Duodenal and gastric ulcers and gastric cancer are common and serious diseases but occur in only a minority of people infected with Helicobacter pylori. Mass eradication of H pylori is impractical because of the cost and the danger of generating antibiotic resistance, so we need to know how to target prophylaxis. Knowledge of the mechanisms that lead to ulcer formation or to gastric cancer in the presence of H pylori infection is therefore valuable.
Various factors affect the outcome of H pylori infection, including the host response and particularly the extent and severity of gastric inflammation and thus the amount of acid secreted by parietal cells. H pylori can elevate acid secretion in people who develop duodenal ulcers, decrease acid through gastric atrophy in those who develop gastric ulcers or cancer, and leave acid secretion largely unchanged in those who do not develop these diseases.
Regulation of gastric acid secretion
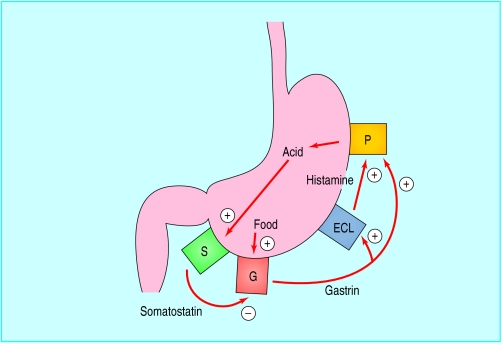
Several specialised cells in the gastric mucosa contribute to the control of acid secretion. G cells in the gastric antrum release the hormone gastrin. Gastrin acts on the enterochromaffin-like cells in the gastric corpus to release histamine, which stimulates parietal cells to secrete acid. Gastrin also stimulates parietal cells directly and promotes growth of enterochromaffin-like and parietal cells.
Histamine H2 receptor antagonists act by blocking the effect of histamine on parietal cells. Proton pump inhibitors act by inhibiting the enzyme in parietal cells that catalyses acid production for release into the gastric lumen. G cells, enterochromaffin-like cells, and parietal cells are all regulated by release of the inhibitory peptide somatostatin from somatostatin cells, which are distributed throughout the stomach. The effect of H pylori infection on acid secretion depends on which part of the stomach is most inflamed because this determines which of these cells are affected most.
H pylori related acid secretion
Hypersecretion in duodenal ulcer disease
Before the discovery of H pylori it was known that patients with duodenal ulcers secrete about twice as much acid as controls because they have twice as many parietal cells. Patients with gastric ulcer and those with functional dyspepsia have normal acid output and parietal cell count. Thus there was good evidence that acid played a major role in ulcer formation. Duodenal ulcers did not occur in achlorhydric people or in those secreting <15 mmol/h of acid. Duodenal ulcers can be healed, but not cured, by pharmacological suppression of acid secretion below this threshold.
Causes of duodenal ulcer
Helicobacter pylori antral gastritis
Non-steroidal anti-inflammatory drugs
Rare causes
Crohn's disease
HypergastrinaemiaIdiopathicGastrinoma
Hyperparathyroidism
Areas of gastric metaplasia in the duodenum can be colonised by H pylori, causing inflammation (duodenitis) and leading to further damage of the mucosa. The extent of gastric metaplasia is related to the amount of acid entering the duodenum—lowest in patients with pernicious anaemia who secrete no acid and highest in patients with acid hypersecretion due to gastrin-secreting tumours (Zollinger-Ellison syndrome). Acid hypersecretion in duodenal ulcer disease is virtually always due to H pylori infection because secretion returns to normal after the infection is eradicated. The predominantly antral gastritis in duodenal ulcer disease leads to acid hypersecretion by suppressing somatostatin cells and increasing gastrin release from the G cells in the gastric antrum.
Hyposecretion in patients at risk of gastric cancer
H pylori infection predisposes to distal gastric cancer, but patients who develop this complication have diminished acid secretion. Low acid secretion in gastric cancer was, until recently, thought to be predominantly due to gastric corpus gastritis, the associated gastric atrophy leading to loss of parietal cells. However, H pylori associated acid hyposecretion can in part be reversed by eradicating H pylori, suggesting that hyposecretion is due to inflammation rather than to permanent loss of cells.
H pylori associated acid hyposecretion may also be due to incomplete recovery from the loss of acid secretion that occurs with acute infection or may be in part genetically determined because it is more common in the first degree relatives of patients with gastric cancer.
Low acid secretion predisposes to gastric cancer by several mechanisms, including impaired absorption of vitamin C and overgrowth of salivary and intestinal bacteria within the stomach. By contrast, proximal gastric cancer (at the gastro-oesophageal junction) is associated with normal acid output. The rising incidence of this type of gastric cancer may reflect the decreasing prevalence of H pylori infection in Western societies.
Relation between distribution of gastritis and acid secretion
Thus distribution of H pylori gastritis determines acid secretion and the clinical outcome of H pylori infection, be that duodenal ulcer, gastric ulcer, gastric cancer, or asymptomatic infection. Positive feedback may perpetuate the different patterns of gastritis; for example, suppression of acid with a proton pump inhibitor diminishes antral gastritis but allows H pylori to colonise the corpus, which then becomes inflamed. This shows that acid secretion normally protects the corpus from H pylori infection. This effect has several important consequences:
High acid secretion in people with duodenal ulcers may be self perpetuating because it restricts gastritis to the antrum, leaving a healthy corpus to continue secreting acid
Low acid secretion may be self perpetuating because it increases corpus gastritis, which further depresses acid secretion
Proton pump inhibitors may be more effective in patients with H pylori infection than in those without because they promote corpus gastritis, which further inhibits acid secretion.
Aspects of the environment, bacterium, or host that affect acid output or the severity of corpus gastritis might steer a person infected with H pylori to a state of high acid secretion (predominantly antral gastritis) or to low acid secretion (predominantly corpus gastritis). This model is attractive because it allows studies of gastric physiology to be integrated with other equally important determinants of disease outcome.
Other factors that might affect gastric physiology and disease outcome
The pathogenic importance of H pylori depends on the interaction between bacterial virulence, the host, and the environment.
Host
Studies of identical and non-identical twins have shown that host factors are important in determining the outcome of infection. Duodenal ulcer is twice as common in those who are blood group O non-secretors. Studies in the mouse model of Helicobacter infection have shown that different strains of mice developed either severe gastritis or hardly any gastritis when infected with the same strain of Helicobacter. The genes responsible for the different outcomes are not known, but preliminary evidence suggests that a variety of genes involved in the inflammatory response affect the likelihood of H pylori infection progressing to duodenal ulcer disease.
Bacterium
In contrast, some investigators believe that H pylori is mainly responsible for disease because gastric mucosal inflammation is always present and fully resolves only when infection is successfully treated. Most strains of H pylori can be divided into two distinct phenotypes based on the presence or absence of a vacuolating toxin (Vac A toxin) and the products of the cag pathogenicity island (cagPI), a large chromosomal region that encodes virulence genes and is similar to that found in other enteric pathogens such as Escherichia coli and Salmonella typhi. People infected with strains of H pylori with the cagPI have more severe mucosal damage and are more likely to have duodenal ulcers or gastric cancer.
However, research has not so far identified H pylori genes that predispose to either duodenal ulcer or gastric cancer. Furthermore, in developing countries, where H pylori infects most of the population, cagPI strains of H pylori are present in almost all infected people but only a few develop clinical disease.
Summary points
Both duodenal ulcer and gastric ulcer are essentially gastric ulcers
They occur in gastric mucosa in the stomach or in gastric metaplasia mucosa in the duodenum
The mucosa may be attacked bySecretagogues (excess gastrin, histamine, or calcium producing excess of acid)Bacteria (H pylori)Drugs (non-steroidal anti-inflammatory drugs)
Identifying these and other bacterial virulence factors associated with more severe disease may allow screening tests to be developed. These may then permit identification of patients infected with “bad” bacteria so that eradication treatment can be targeted to them.
Environment
Gastric cancer is epidemiologically linked with diets high in salt and low in fresh fruit. Salt may change acid secretion because it suppresses parietal cells, and salty diets cause gastric atrophy. Conversely, the antioxidant vitamins in fresh fruit might protect specialised gastric cells from the reactive oxygen species released by inflammatory cells. A diet high in salt and lacking antioxidant vitamins might thus promote low acid secretion with corpus gastritis. Cigarette smoking strongly predisposes to both duodenal ulcer and gastric cancer.
Further reading
Calam J. Clinicians guide to Helicobacter pylori. London: Chapman and Hall, 1996
El-Omar EM, Penman ID, Ardill JES, Chittajallu RS, Howie C, McColl KEL. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 1995;109:681-91
Harris AW, Grummett PA, Misiewicz JJ, Baron JH. Eradication of Helicobacter pylori in patients with duodenal ulcers lowers basal and peak acid outputs in response to gastrin releasing peptide and pentagastrin. Gut 1996;38:663-7
Logan RPH, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, Baron JH. Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole. Gut 1995;36:12-6
Saponin P, Hyvarinen H, Psoralea M. H pylori corpus gastritis— relation to acid output. J Physiol Pharmacol 1996;47:151-9
Time and geographical trends
The factors described above might explain some geographical differences and changes with time in the prevalence of the different upper gastrointestinal diseases. For example, a high prevalence of H pylori infection plus a traditional salty diet might explain the high prevalence of gastric cancer in Japan and China. Rates of acid secretion have risen recently in Japan, perhaps because of a fall in the prevalence of H pylori or some Westernisation of the diet. In the United Kingdom the replacement of salt with refrigeration to preserve food might have accounted for the rise in the prevalence of duodenal ulcer disease in the middle of this century, as the gastric corpus became healthier and acid secretion higher.
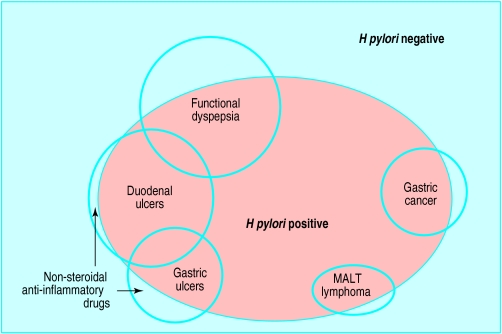
Figure.
Relation of H pylori infection to upper gastrointestinal conditions
Figure.
Autoregulation of acid secretion. Food stimulates release of gastrin from antral G cells (G). Gastrin stimulates enterochromaffin-like cells (ECL) to release histamine, which stimulates parietal cells (P) in the gastric corpus to secrete acid. Acid stimulates release of somatostatin from somatostatin cells (S) in the antrum, inhibiting further gastrin release
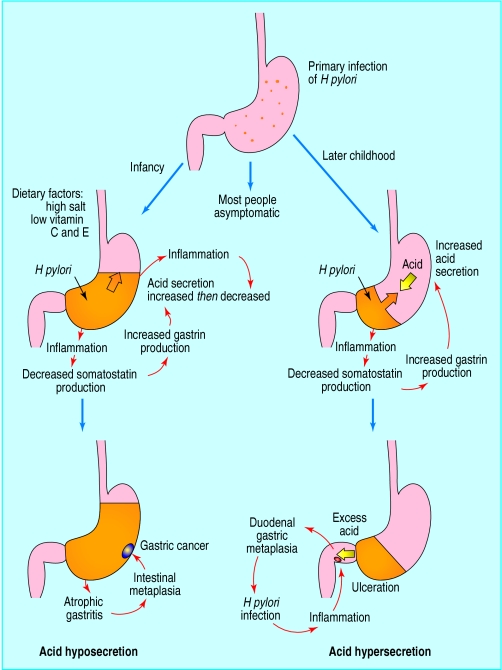
Figure.
With acid hyposecretion (left), the main effect of H pylori gastritis affecting the gastric body is to suppress parietal cells, leading to low acid secretion, which is associated with gastric cancer. With acid hypersecretion (right), antral H pylori gastritis increases acid secretion by suppressing somatostatin and elevating gastrin release, increasing the risk of duodenal ulceration. Orange areas indicate extent and location of gastritis
Figure.
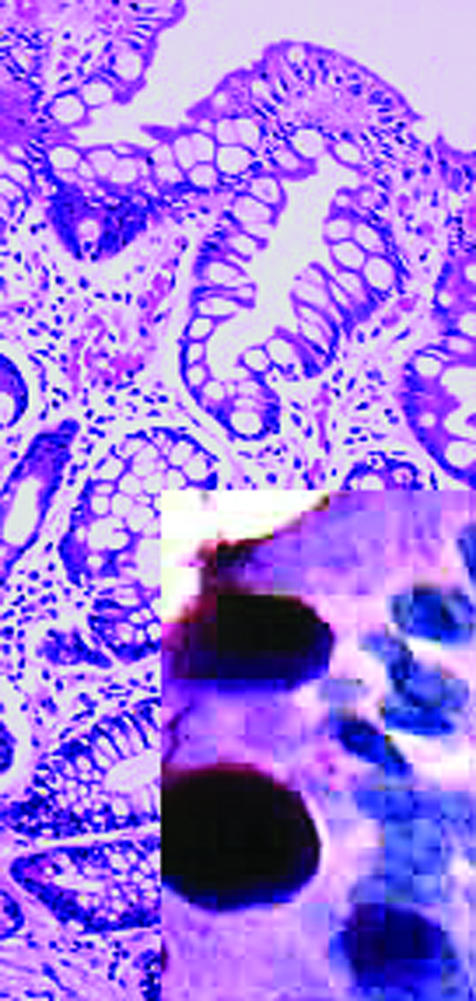
Intestinal metaplasia of antral mucosa. Inset shows large goblet cells packed with mucin (shown by Alcian blue staining)
Figure.
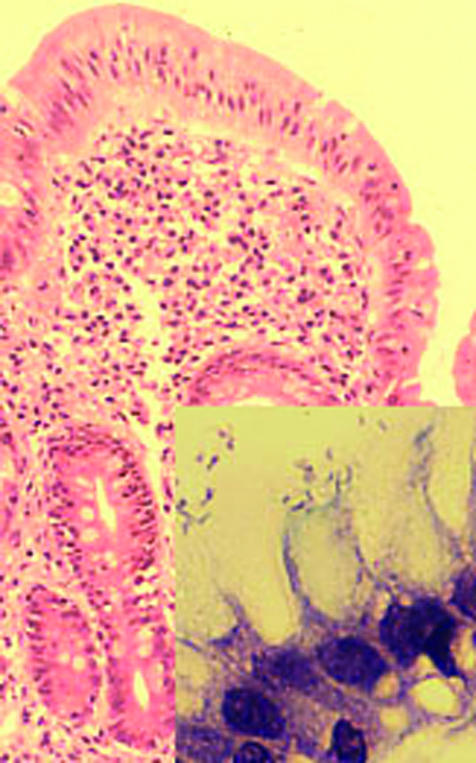
Duodenum with gastric metaplasia and mild inflammation. Inset shows H pylori adhering to surface epithelial cells
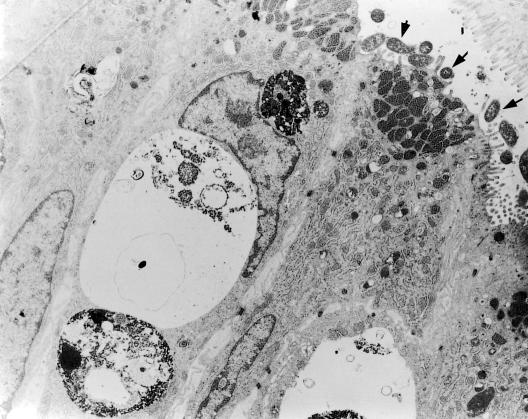
Figure.
Transmission electron micrograph of duodenal gastric metaplasia with H pylori attached to the apical surface (arrows)
Footnotes
John Calam was professor of gastroenterology at Imperial College School of Medicine, Hammersmith Hospital, London. J H Baron is honorary professorial lecturer at Mount Sinai School of Medicine, New York, USA, and former consultant gastroenterologist, St Mary's Hospital, London.
The ABC of the upper gastrointestinal tract is edited by Robert Logan, senior lecturer in the division of gastroenterology, University Hospital, Nottingham, Adam Harris, consultant physician and gastroenterologist, Kent and Sussex Hospital, Tunbridge Wells, J J Misiewicz, honorary consultant physician and honorary joint director of the department of gastroenterology and nutrition, Central Middlesex Hospital, London, and J H Baron. The series will be published as a book in Spring 2002.