchi was considered as 1, regardless of whether mucus plugging was observed in the proximal segmental bronchus. The mucus plugging scoring criteria were defined as follows: a score of 0 points for absence, 1 for involvement of 1–5 segments, 2 points for involvement of 6–9 segments, and 3 points for involvement of over 9 segments. We used both the total Modified Bhalla (bronchiectasis scores) and mucus plugging extent scores for the analysis.
Quantitative CT Analysis: Air Trapping and Airways
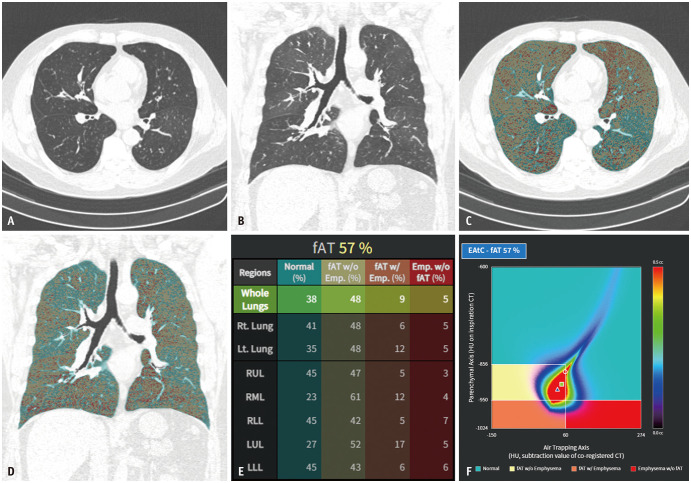
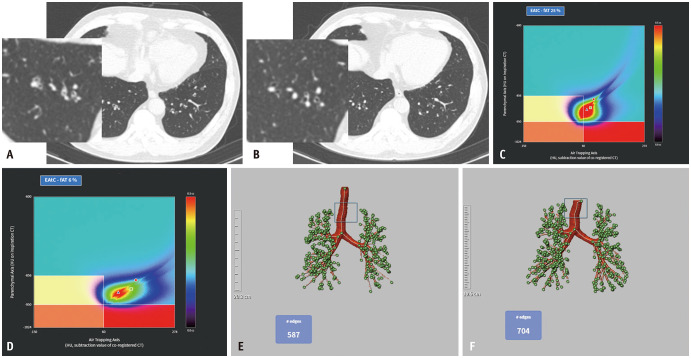
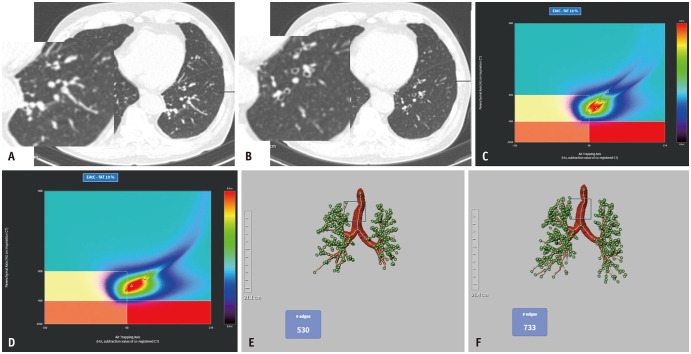
Quantitative CT analysis was performed regarding air trapping (AT) and airways using an automatic segmentation software (Aview; Coreline Soft, Seoul, South Korea). For AT analysis, inspiration and expiration CT images were registered using a non-rigid method [19]. Lung segmentation was performed for emphysema AT composite mapping, classifying the lung parenchyma into three lung areas: areas with functional AT (fAT), areas with emphysema (emph), areas with normal lung parenchyma (normal) (Fig. 1). Emphysema was defined as lung areas with CT attenuation values < -950 Hounsfield unit (HU) during inspiration in the entire lung. fAT was defined as lung areas with a density change < 60 HU between the inspiration and registered expiration CT scans [20] in the lung parenchyma, exhibiting a density < -856 HU on the inspiration CT scan. This 60-HU threshold for the quantification of AT was determined based on correlations with pulmonary function parameters in a previous study [20]. fAT area was divided into AT without emphysema (AT without emph), AT with emphysema (AT with emph), according to -950 HU at inspiration. The remaining lung area was defined as normal [21]. For airway analysis, the wall area percentage, standardized airway wall thickness (Pi10 mm), and total branch count were calculated. The integral-based half-band method was used to measure peripheral airway dimensions [22]. The total branch count was calculated by summing the number of bronchi, considering the area between the start of one branch and the beginning of the next branch as one bronchial count (Figs. 2, 3). All quantitative parameters were measured by radiological technicians with 3–6 years of experience in image analysis and post-processing using a software.
Fig. 1. An example of emphysema and AT composite. A-F: Axial (A) and coronal (B) chest CT images of a patient with asthma are shown. Axial (C) and coronal (D) images with an overlay of the density mask (shown in different colors, indicating areas of AT without emphysema, AT with emphysema, emphysema without AT, and normal), allowing a robust assessment of AT. The results of the analysis (E) provide the percentage volume (relative to the total lung volume) of the aforementioned four areas. The EAtC map (F) displays various colors corresponding to different voxel volumes (indicated by the color bar), correlating with the Hounsfield units of inspiration on the y-axis and the subtraction value of co-registered image on the x-axis. fAT = functional air trapping, EAtC = emphysema air-trapping composite.
Fig. 2. Clinical data from a patient with improvement in AT and total branch count on quantitative CT analysis. A: Diffuse bronchial wall thickening with mucus plugging in both lower lungs on an initial chest CT of a 61-year-old female. B: Follow-up chest CT performed 1 year later reveals improvement in mucus plugging and bronchial wall thickening. C, D: The percentage of the area of fAT is reduced from 25.0% to 6.0% after treatment. E, F: The total bronchial count is increased from 587 to 704. This patient also shows improved lung function with an increase in FEV1 (% predicted) from 80% to 98% and an increase in FEV1/FVC from 0.71 to 0.73. fAT = functional air trapping, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, EAtC = emphysema air-trapping composite.
Fig. 3. Clinical data from a patient with chest CT parameter improvement limited to the total branch count on quantitative CT analysis. A: A 58-year-old male presenting diffuse bronchial wall thickening with mucus-plugging in both lower lobes on the initial CT. B: Follow-up chest CT 1 year later reveals improvement of mucus plugging. C, D: Percentage of the area of fAT without significant improvement, remains at 18% to 19%. E, F: Pi10 improvement is absent, ranging from 3.3 to 3.4 (not shown), whereas the total branch count has increased from 530 to 733. fAT = functional air trapping, EAtC = emphysema air-trapping composite.
Statistical Analysis
Data normality was assessed using the Shapiro–Wilk test, and nonparametric tests were performed when the data did not exhibit a normal distribution. The intraclass correlation coefficient (ICC) was calculated to evaluate the agreement of the decision between the two radiologists (reader 1 [R1] and reader 2 [R2]) on the visual assessment of the bronchiectasis and mucus plugging extent scores. The median visual, quantitative CT, and clinical parameters at baseline were compared with those at follow-up using a Wilcoxon signed rank test. Correlations between visual and quantitative CT parameters on the initial chest CT were assessed using Spearman correlation method. Visual and quantitative CT parameters from the initial chest CT were correlated with clinical parameters, including pulmonary function tests (FEV1 and FEV1/forced vital capacity [FVC]), sputum eosinophil and neutrophil counts, and blood eosinophil counts, using Pearson or Spearman correlation. Additionally, changes (Δ) in visual and quantitative CT parameters before and after treatment were correlated with changes in clinical parameters and changes in the mucus plugging extent scores were correlated with changes in quantitative CT parameters using Pearson or Spearman correlation. Changes in visual, quantitative CT, and clinical parameters were defined as the differences between follow-up and initial data [23]. P values < 0.05 indicated statistical significance. Statistical analyses were performed using commercial software ver. 21 (IBM Corp., Armonk, NY, USA) and R (version 4.2.2; The R Foundation for Statistical Computing; https://cran.r-project.org/bin/windows/base/old/4.2.2).
RESULTS
Characteristics of Participants
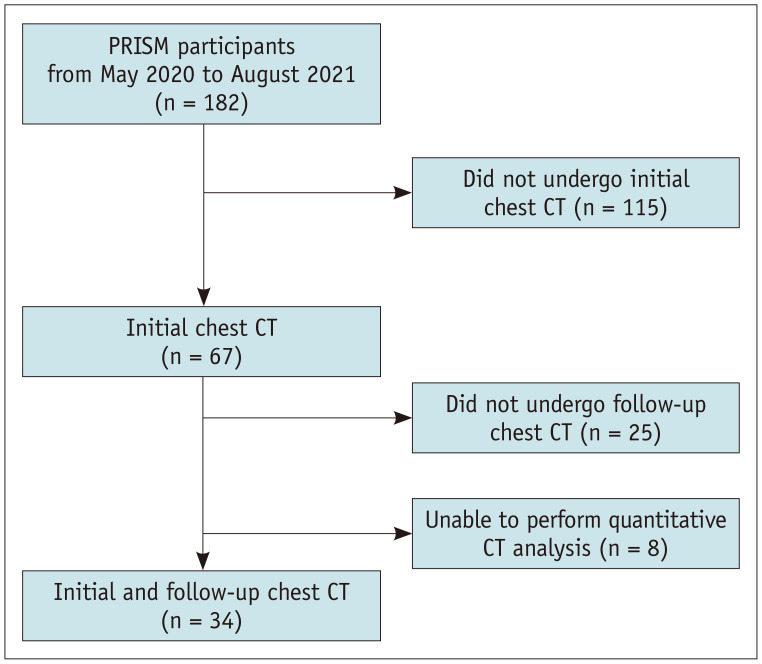
The final sample, comprising 34 patients from three centers, was analyzed (Fig. 4). Out of the 182 participants enrolled in the PRISM between May 2020 and August 2021, 67 underwent initial chest CT scans. Among them, follow-up chest CT scans were not performed in 25 due to the early termination of the study, whereas 8 patients were excluded due to technical issues related to the acquisition and/or analysis of CT images. The participant characteristics are detailed in Table 1.
Fig. 4. Flowchart depicting the process of inclusion of the participants. PRISM = Precision Medicine Intervention in Severe Asthma.
Table 1. Baseline characteristics.
| Characteristics | Value | |
|---|---|---|
| Age, yr | 50.5 (42.3, 62.0) | |
| Sex, female | 20 (58.8) | |
| BMI, kg/m2 | 24.0 (21.8, 27.1) | |
| Smoking | ||
| None | 12 (35.3) | |
| Ex-smoker | 19 (55.9) | |
| Current smoker | 3 (8.8) | |
| Duration of asthma, yr | 9.5 (5.0, 18.5) | |
| History of biologic treatment* | 11 (32.4) | |
| Treatment of asthma | ||
| Biologic treatment† | 28 (82.4) | |
| Conventional treatment | 6 (17.6) | |
Data are presented as median with interquartile range or numbers with percentage in parentheses.
*Indicates prior use of biologic agents with a discontinuation period of at least 3 months before study enrollment, †Indicates treatment during the study period and includes omalizumab (n = 1), mepolizumab (n = 9), reslizumab (n = 15), dupilumab (n = 3).
BMI = body mass index
Table 2 shows the clinical and CT parameters from the initial and follow-up chest CT and their changes (Δ). The FEV1 and FEV1/FVC increased significantly following treatment, and the asthma control test scores significantly increased after treatment, from 18.0 to 22.5 points. All median values of visual and quantitative CT parameters, except for emphysema without AT, presented significant improvement after treatment (all P < 0.05). The ICC denoting the agreement on the bronchiectasis and mucus plugging extent scores was 0.808 (95% confidence interval [CI], 0.616–0.904) and 0.937 (95% CI, 0.874–0.969), respectively. The distribution of the mucus plugging extent scores by the smoking status of the participants is presented in Supplementary Figure 2.
Table 2. Results of clinical parameters and chest CT analysis.
| Clinical and quantitative CT parameters | Initial | Follow-up | Changes (Δ) between follow-up and initial data | P | ||
|---|---|---|---|---|---|---|
| Clinical parameters | ||||||
| Pulmonary function* | ||||||
| FEV1, % predicted | 65.5 (58.0, 77.3) | 76.5 (66.8, 87.3) | 9.0 (3.0, 21.0) | < 0.001 | ||
| FEV1/FVC | 0.7 (0.6, 0.8) | 0.7 (0.6, 0.8) | 0.1 (0.0, 0.1) | 0.002 | ||
| FeNO, ppb | 56.5 (25.0, 90.3) | 34.5 (20.0, 67.5) | -8.0 (-34.0, 4.5) | 0.024 | ||
| Blood eosinophils, % | 9.0 (2.5, 11.4) | 0.8 (0.5, 2.1) | -6.2 (-10.1, -0.6) | < 0.001 | ||
| Sputum eosinophils, % | 5.5 (0.0, 31.3) | 1.0 (0.0, 20.8) | -0.5 (-33.5, 12.3) | 0.219 | ||
| Sputum neutrophil, % | 75.0 (48.0, 92.0) | 62.5 (10.5, 95.0) | -2.5 (-44.0, 37.8) | 0.641 | ||
| Asthma control test score | 18.0 (12.0, 20.0) | 22.5 (19.0, 24.0) | 3.0 (0.0, 8.0) | < 0.001 | ||
| Visual CT scores | ||||||
| Bronchiectasis, R1 | 8.0 (4.0, 12.0) | 6.0 (3.0, 10.0) | -1.0 (-3.0, 0.0) | < 0.001 | ||
| Bronchiectasis, R2 | 5.8 (10.0, 12.3) | 4.5 (8.0, 11.0) | -2.0 (-1.0, 0.0) | 0.001 | ||
| Mucus plugging extent, R1 | 2.0 (1.0, 3.0) | 0.0 (1.0, 2.0) | -2.0 (-1.0, 0.0) | < 0.001 | ||
| Non-smoker | 2.0 (1.0, 3.0) | 0.5 (0.0, 1.0) | -1.0 (-2.0, 0.0) | 0.016 | ||
| Ever-smoker | 2.0 (0.8, 3.0) | 1.0 (0.0, 1.0) | -1.0 (-2.0, 0.0) | 0.002 | ||
| Mucus plugging extent, R2 | 2.0 (1.0, 3.0) | 0.0 (1.0, 2.0) | -0.5 (-1.0, 0.0) | 0.001 | ||
| Non-smoker | 3.0 (1.0, 3.0) | 1.0 (0.3, 2.0) | -0.5 (-1.8, 0.0) | 0.026 | ||
| Ever-smoker | 2.0 (1.8, 3.0) | 1.0 (0.0, 3.0) | -0.5 (-1.0, 0.0) | 0.018 | ||
| Quantitative CT parameters | ||||||
| Air trapping | ||||||
| Normal lung area, % | 81.4 (61.9, 93.8) | 87.1 (78.2, 95.5) | 3.9 (0.8, 15.0) | 0.001 | ||
| Air trapping without emphysema, % | 16.6 (4.6, 30.6) | 9.2 (4.2, 17.8) | -2.7 (-15.2, -0.7) | < 0.001 | ||
| Air trapping with emphysema, % | 0.3 (0.1, 2.5) | 0.3 (0.1, 1.1) | -0.1 (-0.9, 0.1) | 0.021 | ||
| Emphysema without air trapping, % | 1.8 (0.5, 2.9) | 1.5 (0.6, 4.7) | 0.3 (-1.1, 1.8) | 0.242 | ||
| Airway | ||||||
| Total branch count | 481.0 (380.0, 589.0) | 541.0 (432.0, 696.5) | 59.5 (-22.8, 103.8) | 0.007 | ||
| Pi10, mm | 4.2 (3.6, 4.8) | 3.76 (3.5, 4.3) | -0.2 (-0.6, 0.1) | 0.014 | ||
| Wall area, % | 62.0 (57.6, 69.8) | 59.4 (55.7, 65.7) | -1.7 (-7.1, 2.2) | 0.041 | ||
Data are presented as median with interquartile range in parentheses.
*Indicates value before bronchodilator.
FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity, FeNO = fractional exhaled nitric oxide, R1 = reader 1, R2 = reader 2
Correlation between Visual and Quantitative CT Parameters
Supplementary Table 2 shows the correlation between visual and CT quantitative analysis on the initial chest CT. The bronchiectasis scores and mucus plugging extent scores showed similar correlation with CT parameters; both were negatively correlated with the normal lung area (ρ: from -0.683 to -0.516, all P < 0.05) and positively correlated with AT without emph (ρ: from 0.500 to 0.685, P ≤ 0.05) and AT with emph (ρ: from 0.563 to 0.672, P ≤ 0.001). Regarding airway analysis, only the mucus plugging extent scores were negatively correlated with total branch count (R1: ρ = -0.537, P = 0.001; R2: ρ = -0.587, P < 0.001).
Correlation of Visual and Quantitative CT Parameters with Clinical Parameters
The associations between visual and quantitative parameters from the initial CT with clinical parameters, including pulmonary function tests (FEV1, FEV1/FVC), sputum eosinophil and neutrophil counts, and blood eosinophil counts, are presented in Table 3. The mucus plugging extent scores showed a stronger correlation with clinical parameters, compared with the bronchiectasis scores. Specifically, the mucus plugging extent scores demonstrated significant negative correlations with FEV1 (R1: ρ = -0.392, P = 0.022; R2: ρ = -0.427, P = 0.012) and FEV1/FVC (R1: ρ = -0.521, P = 0.002; R2: ρ = -0.538, P = 0.001) and positive correlations with sputum eosinophil count (R1: ρ = 0.566; R2: ρ = 0.551, all P = 0.001).
Table 3. Correlation of visual and quantitative CT analysis from the initial CT with clinical parameters.
| Clinical parameter | Visual assessment scores | Quantitative analysis: air trapping | Quantitative analysis: airway | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bronchiectasis* | Mucus plugging extent* | Normal lung area | Air trapping without emphysema | Air trapping with emphysema* | Emphysema without air trapping | Total branch count | Pi10 | Wall area | |||
| R1 | R2 | R1 | R2 | ||||||||
| FEV1 | -0.251 (0.153) | -0.344† (0.047) | -0.392† (0.022) | -0.427† (0.012) | 0.622† (< 0.001) | -0.585† (< 0.001) | -0.509† (0.002) | -0.252 (0.151) | 0.444† (0.009) | -0.387† (0.024) | -0.427† (0.012) |
| FEV1/FVC | -0.317 (0.067) | -0.406† (0.017) | -0.521† (0.002) | -0.538† (0.001) | 0.803† (< 0.001) | -0.760† (< 0.001) | -0.684† (< 0.001) | -0.378† (0.027) | 0.579† (< 0.001) | -0.454† (0.007) | -0.465† (0.006) |
| Sputum eosinophils* | 0.220 (0.225) | 0.315 (0.079) | 0.566† (0.001) | 0.551† (0.001) | -0.356† (0.049) | 0.349 (0.054) | 0.339 (0.062) | 0.209 (0.259) | -0.315 (0.084) | 0.171 (0.358) | 0.257 (0.156) |
| Sputum neutrophils | -0.0222 (0.905) | -0.255 (0.159) | -0.259 (0.152) | -0.351† (0.049) | 0.300 (0.096) | -0.278 (0.124) | -0.219 (0.236) | -0.136 (0.459) | 0.435† (0.013) | -0.332 (0.063) | -0.338 (0.059) |
| Blood eosinophils | -0.052 (0.772) | -0.083 (0.640) | 0.269 (0.124) | 0.208 (0.238) | 0.115 (0.516) | -0.069 (0.697) | -0.212 (0.236) | -0.270 (0.122) | -0.354† (0.040) | 0.178 (0.315) | 0.128 (0.469) |
Data are persented as correlation coefficients determed using the Pearson’s correlation test or *Spearman’s correlation test with corresponding P value in parentheses, †P < 0.05.
R1 = reader 1, R2 = reader 2, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity
Quantitative CT parameters of AT, normal lung area, AT without emph, and AT with emph were correlated with pulmonary function tests, with the strongest correlation observed with FEV1/FVC (normal: r = 0.803, AT without emph: r = -0.760, AT with emph = -0.684, all P < 0.001). Total branch count, Pi10, and wall area were correlated with pulmonary function tests. The total branch count was also correlated with sputum neutrophil (r = 0.435, P = 0.013) and blood eosinophil counts (r = -0.354, P = 0.040).
Correlation of Changes in Visual and Quantitative CT Parameters with Changes in Clinical Parameters
Changes in the bronchiectasis scores and mucus plugging extent scores were negatively correlated with changes in FEV1 and FEV1/FVC (ρ: from -0.544 to -0.368, P < 0.05), except the correlation between the bronchiectasis score (R1) and FEV1/FVC (Table 4).
Table 4. Correlations of changes in visual and quantitative CT parameters before and after treatment with clinical characteristics.
| ΔClinical parameters | Visual assessment scores | Quantitative analysis: air trapping | Quantitative analysis: airway | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ΔBronchiectasis* | ΔMucus plugging extent* | ΔNormal lung area | ΔAir trapping without emphysema | ΔAir trapping with emphysema* | ΔEmphysema without air trapping | ΔTotal branch count | ΔPi10 | |||
| ΔR1 | ΔR2 | ΔR1 | ΔR2 | |||||||
| ΔFEV1 | -0.368† (0.032) | -0.544† (0.001) | -0.511† (0.002) | -0.519† (0.002) | 0.373† (0.030) | -0.351† (0.042) | -0.187 (0.288) | 0.118 (0.505) | 0.349† (0.043) | 0.252 (0.151) |
| ΔFEV1/FVC | -0.224 (0.204) | -0.507† (0.002) | -0.373† (0.030) | -0.420† (0.013) | 0.390† (0.022) | -0.370† (0.031) | -0.210 (0.233) | 0.042 (0.815) | 0.161 (0.363) | 0.151 (0.394) |
| ΔSputum eosinophils | 0.137 (0.487) | 0.318 (0.099) | 0.294 (0.129) | 0.175 (0.374) | -0.196 (0.317) | 0.165 (0.402) | 0.165 (0.400) | 0.089 (0.653) | -0.062 (0.755) | -0.254 (0.192) |
| ΔBlood eosinophils | 0.159 (0.368) | 0.284 (0.104) | 0.085 (0.631) | 0.317 (0.068) | 0.130 (0.464) | -0.075 (0.673) | -0.157 (0.374) | -0.208 (0.239) | -0.107 (0.548) | -0.147 (0.406) |
Data are persented as correlation coefficients determed using the Pearson’s correlation test or *Spearman’s correlation test with corresponding P value in parentheses, †P < 0.05.
Δ = interval change between initial and follow up, R1 = reader 1, R2 = reader 2, FEV1 = forced expiratory volume in 1 s, FVC = forced vital capacity
Among the quantitative CT parameters of AT, changes in normal lung area were positively correlated with changes in FEV1 (r = 0.373, P = 0.030) and FEV1/FVC (r = 0.390, P = 0.022). Changes in AT without emph were negatively correlated with FEV1 (ρ = -0.351, P = 0.042) and FEV1/FVC (ρ = -0.370, P = 0.031). Regarding the airway, only the change in total branch count showed a significant positive correlation with the change in FEV1 (r = 0.349, P = 0.043), whereas Pi10 did not show any correlation with any clinical parameter (all P > 0.05).
When assessing the correlation between changes in the mucus plugging extent scores and changes in quantitative CT measures, the following correlations were observed: normal lung area (R1: ρ = -0.661 P < 0.001; R2: ρ = -0.389 P = 0.023), AT without emph (R1: ρ = 0.659, P < 0.001; R2: ρ = 0.425, P = 0.012), AT with emph (R1: ρ = 0.683, P < 0.001; R2: ρ = 0.407, P = 0.017), and total branch count (R1: ρ = -0.481, P = 0.004; R2: ρ = -0.415, P = 0.015) (Supplementary Fig. 3).
DISCUSSION
This study showed that changes in visual and quantitative CT parameters of AT and total branch count are correlated with improved pulmonary function tests. In addition, improved mucus plugging extent presented a good correlation with ameliorated quantitative CT parameters. These results suggest the potential role of quantitative CT analysis in assessing the functional response after biologic treatment in patients with severe asthma.
Among patients with severe asthma, those with mucus plugging had worse lung function, more frequent severe exacerbations, and higher T2 biomarkers than did those without [9]. Tang et al. [24] demonstrated that in the analysis of initial CT and follow-up CT after 3 years, changes in the mucus plugging scores were associated with changes in lung function and CT AT parameters. We observed similar results in our study, with the changes in the mucus plugging extent scores showing a correlation with the changes in CT parameters of AT and pulmonary function tests. This finding suggests that improved mucus plugging plays an important role in mechanisms of treatment-related changes. Therefore, evaluation of mucus plugging extent with chest CT is important for making an appropriate treatment choice, such as a mucoactive drug or biologic medication, rather than conventional treatment in this phenotype of asthma.
In our study, visual assessment was performed separately for R1 and R2 rather than using a consensus result, because we aimed to demonstrate how each result correlates differently with the CT quantitative analysis. Although the correlation coefficients varied between the readers, the mucus plugging extent scores assessed by both R1 and R2 exhibited similar correlations with clinical parameters. To confirm these results, further studies employing quantitative parameters of bronchiectasis severity and mucus plugging extent are needed.
Total airway count correlates with respiratory function in chronic obstructive pulmonary disease [25,26], and a few recent studies reported total airway count in asthma. Eddy et al. [12] demonstrated that the total airway count was reduced in advanced stage of asthma, which was related to airway wall thickness and lumen area. Additionally, this count was moderately related to FEV1. Another study showed that the total airway count significantly increased after treatment with benralizumab in 11 patients with severe eosinophilic asthma [27]. We also observed that the mean total branch count increased from 480.0 ± 164.6 to 540.2 ± 178.0 after treatment, and these changes were correlated with improved FEV1. The reduced number of airways may occur due to airway wall thickening and obstruction by mucus plugging [12], and it may be associated with the susceptibility or pathogenesis of severe asthma. Our results further support the idea that total airway count could be used to assess treatment response in patients with severe asthma.
According to findings from our study, Pi10 was not a useful parameter for the assessment of treatment response, although it was correlated with initial pulmonary function parameters. It is postulated that in the bronchial analysis on the initial chest CT using the software, airways obstructed by mucus were excluded from the analysis for Pi10 assessment. However, as mucus plugging was associated with severe airway thickening, excluding these bronchi may have led to an underestimation of the initial Pi10 value. This is considered a limitation inherent to the current CT analysis. Therefore, a method that reflects bronchi obstructed by mucus needs to be developed for Pi10 calculation. Tsubokawa et al. [27] found an additional reason explaining the lack of correlation between Pi10 and baseline pulmonary function parameters. They observed that bronchial wall thickening did not significantly improve except for the third and sixth generation bronchi following biologic treatment in severe eosinophilic asthma. Findings of this study suggested that the partial improvement in the airways may be primarily attributed to the clearing of mucus rather than changes in the bronchial wall itself.
In a previous study, 57% of smokers exhibited mucus plugging on CT [28]. In our study, 3 patients were current smokers, and 19 were ex-smokers. Although we could not differentiate mucus plugging related to smoking and asthma, the initial mucus plugging extent scores did not significantly vary based on smoking status. Additionally, as the smoking status remained unchanged between the initial and follow-up periods, we hypothesized that alterations in mucus plugging post-treatment could primarily be attributed to treatment response rather than being an effect of smoking.
This study has some limitations. First, the sample size was small; thus the findings can be potentially affected by a selection bias. Further research is required to validate the clinical reliability of CT analysis. Second, although most of the patients in our study received biologic treatment, we also included six patients treated with conventional medication in the analysis. Subsequently, our goal was to collect additional data and analyze treatment responses based on biologic medication. Third, we could not evaluate quantitative parameters of mucus plugging extent itself, and further study using this measurement would be needed for objective evaluation. Lastly, we could not perform a location-specific correlation between visual and quantitative CT parameters.
In conclusion, visual and quantitative CT analysis of AT and total branch count could represent an effective method for evaluating treatment response in patients with severe asthma. Additionally, we demonstrated that mucus plugging extent is an important factor related to the post-treatment changes in quantitative CT parameters.
Footnotes
Conflicts of Interest: Joon Beom Seo, who holds the respective position of Editorial Board Member of the Korean Journal of Radiology, was not involved in the editorial evaluation or decision to publish this article. The remaining author has declared no conflicts of interest.
Joon Beom Seo and Sang Min Lee are stockholders of Coreline Soft and received royalties for licensing the patent and knowhow of image quantification.
- Conceptualization: Sang Min Lee, Tae-Bum Kim.
- Data curation: Miji Lee, Hye Jeon Hwang, Sang Min Lee.
- Formal analysis: Miji Lee, Hye Jeon Hwang.
- Funding acquisition: Sang Min Lee, Tae-Bum Kim.
- Investigation: Miji Lee, Hye Jeon Hwang, Jin An, Ji-Hyang Lee.
- Methodology: Han Na Lee, Sang Min Lee, Tae-Bum Kim.
- Project administration: Sang Min Lee, Tae-Bum Kim.
- Resources: Ji-Hyang Lee, Min-Hye Kim, Young-Joo Cho.
- Software: Jooae Choe, Jihye Yoon.
- Supervision: Sang Min Lee, Tae-Bum Kim, Joon Beom Seo.
- Validation: Han Na Lee, Sang Min Lee.
- Visualization: Miji Lee, Hye Jeon Hwang.
- Writing—original draft: Han Na Lee, Sang Min Lee.
- Writing—review & editing: Han Na Lee, Sang Min Lee, Tae-Bum Kim, Joon Beom Seo.
Funding Statement: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI22C1723). This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. 2019M3E5D3073365). This work was supported by the NRF grant funded by the MSIT (No. RS-2023-00211367).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2024.0110.
References
- 1.Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med. 2022;205:17–35. doi: 10.1164/rccm.202109-2205PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kavanagh JE, Hearn AP, Jackson DJ. A pragmatic guide to choosing biologic therapies in severe asthma. Breathe (Sheff) 2021;17:210144. doi: 10.1183/20734735.0144-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen S, von Bulow A, Sandin P, Ernstsson O, Janson C, Lehtimaki L, et al. Prevalence and management of severe asthma in the Nordic countries: findings from the NORDSTAR cohort. ERJ Open Res. 2023;9:00687–02022. doi: 10.1183/23120541.00687-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maspero J, Adir Y, Al-Ahmad M, Celis-Preciado CA, Colodenco FD, Giavina-Bianchi P, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res. 2022;8:00576–02021. doi: 10.1183/23120541.00576-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragonieri S, Carpagnano GE. Biological therapy for severe asthma. Asthma Res Pract. 2021;7:12. doi: 10.1186/s40733-021-00078-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoettler N, Strek ME. Recent advances in severe asthma: from phenotypes to personalized medicine. Chest. 2020;157:516–528. doi: 10.1016/j.chest.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busse WW, Kraft M, Rabe KF, Deniz Y, Rowe PJ, Ruddy M, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J. 2021;58:2003393. doi: 10.1183/13993003.03393-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan R, Duraikannu C, Lipworth B. Clinical associations of mucus plugging in moderate to severe asthma. J Allergy Clin Immunol Pract. 2023;11:195–199.e2. doi: 10.1016/j.jaip.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Verbanck S. Quantitative computed tomography in asthma: for good measure. Am J Respir Crit Care Med. 2020;201:885–886. doi: 10.1164/rccm.201912-2481ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svenningsen S, Haider E, Boylan C, Mukherjee M, Eddy RL, Capaldi DPI, et al. CT and functional MRI to evaluate airway mucus in severe asthma. Chest. 2019;155:1178–1189. doi: 10.1016/j.chest.2019.02.403. [DOI] [PubMed] [Google Scholar]
- 12.Eddy RL, Svenningsen S, Kirby M, Knipping D, McCormack DG, Licskai C, et al. Is computed tomography airway count related to asthma severity and airway structure and function? Am J Respir Crit Care Med. 2020;201:923–933. doi: 10.1164/rccm.201908-1552OC. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh MJ, Kooner HK, Eddy RL, Jeimy S, Licskai C, Mackenzie CA, et al. Asthma control, airway mucus, and 129Xe MRI ventilation after a single benralizumab dose. Chest. 2022;162:520–533. doi: 10.1016/j.chest.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 15.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, et al. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129(3 Suppl):S24–S33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Dixey P, Bhavsar P, Raby K, Kermani N, Chadeau-Hyam M, et al. Precision medicine intervention in severe asthma (PRISM) study: molecular phenotyping of patients with severe asthma and response to biologics. ERJ Open Res. 2023;9:00485–02022. doi: 10.1183/23120541.00485-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179:783–788. doi: 10.1148/radiology.179.3.2027992. [DOI] [PubMed] [Google Scholar]
- 18.Tulek B, Kivrak AS, Ozbek S, Kanat F, Suerdem M. Phenotyping of chronic obstructive pulmonary disease using the modified Bhalla scoring system for high-resolution computed tomography. Can Respir J. 2013;20:91–96. doi: 10.1155/2013/727523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EY, Seo JB, Lee HJ, Kim N, Lee E, Lee SM, et al. Detailed analysis of the density change on chest CT of COPD using nonrigid registration of inspiration/expiration CT scans. Eur Radiol. 2015;25:541–549. doi: 10.1007/s00330-014-3418-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee SM, Seo JB, Lee SM, Kim N, Oh SY, Oh YM. Optimal threshold of subtraction method for quantification of airtrapping on coregistered CT in COPD patients. Eur Radiol. 2016;26:2184–2192. doi: 10.1007/s00330-015-4070-z. [DOI] [PubMed] [Google Scholar]
- 21.Hwang HJ, Seo JB, Lee SM, Kim N, Yi J, Lee JS, et al. New method for combined quantitative assessment of air-trapping and emphysema on chest computed tomography in chronic obstructive pulmonary disease: comparison with parametric response mapping. Korean J Radiol. 2021;22:1719–1729. doi: 10.3348/kjr.2021.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YH, Seo JB, Kim N, Lee HJ, Hwang HJ, Kim EY, et al. Comparison of a new integral-based half-band method for CT measurement of peripheral airways in COPD with a conventional full-width half-maximum method using both phantom and clinical CT images. J Comput Assist Tomogr. 2015;39:428–436. doi: 10.1097/RCT.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 23.Lee SW, Lee SM, Shin SY, Park TS, Oh SY, Kim N, et al. Improvement in ventilation-perfusion mismatch after bronchoscopic lung volume reduction: quantitative image analysis. Radiology. 2017;285:250–260. doi: 10.1148/radiol.2017162148. [DOI] [PubMed] [Google Scholar]
- 24.Tang M, Elicker BM, Henry T, Gierada DS, Schiebler ML, Huang BK, et al. Mucus plugs persist in asthma, and changes in mucus plugs associate with changes in airflow over time. Am J Respir Crit Care Med. 2022;205:1036–1045. doi: 10.1164/rccm.202110-2265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat M, Hague CJ, et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am J Respir Crit Care Med. 2018;197:56–65. doi: 10.1164/rccm.201704-0692OC. [DOI] [PubMed] [Google Scholar]
- 26.Wu F, Jiang C, Zhou Y, Zheng Y, Tian H, Li H, et al. Association of total airway count on computed tomography with pulmonary function decline in early-stage COPD: a population-based prospective cohort study. Int J Chron Obstruct Pulmon Dis. 2021;16:3437–3448. doi: 10.2147/COPD.S339029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsubokawa F, Koya T, Murai Y, Tanaka K, Tsutsui Y, Naramoto S, et al. Effects of benralizumab on three-dimensional computed tomography analysis in severe eosinophilic asthma. Int Arch Allergy Immunol. 2023;184:243–251. doi: 10.1159/000525846. [DOI] [PubMed] [Google Scholar]
- 28.Dunican EM, Elicker BM, Henry T, Gierada DS, Schiebler ML, Anderson W, et al. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med. 2021;203:957–968. doi: 10.1164/rccm.202006-2248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.