Abstract
In vascular neurosurgery, dural arteriovenous fistulas (DAVFs) are a difficult, challenging condition whose natural history and therapy are still debated. This case report presented a 30-year-old male patient who experienced intermittent headaches for two months, along with gradual weakness in all four limbs, resulting in quadriplegia. Magnetic resonance imaging (MRI), computed tomography (CT), and digital subtraction angiography (DSA) played a significant role in the diagnosis of the patient, in which the final diagnosis was vascular myelopathy due to Dural arteriovenous fistula (DAVF). A successful embolization procedure of arteriovenous fistula using balloon-assisted liquid embolic agents, through branches of the right occipital artery was performed, resulting in complete obliteration of the fistula. In order to improve the neurovascular symptoms that had previously been reported, the patient was effectively undergoing rehabilitation, with notable progress.
Keywords: Intracranial, Dural arteriovenous fistula, MRA, CTA
Introduction
A pathological shunt is observed between dural arteries and dural venous sinuses, cortical veins, or meningeal veins, a condition known as intracranial dural arteriovenous fistulae (DAVF) [1].
10% to 15% of all cerebral arteriovenous malformations are caused by pathologic dural-based shunts or intracranial DAVFs. Meningeal vessels provide the majority of the venous drainage for these abnormalities, while the dural venous sinuses or cortical veins handle the venous supply [2].
More than one DAVF, localized in different intracranial regions, was noted in approximately 8% of the patients [3].
DAVFs are predominantly idiopathic; dural sinus thrombosis, trauma, and a history of previous craniotomies are connected to a small percentage of patients [4,5]. In pediatric patients, DAVF is reported due to congenital causes or results from infection, birth trauma, in-utero venous thrombosis, or maternal hormones [6].
To distinguish the DAVF, a different classification system was developed: the Borden-Shucart classification, which is based on venous drainage characteristics [7,8], the Cognard classification, which is predicated on shunt location, venous drainage characteristics, and venous outflow angioarchitecture [8,9], and Zipfel et al., who modified the Borden-Shucart model based on new natural history data, where the mode of presentation markedly impacts the future risk of intracranial hemorrhage (ICH) and None Hemorrhagic Neurological Deficit (NHND) was indicated [10].
IDVFS diagnoses are difficult because the symptoms vary depending on where the fistula is located. These are rare, complex diseases with a variety of presentations [11], including the presentation of symptoms leading to diagnosis at any age with a mean age at the time of diagnosis of 50 to 60 years [9]. Because the patient's symptoms can change based on several factors, such as the location of the fistula and the existence of cerebral venous reflux, this could result in inappropriate care of the patient's condition. In high-grade DAVFs with cortical venous reflux, the annual risk of bleeding can reach 20% [12].
Different diagnostic modalities can be employed for the assessment and diagnosis of DAVFs; conventional digital subtraction angiography (DSA) is the gold standard, although it lacks proper identification of brain morphology. To evaluate DAVFs, magnetic resonance imaging (MRI) is also utilized. This advances methods by providing MR angiography (MRA) without the use of invasive procedures or ionizing radiation. Advanced magnetic resonance imaging (MRI) techniques like as susceptibility-weighted imaging (SWI) and dynamic contrast-enhanced magnetic resonance imaging (dCE-MRA) provide valuable real-time physio-pathological information regarding the hemodynamics of DAVFs [13], Narvid et al., [14] evaluated CTA imaging with more than 86% sensitivity to identify the arterial feeders, while Kwon et al. [15] evaluated the time of flight MRA in the detection of DAVF with more than 91% sensitivity in abnormal venous flow-related enhancement.
Early refluxes and treatment of these diseases are critical since high-grade DAVFs with cortical venous reflux carry substantial dangers, including brain injury, strokes, and even death if left untreated.
Case report
A 30-year-old male patient, presented to the emergency room at (X) Hospitals. He had served in the military, was married, and had given up smoking six months prior. Two months before the hospital admission, he had been complaining of a persistent headache. Over the previous two months, the headache had gotten worse along with dizziness and vomiting, which was followed by lower limb numbness, which eventually turned into weakness in all four limbs, especially in the last four days.
Getting back to his medical history, he previously had stomach discomfort and abdominal pain, for which he received initial diagnoses of gastritis and irritable bowel syndrome (IBS). To rule out the causes of severe headaches and provide an explanation for the lower limb weakness, an MRI brain scan was requested. MRI result shows minimal enhancement in the left periventricular and inferior pons lesions, as well as dispersed, nonspecific periventricular and subcortical white matter foci with high T2/FLAIR signal intensities. The radiologist's differential diagnosis was a demyelinating disease, recommended for further clinical and laboratory correlations. Following a successful brain imaging procedure, he sought medical advice in the hospital and was released.
Two days later, he was admitted to (XX) hospital due to significantly worsened weakness in his four limbs, particularly in upper limbs with limited mobility. Additionally, dysphagia, bowel incontinence, and urinary retention were developed. He didn't experience shortness of breath or facial weakness, along with normal vital signs such as respiratory rate (RR), pulse rate (PR), blood pressure (BP), and absence of fever. On clinical examination (O/E), he was fully conscious, and oriented, with normal extraocular movements (EOM), and no double vision observed. Facial muscles, including frontalis, orbicularis oris, and buccinators muscles were all intact. However, the patient displays mild ptosis in the right eye without any abnormalities in the pupil or restriction in EOM. The gag reflex exhibited bilateral weakness. The upper right eyelid displayed a normal tone and a power rating of 4/5 for both proximal and distal muscles also a positive spastic catch was observed, while deep tendon reflex (DTR) was absent bilaterally. The lower lids on both the right and left sides were found to be hypotonic with an absent DTR.
The patient's musculature was graded at 2/5 bilaterally for hip flexion and 3/5 for hip extension. The patient also showed decreased strength in the distal muscle groups of the lower limbs, including plantar flexion, extension, eversion, and inversion bilaterally. The normal proprioceptive function was also noted. As a result of these clinical findings, the patient was admitted to a ward for monitoring.
Along with unimproved conditions of headache and dizziness for 10 days. During hospitalization at XX hospital, there was a rapid deterioration in brain stem functions, with quadriparesis and sphincter control-related issues, leading to intubation. CSF analysis after successful lumber puncture showed no pleocytosis and normal protein levels. The patient remained on fentanyl at a rate of 200 mg/h and midazolam at 10 mg/h. The patient's temperature started to elevate but was responsive to simple commands while on a ventilator with full support, and no inotropes were required. Further clinical examinations reveal restricted pupils measuring at 1 mm, sluggish but intact pupillary responses, the limbs didn't exhibit movement, and the plantar reflexes were absent.
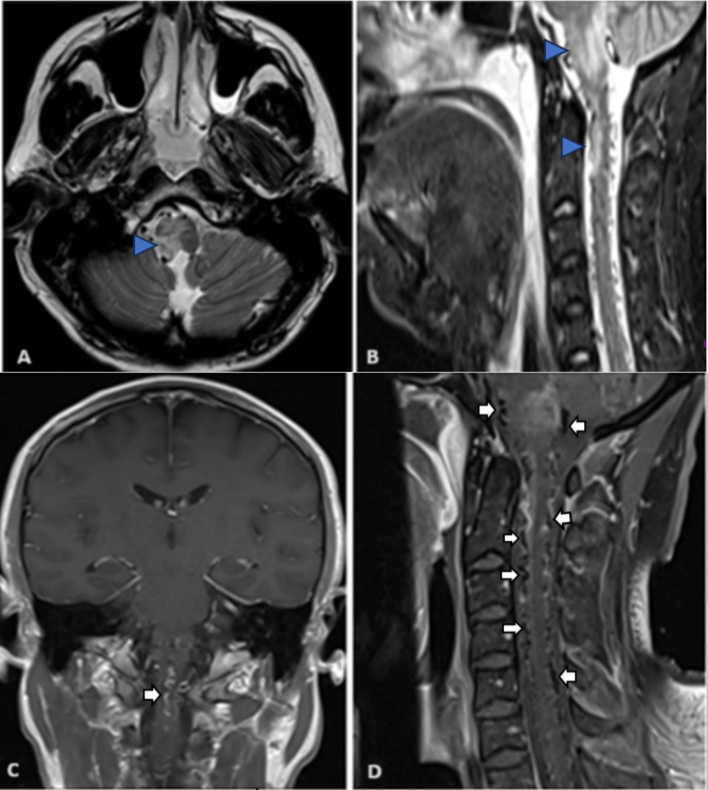
Brain MRI and cervical spine, as in Fig. 1 were requested and the results show, indicated a non-specific white matter signal change and an extensive flow void signal from the lower cervical to the cervical-medullary junction level as in Fig. 1(D).
Fig. 1.
Brain MRI hyperintense lesion in the right medulla (arrow) at axial T2 image (A), also right medullar and upper cervical cord (arrowed) as in sagittal STIR in (B). post-contrast MRI showed a faint enhancement of the medullary lesion along with the presence of an enhancing vein (arrow) as in the coronal section (C). Venous congestion with multiple cervical intradural flow voids (white arrows) as in sagittal image (D).
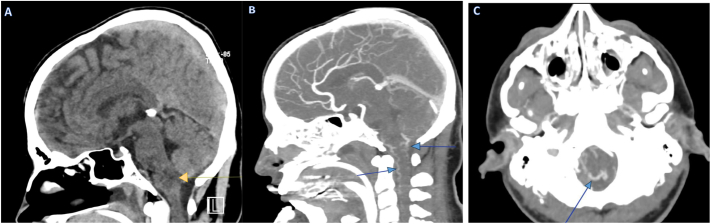
Multiple abnormally enlarged small blood vessels were observed in the craniocervical junction and the upper cervical spinal canal. In addition to a small diffusion-restricted area in the left posterior medullary region. No enhancing lesions were detected. These findings suggest vascular myelopathy is associated with a Dural Arteriovenous Fistula. Further diagnostic advice was provided for computed tomography angiography (CTA) of the brain as in Fig. 2.
Fig. 2.
Expansible hypodense ill-defined pathological lesion (arrow) at the cervical-occipital junction, as demonstrated in Sagittal CT scan of the brain without contrast media (A), Subfigures (B) and (C), illustrate sagittal and axial CT scans with contrast, revealing an arteriovenous fistula (arrowed) at the craniocervical junction.
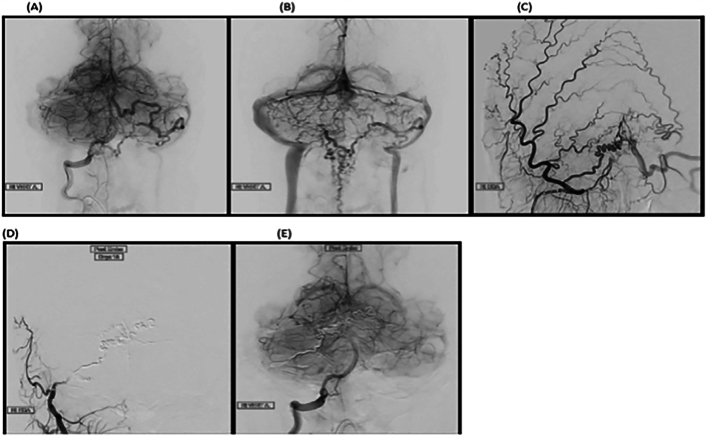
After establishing the patient's diagnosis, the patient was immediately admitted to the ICU due to a critical need for neurovascular intervention. During the assessment by the neurologist, a high-grade arteriovenous fistula at the craniocervical junction with a large retrogradely draining vein into the anterior and posterior spinal veins was identified. Arterial feeders coursed through the branches of the bilateral occipital artery and the posterior meningeal branches of the right vertebral artery. Using balloon-assisted liquid embolic agents, a successful embolization of the arteriovenous fistula through branches of the right occipital artery was performed, resulting in complete obliteration of the fistula. This procedure was confirmed through cerebral angiography (see Fig. 3[A, B, C, D, E]). Finally, according to the cerebral angiography, there was no residual filling of the draining vein upon completion of the intervention.
Fig. 3.
Selective digital subtraction angiograms demonstrating a high-grade arteriovenous fistula at the craniocervical junction with arterial feeders from branches of the occipital artery and posterior meningeal branches of the right vertebral artery as in (A, and B) and external carotid arteries as in (C), connected to a large retrograde draining vein that extends through the anterior and posterior spinal veins. After a successful embolization procedure of the arteriovenous fistula through branches of the right occipital artery, a selective right external (D) and right vertebral arteries (E) DSA demonstrated complete obliteration of the fistula with no residual filling.
The patient was successfully undergoing rehabilitation to improve the neurovascular manifestations as presented earlier, with significant improvements.
A nasogastric (NG) tube was inserted for proper nutritional assessment. Post-procedure prescriptions of the medication, such as Levetiracetam 500mg given twice daily for one week, were stopped; also, Pregabalin at a dose of 75mg twice daily, then the dose increased to 150mg twice daily; and Risperidone 0.5mg one time per day given orally. a preventative treatment for deep vein thrombosis (DVT) in addition to appropriate management of decubitus ulcers, provided as a result of the patient's bed score. Lastly, the Peptamen feeding regimen was maintained, along with rigorous rehabilitation that included frequent chest physical therapy sessions and suction when necessary.
Discussion
The dural arteriovenous fistula (DAVF) can result from meningeal and pial arteries connecting abnormally to cortical veins, meningeal veins, or dural venous sinuses. This indicates a particular subtype of abnormalities related to the blood vessels in the brain [13]. Brainstem dysfunction has been noted as the initial symptom in many intracranial DAVF cases. Furthermore, venous hypertension-induced congestion is considered to be a likely pathogenic etiology [16]. Depending on the drainage patterns, DAVFs can manifest a wide range of symptoms that may impact the orbit, eyes, cranial nerves, and brain. Cerebral symptoms are present in 3–5 % of cases [17].
Clinical symptoms, indicators, and imaging, primarily DSA supplemented by CT, CTA, 3D-CT, magnetic resonance imaging (MRI), and/or MRA, are used to diagnose DAVF. The blood-supplying arteries, the location, size, and type of fistulas, the drainage veins and sinuses, the direction of drainage, the blood flow velocity, the intracranial steal, and potentially hazardous vascular anastomoses may all be seen with DSA. The most reliable diagnostic technique is still fluoroscopic examination, although it can be intrusive, and patients may not always tolerate it well. Color Doppler flow imaging is a useful DAVF diagnostic adjunct [18].
Magnetic resonance imaging is the modality of choice in diagnosing DAVF with an increased diffusion-weighted signal; it can show a fistula close to the dural sinuses as well as a “flow void” that may indicate DAVF. The application of MRA provides a non-invasive screening option. Without the need for contrast material or the accompanying risk of radiation exposure, it may locate the fistula, identify the blood-supplying arteries, ascertain the direction of draining veins, and evaluate the state of the surrounding brain tissue [18].
In terms of therapy, both a comprehensive embolic examination and empirical antiplatelet therapy proved ineffective [19].
Conclusion
This case report presented DAVF complicated with a rare manifestation of brainstem dysfunction. Initial misdiagnosis and delaying of definitive diagnosis are both common in the past decades, in this case, and generally, Magnetic resonance imaging played a vital role in the diagnosis, revealing T2/FLAIR signal intensities. As a result of such, a rapid and successful intervention was conducted, involving balloon-assisted embolization of the arteriovenous fistula. Early diagnosis and management using up-to-date diagnostic tools are most important in terms of better patient care.
Patient consent
A written informed consent for the publication of this case report was obtained from the patient.
CRediT authorship contribution statement
Nagwan Alhussein: Writing – review & editing, Writing – original draft, Visualization, Funding acquisition, Conceptualization. Mohammed Alharbi: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization. Zuhal Y. Hamd: Writing – review & editing, Writing – original draft, Investigation, Funding acquisition, Conceptualization. Amal I. Alorainy: Writing – review & editing, Writing – original draft, Funding acquisition. Mansour Elshinwani: Writing – review & editing, Writing – original draft, Resources, Funding acquisition. Eyas Alsuhabani: Writing – review & editing, Writing – original draft. Bader Alhariqi: Writing – original draft. Basim Alhomida: Writing – original draft. A.B. Abdoelrahman Hassan: Writing – review & editing, Validation, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wen H.-Y., Chen H.-C., Yang S.-T. Risk factors of aggressive clinical presentation in patients with angiographically aggressive cranial dural arteriovenous fistulas. J. Clin. Med. 2021;10(24):5835. doi: 10.3390/jcm10245835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandhi D., et al. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. Am. J. Neuroradiol. 2012;33(6):1007–1013. doi: 10.3174/ajnr.A2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Dijk J.M.C., et al. Multiplicity of dural arteriovenous fistulas. J. Neurosurg. 2002;96(1):76–78. doi: 10.3171/jns.2002.96.1.0076. [DOI] [PubMed] [Google Scholar]
- 4.Chung S.J., et al. Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc. Dis. 2002;13(2):79–88. doi: 10.1159/000047755. [DOI] [PubMed] [Google Scholar]
- 5.Izumi T., et al. Thrombophilic abnormalities among patients with cranial dural arteriovenous fistulas. Neurosurgery. 2007;61(2):262–269. doi: 10.1227/01.NEU.0000255529.46092.7C. [DOI] [PubMed] [Google Scholar]
- 6.Morita A., et al. Childhood dural arteriovenous fistulae of the posterior dural sinuses: three case reports and literature review. Neurosurgery. 1995;37(6):1193–1200. doi: 10.1227/00006123-199512000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Borden J.A., Wu J.K., Shucart W.A. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J. Neurosurg. 1995;82(2):166–179. doi: 10.3171/jns.1995.82.2.0166. [DOI] [PubMed] [Google Scholar]
- 8.Della Pepa G.M., et al. Angio-architectural features of high-grade intracranial dural arteriovenous fistulas: correlation with aggressive clinical presentation and hemorrhagic risk. Neurosurgery. 2017;81(2):315–330. doi: 10.1093/neuros/nyw175. [DOI] [PubMed] [Google Scholar]
- 9.Cognard C., et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194(3):671–680. doi: 10.1148/radiology.194.3.7862961. [DOI] [PubMed] [Google Scholar]
- 10.Zipfel G.J., et al. Cranial dural arteriovenous fistulas: modification of angiographic classification scales based on new natural history data. Neurosurg. Focus. 2009;26(5):E14. doi: 10.3171/2009.2.FOCUS0928. [DOI] [PubMed] [Google Scholar]
- 11.Baek H.-G., et al. Stereotactic radiosurgery for dural arteriovenous fistulas involving the transverse-sigmoid sinus: a single center experience and review of the literatures. J. Korean Neurosurg. Soc. 2019;62(4):458. doi: 10.3340/jkns.2018.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura Y., et al. Targeted transvenous embolization of cavernous sinus dural arteriovenous fistula with liquid materials using a dual-lumen balloon microcatheter. Cureus. 2021;13(3) doi: 10.7759/cureus.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou K., et al. Intracranial dural arteriovenous fistulas with brainstem engorgement: an under-recognized entity in diagnosis and treatment. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.526550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narvid J., et al. CT angiography as a screening tool for dural arteriovenous fistula in patients with pulsatile tinnitus: feasibility and test characteristics. Am. J. Neuroradiol. 2011;32(3):446–453. doi: 10.3174/ajnr.A2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon B.J., et al. MR imaging findings of intracranial dural arteriovenous fistulas: relations with venous drainage patterns. Am. J. Neuroradiol. 2005;26(10):2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q., Wang H.D., Shin Y.S., Zhang X. Brainstem congestion due to dural ateriovenous fistula at the craniocervical junction. Journal of Korean Neurosurgical Society. 2014 Mar;55(3):152. doi: 10.3340/jkns.2014.55.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka Y., Komatsu K., Abe S., Yoshimoto N., Taki J., Matsumoto S. Acute brainstem dysfunction caused by cavernous sinus dural arteriovenous fistula. Case Reports in Neurological Medicine. 2020;2020(1) doi: 10.1155/2020/2630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y., Ou S.X., Qian M., Zeng X., Li B. Dual-energy CT angiography for the diagnosis of intracranial dural arteriovenous fistula. International Journal of Clinical and Experimental Medicine. 2015;8(5):7802. [PMC free article] [PubMed] [Google Scholar]
- 19.Kulwin C., Bohnstedt B.N., Scott J.A., Cohen-Gadol A. Dural arteriovenous fistulas presenting with brainstem dysfunction: diagnosis and surgical treatment. Neurosurgical Focus. 2012 May 1;32(5):E10. doi: 10.3171/2012.2.FOCUS1217. [DOI] [PubMed] [Google Scholar]