Abstract
Sindbis virus (SV) causes acute encephalomyelitis by infecting and inducing the death of neurons. Induction of apoptosis occurs during virus entry and involves acid-induced conformational changes in the viral surface glycoproteins and sphingomyelin (SM)-dependent fusion of the virus envelope with the endosomal membrane. We have studied neuroblastoma cells to determine how this entry process triggers cell death. Acidic sphingomyelinase was activated during entry followed by activation of neutral sphingomyelinase, SM degradation, and a sustained increase in ceramide. Ceramide-induced apoptosis and SV-induced apoptosis could be inhibited by treatment with Z-VAD-fmk, a caspase inhibitor, and by overexpression of Bcl-2, an antiapoptotic cellular protein. Acid ceramidase, expressed in a recombinant SV, decreased intracellular ceramide and protected cells from apoptosis. The data suggest that acid-induced SM-dependent virus fusion initiates the apoptotic cascade by inducing SM degradation and ceramide release.
Alphaviruses, enveloped plus-strand RNA viruses, are important causes of mosquito-borne viral arthritis and encephalitis worldwide (25). Neurons are the primary target cell in the central nervous system of hosts that develop encephalitis. Sindbis virus (SV), the prototypic alphavirus, causes neuronal infection in mice (22), and studies of this infection have provided important insights into the molecular mechanisms underlying virus-induced encephalomyelitis.
Like other alphaviruses, SV has three major structural proteins, two surface glycoproteins, E1 and E2, and a capsid protein that surrounds the genome. E1 and E2 heterodimerize and then trimerize to form spikes on the virion surface that mediate virus binding and entry (55). E2 is the primary determinant of binding to cellular receptors, and E1 is responsible for cholesterol-dependent binding to liposomal membranes and contains the hydrophobic domain essential for virus-cell fusion (8, 43). Alphavirus fusion occurs in the endosome and requires an acid-induced conformational change in the E1-E2 heterodimer and a target membrane that contains sphingomyelin (SM) (8, 46, 59).
SV infects many types of cells in culture and induces apoptosis in neurons and other vertebrate cells in vitro and in vivo (31, 32). The study of SV-induced apoptosis offers a powerful system for defining important cellular mechanisms regulating virus-induced cell death since recombinant viruses can be used to express modifiers of the apoptotic process in virus-infected cells. Studies using these recombinant SVs have shown that SV-induced apoptosis can be slowed or prevented by caspase inhibitors and by Bcl-2 family member proteins (10, 30, 31, 45). However, the mechanism of induction of apoptosis by SV is largely unknown.
In the best-defined systems, tumor necrosis factor (TNF)- and Fas ligand-induced cell death, apoptosis is initiated at the cell membrane through cross-linking of a transmembrane protein belonging to the TNF receptor family. Cross-linking initiates a cascade of intracellular events resulting in death of susceptible cells. Many viruses that cause acute infections and host cell destruction induce apoptosis. Some viruses initiate this process at the time of binding or entry (7, 20, 50), while others initiate the process after infection is established and viral proteins are produced (4). Previous studies of SV-induced apoptosis have shown that cell death is most efficiently induced when the structural proteins are present (17), that transient overexpression of the transmembrane portions of either E1 or E2 induces apoptosis (24), and that induction of apoptosis does not require virus replication but does require virus fusion with the cell membrane (23).
Since alphaviruses require sphingolipids containing ceramide (i.e., SM) to be present in the cell membrane as a cofactor for fusion (46), we investigated the role of the SM pathway in SV-induced apoptosis and have found that SV infection rapidly induced activation of acidic sphingomyelinase (aSMase) to hydrolyze SM and release ceramide, a well-defined intracellular mediator of apoptosis (47). Mg2+-dependent neutral sphingomyelinase (nSMase) was activated at later times, leading to a prolonged increase in intracellular ceramide. Recombinant SV expressing acid ceramidase (AC) decreased levels of ceramide and delayed virus-induced cell death. Our results suggest that SV-induced apoptosis can be triggered by activation of aSMase associated with the endosomal membrane during the SM-requiring process of virus-cell fusion, leading to the release of ceramide and induction of apoptosis.
MATERIALS AND METHODS
Cell culture and biological reagents.
Mouse neuroblastoma cell line N18 (3), baby hamster kidney cell line BHK-21, type A Niemann-Pick disease (NPD) and control fibroblasts, and rat prostate carcinoma cell line AT3 transfected with the expression vector pZipNeo (AT3Neo) or the recombinant vector pZipBcl-2 (AT3Bcl-2) (31) were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. Cell viability was assessed by trypan blue exclusion. Fumonisin B1, C2-ceramide, diacylglycerol, phosphatidic acid, bis-benzimide (Hoechst 33258), 6-dimethylaminopurine (DMAP), and okadaic acid (OKA) were obtained from Sigma Chemical Company (St. Louis, Mo.). Z-VAD-fmk was obtained from Alexis (San Diego, Calif.).
Virus preparation.
Neuroadapted SV (NSV), derived by serial passage of wild-type SV (strain AR339) in mouse brain (18), was plaque purified, grown, and assayed in BHK-21 cells. For purification, virus was precipitated in 10% (wt/vol) polyethylene glycol 8000 in 0.5 M NaCl, pelleted, suspended in NET buffer (10 mM Tris, 3 mM EDTA, 150 mM NaCl, pH 7.4), and banded in a continuous 15-to-40% potassium tartrate gradient. Banded virus was dialyzed against 0.05M Tris-Cl (pH 7.4) and stored in aliquots at −70°C. Virus was UV inactivated at 4°C with a germicidal lamp (254 nm) at a distance of 5 cm for 30 min. Inactivation was confirmed by plaque assay on monolayers of BHK-21 cells.
Lipid studies.
N18 cells were pelleted, washed twice with ice-cold phosphate-buffered saline (PBS), and extracted with chloroform–methanol–1 N HCl (100:100:1, vol/vol/vol). Lipids in the organic phase were dried in a vacuum dryer and subjected to mild alkaline hydrolysis (0.1 N methanolic KOH for 1 h at 37°C) to remove glycerophospholipids. Samples were reextracted, and the organic phase was dried. Detergent solution (20 μl of 7.5% n-octyl-β-glucopyranoside with 5 mM cardiolipin in 1 mM diethylenetriamine-pentaacetic acid) was added, and the sample was sonicated. Ceramide was measured using the sn-1,2-diacylglycerol kinase assay reagent system and labeling for 30 min with 1 mCi of [γ-32P]ATP in 10 μl of 5 mM ATP (57) (Amersham, Arlington Heights, Ill.). Ceramide-1-phosphate and sphingosine-1-phosphate were resolved by thin-layer chromatography on Silica Gel 60 plates (Whatman, Clinton, N.J.) using a solvent of chloroform-methanol-acetic acid (65:15:5) and detected by autoradiography. Incorporated 32P was quantified by scraping the spots and counting radioactivity in a liquid scintillation counter. Diacylglycerol was quantified in a similar manner to ceramide, except that the alkaline hydrolysis step was omitted.
Changes in SM levels were measured by labeling cells to isotopic equilibrium with [3H]choline chloride (79.2 Ci/mmol; 1.0 μCi/ml; Dupont New England Nuclear) for at least four cell doublings. After infection with NSV, cellular lipids were extracted, dried, and subjected to alkaline hydrolysis as described for ceramide measurement. SM was resolved from residual phosphatidylcholine and lysophosphatidylcholine by thin-layer chromatography using a solvent of chloroform-methanol-acetic acid-water (50:30:8:4), identified by iodine vapor staining, and quantified by liquid scintillation counting.
SMase assays.
Activities of aSMase and nSMase were measured according to previously described methods with minor modifications (49). In brief, 5 × 106 N18 cells were scraped from the culture plates, washed with PBS, and disrupted by repeated passage through a 25-gauge needle. Nuclei and cell debris were pelleted at 800 × g for 5 min. Supernatant fluid was collected, and the protein concentration was measured using the Bio-Rad (Hercules, Calif.) protein detection kit. To measure SMase activity, 50 μg of protein was incubated for 90 min (the reaction was linear for up to 120 min) at 37°C in buffer (200-μl final volume) containing 250 mM sodium acetate and 1 mM EDTA, pH 5.0, for aSMase or 250 mM Tris-Cl, pH 7.4, with or without 6 mM MgCl2 for nSMase, and 0.75 μl of [methyl-14C]SM (0.2 mCi/ml; 56.6 mCi/mmol). Radioactive phosphorylcholine produced from [14C]SM was extracted with 800 μl of chloroform-methanol (2:1, vol/vol) and 100 μl of H2O. [14C]phosphorylcholine in the aqueous phase was measured by scintillation counting.
Assessment of apoptosis.
A total of 5 × 106 NSV-infected or C2-ceramide-treated N18 cells were scraped from the culture plates and washed with PBS. Genomic DNA was isolated with DNAZOL and incubated with RNase (1μg/ml) at 50°C for 1 h. DNA (10 μg) from each sample was electrophoresed through a 2.0% agarose gel in TAE buffer (40 mM Tris acetate, 2 mM EDTA) and stained with ethidium bromide.
Morphological changes in the nuclear chromatin were visualized by staining with the DNA-binding fluorochrome bis-benzimide. In brief, cells were pelleted, washed with PBS, and resuspended in 50 μl of 3% paraformaldehyde in PBS. After 10 min cells were washed and resuspended in 15 μl of PBS containing 16 μg of bis-benzimide per ml. Five hundred cells were scored for the presence of apoptotic chromatin changes using a Nikon Eclipse E800 fluorescence microscope. Cells with two or more chromatin fragments were considered apoptotic.
Construction of recombinant SV vectors encoding the AC gene.
A plasmid, pACFL, encoding the full-length human AC gene was provided by Konrad Sandhoff (Institute of Organic Chemistry and Biochemistry, Bonn, Germany). The plasmid was digested with BamHI and SalI. The fragment containing the 1,185-bp AC open reading frame was blunt end-ligated into the BstEII site of the double subgenomic SV vector (dsTE12) (10) in forward and reverse orientations. dsTE12 DNA containing AC was linearized with XhoI and transcribed in vitro using SP6 RNA polymerase. Stocks of recombinant viruses were generated by transfecting the full-length RNA into BHK-21 cells and collecting the supernatant fluid when more than 70% cytopathic effect was observed. Virus titers were determined by plaque assay on BHK-21 cells.
RESULTS
SV infection induces ceramide release.
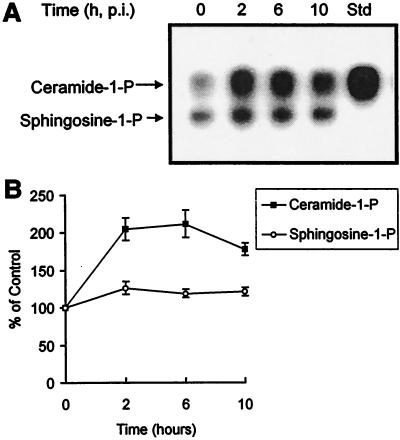
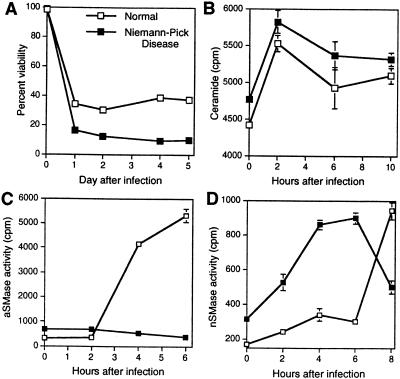
To determine whether SV infection leads to increased ceramide production, N18 cells were infected with NSV at a multiplicity of infection (MOI) of 50, which results in synchronous infection of all the cells. Levels of intracellular ceramide were measured, and an increase was detectable by 2 h after initiation of infection (Fig. 1A) and peaked between 2 and 6 h at 2.2 times control levels (Fig. 1B). Diacylglycerol levels remained at 80 to 100% of control levels. Sphingosine levels increased slightly (Fig. 1A), possibly due to in vitro catabolism of ceramide. Levels of ceramide decreased between 6 and 10 h after NSV infection (Fig. 1B), a time of rapid increase in virus production (56).
FIG. 1.
Generation of ceramide in response to NSV infection. (A) N18 cells were infected with NSV (MOI = 50), and at the indicated times (p.i., postinfection), lipid extracts of the cells were assayed for ceramide and sphingosine by the diacylglycerol kinase reaction and resolved by thin-layer chromatography followed by autoradiography. Std, C2-ceramide, 5 nmol. (B) Quantitation of ceramide-1-P and sphingosine-1-P in NSV-infected cells. Values are percentages of the levels present in mock-infected cells ± standard deviations (error bars).
Ceramide mimics SV infection in inducing apoptosis.
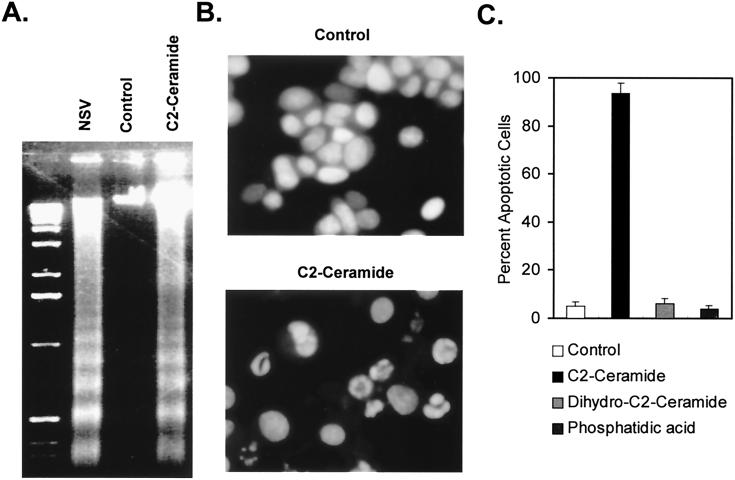
To determine whether ceramide alone could induce apoptosis, N18 cells were treated with a ceramide analog, C2-ceramide, which has two carbons rather than the 18 carbons of ceramide and is cell permeable, and assessed for apoptosis. C2-ceramide mimicked SV infection by inducing oligonucleosomal DNA fragmentation (Fig. 2A) and the appearance of typical apoptotic nuclear changes (Fig. 2B). The induction and extent of apoptotic morphology depended on the dose of ceramide, with 40 μM C2-ceramide being the minimum required for inducing apoptosis of all cells (data not shown). Treating cells with 50 μM dihydroceramide, the immediate precursor of ceramide, which lacks the double bond at C-4–C-5 of the sphingosine backbone, did not result in apoptosis (Fig. 2C). Furthermore, other cell-permeable analogs of lipid second messengers, including sphingosine, 1,2-diacylglycerol, and phosphatidic acid, did not induce apoptosis (Fig. 2C).
FIG. 2.
Induction of apoptosis in N18 cells by NSV and C2-ceramide. (A) Gel electrophoresis of DNA from cells infected with NSV (MOI = 5) or treated with 50 μM C2-ceramide or diluent (0.1% ethanol) (control). After 36 h (NSV) and 48 h (C2-ceramide), genomic DNA was analyzed by agarose gel electrophoresis and staining with ethidium bromide. (B) Morphological alterations of the chromatin of N18 cells treated with 0.1% ethanol (control) or 50 μM C2-ceramide. After 36 h cells were fixed and stained with bis-benzimide. (C) Quantitation of nuclear morphological changes of N18 cells 48 h after treatment with 0.1% ethanol (control) or 50 μM C2-ceramide, dihydro-C-2-ceramide, or phosphatidic acid. A total of 500 cells per slide were scored for apoptotic chromatin changes. Values represent means ± standard deviations (error bars).
Mechanism of ceramide generation.
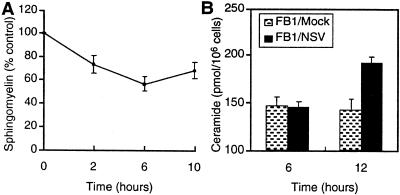
Ceramide can be generated by hydrolysis of membrane SM by SMase or by biosynthesis from sphingosine by ceramide synthase (40), and both pathways can lead to apoptosis. TNF alpha (TNF-α), Fas ligand, and ionizing radiation activate the apoptotic pathway by increasing intracellular ceramide through hydrolysis of SM (11, 28), while the chemotherapeutic agent daunorubicin activates ceramide synthase (5). To determine the mechanism of SV-induced ceramide generation, we measured SM levels during NSV infection. As the ceramide level increased, there was a concomitant decrease in content of cellular SM (Fig. 1B and 3A). Cellular SM levels decreased soon after infection and reached a minimum of 4 to 6 h, suggesting that ceramide generated in the infected cells was derived from hydrolysis of membrane SM.
FIG. 3.
Changes in SM levels and effect of fumonisin B1 on ceramide generation in response to NSV infection. (A) SM degradation in N18 cells labeled with [3H]choline and then infected with NSV (MOI = 50). Lipids were extracted, and SM was resolved by thin-layer chromatography and quantified by liquid scintillation counting. The values represent means ± standard deviations (SD) (error bars) of the SM levels in NSV-infected cells as a percentage of mock-infected cells × 100. (B) N18 cells treated with 100 μM fumonisin B1 (FB1) and then mock infected or infected with NSV (MOI = 50). At 6 and 12 h after infection, cellular lipids were extracted and assayed for ceramide as described in the legend to Fig. 1. Values represent means ± SD (error bars).
To confirm this observation cells were treated with fumonisin B1, a potent inhibitor of ceramide synthase (41). Preincubation of N18 cells with fumonisin B1 delayed, but did not prevent, NSV-induced ceramide elevation (Fig. 3B). Intracellular ceramide levels increased by 12 h after NSV infection in the presence of fumonisin B1, whereas fumonisin B1 alone did not induce the elevation of ceramide levels. Fumonisin B1 may delay NSV-induced ceramide generation by inhibiting virus entry or by having a toxic effect on N18 cells. This result, combined with the slightly increased sphingosine level (Fig. 1B), indicated that ceramide in infected cells was not generated by synthesis from sphingosine.
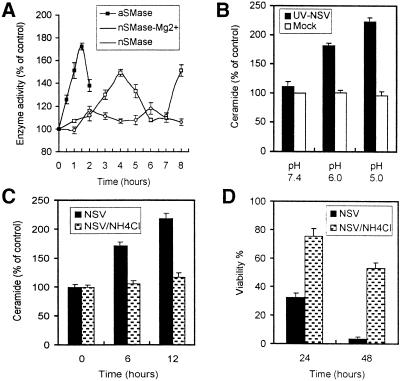
To determine the involvement of SMase in the generation of ceramide, a micellar assay system was used. aSMase and nSMase were distinguished by adjusting the buffer pH to the activation requirements of the SMase. Membrane-associated, Mg2+-dependent and cytosolic, Mg2+-independent nSMases (9, 48) were distinguished by using buffers with and without Mg2+. aSMase activity increased quickly and peaked at a maximum of 1.7-fold control 90 min after infection. Mg2+-dependent nSMase activity also increased, but with a peak (1.5-fold control) at 4 h (Fig. 4A). An additional peak at 8 h may represent activity of a different nSMase or secondary activation by downstream mediators. Mg2+-independent nSMase activity increased only slightly after NSV infection.
FIG. 4.
Activation of aSMase and nSMase during NSV infection. (A) NSV (MOI = 50) was bound to N18 cells at 4°C for 2 h. The temperature was shifted to 37°C to initiate entry and at the indicated times lysates were assayed for SMase activity at pH 5.0 (aSMase) and pH 7.4 (nSMase) with and without Mg2+. Data are expressed as a percentage of control values (NSV infected/mock infected × 100) (means ± standard deviations [SD] [error bars]). (B) UV-inactivated NSV induces generation of ceramide. N18 cells with or without UV-inactivated NSV (MOI equivalent = 500) bound at 4°C were shifted to 37°C and incubated with medium of pH 5.0, pH 6.0, or pH 7.4. Five hours later, lipids were extracted and ceramide levels were determined. (C) NH4Cl blocks ceramide elevation in response to NSV infection. Lipids were extracted from N18 cells treated with 20 mM NH4Cl for 1 h prior to infection with NSV (MOI = 50), and ceramide levels were determined. (D) NH4Cl protects against NSV-induced cell death. N18 cells pretreated with 20 mM NH4Cl for 1 h were infected with NSV (MOI = 5). Cell viabilities were determined by trypan blue exclusion. (B to D) Values represent means ± SD (error bars).
UV-inactivated NSV can trigger apoptosis when cells with virus bound to the surface are transiently exposed to a low-pH environment to induce virus-cell fusion (23). To determine whether ceramide was generated during such fusion, intracellular ceramide levels were assessed (Fig. 4B). Ceramide was generated when N18 cells with bound replication-defective UV-inactivated NSV were exposed to low pH, with pH 5.0 being more efficient than pH 6.0. Generation of ceramide was dependent on the acid-induced fusion event since it did not occur if endosomal acidification was blocked by treatment of NSV-infected N18 cells with NH4Cl (Fig. 4C). By 48 h after NSV infection, more than 40% of the NH4Cl-treated cells were alive, while all untreated cells were dead (Fig. 4D). NSV infection did not stimulate the release of choline or increase diacylglycerol (data not shown), indicating that phosphatidyl choline-specific phospholipase C was not activated. The tight coupling of NSV infection, aSMase activation, and ceramide generation within 1 to 2 h; dependence of ceramide generation on endosomal acidification; and the fact that pH 4.5 to 5.0 is optimal for aSMase activity suggest that activation of aSMase occurs in the endosome during the viral fusion event.
Activation of nSMase is sufficient for SV to induce apoptosis.
To determine whether activation of aSMase is necessary for SV to induce apoptosis, fibroblasts from a patient with type A NPD, an inherited deficiency of aSMase (29), were studied. Surprisingly, NPD fibroblasts died more rapidly after NSV infection than control fibroblasts (Fig. 5A). This was accompanied by an increase in the level of ceramide (Fig. 5B). There was no aSMase activity induced in NPD fibroblasts (Fig. 5C), but nSMase was activated early to a greater extent than in control fibroblasts (Fig. 5D), suggesting the presence of compensatory SMase mechanisms.
FIG. 5.
NSV infection of type A NPD and control fibroblasts. Fibroblasts were infected with NSV (MOI = 50) and monitored for viability by trypan blue exclusion (A), levels of intracellular ceramide (B), aSMase activity (C), and nSMase activity (D) at various times after infection. (B to D) Values represent means ± standard deviations (error bars).
Inhibitors of protein kinases and phosphatases protect SV-infected cells against death.
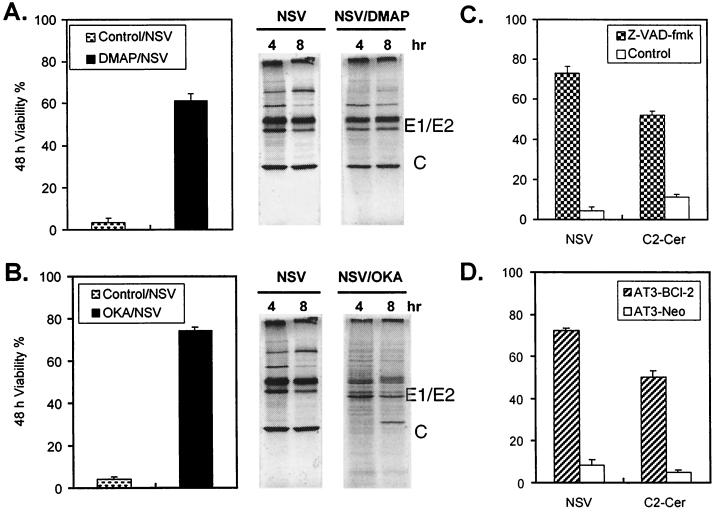
Ceramide influences protein phosphorylation by directly activating at least two target enzymes, ceramide-activated protein kinase (CAPK) and ceramide-activated protein phosphatase (CAPP) (16, 35). An inhibitor of protein kinase, DMAP (38), and an inhibitor of protein phosphatase, OKA (51), were used to evaluate the roles of protein phosphorylation and dephosphorylation in SV-induced apoptosis triggered by an increase in ceramide. Both DMAP and OKA were protective against SV-induced cell death (Fig. 6A and 6B). By 48 h after NSV infection, most untreated cells were dead, while 62% of DMAP-treated cells and 75% of OKA-treated cells survived. To determine whether DMAP or OKA has an effect on SV entry or intracellular replication, we examined viral protein synthesis by labeling infected cells with [35S]methionine and determined virus titers in the culture medium. DMAP had no effect on viral protein synthesis or virus production, but OKA delayed viral protein synthesis and inhibited virus production possibly due to effects on E2 processing (36). These results imply that protein phosphorylation is an important determinant of SV-induced cell death and ceramide may trigger these reactions.
FIG. 6.
Effects of various modulators on the viability of NSV-infected or ceramide-treated cells. N18 cells were or were not pretreated with 5 mM DMAP (A) or 5 μM OKA (B) for 1 h and then infected with NSV (MOI = 5). (C) N18 cells were treated with 100 μM Z-VAD-fmk for 6 h and then infected with NSV or treated with C2- ceramide (C2-Cer) (50 μM). (D) AT3-Bcl-2 and AT3-Neo cells were infected with NSV or treated with C2-Cer. Cell viabilities were determined 48 h later by trypan blue exclusion. Values represent means ± standard deviations (error bars).
Caspase inhibition and Bcl-2 expression protect cells against SV- and ceramide-induced death.
To determine whether caspase is involved in induction of death of SV-infected and ceramide-treated N18 cells, we preincubated cells with a caspase inhibitor, Z-VAD-fmk, prior to infection with NSV or treatment with C2-ceramide. Z-VAD-fmk effectively blocked both NSV- and ceramide-induced cell death (Fig. 6C). To investigate the contribution of Bcl-2 to protection from these inducers of apoptosis, AT3 cells overexpressing Bcl-2 and control AT3-Neo cells (31) were examined for their resistance to NSV- and ceramide-induced cell death. Bcl-2 protein protected against apoptosis induced by both NSV infection and C2-ceramide treatment (Fig. 6D).
AC protects NSV-infected cells against death by decreasing intracellular ceramide.
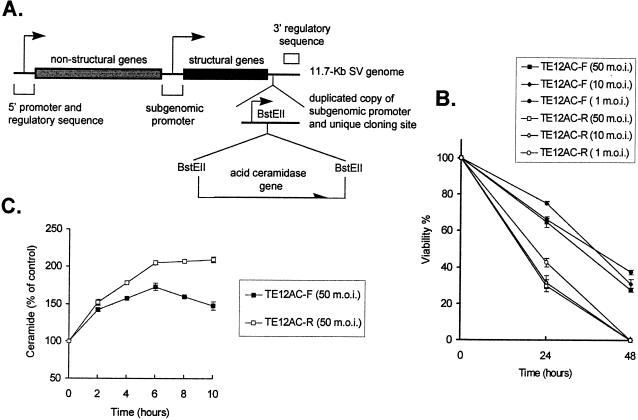
AC specifically catalyzes the hydrolysis of ceramide into sphingosine and free fatty acid (27). The AC gene was inserted in both forward and reverse orientations into a double subgenomic SV vector (10) (Fig. 7A). Viabilities of N18 cells infected with recombinant SV encoding AC in the forward orientation (TE12AC-F) were higher than those infected with recombinant SV carrying the reverse AC gene (TE12AC-R) at all MOIs tested (Fig. 7B). To confirm the expression and activity of the encoded ceramidase, ceramide levels in these cells were measured. Early in infection ceramide in TE12AC-F-infected cells was similar to that in TE12AC-R-infected cells, consistent with predicted translation of the subgenomic RNA beginning 3 to 4 h after infection. However, by 4 to 6 h the level of ceramide in TE12AC-F-infected cells was lower than that in TE12AC-R-infected cells (Fig. 7C). These results demonstrate that SV-infected cells can be protected from death by decreasing intracellular ceramide levels and further demonstrate that ceramide is an important mediator in inducing SV-induced cell death.
FIG. 7.
Effects of AC on NSV-infected cells. (A) Diagram of the double subgenomic SV vector containing the AC gene. (B) N18 cells were infected with recombinant SV TE12AC-F (forward AC gene) or TE12AC-R (reverse AC gene) at various MOIs. Cell viabilities were determined by trypan blue exclusion at 24 and 48 h after infection. (C) N18 cells were infected with recombinant TE12AC-F and TE12AC-R (MOI = 50), and levels of ceramide were determined. Values represent means ± standard deviations (error bars). Some error bars are too small to be visible.
DISCUSSION
SV activates aSMase to generate ceramide during the process of fusion with the cell membrane.
Studies of alphavirus fusion have shown that acid-induced fusion with liposomal membranes requires the presence of sphingolipids in the target membrane. The need for sphingolipid is confined to the actual fusion event, with cholesterol being necessary and sufficient for low-pH-dependent binding of virus to the target membrane (8, 46). Thus, the virus membrane, and presumably the transmembrane viral surface glycoproteins, must interact with SM present in the outer leaflet of the membrane during the process of fusion and entry. Only small amounts of SM are required, suggesting that SM acts as a cofactor for activation of the viral fusion protein (46). The interaction is stereospecific, and the minimal molecular characteristic of SM essential for alphavirus fusion is presence of the ceramide portion of the sphingosine backbone, which must have a 3-hydroxyl group and a 4,5-transcarbon double bond (12, 42). Importantly, this 4,5-transcarbon double bond is also required for ceramide-induced apoptosis, since a ceramide analog, C2-dihydroceramide, which lacks this double bond, is inefficient in inducing apoptosis (47).
All three forms of SMase are capable of initiating signaling through production of ceramide (60). TNF-α-induced apoptosis has been particularly well studied. TNF-α binding to the 55-kDa TNF receptor activates aSMase and nSMase through distinct pathways initiated by separate cytoplasmic domains of TNF-R55 (1, 2, 53, 60). Membrane-associated nSMase activation is initiated by interaction of a novel WD repeat protein, FAN, with the nSMase domain of TNF-R55 (1). Activation of aSMase occurs later and is initiated through interaction of the proapoptotic adapter proteins TRADD and FADD with the death domain of TNF-R55 and involves the activation of phosphatidyl choline-specific phospholipase C and an interleukin-1 (IL-1) cleavage enzyme (ICE)-like protease (6, 14, 19, 54).
Like TNF-α, SV also activates both aSMase and nSMase, but aSMase activation occurs first and nSMase activation is later. Studies in aSMase-deficient NPD fibroblasts show that activation of nSMase by SV can also lead to early increases in ceramide and apoptosis. Cell type differences may also play a role since in the normal fibroblasts aSMase was not induced until 4 h after infection when there was also a detectable increase in nSMase activity, which was greatly exaggerated in the NPD fibroblasts. SV activation of aSMase may be more analogous to the activation of aSMase by IL-1, which is linked to the internalization of IL-1 receptor 1 mediated by the IL-1 receptor activating protein (21). After binding to a cellular receptor SV is rapidly internalized and fusion of the viral envelope and the endosomal membrane occurs within 30 min (39). Preincubating cells with NH4Cl, which has no effect on virus binding, prevents virus fusion by blocking the decrease of endosomal pH and inhibits the generation of ceramide. It is possible that either the E1 or E2 protein directly interacts with SM and induces activation of aSMase during the fusion process. Studies of transient expression of various viral proteins have suggested that the transmembrane regions of either E1 or E2 can induce apoptosis (24), possibly through their participation in this process.
Ceramide is an early mediator for triggering SV-induced apoptosis.
Ceramide has been shown to be involved in cell differentiation, cell cycle arrest, and apoptosis (19). Ceramide is implicated as a mediator in the apoptotic signaling of a variety of inducers of apoptosis, including TNF-α, Fas ligand, neurotrophins, ionizing radiation, cytokines, heat shock, UV light, antitumor drugs, and oxidative stress (11, 15, 19, 52, 58). In most of these cases, ceramide is generated by hydrolysis of membrane-associated SM and the elevation of ceramide precedes apoptosis as it does in SV-induced apoptosis. Treatment of N18 cells with C2-ceramide induced apoptosis. Furthermore, AC, which degrades intracellular ceramide, protected NSV-infected cells from death.
The details of the apoptotic pathway downstream of ceramide are not fully known because the range of immediate ceramide targets has not been completely elucidated. Direct targets for ceramide action include membrane-associated CAPK, a member of the proline-directed family of serine/threonine protein kinases (26), and CAPP, a member of the heterotrimeric protein phosphatase 2A family (16). CAPK, in response to TNF-α activation of nSMase, phosphorylates Raf1, activating the mitogen-activated protein kinase cascade (61). DMAP, a protein kinase inhibitor, abrogates or significantly attenuates TNF-α-induced cytotoxicity (38). Functions of CAPP are inhibited by the phosphatase 2A inhibitor OKA (16). Both DMAP and OKA are protective against SV-induced cell death, indicating that a series of kinase and phosphatase reactions occur in the SV-triggered apoptotic pathway. Ceramide can also activate protein kinase C-ζ and link cytokine receptors to NF-κB activation (37, 44). In AT3 but not N18 cells, antisense RNA of NF-κB blocks SV-induced apoptosis through inhibition of constitutive NF-κB expression (33, 34). However, ceramide induced by TNF does not lead to transcription of NF-κB-dependent genes (13). Further studies of the ceramide targets, their functions, compartmentalization, and consequent effects are necessary for the understanding of the downstream pathway of SV-triggered, ceramide-induced apoptosis.
ACKNOWLEDGMENTS
Jia-Tsrong Jan was supported by a predoctoral scholarship from the National Defense Medical Center, Taiwan. The research was supported by grants DK31722 (S.C.) and NS 18596 (D.E.G) from the National Institutes of Health.
NPD and control fibroblasts were supplied by the Kennedy/Hopkins NICHD Mental Retardation Research Center Core (HD24061).
REFERENCES
- 1.Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Kronke M. Fan, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/s0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 2.Adams-Klages S, Schwandner R, Adam D, Kreder D, Bernardo K, Kronke M. Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J Leukoc Biol. 1998;63:678–682. doi: 10.1002/jlb.63.6.678. [DOI] [PubMed] [Google Scholar]
- 3.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anouja F, Watiez R, Mousset S, Caillet-Fauquet P. The cytotoxicity of the parvovirus minute virus of mice nonstructural protein NS1 is related to changes in the synthesis and phosphorylation of cell proteins. J Virol. 1997;71:4671–4678. doi: 10.1128/jvi.71.6.4671-4678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bose R, Verheij M, Haimovitzfriedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis - an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 6.Bourteele S, Hauber A, Doopler H, Horn-Muller J, Ropke C, Schwarzmann G, Pfizenmaier K, Muller G. Tumor necrosis factor induces ceramide oscillations and negatively controls sphingolipid synthases by caspases in apoptotic Kmy-1 cells. J Biol Chem. 1998;273:31245–31251. doi: 10.1074/jbc.273.47.31245. [DOI] [PubMed] [Google Scholar]
- 7.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 8.Bron R, Wahlberg J M, Garoff H, Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993;12:693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S. Neutral sphingomyelinase. Adv Lipid Res. 1993;26:25–48. [PubMed] [Google Scholar]
- 10.Cheng E H, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 11.Cifone M G, De Maria R, Roncaioli P, Rippo M R, Azuma M, Lanier L L, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corver J, Moesby L, Erukulla R K, Reddy K C, Bittman R, Wilschut J. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J Virol. 1995;69:3220–3223. doi: 10.1128/jvi.69.5.3220-3223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dbaibo G S, Obeid L M, Hannun Y A. Tumor necrosis factor-α (TNF-α) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-α from activation of nuclear factor-kB. J Biol Chem. 1993;268:17762–17766. [PubMed] [Google Scholar]
- 14.Dbaibo G S, Perry D K, Gamard C J, Platt R, Poirier G G, Obeid L M, Hannun Y A. Cytokine response modifier A (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-alpha: CrmA and Bcl-2 target distinct components in the apoptotic pathway. J Exp Med. 1997;185:481–490. doi: 10.1084/jem.185.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrowsky R T, Carter B D. Coupling of the p75 neutrotrophin receptor to sphingolipid signaling. Ann N Y Acad Sci. 1998;845:32–45. doi: 10.1111/j.1749-6632.1998.tb09660.x. [DOI] [PubMed] [Google Scholar]
- 16.Dobrowsky R T, Hannun Y A. Ceramide-activated protein phosphatase: partial purification and relationship to protein phosphatase 2A. Adv Lipid Res. 1993;25:91–104. [PubMed] [Google Scholar]
- 17.Frolov I, Schlesinger S. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J Virol. 1994;68:1721–1727. doi: 10.1128/jvi.68.3.1721-1727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin D E, Johnson R T. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J Immunol. 1977;118:1070–1075. [PubMed] [Google Scholar]
- 19.Hannun Y A, Obeid L M. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995;20:73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- 20.Hanon E, Meyer G, Vanderplasschen A, Dessy-Doize C, Thiry E, Pastoret P-P. Attachment but not penetration of bovine herpesvirus 1 is necessary to induce apoptosis in target cells. J Virol. 1998;72:7638–7641. doi: 10.1128/jvi.72.9.7638-7641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmeister R, Wiegmann K, Korherr C, Bernardo K, Kronke M, Falk W. Activation of acid sphingomyelinase by interleukin-1 (IL-1) requires the IL-1 receptor accessory protein. J Biol Chem. 1997;272:27730–27736. doi: 10.1074/jbc.272.44.27730. [DOI] [PubMed] [Google Scholar]
- 22.Jackson A C, Moench T R, Trapp B D, Griffin D E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab Investig. 1988;58:503–509. [PubMed] [Google Scholar]
- 23.Jan J-T, Griffin D E. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J Virol. 1999;73:10296–10302. doi: 10.1128/jvi.73.12.10296-10302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joe A K, Foo H, Kleeman L, Levine B. The transmembrane domains of Sindbis virus envelope glycoproteins induce cell death. J Virol. 1998;72:3935–3943. doi: 10.1128/jvi.72.5.3935-3943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston R E, Peters C J. Alphaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Virology. New York, N.Y: Lippincott-Raven Press; 1996. pp. 843–898. [Google Scholar]
- 26.Joseph C K, Byun H S, Bittman R, Kolesnick R N. Substrate recognition by ceramide-activated protein kinase. Evidence that kinase activity is proline-directed. J Biol Chem. 1993;268:20002–20006. [PubMed] [Google Scholar]
- 27.Koch J, Gartner S, Li C M, Quintern L E, Bernardo K, Levran O, Schnabel D, Desnick R J, Schuchman E H, Sandhoff K. Molecular cloning and characterization of a full-length complementary DNA encoding human acid ceramidase. Identification of the first molecular lesion causing Farber disease. J Biol Chem. 1996;271:33110–33115. doi: 10.1074/jbc.271.51.33110. [DOI] [PubMed] [Google Scholar]
- 28.Kolesnick R, Golde D W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 29.Levade T, Salvayre R, Douste-Blazy L. Sphingomyelinases and Niemann-Pick disease. J Clin Chem Clin Biochem. 1986;24:205–220. [PubMed] [Google Scholar]
- 30.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 32.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin K-I, DiDonato J A, Hoffman A, Hardwick J M, Ratan R R. Suppression of steady-state, but not stimulus-induced NF-κB activity inhibits alphavirus-induced apoptosis. J Cell Biol. 1998;141:1479–1487. doi: 10.1083/jcb.141.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin K-I, Lee S H, Narayanan R, Baraban J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-kappa B. J Cell Biol. 1995;131:1–14. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Mathias S, Yang Z, Kolesnick R N. Renaturation and tumor necrosis factor-alpha stimulation of a 97-kDa ceramide-activated protein kinase. J Biol Chem. 1994;269:3047–3052. [PubMed] [Google Scholar]
- 36.Liu N, Brown D T. Phosphorylation and dephosphorylation events play critical roles in Sindbis virus maturation. Virology. 1993;196:703–711. doi: 10.1006/viro.1993.1527. [DOI] [PubMed] [Google Scholar]
- 37.Lozano J, Berra E, Municio M M, Diaz-Meco M T, Dominguez I, Sanz L, Moscat J. Protein kinase C zeta isoform is critical for kappa B-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 38.Marino M W, Dunbar J D, Wu L W, Ngaiza J R, Han H M, Guo D, Matsushita M, Nairn A C, Zhang Y, Kolesnick R, Jaffe E A, Donner D B. Inhibition of tumor necrosis factor signal transduction in endothelial cells by dimethylaminopurine. J Biol Chem. 1996;271:28624–28629. doi: 10.1074/jbc.271.45.28624. [DOI] [PubMed] [Google Scholar]
- 39.Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984;218:1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merrill A H, Jr, Jones D D. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990;1044:1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- 41.Merrill A H, Jr, Liotta D C, Riley R T. Fumonisins: fungal toxins that shed light on sphingolipid function. Trends Cell Biol. 1996;6:218–223. doi: 10.1016/0962-8924(96)10021-0. [DOI] [PubMed] [Google Scholar]
- 42.Moesby L, Corver J, Erukulla R K, Bittman R, Wilschut J. Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner. Biochemistry. 1995;34:10319–10324. doi: 10.1021/bi00033a001. [DOI] [PubMed] [Google Scholar]
- 43.Mooney J J, Dalrymple J M, Alving C R, Russell P K. Interaction of Sindbis virus with liposomal model membranes. J Virol. 1975;15:225–231. doi: 10.1128/jvi.15.2.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKC zeta is a molecular switch in signal transduction of TNF-alpha, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieva J-L, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obeid L M, Linardic C M, Karolak L A, Hannun Y A. Programmed cell death induced by ceramide. Science. 1993;259:1769–1772. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 48.Okazaki T, Bielawska A, Domae N, Bell R M, Hannun Y A. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutral sphingomyelinase activated in the early signal transduction of 1 alpha, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1994;269:4070–4077. [PubMed] [Google Scholar]
- 49.Quintern L E, Sandhoff K. Human acid sphingomyelinase from human urine. Methods Enzymol. 1991;197:536–540. doi: 10.1016/0076-6879(91)97180-7. [DOI] [PubMed] [Google Scholar]
- 50.Ramsey-Ewing A, Moss B. Apoptosis induced by a postbinding step of vaccinia virus entry into Chinese hamster ovary cells. Virology. 1998;242:138–149. doi: 10.1006/viro.1997.8985. [DOI] [PubMed] [Google Scholar]
- 51.Reyes J G, Robayna I G, Delgado P S, Gonzalez I H, Aguiar J Q, Rosas F E, Fanjul L F, Galarreta C M R. c-Jun is a downstream target for ceramide-activated protein phosphatase in A431cells. J Biol Chem. 1996;271:21375–21380. doi: 10.1074/jbc.271.35.21375. [DOI] [PubMed] [Google Scholar]
- 52.Santana P, Pena L A, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman E H, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 53.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 54.Schwandner R, Wiegmann K, Bernardo K, Kreder D, Kronke M. TNF receptor death domain-associated proteins TRADD and FADD signal activation of acid sphingomyelinase. J Biol Chem. 1998;273:5916–5922. doi: 10.1074/jbc.273.10.5916. [DOI] [PubMed] [Google Scholar]
- 55.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ubol S, Park S, Budihardjo I, Desnoyers S, Montrose M H, Poirier G G, Kaufmann S H, Griffin D E. Temporal changes in chromatin, intracellular calcium, and poly (ADP-ribose) polymerase during Sindbis virus-induced apoptosis of neuroblastoma cells. J Virol. 1996;70:2215–2220. doi: 10.1128/jvi.70.4.2215-2220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Veldhoven P P, Bishop W R, Yurivich D A, Bell R M. Ceramide quantitation: evaluation of a mixed micellar assay using E. coli diacylglycerol kinase. Biochem Mol Biol Int. 1995;36:21–30. [PubMed] [Google Scholar]
- 58.Verheij M, Bose R, Lin X-H, Yao B, Jarvis W D, Grant S, Birrer M, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–78. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 59.Wahlberg J M, Garoff H. Membrane fusion process of Semliki Forest virus I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992;116:339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiegmann K, Schütze S, Machleidt T, Witte D, Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 61.Yao B, Zhang Y, Delikat S, Mathias S, Basu S, Kolesnick R. Phosphorylation of Raf by ceramide-activated protein kinase. Nature. 1995;378:307–310. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]