Abstract
Introduction
Diabetic kidney disease (DKD) is an important complication of diabetes as it results in end-stage renal disease; hence, several drugs have been developed for its treatment. However, even with treatment with renin-angiotensin system inhibitors and sodium-glucose cotransporter-2 inhibitors, the residual risk of DKD remains. While this risk is an issue, the renoprotective effects of finerenone, a novel non-steroidal mineralocorticoid receptor antagonist, are becoming evident. High proteinuria increases the risk of cardiovascular death as well as renal failure. Hence, it is especially important to address cases of urine protein to nephrotic levels in DKD, however, no previous studies have assessed the safety and efficacy of finerenone in patients with DKD and nephrotic syndrome. Therefore, this study aimed to assess whether finerenone has a renoprotective effect in advanced DKD complicated by nephrotic syndrome.
Methods
Nine patients with DKD and nephrotic syndrome who received 10–20 mg/day of finerenone were retrospectively analyzed. The average observation period was 9.7 ± 3.4 months. Patients with serum potassium levels greater than 5.0 mEq/L at the start of finelenone were excluded. Changes in urinary protein levels, estimated glomerular filtration rate (eGFR), and serum potassium levels were studied before and after finerenone administration.
Results
The mean changes in the urinary protein creatinine ratio (UPCR) at baseline were 6.6 ± 2.0. After finerenone treatment, the mean UPCR decreased to −0.6 ± 3.9; however, this change was not statistically significant.
The eGFR decline slope also tended to decrease with finerenone treatment (before vs. after: 3.1 ± 4.9 vs. −1.7 ± 3.2 mL/min/1.73 m2. Furthermore, finerenone did not increase serum potassium levels.
Conclusions
Patients treated with finerenone showed decreased UPCR; hence, it is suggested that finerenone may be effective in treating nephrotic syndrome in patients with DKD. These findings may be applicable to real-world clinical settings. Nonetheless, it is important to note that this study was a retrospective analysis of a single-center cohort and had a limited sample size, highlighting the need for additional large-scale investigations.
Keywords: Diabetic kidney disease, Estimated glomerular filtration rate, Finerenone, Nephrotic syndrome, UPCR
1. Introduction
Type 2 diabetes is the leading cause of diabetic kidney disease (DKD), which is the most important important chronic kidney disease (CKD) and a significant microvascular complication of diabetes that escalates the overall disease burden worldwide [1,2]. The renin-angiotensin system (RAS) is known to pivotal role in developing DKD. The blockade of the RAS, using angiotensin-converting enzyme inhibitors (ACEIs) and later transitioning to angiotensin receptor blockers (ARBs), as the standard of care has effectively slowed chronic kidney disease (CKD) progression in individuals with diabetes and decreased the occurrence of renal failure since the early 2000s; however, there have been no subsequent therapeutic breakthroughs in this regard [3,4].
Additionally, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose cotransporter-2 (SGLT2) inhibitors are used in patients with DKD; The Empagliflozin and Progression of Kidney Disease in type 2 diabetes (The EMPA-REG Renal OUTCOME trial) and the CANagliflozin cardioVascular Assessment Study (CANVAS) program distinctly demonstrated the renal protective benefits of SGLT2 inhibitors in individuals with type 2 diabetes. These benefits encompass reductions in albuminuria, nephropathy progression, doubling of serum creatinine levels, and the onset of renal replacement therapy [[5], [6], [7], [8], [9]].
Incretin-related drugs exert vasotropic effects, mitigate oxidative stress or inflammation, and improve DKD [10]. Recently, large-scale clinical trials have revealed that SGLT2 inhibitors effectively decelerate DKD progression [7,11]. Despite significant advances, there has been limited progress in the prevention and treatment of DKD; hence, residual risks remain.
The hyperactivation of mineralocorticoid receptors contributes to increased fibrosis, inflammation, and decreased renal function. Therefore, mineralocorticoid receptor antagonists (MRAs), whether used independently or in combination with ACEIs, have been found to decrease albuminuria and slow renal function decline in individuals with DKD [12]. However, while the renoprotective benefits of MRAs have been acknowledged, they have not been recognized as a viable treatment for DKD because of concerns about potential risks such as hyperkalemia and acute kidney injury (AKI). On the other hand, finerenone, a selective non-steroidal MRA, has proven to be effective in diminishing albuminuria and halting the transition from acute kidney injury (AKI) to chronic kidney disease (CKD) [13].
Moreover, finerenone has shown efficacy in attenuating the deterioration of estimated glomerular filtration rate (eGFR) and reducing albuminuria. The Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD) trial illustrated that finerenone decreased the incidence of cardiovascular events among those with DKD, whereas in the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial, it enhanced cardiovascular and renal outcomes in individuals with advanced CKD [14,15]. Furthermore, our study showed that finerenone slows down the rate of eGFR decline in patients with DKD with an eGFR of less than 25 [16]. Therefore, the main treatment options for DKD comprise RAS inhibitors, SGLT2 inhibitors, therapies based on incretin, and non-steroidal MRAs, collectively referred to as the “Fantastic Four” for DKD [7].
In DKD, renal prognosis is poor when individuals present with large amounts of urinary protein [17]. However, the safety and efficacy of finerenone in patients with DKD complicated by nephrotic syndrome remains unclear.
Furthermore, even FIGARO-DKD and FIDELIO-DKD do not have data restricted to patients with nephrotic syndrome. Therefore, this study aimed to determine whether finerenone has a renoprotective effect in advanced DKD complicated by nephrotic syndrome. To the best of our knowledge, this is the first study to investigate the efficacy and safety of finerenone in patients with DKD and nephrotic syndrome.
2. Patients and methods
2.1. Study design and collection of medical data
We conducted a retrospective examination and analysis of the medical documentation of nine individuals who received outpatient treatment at the Department of Nephrology, Osaka Medical and Pharmaceutical University (Osaka, Japan), was reviewed. Eligible participants were individuals diagnosed with DKD who were 20 years old or more with nephrotic syndrome. The diagnosis of DKD followed the National Health and Nutrition Examination Surveys (NHANES) criteria [18]. Urinary protein creatinine ratio (UPCR) was calculated by dividing the level of protein (mg/dL) in a spot urine test by the creatine (Cr) level (mg/dL) and nephrotic syndrome was defined as a UPCR of 3.5 g/g Cr or higher [19]. With reference to FIGALO-DKD and FIDELIO-DKD, prospective participants had to exhibit serum potassium levels below 4.9 mEq/L during the screening visit, preferably without any modifications to dosage, drug choice, or other interventions associated with antihypertensive or antidiabetic therapies.
All procedures involving human participants adhered to the ethical standards of the National Research Committee and the 1964 Declaration of Helsinki, along with its subsequent amendments or comparable ethical standards.
The requirement for informed consent was waived, our institution does not require approval from the university's ethics committee for clinical study with small sample numbers, as per the Ethical Guidelines for Medical and Health Research Involving Human Subjects from the Japanese Ministry of Health, Labour and Welfare. The patient provided written informed consent.
Data for the study was gathered retrospectively and assessed utilizing the electronic medical records upheld by Osaka Medical and Pharmaceutical University. To ensure the accuracy and reliability of the data collected, our multi-person research team performed data verification. Blood and urine biochemical analyses were conducted utilizing a Labospect 008 autoanalyzer (Hitachi, Tokyo, Japan) [16,20]. Information from the electronic medical records included data on serum Cr, serum potassium, total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, UPCR, and patient attributes such as age, sex, blood pressure, and medication. The eGFR of each patient was calculated using the isotope dilution mass spectrometry (IDMS) traceable 4-variable MDRD (Modification of Diet in Renal Disease) (IDMS-MDRD Study formula) formula with the 3-variable Japanese equation: 194 × serum Cr - 1.094 × age - 0.287 × 0.739 (if female) [[21], [22], [23], [24]]. These research methods are similar to our previous study with finerenone [16].
2.2. Data analysis
The mean changes in proteinuria, mean eGFR decline slope, and changes in serum potassium levels were compared before, at the time of, and after finerenone treatment. Statistical significance was determined using the Wilcoxon test. All analyses were performed using StatView (SAS Institute) and Excel. Statistical significance was set at a P-value of less than 0.05.
3. Results
Table 1 shows the baseline characteristics of the nine patients (five males and four females). The average age at onset was 69.2 ± 9.2 years. The median duration of diabetes was 12 years. The mean baseline systolic blood pressure was 130 ± 18 mmHg. The baseline blood parameters were: glycosylated hemoglobin level 6.3 ± 0.5 %, serum potassium 4.3 ± 0.4 mEq/L, total cholesterol 181 ± 45 mg/dL, triglyceride 149 ± 100 mg/dL, and HDL cholesterol 55 ± 18 mg/dL. The average baseline renal function was: eGFR 23.6 ± 9.4 mL/min/1.73 m2. All patients were treated with finerenone (10–20 mg/day). Concomitant medications included: biguanide (11.1 %), imeglimin (11.1 %), dipeptidyl peptidase 4 inhibitors (22.2 %), SGLT2 inhibitors (66.7 %), GLP-1 receptor agonists (44.4 %), insulin (11.1 %), statins (55.6 %), eicosapentaenoic acid (22.2 %), ARBs (22.2 %), angiotensin receptor neprilysin inhibitors (27.4 %), calcium channel blockers (88.9 %), α-blockers (22.2 %), β-blockers (11.1 %), αβ-blockers (27.3 %), thiazides (22.2 %), loop diuretics (27.3 %), tolvaptan (44.4 %), and hypoxia inducible factors-prolyl hydroxylase inhibitors (27.3 %).
Table 1.
Clinical baseline characteristics of the patients.
| Variables | |
|---|---|
| Number of patients, n | 9 |
| Men/women, n | 4/5 |
| Age, years (mean) | 69.2 (9.2) |
| Duration of diabetes, years (median) | 12 [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18]] |
| HbA1c, % (mean) | 6.3 (0.5) |
| Serum potassium, mEq/L (mean) | 4.3 (0.4) |
| eGFR, mL/min/1.73m2 (mean) | 23.6 (9.4) |
| UPCR, g/gCr (mean) | 6.6 (2.0) |
| Systolic BP, mmHg (mean) | 130 (18) |
| TC, mg/dL (mean) | 181 (45) |
| TG, mg/dL (mean) | 149 (100) |
| HDL-C, mg/dL (mean) | 55 (18) |
| Medications | |
| Biguanide, n (%) | 1 (11.1) |
| Imeglimin, n (%) | 1 (11.1) |
| DPP4 inhibitor, n (%) | 2 (22.2) |
| SGLT2 inhibitor, n (%) | 6 (66.7) |
| GLP-1RA, n (%) | 4 (44.4) |
| Insulin, n (%) | 1 (11.1) |
| Statin, n (%) | 5 (55.6) |
| EPA, n (%) | 2 (22.2) |
| ARB, n (%) | 2 (22.2) |
| ARNI, n (%) | 3 (27.3) |
| Ca-blocker, n (%) | 8 (88.9) |
| α-blocker, n (%) | 2 (22.2) |
| β-blocker, n (%) | 1 (11.1) |
| αβ-blocker, n (%) | 4 (44.4) |
| Thiazide, n (%) | 2 (22.2) |
| Loop diuretics, n (%) | 3 (27.3) |
| Tolvaptan, n (%) | 4 (44.4) |
| HIF–PHI | 3 (27.3) |
HbA1c, glycated hemoglobin; eGFR, estimated glomerular filtration rate; UPCR, urine protein-creatinine ratio; BP, blood pressure; TC, total cholesterol; TG, triglyceride, HDL-C, high-density lipoprotein cholesterol; DPP4, dipeptidyl peptidase 4; SGLT2, sodium-glucose cotransporter 2; GLP-1RA, glucagon-like peptide-1 receptor agonist; EPA, eicosapentaenoic acid; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; Ca, calcium; HIF–PHI, Hypoxia inducible factors-prolyl hydroxylase inhibitor.
Age, HbA1c, serum potassium, eGFR, UPCR, systolic BP, TC, TG, HDL-C were expressed as mean ± standard deviation. Duration of diabetes was expressed as median.
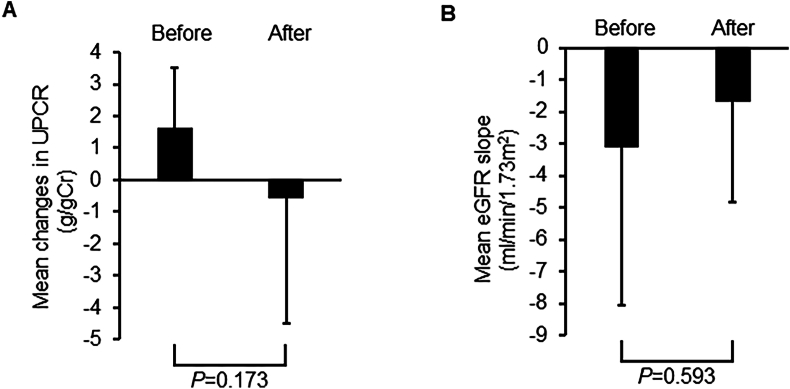
The mean baseline UPCR was 6.6 ± 2.0 g/g Cr. UPCR, which was increased before finerenone administration, tended to decrease with finerenone treatment, albeit without statistical significance (before vs. after: 1.6 ± 1.9 vs. −0.6 ± 3.9 g/g Cr, respectively, P = 0.593, Fig. 1A). Moreover, while the eGFR decline slope showed a tendency to decrease with finerenone treatment, this change did not reach statistical significance (before vs. after: 3.1 ± 4.9 vs. −1.7 ± 3.2 mL/min/1.73 m2, respectively, P = 0.173, Fig. 1B).
Fig. 1.
Mean changes in (A) urinary protein creatinine ratio (UPCR) and (B) estimated glomerular filtration rate (eGFR) before and after administration of finerenone. Cr: creatinine.
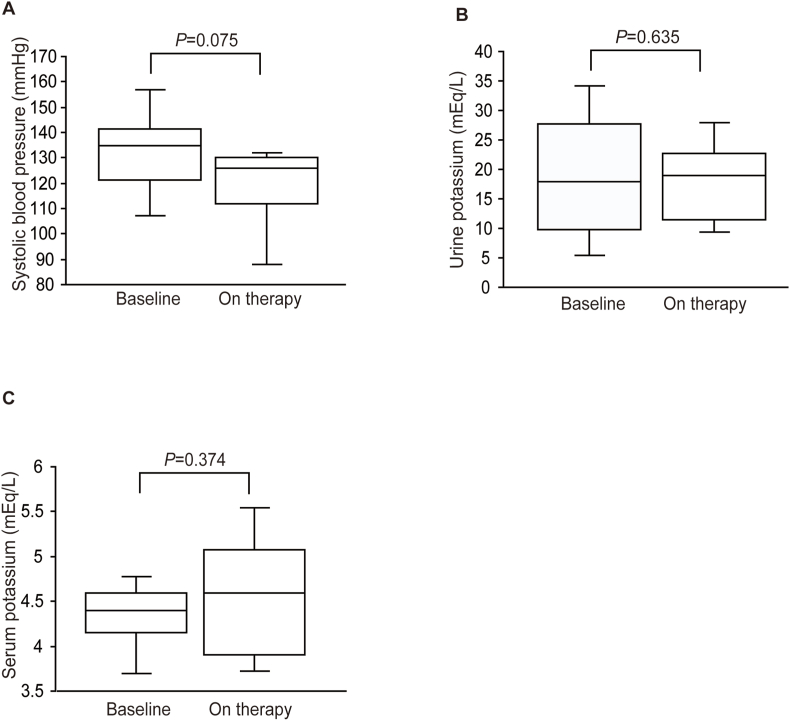
Finerenone treatment did not change systolic blood pressure (before vs. after: 130 ± 18 vs. 118 ± 18 mmHg, respectively; P = 0.075; Fig. 2A). Furthermore, finerenone did not affect urinary excretion of potassium (before vs. after: 19.1 ± 10.8 vs. 17.8 ± 7.4 mEq/L, respectively, P = 0.635, Fig. 2B). Finally, there was no discernible distinction noted in serum potassium levels before and after finerenone administration from baseline (4.3 ± 0.3 vs. 4.6 ± 0.7 mEq/L, respectively, P = 0.374, Fig. 2C).
Fig. 2.
Box plots showing changes in (A) systolic blood pressure, (B) urine potassium, and (C) serum potassium upon finerenone treatment during the observation period.
4. Discussion
To the best of our knowledge, this is the first study to reveal the renal effect of finerenone in individuals with DKD and nephrotic syndrome. Our findings suggest that the use of finerenone results in reduced proteinuria in individuals with DKD.
In our previous study, we have shown that finerenone moderates the eGFR lowering slope in DKD patients with eGFR below 25. However, the urinary protein reduction was not clear in that no statistically significant differences occurred [16]. The present study is similar to the previous one, except that only the group of DKD patients presenting with nephrotic syndrome was included.
The aim of the FIDELIO-DKD trial was to assess whether finerenone could slow CKD progression or reduce the occurrence of cardiovascular complications and mortality in individuals with type 2 diabetes who were prescribed the maximum recommended dose of ACEIs or ARBs. The primary goals of this research were to evaluate renal impairment, characterized by end-stage renal disease or eGFR falling below 15 mL/min/1.73 m2, a decline in eGFR exceeding 40 % from the baseline, and death from renal causes. The primary outcome was a statistically significant reduction in the finerenone group [15]. However, unlike our study, this trial omitted patients with a urine albumin-to-creatinine ratio (UACR) greater than 5000 mg/g Cr [15].
The FIGARO-DKD trial investigated the efficacy and safety of finerenone in enhancing cardiovascular and renal outcomes in individuals with CKD and type 2 diabetes. The study participants were categorized into two groups: those with a UACR ranging 30–300 mg/g Cr and those with an eGFR exceeding 60 mL/min/1.73 m2. This suggests that the FIGARO-DKD trial enrolled a higher proportion of individuals with DKD than did the FIDELIO-DKD trial, with rates of 9.5 % in the finerenone group and 10.8 % in the placebo group (hazard ratio [HR]: 0.87; 95 % confidence interval [CI]: 0.76–1.01) [14]. Their findings demonstrated that finerenone exerts protective effects on the kidneys of patients with DKD. However, the degree of albuminuria was not significantly different in either study. This underscores the importance of this study as the first to identify the renoprotective effects of finerenone in patients diagnosed with DKD and nephrotic syndrome.
The Finerenone in Chronic Kidney Disease and Type 2 Diabetes (FIDELITY) study, which integrated data from the FIDELIO-DKD and FIGARO-DKD trials, combined both trials to assess the impact of finerenone on renal and cardiac health. This comprehensive analysis involved approximately 13,000 participants with a median follow-up of 3 years. Results from this study revealed that finerenone significantly reduced the risk compared to the placebo across all combined cardiovascular and renal endpoints. Specifically, there was a notable 14 % reduction in the risk of composite cardiovascular events (HR: 0.86; 95 % CI: 0.78–0.95) and a significant 23 % reduction in composite renal outcomes (HR: 0.77; 95 % CI: 0.67–0.88). Of particular importance was the finding of a 20 % decrease in the risk of end-stage renal disease requiring dialysis (HR: 0.80; 95 % CI: 0.64–0.99) [25].
The slight alterations in blood pressure noted among the study participants imply that the cardiovascular and renal protective benefits of finerenone may not be solely linked to blood pressure modifications. Furthermore, it is important to underscore the originality and distinctiveness of our study, particularly akin to the FIGARO-DKD and FIDELIO-DKD trials mentioned earlier as they omitted individuals in the nephrotic range from the analysis.
Recent large-scale clinical trials, such as the Empagliflozin and Progression of Kidney Disease in type 2 diabetes (EMPA-REG Renal OUTCOME), CANagliflozin cardioVascular Assessment Study (CANVAS) program, and Dapagliflozin Effect on Cardiovascular Events-Thromolysis in Myocardial Infarction 58 (DECLARE-TIMI 58), involving SGLT2 inhibitors, have demonstrated enhanced renal outcomes among patients with type 2 diabetes. Nonetheless, these trials were not specifically structured to assess the renoprotective effects of SGLT2 inhibitors, and the patients included had a comparatively low risk of exacerbating CKD [6,26,27]. On the other hand, the CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) trial showed a protective effect of canagliflozin in patients with type 2 diabetes mellitus [28,29].
GLP-1 exerts renoprotective effects against DKD. Specifically, GLP-1 reduces inflammation and oxidative stress markers in glomerular endothelial cells [9]. GLP-1 receptors are primarily detected in renal glomeruli rather than in kidney tubules [30]. Notably, the findings of our previous study indicated a decrease in GLP-1 receptor expression within the renal cortex of individuals with long-standing type 1 diabetes (The Joslin 50-Year Medalist Study; Mima A and King GL, unpublished observation). This study demonstrated that GLP-1 receptor agonists activate the cyclic adenosine monophosphate/protein kinase A transduction pathway, leading to increased phospho-c-Raf (Ser259) levels, through protein kinase C (PKC) β activity which inhibits phopho-c-Raf (Ser338)/PAI-1. Furthermore, we showed that activation of PKCβ induced by hyperglycemia can inhibit the protective effect of GLP-1 and induce an inflammatory response in the glomerular endothelial cells through two independent mechanisms: PKCβ enhanced action of the angiotensin II and reduced GLP-1 receptors [30]. Although the renoprotective effect of GLP-1 receptor agonists through such anti-inflammatory effects may require a longer administration period, they may be obtained even with relatively short-term administration of GLP-1. This could occur via the diuretic actions of inhibiting sodium-hydrogen exchanger 3 and SGLT2, along with dampening the activity of the sympathetic nervous system [31].
There are several possible mechanisms of renal protection by finerenone; it has been reported that finerenone could decrease the expression of fibrosis markers in rat models of CKD and to confer renoprotective effects by increasing renal blood flow [32,33].
In the FIDELIO-DKD study, the efficacy of SGLT2 inhibitors and GLP-1 receptor agonists combined with finerenone was unclear [15]. In our study, both drugs were administered; however, their efficacy was similar to that of the FIDELIO-DKD study. However, the small number of combinations in this study and the FIDELIO-DKD study warrant further investigation.
The 2023 clinical guidelines of the American Diabetes Association (ADA) in 2023 suggest prescribing finerenone to individuals with DKD who exhibit albuminuria and are already receiving the highest recommended doses of RAS inhibitors [34]. This recommendation aims to enhance cardiovascular health and mitigate DKD progression. Furthermore, the Kidney Disease Improving Global Outcomes 2024 clinical practice guidelines for the management of CKD with type 2 diabetes recommend considering the utilization of nonsteroidal MRAs [35].
This study had a few limitations. First, this was a retrospective analysis of a single-center cohort with a limited number of patients. Second, the study had a short follow-up period and was of a non-randomized controlled trial design. These are considered potential biases, further affecting the reliability of the results.
5. Conclusion
In this study we found that patients with DKD and nephrotic syndrome had a decrease in the UPCR in the finerenone therapy group, albeit without statistical significance. Although this study demonstrated the safety and efficacy of finerenone in patients with DKD and nephrotic syndrome, further studies with larger sample sizes are needed.
Funding
This study was supported by JSPS KAKENHI (grant number: 22K08368) (to A. Mima).
Medical writing, editorial, and other assistance
The authors would like to thank Ms. Ayumi Noguchi for her contribution in preparing the clinical data and her insights into the statistical analysis. In addition, we extend our appreciation to Honyaku Center, Inc., for their assistance with English language editing.
Ethics/ethical approval
All procedures involving human participants adhered to the ethical standards of the National Research Committee and the 1964 Declaration of Helsinki, along with its subsequent amendments or comparable ethical standards. The requirement for informed consent was waived, and the institutional review board did not perform an ethical review due to the small sample size.
Data availability
Data are available from the author upon reasonable request.
CRediT authorship contribution statement
Akira Mima: Writing – original draft, Software, Methodology, Data curation, Conceptualization. Yuta Saito: Visualization, Validation, Software, Investigation. Keishi Matsumoto: Writing – review & editing, Visualization. Takahiro Nakamoto: Writing – review & editing, Visualization, Investigation. Shinji Lee: Writing – review & editing, Visualization, Supervision, Investigation.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used ChatGPT in order to text proofreading. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Declaration of competing interest
A. Mima received speaker honorariums from Novartis, Kyowa Kirin, Bayer, Eli Lilly, Mochida, and Boehringer Ingelheim. A. Mima has also received research grants from Sumitomo Pharma, Chugai, Torii, and Mochida.
References
- 1.Diabetes C., Complications Trial Research G., Nathan D.M., Genuth S., Lachin J., Cleary P., et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C.J., Grant P.J. The UK prospective diabetes study. Lancet. 1998;352:1932. doi: 10.1016/S0140-6736(98)00090-7. author reply 4. [DOI] [PubMed] [Google Scholar]
- 3.Brenner B.M., Cooper M.E., de Zeeuw D., Keane W.F., Mitch W.E., Parving H.H., et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.Lewis E.J., Hunsicker L.G., Clarke W.R., Berl T., Pohl M.A., Lewis J.B., et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 5.Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 6.Neal B., Perkovic V., Matthews D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 7.Mima A. A narrative review of diabetic kidney disease: previous and current evidence-based therapeutic approaches. Adv Ther. 2022;39:3488–3500. doi: 10.1007/s12325-022-02223-0. [DOI] [PubMed] [Google Scholar]
- 8.Mima A., Yasuzawa T., Nakamura T., Ueshima S. Linagliptin affects IRS1/Akt signaling and prevents high glucose-induced apoptosis in podocytes. Sci Rep. 2020;10:5775. doi: 10.1038/s41598-020-62579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mima A., Nomura A., Fujii T. Current findings on the efficacy of incretin-based drugs for diabetic kidney disease: a narrative review. Biomed Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115032. [DOI] [PubMed] [Google Scholar]
- 10.Mima A., Gotoda H., Lee R., Murakami A., Akai R., Lee S. Effects of incretin-based therapeutic agents including tirzepatide on renal outcomes in patients with type 2 diabetes: a systemic review and meta-analysis. Metabol Open. 2023;17 doi: 10.1016/j.metop.2023.100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mima A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabet Complicat. 2018;32:720–725. doi: 10.1016/j.jdiacomp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Goenka L., Padmanaban R., George M. The ascent of mineralocorticoid receptor antagonists in diabetic nephropathy. Curr Clin Pharmacol. 2019;14:78–83. doi: 10.2174/1574884713666181116100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitt B., Kober L., Ponikowski P., Gheorghiade M., Filippatos G., Krum H., et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitt B., Filippatos G., Agarwal R., Anker S.D., Bakris G.L., Rossing P., et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 15.Bakris G.L., Agarwal R., Anker S.D., Pitt B., Ruilope L.M., Rossing P., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 16.Mima A., Lee R., Murakami A., Gotoda H., Akai R., Kidooka S., et al. Effect of finerenone on diabetic kidney disease outcomes with estimated glomerular filtration rate below 25 mL/min/1.73 m(2) Metabol Open. 2023;19 doi: 10.1016/j.metop.2023.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iimori S., Naito S., Noda Y., Sato H., Nomura N., Sohara E., et al. Prognosis of chronic kidney disease with normal-range proteinuria: the CKD-ROUTE study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afkarian M., Zelnick L.R., Hall Y.N., Heagerty P.J., Tuttle K., Weiss N.S., et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginsberg J.M., Chang B.S., Matarese R.A., Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 20.Mima A. Enarodustat treatment for renal anemia in patients with non-dialysis chronic kidney disease. In Vivo. 2023;37:825–829. doi: 10.21873/invivo.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai E., Horio M., Nitta K., Yamagata K., Iseki K., Tsukamoto Y., et al. Modification of the modification of Diet in renal disease (MDRD) study equation for Japan. Am J Kidney Dis. 2007;50:927–937. doi: 10.1053/j.ajkd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S., Imai E., Horio M., Yasuda Y., Tomita K., Nitta K., et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Mima A. Prediction of decreased estimated glomerular filtration rate using liver fibrosis markers: a renal biopsy-based study. Sci Rep. 2022;12 doi: 10.1038/s41598-022-22636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mima A., Murakami A., Lee R., Lee S. Predictive significance of glomerular insulin receptor substrate-1 in patients with diabetic kidney disease. Metabol Open. 2023;18 doi: 10.1016/j.metop.2023.100240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal R., Filippatos G., Pitt B., Anker S.D., Rossing P., Joseph A., et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474–484. doi: 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wanner C., Inzucchi S.E., Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–1802. doi: 10.1056/NEJMc1611290. [DOI] [PubMed] [Google Scholar]
- 27.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 28.Perkovic V., Jardine M.J., Neal B., Bompoint S., Heerspink H.J.L., Charytan D.M., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 29.Mima A. Sodium-glucose cotransporter 2 inhibitors in patients with non-diabetic chronic kidney disease. Adv Ther. 2021;38:2201–2212. doi: 10.1007/s12325-021-01735-5. [DOI] [PubMed] [Google Scholar]
- 30.Mima A., Hiraoka-Yamomoto J., Li Q., Kitada M., Li C., Geraldes P., et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes. 2012;61:2967–2979. doi: 10.2337/db11-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muskiet M.H.A., Tonneijck L., Smits M.M., van Baar M.J.B., Kramer M.H.H., Hoorn E.J., et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13:605–628. doi: 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 32.Kolkhof P., Delbeck M., Kretschmer A., Steinke W., Hartmann E., Barfacker L., et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78. doi: 10.1097/FJC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 33.Lattenist L., Lechner S.M., Messaoudi S., Le Mercier A., El Moghrabi S., Prince S., et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension. 2017;69:870–878. doi: 10.1161/HYPERTENSIONAHA.116.08526. [DOI] [PubMed] [Google Scholar]
- 34.ElSayed N.A., Aleppo G., Aroda V.R., Bannuru R.R., Brown F.M., Bruemmer D., et al. 10. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46:S158–S190. doi: 10.2337/dc23-S010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidney Disease: Improving Global Outcomes CKDWG KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105:S117–S314. doi: 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the author upon reasonable request.